Summary
China is the world's second‐largest maize producer and consumer. In recent years, the invasive fall armyworm Spodoptera frugiperda (J.E. Smith) has adversely affected maize productivity and compromised food security. To mitigate pest‐inflicted food shortages, China's Government issued biosafety certificates for two genetically modified (GM) Bt maize hybrids, Bt‐Cry1Ab DBN9936 and Bt‐Cry1Ab/Cry2Aj Ruifeng 125, in 2019. Here, we quantitatively assess the impact of both Bt maize hybrids on pest feeding damage, crop yield and food safety throughout China's maize belt. Without a need to resort to synthetic insecticides, Bt maize could mitigate lepidopteran pest pressure by 61.9–97.3%, avoid yield loss by 16.4–21.3% (range −11.9–99.2%) and lower mycotoxin contamination by 85.5–95.5% as compared to the prevailing non‐Bt hybrids. Yield loss avoidance varied considerably between experimental sites and years, as mediated by on‐site infestation pressure and pest identity. For either seed mixtures or block refuge arrangements, pest pressure was kept below established thresholds at 90% Bt maize coverage in Yunnan (where S. frugiperda was the dominant species) and 70% Bt maize coverage in other sites dominated by Helicoverpa armigera (Hübner) and Ostrinia furnacalis (Guenée). Drawing on experiences from other crop/pest systems, Bt maize in se can provide area‐wide pest management and thus, contribute to a progressive phase‐down of chemical pesticide use. Hence, when consciously paired with agroecological and biodiversity‐based measures, GM insecticidal crops can ensure food and nutrition security, contribute to the sustainable intensification of China's agriculture and reduce food systems' environmental footprint.
Keywords: Bt maize, lepidopteran pest pressure, seed mixtures, block refuge, crop yield, mycotoxin contamination
Introduction
As the world's most widely cultivated cereal, maize plays a pivotal role in sustaining global food security. Maize provides up to 20% of food calories in Africa and Meso America, while constituting a prime fodder crop for livestock across the globe (Murray‐Tortarolo et al., 2018; Shiferaw et al., 2011). China is the world's second largest maize producer and consumer, cultivating 43.3 million hectares of maize (equivalent to 273 million tons of harvested grain) in 2021 (Wu et al., 2021a). Yet, to account for shortfalls in production, China imported 28 million tons of maize in 2021. Those production shortfalls have been ascribed to a loss of arable land, overuse of agrochemicals and pest‐inflicted yield losses (Lu, 2016). Pests and pathogens lower maize yields by 16–41% globally (Savary et al., 2019), and these losses are bound to increase by 10–25% under global warming (Deutsch et al., 2018).
China's maize crop is affected by multiple lepidopteran herbivores including the corn borer Ostrinia furnacalis Guenée (Crambidae), armyworm Mythimna separata Walker (Noctuidae) and cotton bollworm Helicoverpa armigera Hübner (Noctuidae). In late 2018, the invasive fall armyworm Spodoptera frugiperda J.E. Smith (Lepidoptera: Noctuidae) complemented the suite of resident herbivores, proliferated across China's maize cropping areas and further inflicted substantial losses in the country's maize crop (Jing et al., 2020; Song et al., 2021; Yang et al., 2021). Until recently, local S. frugiperda management has primarily relied on chemical insecticides. The S. frugiperda invasion led to threefold increases in pesticide usage intensity and an associated decline in farm‐level revenue, exacerbating insecticide resistance evolution and causing a suite of environmental, socio‐economic and human health problems (Yang et al., 2021). A transition towards less pesticide‐dependent approaches can spawn multiple societal benefits (Wyckhuys et al., 2022b). Much is to be gained from consolidating the agro‐ecological foundation of integrated pest management (IPM) and by preventing pest attacks through sanitary practices, varietal resistance, diversification tactics or biological control (Bommarco et al., 2013; Deguine et al., 2021; Dively et al., 2018).
Natural, bred or genetically engineered varietal resistance to plant pathogens and pests is a core constituent of IPM (Naranjo et al., 2020; Wyckhuys et al., 2022a). In this regard, genetically modified (GM) crops expressing Bacillus thuringiensis (Bt) insecticidal proteins can provide reduced pesticide use in controlling herbivorous pests (Edgerton et al., 2012; Lu et al., 2012; Perry et al., 2016). Transgenic Bt crops were initially commercialized in the United States during the 1990s and have since been adopted in multiple other countries. While instances of resistance evolution and secondary pest outbreaks have been recorded in Bt cropping systems (Tabashnik and Carriere, 2019; Zeilinger et al., 2016; Zhang et al., 2018), Bt crops can reduce crop yield losses and increase farmer income (Elbehri and MacDonald, 2004; Hutchison et al., 2010; Kiresur and Manjunath, 2011). Their establishment—even in comparatively small areas—can reduce insecticidal pollution of farmland, enhance biological control and generate societal dividends over extensive geographic areas (Dively et al., 2018; Hutchison et al., 2010; Zhang et al., 2018). When judiciously implemented, Bt crops represent markedly lower human and environmental health risks than pesticide‐based approaches (Mendelsohn et al., 2003).
Since the 1980s, China's Government has supported research on GM crops. In 2019, biosafety certificates were issued for two herbicide‐tolerant and insect‐resistant Bt maize varieties, i.e. DBN9936 and Ruifeng 125. Considering how maize‐feeding lepidopterans such as S. frugiperda are susceptible to Bt toxins (Li et al., 2021), these maize varieties can lower pesticide usage intensity but concurrently trigger Bt resistance evolution. Non‐Bt maize refugia can delay resistance evolution (Hutchison et al., 2010; Tellez‐Rodriguez et al., 2014) and are implemented through seed mixtures of Bt and non‐Bt cultivars at the appropriate ratio also termed ‘refuge‐in‐the‐bag’ or structured refuges, i.e. blocks or strips of non‐Bt maize within or adjacent to the Bt portion of the field. However, the scientific underpinnings of Bt crop refuges need to be strengthened and the required stewardship may pose challenges in China (Li et al., 2018). By mitigating pest infestation pressure, Bt maize can also resolve food safety issues that emanate from mycotoxin or pesticide contamination of harvested maize grain (Abbas et al., 2013; Koch et al., 2015; Pellegrino et al., 2018; Wyckhuys et al., 2020). Conversely, as compared to (Bt) insecticidal cultivars, herbicide‐tolerant ones tend to receive higher pesticide application rates, often lead to ecosystem simplification (e.g. by lowering the incidence of farmland weeds) and can increase farmers' input dependencies (Bonny, 2016; Tsatsakis et al., 2017). Nation‐wide adoption of glyphosate‐tolerant varieties may thus entail important risks for human well‐being and environmental health (Van Bruggen et al., 2018) and aggravate China's weed resistance issues (Zhu et al., 2020). Given the above, transgene stacking in maize not only represents opportunities but also major challenges for China's transition towards more sustainable food systems. Field trials are thus required to comprehensively gauge its field performance and environmental risks, and to contrast those with its societal benefits in terms of food or nutrition security.
In this study, we conducted (i) an assessment of lepidopteran infestation levels and the resulting grain yield or quality of the two biosafety‐certified Bt maize hybrids across China's main maize cropping areas, and (ii) a pilot evaluation of the performance of seed mixtures and block refuges with non‐Bt plants. As such, this work comprises a large‐scale evaluation of the relative contribution of Bt transgenic maize hybrids to lepidopteran pest control and a critical examination of their social‐environmental benefits in China.
Results
Pest infestation level
Lepidopteran pest complex
Experimental sites were established throughout China's main maize‐growing areas (Figure 1). Across maize phenological stages and sites, overall species composition and relative abundance of the main lepidopteran herbivores were similar on DBN9936's non‐Bt hybrid DBN9858 and Ruifeng 125's conventional hybrid Hongshuo 899 (Figure 2, Figure S1 and Table S1). Across sites for DBN9858, S. frugiperda was the most abundant pest across four stages (average 18.2 larvae/100 plants), followed by O. furnacalis (2.8 larvae/100 plants), M. separata (2.2 larvae/100 plants), H. armigera (1.5 larvae/100 plants), shoot borer Dichocrocis punctiferalis Guenée (Lepidoptera: Pyraustidae) (0.4 larvae/100 plants) and Athetis lepigone Möschler (Lepidoptera: Noctuidae) (0.1 larvae/100 plants). For specific maize hybrids or phenological stages, lepidopteran infestation pressure (number of larvae/100 plants) differed markedly between sites (DBN9858 V5: F 10,32 = 5.978, P < 0.001, R1: F 10,32 = 18.769, P < 0.001; Hongshuo 899 V5: F 10,32 = 26.996, P < 0.001, R1: F 10,32 = 20.364, P < 0.001). Spodoptera frugiperda was the dominant species in south‐western China, attaining plant‐level incidence of 100.0% in Yunnan (max. 227.3 larvae/100 plants at V10‐V12) and Guizhou (max. 123.5 larvae/100 plants at V10‐V12). Meanwhile, S. frugiperda attained comparatively low incidence in Hubei (max. 8.7 larvae/100 plants at VT‐R1), Jiangsu (max. 42.2 larvae/100 plants at V5‐V7) and Henan (max. 13.3 larvae/100 plants at R3‐R5) (Figure 2). In Guizhou, Yunnan (2019 and 2020) and Jiangsu (2020), other species attained infestation levels below 1 larva/100 plants. In northern and north‐eastern China, O. furnacalis and H. armigera were the dominant herbivores—usually attaining infestation levels below 50 larvae/100 plants. Lastly, H. armigera was the dominant herbivore in Xinjiang, north‐western China, where it attained an infestation level of 3.7 larvae/100 plants.
Figure 1.
Experimental locations across China's main maize‐growing regions. Different colours are indicative of province‐level maize production in 2021, as sourced from Statistical Yearbook China (2021).
Figure 2.
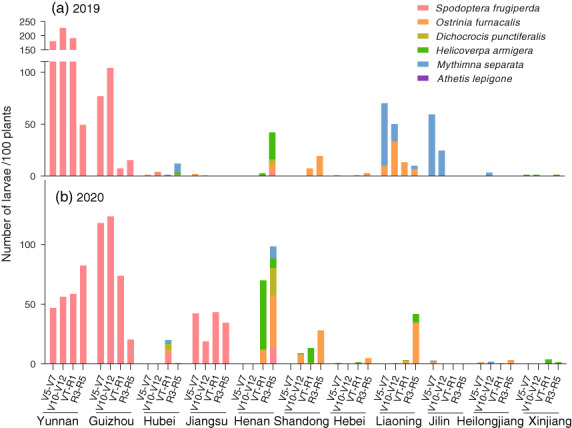
Infestation levels for six lepidopteran herbivores at different phenological stages of non‐Bt maize DBN9858 during 2019 and 2020. Larval infestation levels are shown for each of the 11 experimental sites in China's primary maize‐growing areas.
Bt maize control efficacy
Across experimental treatments and years, Bt‐maize DBN9936 and Ruifeng 125 effectively suppressed the prevailing lepidopteran herbivores. In seed mixture field trials, larval densities on DBN9936 (0% non‐Bt maize) at V5‐V7 stage attained 0.5 ± 0.3 (2019) and 0.9 ± 0.5 (2020) larvae per 100 plants across all sites. These infestation levels were significantly lower than those of the corresponding non‐Bt hybrids (DBN9858, 100% non‐Bt maize) of 21.1 ± 10.5 (t = 9.29, P < 0.001) and 19.2 ± 11.2 (t = 64.04, P < 0.001) larvae/100 plants during either year (Figure 3a). In block refuge field trials, larval densities on Ruifeng 125 (0% non‐Bt maize) at the V5 stage attained 1.1 ± 0.7 (2019) and 2.6 ± 1.3 (2020) larvae/100 plants across all sites. Larval densities were lower than those of Hongshuo 899 (100% non‐Bt maize) at 17.9 ± 10.2 (t = 39.85, P < 0.001) and 14.7 ± 9.1 (t = 12.14, P < 0.001) larvae/100 plants during either year (Figure 3b, Table S2).
Figure 3.
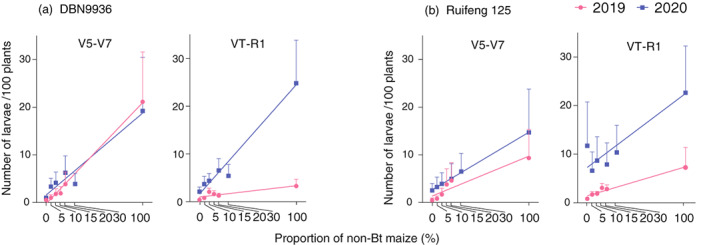
Larval infestation level increases with the relative cover of non‐Bt maize, as exemplified for seed mixture field trials with Bt‐Cry1Ab maize DBN9936 (a) and block refuge field trials with Bt‐Cry1Ab/Cry2Aj maize Ruifeng 125 (b). Each point on the graph represents the average larval infestation level of all locations. Patterns are plotted for DBN9936 and Ruifeng 125 maize plants at V5‐V7 and VT‐R1 stages. Error bars are only shown in an upwards direction.
For the two Bt maize hybrids, control efficacy did not differ among locations except for Ruifeng 125 in 2020 (Ruifeng 125: F 8,75 = 1.175, P = 0.333 (2019); F 10,96 = 14.785, P < 0.001 (2020); DBN9936: F 10,75 = 0.753, P = 0.672 (2019); F 10,98 = 1.885, P = 0.063 (2020)). Also, control efficacy did not differ between maize phenological stages for either year (Ruifeng 125: F 3,75 = 2.323, P = 0.086 (2019); F 3,96 = 2.941, P = 0.04 (2020); DBN9936: F 3,75 = 0.849, P = 0.472 (2019); F 3,98 = 1.885, P = 0.063 (2020)). DBN9936 attained an overall control efficacy of 86.6% (2019) and 97.3% (2020) at V5‐V7 stage, 78.9% and 74.5% at V10‐V12 stage, 79.0% and 93.8% at VT‐R1 stage and 61.9% and 92.1% at R3‐R5 stage across all sites. Ruifeng 125 attained control efficacy levels of 97.3% (2019) and 83.1% (2020) at V5‐V7 stage, 76.3% and 91.8% at V10‐V12 stage, 75.1% and 80.2% at VT‐R1 stage and 79.4% and 87.1% at R3‐R5 stage. In Yunnan and Guizhou where S. frugiperda was the main herbivore, Bt maize attained high control efficacy at V5‐V7 stage. DBN9936 had efficacy levels of 95.6–97.7% in Guizhou and 96.6–99.2% in Yunnan, while Ruifeng 125 reached efficacy levels of 90.2–87.4% in Guizhou and 93.2–79.7% in Yunnan during 2019 and 2020 respectively. In Guizhou, non‐Bt hybrids DBN9858 and Hongshuo 899 experienced high levels of S. frugiperda feeding damage prior to the V10 stage in 2019. As a result, few tassels and ears were produced on these plants and low larval infestation levels were recorded. In 2020, DBN9936 and Ruifeng 125 attained efficacy levels of 86.7% and −8.1% at the VT‐R1 stage. At the same stage, DBN9936 attained efficacy levels of 98.6–92.0%, while Ruifeng 125 reached efficacy levels of 36.2–77.2% in Yunnan during 2019 and 2020 (Table S2). At the R3‐R5 ear stage, DBN9936 attained efficacy levels of 63.0% (2020) in Guizhou and 60.7% (2019) and 98.4% (2020) in Yunnan, while Ruifeng 125 reached levels of 32.6% (2020) in Guizhou and 6.0% (2019) and 84.3% (2020) in Yunnan. In Henan where H. armigera and O. furnacalis were the dominant species, DBN9936 and Ruifeng 125 attained respective efficacy levels of 100.0–92.3% and 100.0–76.8% during the VT‐R1 development stage, and 39.6–85.8% and 47.7–85.9% during the R3‐R5 stage in either year.
Throughout the maize cropping cycle, larval infestation levels were positively associated with the relative coverage (proportion) of non‐Bt maize in the seed mixture field trials with DBN9936 (V5‐V7, F 1,58 = 16.97, P < 0.001 (2019), F 1,64 = 8.556, P = 0.005 (2020); VT‐R1, F 1,58 = 6.578, P = 0.013 (2019), F 1,64 = 23.02, P < 0.001 (2020)) and the block refuge field trials with Ruifeng 125 (V5‐V7, F 1,58 = 4.955, P = 0.030 (2019), F 1,64 = 5.411, P = 0.023 (2020); VT‐R1, F 1,58 = 8.812, P = 0.006 (2019), F 1,64 = 3.819, P = 0.055 (2020)). Hence, irrespective of whether non‐Bt maize was established in mixtures or as well‐delineated block refuges, more lepidopteran larvae were recorded at higher non‐Bt maize coverage.
For the seed mixture and block refuge field trials in Yunnan (where S. frugiperda was the dominant species), pest infestation pressure on non‐Bt maize in each proportion was not significantly different in 2020 (DBN9858: F 4,10 = 2.515, P = 0.108 at V5‐V7, F 4,10 = 3.536, P = 0.048 at R3‐R5; Hongshuo 899: F 4,10 = 0.902, P = 0.498 at V5‐V7, F 4,10 = 1.995, P = 0.171 at R3‐R5, Figure S2). But the pest infestation level (number of larvae per 100 plants) on Bt maize (0% non‐Bt maize) was significantly lower than that planted with non‐Bt maize (5–30%) (DBN9936: F 4,10 = 6.244, P = 0.009 at V5‐V7, F 4,10 = 1.038, P = 0.435 at R3‐R5) in seed mixture trials (Figure 4a) but not in block refuges (Ruifeng 125: F 4,10 = 1.527, P = 0.267 at V5‐V7, F 4,10 = 0.289, P = 0.879 at R3‐R5, Figure 4b). This is possibly due to the S. frugiperda larval dispersal between neighbouring Bt and non‐Bt maize plants. In Henan where H. armigera and O. furnacalis were the dominant species, larval infestation pressure on non‐Bt maize in each proportion was also not significantly different (DBN9858: F 4,10 = 0.599, P = 0.672 at VT‐R1, F 4,10 = 0.267, P = 0.892 at R3‐R5; Hongshuo 899: F 4,10 = 1.977, P = 0.174 at VT‐R1, F 4,10 = 0.661, P = 0.633 at R3‐R5, Figure S2), similar to that on Bt maize (DBN9936: F 4,10 = 1.932, P = 0.182 at VT‐R1, F 4,10 = 0.890, P = 0.504 at R3‐R5; Ruifeng 125: F 4,10 = 1.276, P = 0.342 at VT‐R1, F 4,10 = 0.574, P = 0.688 at R3‐R5) (Figure 4c,d).
Figure 4.
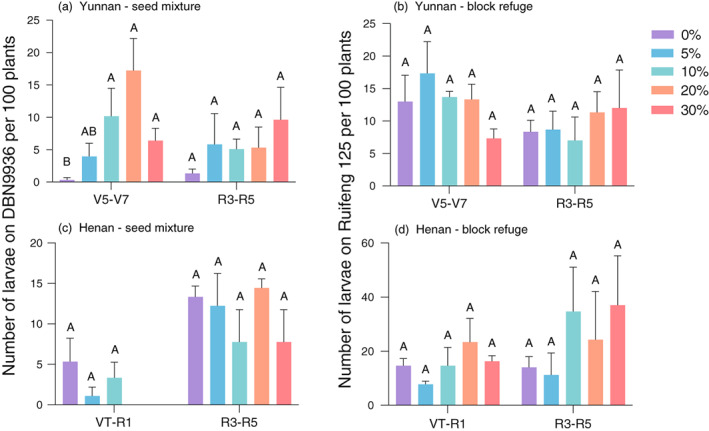
Larval infestation level on Bt maize for seed mixture (DBN9936, panels a and c) and block refuge (Ruifeng 125, panels b and d) field trials with different non‐Bt maize proportions in Yunnan (panels a and b) and Henan (panels c and d). Different capital letters within the same maize development stage for each site indicate significant differences in pest abundance (ANOVA, P < 0.05).
Similarly, the plant damage rate of Bt plants at V5‐V7 stage was lower than that of corresponding non‐Bt hybrids. More specifically, lepidopteran feeding damage on DBN9936 in 2020 attained 3.7 ± 1.8% in Yunnan and 0.0 ± 0.0% in Shandong; damage on Ruifeng 125 in 2020 reached 21.0 ± 2.6% in Yunnan and 0.0 ± 0.0% in Shandong (Table S3). Meanwhile, the non‐Bt DBN9858 hybrids experienced 56.0 ± 5.5% and 8.9 ± 4.2% feeding damage in Yunnan and Shandong, respectively; damage on Hongshuo 899 reached 67.3 ± 10.7% and 3.6 ± 1.9% in Yunnan and Shandong. Similar patterns were found for kernel damage at R1‐R3 stage. Kernel damage on DBN9936 in 2020 attained 23.3 ± 3.0% in Yunnan and 0.0 ± 0.0% in Shandong, while Ruifeng 125 experienced kernel damage rates of 26.7 ± 5.8% in Yunnan and 3.2 ± 1.3% in Shandong. These rates were lower than those of corresponding non‐Bt hybrids, i.e. DBN9858, with damage rates of 93.0 ± 2.0% (t = 19.49, P < 0.001) in Yunnan and 24.4 ± 3.1% (t = 7.798, P < 0.001) in Shandong; or Hongshuo 899 with 84.7 ± 7.0% (t = 6.383, P = 0.003) damage in Yunnan and 19.1 ± 9.9% (t = 1.925, P = 0.127) in Shandong.
Pest pressure under experimental treatments was also compared to the established economic threshold for S. frugiperda of 20% infested plants per field or approx. 20 larvae/100 plants in early whorl‐stage maize (Overton et al., 2021; Pitre et al., 1997). In Yunnan and Guizhou, seed mixtures (DBN9936) with 90% Bt coverage yielded plot‐level damage incidence (%) of 11.0 ± 3.5% in Yunnan and 16.0 ± 3.5% in Guizhou at V5‐V7 stage (Table S3). Meanwhile, seed mixtures at other proportions larger than 10% all resulted in damage incidence levels above 20%. In the block refuge field trials with Ruifeng 125, only 95% Bt coverage yielded damage incidence levels below the threshold in Yunnan, i.e. 11.2 ± 5.4%. In Shandong—where H. armigera and O. furnacalis were the dominant species—70% Bt coverage plots resulted in damage incidence levels below the 20% threshold, i.e. 0.0 ± 0.0% incidence for seed mixture and 2.0 ± 1.2% for block refuge trials in 2020. In addition, 90% Bt seed mixtures resulted in larval pest pressure below the economic threshold, i.e. 14.7 ± 4.3 larvae/100 plants in Yunnan and 11.1 ± 4.2 larvae/100 plants in Guizhou. Meanwhile, block refuge fields with Ruifeng 125 at 90% Bt coverage resulted in infestation levels of 18.5 ± 3.8 larvae/100 plants in Guizhou while 95% Bt coverage resulted in 19.3 ± 7.1 larvae/100 plants in Yunnan. In Henan, Shandong, Hebei and Liaoning—where H. armigera and O. furnacalis were the dominant species—pest pressure in all plots was below the respective economic thresholds of 2–3.2 larvae/plant (Sun et al., 2020).
Maize yield
Over 2019–2020, DBN9936 and Ruifeng 125 attained higher yields than their respective non‐Bt isoline or conventional hybrids in 16 of 19 and 12 of 18 instances (Table 1 and Table S4). Across years and locations, yield‐loss avoidance (i.e. per cent reduction in yield loss) equalled 19.3% (1.67 tons/ha, 2019) and 21.3% (1.39 tons/ha, 2020) for DBN9936 versus 18.3% (1.50 tons/ha, 2019) and 16.4% (0.68 tons/ha, 2020) for Ruifeng 125 as compared to non‐Bt hybrids (Table 1 and Table S4). Yield‐loss avoidance levels correlated with larval infestation pressure (DBN9936: F 1,18 = 11.052, P = 0.040; Ruifeng 125: F 1,16 = 5.009, P = 0.041; Figure 5a), but varied greatly between sites. For DBN9936, avoided losses varied between 0.4% (Hebei) and 99.2% (Guizhou) in 2019 and −6.7% (Shandong) to 69.4% (Jiangsu) in 2020. For Ruifeng 125, avoided losses ranged from −8.3% (Shandong) to 90.1% (Guizhou) in 2019 and from −11.9% (Hebei) to 45.8% (Guizhou) in 2020.
Table 1.
Avoided yield loss of seed mixture (DBN9936) and block refuge (Ruifeng 125) field trials at each of the experimental sites during 2019 and 2020
| Maize | Year | Non‐Bt Percent | Yunnan | Guizhou | Hubei | Jiangsu | Henan | Shandong | Hebei | Jilin | Heilongjiang | Xinjiang | Means |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DBN9936 | 2019 | 0% | 44.8 ± 2.5% | 99.2 ± 0.0% | 9.4 ± 3.3% | 3.1 ± 8.1% | 1.5 ± 12.8% | 14.4 ± 5.4% | 0.4 ± 7.2% | 10.7 ± 2.7% | 2.7 ± 3.1% | 6.5 ± 4.5% | 19.3 ± 9.5% |
| 5% | 37.6 ± 0.7% | 99.2 ± 0.0% | 9.2 ± 1.9% | −7.6 ± 19.1% | 4.9 ± 12.4% | 14.4 ± 0.3% | 4.5 ± 6.0% | −0.2 ± 1.5% | −3.5 ± 3.1% | 13.1 ± 0.5% | 17.2 ± 9.9% | ||
| 10% | 36.4 ± 1.0% | 99.1 ± 0.0% | 3.5 ± 7.0% | 6.9 ± 11.3% | −8.1 ± 12.8% | 10.4 ± 5.8% | −6.4 ± 11.3% | 0.6 ± 3.0% | 5.6 ± 6.8% | 7.9 ± 3.9% | 15.6 ± 10.0% | ||
| 15% | 34.8 ± 1.0% | 99.1 ± 0.0% | 6.2 ± 5.5% | 2.2 ± 11.1% | −2.9 ± 18.0% | 9.8 ± 6.2% | −14.8 ± 10.0% | 2.6 ± 1.9% | −1.9 ± 3.3% | 4.3 ± 2.8% | 13.9 ± 10.3% | ||
| 20% | 36.0 ± 1.8% | 99.0 ± 0.0% | 10.8 ± 1.1% | −0.6 ± 9.4% | −12.9 ± 17.4% | 16.9 ± 3.2% | 10.9 ± 0.7% | 2.9 ± 4.0% | −0.7 ± 9.1% | 4.2 ± 8.3% | 16.7 ± 10.0% | ||
| 2020 | 0% | 38.4 ± 1.5% | 48.0 ± 2.8% | – | 69.4 ± 4.7% | 21.5 ± 0.4% | −6.7 ± 1.5% | 14.5 ± 7.3% | −1.5 ± 4.5% | 11.3 ± 2.9% | −2.8 ± 5.9% | 21.3 ± 8.6% | |
| 5% | 31.9 ± 2.3% | 43.0 ± 2.5% | – | 68.5 ± 5.9% | 24.6 ± 0.8% | −0.1 ± 1.7% | 18.7 ± 4.9% | 0.1 ± 4.8% | 3.2 ± 0.6% | 0.7 ± 12.0% | 21.2 ± 7.9% | ||
| 10% | 25.1 ± 3.4% | 37.8 ± 2.1% | – | 65.2 ± 11.8% | 14.7 ± 4.1% | 1.6 ± 1.0% | 9.3 ± 7.7% | 1.1 ± 5.7% | 7.9 ± 7.6% | 11.2 ± 0.7% | 19.3 ± 6.9% | ||
| 20% | 17.9 ± 2.1% | 41.5 ± 3.3% | – | 67.5 ± 6.2% | 3.7 ± 3.1% | 1.5 ± 0.6% | 7.6 ± 6.0% | −3.7 ± 2.1% | −7.2 ± 0.1% | 13.0 ± 4.5% | 15.8 ± 8.1% | ||
| 30% | 11.7 ± 4.2% | 38.7 ± 2.8% | – | 68.1 ± 4.4% | 12.7 ± 5.3% | 0.2 ± 5.0% | 9.7 ± 14.6% | 1.4 ± 4.8% | −11.7 ± 5.4% | 0.0 ± 13.7% | 14.5 ± 8.1% | ||
| Ruifeng 125 | 2019 | 0% | 40.6 ± 2.9% | 90.1 ± 0.1% | −1.1 ± 2.8% | 27.0 ± 7.2% | 25.5 ± 2.9% | −8.3 ± 6.5% | 2.5 ± 0.7% | 1.9 ± 3.3% | −7.2 ± 14.6% | 11.9 ± 4.8% | 18.3 ± 9.5% |
| 5% | 37.3 ± 0.4% | 89.8 ± 0.2% | −13.0 ± 9.0% | 24.2 ± 9.9% | 9.2 ± 2.6% | −0.5 ± 1.3% | −8.6 ± 7.1% | −10.5 ± 7.3% | −5.6 ± 3.3% | −6.8 ± 2.6% | 11.5 ± 10.1% | ||
| 10% | 34.5 ± 1.9% | 89.8 ± 0.1% | 9.4 ± 2.0% | 18.5 ± 3.6% | −4.8 ± 14.2% | 2.3 ± 1.2% | −7.5 ± 7.2% | −8.2 ± 3.8% | −4.7 ± 6.0% | −3.7 ± 6.3% | 12.6 ± 9.6% | ||
| 15% | 36.0 ± 2.2% | 89.3 ± 0.3% | 15.3 ± 2.9% | 24.1 ± 6.6% | 20.4 ± 0.9% | −1.6 ± 1.7% | −5.5 ± 2.2% | −2.0 ± 3.5% | 5.6 ± 15.4% | −12.3 ± 4.8% | 16.9 ± 9.3% | ||
| 20% | 32.0 ± 3.4% | 88.2 ± 0.4% | 18.1 ± 3.0% | 18.5 ± 12.3% | 20.1 ± 4.9% | −8.7 ± 6.9% | −10.7 ± 4.5% | −5.3 ± 1.6% | −3.8 ± 6.5% | −25.9 ± 19.2% | 12.3 ± 10.2% | ||
| 2020 | 0% | 33.6 ± 3.5% | 45.8 ± 2.2% | 5.3 ± 2.9% | 36.8 ± 1.4% | −3.9 ± 4.5% | −4.4 ± 1.6% | −11.9 ± 10.5% | – | – | 9.3 ± 5.1% | 16.4 ± 4.5% | |
| 5% | 23.5 ± 2.9% | 44.5 ± 3.1% | −5.8 ± 16.0% | 17.5 ± 16.1% | −4.9 ± 5.9% | −0.9 ± 2.8% | 4.4 ± 5.4% | – | – | 10.5 ± 5.7% | 12.8 ± 7.4% | ||
| 10% | 22.7 ± 1.7% | 45.3 ± 0.9% | −3.6 ± 13.0% | 17.4 ± 16.2% | 0.6 ± 1.0% | 0.4 ± 0.7% | 6.2 ± 3.3% | – | – | 12.3 ± 6.8% | 14.4 ± 6.1% | ||
| 20% | 25.8 ± 1.6% | 41.2 ± 1.8% | −13.2 ± 10.6% | 24.3 ± 9.5% | 6.2 ± 2.1% | −1.6 ± 2.1% | −7.4 ± 6.7% | – | – | 4.1 ± 7.5% | 11.6 ± 6.3% | ||
| 30% | 23.9 ± 2.2% | 42.9 ± 3.4% | −6.0 ± 11.5% | −7.8 ± 30.4% | 11.6 ± 3.0% | 3.0 ± 2.3% | −5.6 ± 16.0% | – | – | −0.5 ± 2.8% | 8.3 ± 8.3% |
Figure 5.
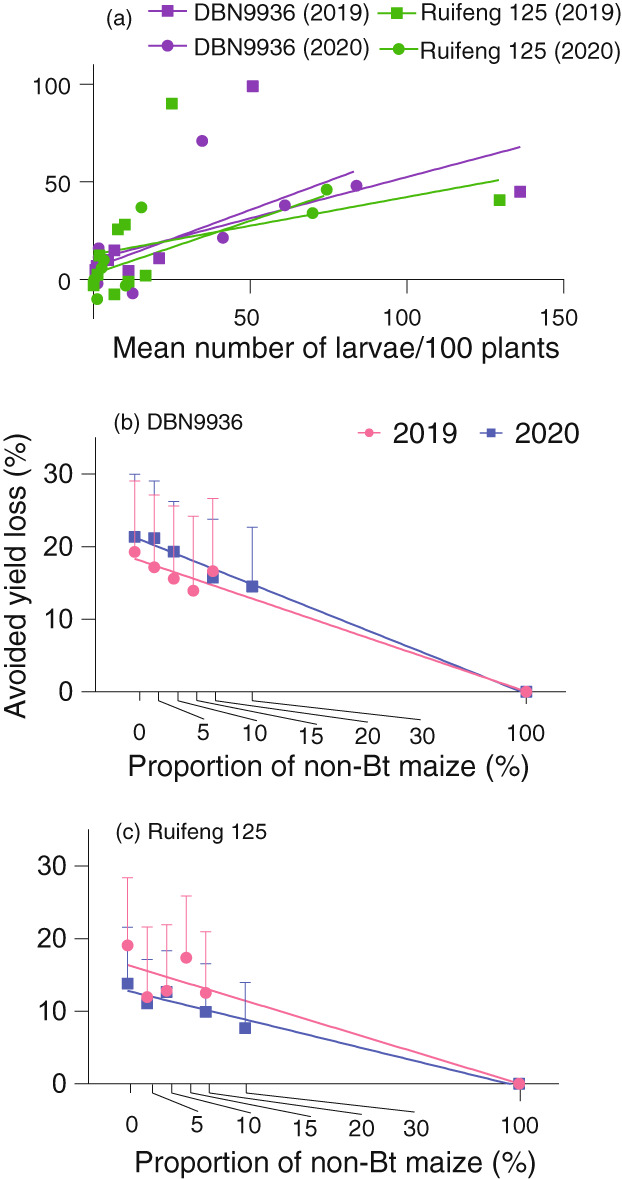
Yield loss avoidance under varying lepidopteran pest pressure or non‐Bt maize coverage. Panel (a) shows a correlation between lepidopteran larval infestation pressure on non‐Bt maize and avoided yield loss for two different maize hybrids Bt‐Cry1Ab DBN9936 and Bt‐Cry1Ab/Cry2Aj Ruifeng 125. Panels (b) and (c) reveal the extent of yield loss avoidance (mean ± SE) for either seed mixtures with hybrid DBN9936 (b) and block refuges with hybrid Ruifeng 125 (c) established at different non‐Bt maize coverage rates. Avoided yield loss data are averaged across all locations for each given year. Error bars are only shown in an upwards direction.
In Guizhou, Yunnan and Jiangsu, where S. frugiperda was the main herbivore, avoided losses attained 99.2% (2019) and 48.0% (2020) in Guizhou, 44.8% (2019) and 38.4% (2020) in Yunnan and 69.4% (2020) in Jiangsu in the seed mixture field trial with DBN9936. Meanwhile, avoided losses reached 90.1% (2019) and 45.8% (2020) in Guizhou, 40.6% (2019) and 33.6% (2020) in Yunnan and 36.8% (2020) in Jiangsu in the block refuge field trial with Ruifeng 125. In Henan province where H. armigera and O. furnacalis were the main species, avoided losses attained 1.5% (2019) and 21.5% (2020) in the seed mixture field trials and 25.5% (2019) and − 3.9% (2020) in the block refuge field trials. However, in sites with low infestation pressure, yield loss avoidance regularly was below 15% (Table 1). Overall plot‐level yield decreased with increasing non‐Bt maize cover in both the seed mixture (F 1,52 = 5.546, P = 0.023 (Figure 5b)) and block refuge field trials (F 1,52 = 6.754, P = 0.013 (Figure 5c)) in 2020 but not 2019.
Mycotoxin contamination
Seventeen of 540 samples, i.e. 3% tested positive for aflatoxin; of these, 13 were non‐Bt maize grain samples. For fumonisin, 422 of 576 samples, i.e. 73.3% tested positive, and the fumonisin content significantly correlated with the number of larvae per ear for both seed mixture (R 2 = 0.293, P < 0.001) and block refuge (R 2 = 0.252, P = 0.001) field trials. Across sites, fumonisin contamination was 10‐fold higher on non‐Bt maize plants than Bt maize hybrids (Figure 6). In seed mixture field trials, fumonisin titres were 0.56 ± 0.30 mg/kg (2019) and 1.69 ± 1.23 mg/kg (2020) on DBN9936, as compared to 6.68 ± 5.60 mg/kg (2019) and 15.68 ± 10.71 mg/kg (2020) on its non‐Bt analogue (Figure 6a; Table 2). In block refuge field trials, fumonisin titres reached 0.74 ± 0.43 mg/kg (2019) and 0.25 ± 0.10 mg/kg (2020) on Ruifeng 125 as compared to 5.12 ± 2.21 mg/kg (2019) and 5.59 ± 2.29 mg/kg (2020) on the conventional hybrid Hongshuo 899 (Figure 6b). On non‐Bt maize, average fumonisin content exceeded established WHO maximum residue limits (4 mg/kg) by 67.0% (2019) and 292.0% (2020) for DBN9936, and by 28.0% (2019) and 39.8% (2020) for Ruifeng 125. More specifically, fumonisin titres on DBN9858 in Guizhou (44.79 mg/kg in 2019, 104.98 mg/kg in 2020), Jiangsu (11.75 mg/kg in 2020) and Shandong (13.94 mg/kg in 2020) surpassed the WHO residue limits. On Hongshuo 899, fumonisin titres in Guizhou reached 19.71 mg/kg in 2019 and 181.51 mg/kg in 2020, while they attained 12.34 mg/kg in 2019 and 22.65 mg/kg in 2020 in Jiangsu. In 2020, fumonisin titres were 6.37 mg/kg in Shandong, 5.10 mg/kg in Henan and 4.53 mg/kg in Hebei. All the above values surpassed the WHO residue limits.
Figure 6.
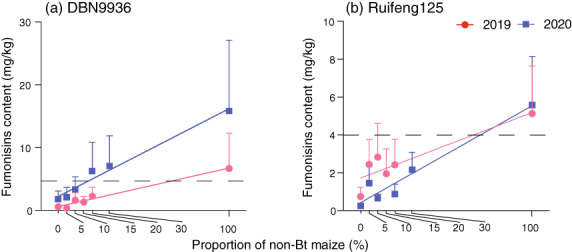
The extent of mycotoxin contamination in harvested maize grains for the seed mixture (DBN9936, Panel a) or block refuge field trials (Ruifeng 125, Panel b). Per year, mycotoxin titres are averaged across all locations and plotted against non‐Bt maize coverage rate for seed mixture (a) and block refuge (b). Dashed lines indicate the established WHO thresholds of fumonisin, i.e. 4 mg/kg in unprocessed maize grain. Mycotoxin data from block refuges in Guizhou from 2020 were not included in the analysis due to its extremely high dose. Error bars are only shown in an upwards direction.
Table 2.
Plot‐level fumonisin content (mg/kg) on harvested maize grain at each of the experimental sites during 2019 and 2020
| Maize | Year | Non‐Bt proportion | Guizhou | Hubei | Jiangsu | Henan | Shandong | Hebei | Jilin | Heilongjiang | Xinjiang | Means |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DBN9936 | 2019 | 0% | 2.01 ± 0.87CD* | 1.65 ± 1.29 | – | 1.36 ± 1.31 | 0.00 ± 0.00B | 0.32 ± 0.24 | 0.10 ± 0.10 | 0.40 ± 0.21 | 0.00 ± 0.00 | 0.56 ± 0.30 |
| 5% | 1.00 ± 0.23D | 0.06 ± 0.03 | – | 0.95 ± 0.95 | 0.20 ± 0.19AB | 0.22 ± 0.22 | 0.22 ± 0.01 | 0.10 ± 0.05 | 0.00 ± 0.00 | 0.35 ± 0.17 | ||
| 10% | 8.98 ± 0.70B † | 0.10 ± 0.09 | – | 1.97 ± 1.95 | 0.01 ± 0.01B | 0.23 ± 0.23 | 0.79 ± 0.04 | 0.02 ± 0.02 | 0.32 ± 0.32 | 1.62 ± 1.20 | ||
| 15% | 4.87 ± 1.94 BC | 0.10 ± 0.9 | – | 0.05 ± 0.05 | 0.00 ± 0.00B | 2.02 ± 1.01 | 0.21 ± 0.03 | 0.24 ± 0.12 | 0.62 ± 0.51 | 1.34 ± 0.88 | ||
| 20% | 10.13 ± 2.83B | 1.43 ± 0.71 | – | 2.90 ± 2.40 | 1.02 ± 0.46A | 0.13 ± 0.13 | 0.41 ± 0.2 | 0.37 ± 0.21 | 0.01 ± 0.01 | 2.27 ± 1.40 | ||
| 100% | 44.79 ± 16.11A | 1.81 ± 0.91 | – | 2.87 ± 1.77 | 0.35 ± 0.15AB | 0.96 ± 0.25 | 0.60 ± 0.37 | 0.20 ± 0.12 | 0.04 ± 0.04 | 6.68 ± 5.60 | ||
| F | 5.985 | 1.458 | 0.491 | 3.456 | 2.597 | 1.870 | 1.132 | 1.055 | 1.889 | |||
| P value | 0.005 | 0.274 | 0.777 | 0.036 | 0.082 | 0.174 | 0.395 | 0.431 | 0.117 | |||
| 2020 | 0% | 12.03 ± 6.48C | 0.67 ± 0.57 BC | 1.09 ± 0.23AB | 0.20 ± 0.10 | 1.09 ± 1.08B | 0.01 ± 0.01C | 0.02 ± 0.02 | 0.00 ± 0.00C | 0.12 ± 0.12AB | 1.69 ± 1.23D | |
| 5% | 14.32 ± 3.20 BC | 0.16 ± 0.07C | 0.69 ± 0.53B | 0.07 ± 0.05 | 0.37 ± 0.30B | 0.16 ± 0.05B | 1.56 ± 1.53 | 0.01 ± 0.01 BC | 0.01 ± 0.01B | 1.93 ± 1.48 BC | ||
| 10% | 19.63 ± 0.78 BC | 0.31 ± 0.14 BC | 1.94 ± 0.79AB | 1.39 ± 0.69 | 1.86 ± 1.85B | 1.74 ± 0.76AB | 0.16 ± 0.06 | 0.03 ± 0.01AB | 0.04 ± 0.04B | 3.01 ± 1.99 BC | ||
| 20% | 43.20 ± 7.51AB | 0.72 ± 0.10 BC | 2.76 ± 0.07AB | 2.96 ± 1.18 | 1.73 ± 0.81B | 2.46 ± 1.11AB | 0.04 ± 0.03 | 0.83 ± 0.40A | 0.09 ± 0.08AB | 6.09 ± 4.58 BC | ||
| 30% | 45.37 ± 7.89AB | 2.49 ± 0.24A | 2.69 ± 1.11AB | 2.36 ± 1.92 | 3.72 ± 2.44B | 3.58 ± 3.31AB | 1.69 ± 1.69 | 0.04 ± 0.04 BC | 0.43 ± 0.06A | 6.89 ± 10.71B | ||
| 100% | 104.98 ± 16.94A | 1.75 ± 0.53AB | 11.75 ± 6.55A | 3.28 ± 2.02 | 13.94 ± 3.89A | 3.91 ± 2.11A | 0.94 ± 0.69 | 0.28 ± 0.28AB | 0.70 ± 0.33A | 15.68 ± 10.71A | ||
| F | 16.011 | 7.052 | 3.570 | 1.185 | 5.898 | 8.815 | 0.621 | 3.120 | 3.443 | 13.799 | ||
| P value | <0.001 | 0.003 | 0.033 | 0.372 | 0.006 | 0.001 | 0.687 | 0.048 | 0.037 | < 0.001 | ||
| Ruifeng 125 | 2019 | 0% | 2.00 ± 2.00B | 0.06 ± 0.03B | 3.64 ± 2.19 | 0.02 ± 0.02B | 0.19 ± 0.14 | 0.03 ± 0.03B | 0.00 ± 0.00 | 0.08 ± 0.07 | 0.00 ± 0.00C | 0.74 ± 0.53 |
| 5% | 7.67 ± 6.21AB | 0.08 ± 0.05B | 9.06 ± 2.74 | 0.89 ± 0.41A | 1.34 ± 1.14 | 0.50 ± 0.24AB | 0.00 ± 0.00 | 0.05 ± 0.03 | 0.01 ± 0.01 BC | 2.44 ± 1.17 | ||
| 10% | 4.84 ± 0.98AB | 0.12 ± 0.08B | 14.75 ± 4.27 | 0.20 ± 0.13AB | 0.71 ± 0.61 | 1.34 ± 0.67A | 0.05 ± 0.03 | 0.26 ± 0.10 | 0.57 ± 0.57AB | 2.82 ± 1.60 | ||
| 15% | 1.87 ± 1.10AB | 0.17 ± 0.14B | 11.04 ± 3.22 | 1.17 ± 0.51A | 0.55 ± 0.31 | 0.79 ± 0.31A | 0.00 ± 0.00 | 0.06 ± 0.04 | 0.01 ± 0.01 BC | 1.95 ± 1.18 | ||
| 20% | 2.83 ± 0.93AB | 0.10 ± 0.06B | 11.55 ± 3.17 | 0.69 ± 0.24AB | 2.53 ± 2.02 | 1.16 ± 0.69A | 0.40 ± 0.40 | 1.03 ± 0.72 | 0.08 ± 0.03AB | 2.42 ± 1.21 | ||
| 100% | 19.71 ± 10.69A | 0.90 ± 0.40A | 12.34 ± 3.54 | 4.17 ± 2.43A | 0.93 ± 0.92 | 1.73 ± 0.69A | 0.06 ± 0.06 | 0.07 ± 0.07 | 1.12 ± 0.59A | 5.12 ± 2.25 | ||
| F | 3.119 | 3.273 | 1.360 | 3.230 | 0.608 | 5.456 | 0.898 | 1.672 | 3.392 | 2.189 | ||
| P value | 0.049 | 0.043 | 0.306 | 0.048 | 0.696 | 0.008 | 0.513 | 0.216 | 0.039 | 0.069 | ||
| 2020 | 0% | 39.48 ± 9.21B | 0.04 ± 0.04B | 0.70 ± 0.32B | 0.70 ± 0.38AB | 0.38 ± 0.38 | 0.00 ± 0.00B | 0.05 ± 0.05 | 0.00 ± 0.00 | 0.10 ± 0.31B | 0.25 ± 0.10C | |
| 5% | 53.48 ± 25.70B | 1.05 ± 0.81A | 7.46 ± 6.10B | 0.18 ± 0.09B | 1.84 ± 1.75 | 0.27 ± 0.12A | 0.02 ± 0.01 | 0.02 ± 0.02 | 0.43 ± 0.38AB | 1.45 ± 0.79C | ||
| 10% | 45.88 ± 12.19B | 0.22 ± 0.04A | 0.79 ± 0.51B | 1.24 ± 0.16AB | 1.64 ± 0.76 | 0.96 ± 0.49A | 0.03 ± 0.03 | 0.10 ± 0.10 | 0.37 ± 0.18AB | 0.66 ± 0.19C | ||
| 20% | 74.33 ± 28.99B | 0.27 ± 0.05A | 4.48 ± 1.67B | 0.98 ± 0.54AB | 0.50 ± 0.22 | 0.37 ± 0.30A | 0.01 ± 0.01 | 0.06 ± 0.06 | 0.14 ± 0.09AB | 0.87 ± 0.47C | ||
| 30% | 79.20 ± 18.14B | 0.68 ± 0.29A | 8.38 ± 2.38B | 1.75 ± 0.50A | 2.80 ± 1.99 | 0.79 ± 0.35A | 1.12 ± 0.60 | 0.15 ± 0.15 | 1.38 ± 0.75A | 2.16 ± 0.83B | ||
| 100% | 181.51 ± 10.05A | 2.17 ± 0.71A | 22.65 ± 2.38A | 5.10 ± 2.65A | 6.37 ± 6.08 | 4.53 ± 2.29A | 0.37 ± 0.37 | 0.90 ± 0.19 | 3.40 ± 3.07A | 5.59 ± 2.29A | ||
| F | 7.673 | 6.064 | 7.763 | 2.668 | 0.654 | 9.408 | 2.304 | 0.409 | 2.055 | 42.649 | ||
| P value | 0.002 | 0.005 | 0.002 | 0.076 | 0.665 | 0.001 | 0.110 | 0.834 | 1.142 | < 0.001 |
Different capital letters within the same row for each location and year indicate statistically significant inter‐plot differences in fumonisin content (P < 0.05, analysis of variance (ANOVA) with Tukey HSD test).
Values in bold indicate fumonisin content exceeded established WHO maximum residue limits (4 mg/kg).
Overall, simple linear regression analysis showed that plot‐level mycotoxin content increased with non‐Bt maize cover for both the seed mixture (2019: y = 61.46x + 602.3, F 1,40 = 4.931, P = 0.032; 2020: y = 139.8x + 2211, F 1,52 = 4.861, P = 0.032) and block refuge field trials (2019: y = 34.67x + 1715, F 1,46 = 3.603, P = 0.064; 2020: y = 51.22x + 419.5, F 1,46 = 4.931, P < 0.01). As indicated by the above analysis, plot‐level mycotoxin content surpasses the established WHO thresholds once non‐Bt maize coverage exceeds 55.3% (2019) and 12.8% (2020) in the seed mixture field trial, and 65.9% (2019) and 69.9% (2020) in the block refuge field trial. The low percentage of 12.8% in 2020 was biased by the high fumonisin contamination in seed mixture samples in Guizhou. Across all sites, plot‐level mycotoxin content in the 5% and 10% non‐Bt maize coverage treatments consistently remained below WHO residue limits except in Guizhou and Jiangsu (Table 2). For Hubei, Henan, Shandong, Hebei, Jilin, Heilongjiang and Xinjiang, plot‐level mycotoxin content in all plots with 5–30% non‐Bt maize coverage remained below residue limits.
Discussion
In this study, we show how two transgenic Bt maize hybrids DBN9936 and Ruifeng 125 mitigate lepidopteran feeding damage, restore pest‐induced crop losses and lower mycotoxin contamination of harvested kernels. These benefits are especially pronounced at elevated infestation pressure, e.g. in southern China. By employing seed mixtures or block refuges, clear benefits can be gained at comparatively low rates of Bt crop coverage, e.g. 10–30% in most sites and years. Although the stacked herbicide tolerance traits of both hybrids still require a full‐fledged environmental impact assessment, our studies underline how Bt insecticidal maize hybrids in se can suppress pest population, raise China's food security and ameliorate food safety.
Multiple herbivorous insects occur in China's maize agro‐ecosystem including prominent pests such as O. furnacalis, H. armigera or M. separata (Song et al., 2021; Wang and Wang, 2019). Since 2018, the invasive S. frugiperda has complemented this herbivore complex and now annually colonizes all of China's maize‐cropping areas (Wu et al., 2021b,c). In its native and invaded range, yield losses due to S. frugiperda exhibit major variability over geographic and farming contexts, averaging 21–25% for pesticide‐based or unmanaged scenarios (Overton et al., 2021). In Africa, S. frugiperda thus causes losses worth $9.4 billion/year (Eschen et al., 2021). In response to the S. frugiperda invasion, African farmers have largely reverted to synthetic insecticides including banned and highly hazardous products—with major implications for occupational exposure, food safety and environmental pollution (Jepson et al., 2020; Tambo et al., 2020). Similarly, resource‐poor smallholders in China's Yunnan province now annually expend more than US$ 250 per hectare on synthetic insecticides for S. frugiperda control (Yang et al., 2021). Such pesticide‐centred regimes tend to be economically unsound, degrade farm‐level profit and negatively impact non‐target biota far beyond the farm edge (Sanchez‐Bayo and Wyckhuys, 2021; Schreinemachers et al., 2020). Insect feeding injury of maize ears further raises levels of aflatoxin and fumonisin contamination, which entails important food safety hazards (Pruter et al., 2019) as also confirmed by our data. Our study shows that Bt‐Cry1Ab and Bt‐Cry1Ab/Cry2Aj transgenic maize hybrids could alleviate the above impacts—as also demonstrated in the United States and Brazil (Overton et al., 2021). The extent of pest suppression was more pronounced for the single toxin DBN9936 hybrid, possibly due to elevated Bt toxin expression (Liang et al., 2021). As such, different Bt maize hybrids could reconstitute primary productivity by 16.4–21.3% within individual production fields. At high lepidopteran infestation pressure, Bt/non‐Bt seed mixtures of DBN9936 outperformed structural refuges of Ruifeng 125 in terms of yield loss avoidance, larval infestation pressure or damage incidence, possibly ascribed to the difference in their effectiveness for controlling target pests. These impacts, however, exhibited variation across sites and years due to ambient pest pressure, climatic parameters, edaphic conditions and farm‐level management, e.g. plant nutrition, soil organic matter content or pesticide application frequency. Notwithstanding the above variability, Bt maize can raise the yield distribution bracket and lower the incidence of pest‐induced yield loss (Edgerton et al., 2012). Insecticidal varieties, even when deployed on a fraction of cropping areas, may also provide area‐wide management of polyphagous lepidopteran pests—as demonstrated in cotton systems in the United States and China (Lu et al., 2022; Tabashnik et al., 2021; Wan et al., 2017).
Our work shows that Bt maize hybrids lower mycotoxin contamination by at least 70% under high pest pressure, e.g. in Guizhou and Yunnan. These benefits emanate from a reduction in larval feeding damage in the maize ear (Hammond et al., 2004), and are less pronounced for maize hybrids that solely express Cry1Ab (Bowers et al., 2013). The Bt‐mediated reduction in pest pressure can enable a drastic reduction in pesticide usage intensity and in se raise farm‐level profit. Those benefits are not solely confined to farmers who cultivate Bt crops but also nearby growers of non‐Bt crops and cultivated plants that are susceptible to polyphagous pest attacks (Hutchison et al., 2010; Wu et al., 2008). Such off‐site benefits can be substantial, accrue over time and are mirrored in an overall alleviation of the environmental footprint of agriculture (Dively et al., 2018). The establishment of Bt insecticidal crops within the agro‐landscape can thus lower insecticidal pollution, conserve ecosystem functioning and generate a broad suite of societal benefits (Dainese et al., 2019). Under conditions of climate change and precipitative biodiversity decline (Deutsch et al., 2018; Riegler, 2018; Wang et al., 2022), these landscape‐level benefits are exceptionally valuable.
Aside from the above social‐environmental benefits, sole reliance upon Bt transgenic crops entails important risks (Tabashnik et al., 2013; Tabashnik and Carriere, 2017). In the absence of credible stewardship, the effectiveness of Bt insecticidal crops can be compromised by a rapid surge in Bt‐resistant populations of the target pest (Tabashnik and Carriere, 2017). Field‐evolved resistance to Bt toxins has been documented for multiple maize‐feeding lepidopterans, i.e. Busseola fusca (Fuller), Diatraea saccharalis (Fabricius), Diatraea virgifera (LeConte), S. frugiperda and Striacosta albicosta (Smith). For S. frugiperda in particular, these risks cannot be neglected; Bt resistance has already been detected against Cry1F and Cry1Ab toxins in Puerto Rico, Brazil, the United States and Argentina (Omoto et al., 2016). Mandatory refuges with non‐Bt crops can retain populations of Bt‐susceptible pests and substantially delay the onset of resistance evolution (Carriere et al., 2012; Jin et al., 2015; Tabashnik and Carriere, 2017). Our field work shows that lepidopteran infestation pressure progressively increases with non‐Bt maize coverage, although key pests such as S. frugiperda are kept below economic thresholds in experimental fields with 10% non‐Bt maize coverage (Yang et al., 2021). Non‐Bt crop cover likely can be augmented, although supportive experimental trials need to be conducted. We are conscious that Chinese maize growers may be inclined to use Bt maize and that the enforcement of mandatory refuges may face several obstacles in China's countryside (Li et al., 2018). Hence, in areas where growers do not prefer local landraces or farm‐produced hybrids, one could consider promoting Bt/non‐Bt seed mixtures or retaining non‐Bt cultivars. This applies to maize growers in Northern China where several key pests occur at background levels and Bt‐mediated yield loss avoidance is low or nil. In southern China where the maize lepidopteran complex is dominated by S. frugiperda, it is better to adopt block refuge strategies as its high mobility between neighbouring Bt and non‐Bt plants, which might increase the risk of resistance development, and/or increasing survival of late instar larvae that move from non‐Bt maize to Bt maize (Carriere et al., 2020b). This phenomenon was also observed in Yunnan in the current study. Another way to mitigate resistance evolution is by ‘pyramiding’ Bt genes or incorporating structurally distinct toxins, e.g. Cry and Vip toxins (Carriere et al., 2016; Van den Berg et al., 2021). Yet, as Vip3Aa20 resistance alleles have already been picked up in natural lepidopteran populations (Amaral et al., 2020), the odds of rapid resistance evolution may be especially high under deficient stewardship. As a second major risk, wide‐scale adoption of DBN9936 and Ruifeng 125 can escalate herbicide use among Chinese maize growers and deepen issues with herbicide‐resistant weeds (Bonny, 2016; Tsatsakis et al., 2017; Zhu et al., 2020). While these issues can be partially countered through Integrated Weed Management (IWM), its benefits are seldom realized due to various technical and socio‐economic obstacles (Lamichhane et al., 2017). Recognizing how our study does not address herbicide‐tolerance traits, the related social‐environmental risks need to be independently assessed in a serious and holistic manner.
Exclusively accounting for its Bt insecticidal traits, our work accentuates how transgenic maize can mitigate (lepidopteran) pest pressure, improve food and nutrition security, and reduce food safety concerns throughout China. While sound stewardship, e.g. through mandatory refuges or on‐farm deployment of agro‐ecological measures can counter future risks of resistance evolution, socio‐technical barriers can hamper their adoption among local smallholders. Hence, we emphatically support pairing Bt insecticidal maize with a full repertoire of agro‐ecological measures at multiple organizational scales or tiers of influence (Wyckhuys et al., 2022b). Pest populations can be suppressed over extensive geographic areas at low Bt crop coverage rates, possibly far below the 65% cover that was empirically determined by Carriere et al. (2003). This could be achieved by systematically integrating crop diversification, egg parasitoid releases, fungal seed coatings, spray applications of baculoviruses or conservation biological control schemes (Carriere et al., 2020a; de Lira et al., 2020; Gurr et al., 2017; Huang et al., 2020; Wang et al., 2014). Sterile insect releases have also shown promise to bring down lepidopteran population levels (Tabashnik et al., 2021), while landscape heterogeneity is a key mediating factor for several of these curative or preventative measures (Martin et al., 2019). Lastly, agro‐ecological approaches can equally lower weed pressure and suspend a need for herbicide spray applications even in glyphosate‐tolerant cultivars (Petit et al., 2018). When implemented at a macro‐scale, the joint deployment of Bt transgenics and agro‐ecological measures also carries the benefit of slowing or averting Bt resistance evolution. As such, biodiversity‐driven tactics can reconstitute China's domestic maize production levels (Edgerton et al., 2012), reduce its reliance on grain imports and bolster the ecological resilience of national farming systems in the face of global change (Feit et al., 2021; Wyckhuys et al., 2018). In addition, a broad suite of social‐environmental benefits can be gained. By consciously deploying Bt transgenic maize under an IPM umbrella and harnessing biodiversity for pest control, one can attain a sustainable intensification of China's maize crop with little (or no) need for insecticides.
This work centred on the Bt insecticidal traits of two maize hybrids, without explicitly taking into consideration its (stacked) herbicide‐tolerance transgenes. Hence, our statements regarding the environmental benefits of these Bt maize hybrids cannot be viewed in isolation, and instead need to be complemented with credible, multi‐criteria impact assessments of their herbicide‐tolerance traits.
Experimental procedures
Plants and experimental design
Plants
Two insect‐resistant GM varieties were evaluated in this study, i.e. DBN9936 and Ruifeng 125. DBN9936 is an insect‐resistant and glyphosate‐tolerant maize variety developed by Beijing Dabeinong (DBN) Biotechnology Co., Ltd. that expresses EPSPS (5‐enolpyruvylshikimate‐3‐phosphate synthase for glyphosate resistance) and Cry1Ab genes, and received biosafety certification in 2019. As a non‐Bt control, we used DBN9858, i.e. a non‐Bt variety of DBN9936 that expresses the EPSPS gene. The following conventional recipient hybrids of DBN9936 and DBN9858 were used at different experimental sites—as indicated between parentheses: Nonghua 106 (Heilongjiang), Zhengdan 958 (Hubei, Hebei, Shandong, Xinjiang, Henan), Huanong 887 (Jilin, Liaoning), Wugu 3861 (Yunnan), Dongdan 6531 (Guizhou), Longping 206 (Jiangsu). The above hybrids are adapted to the specific agro‐climatic conditions of each site. A second insect‐resistant and glyphosate‐tolerant Bt maize hybrid was evaluated, i.e. Ruifeng 125 which expresses Cry1Ab/Cry2Aj and G10evo‐epsps, and which was developed by Hangzhou Ruifeng Biotech Co., Ltd. Ruifeng 125 also received biosafety certification in 2019. As a non‐Bt control for Ruifeng 125, we employed the conventional recipient hybrid Hongshuo 899, which carries no insect‐resistant or glyphosate‐tolerant traits.
Experimental sites
Over 2019–2020, DBN9936 and Ruifeng 125 were cultivated using locally prevailing management practices at 11 experimental sites: Pu′er (22°40´N, 101°38′ E), Yunnan Province; Guiyang (26°24´N, 106°41′ E), Guizhou Province; Wuhan (30°44´N, 114°46′ E), Hubei Province; Yancheng (33°52´N, 120°20′ E), Jiangsu Province; Xinxiang (35°10´N, 113°41′ E), Henan Province; Yantai (37°29´N, 121°16′ E), Shandong Province; Langfang (39°51´N, 116°60′ E), Hebei Province; Shenyang (41°49´N, 123°33′ E), Liaoning Province; Changchun (43°48´N, 125°24′ E), Jilin Province; Harbin (45°44´N, 126°42′ E), Heilongjiang Province; and Kuerle (41°45´N, 85°49′ E), Xinjiang Uyghur Autonomous Region. Experimental sites were situated within China's main maize‐growing areas (Figure 1). At each location, maize was planted during April–May, and the below field experiments were conducted.
Seed mixtures and block refuge field trials
As both DBN9936 and DBN9858 are glyphosate resistant, they could in principle be commercially deployed as seed mixtures, i.e. so‐called ‘refuge‐in‐the‐bag’. Meanwhile, Hongshuo 899 does not exhibit herbicide‐tolerance and was thus evaluated with Ruifeng 125 under block refuge arrangements.
First, 200 m2 (around 10 m × 20 m) experimental plots were established to investigate whether Bt/non‐Bt seed mixtures affect the infestation levels of maize‐feeding lepidopteran herbivores. Specifically, we evaluated the performance in terms of grain yield, larval infestation pressure and damage incidence of seed mixtures with DBN9936 Bt maize hybrids and the non‐Bt hybrid DBN9858. In experimental fields, randomized complete block trials were conducted in which six different treatments were evaluated: 100% DBN9936 Bt maize, 100% DBN9858 non‐Bt maize and four mixtures, i.e. at 95, 90, 85, 80% rates of DBN9936 (and a corresponding 5, 10, 15 and 20% DBN9858). In 2020, the seed mixtures were modified to 95, 90, 80 and 70% DBN9936 and a corresponding 5, 10, 20 and 30% DBN9858. In most sites, Bt and non‐Bt seeds (at specific ratios) were mixed before planting and no gene‐checks and no specific planting configurations were used during the experiment course. In Yunnan and Henan in 2020, Bt and non‐Bt seeds coated with different colours were mixed at a specific ratio and specific configuration was used. Experimental fields were established at a planting density of 57 000 plants ha−1 and 80‐cm row spacing. Between individual plots, a 1.5 m weed and maize‐free space were maintained to minimize larval dispersal. This configuration also helped to mitigate the eventual confounding effects of pollen shedding and deposition, as pollen grains generally spread at least two rows to either side of the donor row or 1.5 m in either direction (Burkness and Hutchison, 2012). In this inter‐plot space, weeds were removed mechanically or manually. Per site, treatments were replicated three times. Plots with Bt and non‐Bt maize hybrids were primarily rainfed except Xinjiang where maize mainly relied on irrigation.
Second, block refuges were evaluated using Bt maize Ruifeng 125 and non‐Bt maize Hongshuo 899. More specifically, 200 m2 experimental plots were established at a planting density of 57 000 plants ha−1 and a wide (80 cm) and narrow (40 cm) row planting pattern with 30 cm inter‐plant distance within a row. Per experimental site, randomized complete block trials were set up in 2019 with the following six treatments: 100% Ruifeng 125 Bt maize, 100% Hongshuo 899 and four treatments with refuge blocks of varying size, i.e. 95, 90, 85 and 80% Ruifeng 125 and a corresponding block refuge of 5, 10, 15 and 20% Hongshuo 899. In 2020, we evaluated arrangements with 95, 90, 80 and 70% Ruifeng 125 and corresponding refuge blocks of 5, 10, 20 and 30% Hongshuo 899. Per site, treatments were replicated three times with a 1.5 m inter‐plot distance.
Pest infestation level
At each site, surveys were conducted to assess the relative abundance of S. frugiperda larvae and other lepidopteran herbivores on transgenic and conventional maize hybrids. Within each plot, a total of 100 plants were randomly selected along five linear transects (20 plants per transect). In the block refuge field trials, 20 non‐Bt plants and 100 Bt plants were examined for all plots as certain treatments, e.g. 5% coverage refuges included as little as 52 non‐Bt plants. Further assessments showed that larval infestation pressure or feeding damage on 20 non‐Bt plants per plot reflected the overall situation in the field. Each plant was visually inspected and the plant‐level feeding damage, incidence and identity of foliage‐dwelling herbivores were recorded. Plant damage rate was only recorded for sites in Yunnan, Guizhou and Shandong. The two border rows in each plot were not checked to minimize the effect of pollen dispersal and kernel mosaics. On maize plants at early developmental stages, the leaf whorl was carefully inspected for lepidopteran larvae. On maize plants at the reproductive stage, i.e. R1 onwards (Nleya et al., 2016), the maize tassel, leaf axil, silks and ear were closely examined. Field visits were conducted every 2 weeks throughout the entire maize‐growing season, covering April–November depending on the local planting date. During each visit, the phenological stage of maize plants was recorded as follows: V5‐V7, V10‐V12, VT ‐R1 or R3‐R5 stages (Nleya et al., 2016). Caterpillars usually damage maize foliage at V5‐V12 and affect ears at R3‐R5 stage. Hence, the R3‐R5 data specifically referred to caterpillars feeding on ears. For seed mixture field trials, the infestation pressure of a specific lepidopteran herbivore was expressed as the number of its larvae per 100 (unidentified Bt and non‐Bt) plants. For the block refuge field trial, the infestation pressure of a specific lepidopteran herbivore was calculated by averaging the number of larvae on the respective proportion of Bt and non‐Bt plants in each plot, e.g. 80 Bt plants and 20 non‐Bt plants in a 20% coverage field trial. The control efficacy of a given treatment was expressed as larval density ((non‐Bt – Bt)/non‐Bt) for each target lepidopteran pest. Damage incidence was expressed as the proportion of damaged plants in the whole plot. Pest occurrence was surveyed in all locations during either year.
Maize yield
In each experimental plot, all maize ears (i.e. Bt plus non‐Bt plants) were harvested and grain yield was calculated at 14% moisture content. Under a given experimental treatment, yield estimates were obtained by averaging the actual yield of the three respective plots. In both the seed mixture and block refuge field trials, plot‐level yields thus reflect the combined yields of Bt and non‐Bt plants at their respective coverage. Given that crop yield is affected by variety x environment interplay, avoided losses were estimated for the two Bt maize hybrids as such: avoided yield loss of (experimental plots – 100% non‐Bt maize plots)/yield of 100% Bt maize. Extreme weather events during 2019 and 2020 led to the loss of yield data from one or more experimental locations such as Liaoning or Heilongjiang.
Mycotoxin contamination
For the seed mixture field trial, 1 kg grain was randomly harvested from maize plants within each experimental plot. In the block refuge plot, 1 kg grain was collected from either Bt or non‐Bt plants in each plot. Seed samples were sent to the laboratory in paper bags, ground to 20 mesh fineness in a laboratory mill and stored at 4°C for max. 7 days until analysis. For each grain sample, contamination with mycotoxins, i.e. fumonisin, aflatoxin was assessed at the Institute of Food Safety and Nutrition, Jiangsu Academy of Agricultural Sciences. Briefly, 10 g of powered grain sample was shaken and extracted with 40 mL of acetonitrile: water: acetic acid (79:20:1 v/v/v) at 180 rpm for 30 min. After centrifugation at 3000 rpm for 10 min, 0.5 mL of each final extract was diluted with acetonitrile: water: acetic acid (79:20:1 v/v/v) and filtered through a nylon filter (13 mm in diameter, 0.22–l m pore size). A high‐pressure liquid chromatography/electrospray ionization–tandem mass spectrometer (LC–MS/MS) was used to simultaneously quantify different metabolites (Qiu et al., 2020). For each experimental plot, mycotoxin contamination was expressed as mg per kilogram. For block refuge field trials, mycotoxin contamination was expressed as mycotoxin titres on the respective proportion of Bt and non‐Bt maize grains, e.g. samples including 80% Bt and 20% non‐Bt maize grains in 20% field trial. The established EU and WHO thresholds of fumonisin were 5 mg FBs (B1 + B2)/kg for complete feed for swine and 4 mg/kg in unprocessed maize grain respectively. Harvested maize grains from Yunnan and Liaoning were not well preserved and were not included in further laboratory evaluations of mycotoxin contamination.
Statistical analysis
For the seed mixture field trials, linear regression analysis was used to relate (plot‐level) infestation pressure, avoided losses and mycotoxin contamination with the specific coverage of non‐Bt maize plants. Meanwhile, for the block refuge field trials, analysis of variance (ANOVA) was used to assess differences in (plot‐level) infestation pressure, avoided yield loss and mycotoxin contamination between plots with varying coverage of non‐Bt plants, followed by Tukey's honestly significant differences (HSD) post hoc test. Prior to analysis, raw data were log‐transformed (log (n + 1)) to meet assumptions of normality and homogeneity of variance.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
K.‐M.W.: conceived the idea. X.‐M.Y., S.‐Y.Z., B.L., Y.G., C.‐X.H., W.‐J.L., Y.‐Z.Y., G.‐P.L., L.‐L.W., X.‐Q.Y., H.‐B.Y., J.L. and D.‐Z.L.: performed the research. X.‐M.Y., S.‐Y.Z., X.‐J.S., B.L. and K.A.G.W.: analysed the data. X.‐M.Y. and K.A.G.W.: wrote the draft. All authors contributed to the revision of the manuscript.
Supporting information
Figure S1 Larval infestation pressure for six lepidopteran herbivores at different phenological stages of non‐Bt maize Hongshuo 899. Larval infestation levels are shown for each of the 11 experimental locations during 2019 and 2020.
Figure S2 Larval infestation level on non‐Bt maize in seed mixtures (DBN9936) and block refuges (Ruifeng 125) field trials with different non‐Bt maize proportions. Different capital letters within the same maize development stage for each site indicate significant differences in pest abundance (P < 0.05).
Table S1 Larval infestation pressure (mean number of larvae per 100 plants) of six lepidopteran herbivores at different phenological stages of non‐Bt maize DBN9858 for each of the 11 experimental sites during 2019 and 2020.
Table S2 Larval infestation pressure of all lepidopteran herbivores and their respective degree of control on Bt maize.
Table S3 Damage incidence on maize in experimental sites where either Spodoptera frugiperda (Yunnan and Guizhou) or Helicoverpa armigera and Ostrinia furnacalis (Shandong) were the prevailing lepidopteran species in 2020.
Table S4 Maize grain yield (tons/hectares) in seed mixtures and block refuge field trials, for each of the experimental sites during 2019 and 2020.
Acknowledgements
This study was funded by the National Key R&D Program of China (2021YFD1400702), the National Modern Agricultural Industry Technology System Construction Fund of China (CARS‐02) and the Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences.
References
- Abbas, H.K. , Zablotowicz, R.M. , Weaver, M.A. , Shier, W.T. , Bruns, H.A. , Bellaloui, N. , Accinelli, C. et al. (2013) Implications of Bt traits on mycotoxin contamination in maize: Overview and recent experimental results in southern United States. J. Agric. Food Chem. 61, 11759–11770. [DOI] [PubMed] [Google Scholar]
- Amaral, F.S.A. , Guidolin, A.S. , Salmeron, E. , Kanno, R.H. , Padovez, F.E.O. , Fatoretto, J.C. and Omoto, C. (2020) Geographical distribution of Vip3Aa20 resistance allele frequencies in Spodoptera frugiperda (Lepidoptera: Noctuidae) populations in Brazil. Pest Manag. Sci. 76, 169–178. [DOI] [PubMed] [Google Scholar]
- Bommarco, R. , Kleijn, D. and Potts, S.G. (2013) Ecological intensification: harnessing ecosystem services for food security. Trends Ecol. Evol. 28, 230–238. [DOI] [PubMed] [Google Scholar]
- Bonny, S. (2016) Genetically modified herbicide‐tolerant crops, weeds, and herbicides: Overview and impact. Environ. Manag. 57, 31–48. [DOI] [PubMed] [Google Scholar]
- Bowers, E. , Hellmich, R. and Munkvold, G. (2013) Vip3Aa and Cry1Ab proteins in maize reduce Fusarium ear rot and fumonisins by deterring kernel injury from multiple Lepidopteran pests. World Mycotoxin J. 6, 127–135. [Google Scholar]
- Burkness, E.C. and Hutchison, W.D. (2012) Bt pollen dispersal and Bt kernel mosaics: integrity of non‐Bt refugia for lepidopteran resistance management in maize. J. Econ. Entomol. 105, 1773–1780. [DOI] [PubMed] [Google Scholar]
- Carriere, Y. , Brown, Z. , Aglasan, S. , Dutilleul, P. , Carroll, M. , Head, G. , Tabashnik, B.E. et al. (2020a) Crop rotation mitigates impacts of corn rootworm resistance to transgenic Bt corn. Proc. Natl. Acad. Sci. U. S. A. 117, 18385–18392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriere, Y. , Degain, B. , Harpold, V.S. , Unnithan, G.C. and Tabashnik, B.E. (2020b) Gene flow between Bt and non‐Bt plants in a seed mixture increases dominance of resistance to pyramided Bt corn in Helicoverpa zea (Lepidoptera: Noctuidae). J. Econ. Entomol. 113, 2041–2051. [DOI] [PubMed] [Google Scholar]
- Carriere, Y. , Ellers‐Kirk, C. , Hartfield, K. , Larocque, G. , Degain, B. , Dutilleul, P. , Dennehy, T.J. et al. (2012) Large‐scale, spatially‐explicit test of the refuge strategy for delaying insecticide resistance. Proc. Natl. Acad. Sci. U. S. A. 109, 775–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriere, Y. , Ellers‐Kirk, C. , Sisterson, M. , Antilla, L. , Whitlow, M. , Dennehy, T.J. and Tabashnik, B.E. (2003) Long‐term regional suppression of pink bollworm by Bacillus thuringiensis cotton. Proc. Natl. Acad. Sci. U. S. A. 100, 1519–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriere, Y. , Fabrick, J.A. and Tabashnik, B.E. (2016) Can pyramids and seed mixtures delay resistance to Bt crops? Trends Biotechnol. 34, 291–302. [DOI] [PubMed] [Google Scholar]
- Dainese, M. , Martin, E.A. , Aizen, M.A. , Albrecht, M. , Bartomeus, I. , Bommarco, R. , Carvalheiro, L.G. et al. (2019) A global synthesis reveals biodiversity‐mediated benefits for crop production. Sci. Adv. 5, eaax0121,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lira, A.C. , Mascarin, G.M. and Delalibera, I. (2020) Microsclerotia production of Metarhizium spp. for dual role as plant biostimulant and control of Spodoptera frugiperda through corn seed coating. Fungal Biol. 124, 689–699. [DOI] [PubMed] [Google Scholar]
- Deguine, J.P. , Aubertot, J.N. , Flor, R.J. , Lescourret, F. , Wyckhuys, K.A.G. and Ratnadass, A. (2021) Integrated pest management: good intentions, hard realities. A review. Agron. Sustain. Dev. 41, 38. [Google Scholar]
- Deutsch, C.A. , Tewksbury, J.J. , Tigchelaar, M. , Battisti, D.S. , Merrill, S.C. , Huey, R.B. and Naylor, R.L. (2018) Increase in crop losses to insect pests in a warming climate. Science 361, 916–919. [DOI] [PubMed] [Google Scholar]
- Dively, G.P. , Venugopal, P.D. , Bean, D. , Whalen, J. , Holmstrom, K. , Kuhar, T.P. , Doughty, H.B. et al. (2018) Regional pest suppression associated with widespread Bt maize adoption benefits vegetable growers. Proc. Natl. Acad. Sci. U. S. A. 115, 3320–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton, M.D. , Fridgen, J. , Anderson, J.R. , Ahlgrim, J. , Criswell, M. , Dhungana, P. , Gocken, T. et al. (2012) Transgenic insect resistance traits increase corn yield and yield stability. Nat. Biotechnol. 30, 493–496. [DOI] [PubMed] [Google Scholar]
- Elbehri, A. and MacDonald, S. (2004) Estimating the impact of transgenic Bt cotton on West and Central Africa: a general equilibrium approach. World Dev. 32, 2049–2064. [Google Scholar]
- Eschen, R. , Beale, T. , Bonnin, J.M. , Constantine, K.L. , Duah, S. , Finch, E.A. , Makale, F. et al. (2021) Towards estimating the economic cost of invasive alien species to African crop and livestock production. CABI Agri. Bio. 2, 18. [Google Scholar]
- Feit, B. , Blüthgen, N. , Daouti, E. , Straub, C. , Traugott, M. and Jonsson, M. (2021) Landscape complexity promotes resilience of biological pest control to climate change. P. Roy. Soc. B‐Biol. Sci. 288, 20210547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurr, G.M. , Wratten, S.D. , Landis, D.A. and You, M.S. (2017) Habitat management to suppress pest populations: progress and prospects. Annu. Rev. Entomol. 62, 91–109. [DOI] [PubMed] [Google Scholar]
- Hammond, B.G. , Campbell, K.W. , Pilcher, C.D. , Degooyer, T.A. , Robinson, A.E. , McMillen, B.L. , Spangler, S.M. et al. (2004) Lower fumonisin mycotoxin levels in the grain of Bt corn grown in the United States in 2000–2002. J. Agric. Food Chem. 52, 1390–1397. [DOI] [PubMed] [Google Scholar]
- Huang, N.X. , Jaworski, C.C. , Desneux, N. , Zhang, F. , Yang, P.Y. and Wang, S. (2020) Long‐term and large‐scale releases of Trichogramma promote pesticide decrease in maize in northeastern China. Entomol. Gen. 40, 331–335. [Google Scholar]
- Hutchison, W.D. , Burkness, E.C. , Mitchell, P.D. , Moon, R.D. , Leslie, T.W. , Fleischer, S.J. , Abrahamson, M. et al. (2010) Areawide suppression of European corn borer with Bt maize reaps savings to non‐Bt maize growers. Science 330, 222–225. [DOI] [PubMed] [Google Scholar]
- Jepson, P.C. , Murray, K. , Bach, O. , Bonilla, M.A. and Neumeister, L. (2020) Selection of pesticides to reduce human and environmental health risks: a global guideline and minimum pesticides list. Lancet Planet. Health 4, E56–E63. [DOI] [PubMed] [Google Scholar]
- Jin, L. , Zhang, H.N. , Lu, Y.H. , Yang, Y.H. , Wu, K.M. , Tabashnik, B.E. and Wu, Y.D. (2015) Large‐scale test of the natural refuge strategy for delaying insect resistance to transgenic Bt crops. Nat. Biotechnol. 33, 169–174. [DOI] [PubMed] [Google Scholar]
- Jing, D.P. , Guo, J.F. , Jiang, Y.Y. , Zhao, J.Z. , Sethi, A. , He, K.L. and Wang, Z.Y. (2020) Initial detections and spread of invasive Spodoptera frugiperda in China and comparisons with other noctuid larvae in cornfields using molecular techniques. Insect Sci. 27, 780–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiresur, V.R. and Manjunath, I. (2011) Socio‐economic impact of Bt cotton ‐ a case study of Karnataka. Agric. Econ. Res. Rev. 24, 67–82. [Google Scholar]
- Koch, M.S. , Ward, J.M. , Levine, S.L. , Baum, J.A. , Vicini, J.L. and Hammond, B.G. (2015) The food and environmental safety of Bt crops. Front. Plant Sci. 6, 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane, J.R. , Devos, Y. , Beckie, H.J. , Owen, M.D.K. , Tillie, P. , Messean, A. and Kudsk, P. (2017) Integrated weed management systems with herbicide‐tolerant crops in the European Union: lessons learnt from home and abroad. Crit. Rev. Biotechnol. 37, 459–475. [DOI] [PubMed] [Google Scholar]
- Li, G.P. , Feng, H.Q. , Ji, T.J. , Huang, J.R. and Tian, C.H. (2021) What type of Bt corn is suitable for a region with diverse lepidopteran pests: a laboratory evaluation. GM Crops Food 12, 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y.H. , Hallerman, E.M. and Peng, Y.F. (2018) How can China prepare for the domestic cultivation of Bt maize? Trends Food. Sci. Dent. Tech. 73, 87–88. [Google Scholar]
- Liang, J.G. , Zhang, D.D. , Li, D.Y. , Zhao, S.Y. , Wang, C.Y. , Xiao, Y.Y. , Xu, D. et al. (2021) Expression profiles of Cry1Ab protein and its insecticidal efficacy against the invasive fall armyworm for Chinese domestic GM maize DBN9936. J. Integr. Agric. 20, 792–803. [Google Scholar]
- Lu, B.R. (2016) Challenges of transgenic crop commercialization in China. Nat. Plants 2, 16077. [DOI] [PubMed] [Google Scholar]
- Lu, Y. , Wu, K. , Jiang, Y. , Guo, Y. and Desneux, N. (2012) Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 487, 362–365. [DOI] [PubMed] [Google Scholar]
- Lu, Y.H. , Wyckhuys, K.A.G. , Yang, L. , Liu, B. , Zeng, J. , Jiang, Y.Y. , Desneux, N. et al. (2022) Bt cotton area contraction drives regional pest resurgence, crop loss, and pesticide use. Plant Biotechnol. J. 20, 390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, E.A. , Dainese, M. , Clough, Y. , Baldi, A. , Bommarco, R. , Gagic, V. , Garratt, M.P.D. et al. (2019) The interplay of landscape composition and configuration: new pathways to manage functional biodiversity and agroecosystem services across Europe. Ecol. Lett. 22, 1083–1094. [DOI] [PubMed] [Google Scholar]
- Mendelsohn, M. , Kough, J. , Vaituzis, Z. and Matthews, K. (2003) Are Bt crops safe? Nat. Biotechnol. 21, 1003–1009. [DOI] [PubMed] [Google Scholar]
- Murray‐Tortarolo, G.N. , Jaramillo, V.J. and Larsen, J. (2018) Food security and climate change: the case of rainfed maize production in Mexico. Agric. For. Meteorol. 253, 124–131. [Google Scholar]
- Naranjo, S.E. , Hellmich, R.L. , Romeis, J. , Shelton, A.M. and Velez, A.M. (2020) The role and use of genetically engineered insect‐resistant crops in integrated pest management systems. In: Integrated Management of Insect Pests: Current and Future Developments ( Kogan, M. and Heinrichs, E.A. eds). Cambridge: Burleigh Dodds Science Publishing, pp. 283‐340. [Google Scholar]
- Nleya, T. , Chungu, C. and Kleinjan, J. (2016) Chapter 5: Corn growth and development. In iGrow Corn: Best Management Practices( Clay, D.E. , Carlson, C.G. , Clay, S.A. and Byamukama, E. , eds). South Dakota State: South Dakota State University. [Google Scholar]
- Omoto, C. , Bernardi, O. , Salmeron, E. , Sorgatto, R.J. , Dourado, P.M. , Crivellari, A. , Carvalho, R.A. et al. (2016) Field‐evolved resistance to Cry1Ab maize by Spodoptera frugiperda in Brazil. Pest Manag. Sci. 72, 1727–1736. [DOI] [PubMed] [Google Scholar]
- Overton, K. , Maino, J.L. , Day, R. , Umina, P.A. , Bett, B. , Carnovale, D. , Ekesi, S. et al. (2021) Global crop impacts, yield losses and action thresholds for fall armyworm (Spodoptera frugiperda): A review. Crop Prot. 145, 105641. [Google Scholar]
- Pellegrino, E. , Bedini, S. , Nuti, M. and Ercoli, L. (2018) Impact of genetically engineered maize on agronomic, environmental and toxicological traits: a meta‐analysis of 21 years of field data. Sci. Rep. 8, 3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, E.D. , Ciliberto, F. , Hennessy, D.A. and Moschini, G. (2016) Genetically engineered crops and pesticide use in U.S. maize and soybeans. Sci. Adv. 2, e1600850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit, S. , Cordeau, S. , Chauvel, B. , Bohan, D. , Guillemin, J.‐P. and Steinberg, C. (2018) Biodiversity‐based options for arable weed management. A review. Agron. Sustain. Dev. 38, 48. [Google Scholar]
- Pitre, H.N. , Portillo, H.E. , Meckenstock, D.H. , Castro, M.T. , Lopez, J.I. , Andrews, K.L. , Gomez, F. et al. (1997) A complex of lepidopterous defoliators on sorghum and maize in Honduras: some management tactics. Ceiba 38, 109–119. [Google Scholar]
- Pruter, L.S. , Brewer, M.J. , Weaver, M.A. , Murray, S.C. , Isakeit, T.S. and Bernal, J.S. (2019) Association of insect‐derived ear injury with yield and aflatoxin of maize hybrids varying in Bt transgenes. Environ. Entomol. 48, 1401–1411. [DOI] [PubMed] [Google Scholar]
- Qiu, J.B. , Lu, Y.N. , He, D. , Lee, Y.W. , Ji, F. , Xu, J.H. and Shi, J.R. (2020) Fusarium fujikuroi species complex associated with rice, maize, and soybean from Jiangsu province, China: phylogenetic, pathogenic, and toxigenic analysis. Plant Dis. 104, 2193–2201. [DOI] [PubMed] [Google Scholar]
- Riegler, M. (2018) Insect threats to food security. Science 361, 846. [DOI] [PubMed] [Google Scholar]
- Sanchez‐Bayo, F. and Wyckhuys, K.A.G. (2021) Further evidence for a global decline of the entomofauna. Austral. Entomol. 60, 9–26. [Google Scholar]
- Savary, S. , Willocquet, L. , Pethybridge, S.J. , Esker, P. , McRoberts, N. and Nelson, A. (2019) The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 3, 430–439. [DOI] [PubMed] [Google Scholar]
- Schreinemachers, P. , Grovermann, C. , Praneetvatakul, S. , Heng, P. , Nguyen, T.T.L. , Buntong, B. , Le, N.T. et al. (2020) How much is too much? Quantifying pesticide overuse in vegetable production in Southeast Asia. J. Clean. Prod. 244, 118738. [Google Scholar]
- Shiferaw, B. , Prasanna, B.M. , Hellin, J. and Banziger, M. (2011) Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Security 3, 307–327. [Google Scholar]
- Song, Y.F. , Yang, X.M. , Zhang, H.W. , Zhang, D.D. , He, W. , Wyckhuys, K.A.G. and Wu, K.M. (2021) Interference competition and predation between invasive and native herbivores in maize. J. Pest. Sci. 94, 1053–1063. [Google Scholar]
- Sun, L.J. , Chen, S.L. and Li, Y.Y. (2020) Overview of the damage loss and control index of corn borer. China Plant Protect. 40, 55–59 +66. [Google Scholar]
- Tabashnik, B.E. , Brevault, T. and Carriere, Y. (2013) Insect resistance to Bt crops: lessons from the first billion acres. Nat. Biotechnol. 31, 510–521. [DOI] [PubMed] [Google Scholar]
- Tabashnik, B.E. and Carriere, Y. (2017) Surge in insect resistance to transgenic crops and prospects for sustainability. Nat. Biotechnol. 35, 926–935. [DOI] [PubMed] [Google Scholar]
- Tabashnik, B.E. and Carriere, Y. (2019) Global patterns of resistance to Bt crops highlighting pink bollworm in the United States, China, and India. J. Econ. Entomol. 112, 2513–2523. [DOI] [PubMed] [Google Scholar]
- Tabashnik, B.E. , Liesner, L.R. , Ellsworth, P.C. , Unnithan, G.C. , Fabrick, J.A. , Naranjo, S.E. , Li, X.C. et al. (2021) Transgenic cotton and sterile insect releases synergize eradication of pink bollworm a century after it invaded the United States. Proc. Natl. Acad. Sci. U. S. A. 118, e2019115118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambo, J.A. , Kansiime, M.K. , Mugambi, I. , Rwomushana, I. , Kenis, M. , Day, R.K. and Lamontagne‐Godwin, J. (2020) Understanding smallholders' responses to fall armyworm (Spodoptera frugiperda) invasion: evidence from five African countries. Sci. Total Environ. 740, 140015. [DOI] [PubMed] [Google Scholar]
- Tellez‐Rodriguez, P. , Raymond, B. , Moran‐Bertot, I. , Rodriguez‐Cabrera, L. , Wright, D.J. , Borroto, C.G. and Ayra‐Pardo, C. (2014) Strong oviposition preference for Bt over non‐Bt maize in Spodoptera frugiperda and its implications for the evolution of resistance. BMC Biol. 12, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsatsakis, A.M. , Nawaz, M.A. , Kouretas, D. , Balias, G. , Savolainen, K. , Tutelyan, V.A. , Golokhvast, K.S. et al. (2017) Environmental impacts of genetically modified plants: A review. Environ. Res. 156, 818–833. [DOI] [PubMed] [Google Scholar]
- Van Bruggen, A.H.C. , He, M.M. , Shin, K. , Mai, V. , Jeong, K.C. , Finckh, M.R. and Morris, J.G., Jr. (2018) Environmental and health effects of the herbicide glyphosate. Sci. Total Environ. 616‐617, 255–268. [DOI] [PubMed] [Google Scholar]
- Van den Berg, J. , Prasanna, B.M. , Midega, C.A.O. , Ronald, P.C. , Carriere, Y. and Tabashnik, B.E. (2021) Managing fall armyworm in Africa: can Bt maize sustainably improve control? J. Econ. Entomol. 114, 1934–1949. [DOI] [PubMed] [Google Scholar]
- Wan, P. , Xu, D. , Cong, S. , Jiang, Y. , Huang, Y. , Wang, J. , Wu, H. et al. (2017) Hybridizing transgenic Bt cotton with non‐Bt cotton counters resistance in pink bollworm. Proc. Natl. Acad. Sci. U. S. A. 114, 5413–5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. , Wang, X. , Jin, Z. , Müller, C. , Pugh, T.A.M. , Chen, A. , Wang, T. et al. (2022) Occurrence of crop pests and diseases has largely increased in China since 1970. Nat. Food 3, 57–65. [DOI] [PubMed] [Google Scholar]
- Wang, Z.Y. , He, K.L. , Zhang, F. , Lu, X. and Babendreier, D. (2014) Mass rearing and release of Trichogramma for biological control of insect pests of corn in China. Biol. Control 68, 136–144. [Google Scholar]
- Wang, Z.Y. and Wang, X.M. (2019) Current status and management strategies for corn pests and diseases in China. Plant Prot. 45, 1–11. [Google Scholar]
- Wu, J.Z. , Zhang, J. , Ge, Z.M. , Xing, L.W. , Han, S.Q. , Shen, C. and Kong, F.T. (2021a) Impact of climate change on maize yield in China from 1979 to 2016. J. Integr. Agric. 20, 289–299. [Google Scholar]
- Wu, K.M. , Lu, Y.H. , Feng, H.Q. , Jiang, Y.Y. and Zhao, J.Z. (2008) Suppression of cotton bollworm in multiple crops in China in areas with Bt toxin‐containing cotton. Science 321, 1676–1678. [DOI] [PubMed] [Google Scholar]
- Wu, Q.L. , Jiang, Y.Y. , Liu, J. , Hu, G. and Wu, K.M. (2021b) Trajectory modeling revealed a southwest‐northeast migration corridor for fall armywormSpodoptera frugiperda (Lepidoptera: Noctuidae) emerging from the North China Plain. Insect Sci. 28, 649–661. [DOI] [PubMed] [Google Scholar]
- Wu, Q.L. , Shen, X.J. , He, L.M. , Jiang, Y.Y. , Liu, J. , Hu, G. and Wu, K.M. (2021c) Windborne migration routes of newly‐emerged fall armyworm from Qinling Mountains–Huaihe River region, China. J. Integr. Agric. 20, 694–706. [Google Scholar]
- Wyckhuys, K.A.G. , Aebi, A. , Bijleveld van Lexmond, M. , Bojaca, C.R. , Bonmatin, J.M. , Furlan, L. , Guerrero, J.A. et al. (2020) Resolving the twin human and environmental health hazards of a plant‐based diet. Environ. Int. 144, 106081. [DOI] [PubMed] [Google Scholar]
- Wyckhuys, K.A.G. , Zhang, W. , Colmenarez, Y.C. , Simelton, E. , Sander, B.O. and Lu, Y. (2022a) Tritrophic defenses as a central pivot of low‐emission, pest‐suppressive farming systems. Curr. Opin. Environ. Sustain. 58, 101208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckhuys, K.A.G. , Zhang, W. , Prager, S.D. , Kramer, D.B. , Delaquis, E. , Gonzalez, C.E. and van der Werf, W. (2018) Biological control of an invasive pest eases pressures on global commodity markets. Environ. Res. Lett. 13, 094005. [Google Scholar]
- Wyckhuys, K.A.G. , Zou, Y. , Wanger, T.C. , Zhou, W. , Gc, Y.D. and Lu, Y. (2022b) Agro‐ecology science relates to economic development but not global pesticide pollution. J. Environ. Manag. 307, 114529. [DOI] [PubMed] [Google Scholar]
- Yang, X.M. , Wyckhuys, K.A.G. , Jia, X.P. , Nie, F.Y. and Wu, K.M. (2021) Fall armyworm invasion heightens pesticide expenditure among Chinese smallholder farmers. J. Environ. Manag. 282, 111949. [DOI] [PubMed] [Google Scholar]
- Zeilinger, A.R. , Olson, D.M. and Andow, D.A. (2016) Competitive release and outbreaks of non‐target pests associated with transgenic Bt cotton. Ecol. Appl. 26, 1047–1054. [DOI] [PubMed] [Google Scholar]
- Zhang, W. , Lu, Y.H. , van der Werf, W. , Huang, J.K. , Wu, F. , Zhou, K. , Deng, X.Z. et al. (2018) Multidecadal, county‐level analysis of the effects of land use, Bt cotton, and weather on cotton pests in China. Proc. Natl. Acad. Sci. U. S. A. 115, E7700–E7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, J.W. , Wang, J. , DiTommaso, A. , Zhang, C.X. , Zheng, G.P. , Liang, W. , Islam, F. et al. (2020) Weed research status, challenges, and opportunities in China. Crop Prot. 134, 104449. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Larval infestation pressure for six lepidopteran herbivores at different phenological stages of non‐Bt maize Hongshuo 899. Larval infestation levels are shown for each of the 11 experimental locations during 2019 and 2020.
Figure S2 Larval infestation level on non‐Bt maize in seed mixtures (DBN9936) and block refuges (Ruifeng 125) field trials with different non‐Bt maize proportions. Different capital letters within the same maize development stage for each site indicate significant differences in pest abundance (P < 0.05).
Table S1 Larval infestation pressure (mean number of larvae per 100 plants) of six lepidopteran herbivores at different phenological stages of non‐Bt maize DBN9858 for each of the 11 experimental sites during 2019 and 2020.
Table S2 Larval infestation pressure of all lepidopteran herbivores and their respective degree of control on Bt maize.
Table S3 Damage incidence on maize in experimental sites where either Spodoptera frugiperda (Yunnan and Guizhou) or Helicoverpa armigera and Ostrinia furnacalis (Shandong) were the prevailing lepidopteran species in 2020.
Table S4 Maize grain yield (tons/hectares) in seed mixtures and block refuge field trials, for each of the experimental sites during 2019 and 2020.


