Abstract
Aim
The present study aimed to identify risk factors for venous thromboembolism (VTE) after pancreaticoduodenectomy (PD) and to develop and internally validate a predictive model for the risk of venous thrombosis.
Methods
We retrospectively collected data from 352 patients who visited our hospital to undergo PD from January 2018 to March 2022. The number of patients recruited was divided in an 8:2 ratio by using the random split method, with 80% of the patients serving as the training set and 20% as the validation set. The least absolute shrinkage and selection operator (Lasso) regression model was used to optimize feature selection for the VTE risk model. Multivariate logistic regression analysis was used to construct a prediction model by incorporating the features selected in the Lasso model. C-index, receiver operating characteristic curve, calibration plot, and decision curve were used to assess the accuracy of the model, to calibrate the model, and to determine the clinical usefulness of the model. Finally, we evaluated the prediction model for internal validation.
Results
The predictors included in the prediction nomogram were sex, age, gastrointestinal symptoms, hypertension, diabetes, operative method, intraoperative bleeding, blood transfusion, neutrophil count, prothrombin time (PT), activated partial thromboplastin time (APTT), aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio (AST/ALT), and total bilirubin (TBIL). The model showed good discrimination with a C-index of 0.827, had good consistency based on the calibration curve, and had an area under the ROC curve value of 0.822 (P < 0.001, 95%confidence interval:0.761–0.882). A high C-index value of 0.894 was reached in internal validation. Decision curve analysis showed that the VTE nomogram was clinically useful when intervention was decided at the VTE possibility threshold of 10%.
Conclusion
The novel model developed in this study is highly targeted and enables personalized assessment of VTE occurrence in patients who undergo PD. The predictors are easily accessible and facilitate the assessment of patients by clinical practitioners.
Keywords: Pancreaticoduodenectomy, Venous thromboembolism, Prediction model, Nomogram
Introduction
Pancreaticoduodenectomy (PD) is the primary treatment for benign and malignant tumors involving the head of the pancreas and ampulla of the duodenum and for severe pancreatic and duodenal injuries [1, 2]. It was first performed and reported by Whippe [3] in 1935. It is known as the ceiling of surgery because of the anatomical complexity of the surgical site, the wide range of the operative area, the difficulty of anastomosis, and the high anatomical and technical requirements for the surgeon. With the constant update of surgeons’ knowledge and advancements in surgical techniques, the safety of surgery has greatly improved, and the mortality rate associated with the surgery has gradually reduced, with a reported mortality rate of < 6% [4]; moreover, the scope of application has gradually widened. However, the postoperative complications of PD include postoperative pancreatic fistula, biliary fistula, hemorrhage, abdominal infection, delayed gastric emptying after surgery, and venous thrombosis [5], which lead to longer hospitalization days and higher hospitalization costs, thereby delaying patients’ recovery and increasing their financial burden.
Venous thromboembolism (VTE) is a common disease that mainly includes deep vein thromboembolism (DVT) and pulmonary embolism (PE), but it may also occur in visceral veins and cerebral veins [6]. Risk factors of the above diseases are common, including any factor can cause venous blood stasis, venous endothelial injury, and thrombophilia, are common and classified either hereditary or acquired. Among the hereditary risk factors, attention should be paid to factors that are relevant to embolic disorders.For instance,whether there are factor V leiden and prothrombin gene mutations,or deficiencies of protein C and antithrombin in the patients. Acquired risk factors are also more frequently observed. The identified risk factors include age, smoking, obesity, recent surgery, trauma, fracture, long-term bed rest, long-distance travel, acute infectious diseases, chronic obstructive pulmonary diseases, malignant tumors, cardiovascular and cerebrovascular diseases, hematological disorders, metabolic diseases, and rheumatic immune diseases [6–9], These risk factors may be present alone or in combination. Strong inducing factors for VTE include surgery and active cancer, but most events are unprovoked. Surgery is a common pathogenic factor of VTE, which may be due to the combination of vascular endothelial injury caused by surgery, hemodynamic changes, operation method, operation time, vascular reconstruction, blood transfusion during operation, and patients’ own factors. Cancer-associated thromboembolism (CAT) is associated with worse prognosis in cancer patients [10]. The incidence of VTE in cancer patients also depends on the type of cancer (pancreatic cancer, gastric cancer, and primary brain tumor have the highest risk) [11] as well as the patient’s own factors and cancer treatment-related factors.
Currently, most of the domestic and international VTE risk assessment scales are designed for surgical and orthopedic patients. However, when assessing the risk of VTE in surgical patients, not only patient-specific factors but also surgery-specific factors should be considered. There is, unfortunately, no similar scale specifically for patients undergoing PD. Surgeons mostly evaluate the risk of thromboembolism after PD on the basis of the Caprini scale [12, 13], the American College of Chest Physicians (ACCP) VTE prevention guidelines, and other available scoring models [14, 15] and empirically use limb pneumatic pumps, low-molecular-weight heparin, and other anticoagulant agents to prevent thrombosis. VTE occurs in patients undergoing PD because of many factors, and therefore, the preoperative and postoperative evaluation scales should be different. Postoperative patients are affected by many factors, such as the operation method, operation time, and other surgical factors. Moreover, the patient’s own factors could greatly increase the risk of VTE after PD. Hence, it is necessary to integrate the relevant VTE prevention and treatment guidelines and the characteristics of PD to establish a database of specific risk factors and acquired risk factors of PD patients and the interaction of various factors to improve the existing risk assessment model; this knowledge can then be used to establish a VTE score scale for PD patients, which can effectively differentiate high-, medium-, and low-risk VTE patients, provide accurate prediction, and enable individualized anticoagulant treatment. This approach can effectively reduce the incidence of VTE and bleeding after anticoagulation, thereby improving the quality of life and prognosis of PD patients to some extent.
The present study aimed to develop an effective and simple prediction tool on the basis of single factor and multifactor regression analyses, integrate multiple prediction indicators, and use nomograms to transform complex regression equations into visual graphs, which can directly reflect the predicted value of individual outcome events, that is, the probability of VTE. The purpose here is to provide a reliable judgment for the occurrence of VTE events after PD and, to a certain extent, to provide a reliable tool for clinicians to judge whether VTE prevention and intervention treatments are required; simultaneously, the prediction tool could facilitate to provide effective help for reducing adverse events such as VTE and bleeding after PD, thereby improving the quality of life and prognosis of PD patients to some extent.
Patients and methods
Patients and data collection
By using the case retrieval system, patients who underwent PD in the First Affiliated Hospital of Xinjiang Medical University from January 2018 to March 2022 were retrieved. The investigators included clinical staff of the Department of Pancreatic Surgery of the First Affiliated Hospital of Xinjiang Medical University and those with clinical experience after professional training. Patients treated for PD with a complete history and description of pathological findings and a clear imaging diagnosis of VTE in our ultrasound or radiology department were included in our study. We excluded (1) patients with a preoperative or previous history of VTE (n = 3), (2) patients with other recent surgical comorbidities (n = 1), (3) patients with severe liver and kidney diseases (n = 5), or (4) patients with hematological diseases and those with missing key clinical information (n = 0). Ultimately, 352 PD patients were included in this study. All data, including demographic, disease characteristics, and treatment characteristics, were collected.
Statistical analysis
The number of patients recruited was divided randomly in the ratio of 8:2, of which 80% of the patients were used as the training set to establish the prediction model, and the remaining 20% of the patients were used as the validation set to verify the accuracy of the model. All data, including demographic, disease characteristics, and treatment characteristics, were expressed by count (%). The data were entered into an Excel spreadsheet. Statistical analysis was performed using SPSS version 26.0 software (IBM, Armonk, NY, USA) and the R studio software (version 4.2.1; https://www.rstudio.com).
The Least Absolute Shrinkage and Selection Operator (Lasso) [16, 17] method was suitable for shrinking high-dimensional data and filtering out the optimal predictive features in risk factors from PD patients with VTE. Characterised by variable selection and regularization in the same process as fitting a generalised linear model, Lasso regression selectively adjusts variables in models, especially those showing high levels of multicollinearity, to obtain optimal performance parameters and avoids overfitting (through regularization) at the same time.A multivariate logistic regression analysis was then used to construct a new predictive model by incorporating the features selected in the Lasso regression model. The features were regarded as odds ratio (OR) with 95% confidence interval (CI) and as P-value. Subsequently, a nomogram was plotted according to the results of logistic regression.
Calibration curves were drawn to assess the calibration of the VTE nomogram, which was then used to compare the consistency of the model prediction with the observed values. To estimate the discrimination capability of the VTE nomogram, the C-index and the area under the ROC curve were measured. The VTE nomogram was internally validated to calculate a relatively corrected C-index. Decision curve analysis was performed to determine the clinical usefulness of the VTE nomogram by quantifying the net benefit at different threshold probabilities. The net benefit was calculated by subtracting the proportion of true-positive patients from the proportion of false-positive patients among all patients and by weighing the relative harms of excluding the intervention against the negative consequences of an unnecessary intervention [18].
Results
Patients’ characteristics
Based on the defined inclusion criteria in this study, 352 patients with PD were included as subjects and categorised taking the similar rate of venous thromboembolism (VTE) in each group into consideration as follows: 56 of 282 patients (about 22.2%) in the training set developed VTE while approximately 22.8% in the validation set, that is, 16 of 70 patients, had been suffering this disease, as shown in Fig. 1.
Fig. 1.
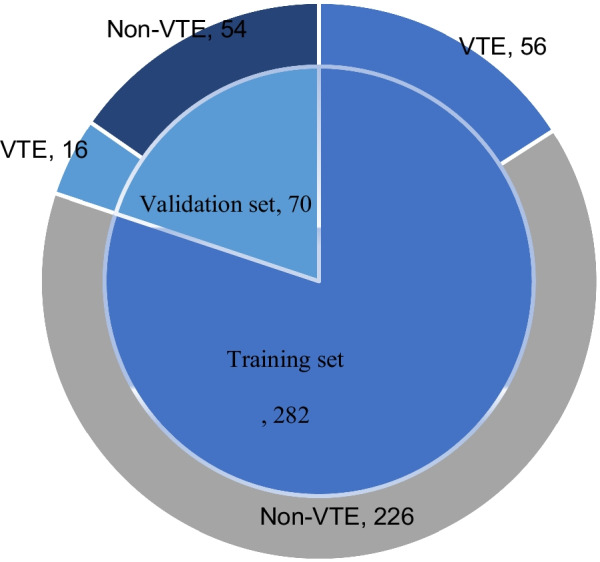
Composition of the two groups
We evaluated 43 candidate variables and compared each detection index between the training set and the validation set. The results confirmed that the training set and the validation set showed statistically significant differences in the blood group type, invasion outside the primary site, and PT% (P < 0.05), but no significant differences were observed in the remaining 40 variables, (P > 0.05). However, the blood group type, invasion outside the primary site, and PT% are laboratory indices and cannot represent the characteristics of the population; hence, they were not excluded in the follow-up study. Table 1 shows all data of the study patients, including the demographic, disease, and treatment features of the two groups.
Table 1.
Comparison of general information of patients between the two groups
| Demographic characteristics | Traning (n = 282) | Validation (n = 70) | P |
|---|---|---|---|
| Age (years) | |||
| ≦ 60 | 143 (50.71%) | 39 (55.71%) | 0.453 |
| > 60 | 139 (49.29%) | 31 (44.29%) | |
| Sex | |||
| Female | 50 (17.73%) | 8 (11.43%) | 0.203 |
| Male | 232 (82.26%) | 62 (88.57%) | |
| Tumor site | |||
| Head of pancreas | 145 (51.41%) | 31 (44.29%) | 0.193 |
| The ampulla of vater | 68 (24.11%) | 17 (24.29%) | |
| Duodenum | 27 (9.58%) | 13 (18.57%) | |
| Biliary tract | 42 (14.90%) | 9 (12.85%) | |
| Degree of tumor differentitation | |||
| Benign | 41 (14.54%) | 12 (17.14%) | 0.620 |
| Highly differentitated | 23 (8.16%) | 4 (5.72%) | |
| Highly-moderately differentitated | 12 (4.25%) | 4 (5.72%) | |
| Moderately differentitated | 103 (36.53%) | 26 (37.14%) | |
| Moderately-poorly differentitated | 77 (27.30%) | 14 (20.00%) | |
| Poorly differentitated | 26 (9.22%) | 10 (14.28%) | |
| Blood type | |||
| A | 70 (24.82%) | 26 (37.14%) | 0.047 |
| B | 100 (35.46%) | 17 (24.29%) | |
| O | 68 (24.11%) | 21 (30.00%) | |
| AB | 44 (15.61%) | 6 (8.57%) | |
| Pian | |||
| No | 169 (59.93%) | 46 (65.71%) | 0.374 |
| Yes | 113 (40.07%) | 24 (34.29%) | |
| Jaundice | |||
| No | 139 (49.29%) | 41 (58.57%) | 0.164 |
| Yes | 143 (50.71%) | 29 (41.43%) | |
| Lost weight | |||
| No | 111 (39.36%) | 25 (35.71%) | 0.589 |
| Yes | 171 (60.64%) | 45 (64.29%) | |
| Gastrointestinal symptoms | |||
| No | 233 (82.62%) | 52 (74.79%) | 0.112 |
| Yes | 49 (17.38%) | 18 (25.71%) | |
| Drinking history | |||
| No | 220 (78.01%) | 52 (74.79%) | 0.505 |
| Yes | 62 (21.89%) | 18 (25.71%) | |
| Hypertension | |||
| No | 195 (69.15%) | 49 (70.00%) | 0.890 |
| Yes | 87 (30.85%) | 21 (30.00%) | |
| Diabetes | |||
| No | 228 (80.85%) | 59 (84.29%) | 0.507 |
| Yes | 54 (19.15%) | 11 (15.71%) | |
| Hepatitis B | |||
| No | 276 (97.87%) | 68 (97.14%) | 0.714 |
| Yes | 6 (2.13%) | 2 (2.86%) | |
| Hepatitis C | |||
| No | 280 (99.29%) | 69 (98.57%) | 0.487 |
| Yes | 2 (0.71%) | 1 (1.43%) | |
| Operative method | |||
| PD | 217 (76.95%) | 56 (80.00%) | 0.458 |
| LPD | 39 (13.83%) | 6 (8.57%) | |
| LPD + PD | 26 (9.22%) | 8 (11.43%) | |
| Intraoperative bleeding (> 500 ml) | |||
| No | 157 (55.67%) | 39 (55.71%) | 0.995 |
| Yes | 125 (44.33%) | 31 (44.29%) | |
| Operation time (> 600 min) | |||
| No | 184 (65.25%) | 44 (62.86%) | 0.708 |
| Yes | 98 (34.75%) | 26 (37.14%) | |
| Blood transfusion | |||
| No | 169 (59.93%) | 40 (57.14%) | 0.671 |
| Yes | 113 (40.07%) | 30 (42.86%) | |
| Minus jaundice measures | |||
| No | 224 (79.43%) | 57 (81.43%) | 0.710 |
| Yes | 58 (50.57%) | 13 (18.57%) | |
| Vascular resection | |||
| No | 250 (88.65%) | 64 (91.43%) | 0.503 |
| Yes | 32 (11.35%) | 6 (8.57%) | |
| History of abdominal surgery | |||
| No | 202 (71.63%) | 54 (77.14%) | 0.354 |
| Yes | 80 (28.37%) | 16 (22.86%) | |
| Invasion outside the primary site | |||
| No | 172 (60.99%) | 40 (57.14%) | 0.012 |
| Yes | 110 (39.01%) | 30 (42.86%) | |
| Tumor size (≧ 3 cm) | |||
| No | 134 (47.52%) | 30 (42.86%) | 0.484 |
| Yes | 148 (52.48%) | 40 (57.14%) | |
| Blood vessel invasion | |||
| No | 163 (57.80%) | 45 (64.29%) | 0.323 |
| Yes | 119 (42.20%) | 25 (35.71%) | |
| Nerve invasion | |||
| No | 103 (36.52%) | 31 (44.29%) | 0.231 |
| Yes | 179 (63.48%) | 39 (55.71%) | |
| Lymphatic metastasis | |||
| No | 198 (70.21%) | 51 (72.86%) | 0.663 |
| Yes | 84 (29.79%) | 19 (27.14%) | |
| R1 resection | |||
| No | 245 (86.88%) | 50 (71.43%) | 0.002 |
| Yes | 37 (13.12%) | 20 (28.57%) | |
| Pancreatic duct dilatation | |||
| No | 122 (43.26%) | 29 (41.43%) | 0.781 |
| Yes | 160 (56.74%) | 41 (58.57%) | |
| BMI (≧ 25 kg/m2) | |||
| No | 179 (63.48%) | 42 (60.00%) | 0.590 |
| Yes | 103 (36.52%) | 28 (40.00%) | |
| HGB (≧ 120 g/L) | |||
| No | 101 (35.82%) | 29 (41.43%) | 0.384 |
| Yes | 181 (64.18%) | 41 (58.57%) | |
| WBC (≦ 10 × 109/L) | |||
| No | 28 (9.93%) | 5 (7.14%) | 0.474 |
| Yes | 254 (90.07%) | 65 (92.86%) | |
| Neutrophil count (< 6.3 × 109/L) | |||
| No | 55 (19.05%) | 15 (21.43%) | 0.718 |
| Yes | 227 (80.50%) | 55 (78.57%) | |
| Lymphocyte count (< 3.2 × 109/L) | |||
| No | 5 (1.77%) | 1 (1.45%) | 1 |
| Yes | 277 (98.23%) | 69 (98.55%) | |
| PLT (109/L) | |||
| ≦ 125 | 4 (1.42%) | * | 0.092 |
| 125–350 | 234 (82.98%) | 52 (74.79%) | |
| ≧ 350 | 44 (15.60%) | 18 (25.71%) | |
| CA19-9 (> 25 U/mL) | |||
| No | 119 (42.20%) | 30 (42.86%) | 0.920 |
| Yes | 163 (57.80%) | 40 (57.14%) | |
| PT(s) | |||
| ≦ 9 | 7 (2.49%) | 2 (2.86%) | 0.855 |
| 9–15 | 263 (93.26%) | 66 (94.28%) | |
| ≧ 15 | 12 (4.25%) | 2 (2.86%) | |
| APTT(s) | |||
| ≦ 23 | 1 (0.35%) | 1 (1.43%) | 0.546 |
| 23–38 | 258 (91.48%) | 64 (91.43%) | |
| ≧ 38 | 23 (8.16%) | 5 (7.14%) | |
| PT% | |||
| ≦ 75 | 66 (23.40%) | 7 (10.00%) | 0.041 |
| 75–120 | 187 (66.31%) | 53 (75.71%) | |
| ≧ 120 | 29 (10.28%) | 10 (14.28%) | |
| AST/ALT | |||
| ≦ 1 | 148 (52.48%) | 42 (60.00%) | 0.259 |
| > 1 | 134 (47.52%) | 28 (40.00%) | |
| Albumin (> 35 g/L) | |||
| No | 88 (31.21%) | 28 (40.00%) | 0.161 |
| Yes | 194 (68.79%) | 42 (60.00%) | |
| TBIL (> 22 µmmol/L) | |||
| No | 91 (32.27%) | 28 (40.00%) | 0.221 |
| Yes | 191 (67.73%) | 42 (60.00%) | |
| TG (≧1.69 mmol/L) | |||
| No | 126 (44.68%) | 32 (45.71%) | 0.876 |
| Yes | 156 (55.32%) | 38 (54.29%) | |
| PCT (< 0.05 ng/mL) | |||
| No | 237 (84.04%) | 62 (88.57%) | 0.343 |
| Yes | 45 (15.96%) | 8 (11.43%) |
LPD laparoscopic pancreatoduodenectomy, PD open pancreatoduodenectomy, PT prothrombin time, BMI body mass index, HGB hemoglobinopathies, WBC white blood cells, PLT platelet, APTT activated partial thromboplastin time, AST/ALT the aspartat aminotransferase (AST)/alanin aminotransferase (ALT) ratio, TBIL total bilirubin, TG triglyceride, PCT procalcitonin
Feature selection
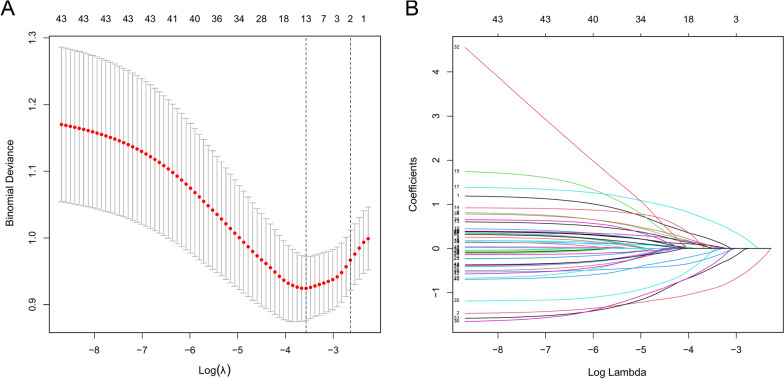
In the training set, Lasso regression screened 13 potential predictors out of 43 candidate variables. These factors were characterized by nonzero coefficients in the Lasso regression model (Fig. 2A, B). These features included sex, age, gastrointestinal symptoms, hypertension, diabetes, operative method, intraoperative bleeding, blood transfusion, neutrophil count, PT, APTT, AST/ALT, and TBIL.
Fig. 2.
A Optimal parameter (lambda) selection in the Lasso model used five-fold cross-validation through the minimum criteria. Dotted vertical lines were drawn at the optimal values by using the minimum criteria and the 1 SE of the minimum criteria (the 1-SE criteria). B Lasso coefficient profiles of the 43 features. A coefficient profile plot was generated against the log(lambda) sequence. A vertical line was drawn at the value selected using five-fold cross-validation, where optimal lambda resulted in 13 features with nonzero coefficients. Lasso least absolute shrinkage and selection operator, SE standard error
Development of an individualized prediction model
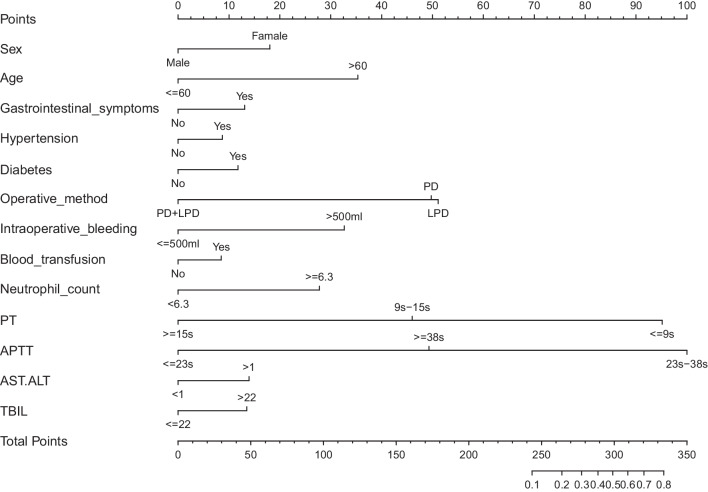
Table 2 shows the results of the logistic regression analysis for sex, age, gastrointestinal symptoms, hypertension, diabetes, operative method, intraoperative bleeding, blood transfusion, neutrophil count, PT, APTT, AST/ALT, and TBIL. A model that incorporated these independent predictors was developed and presented as the nomogram (Fig. 3).
Table 2.
Prediction factors for VTE in patients undergoing pancreatoduodenectomy
| Intercept and variable | Prediction model | 95%CI | |||
|---|---|---|---|---|---|
| β | P-value | Odds ratio | Lower | Upper | |
| Intercept | − 10.8864 | 0.9901 | 1.87E−05 | NA | 4.00E+60 |
| Sex | 0.7131 | 0.1018 | 2.04 | 0.859 | 4.798 |
| Age | − 1.3973 | 0.0003 | 2.472 | 0.111 | 0.52 |
| Gastrointestinal symptoms | 0.5174 | 0.2401 | 1.677 | 0.695 | 3.953 |
| Hypertension | 0.3451 | 0.3705 | 1.412 | 0.658 | 3.003 |
| Diabetes | 0.4653 | 0.2724 | 1.593 | 0.684 | 3.639 |
| Operative method | |||||
| LPD | 0.0553 | 0.9186 | 1.056 | 0.345 | 2.959 |
| PD + LPD | − 1.9675 | 0.0669 | 0.139 | 0.007 | 0.771 |
| Intraoperative bleeding | 1.2920 | 0.0005 | 3.64 | 1.771 | 7.787 |
| Blood transfusion | 0.3361 | 0.3658 | 1.399 | 0.673 | 2.909 |
| Neutrophil count | − 1.0978 | 0.0069 | 0.334 | 0.149 | 0.742 |
| PT(s) | |||||
| 9–15 | − 1.9420 | 0.1871 | 0.143 | 0.008 | 4.207 |
| ≧ 15 | − 3.7631 | 0.0514 | 0.023 | 0.0004 | 1.222 |
| APTT(s) | |||||
| 23–38 | 11.7717 | 0.9893 | 1.30E+05 | 1.83E−61 | NA |
| ≧ 38 | 9.7665 | 0.9911 | 1.74E+04 | 4.88E−53 | NA |
| AST/ALT | 0.5509 | 0.1224 | 1.734 | 0.868 | 3.537 |
| TBIL | − 0.5344 | 0.1618 | 0.589 | 0.275 | 1.243 |
β is the regression coefficient
CI confidence interval, LPD laparoscopic pancreatoduodenectomy, PD open pancreatoduodenectomy, PT prothrombin time, APTT activated partial thromboplastin time, AST/ALT The aspartat aminotransferase (AST)/alanin aminotransferase (ALT) ratio, TBIL total bilirubin
Fig. 3.
The Developed VTE nomogram
Performance of the VTE risk nomogram in the study cohort
The calibration curve of the VTE risk nomogram for the prediction of VTE risk in patients undergoing PD showed a good agreement in the training set (Fig. 4). The area under the receiver operating characteristic curve (AUC) for the prediction nomogram was 0.8218 (P < 0.001, 95%confidence interval:0.761–0.882), with a sensitivity of 0.839 and a specificity of 0.717(Fig. 5), which showed that the prediction model had good accuracy. The C-index for the prediction nomogram was 0.827 for the training set, and it was confirmed to be 0.894 through internal validation; this finding suggested that the model had good discrimination ability. Thus, the performance of the VTE risk nomogram indicated that it had a good prediction capability.
Fig. 4.
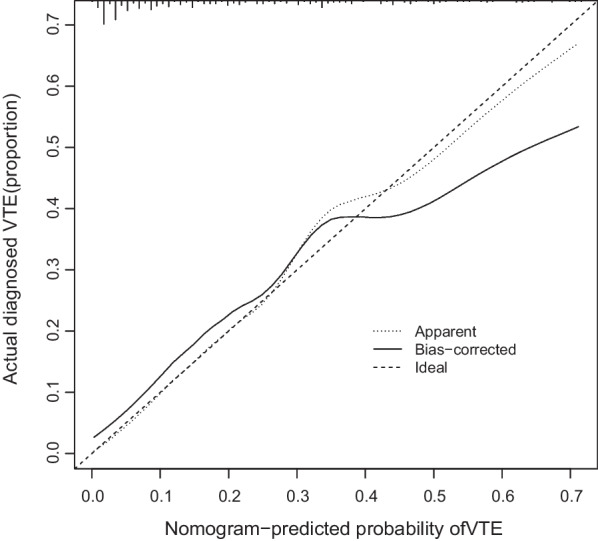
Calibration curves of the VTE prediction nomogram. The x-axis represents the predicted medication nonadherence risk. The y-axis represents the actual diagnosed nonadherence. The diagonal dotted line represents a perfect prediction by an ideal model. The solid line represents the performance of the nomogram, and a closer fit to the diagonal dotted line represents a better prediction
Fig. 5.
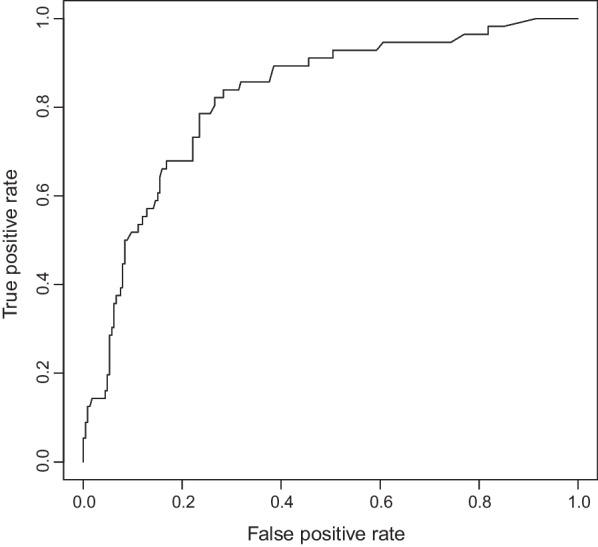
The area under the receiver operating characteristic curve (AUC) for the prediction nomogram. The ROC curve was measured to discriminate the performance of the VTE nomogram. The area under the receiver operating characteristic (ROC) curve (AUC) for the prediction nomogram was 0.822 (P < 0.001, 95%confidence interval:0.761–0.882), with a sensitivity of 0.839 and a specificity of 0.717
Clinical application
The decision curve analysis for the medication nonadherence nomogram is presented in Fig. 6. The decision curve showed that if the threshold probability of a PD patient was > 10%, then the use of this VTE nomogram to predict VTE risk adds more benefit than the scheme. Within this range, the net benefit was comparable with several overlaps on the basis of the VTE risk nomogram.
Fig. 6.
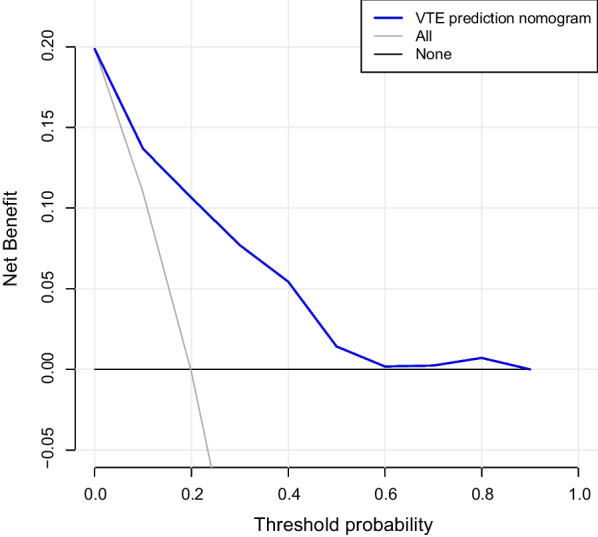
Decision curve analysis for the VTE prediction nomogram. The y-axis represents the net benefit. The blue line indicates the VTE risk Nomogram: the thin line represents all PD patients who developed VTE, while the thick line represents all PD patients who did not develop VTE
The decision curve shows that the predictive model has a high clinical application if the threshold probability for PD patients is > 10%, respectively.
Discussion
In our present study, we combined the relevant domestic and international guidelines for the prevention and treatment of VTE after PD. According to the characteristics of PD, 43 candidate variables were chosen. Of these, 13 variables with nonzero coefficient characteristics were screened out by Lasso regression. These 13 variables were integrated, and nomograms were drawn accordingly. The complex regression equations were transformed into visual graphs to visually reflect the predicted value of individual outcome events, that is, the probability of VTE. The model evaluation showed the following results: C-index was 0.827, the calibration curve showed good consistency, the AUC of the ROC curve was 0.822 (P < 0.001, 95%confidence interval:0.761–0.882), with a sensitivity of 0.839 and a specificity of 0.717, and the internal validation C-index was 0.894. These indicators showed that the model was relatively accurate. The prediction model could provide a reliable judgment for the occurrence of VTE events after PD. The model also achieved individualized predictions, and thus, clinicians can use this tool to assess patients for their participation in clinical trials. Moreover, this study was the first to apply a nomogram to predict VTE after PD.
The risk factor analysis revealed that female gender, age > 60 years, presence of preoperative gastrointestinal symptoms, complications of hypertension and diabetes, use of laparoscopic pancreaticoduodenectomy (LPD) as the operation method, intraoperative hemorrhage > 500 mL and blood transfusion; preoperative neutrophil count ≥ 6.3 × 109/L; PT ≤ 9 s, APTT of 23–38 s, AST/ALT > 1, and TBIL > 22 µmol/L may be the risk factors for VTE in patients undergoing PD.
In this study, we found that the female gender might be a risk factor for VTE after PD. A significant difference is observed in the patient composition ratio between male and female patients, and this finding may be attributed to the high incidence of pancreatic cancer and other diseases that require treatment with PD in males [19, 20]. Therefore, the prevalence rate of VTE in the two groups was compared. As shown in Table 3, the prevalence rate of VTE in female patients in the training and validation sets was 30% and 37.5%, respectively, which was much higher than those for male patients.
Table 3.
Comparison of the prevalence of VTE in sex between two groups
| Training set | Validation set | |||||
|---|---|---|---|---|---|---|
| VTE | Non-VTE | Prevalence rate (%) | VTE | Non-VTE | Prevalence rate (%) | |
| Female | 15 | 35 | 30.0 | 3 | 5 | 37.5 |
| Male | 41 | 191 | 17.7 | 6 | 56 | 9.7 |
| Total | 56 | 226 | 19.8 | 9 | 61 | 12.8 |
Previous studies [8] have shown that the incidence rate of VTE increases markedly with age for both male and female patients and for both DVT and PE. The incidence rates are somewhat higher in females during childbearing years, while the incidence rates are generally higher in males after the age of 45 years; thus, the incidence rate may vary by gender according to age and exposure to gender-specific triggers [21]. Interestingly, a large cohort study [22] in Sweden found that males have a higher incidence of first-ever VTE than females. Risk factors for VTE may differ between male and female patients. The Swedish cohort study also found that hypertension seems to have a protective effect on the future incidence of VTE in males, but not in females. Our present study showed that hypertension might be a risk factor for postoperative VTE, and in agreement with the Swedish cohort study, male patients with hypertension had a lower risk of future incidence of VTE, while female patients with hypertension had a significantly higher risk for VTE than male patients. This finding indicates that hypertension may play different roles according to gender in patients with VTE, and future research should focus on understanding this difference in detail.
We hypothesized that the choice of surgical method, the amount of intraoperative bleeding, and the volume of blood transfusion are risk factors for the development of VTE after PD. We found that the choice of LPD as the surgical method may be a risk factor for postoperative VTE in PD patients, while conversion to open laparoscopic surgery (LPD + PD) was not a risk factor for postoperative VTE in PD patients; this finding may be related to the longer duration of LPD and compromised blood return due to increased abdominal pressure from the establishment of the pneumoperitoneum. In a retrospective study, Brent et al. [23] found that as compared to open distal pancreatectomy, minimally invasive distal pancreatectomy was associated with a higher incidence of postoperative VTE in patients. However, no similar studies have reported the relationship between the choice of surgical approach for PD and the development of VTE after PD. Furthermore, our study reached the same conclusion as previous studies [24, 25] that perioperative RBC transfusion may be significantly related to the development of new or progressive postoperative VTE, independent of several hypothetical confounding factors. These findings, if confirmed, should reinforce the significance of strict management of perioperative transfusion practices. Heavy intraoperative bleeding is correlated with blood transfusion; this clearly indicates that intraoperative bleeding is a risk factor for VTE after PD. This implies that surgeons should closely monitor such patients. Moreover, because abdominal bleeding is also a common postoperative complication of PD, it is clear that the development of VTE is a cumulative effect of various factors. In summary, surgical factors play a crucial role in the development of VTE in PD patients postoperatively.
There is an increasing consensus that thromboembolism is closely linked to inflammation, and it is possible that inflammation is involved in the initiation of thrombosis. We also found that preoperative neutrophil counts may be relevant to the formation of VTE after PD. Mauracher et al. [26] showed that neutrophils may enhance thromboembolism by releasing neutrophil extracellular traps (NETs) to enhance thrombosis. In addition, activated neutrophils release proteases that degrade anticoagulant tissue factor (TF) pathway inhibitors, thereby increasing TF activity and activation of the coagulation pathway. Plasma guanosine levels of plasma citrullinated histone H3, a biomarker of NET formation, are associated with VTE in patients with pancreatic cancer (PC). Elevated neutrophil count is a non-specific biomarker of inflammation [27]. A multicentre retrospective study [28] showed that postoperative intraperitoneal infection increased the risk of Splanchnic vein thrombosis (SVT). The study also drew our attention to the fact that local inflammation played an important role in the development of SVT, which was associated with mortality at 90 days postoperatively. In summary, inflammation may be a precursor of thrombosis; hence, anti-inflammatory treatment may be a protective factor for VTE and could provide new strategies for thromboembolism prevention. This hypothesis needs to be confirmed in prospective studies.
Obstructive jaundice [29] is a common clinical symptom of a lesion occupying the head of the pancreas, lower bile duct, and duodenal papilla. In the present study, we included TBIL, PT, and APTT, which are indicators of liver function to a certain extent, to investigate whether these factors could be used as predictors of VTE after PD. We found that elevated TBIL level was associated with postoperative VTE development in PD patients. Interestingly, in other studies [30], bilirubin was found to have anti-inflammatory effects along with antioxidant effects. A retrospective case–control study by Cervellin et al. [31] established a significant relationship between low TBIL levels and PE. Mustafa et al. [32] showed that the presence of a genetic variant of the heme oxygenase 1 gene increased the risk of VTE recurrence. Bilirubin inhibits collagen-induced platelet activation, vascular smooth muscle cell migration and proliferation, platelet aggregation, and neoplastic endothelial formation. Does this indicate that we obtained a wrong result? We, however, are not convinced that our result might be inappropriate. For example, although bilirubin has anti-inflammatory properties and inhibits platelet aggregation, it is undeniable that most patients undergoing PD have obstructive jaundice, and some of these patients often have varying levels of elevation of the tumor marker CA19-9. The tumor marker CA19-9 has been shown to predict the incidence of VTE in PC patients [33]. This may be related to the fact that elevated CA19-9 level may activate platelets and neutrophils, among other mechanisms, to activate the coagulation pathways and increase blood hypercoagulability. This indicates that VTE events occur following the interaction of multiple factors. Further experiments are required to verify whether bilirubin plays a protective or promotional role in the development of VTE in PD patients.
Our study is the first to use AST/ALT to assess the occurrence of VTE. AST and ALT are well-known circulating biomarkers for reflecting liver injury. The AST/ALT ratio is also known as the De Ritis ratio [34]. It was first proposed for studying the etiology of hepatitis and is often used to differentiate between the different causes of liver diseases, such as the fatty liver. Currently, the AST/ALT ratio is used as a validated biomarker for nonliver diseases such as cardiovascular diseases, cancer, type 2 diabetes, and other diseases [35, 36]. The AST/ALT ratio reflects impaired liver function; however, its elevation does not necessarily accurately reflect the severity of liver diseases. Hepatic impairment leads to a decrease in the levels of coagulation factors II, V, VII, IX, and X. This reduces coagulation and predisposes to bleeding tendencies. This is, however, contradictory to the results of our present study. The increase in the De Ritis ratio, on the one hand, increases the viscosity of the blood, and on the other hand, indicates the possibility of inflammation; thus, these parameters are associated with an increased risk of VTE. The AST/ALT ratio is an economical and readily available laboratory indicator that can be used in clinical monitoring of postoperative VTE in PD patients and also demonstrates the feasibility of our predictive model.
The shortened activated partial thromboplastin time (APTT) and prothrombin time (PT) are increasingly and frequently referred to as potential risk factors [37] for VTE. But our nomogram showed that this generally acknowledged point of view may be biased to a certain extent. In our study, the PT shortening was shown to be associated with the development of VTE whereas APTT was found to be the opposite. It seemed that APTT shortening is incorrelate with the development of VTE. The multivariate analysis demonstrated a comparatively wide confidence interval for APTT, which we considered as a consequence of the insufficient sample size, as shown in Table 4. However, we did not integrate the groups because APTT and VTE directly reflect the abnormalities in the coagulation system. The APTT has been used clinically to monitor the efficacy of anticoagulation [38], but whether prolongation of APTT is an absolute contraindication to anticoagulation deserves contemplation. As stated by Bachler et al. [39], the prolongation of APTT may lead to the deficient anticoagulation amount prescribed or even the absence of anticoagulation therapy, so the clinical significance of the APTT must be re-evaluated.
Table 4.
Comparison of the prevalence of VTE in PT and APTT between two groups
| Training set | VTE (%) | Validation set | VTE (%) | |||
|---|---|---|---|---|---|---|
| VTE | Non-VTE | VTE | Non-VTE | |||
| PT(s) | ||||||
| ≦ 9 | 1 | 2 | 33.3 | 2 | 0 | 100.0 |
| 9–15 | 54 | 213 | 20.2 | 14 | 52 | 21.2 |
| ≧ 15 | 1 | 11 | 7.7 | 0 | 2 | 0.0 |
| APTT(s) | ||||||
| ≦23 | 0 | 1 | 0.0 | 1 | 0 | 100.0 |
| 23–38 | 55 | 203 | 21.3 | 14 | 50 | 21.9 |
| ≧ 38 | 1 | 22 | 4.5 | 1 | 4 | 20.0 |
The essential goal of surgical extirpation of cancer is to achieve R0 resection, as margin-positive specimens are associated with poor long-term survival [40]. In addition, positive margins are strongly associated with disease recurrence and metastasis [41]. Therefore, we included the surgical resection margin as one of the factors to be investigated in the study and compared the prevalence rate of VTE in the two groups.. As shown in Table 1, the composition ratios of the two groups are statistically different (P < 0.05). When Lasso was applied to filter variables, surgical margins failed to remain their correlation with VTE. However, the retrospective nature of this study could not allow us to assume incoherence with the occurrence of VTE. Previous studies [42, 43] were keen to explore the relationships between positive surgical margins and overall survival, for instance, the study performed by Eurola et al. [42], which unfortunately discovered no associations in their univariate analysis. Although the results were not satisfactory, this might provide a new path for future large-population prospective studies.
Thus, VTE after PD occurs due to a combination of various factors. Hence, a relatively accurate assessment will help physicians to determine the probability of VTE in patients and to intervene in a timely manner to avoid adverse outcomes. The development of models for surgery-related VTE is a reflection of individualization and precision, which is a future trend. There are, however, several risk factors for VTE; therefore, the determination of the underlying cause is the key to prevent VTE events, and this aspect will be the topic of future research.
Limitations
Our present study has several limitations. First, our study is retrospective in nature and cannot eliminate bias in the data, the sample size was small and did not achieve a minimum of 10 patients for each risk factor. Second, our data are obtained from a single institution. Thus, the obtained data are not representative of all Chinese PD patients. Third, data collection was incomplete, and the risk factor analysis did not include all potential factors affecting VTE. Finally, although we assessed several variables, the effects of undetermined confounders cannot be ignored. To overcome these limitations, external validation and prospective multicenter studies with a large number of patients are required.
Conclusion
In summary, the predictors required for the model developed in the present study are clinically accessible; moreover, the previous complex regression equations have been transformed into intuitive graphs, thus making the predictive model more readable and convenient for clinicians to assess patients. The application of the model needs to be validated in future prospective studies.
Acknowledgements
None.
Author contributions
All authors contributed to data analysis, drafting, and revising the paper and agree to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Funding
This study was supported by the Open Project of the State Key Laboratory of Pathogenesis, Prevention and Treatment of High Incidence Diseasees in Central Asia (SKL-HIDCA-2022-JZ4), which paid all editing and publication fees.
Availability of data and materials
The datasets generated and analysed during the current study are not publicly available due to patient privacybut are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the institutional review board of The First Affiliated Hospital of Xinjiang Medical University (IRB number: K202209-03) and was performed in accordance with the Declaration of Helsinki. We informed all patients included in the trial and obtained their informed consent. This study was conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors report no competing interests in this work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ban D, et al. International Expert Consensus on Precision Anatomy for minimally invasive distal pancreatectomy: PAM-HBP Surgery Project. J Hepatobil Pancreat Sci. 2022;29:161–173. doi: 10.1002/jhbp.1071. [DOI] [PubMed] [Google Scholar]
- 2.Mihaljevic AL, et al. Not all Whipple procedures are equal: proposal for a classification of pancreatoduodenectomies. Surgery. 2021;169:1456–1462. doi: 10.1016/j.surg.2020.11.030. [DOI] [PubMed] [Google Scholar]
- 3.Whipple AO. Pancreaticoduodenectomy for islet carcinoma : a five-year follow-up. Ann Surg. 1945;121:847–852. doi: 10.1097/00000658-194506000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Are C, Dhir M, Ravipati L. History of pancreaticoduodenectomy: early misconceptions, initial milestones and the pioneers. HPB (Oxford) 2011;13:377–384. doi: 10.1111/j.1477-2574.2011.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kokkinakis S, et al. Complications of modern pancreaticoduodenectomy: a systematic review and meta-analysis. Hepatobil Pancreat Dis Int HBPD INT. 2022 doi: 10.1016/j.hbpd.2022.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Khan F, Tritschler T, Kahn SR, Rodger MA. Venous thromboembolism. The Lancet. 2021;398:64–77. doi: 10.1016/s0140-6736(20)32658-1. [DOI] [PubMed] [Google Scholar]
- 7.Cohen D. Current issues in venous thromboembolism. Postgrad Med. 2021;133:1–2. doi: 10.1080/00325481.2021.1955541. [DOI] [PubMed] [Google Scholar]
- 8.Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. 2016;41:3–14. doi: 10.1007/s11239-015-1311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paul JD, Cifu AS. Prevention and management of venous thromboembolism. JAMA. 2019;322:1602–1603. doi: 10.1001/jama.2019.13853. [DOI] [PubMed] [Google Scholar]
- 10.Khorana AA, et al. Cancer-associated venous thromboembolism. Nat Rev Dis Primers. 2022;8:11. doi: 10.1038/s41572-022-00336-y. [DOI] [PubMed] [Google Scholar]
- 11.Hisada Y, Mackman N. Cancer-associated pathways and biomarkers of venous thrombosis. Blood. 2017;130:1499–1506. doi: 10.1182/blood-2017-03-743211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu X, et al. Application of the Caprini risk assessment model for deep vein thrombosis among patients undergoing laparoscopic surgery for colorectal cancer. Medicine. 2021;100:e24479. doi: 10.1097/md.0000000000024479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterbling HM, et al. Caprini risk model decreases venous thromboembolism rates in thoracic surgery cancer patients. Ann Thorac Surg. 2018;105:879–885. doi: 10.1016/j.athoracsur.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Stevens, H., Peter, K., Tran, H. & McFadyen, J. Predicting the risk of recurrent venous thromboembolism: current challenges and future opportunities. J Clin Med 2020;9. 10.3390/jcm9051582. [DOI] [PMC free article] [PubMed]
- 15.Godinho J, et al. ONKOTEV score as a predictive tool for thromboembolic events in pancreatic cancer-a retrospective analysis. Oncologist. 2020;25:e284–e290. doi: 10.1634/theoncologist.2019-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venkatesh KK, et al. Machine learning and statistical models to predict postpartum hemorrhage. Obstet Gynecol. 2020;135:935–944. doi: 10.1097/aog.0000000000003759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Egmond MB, et al. Privacy-preserving dataset combination and Lasso regression for healthcare predictions. BMC Med Inform Decis Mak. 2021;21:266. doi: 10.1186/s12911-021-01582-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, et al. Predicting medication nonadherence risk in a Chinese inflammatory rheumatic disease population: development and assessment of a new predictive nomogram. Patient Prefer Adherence. 2018;12:1757–1765. doi: 10.2147/ppa.S159293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lippi G, Mattiuzzi C. The global burden of pancreatic cancer. Arch Med Sci AMS. 2020;16:820–824. doi: 10.5114/aoms.2020.94845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 21.Faioni EM, Zighetti ML, Vozzo NP. Sex, gender and venous thromboembolism: do we care enough? Blood Coagul Fibrinol Int J Haemos Thromb. 2018;29:663–667. doi: 10.1097/mbc.0000000000000773. [DOI] [PubMed] [Google Scholar]
- 22.Lind MM, Johansson M, Själander A, Johansson L. Incidence and risk factors of venous thromboembolism in men and women. Thromb Res. 2022;214:82–86. doi: 10.1016/j.thromres.2022.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Willobee BA, et al. Minimally invasive surgery is associated with an increased risk of postoperative venous thromboembolism after distal pancreatectomy. Ann Surg Oncol. 2020;27:2498–2505. doi: 10.1245/s10434-019-08166-1. [DOI] [PubMed] [Google Scholar]
- 24.Goel R, et al. Association of perioperative red blood cell transfusions with venous thromboembolism in a north American Registry. JAMA Surg. 2018;153:826–833. doi: 10.1001/jamasurg.2018.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheth BK, et al. Red blood cell transfusions and risk of postoperative venous thromboembolism. J Am Acad Orthop Surg. 2022;30:e919–e928. doi: 10.5435/jaaos-d-22-00043. [DOI] [PubMed] [Google Scholar]
- 26.Mauracher LM, et al. Citrullinated histone H3, a biomarker of neutrophil extracellular trap formation, predicts the risk of venous thromboembolism in cancer patients. J Thromb Haemost. 2018;16:508–518. doi: 10.1111/jth.13951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu J, Cai Z, Zhou Y. The association of neutrophil-lymphocyte ratio with venous thromboembolism: a systematic review and meta-analysis. Clin Appl Thromb Hemost. 2022;28:10760296221130061. doi: 10.1177/10760296221130061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duceppe E, et al. Incidence and predictors of splanchnic vein thrombosis and mortality following hepatobiliary and pancreatic surgery. J Thromb Haemost. 2021;19:797–804. doi: 10.1111/jth.15198. [DOI] [PubMed] [Google Scholar]
- 29.Satoh D, Matsukawa H, Shiozaki S. The optimal type and management of biliary drainage in patients with obstructive jaundice who undergo pancreaticoduodenectomy. In Vivo (Athens, Greece) 2022;36:391–397. doi: 10.21873/invivo.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao Q, et al. Bilirubin improves the quality and function of hypothermic preserved islets by its antioxidative and anti-inflammatory effect. Transplantation. 2019;103:2486–2496. doi: 10.1097/tp.0000000000002882. [DOI] [PubMed] [Google Scholar]
- 31.Cervellin G, Buonocore R, Sanchis-Gomar F, Lippi G. Low serum bilirubin values are associated with pulmonary embolism in a case–control study. Clin Chem Lab Med (CCLM) 2016;54:e229–e230. doi: 10.1515/cclm-2015-1156. [DOI] [PubMed] [Google Scholar]
- 32.Mustafa S, et al. Genetic variation in heme oxygenase 1 (HMOX1) and the risk of recurrent venous thromboembolism. J Vasc Surg. 2008;47:566–570. doi: 10.1016/j.jvs.2007.09.060. [DOI] [PubMed] [Google Scholar]
- 33.Peippo MH, Kurki S, Seppänen H, Lassila R, Carpén O. CA 19-9 doubling time in pancreatic cancer as a predictor of venous thromboembolism: a hospital database study. Acta Oncolog (Stockholm, Sweden) 2020;59:237–241. doi: 10.1080/0284186x.2019.1679881. [DOI] [PubMed] [Google Scholar]
- 34.Liu H, et al. AST/ALT ratio and peripheral artery disease in a Chinese hypertensive population: a cross-sectional study. Angiology. 2021;72:916–922. doi: 10.1177/00033197211004410. [DOI] [PubMed] [Google Scholar]
- 35.Chen W, et al. Elevated AST/ALT ratio is associated with all-cause mortality and cancer incident. J Clin Lab Anal. 2022;36:e24356. doi: 10.1002/jcla.24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou J, He Z, Ma S, Liu R. AST/ALT ratio as a significant predictor of the incidence risk of prostate cancer. Cancer Med. 2020;9:5672–5677. doi: 10.1002/cam4.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ono R, Fukushima K, Yamazaki T, Takahashi H, Hori Y. The distribution of anti-factor Xa activity value, PT and APTT at peak and trough times in patients with direct anti-factor Xa inhibitors. Eur Heart J. 2020;41:ehaa946.3368. doi: 10.1093/ehjci/ehaa946.3368. [DOI] [Google Scholar]
- 38.Toulon P, Smahi M, De Pooter N. APTT therapeutic range for monitoring unfractionated heparin therapy. Significant impact of the anti-Xa reagent used for correlation. J Thromb Haemost. 2021;19:2002–2006. doi: 10.1111/jth.15264. [DOI] [PubMed] [Google Scholar]
- 39.Bachler M, et al. Influence of factor XII deficiency on activated partial thromboplastin time (aPTT) in critically ill patients. J Thromb Thrombolysis. 2019;48:466–474. doi: 10.1007/s11239-019-01879-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tempero MA, et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Nat Comprehen Cancer Netw JNCCN. 2021;19:439–457. doi: 10.6004/jnccn.2021.0017. [DOI] [PubMed] [Google Scholar]
- 41.Li B, et al. Risk factors of positive resection margin differ in pancreaticoduodenectomy and distal pancreatosplenectomy for pancreatic ductal adenocarcinoma undergoing upfront surgery. Asian J Surg. 2022 doi: 10.1016/j.asjsur.2022.09.156. [DOI] [PubMed] [Google Scholar]
- 42.Eurola A, et al. Preoperative oncologic therapy and the prolonged risk of venous thromboembolism in resectable pancreatic cancer. Cancer Med. 2022;11:1605–1616. doi: 10.1002/cam4.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmocker RK, et al. Impact of margin status on survival in patients with pancreatic ductal adenocarcinoma receiving neoadjuvant chemotherapy. J Am Coll Surg. 2021;232:405–413. doi: 10.1016/j.jamcollsurg.2020.11.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during the current study are not publicly available due to patient privacybut are available from the corresponding author on reasonable request.