Abstract
Purpose
To determine the prevalence of anemia and its association with Helicobacter pylori infection among adult dyspeptic patients.
Patients and Methods
A cross-sectional study was conducted among 283 dyspeptic patients at Kiryandongo General Hospital, in Uganda. A structured questionnaire was administered to capture demographic and clinical characteristics of study participants. Four milliliters of blood were then collected into an EDTA vacutainer for Complete Blood Count (CBC) and analyzed using HUMA COUNT 30TS, and peripheral blood smears were made and stained using Giemsa stain. Anemia was defined as hemoglobin levels <12g/dl in females and <13g/dl in men according to the World Health Organization (WHO). Helicobacter pylori (H. pylori) stool antigen test was performed using Whole power H. pylori Ag rapid test device, and saline stool preparation was examined for intestinal parasites. Chi-squared test and Logistic regression were performed to determine association, and a p-value of ≤0.05 was considered statistically significant.
Results
The overall prevalence of Helicobacter pylori infection was 42.4% (120/283). The prevalence of anemia among H. pylori-infected patients was 25.8% (31/120) and 15.3% (25/163) among H. pylori-negative counterparts. H. pylori infection was significantly associated with anemia (p-value 0.042), age (p-value 0.02, 0.009), water sources (p-value 0.0049,) and intestinal parasitic infestation (p-value 0.02), respectively.
Conclusion
This study has shown that the prevalence of H. pylori infection and anemia is high among dyspeptic patients at Kiryandongo General Hospital. H. pylori infection was found associated with anemia, age, water sources, and intestinal parasitic infestation. Routine screening of anemia in H. pylori-infected individuals and further studies to explore the relationship between anemia and H. pylori disease is highly recommended.
Keywords: anemia, prevalence, Helicobacter pylori infection
Introduction
Helicobacter pylori (H. pylori) infection is a global public health problem affecting both developed and developing countries1,2 with a higher burden of 50.8% reported in developing countries compared to 34.7% in developed countries.3 Helicobacter pylori is a helix-shaped, curved rod and gram-negative bacteria. It causes gastritis, peptic ulcer disease, ggastroduodenal ulcer, atrophic gastritis, gastric cancers, and dyspeptic symptom.4,5 However, more than 80% of persons who become infected with H. pylori are usually asymptomatic. H. pylori plays a vital role in the natural stomach ecology.6
H. pylori infection affects about 4.4 billion people worldwide.1 Africa has the highest prevalence of the infection, 70.1%, and the prevalence ranged from 18.9% in Switzerland to 87.7% in Nigeria.1 In Southern Asia, Pakistan and India showed the highest prevalence and in Western Asia, Turkey reported the leading prevalence, 77.2%.6 In Uganda, the prevalence of H. pylori among dyspeptic patients was 74%.7
H. pylori infection has been implicated in hematological manifestations such as anemia and micronutrient deficiency (iron and vitamin B12).8 It has also been related to extra-gastric manifestations, eg thrombocytopenic purpura, reduction in growth velocity, iron deficiency, and/or anemia.9,10 Several studies described that by eliminating H. pylori bacteria, the iron nutritional status becomes normal without the necessity for iron supplementation.11–14
The mechanism or mechanisms through which H. pylori infection may cause iron deficiency and/or anemia are not fully understood but probable mechanisms comprise an upsurge in intragastric pH; reduced concentration of ascorbic acid in gastric juices, which disturbs iron absorption from the diet; chronic bleeding caused by the increase of micro-erosions in gastric mucous; production of lactoferrins by neutrophils; and capture of iron by the bacteria.15 Another way could be the rise in the synthesis of hepcidin, an essential regulator of iron metabolism that inhibits iron absorption in the small intestine.16
Therefore, we aimed to determine the prevalence of anemia and its association with H. pylori infection among adult dyspeptic patients.
Materials and Methods
Study Area
The study was carried out at Kiryandongo General Hospital which is located in Kiryandongo District. The hospital is approximately 225 km from Kampala along the Kampala-Gulu highway. It is the biggest public hospital along the Kampala-Gulu highway serving Kiryandongo and parts of Masindi, Nakasongola, Apac, Amuru, and Oyam districts with an estimated catchment population of 47,155 people. Kiryandongo General Hospital laboratory is accredited by the South African National Accreditation System (SANAS) with the capacity to diagnose H. pylori infection and anemia.
Study Design and Period
This was a prospective cross-sectional study conducted from November 2021 to February 2022.
Sample Size Calculation
This was done according to a similar study carried out in Southwest Ethiopia where the prevalence of anemia was 24.3%17 using the Kish and Leslie formula.
n = Z2 P(1-P)/d2 (1)
Where.
n = the desired sample size.
Z = critical values of normal distribution at 95%, which corresponds to 1.96
P = the proportion of the target population estimated to have H. pylori infection and anemia 24.3%
Hence n = 283 study participants.
Sampling Technique
A random sampling procedure was done where every adult dyspeptic patient identified was considered for the study. Study participants who met the inclusion criteria were allowed to pick numbers from the box and those that picked odd numbers were enrolled in the study.
Selection Criteria
All adult dyspeptic patients aged 18 years and above attending Kiryandongo General Hospital who consented to participate in the study were included. Patients who had undergone gastrectomy and iron supplements were excluded from the study. Adults who had a history of chronic disease or severely ill patients were also excluded from the study.
Data Collection
Demographic data (age, sex, occupation, household income, marital status, level of education) and risk factors associated with anemia, dietary supplements, history of alcohol consumption, deworming status and drinking boiled or treated water were collected from the participants using a questionnaire.
Dyspepsia was defined as upper abdominal discomfort, often chronic or persistent indigestion, and symptoms include fullness, bloating, nausea, loss of appetite or upper abdominal pain. All study participants with the above symptoms were enrolled in the study.
Four mL (4mL) of the venous blood sample was then collected from each dyspeptic patient into ethylenediamine tetraacetic acid (EDTA) vacutainers. Complete blood count (CBC) was determined using an automated hematology analyzer (HUMA COUNT 30TS, Germany). Thin blood films were made on all blood samples with anemia (Hb concentration <12g/dl for females and <13g/dl for males). Thin and thick blood films were made, thin films were fixed with absolute methanol, and both were stained using Giemsa stain for 20 minutes. Thick films were examined at ×1000 magnification for malaria parasites, and thin films were examined for morphological classification of anemia. Sterile stool containers were given to the participants with an explanation on how to collect approximately 1 gram of stool specimen and tested for the presence of H. pylori by stool antigen rapid test strips (Whole Power H. pylori antigen rapid test device, Zhejiang Orient Gene Biotech Co, LTD, China) with the sensitivity and specificity of >95 and 95.7%, respectively. Saline stool preparations were also done and examined for the presence of intestinal parasites.
Data Analysis
Data collected were entered into an Excel spreadsheet, cleaned and checked fo,r completeness and then exported to STATA software version 14 for analysis. Demographic data were analyzed and presented in the form of percentages and frequencies. The association between H. pylori infection and anemia was studied using Pearson’s chi-squared test. Association between H. pylori infection and risk factors was studied using logistic regression. Odds ratio >1 and p-value ≤0.05 were considered statistically significant.
Ethical Consideration
This study was approved by the Faculty Research Committee (FRC) of Mbarara University of Science and Technology with approval number (MUST/MLS/30). Ethical clearance was also got from the management of Kiryandongo General Hospital before conducting the study. Informed consent was obtained from all study participants before their involvement in the study and was confirmed by their signature or thumbprint on the consent form. This study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki (1964). Confidentiality of the study participants was observed by giving each participant a study code that was not traceable to them. Participants were also informed that their participation is free and voluntary and that they had a right to withdraw from the study at any time and that their withdrawal would not affect their access to medical care.
Results
Socio-Demographic Characteristics of Study Participants
We recruited 283 dyspeptic patients, and 193 (68.2%) were females. The mean age of the study participants was 31.9 years and a standard deviation of 12.7 years. The majority of them were aged 18–22 years (26.8%), had primary education (53.7%), were living in the rural area (73.5%), were not smoking (97.2%), were not taking alcohol (88%) and were drinking piped water (52.3%) as indicated in Table 1.
Table 1.
Baseline Characteristics of the Study Participants
| Variable | Category | Frequency (n) | Percentage (%) |
|---|---|---|---|
| Gender | |||
| Male | 90 | 31.8 | |
| Female | 193 | 68.2 | |
| Age (years) | |||
| 18–22 | 76 | 26.8 | |
| 23–27 | 51 | 18.0 | |
| 28–32 | 52 | 18.4 | |
| 33–37 | 32 | 11.3 | |
| 38–42 | 22 | 7.8 | |
| ≥42 | 50 | 17.7 | |
| Education level | |||
| None | 30 | 10.6 | |
| Primary | 152 | 53.7 | |
| Secondary | 87 | 30.7 | |
| Tertiary | 14 | 5.0 | |
| Residence | |||
| Rural | 208 | 73.5 | |
| Urban | 75 | 26.5 | |
| Smoking | |||
| No | 275 | 97.2 | |
| Yes | 8 | 2.8 | |
| Alcohol intake | |||
| No | 249 | 88.0 | |
| Yes | 34 | 12.0 | |
| Water source | |||
| Piped | 148 | 52.3 | |
| Spring | 107 | 37.8 | |
| Open water | 28 | 9.9 |
Prevalence of H. pylori Infection Among Dyspeptic Patients
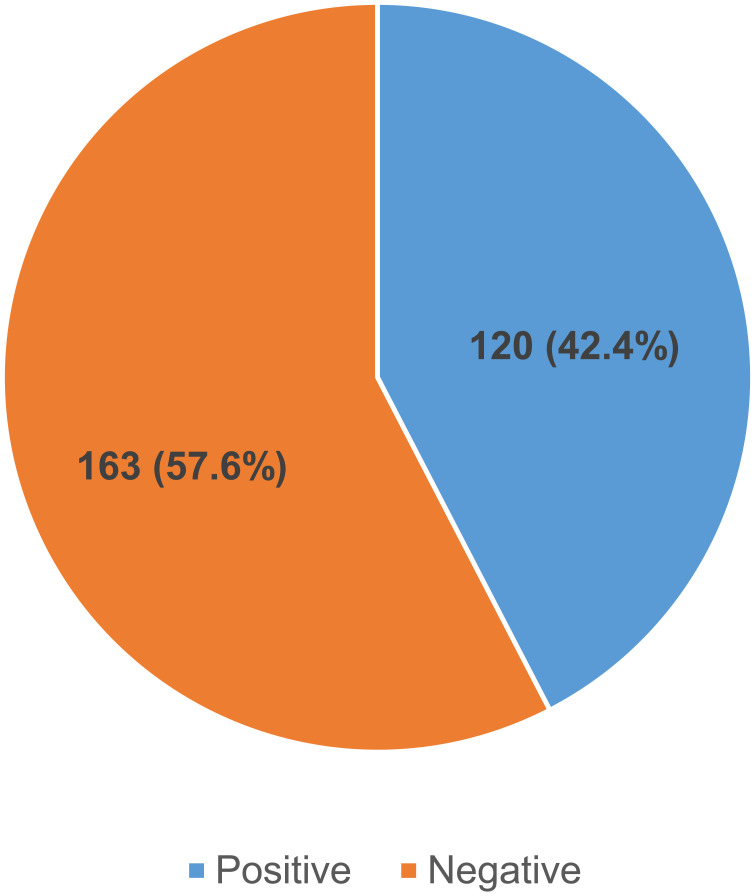
The overall prevalence of H. pylori infection was 42.4% (120/283) (Figure 1).
Figure 1.
Pie chart showing prevalence of H. pylori infection among adult dyspeptic patients at Kiryandongo General Hospital.
Prevalence of Anemia Among Dyspeptic Patients and Its Association with H. pylori Infection
The prevalence of anemia among dyspeptic patients was 19.8% (56/283). The mean hemoglobin was 13.9 g/dL with a Standard Deviation (SD) of 2.7 g/dL. Mean hemoglobin among males and females was 14.9 g/dL and 13.5 g/dL with SD of 2.7 g/dL and 2.6 g/dL, respectively. However, the prevalence of anemia among H. pylori-infected individuals was higher at 25.8% (31/120) compared to H. pylori-negative patient counterparts at 15.3% (25/163). The difference in the prevalence of anemia among H. pylori patients and uninfected patients was statistically significant with a Pearson chi-squared value of 4.8 and a p-value of 0.029 (Table 2).
Table 2.
A 2 × 2 Dyspeptic Patients with H. pylori Infection in Association with Anemia
| H. pylori-Negative | H. pylori-Positive | Total | X2 (p-value) | |
|---|---|---|---|---|
| Non-anemic | 138 | 89 | 227 | |
| Anemic | 25 | 31 | 56 | 4.8 (0.029) |
| Total | 163 | 120 | 283 |
Note: Chi-square 4.8 and p-value 0.029.
Morphological Classification of Anemia Among H. pylori-Infected Dyspeptic Patients
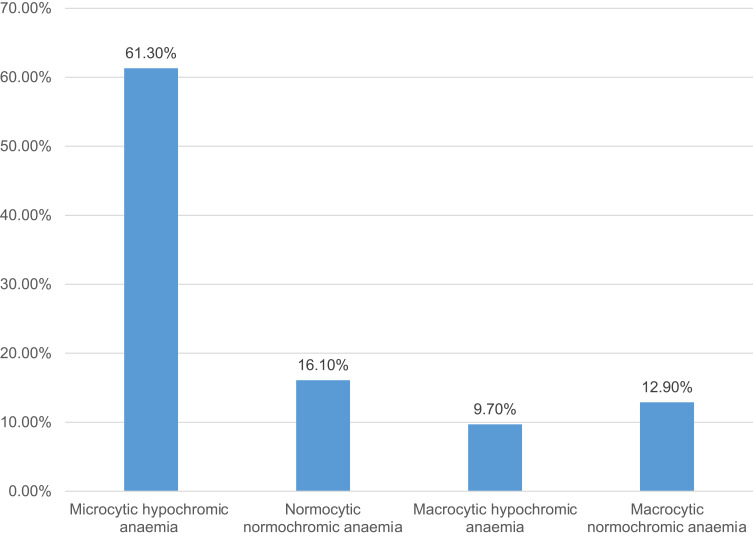
The majority of the H. pylori-infected patients who were anemic had microcytic hypochromic anemia 19 (61.3%), followed by normocytic normochromic anemia 5 (16.1%), macrocytic normochromic anemia 4 (12.9%) and macrocytic hypochromic anemia 3 (9.7%) as shown in Figure 2.
Figure 2.
H. pylori infected patients with different morphological types of anemia.
Factors Associated with H. pylori Infection Among Dyspeptic Patients
Bivariate Analysis of Factors Associated with H. pylori Infection
On bivariate analysis, age (p = 0.08 and 0.009), and stool Parasitemia (p = 0.027) were associated with H. pylori infection (Table 3).
Table 3.
Bivariate Analysis of Factors Associated with H. pylori Infection
| Variables | Odds ratio | 95% Confidence Interval | P value | |
|---|---|---|---|---|
| Gender | ||||
| Male | Ref | |||
| Female | 0.87 | 0.49–1.54 | 0.634 | |
| Age (years) | ||||
| 18–22 | Ref | |||
| 23–27 | 1.44 | 0.66–3.15 | 0.366 | |
| 28–32 | 1.96 | 0.89–4.30 | 0.094 | |
| 33–37 | 3.48 | 1.38–8.76 | 0.008* | |
| 38–42 | 4.18 | 1.42–12.26 | 0.009* | |
| >42 | 1.02 | 0.41–2.53 | 0.961 | |
| Education level | ||||
| None | Ref | |||
| Primary | 0.40 | 0.16–1.04 | 0.060 | |
| Secondary | 0.50 | 0.18–1.41 | 0.188 | |
| Tertiary | 0.34 | 0.07–1.57 | 0.167 | |
| Boil drinking water | ||||
| Yes | Ref | |||
| No | 0.75 | 0.43–1.34 | 0.334 | |
| Washing hands before eating | ||||
| Yes | Ref | |||
| No | 3.93 | 0.87–17.72 | 0.075 | |
| Water sources | ||||
| Piped | Ref | |||
| Spring | 0.61 | 0.34–1.09 | 0.097 | |
| Open well | 0.50 | 0.19–1.27 | 0.144 | |
| Washing facility at the toilet | ||||
| Yes | Ref | |||
| No | 1.33 | 0.39–4.56 | 0.641 | |
| Residence | ||||
| Rural | Ref | |||
| Urban | 1.52 | 0.83–2.80 | 0.169 | |
| Smoking | ||||
| No | Ref | |||
| Yes | 0.86 | 0.17–4.25 | 0.853 | |
| Alcohol | ||||
| No | Ref | |||
| Yes | 0.93 | 0.40–2.17 | 0.871 | |
| Anemia | ||||
| Non-Anemic | Ref | |||
| Anemic | 1.53 | 0.78–3.00 | 0.212 | |
| Stool Parasitemia | ||||
| No | Ref | |||
| Yes | 11.30 | 1.31–97.46 | 0.027* |
Note: *Significant factor associated with H. pylori.
Multivariate Analysis of Factors Associated with H. pylori Infection
On multivariate analysis, age (p = 0.020 and 0.009), water source (p = 0.049) and stool Parasitemia (p = 0.020) were found associated with H. pylori infection as shown in Table 4.
Table 4.
Multivariate Analysis of Factors Associated with H. pylori Infection
| Variable | Adjusted Odds Ratio | 95% Confidence Interval | P value | |
|---|---|---|---|---|
| Age (years) | ||||
| 18–22 | Ref | |||
| 23–27 | 1.37 | 0.64–2.95 | 0.416 | |
| 28–32 | 1.91 | 0.88–4.15 | 0.100 | |
| 33–37 | 2.85 | 1.18–6.90 | 0.020* | |
| 38–42 | 4.06 | 1.42–11.63 | 0.009* | |
| >42 | 0.85 | 0.36–2.05 | 0.725 | |
| Education level | ||||
| None | Ref | |||
| Primary | 0.42 | 0.17–1.05 | 0.064 | |
| Secondary | 0.54 | 0.20–1.48 | 0.231 | |
| Tertiary | 0.42 | 0.10–1.82 | 0.247 | |
| Water sources | ||||
| Piped | Ref | |||
| Spring | 0.57 | 0.33–1.00 | 0.049* | |
| Open well | 0.55 | 0.22–1.39 | 0.207 | |
| Washing hands before eating | ||||
| Yes | Ref | |||
| No | 3.60 | 0.82–15.72 | 0.090 | |
| Stool Parasitemia | ||||
| No | Ref | |||
| Yes | 12.34 | 1.48–103.11 | 0.020* |
Note: *Significant factor associated with H. pylori.
Discussion
Prevalence of Anemia and H. pylori Infection Among Dyspeptic Patients
In our study, the overall prevalence of anemia was 19.8% and H. pylori infection among dyspeptic patients was 42.4%. However, the prevalence of anemia among H. pylori-infected individuals was higher at 11% compared to H. pylori-negative patient counterparts at 8.8%. This prevalence is higher than the reported prevalence of H. pylori infection in Bwera Hospital in Kasese, Uganda, by Tsongo et al at 29.9%,18 in Kampala 35.7%,19 27.3% in eastern Uganda20 and 29.2% in Butembe Health Centre III Kyankwanzi district, Uganda.
Our study finding is not comparable with a study finding from China that found that the burden of H. pylori infection was 35.3%21 but consistent with a study by Baingana et al in Uganda 45.2%7 in Nairobi 46.2%22 43.9% reported in China23 and 41% observed in the United Arab Emirates.6 Our study finding is lower than the reported prevalence of H. pylori infection among dyspeptic patients from Ethiopia 52.4%,24 Nigeria 67.4%,25 and Ibadan 63.5%.26 The observed variation in the burden of H. pylori infection is probably attributed to the lack of clear cut-off for dyspepsia, different diagnostic methods used, sample size, and socioeconomic factors.
Association of Anemia and H. pylori Infection
Anemia was significantly associated with H. pylori with a Chi-squared value of 4.8 and a p-value of 0.029. It also showed at a multivariate level that those who were infected with H. pylori were 1.92 times more likely to be anemic than the H. pylori-negative group. This finding is in line with findings from Ethiopia,24 and China23 that observed an association of H. pylori infection with anemia. Another study from China showed that H. pylori patients had a 2.53 times increased risk of being anemic compared to the H. pylori-negative group.21 Our finding is however not in line with other scholars’ findings from Latin America27 and the rural Haitian population28 that reported no association between H. pylori and anemia. This could be due to the sample size studied, geographical variation of the disease, and methods used in the diagnosis of H. pylori and anemia.
This study observed that H. pylori infection was significantly associated with middle age (33–42 years old) and intestinal parasites. This finding is consistent with the findings in studies in Ethiopia24,29 Kuwait,30 South Africa,31 Brazil and in the United Arab Emirates where H. Pylori infection was observed to Increase with age32 but it differs with the study by Ahmed et al, in Khartoum33 that reported H. pylori infection and its association in individuals >60 years old and a study in eastern Uganda where age was not significantly associated with H. pylori infection.20
A case–control study in Sudan reported a relationship between intestinal parasites and H. pylori infection.34 Another study in Venezuela also showed evidence of an association between H. pylori infection and intestinal parasitic infections,35 and similar finding had been reported in Ethiopia.36 A study by Zylberberg et al in the US reported that H. pylori infected study participants are independently associated with giardiasis,37 and similar finding was reported in Germany.38
This current study observed association between H. pylori infection and different water sources. The risk of acquiring H. pylori infection seems to be multifactorial and potentially contaminated environmental sources, such as local drinking water, swimming in rivers, or the ingestion of fecally contaminated vegetables have been reported as risk factors for H. pylori infection.39,40 A study by Khoder et al similarly demonstrated the association between H. pylori infection and source of drinking water.6 Baingana et al in Uganda demonstrated that H. pylori infection was independently associated with using water from public wells, boreholes or springs and from rivers, lakes or streams.7 However, the association found in this study does not imply causality but it predicts the risk of the occurrence of the disease among the population with the associated variables.
Morphological Types of Anemia Among H. pylori Patients
The major morphological form of anemia in H. pylori-infected patients was microcytic hypochromic anemia followed by normocytic normochromic anemia, macrocytic normochromic anemia, and macrocytic hypochromic anemia. This highly suggests that H. pylori infection causes iron deficiency by reducing iron absorption from the intestine and causes majorly microcytic hypochromic red blood cells with low hemoglobin concentration due to iron deficiency.24 This is not in line with findings from Ethiopia.17 They found that the major morphological type of anemia among H. pylori-infected patients was normocytic normochromic anemia. Another study found that the major morphological class of anemia among H. pylori patients was macrocytic normochromic anemia.23
Limitations
The cross-sectional design coupled with lack of control population for comparison limited the opportunity to establish the association of H. pylori infection and anemia.
Conclusion
The prevalence of H. pylori infection reported among dyspeptic patients in the Kiryandongo district in this study was high. H. pylori infection was independently associated with anemia, increasing age, water sources and intestinal parasitaemia among dyspeptic patients. Microcytic hypochromic anemia was the commonest morphological type of anemia among dyspeptic patients with H. pylori infection. Routine screening of dyspeptic patients for H. pylori infection, hemoglobin estimation of the infected patients and improvement of water sources is advised.
Acknowledgments
We acknowledge the study participants, the Clinicians, the Principal Nursing Officer, and the Laboratory staff of Kiryandongo General Hospital for the support they gave us during the study period.
Data Sharing Statement
The datasets used in the analysis of this research study are available from the corresponding author upon reasonable request.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hooi JK, Lai WY, Ng WK., et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153(2):420–429. doi: 10.1053/j.gastro.2017.04.022 [DOI] [PubMed] [Google Scholar]
- 2.Mendoza E, Duque X, Hernández Franco JI, et al. Association between active H. pylori infection and iron deficiency assessed by serum hepcidin levels in school-age children. Nutrients. 2019;11(9):2141. doi: 10.3390/nu11092141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zamani M, Ebrahimtabar F, Zamani V, et al. Systematic review with meta‐analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. 2018;47(7):868–876. doi: 10.1111/apt.14561 [DOI] [PubMed] [Google Scholar]
- 4.de Brito BB, da Silva FAF, Soares AS, et al. Pathogenesis and clinical management of Helicobacter pylori gastric infection. World J Gastroenterol. 2019;25(37):5578. doi: 10.3748/wjg.v25.i37.5578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaakoush NO, Castaño-Rodríguez N, Mitchell HM, Man SM. Global epidemiology of Campylobacter infection. Clin Microbiol Rev. 2015;28(3):687–720. doi: 10.1128/CMR.00006-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khoder G, Muhammad JS, Mahmoud I, Soliman SS, Burucoa C. Prevalence of Helicobacter pylori and its associated factors among healthy asymptomatic residents in the United Arab Emirates. Pathogens. 2019;8(2):44. doi: 10.3390/pathogens8020044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baingana RK, Kiboko Enyaru J, Davidsson L. Helicobacter pylori infection in pregnant women in four districts of Uganda: role of geographic location, education and water sources. BMC Public Health. 2014;14(1):1–10. doi: 10.1186/1471-2458-14-915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campuzano-Maya G. Hematologic manifestations of Helicobacter pylori infection. World J Gastroenterol. 2014;20(36):12818. doi: 10.3748/wjg.v20.i36.12818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malfertheiner P, Selgrad M. Helicobacter pylori infection and current clinical areas of contention. Curr Opin Gastroenterol. 2010;26(6):618–623. doi: 10.1097/MOG.0b013e32833efede [DOI] [PubMed] [Google Scholar]
- 10.Pacifico L, Osborn JF, Tromba V, Romaggioli S, Bascetta S, Chiesa C. Helicobacter pylori infection and extragastric disorders in children: a critical update. World J Gastroenterol. 2014;20(6):1379. doi: 10.3748/wjg.v20.i6.1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang X, Qu X, Yan W, et al. Iron deficiency anaemia can be improved after eradication of Helicobacter pylori. Postgrad Med J. 2010;86(1015):272–278. doi: 10.1136/pgmj.2009.089987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wenzhen Y, Yumin L, Kehu Y, et al. Iron deficiency anemia in Helicobacter pylori infection: meta-analysis of randomized controlled trials. Scand J Gastroenterol. 2010;45(6):665–676. doi: 10.3109/00365521003663670 [DOI] [PubMed] [Google Scholar]
- 13.Xin-Hua Q. Iron deficiency anemia can be improved after eradication of Helicobacter pylori. Postgrad Med J. 2010;86:272–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hudak L, Jaraisy A, Haj S, Muhsen K. An updated systematic review and meta‐analysis on the association between H elicobacter pylori infection and iron deficiency anemia. Helicobacter. 2017;22(1):e12330. doi: 10.1111/hel.12330 [DOI] [PubMed] [Google Scholar]
- 15.Stein J, Connor S, Virgin G, Ong DEH, Pereyra L. Anemia and iron deficiency in gastrointestinal and liver conditions. World J Gastroenterol. 2016;22(35):7908. doi: 10.3748/wjg.v22.i35.7908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganz T. Systemic iron homeostasis. Physiol Rev. 2013;93(4):1721–1741. doi: 10.1152/physrev.00008.2013 [DOI] [PubMed] [Google Scholar]
- 17.Haile K, Yemane T, Tesfaye G, Wolde D, Timerga A, Haile A. Anemia and its association with Helicobacter pylori infection among adult dyspeptic patients attending Wachemo University Nigist Eleni Mohammad Memorial Referral Hospital, Southwest Ethiopia: a cross-sectional study. PLoS One. 2021;16(1):e0245168. doi: 10.1371/journal.pone.0245168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsongo L, Nakavuma J, Mugasa C, Kamalha E. Helicobacter pylori among patients with symptoms of gastroduodenal ulcer disease in rural Uganda. Infect Ecol Epidemiol. 2015;5(1):26785. doi: 10.3402/iee.v5.26785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Namyalo E, Nyakarahuka L, Afayoa M, et al. Prevalence of Helicobacter pylori among Patients with Gastrointestinal Tract (GIT) Symptoms: a Retrospective Study at Selected Africa Air Rescue (AAR) Clinics in Kampala, Uganda, from 2015 to 2019. J Trop Med. 2021;2021:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nekaka R, Oboth P, Nteziyaremye J, Gavamukulya Y, Ssenyonga LV, Iramiot JS. Sero prevalence and factors associated with Helicobacter pylori infection in a rural population in Eastern Uganda a community cross sectional study. Primary Health Care. 2021;11(4):1–9. [Google Scholar]
- 21.Hou B, Zhang M, Liu M, et al. Association of active Helicobacter pylori infection and anemia in elderly males. BMC Infect Dis. 2019;19(1):1–9. doi: 10.1186/s12879-019-3849-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Said MK. Prevalence of Helicobacter Pylori Infection Among Patients with Peptic Ulcers and the Associated Risk Factors in Mbagathi Level V Hospital. Nairobi County, Kenya: School Of Medicine, Kenyatta University; 2019. [Google Scholar]
- 23.Xu M-Y, Cao B, Yuan B-S, Yin J, Liu L, Lu Q-B. Association of anaemia with Helicobacter pylori infection: a retrospective study. Sci Rep. 2017;7(1):1–7. doi: 10.1038/s41598-016-0028-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kibru D, Gelaw B, Alemu A, Addis Z. Helicobacter pylori infection and its association with anemia among adult dyspeptic patients attending Butajira Hospital, Ethiopia. BMC Infect Dis. 2014;14(1):1–7. doi: 10.1186/s12879-014-0656-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aje A, Otegbayo J, Odaibo G, Bojuwoye B. Comparative study of stool antigen test and serology for among Nigerian dyspeptic patients-a pilot study. Niger J Clin Pract. 2010;13(2):120–124. [PubMed] [Google Scholar]
- 26.Jemilohun AC, Otegbayo JA, Ola SO, Oluwasola OA, Akere A. Prevalence of Helicobacter pylori among Nigerian patients with dyspepsia in Ibadan. Pan Af Med J. 2010;6:1. doi: 10.4314/pamj.v6i1.69063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santos IS, Boccio J, Davidsson L, et al. Helicobacter pylori is not associated with anaemia in Latin America: results from Argentina, Brazil, Bolivia, Cuba, Mexico and Venezuela. Public Health Nutr. 2009;12(10):1862–1870. doi: 10.1017/S1368980009004789 [DOI] [PubMed] [Google Scholar]
- 28.Shak JR, Sodikoff JB, Speckman RA, et al. Anemia and Helicobacter pylori seroreactivity in a rural Haitian population. Am J Trop Med Hyg. 2011;85(5):913. doi: 10.4269/ajtmh.2011.11-0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiferaw G, Abera D. Magnitude of Helicobacter pylori and associated risk factors among symptomatic patients attending at Jasmin internal medicine and pediatrics specialized private clinic in Addis Ababa city, Ethiopia. BMC Infect Dis. 2019;19(1):1–6. doi: 10.1186/s12879-019-3753-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alazmi WM, Siddique I, Alateeqi N, Al-Nakib B. Prevalence of Helicobacter pylori infection among new outpatients with dyspepsia in Kuwait. BMC Gastroenterol. 2010;10(1):1–4. doi: 10.1186/1471-230X-10-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanih N, Okeleye B, Ndip I, et al. Helicobacter pylori prevalence in dyspeptic patients in the Eastern Cape Province–race and disease status. South Af Med J. 2010;100(11):734–737. doi: 10.7196/SAMJ.4041 [DOI] [PubMed] [Google Scholar]
- 32.Escobar-Pardo ML, Godoy A, Machado RS, Rodrigues D, Fagundes Neto U, Kawakami E. Prevalence of Helicobacter pylori infection and intestinal parasitosis in children of the Xingu Indian Reservation. J Pediatr. 2011;87:393–398. doi: 10.2223/JPED.2118 [DOI] [PubMed] [Google Scholar]
- 33.Ahmed NFM. Prevalence rate of Giardia lamblia/Helicobacter pylori co-infections in Khartoum state-Sudan. BMC Public Health. 2016. [Google Scholar]
- 34.Abd Elbagi YY, Abd Alla AB, Saad MBE. The relationship between Helicobacter pylori infection and intestinal parasites in individuals from Khartoum state, Sudan: a case-control study. F1000Research. 2019;8:8. doi: 10.12688/f1000research.17047.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuenmayor-Boscán AD, Hernández IM, Valero KJ, Paz AM, Sandrea LB, Rivero Z. Association between Helicobacter pylori and intestinal parasites in an Añu indigenous community of Venezuela. Indian J Gastroenterol. 2016;35(2):106–112. doi: 10.1007/s12664-016-0641-4 [DOI] [PubMed] [Google Scholar]
- 36.Seid A, Tamir Z, Kasanew B, Senbetay M. Co-infection of intestinal parasites and Helicobacter pylori among upper gastrointestinal symptomatic adult patients attending Mekanesalem Hospital, northeast Ethiopia. BMC Res Notes. 2018;11(1):1–6. doi: 10.1186/s13104-018-3246-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zylberberg HM, Green PH, Turner KO, Genta RM, Lebwohl B. Prevalence and predictors of giardia in the United States. Dig Dis Sci. 2017;62(2):432–440. doi: 10.1007/s10620-016-4447-0 [DOI] [PubMed] [Google Scholar]
- 38.Espelage W, Stark K, Alpers K. Characteristics and risk factors for symptomatic Giardia lamblia infections in Germany. BMC Public Health. 2010;10(1):1–9. doi: 10.1186/1471-2458-10-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leja MAA, Brenner H. Epidemiology of Helicobacter pylori infection. Helicobacter. 2016;21(3–7):2016. doi: 10.1111/hel.12332 [DOI] [PubMed] [Google Scholar]
- 40.Samra ZQ, Javaid U, Ghafoor S, Batool A, Dar N, Athar MA. PCR assay targeting virulence genes of Helicobacter pylori isolated from drinking water and clinical samples in Lahore metropolitan, Pakistan. J Water Health. 2011;9(1):208–216. doi: 10.2166/wh.2010.169 [DOI] [PubMed] [Google Scholar]