Graphical abstract

Keywords: Molecular imprinting, Miniature electrochemical biosensor, Acupuncture needle, SARS-CoV-2 spike protein, Poly(ionic liquids)
Abstract
Accurate detection of SARS-CoV-2 spike (SARS-CoV-2-S) protein is of clinical significance for early diagnosis and timely treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Herein, a surface molecularly imprinted miniature biosensor was fabricated. Au nanoparticles (AuNPs), reduced graphene oxide (rGO), poly(methylene blue)/poly(ionic liquids) and poly(ionic liquids) were successively electrodeposited onto the pinpoint of an acupuncture needle (AN). The molecularly imprinted miniature biosensor was obtained after the template of SARS-CoV-2-S protein was removed, which could be used for sensitive detection of SARS-CoV-2-S protein. The linear range and limit of detection (LOD) were 0.1 ∼ 1000 ng mL−1 and 38 pg mL−1, respectively, which were superior to other molecularly imprinted biosensors previously reported. The developed miniature biosensor also exhibited high specificity and stability. The reliability of the biosensor was evaluated by the detection of SARS-CoV-2-S protein in clinical serum samples.
1. Introduction
The worldwide spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has posed a serious threat to global public health in the past two years [1], [2], and thus early diagnosis of SARS-CoV-2 is of significant importance for human health and global economy. Reverse transcription-polymerase chain reaction (RT-PCR) [3] and the detection of IgG/IgM in whole blood or serum [4] are the two main methods used for the diagnosis of SARS-CoV-2. However, RT-PCR has inevitable limitations such as high cost, time-consumption and trained personnel requirement [5], while the detection of IgG/IgM has little significance for clinical diagnosis due to the positive specific antibodies in vaccinated individuals [6]. Recently, the detection of SARS-CoV-2 specific antigens such as nucleoprotein [7], [8] and spike protein [9], [10] has provided the possibility of early diagnosis of SARS-CoV-2. Especially, SARS-CoV-2 spike (SARS-CoV-2-S) protein plays a crucial role in the cell entry of coronaviruses [11], and thus SARS-CoV-2-S protein is considered as one of the most important specific antigens for the detection of SARS-CoV-2.
Electrochemical methods have attracted considerable attention in medical applications due to their low cost, high sensitivity and ease of operation [12], [13]. Compared with conventional glassy carbon electrodes (GCE) and Au/Pt disk electrodes, stainless steel acupuncture needle (AN) has small size with a superfine pinpoint and good conductivity, which is a competent candidate to be used as the electrode substrate for the construction of miniature electrochemical sensing platforms for in vivo analysis [14], [15], [16].
Molecular imprinting technology can be used for the detection of specific targets based on the imprinted sites formed within the molecularly imprinted polymers (MIPs) [17], [18], and it displays enhanced stability and affinity over conventional aptasensors [19]. Among the developed molecular imprinting strategies, surface imprinting is particularly suitable for protein imprinting due to its high binding capacity and easy removal of protein templates [20].
Room temperature ionic liquids (RTILs) are usually molten salts comprised of anions and cations [21]. Due to their high thermal stability and exceptional chemical properties, RTILs have been widely used for various biochemical applications related to proteins, enzymes and amino acids [22], [23], [24]. More importantly, RTILs containing vinyl or pyrrolidinyl groups can be polymerized, and the resultant poly(ionic liquids) (PILs) can be used as the potential MIPs in the process of surface imprinting [25].
Herein, a molecularly imprinted miniature electrochemical biosensor was developed for the detection of SARS-CoV-2-S protein. Au nanoparticles (AuNPs), reduced graphene oxide (rGO), poly(methylene blue)/PILs and PILs were successively electrodeposited onto the tip of the AN. The use of AuNPs and rGO can increase the electroactive surface area of AN and provide π-π interactions with poly(methylene blue) (PMB)/PILs, respectively. The template of SARS-CoV-2-S protein can be immobilized onto the electrode surface through electrostatic interactions [26], which can be further removed with phosphate buffer saline (PBS) containing 5 % (v/v) methanol. The obtained molecularly imprinted miniature biosensor was used for sensitive and selective detection of the SARS-CoV-2-S protein. In addition, the developed miniature biosensor could be successfully used in the detection of SARS-CoV-2-S protein in the clinical serum samples by standard addition method.
2. Experimental section
2.1. Materials and reagents
Acupuncture needle (AN, 0.25 × 40 mm) was obtained from Suzhou Medical Supplies Co., ltd. (Suzhou, China). Tetrachloroauric(III) acid trihydrate (HAuCl4·3H2O) and l-phenylalanine (l-Phe) were received from Sinopharm Chemical Reagent Co., ltd. (Shanghai, China). Methylene blue (MB), 1-vinyl-3-butylimidazolium bromide ([Bvim]Br) were obtained from Yien Chemical Technology Co., ltd. (Shanghai, China). SARS-CoV-2-S protein dissolved in 0.02 M PBS containing 150 mM NaCl (pH = 7.4) was received from Yibaixin Biotechnology Co., ltd. (Hangzhou, China). Bovine serum albumin (BSA), glutathione (GSH) and l-tryptophan (l-Trp) were purchased from Aladdin Chemical Reagent Co., ltd. (Shanghai, China). Graphene oxide (GO) and immunoglobulins (IgG and IgM) were purchased from Xianfeng Nanomaterial Technology Co., ltd. (Nanjing, China) and Solarbio Technology Co., ltd. (Beijing, China), respectively. Human serum samples were obtained from Changzhou No.3 People’s Hospital, and the experiments involving them were carried out in line with the institutional guidelines of Changzhou/Jiaxing University and consent was obtained for their use from Changzhou No.3 People’s Hospital. Other reagents not mentioned were of analytical grade. All aqueous solutions were prepared with ultrapure water (18.2 MΩ·cm) produced by a Milli-Q purification system (Merck, Germany). Scanning electron microscopy (SEM) characterizations of different samples were conducted on a Sigma 500 scanning electron microscope (Zeiss, Germany). All electrochemical measurements were carried out with a CHI 660E electrochemical workstation (Shanghai Chenhua Instruments Co., ltd., China).
2.2. Fabrication of PMB/PILs/rGO/AuNPs/AN
First, AN was pretreated by the method previously reported [27]. In a typical procedure, AN was cleaned successively in water and ethanol by ultrasonication and dried in ambient air, and then the needle body was covered by a layer of isolated epoxy resin with 5 mm space from the pinpoint left for modification.
Electrodeposition of AuNPs on the AN pinpoint was carried out in a 2.5 mM HAuCl4 solution at a constant potential of –0.8 V for 100 s. The AuNPs modified AN (AuNPs/AN) was used as the substrate for the deposition of rGO, which was conducted in a 1.0 mg mL−1 GO solution at a negative potential of –1.4 V for 600 s, and the obtained electrode was denoted as rGO/AuNPs/AN. After that, the deposition of PMB and PILs on the rGO/AuNPs/AN surface was achieved via simultaneous electropolymerization of MB and [Bvim]Br by cyclic voltammetry (potential range: –0.8 ∼ 1.2 V) for 15 cycles (scan rate: 50 mV s−1), and the electrolyte used was 0.1 M PBS containing 2.0 mM MB, 1.0 mM [Bvim]Br and 0.1 M Na2SO4 (pH = 8.2). The obtained electrode was rinsed with 0.1 M PBS (pH = 8.2) to remove the free [Bvim]Br and MB, which was denoted as PMB/P[Bvim]Br/rGO/AuNPs/AN.
2.3. Fabrication of molecularly imprinted miniature biosensor
The PMB/P[Bvim]Br/rGO/AuNPs/AN was incubated in the solution of SARS-CoV-2-S protein (50 μg mL−1) for 2 h to introduce SARS-CoV-2-S protein as the template, and then the [Bvim]Br was electropolymerized again on the electrode surface by cyclic voltammetry (potential range: –0.5 ∼ 1.2 V) for 5 cycles at a scan rate of 80 mV s−1 (electrolyte: 0.01 M PBS + 20 mM [Bvim]Br). P[Bvim]Br deposited on the surface of PMB/P[Bvim]Br/rGO/AuNPs/AN could function as the MIPs, and the free [Bvim]Br was removed by 0.1 M PBS of pH 7.4. After that, the SARS-CoV-2-S protein template was eluted with 0.1 M PBS (pH = 5.0) containing 5 % (v/v) methanol for 40 min, and the molecularly imprinted miniature biosensor (MI/PMB/P[Bvim]Br/rGO/AuNPs/AN) was obtained. For control experiments, non-imprinted miniature biosensor (NI/PMB/P[Bvim]Br/rGO/AuNPs/AN) was also fabricated by the same procedures without the addition of the SARS-CoV-2-S protein template.
2.4. Detection of SARS-CoV-2-S protein with the miniature biosensor
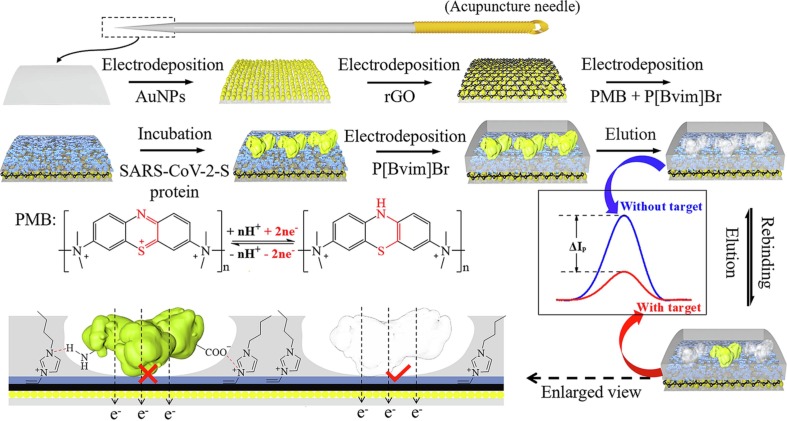
The as-fabricated miniature biosensor was incubated in the solution of 0.02 M PBS (pH = 7.4) containing 150 mM NaCl and different concentrations of SARS-CoV-2-S protein at 4 °C for 30 min for the rebinding of the SARS-CoV-2-S protein, and the unbound SARS-CoV-2-S protein was rinsed with 0.02 M PBS containing 150 mM NaCl. After that, the rebound SARS-CoV-2-S protein was detected by square wave voltammetry in 0.1 M PBS of pH 7.4 at room temperature, and the concentration of the SARS-CoV-2-S protein was obtained according to the decrease in the peak currents of PMB. The fabrication of the miniature electrochemical biosensor and the principle of SARS-CoV-2-S protein detection are illustrated in Schematic 1 .
Schematic 1.
Schematic illustration showing the fabrication of the miniature electrochemical biosensor and the principle of SARS-CoV-2-S protein detection.
3. Results and discussion
3.1. Simultaneous electropolymerization of MB and [Bvim]Br
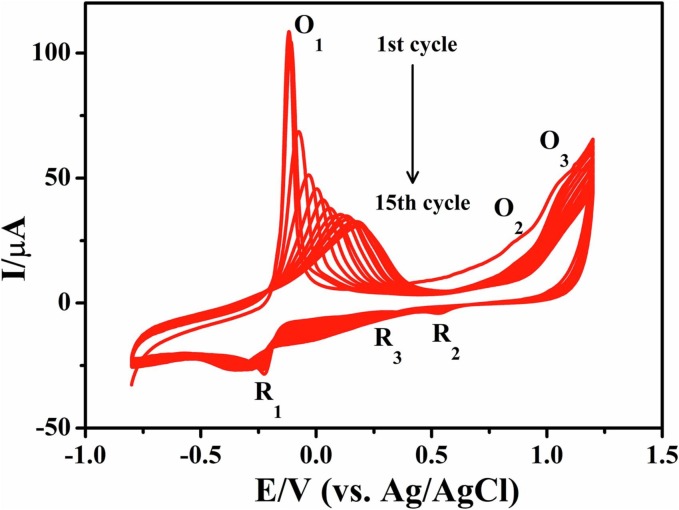
First, AuNPs are anchored to the AN pinpoint through electrodeposition according to the following reactions: (1) HAuCl4 → H+ + AuCl4 −, (2) AuCl4 − + 3e− → Au + 4Cl− [28], and the deposited AuNPs can increase the electroactive surface area of AN. After that, electrochemical reduction of GO with a lamellar structure (Fig. S1) is carried out at –1.4 V [29] for the electrodeposition of rGO on the surface of AuNPs/AN, which can provide π-π interactions with PMB/PILs. The cyclic voltammograms (CVs) during the simultaneous electropolymerization of MB and [Bvim]Br on the surface of rGO/AuNPs/AN are shown in Fig. 1 . There are three pairs of redox peaks on the CVs. Among them, O1/R1 (–0.12/–0.23 V) and O2/R2 (0.87/0.53 V) are related to the redox of MB, while O3/R3 (1.04/0.39 V) is due to the redox of [Bvim]Br. The peak currents decrease gradually when the electropolymerization is carried out from the 1st to the 15th cycle. The product of P[Bvim]Br can be used for the immobilization of the SARS-CoV-2-S protein template through electrostatic attraction, since the SARS-CoV-2-S protein (isoelectric point: 6.78) is negatively charged in PBS of pH 7.4 while the [Bvim] is positively charged. Another product of PMB can function as the signal probe for the following detection of the SARS-CoV-2-S protein.
Fig. 1.
Cyclic voltammograms during the simultaneous electropolymerization of MB and [Bvim]Br on the surface of rGO/AuNPs/AN for 15 cycles in 0.1 M PBS containing 2.0 mM MB, 1.0 mM [Bvim]Br and 0.1 M Na2SO4 (pH = 8.2). Scan rate, 50 mV s−1.
3.2. Electropolymerization of [Bvim]Br on the surface of PMB/P[Bvim]Br/rGO/AuNPs/AN as MIPs
A second electropolymerization of [Bvim]Br is carried out to form the MIPs on the surface of PMB/P[Bvim]Br/rGO/AuNPs/AN, and the CVs are presented in Fig. 2 . The O1/R1 redox peaks on the first circle (–0.22/–0.41 V) correspond to the redox of PMB, which disappear gradually from the 2nd to the 5th cycle owing to formation of P[Bvim]Br on the electrode surface. Another pair of redox peaks (O2/R2) at 1.04/0.39 V is related to the redox of [Bvim]Br, agreeing well with that shown in Fig. 1. The layer of P[Bvim]Br deposited on the surface of PMB/P[Bvim]Br/rGO/AuNPs/AN functions as the MIPs in the process of surface imprinting.
Fig. 2.
Cyclic voltammograms during of a second electropolymerization of [Bvim]Br on the surface of PMB/P[Bvim]Br/rGO/AuNPs/AN for 5 cycles in 0.01 M PBS containing 20 mM [Bvim]Br (pH = 7.4). Scan rate, 80 mV s−1.
3.3. Morphology characterization and elemental analysis
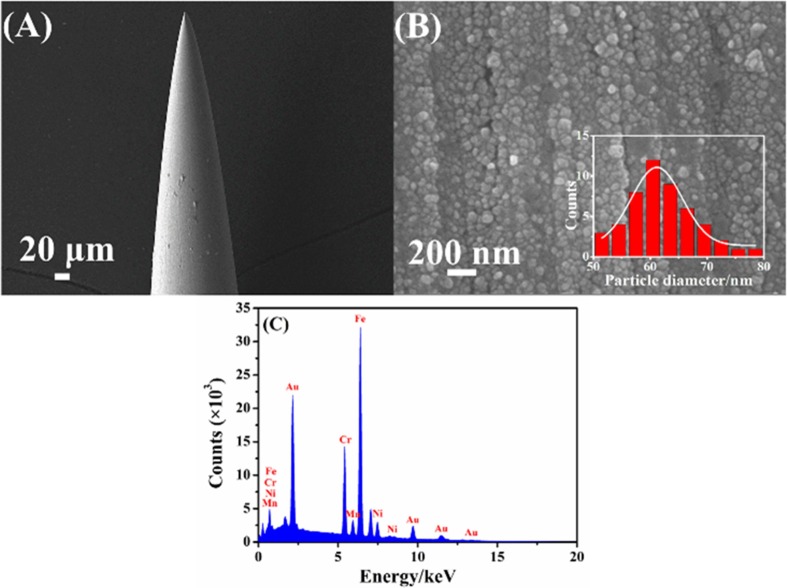
The SEM images of AN and AuNPs/AN are presented in Fig. 3 A and B, respectively. The AN exhibits a highly polished surface, while well-distributed AuNPs with an average size of ∼60 nm can be observed on the surface of AuNPs/AN. Fig. 3C is the energy dispersive X-ray spectroscopy (EDS) of AuNPs/AN, revealing the existence of Cr, Mn, Ni, Fe (from AN itself) and Au elements. Especially, the appearance of Au element further confirms the deposition of AuNPs on the surface of AN, which can provide a layer with large surface area and high electric conductivity for the subsequent modification [30]. The results of elemental mapping also demonstrate the successful introduction of AuNPs to the surface of AN (Fig. S2).
Fig. 3.
SEM images of AN (A) and AuNPs/AN (B). (C) EDS of AuNPs/AN. Inset of (B) is the size distribution of AuNPs.
Fig. S3A shows the SEM image of rGO/AuNPs/AN, and wrinkled and stacked rGO sheets appear on the surface of AuNPs/AN. After the simultaneous electropolymerization of MB and [Bvim]Br, a layer of PMB embedded with the P[Bvim]Br nanospheres can be clearly observed (Fig. S3B). The SEM images of P[Bvim]Br/PMB/P[Bvim]Br/rGO/AuNPs/AN and MI/PMB/P[Bvim]Br/rGO/AuNPs/AN are shown in Fig. S3C and D, respectively. As can be seen, the removal of the SARS-CoV-2-S protein template roughens the surface of MI/PMB/P[Bvim]Br/rGO/AuNPs/AN, which might be ascribed to the formation of imprinted cavities within the miniature biosensor.
3.4. Electrochemical behaviors of different electrodes
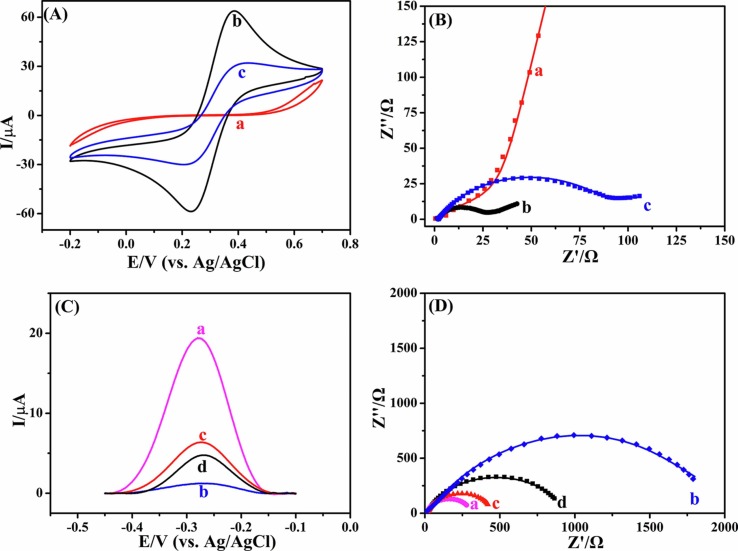
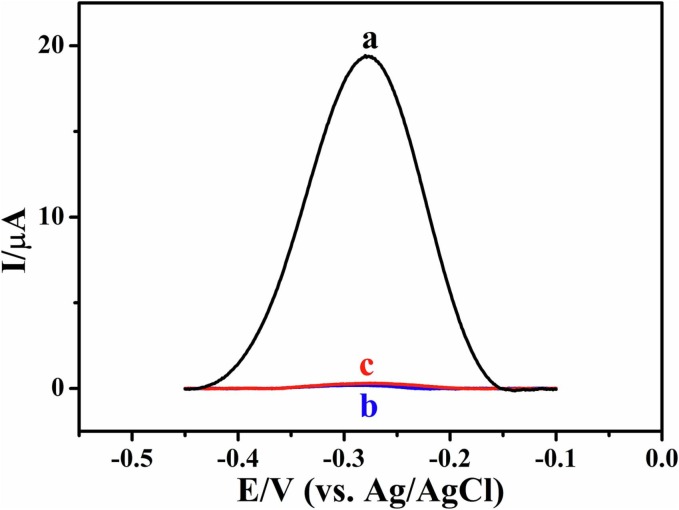
Step-by-step modification of AN can be further confirmed by the CVs and the electrochemical impedance spectroscopy (EIS). Fig. 4 A shows the CVs of AN, AuNPs/AN and rGO/AuNPs/AN in 0.1 M KCl containing 5.0 mM [Fe(CN)6]3−/4−. The AN displays rather low peak currents (curve a) owing to the seriously inhibited heterogeneous electron transfer process of the Fe(CN)6]3−/4− redox probe by the steel material. The peak currents increase significantly at the AuNPs/AN (curve b), which is most likely due to the enhanced heterogeneous electron transfer after the electrodeposition of AuNPs. However, the peak currents decrease a little at the rGO/AuNPs/AN (curve c), which might be ascribed to the fact that only a fraction of GO which has low conductivity is transformed to rGO by electrochemical reduction. The results of EIS (Fig. 4B) agree well with those of the CVs. The charge transfer resistance (Rct) denoted by the diameter of the suppressed semicircle at high frequency of AuNPs/AN (22 Ω) is significantly smaller than those of rGO/AuNPs/AN (95 Ω) and AN (1.8 × 107 Ω). Fig. 4C shows the square wave voltammograms (SWVs) of different electrodes in 0.1 M PBS (pH = 7.4). Due to the oxidation of PMB, there is a huge oxidation peak on the SWVs of PMB/P[Bvim]Br/rGO/AuNPs/AN (curve a). After the immobilization of the SARS-CoV-2-S protein template and the subsequent deposition of P[Bvim]Br, the peak currents decrease remarkably at the P[Bvim]Br/PMB/P[Bvim]Br/rGO/AuNPs/AN due to the poor conductivity of SARS-CoV-2-S protein and P[Bvim]Br (curve b). The peak currents increase after the SARS-CoV-2-S protein template is eluted (curve c), which might be attributed to the facilitated charge transfer caused by the imprinted cavities, and the peak currents decrease again after the SARS-CoV-2-S protein in the solution is captured by the imprinted cavities (curve d). The Rct values of these electrodes are arranged in such an order: PMB/P[Bvim]Br/rGO/AuNPs/AN (258 Ω) < MI/PMB/P[Bvim]Br/rGO/AuNPs/AN (382 Ω) < MI/PMB/P[Bvim]Br/rGO/AuNPs/AN + SARS-CoV-2-S protein (1334 Ω) < P[Bvim]Br/PMB/P[Bvim]Br/rGO/AuNPs/AN (2248 Ω) (Fig. 4D), agreeing well with the results shown in Fig. 4C. For NI/PMB/P[Bvim]Br/rGO/AuNPs/AN, the peak currents of PMB vary little before and after the removal of the template (Fig. 5 ), which is due to the absence of the imprinted cavities within the NI/PMB/P[Bvim]Br/rGO/AuNPs/AN.
Fig. 4.
Cyclic voltammograms (A) and EIS (B) of AN (a), AuNPs/AN (b) and rGO/AuNPs/AN (c) in 0.1 M KCl containing 5.0 mM [Fe(CN)6]3−/4−. (C) Square wave voltammograms of PMB/P[Bvim]Br/rGO/AuNPs/AN (a), P[Bvim]Br/PMB/P[Bvim]Br/rGO/AuNPs/AN (b), MI/PMB/P[Bvim]Br/rGO/AuNPs/AN (c) and MI/PMB/P[Bvim]Br/rGO/AuNPs/AN + SARS-CoV-2-S protein (d) in 0.1 M PBS of pH 7.4. (D) EIS of the electrodes the same as those in (C) in 0.1 M KCl containing 20 mM [Fe(CN)6]3−/4−.
Fig. 5.
Square wave voltammograms of PMB/P[Bvim]Br/rGO/AuNPs/AN (a), NI/PMB/P[Bvim]Br/rGO/AuNPs/AN before (b) and after (c) the removal of the template in 0.1 M PBS of pH 7.4.
3.5. Optimization of parameters
To achieve the sensitive detection of SARS-CoV-2-S protein, several important parameters are optimized. For comparison, the peak currents of PMB/P[Bvim]Br/rGO/AuNPs/AN, MI/PMB/P[Bvim]Br/rGO/AuNPs/AN and MI/PMB/P[Bvim]Br/rGO/AuNPs/AN with rebound SARS-CoV-2-S protein on the SWVs are defined as I1, I2 and I3, respectively. Fig. 6 A shows the relationship between I1 and the cycling number for the simultaneous electropolymerization of MB and [Bvim]Br. The highest I1 is achieved when the polymerization is carried out for 15 cycles, which is most likely due to the blocked electron transfer by the excess PMB thickness after 15 cycles [31]. Since the sensitivity is closely related to the peak currents of PMB, the optimized cycling number is chosen as 15 for the highest sensitivity. The incubation time of PMB/P[Bvim]Br/rGO/AuNPs/AN in the solution of SARS-CoV-2-S protein is also optimized, and the results are shown in Fig. 6B. The value of I2 reaches the maximum at the incubation time of 2 h, which might be ascribed to the saturated imprinted cavities within the MI/PMB/P[Bvim]Br/rGO/AuNPs/AN. That is to say, the immobilization of the SARS-CoV-2-S protein template via electrostatic interactions can reach saturation after the incubation for 2 h. The relationship between I2 and the cycling number for the electropolymerization of [Bvim]Br is presented in Fig. 6C. As can be seen, the value of I2 decreases remarkably when the polymerization exceeds 5 cycles, which is due to the fact that the elution of the template becomes difficult with excess P[Bvim]Br thickness. Therefore, the electropolymerization of [Bvim]Br as the MIPs is carried out for 5 cycles. Fig. 6D shows the influence of elution time on the value of I2. The value of I2 increases with increasing elution time and varies little after 30 min, indicating that 30 min is enough for the removal of the SARS-CoV-2-S protein template. Fig. 6E shows the relationship between I3 and the rebinding time of the SARS-CoV-2-S protein. No decrease in I3 is observed at the rebinding time of 30 min, suggesting that the imprinted cavities are almost completely occupied by the SARS-CoV-2-S protein after 30 min.
Fig. 6.
Optimization of cycling number for simultaneous polymerization of MB and [Bvim]Br (A), incubation time (B), cycling number for polymerization of [Bvim]Br (C), elution time (D) and rebinding time of SARS-CoV-2-S protein (E).
3.6. Detection of SARS-CoV-2-S protein
Under the optimized conditions, the miniature electrochemical biosensor is used for the detection of SARS-CoV-2-S protein. Fig. 7 A shows the SWVs of the miniature biosensor after incubation in the solution containing different concentrations of SARS-CoV-2-S protein. As can be seen, the peak currents of PMB decrease gradually with increasing concentration of SARS-CoV-2-S protein. The decreased currents of PMB can be due to the fact that with increasing concentration of SARS-CoV-2-S protein, the imprinted cavities within the miniature biosensor are gradually occupied by the SARS-CoV-2-S protein in the solution, leading to blocked electron transfer during the oxidation of PMB. There is a good linear relationship between the value of (I2 – I3)/I2 and the logarithm value of the concentration of SARS-CoV-2-S protein (Fig. 7B), and the linear range and limit of detection (LOD) are 0.1 ∼ 1000 ng mL−1 and 38 pg mL−1 (S/N = 3), respectively. The linear regression equation can be expressed as following: (I2 – I3)/I2 = 0.0516 lgC + 0.0648 (R2 = 0.9940). The performances of the developed miniature biosensor are compared with other biosensors used for the detection of SARS-CoV-2-S protein, and the results are listed in Table 1 . Compared with the biosensors previously reported [9], [19], [32], our biosensor displays a higher LOD but a significantly wider linear range, indicating its great potential for the detection of SARS-CoV-2-S protein.
Fig. 7.
(A) Square wave voltammograms of the miniature biosensor after incubation in the solution of 0.02 M PBS (pH = 7.4) containing 150 mM NaCl and different concentrations of SARS-CoV-2-S protein (0, 0.1, 0.5, 1.0, 5.0, 10, 50, 100, 500 and 1000 ng mL−1) at 4 °C for 30 min. (B) Linear relationship between the value of (I2 – I3)/I2 and the logarithm value of the concentration of SARS-CoV-2-S protein.
Table 1.
Comparison of the developed miniature biosensor with other biosensors previously reported for the detection of SARS-CoV-2-S protein.
| Biosensor | Linear range | LOD | Reference |
|---|---|---|---|
| MIP/DTSSPa/4-ATPb/Au-TFMEc | 2.0 ∼ 14.5 pg mL−1 | 1.12 pg mL−1 | [9] |
| AuNPs/carbon cloth | 0 ∼ 1000 ng mL−1 | 110 pg mL−1 | [19]d |
| AuNPs/carbon cloth | 0 ∼ 1000 ng mL−1 | 37.8 ng mL−1 | [19]e |
| MIP/MP-Au-SPEf | 2.0 ∼ 40.0 pg mL−1 | 0.7 pg mL−1 | [32] |
| AN biosensor | 0.1 ∼ 1000 ng mL−1 | 38 pg mL−1 | This work |
DTSSP: 3,3′-dithiobis (sulfosuccinimidyl propionate).
4-ATP: 4-aminothiophenol.
Au-TFME: Au thin-film metal electrode.
by differential pulse voltammetry.
by chronopotentiometry
MP-Au-SPE: macroporous gold screen-printed electrode.
3.7. Specificity and stability of the miniature biosensor
The specificity of the biosensor is investigated by using several possible co-existing substances including BSA, IgG, IgM, GSH, l-Trp and l-Phe (1 μg mL−1) as the interferences for the detection of SARS-CoV-2-S protein of the same concentration, and the results are presented in Fig. 8 A. As can be seen, these inferences yield remarkably lower values of (I2 – I3)/I2 than that of SARS-CoV-2-S protein, suggesting excellent specificity of the biosensor. The stability of the miniature biosensor is also studied, and the results are shown in Fig. 8B. It shows that the value of I2 remains almost unchanged in the first 7 days, and it can still retain 76 % of the initial value after 15 days. This result indicates that the developed biosensor has high stability.
Fig. 8.
Evaluation of specificity (A) and stability (B) of the developed miniature biosensor.
3.8. Detection of SARS-CoV-2-S protein in clinical serum samples
Finally, the developed miniature biosensor is used for the detection of SARS-CoV-2-S protein in three clinical serum samples by standard addition method, and the results are presented in Table 2 . As can be seen, the recoveries are in the range from 94.4 % to 102.0 % and the values of relative standard deviation (RSD) are no more than 1.00 %, demonstrating high reliability of the developed miniature biosensor for clinical diagnosis.
Table 2.
Detection of SARS-CoV-2-S protein in three clinical serum samples.
| Determined (ng mL−1) |
Added (ng mL−1) |
Found (ng mL−1) | RSD (n = 3) |
Recovery |
|---|---|---|---|---|
| 0 | 10 | 9.44 ± 0.04 | 0.41 | 94.4 % |
| 0 | 30 | 30.60 ± 0.20 | 0.66 | 102.0 % |
| 0 | 50 | 50.65 ± 0.51 | 1.00 | 101.3 % |
4. Conclusions
A novel surface molecularly imprinted miniature biosensor is developed for the detection of SARS-CoV-2-S protein. AuNPs, rGO, PMB/PILs and PILs are successively electrodeposited onto the pinpoint of the AN. The template of SARS-CoV-2-S protein can be immobilized on the electrode surface through electrostatic interactions, and the miniature biosensor is obtained after the template is eluted. Compared with the SARS-CoV-2-S protein biosensors previously reported, the developed miniature biosensor displays a higher LOD but a significantly wider linear range, indicating its great potential for the detection of SARS-CoV-2-S protein. Also in this work, the reliability of the molecularly imprinted miniature biosensor for the early diagnosis of SARS-CoV-2 protein is demonstrated by successful detection of SARS-CoV-2-S protein in clinical serum samples. The developed miniature biosensor might be a potential candidate for in vivo analysis of SARS-CoV-2-S protein.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by Science and Technology Program of Zhejiang Province (LGF22B050008), Science and Technology Program of Jiaxing (2022AY10012), Science and Technology Program of Changzhou (CJ20220226), Natural Science Foundation for Colleges and Universities in Jiangsu Province (20KJA150005), Shandong Key Laboratory of Biochemical Analysis (SKLBA2105) and Advanced Catalysis and Green Manufacturing Collaborative Innovation Center (ACGM2022-10-12). We also thank Mr. Wenchang Wang from Analysis and Testing Centre of Changzhou University for the assistance on SEM.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioelechem.2023.108375.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- 1.Liu J.X., Chen P., Hu X.L., Huang L.P., Geng Z., Xu H., Hu W.J., Wang L., Wu P., Liu G.L. An ultra-sensitive and specific nanoplasmonic-enhanced isothermal amplification platform for the ultrafast point-of-care testing of SARS-CoV-2. Chem. Eng. J. 2023;451 doi: 10.1016/j.cej.2022.138822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park S., Jeon C.S., Choi N., Moon J.I., Lee K.M., Pyun S.H., Kang T., Choo J. Sensitive and reproducible detection of SARS-CoV-2 using SERS-based microdroplet sensor. Chem. Eng. J. 2022;446 doi: 10.1016/j.cej.2022.137085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mina M.J., Peto T.E., Garcia-Finana M., Semple M.G., Buchan I.E. Clarifying the evidence on SARS-CoV-2 antigen rapid tests in public health responses to COVID-19. Lancet. 2021;397:1425–1427. doi: 10.1016/S0140-6736(21)00425-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Z.X., Yin Z.Z., Zheng G.J., Zhang H.Y., Zhou M., Li S., Kong Y. Dual-template molecularly imprinted electrochemical biosensor for IgG-IgM combined assay based on a dual-signal strategy. Bioelectrochemistry. 2022;148 doi: 10.1016/j.bioelechem.2022.108267. [DOI] [PubMed] [Google Scholar]
- 5.Liang Q.Z., Huang Y., Wang M.H., Kuang D.Q., Yang J.H., Yi Y.X., Shi H., Li J.L., Yang J., Li G.X. An electrochemical biosensor for SARS-CoV-2 detection via its papain-like cysteine protease and the protease inhibitor screening. Chem. Eng. J. 2023;452 doi: 10.1016/j.cej.2022.139646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward H., Whitaker M., Flower B., Tang S.N., Atchison C., Darzi A., Donnelly C.A., Cann A., Diggle P.J., Ashby D., Riley S., Barclay W.S., Elliott P., Cooke G.S. Population antibody responses following COVID-19 vaccination in 212,102 individuals. Nat. Commun. 2022;13:907. doi: 10.1038/s41467-022-28527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang T., Sun L.A., Zhang Y.Y. Highly sensitive electrochemical determination of the SARS-COV-2 antigen based on a gold/graphene imprinted poly-arginine sensor. Anal. Methods. 2021;13:5772–5776. doi: 10.1039/d1ay01478a. [DOI] [PubMed] [Google Scholar]
- 8.Raziq A., Kidakova A., Boroznjak R., Reut J., Opik A., Syritski V. Development of a portable MIP-based electrochemical sensor for detection of SARS-CoV-2 antigen. Biosens. Bioelectron. 2021;178 doi: 10.1016/j.bios.2021.113029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayankojo A.G., Boroznjak R., Reut J., Opik A., Syritski V. Molecularly imprinted polymer based electrochemical sensor for quantitative detection of SARS-CoV-2 spike protein. Sens. Actuators, B. 2022;353 doi: 10.1016/j.snb.2021.131160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ratautaite V., Boguzaite R., Brazys E., Ramanaviciene A., Ciplys E., Juozapaitis M., Slibinskas R., Bechelany M., Ramanavicius A. Molecularly imprinted polypyrrole based sensor for the detection of SARS-CoV-2 spike glycoprotein. Electrochim. Acta. 2022;403 doi: 10.1016/j.electacta.2021.139581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Z.X., Yin Z.Z., Cai W.R., Wu D.T., Li J.Y., Kong Y. A surface protein−imprinted biosensor based on boronate affinity for the detection of anti−human immunoglobulin G. Microchim. Acta. 2022;189:106. doi: 10.1007/s00604-022-05204-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar N., Shetti N.P., Jagannath S., Aminabhavi T.M. Electrochemical sensors for the detection of SARS-CoV-2 virus. Chem. Eng. J. 2022;430 doi: 10.1016/j.cej.2021.132966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y.T., Tang L.N., Ning Y., Shu Q., Liang F.X., Wang H., Zhang G.J. In vivo monitoring of serotonin by nanomaterial functionalized acupuncture needle. Sci. Rep. 2016;6:28018. doi: 10.1038/srep28018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou J.X., Tang L.N., Yang F., Liang F.X., Wang H., Li Y.T., Zhang G.J. MoS2/Pt nanocomposite-functionalized microneedle for real-time monitoring of hydrogen peroxide release from living cells. Analyst. 2017;142:4322–4329. doi: 10.1039/c7an01446e. [DOI] [PubMed] [Google Scholar]
- 16.Zhou B.B., Ou W.H., Zhao C.H., Shen J.D., Zhang G.B., Tang X.H., Deng Z.Q., Zhu G.Y., Li Y.Y., Lu J. Insertable and reusable SERS sensors for rapid on-site quality control of fish and meat products. Chem. Eng. J. 2021;426 [Google Scholar]
- 17.Li Z.Y., Xu H., Wu D.T., Zhang J., Liu X.R., Gao S.M., Kong Y. Electrochemical chiral recognition of tryptophan isomers based on nonionic surfactant-assisted molecular imprinting sol-gel silica. ACS. Appl. Mater Interfaces. 2019;11:2840–2848. doi: 10.1021/acsami.8b19399. [DOI] [PubMed] [Google Scholar]
- 18.Krishnan H., Gopinath S.C.B., Arshad M.K.M., Zulhaimi H.I., Anbu P., Subramaniam S. Molecularly imprinted polymer enhances affinity and stability over conventional aptasensor for blood clotting biomarker detection on regimented carbon nanohorn and gold nanourchin hybrid layers. Sens. Actuators, B. 2022;363 [Google Scholar]
- 19.Adeel M., Asif K., Alshabouna F., Canzonieri V., Rahman M.M., Ansari S.A., Guder F., Rizzolio F., Daniele S. Label-free electrochemical aptasensor for the detection of SARS-CoV-2 spike protein based on carbon cloth sputtered gold nanoparticles. Biosens. Bioelectron. X. 2022;12 doi: 10.1016/j.biosx.2022.100256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schirhagl R., Ren K.N., Zare R.N. Surface-imprinted polymers in microfluidic devices. Sci. China Chem. 2012;55:469–483. [Google Scholar]
- 21.Paul A., Muthukumar S., Prasad S. Review—room-temperature ionic liquids for electrochemical application with special focus on gas sensors. J. Electrochem. Soc. 2020;167 [Google Scholar]
- 22.Dong K., Zhang S.J., Wang J.J. Understanding the hydrogen bonds in ionic liquids and their roles in properties and reactions. Chem. Commun. 2016;52:6744–6764. doi: 10.1039/c5cc10120d. [DOI] [PubMed] [Google Scholar]
- 23.Upasham S., Banga I.K., Jagannath B., Paul A., Lin K.C., Muthukumar S., Prasad S. Electrochemical impedimetric biosensors, featuring the use of room temperature ionic liquids (RTILs): Special focus on non-faradaic sensing. Biosens. Bioelectron. 2021;177 doi: 10.1016/j.bios.2020.112940. [DOI] [PubMed] [Google Scholar]
- 24.Hu X.P., Tang Y., Xia Y.D., Liu Y.W., Zhao F.Q., Zeng B.Z. Antifouling ionic liquid doped molecularly imprinted polymer-based ratiometric electrochemical sensor for highly stable and selective detection of zearalenone. Anal. Chim. Acta. 2022;1210 doi: 10.1016/j.aca.2022.339884. [DOI] [PubMed] [Google Scholar]
- 25.Wang X., Wang Y.Y., Ye X.X., Wu T., Deng H.P., Wu P., Li C.Y. Sensing platform for neuron specific enolase based on molecularly imprinted polymerized ionic liquids in between gold nanoarrays. Biosens. Bioelectron. 2018;99:34–39. doi: 10.1016/j.bios.2017.07.037. [DOI] [PubMed] [Google Scholar]
- 26.Qian L.W., Hu X.L., Guan P., Gao B., Li J., Wang C.L., Tang Y.M. Preparation of bovine serum albumin imprinting sensitive hydrogels using ionic liquid as co-monomer and stabilizer. Talanta. 2014;121:56–64. doi: 10.1016/j.talanta.2013.12.061. [DOI] [PubMed] [Google Scholar]
- 27.Niu X.L., Wen Z.R., Li X.B., Zhao W.S., Li X.Y., Huang Y.Q., Li Q.T., Li G.J., Sun W. Fabrication of graphene and gold nanoparticle modified acupuncture needle electrode and its application in rutin analysis. Sens. Actuators, B. 2018;255:471–477. [Google Scholar]
- 28.Sharma P., Sablok K., Bhalla V., Suri C.R. A novel disposable electrochemical immunosensor for phenyl urea herbicide diuron. Biosens. Bioelectron. 2011;26:4209–4212. doi: 10.1016/j.bios.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 29.Shi X.M., Yin Z.Z., Xu J.L., Li S., Wang C.C., Wang B.H., Qin Y., Kong Y. Preparation, characterization and the supercapacitive behaviors of electrochemically reduced graphene quantum dots/polypyrrole hybrids. Electrochim. Acta. 2021;385 [Google Scholar]
- 30.Xu C.H., Gu C.C., Xiao Q., Chen J.D., Yin Z.Z., Liu H.Y., Fan K., Li L.H. A highly selective and sensitive biosensor for dopamine based on a surface molecularly imprinted layer to coordinate nano-interface functionalized acupuncture needle. Chem. Eng. J. 2022;436 [Google Scholar]
- 31.Phonklam K., Wannapob R., Sriwimol W., Thavarungkul P., Phairatana T. A novel molecularly imprinted polymer PMB/MWCNTs sensor for highlysensitive cardiac troponin T detection. Sens. Actuators, B. 2020;308 [Google Scholar]
- 32.Tabrizi M.A., Fernandez-Blazquez J.P., Medina D.M., Acedo P. An ultrasensitive molecularly imprinted polymer-based electrochemical sensor for the determination of SARS-CoV-2-RBD by using macroporous gold screen-printed electrode. Biosens. Bioelectron. 2022;196 doi: 10.1016/j.bios.2021.113729. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.