Abstract

Probing multiple proprietary pharmaceutical libraries in parallel via virtual screening allowed rapid expansion of the structure–activity relationship (SAR) around hit compounds with moderate efficacy against Trypanosoma cruzi, the causative agent of Chagas Disease. A potency-improving scaffold hop, followed by elaboration of the SAR via design guided by the output of the phenotypic virtual screening efforts, identified two promising hit compounds 54 and 85, which were profiled further in pharmacokinetic studies and in an in vivo model of T. cruzi infection. Compound 85 demonstrated clear reduction of parasitemia in the in vivo setting, confirming the interest in this series of 2-(pyridin-2-yl)quinazolines as potential anti-trypanosome treatments.
Introduction
Chagas Disease (CD), classified as a neglected tropical disease (NTD) by the World Health Organization, is caused by an infection of the protozoan kinetoplastid parasite Trypanosoma cruzi and demonstrates a broad range in severity including significant morbidity and mortality.1−3 In addition to the high incidence of CD in Brazil and Latin America, prevalence is growing in Central America, southern United States, and elsewhere in the world.4−12 It is assumed that more than 6 million people are currently infected with this parasite, and over 10,000 deaths per year can be attributed to this disease.13,14 Transmission occurs via an insect of the family Reduviidae, trivially known as the “kissing bug”, through the feces of the insect during its blood meal to humans.15−17 In the human host, T. cruzi parasites can multiply in different cell types, as either the dominant, noninvasive, replicative intracellular amastigote form or as the blood-stage invasive, nonreplicative, trypomastigotes.18 There is substantial evidence that post-initial infection, the parasites are widespread in the human host, invading many different organs within the body. As detailed in the life cycle of this parasite reported by Centers for Disease Control and Progression (CDC),19,20 the disease has two stages: an acute stage and a chronic stage; in the acute stage, most infected adults demonstrate only mild symptoms; however, this acute phase demonstrates a significantly higher fatality rate among children, currently approximated at around 5%.20 Following the subsidence of the acute phase, the infection then enters a chronic stage, where the parasite remains dormant for many years or even decades in around 70% of infected individuals. Approximately 30% of patients hosting this chronic infection eventually develop symptoms such as chronic heart disease, megacolon/megaesophagus, or both. Oftentimes, the underlying causative chronic T. cruzi infection is only detectable when the patient is critically ill or even dead.10
Despite the prevalence and mortality rate of CD, pharmacological responses for this disease are still not fit for purpose. There is no available vaccine and the current standard drugs, benznidazole and nifurtimox, demonstrate efficacy only in acute infections, where disease diagnosis is rare.21−25 Pharmacological efficacy of these treatments in chronically infected subjects is less dependable, and suffers from serious side effect profile.13,26,27
Recently, development of a novel class of molecules for CD, CYP51 inhibitors (Posaconazole and E1224, the prodrug of ravuconazole), successfully progressed into clinical development. However, this class of molecules proved to be insufficiently effective in humans, with prevalent treatment failure in patients. General consensus in the scientific Chagas community stresses the need to avoid further development of compounds acting predominantly via this mechanism of action.28 The subsequent attrition of this class of molecules has resulted in a relatively bare R&D pipeline for CD.24,25,29−32 There is a critical urgency for development of new treatment options for this disease, which concerns millions of the world’s poorest people, particularly in low-to-middle income countries (LMIC) across Latin America. Despite many interesting and fruitful approaches to identify new chemical entities for the treatment of T. cruzi infection in the past decade,33−40 further research is still required to address CD, with the clear goal of obtaining an effective, orally bioavailable, affordable, and safe molecule effective in both the acute and chronic phases of the disease.
Results and Discussion
Screening and Initial Hit Identification
Since 2012, one of our organizations (DNDi) has tested over 2 million compounds in high-throughput high-content screening against kinetoplastid parasites including Leishmania donovani and Trypanosoma cruzi, the causative agents of visceral leishmaniasis and CD, respectively, searching for novel and attractive small-molecule starting points for drug discovery campaigns.41 Seeking to improve the efficiency and throughput of the triaging of results from these phenotypic screening efforts led to the creation of the NTD Drug Discovery Booster, a precompetitive virtual screening model comprising key players from industrial pharmaceutical R&D.42,43
NTD Drug Discovery Booster
As an alternative to a commercial-analoguing approach to initial exploration of chemical space around a new high-throughput screening (HTS) hit, the Drug Discovery Booster (“Booster”) engages collaborative, precompetitive in silico screening to extrapolate chemical space around a hit,44,45 searching across multiple proprietary pharmaceutical company databases, seeking both close analogues to the hit as well as structurally differentiated scaffold-hop compounds based on the ligand pharmacophore. A hit structure (“Seed”) is shared with scientists at each partner pharmaceutical company, in silico similarity searches, often proprietary to the partner, are performed probing the proprietary collections of each partner, and up to 100 prospective “similar” molecules per partner are screened against the parasite(s) of interest. The partners are actively encouraged to use their own definition of “ligand-similarity” in an effort to tap into multiple ways of addressing the same overarching question—“what do you have in your library which resembles the “seed”?” This collaborative effort brings a combination of molecules focused on both SAR annotation as well as more general pharmacophore-led exploration. The results of these experimental screens with the providing partners, as well as anonymized sharing of the structure of the most improved molecule from the entire collaborative screening endeavor (“Improved Hit”), allow a second, more refined iteration of in silico screening to be performed, again providing ∼100 molecules per partner. Repetition of this iterative screening/sharing process continues until improvements cease to be made, or until SAR around a chemotype emerges.
The use of orthogonal in silico techniques and clearly differentiated proprietary libraries maximizes both the richness of the SAR explored around the initial seed phenotype and increases the chances of identifying an interesting change of chemotype with similar or improved antiparasitic activity via scaffold hop.46 These newly identified scaffold hops can be subsequently mined using the same Booster approach, thus maximizing the value of the hits coming from HTS.
Hit Identification
2-Arylpyrimidine 1 (Figure 1) was identified as a potential starting point and anti-kinetoplastid Booster seed based on data from a HTS against both T. cruzi and L. donovani (the causative agent of visceral leishmaniasis).41 Compound 1 demonstrated sub-micromolar potency in an intracellular T. cruzi infection assay, and an independent batch of 1 demonstrated similar levels of efficacy in an axenic L. donovani model (see Supporting Information 1).47 Both assays demonstrated a wide selectivity index (SI) between antiparasitic efficacy and cell line cytotoxicity (MRC5 and L-6 cells as controls, respectively). Evaluation of the literature around this 2-arylpyrimidine core revealed extensive general coverage (>25,000 references in scifinder);48 however, only one mention of such a chemotype with efficacy in the kinetoplastid field was identified, the mTOR inhibitor NVP-BEZ235,49,50 which despite sharing a 2-arylpyrimidine motif we considered sufficiently structurally differentiated to validate continued evaluation of 1. Compound 1 did not present any PAINS motif as evaluated by SwissADME;51,52 nevertheless, we were cautious about the potential for metal chelation in the 2-(pyridin-2-yl)pyrimidine core leading to false positive or pan-cytotoxicity results. We therefore targeted early evaluation of a compound from the series in an in vivo proof of concept (PoC) infection model as a key go/no-go decision point for further work.
Figure 1.

Initial hit compound 1 from high-throughput screening (HTS).
Hit Elaboration and Validation via Collaborative In Silico Screening
Compound 1 was submitted as a “seed” to the partners of the NTD Booster consortium: AstraZeneca plc, Eisai Co., Ltd., Shionogi & Co., Ltd., Takeda Pharmaceutical Company Ltd., and Celgene Corporation. Since the precompetitive mechanism of the Booster project requires anonymity and a high degree of confidentiality on the in silico methods and library composition between partners, identities of the partners are presented as blinded in this study as “Companies A–E”. Four of these companies participated in the first cycle of booster screening, these were joined by the fifth company for the second round of screening, this company having joined the consortium in the interim. An overview of the general Booster process and results are shown in Figure 2.
Figure 2.
Overview of the booster virtual screening process.
Partners performed ligand-based virtual screening against their proprietary compound collections using the partner-specific in silico similarity searches approach as described in Table 1 (see Supporting Information 1 for detailed description). The compounds identified via these virtual screening efforts (316 compounds in total, ∼80 compounds per partner) were then investigated in high-content cell-based T. cruzi and L. donovani infection assays using U2OS and THP1 host cells, respectively [Booster Round A (Figure 2)]. A control sample of 1 was included in both assays. The assay read-outs from these high-content approaches demonstrate a compound’s efficacy at total parasite clearance as well as host cell cytotoxicity, yielding an antiparasitic IC50, a host cell CC50, and a selectivity index (SI) between the two read-outs (SI = host cell IC50/parasite IC50; see the Supporting Information for more details). Compounds with a SI > 5 against either L. donovani or T. cruzi were considered as demonstrating legitimate antiparasitic activity, and compounds with SI > 50 against either parasite were classified as being of high potency. The results of the virtual screening and experimental parasitology of this selection of compounds displayed as a 2D chemical space representation generated via principal component analysis of chemical fingerprints (fPCA) and calculated physicochemical properties (PCA) are shown in Figure 2 (RDKit nodes in Knime used to generate chemical descriptors, Morgan 1024 Bit fingerprints, and to run principal component analyses). Despite initial hit 1 demonstrating significantly reduced potency in the high-content assays used for the screening of the virtual screen output relative to the potency seen in the HTS assays via which it had been identified, of the 316 compounds provided, 98 compounds (31%) demonstrated activity, and 18 compounds (6%) were considered highly active. Of the 316 compounds, 58% retained the 2-arylpyrimidine core of the initial seed compound, and the remaining 42% were considered to contain scaffold hops away from the core motif. Within the latter, we identified a small subset of 2-arylquinazolines, exemplified by compound 2, with significant potency against both T. cruzi and L. donovani. Interestingly, despite the relative ubiquity of quinazolines in the med chem literature, the majority of the 2-arylquinazolines within the screening set (>80%) had been provided by a single Booster partner. Rationalizing that the libraries of the other partners would contain a significant number of 2-arylquiazolines, we repeated the booster in silico screening approach with the participating booster companies [Booster Round B (Figure 2)]. For this second cycle, each company received the HTS data from their respective compounds. In addition, the structure and data for 2 was shared with all partners, effectively informing all partners that 2-arylquinazolines were generally more potent that 2-arylpyrimidines. The results of this second round of screening (479 compounds in total) are shown in Figure 2. This second round identified further 180 compounds of 2-arylquinazolines, where 30% were considered as active. In addition, some more highly active compounds provided by partners based on further scaffold hop from the 2-arylquinazoline motif were identified, as exemplified by 3.
Table 1. Overview of the In Silico Approach Taken by Each Partner Company, along with the Number of Compounds Furnished from Each Partner.
| number
of compounds booster round A/B (actives)b |
|||
|---|---|---|---|
| company and computational approacha | total | 2-arylpyrimidinec | 2-arylquinazolined |
| A—the top scoring 150 compounds were selected by Tanimoto similarity calculation using the FCFP4 fingerprint, followed by refinement to 96 compounds based on maximized diversity | 192 | 22/11 (9) | 2/33 (7) |
| B—similarity search using Daylight59 and Chemaxon60 fingerprints and ROCS61 TanimotoCombo scoring | 119 | 27/62 (8) | 1/5 (5) |
| C—ECFP4 similarity (Tanimoto cut-off 0.6) and in-house fingerprint search (Tanimoto cut-off 0.7). Subsequent in-house “quality”, commercial availability, and IP filters | 176 | 83/0 (14) | 3/81 (25) |
| D—series of similarity and substructure-based queries prioritized with Tanimoto similarity calculated by ECFP4 | 182 | 53/0 (24) | 30/3 (24) |
| E—ECFP4 similarity (initial Tanimoto cut-off 0.7 descending incrementally until sufficient compounds had been identified) | 126 | 0/3 (1) | 0/58 (17) |
| all companies | 795 | 185/76 (56) | 36/180 (78) |
Further information is found in Supporting Information 1.
Actives defined as compounds with SI > 5 against one or both parasites.
Compounds with a 2-arylpyrimidine core.
Compounds with a 2-arylquinazoline core.
Combining the results from these two rounds of collaborative virtual screening allowed us to build a strong picture of the SAR around this 2-arylquinazoline motif, itself identified as a scaffold hop via the same virtual screen cycle. The general SAR of the series informed by compounds 4–22 originating directly from Booster virtual screening (Chart 1) encouraged us to initiate further profiling and analoging, and we instigated a strategy to explore the other substituent positions of quinazoline illustrated by compound designs I–IV (Figure 3).
Chart 1. Compounds Identified via the Booster Process—Generic Overview of SAR from Booster Only.

Figure 3.
Overview of the post-screening design strategy.
This strategy entailed variation of (i) the 2-position of quinazoline to understand the importance of the 2-(pyridin-2-yl) motif, (ii) probing the 4-position of the quinazoline core with both acyclic and cyclic amines, (iii) structurally diverse core scaffold replacements with 6,6- or 5,6-membered aromatic ring. In all cases, we evaluated these changes for both T. cruzi activity and pharmacokinetic properties to rapidly identify potential candidates for in vivo PoC studies.
Prior to initiating these further studies and to give confidence that the data obtained from the booster virtual screening was correct, we selected four compounds (4, 13, 18, 20) from the screening for resynthesis and retest in orthogonal assays. This effort served two purposes—first, to ensure that analytically pure samples of the compounds coming from the HTS collections of the pharmaceutical companies could be replicated, indirectly qualifying the booster data set and second, to ensure that the activity we were seeing was not confined to a single antiparasitic assay system. The results of this checking process were uniformly positive, with resynthesized batches of the four compounds giving similar levels of potency, which were then reconfirmed in orthogonal antiparasitic assays against T. cruzi (MRC5 background cell line) and leishmania infantum (PMM background cell line). Full results from this secondary check are provided in Supporting Information 1.
Synthesis
The synthesis of key intermediate 27 was conducted using readily available starting materials and standard synthetic modifications as shown in Scheme 1.53 Amidation of 2-aminobenzamide (23) with pyridine-2-carboxylic acid (24) yielded N-(2-carbamoylphenyl)pyridine-2-carboxamide (25), followed by reaction with methanolic sodium hydroxide heated under reflux condition to afford 2-(pyridin-2-yl)quinazolin-4-ol (26). Treating 26 with POCl3 with TEA yielded 4-chloro-2-(pyridin-2-yl)quinazoline (27). Final compounds A (45–59, 61–63, 65–85, 85-tR1, 85-tR2) were then prepared from 27 by simple SNAr transformation with various amines in step (d).
Scheme 1. Synthesis of Intermediate 27 and Analogue Preparation of Quinazoline Derivatives.
Reagents and conditions: (a) HOBT, EDCI, TEA, DMF, rt, 24 h, 85%; (b) 1 N NaOH (aqueous), MeOH, reflux condition, 1 h, 66%; (c) POCl3, TEA, toluene, reflux condition, overnight, 83%; and (d) amines, n-butanol, 50–110 °C, 12–16 h, or DIEA, solvent (MeCN, DMA or NMP), MW 100–170 °C, 0.5–1 h, 3–98%.
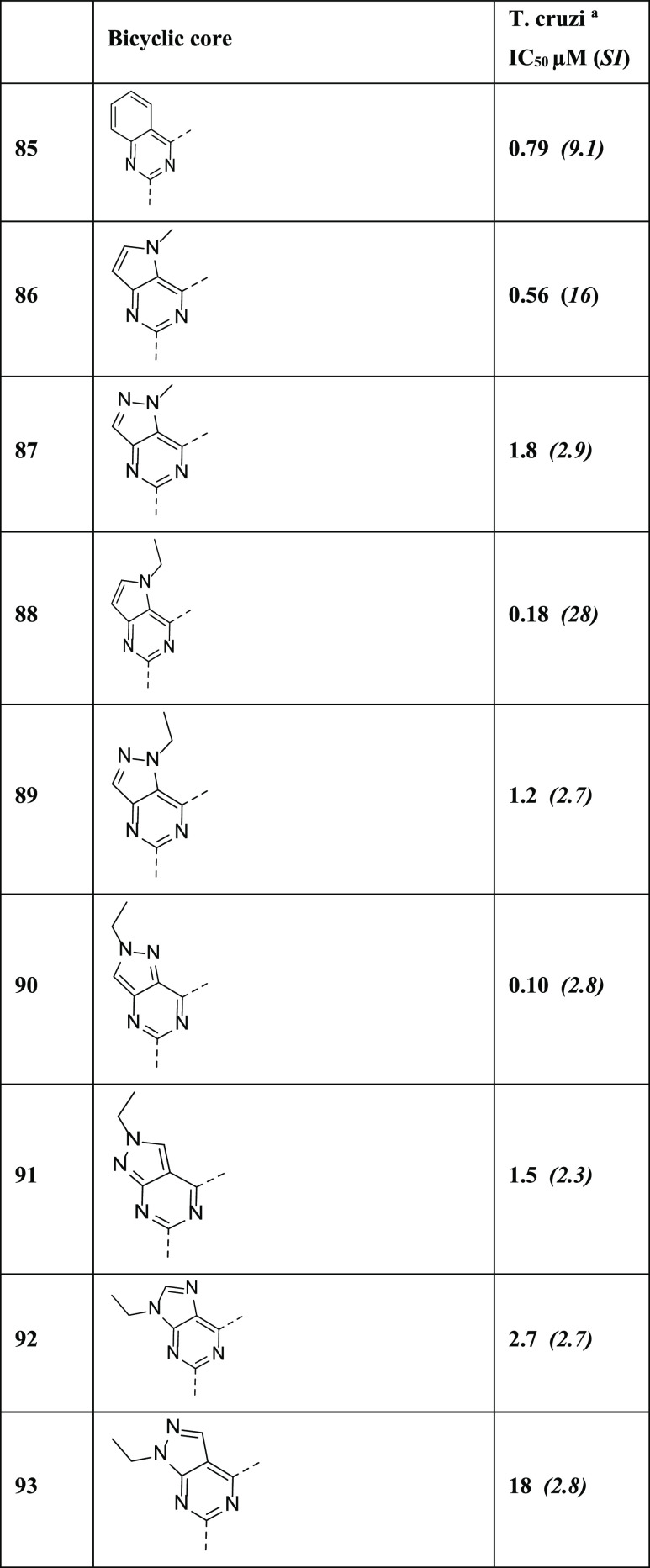
The synthetic routes for core modification to pyrrolo[3,2-d]pyrimidine are exemplified in Scheme 2. From the starting material 2,4-dichloro-5H-pyrrolo[3,2-d]pyrimidine (28), we synthesized N-alkylated 2,4-dichloro-5H-pyrrolo[3,2-d]pyrimidines (29) by the reaction with alkyl iodides and Cs2CO3 in DMF. In the presence of DIEA in THF, 2-(pyrrolidin-3-yl)pyridine (30) was reacted with intermediates 29 to afford 2-chloro-5-alkyl-4-(3-(pyridin-2-yl)pyrrolidin-1-yl)-5H-pyrrolo[3,2-d]pyrimidines (31). Carbon–carbon bond formation via the palladium-catalyzed coupling reaction of (2-pyridine)cyclic-triolborate lithium salt with aryl chlorides 31 furnished the desired compounds 86 and 88.
Scheme 2. Core Modification and Analogue Preparation of Pyrrolo[3,2-d]pyrimidine Derivatives.
Reagents and conditions: (a) alkyl iodides, Cs2CO3, DMF, rt, overnight, 70%; (b) 2-(pyrrolidin-3-yl)pyridine (30), DIEA, THF, rt, 10 h, 71–77%; and (c) (2-pyridine)cyclic-triolborate lithium salt, Pd2(dba)3, cuprous chloride, butyl di-1-adamantylphosphine, potassium tert-butoxide, DME, MW 120 °C, 3 h, 19–43%.
Procedures used to get further core-modified compounds are shown in Scheme 3. Alkylated intermediates 33 were synthesized from commercially available starting material 4-nitro-1H-pyrazole-3-carboxylic acid (32) in the presence of ethyl iodide and K2CO3 in DMF and isolated as a mixture of regioisomers. The reduction of the nitro group of 33 was conducted with iron powder and NH4Cl in EtOH/H2O at reflux condition, and two isomers, 1-ethyl and 2-ethylpyrazole intermediates (34), were successfully isolated separately. We obtained bicyclic intermediates 36 from 34 and picolinimidamide (35) reacted with DIEA in EtOH under reflux condition. We then obtained chloro-substituted intermediates 37 from 36 by treating with POCl3 in toluene under reflux condition. Compounds 87, 89, and 90 were synthesized by SNAr transformation from intermediates 37 as described previously. Another two compounds 91 and 93 were prepared in three steps. Intermediate 39 was provided by SNAr reaction with 4,6-dichloro-1H-pyrazolo[3,4-d]pyrimidine (38) and 30. An ethyl group was introduced to the main core by treating with ethyl iodide and NaH to get 40. In the final step, we introduced the 2-pyridyl group by coupling reaction with 2-(tributylstannyl)pyridine (41) to afford 91 and 93. Compound 92 was also synthesized from 2,6-dichloro-9H-purine (42) using similar conditions for compounds 91 and 93 as described above.
Scheme 3. Further Core Modification and Analogue Preparation of Pyrazolopyrimidine and Purine Derivatives.

Reagents and conditions: (a) ethyl iodide, K2CO3, DMF, rt, 3 h, 70%; (b) iron powder, NH4Cl, EtOH/H2O, reflux condition, 2 h, 70%; (c) picolinimidamide (35), DIEA, EtOH, reflux condition, 12 h, 50–93%; (d) POCl3, toluene, reflux condition, 2 h, 58–87%; (e) 30, n-butanol, 100 °C, overnight, or DIEA, MeCN, MW 120 °C, 2 h, 22–75%; (f) 30, TEA, EtOH, 100 °C, 1 h, or rt, overnight, 27–80%; (g) ethyl iodide, NaH, DMF, rt, 16 h, 37–59%; and (h) 2-(tributylstannyl)pyridine (41), Pd(PPh3)4, 1,4-dioxane, reflux condition, 16 h, 11–31%.
Biological Evaluation
The inhibition of T. cruzi in a whole cell parasite assay (amastigote form) was adopted as the primary in vitro activity screen (see Supporting Information 1 for further details). Primary physicochemical and ADME evaluation included measurement of log D (pH 7.4), kinetic solubility in phosphate buffer (pH 6.8), in vitro microsomal metabolism in mouse and human, and PAMPA permeability (for all compounds see the table in Supporting Information 2).
Results and Discussion
From the output of the Booster screening, we identified many interesting compounds with diverse profiles as shown in Chart 1. Driven predominantly by two of the booster partners (Takeda, DNDi), but with occasional input and oversight from all other partners in the project, we proceeded toward a classical medicinal chemistry optimization based on these preliminary SAR data. As a first approach to build on the SAR revealed by the Booster screening, replacement of the 2-pyridyl group at 2-position of the quinazoline core with diverse substituents was conducted to better understand the importance of this heterocycle motif on activity (see the table in Supporting Information 2, compounds SI01–SI10). A series of compounds were prepared to explore possible substitution of the 2-pyridyl motif, including a “Nitrogen walk” around the pyridyl motif, along with substituted phenyl and other nonpyridyl heteroaryl groups. Unfortunately, all of the modifications away from the 2-pyridyl group resulted in major loss of potency (IC50 > 10 μM) compared to the 2-(pyridin-2-yl)quinazolines identified via the booster screening. These results led us to focus on modification of other parts of the quinazoline core to improve potency based on the initial SAR observations gleaned from surveying the preliminary Booster SAR.
In the second phase of the evaluation strategy, we investigated substituents at the 4-position of the 2-(pyridin-2-yl)quinazoline core, with particular focus on compounds 14 and 20 as interesting starting points for building further SAR. Evaluating the variations of NR′R″ groups reported in Chart 1, it was clear that this substituent position is critical for understanding further SAR and enhancing efficacy. At first, the 4-position with differently substituted 4-anilino residues (R1) was inspected: F, Me, and OMe-substituted compounds 17, 14, and 15 gave T. cruzi IC50 of 1.3, 0.12, and 1.2 μM, respectively, suggesting that a lipophilic moiety in this position is essential for activity against the parasite (Table 2). Since aniline structures are known to potentially have cytotoxicity/genotoxicity liabilities, despite being potent, we also decided to replace the benzene ring with a pyridine ring, with the goal of lipophilicity reduction and mitigating toxicity liability. Of the methylated 2- and 3-pyridine derivatives 45–47, compounds 46 and 47 with 3-pyridyl substitution were less potent, while 2-pyridyl compound 45 retained the T. cruzi activity (IC50 0.10 μM), clearly demonstrating that the pharmacophore is very sensitive to the positioning of heteroatom substituents in this region. This result also validates the possibility to avoid the aniline-type structure at the 4-position of the quinazoline core and that we could adopt other heteroaromatic substitution to maintain the activity. Unfortunately, the low SI value of compound 45 and other compounds in the direct-N-aryl substituted series at 4-position remained as an issue. In the interest of reducing planarity and increasing sp3 nature in our molecules, we decided to investigate the increasing linker length between the core quinazoline and the pendant aryl group at 4-position. Interestingly, insertion of a methylene linker between the core and the aryl substituents at the 4-position (48, 49) afforded moderate potency and recovery of SI. Contrary to the findings in the 4-N-aryl subset, these extended linker compounds demonstrated greater tolerance for the regioisomeric nature of pyridyl groups, although additional lipophilic shielding of the pyridyl nitrogen (50, 51) demonstrated a trend toward increased potency, further confirming the sensitivity toward lipophilicity/polarity balance in this region of the pharmacophore.
Table 2. Inhibitory Activities against T. cruzi: Probing the 4-Position with Acyclic Amines.

| L | R1 | T. cruzia IC50 μM (SI) | |
|---|---|---|---|
| 14 | – | 4-Me-Ph | 0.12 (86) |
| 15 | 4-MeO-Ph | 1.2 (11) | |
| 17 | 4-F-Ph | 1.3 (38) | |
| 45 | 5-Me-pyridin-2-yl | 0.10 (<1) | |
| 46 | 6-Me-pyridin-3-yl | 31 (1.6) | |
| 47 | 2,5-Me-pyridin-3-yl | 14 (3.6) | |
| 48 | –CH2– | pyridin-2-yl | 4.4 (11) |
| 49 | pyridin-3-yl | 6.3 (8.0) | |
| 50 | 3-Me-pyridin-2-yl | 1.6 (15) | |
| 51 | 6-Me-pyridin-2-yl | 2.4 (21) | |
| 52 | –CH2CH2– | pyridin-2-yl | 3.4 (14) |
| 53 | –CH(Me)– | 2.7 (18) | |
| 54 | –CMe2– | 1.0 (25) | |
| 55 | –C(CH2CH2)– | 4.2 (12) | |
| 56 | –CH2– | 1N-Me-pyrazol-4-yl | 9.6 (5.2) |
| 57 | thiazol-2-yl | 2.7 (19) | |
| 58 | 1N-Me-imidazol-2-yl | 3.3 (14) | |
| 59 | –CH2CH2– | imidazol-1-yl | 49 (1.0) |
| 60 | – | indazol-5-yl | 6.2 (3.5) |
| 61 | pyrazolo[1,5-a]pyridin-5-yl | 11 (2.9) | |
| 62 | –CH2– | benzoxazol-2-yl | 2.5 (16) |
| 63 | –CH2CH2– | benzimidazol-1-yl | 8.2 (6.1) |
T. cruzi high-content infected cell assay, IC50 of total parasite count; SI = IC50/CC50 against background U2OS cell line.
Ethylene-linked 2-pyridyl derivative 52 yielded similar activity to that of methylene linker. When exploring heterocyclic variations of R1, we observed that thiazole and imidazole derivatives (57, 58) with the methylene linker retained similar potency to the corresponding pyridyl sidechain, while pyrazole and imidazole analogues (56, 59) with methylene or ethylene linker significantly reduced potency relative to compound 48. These results clearly demonstrated that some variation around the linker is acceptable for this series. We further explored the scope around this methylene linker chain with methyl, dimethyl, and cyclopropyl substitution (53, 54, and 55). Compared with compound 48, these changes resulted in maintained or improved potency, with dimethyl-substituted linker compound 54 demonstrating a well-balanced combination of potency and selectivity index.
Only moderate potency was observed by introduction of 6,5- or 5,6-membered bicyclic substituents (60–63) without any significant improvement of SI over the monocyclic derivatives. This suggested that heteroaromatic-substituted analogues such as pyridine or small heteroaromatic groups, along with an additional linker chain, were the most appropriate option for the 4-position to balance inhibitory activity against T. cruzi. Unfortunately, none of these changes resulted in significant positive impact on metabolic stability, with the exception of compounds 54 and 55 (Table 5). Therefore, we concentrated our efforts elsewhere to find more potent compounds with reasonable SI and improved metabolic stability profiles.
Table 5. In Vitro ADME Properties of Some Analoguesa.
| compound | Clint microsomes (μL/min/mg) mouse/human | solubility pH 6.8 (μg/mL) | PAMPA pH 7.4 (nm/s) |
|---|---|---|---|
| 7 | >500/112 | 59 | 441 |
| 12 | >500/95 | 15 | 291 |
| 47 | 154/<1 | 31 | 201 |
| 54 | ND | 83 | 258 |
| 55 | 24/7 | 84 | 219 |
| 64 | 452/188 | 69 | 364 |
| 67 | 318/205 | 0.1 | 416 |
| 70 | 464/150 | 10 | 408 |
| 80 | >500/NT | 0.7 | NT |
| 81 | >500/361 | 7.7 | 430 |
| 83 | >500/NT | NT | NT |
| 85 | 258/73 | 83 | 319 |
| 86 | ND | 90 | 219 |
| 87 | ND | 99 | 171 |
| 90 | 70/<1 | 94 | 220 |
ND: not determined, NT: not tested.
We attempted to replace the NH linker with a series of closed-ring analogues with the aim of retaining the potencies observed for linear analogues while improving the overall physicochemical properties and SI. The hit screening compounds 2 (IC50 0.90 μM) and 12 (IC50 0.43 μM) provided the initial inspiration for this strategy. Exploring variations of the R2 substituent at 4-position of 2-(pyridin-2-yl)quinazoline core with different heterocyclic rings (piperidine, piperazine, pyrrolidine, and their fused-ring analogues) revealed further SAR trends as depicted in Table 3. Building off compounds 2 and 7, we designed further piperidine analogues to identify the favorable substitutions around the piperidine ring. As expected from previous SAR observations, we found these lipophilic groups retained high potency, but unfortunately in vitro mouse and human clearance remained poor (Table 5). Addition of polar groups such as nitrile at both 3- and 4-positions of the piperidine (65, 66) dramatically reduced both the potency and SI. This data reinforced the view that building in much needed polarity in this area of the molecule would remain challenging. Both 3-phenyl (67) and 3-(pyridin-2-yl) (68) substitution retained moderate potency as expected, but with some decreased SI. In addition, compound 67 also exhibited poor metabolic stability in both mouse and human. Combination of a phenyl and hydroxy group at 4-position (69) as an attempt to building both aromaticity as well as buried polarity in the piperidine subseries unfortunately resulted in reduced potency. A series of fused semisaturated bicyclic analogues 71–74 showed weak activities, with the exception of 1,2,3,4-tetrahydroisoquinoline analogue 70, once again reinforcing the propensity for lipophilicity in this area of the pharmacophore.
Table 3. Inhibitory Activities against T. cruzi: Probing the 4-Position with Cyclic Amines.

T. cruzi high-content infected cell assay, IC50 of total parasite count; SI = IC50/CC50 against background U2OS cell line.
Chiral enantiomer.
Next, we investigated piperazinyl analogues, following up the potency observed in booster-identified compound 12. In general, across all variations of R2, the in vitro activity of the piperazinyl compounds was interesting and a similar pattern emerged to the earlier observation that lipophilic phenyl and benzyl group boosted potency (12, 13). From these observations, we observed again that balance of lipophilicity and a certain size of substituent appeared to be required at the R2 site.
Following on from the piperazines, we decided to check the impact of reducing the ring size linking R2 to the 4-position, since pyrrolidine analogue 4 showed moderate potency. As anticipated by the previously revealed SAR, introduction of polar substituents at 3-position of pyrrolidine, such as carbonitrile, carboxamide, or carboxylic acid (77, 78, and 79), significantly reduced the potency, while substituted aromatic analogues exemplified by 80, 81 demonstrated good activity. Unfortunately, these modifications did not result in improved metabolic stability. A more polar 3-hydroxy analogue of 80 (82) reduced the potency, while addition of fluorine into the phenyl ring of 80 (83) retained potency but failed to stabilize the metabolic profile as well. Introduction of nitrogen into the phenyl ring (85) to reduce log D and in turn improve metabolic stability showed well-balanced profiles of potency and metabolic stability along with better solubility in comparison with phenyl analogue 80 (Table 5). The position of pyridine substitution on pyrrolidine was investigated, with the 3-position of pyrrolidine proving more beneficial (85). Thus, 3-(pyridin-2-yl)pyrrolidine analogue 85 provided a good balance between potency and lipophilicity. The component enantiomers of compound 85 were prepared from enantiopure building blocks (see Supporting Information 1) and both enantiomers 85-tR1 and 85-tR2 were found to be equipotent against T. cruzi, demonstrating IC50 values in line with those of racemic 85. Therefore, further evaluation of 85 proceeded as the racemate. Following this expansion of the SAR, we concluded that, in general, piperidine (compounds 7, 64, 67, 68) and piperazine (compounds 12, 76) analogues were moderate in potency, whereas pyrrolidine analogues (compounds 80, 81, 83, 85) retained the expected higher potency level, possibly due to the favorable introduction of aromatic rings.
To confirm that the series was not exerting an antiparasitic effect via an inhibition of TcCYP51, we tested one of the most potent analogues, compound 83 (0.41 μM), in a direct TcCYP51 inhibitory assay.28,54 Compound 83 demonstrated no inhibitory activity against TcCYP51 at concentration up to and including 100 μM, confirming that this chemical series does not exhibit its trypanocidal effect via a CYP51-mediated pathway. The physicochemical and in vitro DMPK profiles for selected compounds from the overall SAR studies were evaluated to identify compounds, which could be progressed to the critical in vivo PoC studies. In Table 5, the summary of the in vitro mouse and human microsomal metabolism, solubility at pH 6.8, and permeability are presented. Reviewing both these data and in vitro potency (IC50 and SI), we noted compounds 54 and 85 were the molecules with greatest potential for further investigation. Mouse and human microsomal CLint for compound 85 clearly demonstrated only moderate in vitro clearance, whereas for compound 54, unfortunately, the microsomal CLint was not determined due to difficulties with the chromatographic analysis in our assay system. However, both the mouse and human microsomal metabolism of cyclopropyl analogue 55 showed low clearance, suggesting that compound 54 also has good metabolic stability. Both compounds 54 and 85 displayed acceptable kinetic solubility at pH 6.8 (83, 83 μg/mL, respectively). Plasma protein binding measured in mouse species for compound 54 was reported with 94.2%, whereas that for compound 85 with 99.9%. We hypothesized that this significant difference in plasma protein binding was likely to have some impact on the PK study, and both compounds 54 and 85 were worthy of simultaneous progression to maximize understanding of any efficacy observed in in vivo infection models.
Based on the initial SAR findings, we hypothesized that the higher log D of the quinazoline core compounds explored to that point had a negative impact on overall physicochemical and ADME properties for this series. From the lead identification study as described above, compounds 54 and 85 were identified as the most interesting compounds so we decided to target further optimization of lipophilicity by reducing the log D values of the core heterocycle. We initiated a scaffold hop with different variations of heterocyclic core as demonstrated by 86–93 in Table 4. N-Alkylated pyrrolopyrimidine-type core compounds 86 and 88 maintained or enhanced potency up to 4-fold along with reduction of log D by 0.5–0.8 units in comparison with compound 85. But unfortunately, both compounds demonstrated an issue linked to chemical stability when attempting to determine in vitro metabolic profiles, and further investigation of these compounds was therefore not pursued. Other exploratory core shifts with pyrazolopyrimidine and purine ring (87, 89–93) raised concerns for low SI values and were not pursued further (Table 5).
Table 4. Inhibitory Activities against T. cruzi: Investigation of Core Changes.

T. cruzi high-content infected cell assay, IC50 of total parasite count; SI = IC50/CC50 against background U2OS cell line.
Finally, in addition to these scaffold-hop attempts, we decided to return once more and revisit the importance of the pyridin-2-yl group in the 2-position of the core quinazoline, while retaining the 3-(pyridin-2-yl)pyrolidine motif of 85 at the 4-position. Once again, these studies revealed that the 2-pyridyl motif remained essential to achieve both potency and acceptable SI against background cell cytotoxicity (see the table in Supporting Information 2, compounds SI11–SI26).
Initial PK and Tolerability Assessment
After evaluating all other profiles, compounds 54 and 85 were selected for further evaluation to ratify the PoC in a rodent model of acute T. cruzi infection. Compound 54 was dosed intravenously (i.v.) and orally (p.o.) to female Balb/c mice at 1 and 50 mg/kg, respectively. Blood concentration–time profiles after oral dosing gave an AUC 59859 ng·h/mL, with 54 demonstrating excellent oral bioavailability (full PK in Supporting Information 1). No adverse reactions or compound-related side effects were observed following oral administration of this compound at this single dose level, and the compound was progressed for evaluation in a multiday dosing tolerability regimen in healthy mice in which 54 was administered BID orally over 5 days at 10, 50, and 100 mg/kg (1 animal per group). Unfortunately, this preliminary tolerability study ruled out further work with this compound due to abnormal observations in animals at 2 days (100 mg/kg) and 3 days (50 mg/kg) post first dose. These abnormal events included animals being cold to touch, with hunched posture and abnormal gait, as well as decreased activity. To find out potential causes of this toxicity, we investigated off-target selectivity for compound 54 against a panel of 300 kinases (1 μM test concentration) and across a panel of 35 known-liability targets including GPCRs and ion channels in both agonist and antagonist read-out and biochemical functional assay for nuclear hormone receptors and phosphodiesterases (10 μM test concentration). Compound 54 was shown to have no known off-target activity with the exception of 5HT-1A antagonism; however, this was ruled out as the cause of these adverse events due to reports indicating that hypothermia can be elicited by the selective agonism of 5HT-1A in rodent species, whereas antagonists are known to suppress or reverse this agonist-induced hypothermia.55 No clear correlation of toxicity was observed, so we decided to deprioritize further off-target toxicity exploration of this compound; however, one ongoing speculation was that this tolerability issue could be linked to a combination of the high brain penetration profile and moderate free fraction (B/P > 1, Table 6). We therefore decided to discontinue any further profiling of compound 54.
Table 6. Pharmacokinetic and Pharmacodynamic Study in Mice after Intravenous and Oral Administration of 54 and 85.
| PK parameters | 54 | 85 | 85 + ABTa |
|---|---|---|---|
| i.v. dose (mg/kg) | 1 | 0.1b | 2 |
| p.o. dose (mg/kg) | 50 | 50 | 12.5 |
| Cmax (ng/mL) | 6237 | 3363 | 4243 |
| AUC0-last (ng·h/mL) | 59 859 | 9607 | 26 912 |
| plasma CL (mL/min/kg) | 12.4 | 38.5 | 4.9 |
| plasma Vss (L/kg) | 0.909 | 0.77 | 0.94 |
| half-life, i.v./p.o. (h) | 1.13/ND | –/7.2 | 1/3.3 |
| oral bioavailability (%) | >100 | 50 | 67 |
| brain to plasma ratio | 1.6 (0.5 h) | 1.66 (1 h) | ND |
| tolerability (single p.o. dose) | ok | ok | poor |
| tolerability (5 days BID p.o.) | poor | ok | ND |
50 mg/kg 1-aminobenzotriazole administered p.o. 2 h prior to i.v. or p.o. dose.
As part of a five-compound cassette PK study.
We proceeded with pharmacokinetic evaluation of compound 85, which was dosed intravenously and orally to female Balb/c mice at 2 and 12.5 mg/kg, respectively (n = 3 mice/dose route) with same dose formulation described for compound 54, in the presence of 1-aminobenzotiazole (ABT) as a Cytochrome P450 metabolism inhibitor in an effort to boost exposure levels.56 Blood concentration–time profiles following i.v. and oral dosing are shown in Supporting Information 1. Compound 85 demonstrated good oral bioavailability in the presence of ABT; however, at this dose, adverse tolerability events were observed after the single dose, manifesting in similar observations to those seen upon multiday dosing with 54. Compound 85 also demonstrated good oral bioavailability and a moderate-to-high clearance when dosed orally without ABT (0.1 and 50 mg/kg p.o., respectively). Comparing these studies with and without ABT, we concluded that clearance of 85 was likely driven primarily by Cytochrome P450s; however, the bioavailability of 85 when dosed alone was sufficient to merit further investigation. Importantly, no adverse issues were noted during the study in the absence of ABT, so 85 was progressed to a multiday dosing tolerability test. For tolerability test, four female mice were randomly allocated to four groups of one animal/group and administrated with vehicle (0.5% (w/v) HPMC/0.5% (v/v) benzyl alcohol/0.4% (v/v) Tween 80 in purified water) and compound 85 (10, 50, and 100 mg/kg/dose (BID)) for 5 days. As opposed to the findings with 54, there were no treatment-related mortality, clinical observations, body weight and weight gain, food consumption, and gross pathology at ≤100 mg/kg/dose (BID) for 85. This result demonstrated that, contrary to compound 54, compound 85 administered by oral gavage to twice per day to female mice for 5 days was well tolerated. Based on these data, the no-observed-adverse-effect-level (NOAEL) was considered to be at 100 mg/kg/dose (BID). Compared with the properties of compound 54, the desired pharmacokinetic profile of 85 including plasma levels, extended exposure over the dosing interval, and exposure relative to the in vitro IC50 was not quite as attractive as 54, mainly linked to the high plasma protein binding observed for 85. From the previous analysis, compound 85 exhibited a more rapid clearance and had lower plasma concentrations at 24 h, high volume of distribution, long half-life, and high oral bioavailability compared to compound 54. However, the improved tolerability profile of 85 suggested this molecule would provide the best preference for PoC.
In Vivo Efficacy Study
The PoC study employed the bioluminescent T. cruzi CL Brenner strain described previously by Kelly et al.57,58 In this model, parasite burden can be tracked quantifiably in vivo via the tissue-penetrating T. cruzi strain expressing a novel luciferase that emits tissue-penetrating orange-red light. Five-day BID treatment of mice with acute T. cruzi infection with 50 mg/kg 85 from day 14 post-infection reduced the parasite burden by 89.9 ± 5.1% compared with untreated controls by day 18 post infection (Figure 4). Importantly, in line with the pre-PoC tolerability study in healthy mice, no adverse effects were observed during the treatment duration. While the illustration of efficacy in this in vivo PoC study is encouraging and clearly demonstrates that the chemical series antiparasitic effect translates from in vitro to in vivo efficacy, the overall effectiveness of 85in vivo was considered transient. Even if “cure” was not accomplished with compound 85 in this mouse/parasite combination, as the reduction in parasite burden was not maintained by day 28, the level of efficacy demonstrated in such a stringent test of in vivo efficacy was encouraging and follow-up compounds are being considered for further evaluation.
Figure 4.

Assessment of compound 85 as a treatment for acute-stage Trypanosoma cruzi infections. BALB/c mice (n = 3) at the acute stage of infection (14 days) were treated (50 mg/kg, orally, twice daily) for 5 days. The inset graph shows the total body bioluminescence (sum of ventral and dorsal images) of treated (red) and nontreated (black) mice. The blue bar indicates the treatment period. Gray dotted line represents background + SD.
Conclusions
The unique NTD Booster approach consisting of DNDi and pharmaceutical R&D enabled the identification of potent and promising 2-aryl-4-aminoquinazoline derivatives by collaborative in silico screening. A detailed SAR exploration was conducted around the antiparasitic efficacy of a series of 2-(pyridin-2-yl)quinazolines against T. cruzi, and two potential lead compounds 54 and 85 with moderate in vitro potency for use in a PoC study were identified. In the case of compound 54, despite good in vitro efficacy and attractive PK, the 5-day tolerability test in noninfected mice failed, with adverse issues such as hypothermia/death. Despite investigation, no proven correlation for this rapid-onset toxicity of compounds 54 was identified, and we decided to stop any further exploration of compound 54. We hypothesize that these observed toxicity issues could be driven by the as yet unidentified off-target mechanism linked to brain exposure or by higher free plasma fraction relative to compound 85, which did not demonstrate any adverse events. Compound 85 showed an acceptable range of in vitro potency, excellent bioavailability, and acceptable exposure during PK and passed the 5-day tolerability test in healthy animals without any adverse events noted. Compound 85 demonstrated clear, but moderate, activity in an in vivo mouse model of acute T. cruzi infection. Despite this encouraging result, there remain some key concerns to be solved for this chemotype, particularly around in vitro and in vivo toxicity profiles and the continued apparent reliance on a bivalent chelation motif in the SAR. Further exploration of this series is ongoing.
Experimental Section
General Information and Synthetic Methods
Reactions were run using available starting materials and solvents without further purification. Abbreviations of solvents and reagents are used as follows: CDCl3, deuterated chloroform; DCM, dichloromethane; DME, 1,2-dimethoxyethane; DMA, N,N-dimethylacetamide; DMF, N,N-dimethylformamide; DMSO-d6, deuterated dimethyl sulfoxide; EtOAc, ethyl acetate; EtOH, ethanol; H2O, water; MeCN, acetonitrile; MeOH, methanol; NMP, N-methyl-2-pyrrolidone; THF, tetrahydrofuran; DIEA, diisopropylethylamine; EDCI, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide; HOBT, 1-hydroxybenzotriazole; NH4Cl, ammonium chloride; Pd2(dba)3, tris(dibenzylideneacetone)dipalladium(0); Pd(PPh3)4, tetrakis(triphenylphosphine)palladium(0); TEA, triethylamine; Cs2CO3, cesium carbonate; K2CO3, potassium carbonate; MgSO4, magnesium sulfate; NaH, sodium hydride; NaHCO3, sodium hydrogen carbonate; NaOH, sodium hydroxide; Na2SO4, sodium sulfate; POCl3, phosphorus oxychloride. Abbreviations of proton nuclear magnetic resonance (1H NMR) are used as follows: s = singlet, d = doublet, t = triplet, q = quartet, bs = broad singlet, m = multiplet, dd = doublet of doublets, dt = doublet of triplets, tt = triplet of triplets.
Compounds 2–22, 60, and 64 were derived from Booster screening compounds and extracted via cherry picking from pharmaceutical company HTS screening collections. Therefore, no spectrum data nor purity data are available beyond reliance on internal company quality controls on their screening collections. As described earlier, concerns around purity of hit compounds from these libraries were addressed through resynthesis (>95% purity by HPLC) and reconfirmation of activity for a selection of the compounds. Compounds 50–53, 55, 57–59, 61–63, 65, 71, 72, 75, 76, and 84 were synthesized in high-throughput medicinal chemistry (HTMC) parallel synthesis conducted by Takeda Pharmaceutical Company Limited and therefore only MS data were collected as per standard operating practice for this technique (see Supporting Information 1). The majority (over 85%) of these HTMC compounds were later confirmed as >95% purity by HPLC. For all other synthesized compounds, including those advanced to in vivo evaluation, purity was >95% by HPLC.
Method A: Preparation of Compound 25
To a solution of 2-aminobenzamide (23) (10 g, 73.52 mmol) and pyridine-2-carboxylic acid (24) (13.56 g, 110.3 mmol) in DMF (100 mL) were added HOBT (14.9 g, 110.3 mmol), EDCI (21.14 g, 110.3 mmol), and TEA (10.14 mL, 80.88 mmol). The reaction mixture was stirred for 24 h at room temperature. After TLC showed completion of the reaction, the reaction mixture was concentrated, water was added, and the formed precipitate was filtered, washed with water, and dried to afford N-(2-carbamoylphenyl)pyridine-2-carboxamide (25) (15 g, 84.6%) as a white solid. MS (ESI): m/z 242 [M + H]+.
Method B: Preparation of Compound 26
To a solution of 25 (18 g, 74.68 mmol) in methanol, 240 mL of 1 N NaOH (aqueous) was added and refluxed for 1 h. After TLC showed completion of the reaction, the reaction mixture was concentrated and diluted with water and neutralized with conc HCl to give a precipitate that was filtered, washed with water, and dried to afford 2-(pyridin-2-yl)quinazolin-4-ol (26) (11 g, 66.0%) as a white solid. MS (ESI): m/z 224 [M + H]+.
Method C: Preparation of Compound 27
To a solution of 26 (2 g, 8.97 mmol) in toluene (20 mL), TEA (7.5 mL) and POCl3 (13 mL) were added and refluxed at 130 °C overnight. After completion of the reaction (monitored by TLC), toluene and POCl3 were distilled and the residue obtained was diluted with DCM and washed with cold water and cold saturated NaHCO3 solution. The organic layer was dried over Na2SO4, concentrated in vacuo, and purified by column chromatography (using silica gel 100–200 mesh, 15–20% EtOAc/hexane as the eluent) to afford 4-chloro-2-(pyridin-2-yl)quinazoline (27) (1.8 g, 83.1%) as a white solid. 1H NMR (400 MHz, DMSO-d6) δ 8.82–8.81 (d, 1H), 8.53–8.51 (d, 1H), 8.35–8.33 (d, 1H), 8.23–8.15 (m, 2H), 8.06–8.02 (t, 1H), 7.94–7.90 (t, 1H), 7.61–7.58 (m, 1H). MS (ESI): m/z 242 [M + H]+.
Method D: Preparation of Final Compounds (45–59, 61–63, 65–85, 85-tR1, 85-tR2, 87, 89, 90)
Procedure A: 27 (250 mg, 1.04 mmol) and 3-phenylpyrrolidine (153 mg, 1.04 mmol) were mixed together in n-butanol (8 mL), and the reaction mixture was stirred at 110 °C for 12 h. After TLC showed completion of the reaction, the reaction mixture was concentrated and the residue was purified by prep HPLC to afford 4-(3-phenylpyrrolidin-1-yl)-2-(pyridin-2-yl)quinazoline (80) (86 mg, 23.5%) as a white solid. 1H NMR (400 MHz, DMSO-d6) δ 8.72–8.71 (d, 1H), 8.46–8.44 (d, 1H), 8.37–8.35 (d, 1H), 7.94–7.90 (dt, 1H), 7.86–7.78 (m, 2H), 7.50–7.35 (m, 6H), 7.29–7.26 (t, 1H), 4.42–4.37 (m, 1H), 4.18–4.16 (d, 2H), 4.01–3.96 (t, 1H), 3.59–3.55 (m, 1H), 2.43–2.40 (m, 1H), 2.23–2.18 (m, 1H). MS (ESI): m/z 353 [M + H]+. Procedure B: A mixture of 27 (450 mg, 1.86 mmol), 2-(pyrrolidin-3-yl)pyridine (30) (331 mg, 2.23 mmol), and DIEA (0.975 mL, 5.58 mmol) in MeCN (12 mL) was stirred at 120 °C for 30 min under microwave irradiation. The mixture was concentrated in vacuo, and the residue was purified by column chromatography (NH silica gel, 0–20% MeOH in EtOAc as the eluent), then crystallized from EtOAc-hexane to give 2-(pyridin-2-yl)-4-(3-(pyridin-2-yl)pyrrolidin-1-yl)quinazoline (85) (560 mg, 85%) as a white solid. 1H NMR (300 MHz, DMSO-d6) δ 8.74–8.69 (m, 1H), 8.60–8.54 (m, 1H), 8.48–8.42 (m, 1H), 8.37 (d, 1H), 7.96–7.90 (m, 1H), 7.88–7.75 (m, 3H), 7.54–7.43 (m, 3H), 7.33–7.25 (m, 1H), 4.46–4.36 (m, 1H), 4.29–4.12 (m, 3H), 3.83–3.69 (m, 1H), 2.47–2.40 (m, 1H), 2.38–2.24 (m, 1H). 13C NMR (75 MHz, DMSO-d6) δ 160.8, 159.9, 158.8, 156.5, 152.5, 149.7, 137.3, 137.2, 137.1, 132.8, 128.6, 126.3, 125.5, 124.8, 124.0, 123.0, 122.6, 115.4, 56.0, 51.0, 45.4, 31.9. Anal. calcd for C22H19N5: C 74.8, H 5.4, N 19.8. Found: C 75.3, H 4.9, N 20.3. HRMS (ESI): m/z 354.171 [M + H]+ (exact mass 353.164).
Method E: Preparation of Compound 29a
To a solution of 2,4-dichloro-5H-pyrrolo[3,2-d]pyrimidine (28) (1.20 g, 6.38 mmol) and ethyl iodide (2.95 g, 18.91 mmol) in DMF (20 mL), Cs2CO3 (5.20 g, 15.96 mmol) was added at room temperature. The mixture was stirred at room temperature under N2 overnight. After concentration in vacuo, the residue was extracted with EtOAc. The organic layer was separated, washed with water and brine, dried over MgSO4, and concentrated in vacuo. The residue was purified by column chromatography (silica gel, 30–50% EtOAc in hexane as the eluent) to give 2,4-dichloro-5-ethyl-5H-pyrrolo[3,2-d]pyrimidine (29a) (0.956 g, 69.3%) as a white solid. 1H NMR (300 MHz, CDCl3) δ 7.54 (d, 1H), 6.66 (d, 1H), 4.52 (q, 2H), 1.54 (t, 3H). MS (ESI): m/z 216, 218 [M + H]+.
2,4-Dichloro-5-methyl-5H-pyrrolo[3,2-d]pyrimidine (29b)
The title compound (602 mg, 70.0%) was synthesized from 28 according to method E as a white solid. 1H NMR (300 MHz, CDCl3) δ 7.45 (d, 1H), 6.64 (d, 1H), 4.14 (s, 3H). MS (ESI): m/z 202, 204 [M + H]+.
Method F: Preparation of Compound 31a
To a solution of 2-(pyrrolidin-3-yl)pyridine (30) (297 mg, 2.00 mmol) and DIEA (0.875 mL, 5.01 mmol) in THF (8 mL) was added a solution of 29a (360 mg, 1.67 mmol) in THF (4 mL) at room temperature. The mixture was stirred at room temperature for 10 h. After concentration in vacuo, the residue was purified by column chromatography (NH silica gel, 30–100% EtOAc/hexane as the eluent) to give 2-chloro-5-ethyl-4-(3-(pyridin-2-yl)pyrrolidin-1-yl)-5H-pyrrolo[3,2-d]pyrimidine (31a) (421 mg, 77%) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ 8.60–8.55 (m, 1H), 7.67–7.61 (m, 1H), 7.28–7.25 (m, 1H), 7.22 (d, 1H), 7.20–7.14 (m, 1H), 6.51 (d, 1H), 4.36–4.17 (m, 2H), 4.15–3.96 (m, 3H), 3.88–3.76 (m, 1H), 3.63–3.49 (m, 1H), 2.48–2.24 (m, 2H), 1.37 (t, 3H). MS (ESI): m/z 328 [M + H]+.
2-Chloro-5-methyl-4-(3-(pyridin-2-yl)pyrrolidin-1-yl)-5H-pyrrolo[3,2-d]pyrimidine (31b)
The title compound (330 mg, 70.8%) was synthesized from 29b according to method F as a white solid. 1H NMR (300 MHz, CDCl3) δ 8.60–8.54 (m, 1H), 7.67–7.62 (m, 1H), 7.23 (d, 1H), 7.21–7.15 (m, 1H), 7.14 (d, 1H), 6.48 (d, 1H), 4.19–4.01 (m, 3H), 3.95 (s, 3H), 3.88–3.84 (m, 1H), 3.65–3.48 (m, 1H), 2.50–2.24 (m, 2H). MS (ESI): m/z 314 [M + H]+.
6-Chloro-4-(3-(pyridin-2-yl)pyrrolidin-1-yl)-1H-pyrazolo[3,4-d]pyrimidine (39)
The title compound (1.20 g, 80%) was synthesized from 4,6-dichloro-1H-pyrazolo[3,4-d]pyrimidine (38) according to method F as a crude product. 1H NMR (400 MHz, DMSO-d6) δ 13.50 (s, 1H), 8.55–8.52 (t, 1H), 8.19–8.17 (d, 1H), 7.80–7.77 (m, 1H), 7.47–7.41 (m, 1H). 7.30–7.25 (m, 1H), 4.25–3.66 (m, 5H), 2.49–2.33 (m, 2H). MS (ESI): m/z 301 [M + H]+.
2-Chloro-6-(3-(pyridin-2-yl)pyrrolidin-1-yl)purine (43)
The title compound (170 mg, 27%) was synthesized from 2,6-dichloro-9H-purine (42) according to method F as a crude product. 1H NMR (400 MHz, DMSO-d6) δ 13.08 (s, 1H), 8.53–8.52 (d, 1H), 8.10–8.07 (d, 1H), 7.78–7.74 (t, 1H), 7.42–7.40 (d, 1H), 7.28–7.25 (t, 1H), 4.67–3.67 (m, 5H), 2.32–2.17 (m, 2H). MS (ESI): m/z 301 [M + H]+.
Method G: Preparation of Final Compounds (86, 88)
A mixture of 31a (84 mg, 0.26 mmol), (2-pyridine)cyclic-triolborate lithium salt (109 mg, 0.51 mmol), Pd2(dba)3 (24 mg, 0.03 mmol), cuprous chloride (13 mg, 0.13 mmol), butyl di-1-adamantylphosphine (19 mg, 0.05 mmol), and potassium tert-butoxide (86 mg, 0.77 mmol) in DME (2 mL) was heated at 120 °C for 3 h under microwave irradiation. The insoluble material was removed by filtration, and the filtrate was concentrated in vacuo. The residue was purified by column chromatography (NH silica gel, 0–10% MeOH in EtOAc as the eluent), then by preparative HPLC (L-Column 2 ODS, eluted with H2O in acetonitrile containing 10 mM NH4HCO3). The desired fraction was concentrated in vacuo. The residue was dissolved in EtOAc, and the solution was dried over Na2SO4 and concentrated in vacuo to give 5-ethyl-2-(pyridin-2-yl)-4-(3-(pyridin-2-yl)pyrrolidin-1-yl)-5H-pyrrolo[3,2-d]pyrimidine (88) (18 mg, 19.0%) as a colorless amorphous solid. 1H NMR (300 MHz, CDCl3) δ 8.83–8.77 (m, 1H), 8.59 (d, 1H), 8.49 (d, 1H), 7.78–7.76 (m, 1H), 7.68–7.52 (m, 1H), 7.32–7.08 (m, 4H), 6.79–6.65 (m, 1H), 4.43–3.54 (m, 7H), 3.10–2.28 (m, 2H), 1.47–1.36 (m, 3H). MS (ESI): m/z 371 [M + H]+.
5-Methyl-2-(pyridin-2-yl)-4-(3-(pyridin-2-yl)pyrrolidin-1-yl)-5H-pyrrolo[3,2-d]pyrimidine (86)
The title compound (39 mg, 42.9%) was synthesized from 31b according to method G as a colorless amorphous solid. 1H NMR (300 MHz, DMSO-d6) δ 8.66 (d, 1H), 8.54 (d, 1H), 8.33 (d, 1H), 7.91–7.81 (m, 1H), 7.80–7.71 (m, 1H), 7.59 (d, 1H), 7.46–7.34 (m, 2H), 7.26 (dd, 1H), 6.54 (d, 1H), 4.19–3.98 (m, 6H), 3.93–3.80 (m, 1H), 3.72–3.56 (m, 1H), 2.44–2.31 (m, 1H), 2.30–2.15 (m, 1H). MS (ESI): m/z 357 [M + H]+.
Method H: Preparation of Compound 33
To a stirred solution of ethyl iodide (2.08 g, 13.37 mmol) and K2CO3 (2.19 g, 15.92 mmol) in DMF (30 mL) was added 4-nitro-1H-pyrazole-3-carboxylic acid (32) (1.0 g, 6.36 mmol). The reaction mixture was stirred at room temperature for 3 h. After completion of the reaction (monitored by TLC), it was filtered and the filtrate obtained was extracted with EtOAc and the combined organic layer was washed with water (50 mL). The organic layer was dried over Na2SO4 and concentrated in vacuo. The residue was purified by flash chromatography (using silica gel 100–200 mesh, 15–20% EtOAc/hexane as the eluent) to afford compound 33, a mixture of regioisomers (ethyl 1-ethyl-4-nitropyrazole-3-carboxylate and ethyl 2-ethyl-4-nitropyrazole-3-carboxylate) (0.95 g, 70.0%) as a yellow oil. MS (ESI): m/z 214 [M + H]+.
Method I: Preparation of Compounds 34 (a, b)
To a solution of 33 (5 g, 23.34 mmol) in EtOH/H2O (4:1, 20 mL), iron powder (13.03 g, 233.64 mmol) and NH4Cl (1.25 g, 23.36 mmol) were added and the mixture was refluxed for 2 h. After completion of the reaction (monitored by TLC), the insoluble material was removed by filtration through celite and the filtrate was concentrated in vacuo to obtain a solid residue. The residue was purified by flash chromatography (using silica gel 100–200 mesh, 15–20% EtOAc in hexane as the eluent) to afford ethyl 4-amino-2-ethylpyrazole-3-carboxylate (34a) (1.2 g, 28.0%) as a white solid and ethyl 4-amino-1-ethylpyrazole-3-carboxylate (34b) (1.8 g, 42.1%) as a white solid. The structure of 34b was confirmed by NOE experiment. 34a: 1H NMR (400 MHz, DMSO-d6) δ 7.02 (s, 1H), 4.95 (bs, 2H), 4.32–4.25 (m, 4H), 1.32–1.28 (t, 3H), 1.24–1.22 (t, 3H). MS (ESI): m/z 184 [M + H]+. 34b: 1H NMR (400 MHz, DMSO-d6) δ 7.16 (s, 1H), 4.65 (bs, 2H), 4.25–4.19 (q, 2H), 4.05–4.00 (q, 2H), 1.33–1.23 (m, 6H). MS (ESI): m/z 184 [M + H]+.
Method J: Preparation of Compound 36a
To a solution of 34a (1.2 g, 6.55 mmol) and picolinimidamide (35) (1.13 g, 7.2 mmol) in EtOH (30 mL) was added DIEA (4.2 mL, 26.20 mmol) and the mixture was refluxed at 150 °C for 12 h. After completion of the reaction (monitored by TLC), the reaction mixture was cooled and precipitates were filtered off, washed with hexane and water, and dried to give 1-ethyl-5-(pyridin-2-yl)pyrazolo[4,3-d]pyrimidin-7-ol (36a) (1.2 g, 75.9%) as a white solid. 1H NMR (400 MHz, DMSO-d6) δ 11.30 (bs, 1H), 8.73–8.72 (d, 1H), 8.52 (s, 1H), 8.35–8.34 (d, 1H), 8.05–8.02 (t, 1H), 7.61 (t, 1H), 4.42–4.37 (q, 2H), 1.51–1.47 (t, 3H). MS (ESI): m/z 242 [M + H]+.
2-Ethyl-5-(pyridin-2-yl)pyrazolo[4,3-d]pyrimidin-7-ol (36b)
The title compound (2.2 g, 92.8%) was synthesized from 34b according to method J as a white solid. 1H NMR (400 MHz, DMSO-d6) δ 11.74 (bs, 1H), 8.72–8.71 (d, 1H), 8.34–8.32 (d, 1H), 8.10 (s, 1H), 8.04–8.01 (t, 1H), 7.62–7.59 (t, 1H), 4.62–4.57 (q, 2H), 1.44–1.40 (t, 3H). MS (ESI): m/z 242 [M + H]+.
1-Methyl-5-(pyridin-2-yl)pyrazolo[4,3-d]pyrimidin-7-ol (36c)
The title compound (438 mg, 49.8%) was synthesized from ethyl 4-amino-2-methylpyrazole-3-carboxylate (34c) according to method J as an off-white solid. 1H NMR (300 MHz, DMSO-d6) δ 11.78 (bs, 1H), 8.76–8.69 (m, 1H), 8.39–8.31 (m, 1H), 8.12 (s, 1H), 8.08–8.00 (m, 1H), 7.67–7.57 (m, 1H), 4.24 (s, 3H). MS (ESI): m/z 228 [M + H]+.
Method K: Preparation of Compound 37a
To a stirred solution of 36a (1.0 g, 4.149 mmol) in toluene (10 mL) was added POCl3 (15 mL) in ice-cold condition. The resulting solution was heated to reflux for 2 h under N2. The reaction was monitored by TLC and LCMS. After completion, the reaction mixture was concentrated in vacuo. The crude residue was diluted with DCM and quenched with saturated cold NaHCO3 solution (10 mL). The organic layer was separated and the aqueous layer was extracted with DCM (2 × 20 mL), and the combined organic layer was washed with water (30 mL) followed by brine (30 mL). The organic layer was dried over Na2SO4 and concentrated in vacuo. The residue was purified by flash chromatography (using silica gel 100–200 mesh, 15–20% EtOAc/hexane as the eluent) to afford 7-chloro-1-ethyl-5-(pyridin-2-yl)pyrazolo[4,3-d]pyrimidine (37a) (700 mg, 65%) as an off-white solid. 1H NMR (400 MHz, DMSO-d6) δ 8.76–8.75 (d, 1H), 8.63 (s, 1H), 8.41–8.39 (d, 1H), 8.02–7.98 (t, 1H), 7.55–7.53 (t, 1H), 4.79–4.74 (q, 2H), 1.51–1.40 (t, 3H).
7-Chloro-2-ethyl-5-(pyridin-2-yl)pyrazolo[4,3-d]pyrimidine (37b)
The title compound (250 mg, 58%) was synthesized from 36b according to method K as a sticky solid. 1H NMR (400 MHz, DMSO-d6) δ 9.16 (bs, 1H), 8.83 (m, 1H), 8.59 (m, 1H), 8.29 (m, 1H), 7.78 (m, 1H), 4.69–4.63 (q, 2H), 1.61–1.58 (t, 3H).
7-Chloro-1-methyl-5-(pyridin-2-yl)pyrazolo[4,3-d]pyrimidine (37c)
The title compound (1.21 g, 87%) was synthesized from 36c according to method K as an off-white solid. 1H NMR (300 MHz, DMSO-d6) δ 8.79–8.74 (m, 1H), 8.60 (s, 1H), 8.43–8.37 (m, 1H), 8.04–7.95 (m, 1H), 7.58–7.51 (m, 1H), 4.38 (s, 3H). MS (ESI): m/z 246 [M + H]+.
Method L: Preparation of Compounds 40 (a, b)
To a stirred solution of 39 (1.2 g, 4.0 mmol) in DMF (10 mL), NaH (0.32 g, 60%, 13 mmol) and ethyl iodide (0.482 mL) were added at 0 °C. The resulting mixture was stirred at 25 °C for 16 h. After completion (monitored by TLC), the reaction mixture was quenched with ice-cold water and extracted with EtOAc, and the combined organic layers were dried over Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography (using silica gel 100–200 mesh, 10–15% EtOAc/hexane as the eluent) to get 6-chloro-1-ethyl-4-(3-(pyridin-2-yl)pyrrolidin-1-yl)-1H-pyrazolo[3,4-d]pyrimidine (40a) (635 mg, 48%) as a brown solid and 6-chloro-2-ethyl-4-(3-(pyridin-2-yl)pyrrolidin-1-yl)-1H-pyrazolo[3,4-d]pyrimidine (40b) (150 mg, 11%) as a brown solid. Structures of 40a and 40b were assigned based on the NOE experiment on final compound 91. 40a: 1H NMR (400 MHz, DMSO-d6) δ 8.55–8.52 (t, 1H), 8.20–8.19 (d, 1H), 7.80–7.76 (dd, 1H), 7.48–7.41 (m, 1H), 7.30–7.26 (m, 1H). 4.29–3.73 (m, 7H), 2.38–2.18 (m, 2H), 1.37–1.35 (t, 3H). MS (ESI): m/z 329 [M + H]+. 40b: 1H NMR (400 MHz, DMSO-d6) δ 8.67 (s, 1H), 8.55–8.52 (m, 1H), 7.80–7.76 (q, 1H), 7.47–7.41 (dd, 1H), 7.31–7.26 (m, 1H). 4.36–3.66 (m, 7H), 2.38–2.17 (m, 2H), 1.49–1.41 (t, 3H). MS (ESI): m/z 329 [M + H]+.
2-Chloro-9-ethyl-6-(3-(pyridin-2-yl)pyrrolidin-1-yl)purine (44)
The title compound (70 mg, 37%) was synthesized from 43 according to method L as a crude product. MS (ESI): m/z 329 [M + H]+.
Method M: Preparation of Final Compounds (91–93)
To a stirred solution of 40a (635 mg, 1.94 mmol) in 1,4-dioxane (10 mL) was added Pd(PPh3)4 (225 mg, 0.194 mmol) followed by 2-(tributylstannyl)pyridine (41) (1.549 mL, 4.84 mmol) at room temperature. The reaction mixture was stirred at 120 °C for 16 h. After completion (monitored by TLC), the reaction mixture was quenched with ice-cold water and extracted with EtOAc. The combined organic layers were dried over MgSO4 and concentrated in vacuo. The resultant crude material was purified by prep HPLC to afford 1-ethyl-6-(pyridin-2-yl)-4-(3-(pyridin-2-yl)pyrrolidin-1-yl)-1H-pyrazolo[3,4-d]pyrimidine (93) (200 mg, 27.8%) as an off-white solid. 1H NMR (400 MHz, DMSO-d6) δ 8.72–8.70 (d, 1H), 8.55 (s, 1H), 8.44–8.41 (t, 1H), 8.23–8.21 (d, 1H), 7.92–7.88 (t, 1H), 7.78 (t, 1H), 7.49–7.44 (m, 2H). 7.28–7.27 (m, 1H), 4.43–3.73 (m, 7H), 2.49–2.20 (m, 2H), 1.43–1.40 (t, 3H). MS (ESI): m/z 373 [M + H]+.
2-Ethyl-6-(pyridin-2-yl)-4-(3-(pyridin-2-yl)pyrrolidin-1-yl)-2H-pyrazolo[3,4-d]pyrimidine (91)
The title compound (18 mg, 10.6%) was synthesized from 40b according to method M as an off-white solid. The structure of 91 was confirmed by NOE experiment. 1H NMR (400 MHz, DMSO-d6) δ 8.66 (s, 2H), 8.58–8.55 (m, 1H), 8.36 (d, 1H), 7.90–7.78 (m, 2H), 7.50–7.42 (m, 2H), 7.32–7.29 (m, 1H), 4.42–3.80 (m, 7H), 2.49–2.35 (m, 2H), 1.54–1.46 (m, 3H). MS (ESI): m/z 372 [M + H]+.
9-Ethyl-2-(pyridin-2-yl)-6-(3-(pyridin-2-yl)pyrrolidin-1-yl)-9H-purine (92)
The title compound (45 mg, 30.6%) was synthesized from 44 according to method M as an off-white solid. 1H NMR (400 MHz, DMSO-d6) δ 8.70 (s, 1H), 8.55–8.54 (d, 1H), 8.40–8.39 (d, 1H), 8.23–8.22 (m, 1H), 7.91–7.89 (m, 1H), 7.80–7.75 (m, 1H), 7.45–7.43 (m, 2H), 7.29–7.26 (m, 1H), 4.68–3.74 (m, 7H). 2.54–2.24 (m, 2H), 1.46–1.43 (t, 3H). MS (ESI): m/z 372 [M + H]+.
N-(5-Methylpyridin-2-yl)-2-(pyridin-2-yl)quinazolin-4-amine (45)
The title compound (35 mg, 9%) was synthesized from 27 according to method D as a yellow solid. 1H NMR (400 MHz, DMSO-d6) δ 15.92 (bs, 0.5H), 10.42 (bs, 0.5H), 8.89–8.71 (m, 2H), 8.54–7.24 (m, 9H), 2.33 (s, 3H). MS (ESI): m/z 314 [M + H]+.
N-(6-Methylpyridin-3-yl)-2-(pyridin-2-yl)quinazolin-4-amine (46)
The title compound (50 mg, 20%) was synthesized from 27 according to method D as an off-white solid. 1H NMR (400 MHz, DMSO-d6) δ 9.98 (s, 1H), 9.14 (s, 1H), 8.76 (s, 1H), 8.62–8.60 (d, 1H), 8.42–8.37 (t, 2H), 7.99–7.94 (m, 3H), 7.72–7.68 (m, 1H), 7.52–7.49 (m, 1H), 7.34–7.32 (d, 1H), 2.49 (s, 3H). MS (ESI): m/z 314 [M + H]+.
N-(2,5-Dimethylpyridin-3-yl)-2-(pyridin-2-yl)quinazolin-4-amine (47)
The title compound (11 mg, 18.1%) was synthesized from 27 according to method D as a pale yellow solid. 1H NMR (300 MHz, DMSO-d6) δ 9.83 (s, 1H), 8.68 (d, 1H), 8.53 (d, 1H), 8.26 (d, 1H), 8.09 (d, 1H), 7.96–7.83 (m, 3H), 7.74 (d, 1H), 7.71–7.60 (m, 1H), 7.47–7.40 (m, 1H), 2.41 (s, 3H), 2.34 (s, 3H). MS (ESI): m/z 328 [M + H]+.
2-(Pyridin-2-yl)-N-((pyridin-2-yl)methyl)quinazolin-4-amine (48)
The title compound (150 mg, 38%) was synthesized from 27 according to method D as a white solid. 1H NMR (400 MHz, DMSO-d6) δ 9.04–9.01 (t, 1H), 8.70–8.69 (d, 1H), 8.54–8.53 (d, 1H), 8.39–8.36 (d, 1H), 8.29–8.27 (d, 1H), 7.90–7.83 (m, 3H), 7.74–7.69 (m, 1H), 7.60–7.55 (m, 1H), 7.47–7.43 (m, 2H), 7.26–7.23 (m, 1H), 5.01–4.99 (d, 2H). MS (ESI): m/z 314 [M + H]+.
2-(Pyridin-2-yl)-N-((pyridin-3-yl)methyl)quinazolin-4-amine (49)
The title compound (76 mg, 19.5%) was synthesized from 27 according to method D as a light brown solid. 1H NMR (400 MHz, DMSO-d6) δ 8.98–8.96 (t, 1H), 8.73–8.72 (m, 2H), 8.44–8.43 (d, 1H), 8.40–8.38 (d, 1H), 8.32–8.30 (d, 1H), 7.94–7.90 (m, 2H), 7.85–7.80 (m, 2H), 7.58–7.54 (m,1H), 7.48–7.45 (dd, 1H), 7.35–7.32 (dd, 1H), 4.92–4.91 (d, 2H). MS (ESI): m/z 314 [M + H]+.
N-((3-Methylpyridin-2-yl)methyl)-2-(pyridin-2-yl)quinazolin-4-amine (50)
The title compound (20.7 mg, 79%) was synthesized from 27 by HTMC parallel synthesis according to method D as a white solid. MS (ESI): m/z 328 [M + H]+.
N-((6-Methylpyridin-2-yl)methyl)-2-(pyridin-2-yl)quinazolin-4-amine (51)
The title compound (19.9 mg, 74.8%) was synthesized from 27 by HTMC parallel synthesis according to method D as a white solid. MS (ESI): m/z 328 [M + H]+.
2-(Pyridin-2-yl)-N-(2-(pyridin-2-yl)ethyl)quinazolin-4-amine (52)
The title compound (15.8 mg, 53.7%) was synthesized from 27 by HTMC parallel synthesis according to method D as a white solid. MS (ESI): m/z 328 [M + H]+.
2-(Pyridin-2-yl)-N-(1-(pyridin-2-yl)ethyl)quinazolin-4-amine (53)
The title compound (20.9 mg, 79.8%) was synthesized from 27 by HTMC parallel synthesis according to method D as a white solid. MS (ESI): m/z 328 [M + H]+.
2-(Pyridin-2-yl)-N-(2-(pyridin-2-yl)propan-2-yl)quinazolin-4-amine (54)
The title compound (246 mg, 58%) was synthesized from 27 according to method D as a white solid. 1H NMR (300 MHz, DMSO-d6) δ 8.62 (d, 1H), 8.59–8.52 (m, 2H), 8.39 (s, 1H), 7.87–7.78 (m, 2H), 7.77–7.69 (m, 1H), 7.68–7.46 (m, 4H), 7.37 (dd, 1H), 7.16 (dd, 1H), 1.89 (s, 6H). 13C NMR (75 MHz, DMSO-d6) δ 165.5, 158.3, 158.0, 157.6, 155.4, 149.8, 148.8, 147.5, 136.1, 136.0, 132.4, 128.0, 125.4, 123.9, 122.9, 120.8, 119.1, 114.1, 58.0, 27.3, 27.9. Anal. calcd for C21H20N5: C 73.9, H 5.6, N 20.5. Found: C 73.5, H 5.2, N 20.0. HRMS (ESI): m/z 342.171 [M + H]+ (exact mass 341.164).
2-(Pyridin-2-yl)-N-(1-(pyridin-2-yl)cyclopropyl)quinazolin-4-amine (55)
The title compound (17.7 mg, 65.2%) was synthesized from 27 by HTMC parallel synthesis according to method D as a white solid. MS (ESI): m/z 340 [M + H]+.
N-[(1-Methylpyrazol-4-yl)methyl]-2-(pyridin-2-yl)quinazolin-4-amine (56)
The title compound (21 mg, 5.3%) was synthesized from 27 according to method D as a light brown solid. 1H NMR (400 MHz, DMSO-d6) δ 8.76–8.75 (d, 1H), 8.71–8.69 (t, 1H), 8.49–8.47 (d, 1H), 8.28–8.26 (d, 1H), 7.97–7.93 (m, 1H), 7.83–7.77 (m, 3H), 7.53–7.47 (m, 3H), 4.69–4.67 (d, 2H), 3.75 (s, 3H). MS (ESI): m/z 317 [M + H]+.
2-(Pyridin-2-yl)-N-(thiazol-2-ylmethyl)quinazolin-4-amine (57)
The title compound (0.7 mg, 2.7%) was synthesized from 27 by HTMC parallel synthesis according to method D as a white solid. MS (ESI): m/z 320 [M + H]+.
N-((1-Methyl-1H-imidazol-2-yl)methyl)-2-(pyridin-2-yl)quinazolin-4-amine (58)
The title compound (13.8 mg, 54.5%) was synthesized from 27 by HTMC parallel synthesis according to method D as a white solid. MS (ESI): m/z 317 [M + H]+.
N-(2-(1H-Imidazol-1-yl)ethyl)-2-(pyridin-2-yl)quinazolin-4-amine (59)
The title compound (21 mg, 83%) was synthesized from 27 by HTMC parallel synthesis according to method D as a white solid. MS (ESI): m/z 317 [M + H]+.
N-(Pyrazolo[1,5-a]pyridin-5-yl)-2-(pyridin-2-yl)quinazolin-4-amine (61)
The title compound (5.1 mg, 18.8%) was synthesized from 27 by HTMC parallel synthesis according to method D as a white solid. MS (ESI): m/z 339 [M + H]+.
N-(Benzo[d]oxazol-2-ylmethyl)-2-(pyridin-2-yl)quinazolin-4-amine (62)
The title compound (16.5 mg, 58.4%) was synthesized from 27 by HTMC parallel synthesis according to method D as a white solid. MS (ESI): m/z 354 [M + H]+.
N-(2-(1H-Benzo[d]imidazol-1-yl)ethyl)-2-(pyridin-2-yl)quinazolin-4-amine (63)
The title compound (27.9 mg, 97.7%) was synthesized from 27 by HTMC parallel synthesis according to method D as a white solid. MS (ESI): m/z 367 [M + H]+.
1-(2-(Pyridin-2-yl)quinazolin-4-yl)piperidine-3-carbonitrile (65)
The title compound (33 mg, 52.3%) was synthesized from 27 by HTMC parallel synthesis according to method D as a white solid. MS (ESI): m/z 316 [M + H]+.
1-(2-(Pyridin-2-yl)quinazolin-4-yl)piperidine-4-carbonitrile (66)
The title compound (21 mg, 53.2%) was synthesized from 27 according to method D as a white solid. 1H NMR (300 MHz, DMSO-d6) δ 8.75 (d, 1H), 8.46 (dt, 1H), 8.05–7.83 (m, 4H), 7.61–7.47 (m, 2H), 4.08–3.97 (m, 2H), 3.69–3.57 (m, 2H), 3.25 (tt, 1H), 2.18–1.92 (m, 4H). MS (ESI): m/z 316 [M + H]+.
4-(3-Phenylpiperidin-1-yl)-2-(pyridin-2-yl)quinazoline (67)
The title compound (65 mg, 89%) was synthesized from 27 according to method D as a white solid. 1H NMR (300 MHz, DMSO-d6) δ 8.81–8.68 (m, 1H), 8.44 (dt, 1H), 8.05 (d, 1H), 8.00–7.91 (m, 2H), 7.88–7.81 (m, 1H), 7.60–7.53 (m, 1H), 7.51–7.46 (m, 1H), 7.44–7.32 (m, 4H), 7.30–7.22 (m, 1H), 4.49 (t, 2H), 3.33–3.24 (m, 2H), 3.09 (bs, 1H), 2.13–1.80 (m, 4H). MS (ESI): m/z 367 [M + H]+.
2-(Pyridin-2-yl)-4-(3-(pyridin-2-yl)piperidin-1-yl)quinazoline (68)
The title compound (27 mg, 59.2%) was synthesized from 27 according to method D as a white solid. 1H NMR (300 MHz, DMSO-d6) δ 8.73 (d, 1H), 8.55 (d, 1H), 8.45 (d, 1H), 8.06 (d, 1H), 7.98–7.91 (m, 2H), 7.87–7.74 (m, 2H), 7.59–7.53 (m, 1H), 7.51–7.41 (m, 2H), 7.29–7.25 (m, 1H), 4.61–4.49 (m, 2H), 3.47–3.39 (m, 1H), 3.28–3.16 (m, 2H), 2.13–2.07 (m, 1H), 1.99–1.90 (m, 3H). MS (ESI): m/z 368 [M + H]+.
4-Phenyl-1-[2-(pyridin-2-yl)quinazolin-4-yl]piperidin-4-ol (69)
The title compound (50 mg, 19.2%) was synthesized from 27 according to method D as a white solid. 1H NMR (400 MHz, DMSO-d6) δ 8.77–8.76 (m, 1H), 8.49–8.47 (d, 1H), 8.14–8.12 (d, 1H), 7.96–7.94 (m, 2H), 7.87–7.84 (t, 1H), 7.57–7.51 (m, 4H), 7.36–7.32 (t, 2H), 7.25–7.23 (m, 1H), 5.26 (s, 1H), 4.43–4.41 (d, 2H), 3.72–3.66 (t, 2H), 2.32–2.22 (m, 2H), 1.83–1.80 (d, 2H). MS (ESI): m/z 383 [M + H]+.
4-(3,4-Dihydroisoquinolin-2(1H)-yl)-2-(pyridin-2-yl)quinazoline (70)
The title compound (15.0 mg, 35.7%) was synthesized from 27 according to method D as a pale yellow amorphous powder. 1H NMR (300 MHz, DMSO-d6) δ 8.75 (d, 1H), 8.50 (dt, 1H), 8.17 (d, 1H), 8.00–7.83 (m, 3H), 7.62–7.47 (m, 2H), 7.32–7.18 (m, 4H), 5.04 (s, 2H), 4.11–4.07 (m, 2H), 3.16 (t, 2H). MS (ESI): m/z 339 [M + H]+.
4-(5,8-Dihydro-1,7-naphthyridin-7(6H)-yl)-2-(pyridin-2-yl)quinazoline (71)
The title compound (24.4 mg, 81.2%) was synthesized from 27 by HTMC parallel synthesis according to method D as a white solid. MS (ESI): m/z 340 [M + H]+.
4-(7,8-Dihydro-1,6-naphthyridin-6(5H)-yl)-2-(pyridin-2-yl)quinazoline (72)
The title compound (26.1 mg, 95.8%) was synthesized from 27 by HTMC parallel synthesis according to method D as a white solid. MS (ESI): m/z 340 [M + H]+.
4-(5,6-Dihydroimidazo[1,2-a]pyrazin-7(8H)-yl)-2-(pyridin-2-yl)quinazoline (73)
The title compound (57 mg, 69.9%) was synthesized from 27 according to method D as a white solid. 1H NMR (300 MHz, DMSO-d6) δ 8.80–8.75 (m, 1H), 8.55–8.49 (m, 1H), 8.19 (d, 1H), 8.04–7.88 (m, 3H), 7.70–7.60 (m, 1H), 7.57–7.49 (m, 1H), 7.19 (d, 1H), 6.93 (d, 1H), 5.03 (s, 2H), 4.42–4.35 (m, 2H), 4.29–4.21 (m, 2H). MS (ESI): m/z 329 [M + H]+.
2-(Pyridin-2-yl)-4-(3,4,6,7-tetrahydro-5H-imidazo[4,5-c]pyridin-5-yl)quinazoline (74)
The title compound (69 mg, 85%) was synthesized from 27 according to method D as a white solid. 1H NMR (300 MHz, DMSO-d6) δ 12.03–11.77 (m, 1H), 8.77 (dd, 1H), 8.50 (d, 1H), 8.20–8.08 (m, 1H), 8.04–7.82 (m, 3H), 7.66–7.56 (m, 1H), 7.56–7.46 (m, 2H), 4.93–4.77 (m, 2H), 4.12–4.08 (m, 2H), 3.11–2.90 (m, 2H). MS (ESI): m/z 329 [M + H]+.
2-(Pyridin-2-yl)-4-(4-(pyridin-2-yl)piperazin-1-yl)quinazoline (75)
The title compound (23.5 mg, 79.7%) was synthesized from 27 by HTMC parallel synthesis according to method D as a white solid. MS (ESI): m/z 369 [M + H]+.
4-(4-(6-Methylpyridin-2-yl)piperazin-1-yl)-2-(pyridin-2-yl)quinazoline (76)
The title compound (24.5 mg, 80.1%) was synthesized from 27 by HTMC parallel synthesis according to method D as a white solid. MS (ESI): m/z 383 [M + H]+.
1-(2-(Pyridin-2-yl)quinazolin-4-yl)pyrrolidine-3-carbonitrile (77)
The title compound (34 mg, 56.4%) was synthesized from 27 according to method D as a pale yellow solid. 1H NMR (300 MHz, DMSO-d6) δ 8.79–8.67 (m, 1H), 8.47 (dt, 1H), 8.31 (d, 1H), 8.02–7.78 (m, 3H), 7.60–7.43 (m, 2H), 4.36–4.26 (m, 1H), 4.25–4.05 (m, 3H), 3.68–3.61 (m, 1H), 2.48–2.27 (m, 2H). MS (ESI): m/z 302 [M + H]+.
1-(2-(Pyridin-2-yl)quinazolin-4-yl)pyrrolidine-3-carboxamide (78)
The title compound (35 mg, 73.3%) was synthesized from 27 according to method D as an off-white solid. 1H NMR (300 MHz, DMSO-d6) δ 8.73 (d, 1H), 8.45 (d, 1H), 8.33 (d, 1H), 7.99–7.90 (m, 1H), 7.88–7.75 (m, 2H), 7.59 (bs, 1H), 7.54–7.44 (m, 2H), 7.07 (bs, 1H), 4.23–3.97 (m, 4H), 3.17–3.06 (m, 1H), 2.33–2.05 (m, 2H). MS (ESI): m/z 320 [M + H]+.
1-(2-(Pyridin-2-yl)quinazolin-4-yl)pyrrolidine-3-carboxylic Acid (79)
The title compound (33.0 mg, 26.5%) was synthesized from 27 according to method D as an off-white solid. 1H NMR (300 MHz, DMSO-d6) δ 8.74–8.69 (m, 1H), 8.44 (d, 1H), 8.31 (d, 1H), 7.97–7.89 (m, 1H), 7.86–7.73 (m, 2H), 7.52–7.43 (m, 2H), 4.22–3.89 (m, 4H), 3.07–2.93 (m, 1H), 2.26–2.07 (m, 2H). MS (ESI): m/z 321 [M + H]+.
4-(3-Phenoxypyrrolidin-1-yl)-2-(pyridin-2-yl)quinazoline (81)
The title compound (66 mg, 90%) was synthesized from 27 according to method D as a colorless amorphous powder. 1H NMR (300 MHz, DMSO-d6) δ 8.80–8.68 (m, 1H), 8.45 (dt, 1H), 8.36 (d, 1H), 8.02–7.73 (m, 3H), 7.58–7.43 (m, 2H), 7.37–7.25 (m, 2H), 7.10–6.91 (m, 3H), 5.25 (d, 1H), 4.38 (dd, 1H), 4.28–3.95 (m, 3H), 2.43–2.21 (m, 2H). MS (ESI): m/z 369 [M + H]+.
3-Phenyl-1-[2-(pyridin-2-yl)quinazolin-4-yl]pyrrolidin-3-ol (82)
The title compound (80 mg, 50.6%) was synthesized from 27 according to method D as an off-white solid. 1H NMR (400 MHz, DMSO-d6) δ 8.72–8.71 (d, 1H), 8.46–8.44 (d, 1H), 8.37–8.35 (d, 1H), 7.95–7.91 (m, 1H), 7.87–7.78 (m, 2H), 7.66–7.64 (d, 2H), 7.51–7.45 (m, 2H), 7.43–7.39 (m, 2H), 7.33–7.29 (m, 1H), 5.58 (s, 1H), 4.36–4.14 (m, 4H), 2.50–2.45 (m, 1H), 2.27–2.23 (m, 1H). MS (ESI): m/z 369 [M + H]+.
4-[3-(4-Fluorophenyl)pyrrolidin-1-yl]-2-(pyridin-2-yl)quinazoline (83)
The title compound (35 mg, 20.8%) was synthesized from 27 according to method D as an off-white solid. 1H NMR (400 MHz, CDCl3) δ 8.85–8.84 (d, 1H), 8.57–8.55 (d, 1H), 8.18–8.13 (m, 2H), 7.81–7.79 (t, 1H), 7.73–7.69 (t, 1H), 7.41–7.37 (t, 1H), 7.36–7.29 (m, 3H), 7.08–7.04 (t, 2H), 4.47–4.42 (m, 1H), 4.26–4.20 (m, 2H), 4.07–4.01 (t, 1H), 3.57–3.53 (m, 1H), 2.52–2.49 (m, 1H), 2.23–2.20 (m, 1H). MS (ESI): m/z 371 [M + H]+.
2-(Pyridin-2-yl)-4-(2-(pyridin-2-yl)pyrrolidin-1-yl)quinazoline (84)
The title compound (14.6 mg, 51.6%) was synthesized from 27 by HTMC parallel synthesis according to method D as a white solid. MS (ESI): m/z 354 [M + H]+.
Chiral Isomer of 2-(Pyridin-2-yl)-4-(3-(pyridin-2-yl)pyrrolidin-1-yl)quinazoline 3-(Pyridin-2-yl)pyrrolidine-1-carboxylate (85-tR1)
The title compound (118 mg, 90%) was synthesized from 27 and chiral amine 30-tR1 (see Supporting Information 1 for the preparation of chiral amines of 30) according to method D as a white solid. 1H NMR (300 MHz, DMSO-d6) δ 8.75–8.70 (m, 1H), 8.59–8.54 (m, 1H), 8.48–8.42 (m, 1H), 8.36 (d, 1H), 7.97–7.89 (m, 1H), 7.88–7.76 (m, 3H), 7.54–7.43 (m, 3H), 7.32–7.28 (m, 1H), 4.46–4.33 (m, 1H), 4.29–4.13 (m, 3H), 3.83–3.68 (m, 1H), 2.48–2.39 (m, 1H), 2.39–2.24 (m, 1H). MS (ESI): m/z 354 [M + H]+.
Chiral Isomer of 2-(Pyridin-2-yl)-4-(3-(pyridin-2-yl)pyrrolidin-1-yl)quinazoline 3-(Pyridin-2-yl)pyrrolidine-1-carboxylate (85-tR2)
The title compound (82 mg, 86%) was synthesized from 27 and chiral amine 30-tR2 (see Supporting Information 1 for the preparation of chiral amines of 30) according to method D as a white solid. 1H NMR (300 MHz, DMSO-d6) δ 8.75–8.70 (m, 1H), 8.59–8.54 (m, 1H), 8.48–8.42 (m, 1H), 8.37 (d, 1H), 7.97–7.90 (m, 1H), 7.88–7.76 (m, 3H), 7.54–7.43 (m, 3H), 7.32–7.28 (m, 1H), 4.45–4.35 (m, 1H), 4.28–4.13 (m, 3H), 3.83–3.69 (m, 1H), 2.48–2.40 (m, 1H), 2.39–2.24 (m, 1H). MS (ESI): m/z 354 [M + H]+.
1-Methyl-5-(pyridin-2-yl)-7-(3-(pyridin-2-yl)pyrrolidin-1-yl)-1H-pyrazolo[4,3-d]pyrimidine (87)
The title compound (60 mg, 75%) was synthesized from 37c according to method D as a white solid. 1H NMR (300 MHz, DMSO-d6) δ 8.71–8.65 (m, 1H), 8.55 (d, 1H), 8.36 (d, 1H), 8.18 (s, 1H), 7.94–7.84 (m, 1H), 7.82–7.74 (m, 1H), 7.49–7.40 (m, 2H), 7.31–7.28 (m, 1H), 4.32–4.22 (m, 4H), 4.19–3.97 (m, 3H), 3.80–3.64 (m, 1H), 2.47–2.37 (m, 1H), 2.36–2.19 (m, 1H). MS (ESI): m/z 358 [M + H]+.
1-Ethyl-5-(pyridin-2-yl)-7-(3-(pyridin-2-yl)pyrrolidin-1-yl)-1H-pyrazolo[4,3-d]pyrimidine (89)
The title compound (50 mg, 22.5%) was synthesized from 37a according to method D as an off-white solid. 1H NMR (400 MHz, DMSO-d6) δ 8.67 (bs, 1H), 8.54–8.53 (d, 1H), 8.35–8.33 (d, 1H), 8.23 (bs, 1H), 7.92–7.88 (t, 1H), 7.79–7.75 (t, 1H), 7.44–7.42 (d, 2H), 7.28–7.25 (m, 1H), 4.60–4.51 (m, 2H), 4.20–4.09 (m, 3H), 4.00–3.97 (m, 1H), 3.72–3.68 (m, 1H), 2.45–2.35 (m, 1H), 2.31–2.24 (m, 1H), 1.44–1.40 (t, 3H). MS (ESI): m/z 372 [M + H]+.
2-Ethyl-5-(pyridin-2-yl)-7-(3-(pyridin-2-yl)pyrrolidin-1-yl)-2H-pyrazolo[4,3-d]pyrimidine (90)
The title compound (48 mg, 22.3%) was synthesized from 37b according to method D as an off-white solid. 1H NMR (400 MHz, CDCl3) δ 8.77 (bs, 1H), 8.60–8.59 (d, 1H), 8.54–8.50 (t, 1H), 8.01 (bs, 1H), 7.79–7.72 (m, 1H), 7.67–7.63 (m, 1H), 7.30–7.25 (m, 2H), 7.19–7.17 (m, 1H), 4.80–4.25 (m, 5H), 4.11–3.99 (m, 1H), 3.78–3.68 (m, 1H), 2.54–2.38 (m, 2H), 1.63–1.58 (t, 3H). MS (ESI): m/z 372 [M + H]+.
In Vitro Efficacy (Studies Conducted According to Conditions Reported Previously by This Team)34
U2OS (human bone osteosarcoma host cells, ATCC, Manassas, VA) cells were cultured in DMEM-high glucose media (Welgene Inc., Gyeongsangbuk-do, Republic of Korea) supplemented with 10% (v/v) fetal bovine serum (FBS) (Gibco, Waltham, MA), 1% (v/v) penicillin–streptomycin (10,000 U/mL) (Gibco) at 37 °C, 5% CO2. T. cruzi parasites used for the infection were Tissue Culture Trypomastigotes (TCT). To generate this stage of parasites, metacyclic trypomastigotes were obtained from a late-stage epimastigote culture. The metacyclic trypomastigotes were used to infect the monkey kidney cell line LLC-MK2 (ATCC). Parasites 7 days after infection were collected from the supernatant of the LLC-MK2 infected culture and reinfected new culture of LLC-MK2 in DMEM-low glucose media (Welgene Inc.) supplemented with 2% (v/v) FBS (Gibco), 1% (v/v) penicillin–streptomycin (10,000 U/mL) (Gibco). The compounds were prepared in 2-fold serial dilution 10 points from 10 mM (200× stock) with 100% DMSO (v/v) (Sigma-Aldrich, St. Louis, MO) into 384-well polypropylene microplates (Greiner Bio-One, Kremsmünster, Austria). For the controls, benznidazole (Carbosynth Ltd., Berkshire, U.K.) and posaconazole (Carbosynth Ltd.) were used as positive controls and 0.5% DMSO (v/v) as negative control. The 0.3 μL of compounds or reference compounds were dispensed in 10 μL of DPBS (Welgene) containing wells of 384-well tissue culture microplate (Greiner) with CyBi-Well multichannel pipettor (Analytik Jena AG, Jena, Germany). U2OS cells and parasites were mixed in DMEM-low glucose media (Welgene Inc.) supplemented with 2% (v/v) FBS (Gibco) and 1% (v/v) penicillin–streptomycin (10,000 U/mL) (Gibco) at the multiplicity of infection (MOI) values of 12.5:1 parasite to host cell. The prepared cells and parasite mixtures were dispensed into the assay plate at 50 μL/well and incubated for 72 h at 37 °C, 5% CO2. After incubation, the cells and parasites were stained using 5 μM DRAQ5 (Biostatus Ltd., Leicestershire, U.K.) in 4% paraformaldehyde (CureBio, Seoul, Republic of Korea). Cell images were acquired with the Operetta automated confocal microscope (PerkinElmer, Inc., Waltham, MA) at 635 mm excitation filter, 20× lens magnification. Four images per well were captured covering 45% of the well and analyzed with Columbus software (PerkinElmer). The number of cells and intracellular parasites were counted by the DRAQ5 stained nuclei of both host cells and parasites. The large host cell nuclei were first detected and the cytoplasm of the host cells was then identified by a cytoplasm detection script. The intracellular parasites were defined as spots within the host cell cytoplasm using spot analysis and host cells containing more than three spots were considered to be infected. The infection ratio was determined by the number of infected cells divided by the total number of cells and normalized values based on the positive and negative controls to 0 and 100% infection. To estimate EC50 and CC50 values, data were fitted to sigmoid dose–response one-site-fit model 205 (4 Parameter Logistic Model) of XLfit (IDBS, Guildford, U.K.) in Microsoft Excel.
InVivo Efficacy Study
We used in vivo bioluminescence imaging to monitor the therapeutic efficacy of compound 85 against acute-stage T. cruzi infections. Animal work was performed under United Kingdom Home Office project licenses (PPLs 70/8207) and approved by the LSHTM Animal Welfare and Ethical Review Board. Procedures were conducted in accordance with the United Kingdom Animals (Scientific Procedures) Act 1986 (ASPA). BALB/c female mice (aged 7–8 weeks) were purchased from Charles River (U.K.). Animals were maintained under specific pathogen-free conditions in individually ventilated cages. They experienced a 12 h light/dark cycle, with access to food and water ad libitum. Mice were infected via i.p. injection with 1 × 103 bioluminescent T. cruzi strain (CL Brener) that constitutively expressed the red-shifted luciferase PpyRE9h derived from SCID mouse (bred in-house) blood.57 At the peak of parasitemia (14 days after infection), BALB/c mice (n = 3) were treated by the oral route, twice daily at 50 mg/kg for 5 days and monitored by bioluminescence imaging.58
Experimental conditions for in silico screening, additional parasitology, microsomal stability, solubility, PAMPA and mouse PK, and preliminary tolerability studies can be found in Supporting Information 1.
Acknowledgments
The authors acknowledge John Cuff, Leelapavan Tadoori, Jean Robert Ioset, Tatsuro Kuzuki, Daisuke Imoto, Midori Morioka, Dominique Junod, and Jean Pierre Paccaud for their help in implementation and logistics of the Booster project, Chen Min and Jiajia Zhang for ADME-PK studies, Sudin Panja and Ujjwal Pahari for contributions to synthesis, Jen Riley for the TcCYP51 assay data, and Louise Burrows for help in the manuscript preparation. The authors also acknowledge the generous support of Global Health Innovative Technology Fund (GHIT) for financial support of this project, and our collaboration partners at Abbvie Inc., Astellas Pharma Inc., and Merck KGaA for participation in the Booster project outside of this particular series. The authors thank the Abbvie Inc. for the off-target liability screen. The Drugs for Neglected Diseases initiative (DNDi) is grateful to its donors, public and private, who have provided funding to DNDi since its inception in 2003. A full list of DNDi’s donors can be found at http://www.dndi.org/donors/donors/.
Glossary
Abbreviations
- 5HT-1A
serotonin receptor 1A
- BID
bis in die
- CD
Chagas Disease
- HCS
high-content screening
- HTMC
high-throughput medicinal chemistry (parallel synthesis)
- HTS
high-throughput screening
- LCMS
liquid chromatography-mass spectrometry
- NMR
nuclear magnetic resonance
- NTD
neglected tropical disease
- PAMPA
parallel artificial membrane permeability assay
- PoC
proof of concept
- SAR
structure–activity relationship
- SD
standard deviation
Data Availability Statement
Full data set for all compounds explored in this series are available online as sdf file at https://dndi.org/research-development/portfolio/drug-discovery-booster/.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jmedchem.2c00775.
Author Present Address
◆◆ Molecular Devices, 3860 N First Street, San Jose, California 95134, United States
Author Present Address
○○ Exscientia, Oxford Science Park, The Schrödinger Building, Oxford OX4 4GE, U.K.
Author Present Address
∇∇ Loxo Oncology at Lilly, 600 Tech Ct, Louisville, Colorado 80027, United States.
Author Present Address
## Elgia Therapeutics, Inc., 7660 Fay Avenue, La Jolla, California 92037, United States.
Author Present Address
⊥⊥ The University of Tokyo, 7-cho̅me-3-1 Hongo̅, Bunkyo City, Tokyo 113-8654, Japan.
Author Present Address
∥∥ University of Minnesota, Minneapolis, Minnesota 55455, United States.
Author Present Address
§§ Nissan Chemical Corporation, Chiba 274-0069, Japan.
Author Present Address
‡‡ Axcelead Drug Discovery Partners, Inc., Kanagawa 251-0012, Japan
Author Present Address
∞ Medicxi Ventures, 10 Cours de Rive, 1204 Geneva, Switzerland
Author Contributions
O.E., T.K., K.C., K.N., H.N., S.Y., and S.I. performed in silico virtual screening at the respective partners; B.P., T.Y., T.T., A.O., H.X., Y.C., and S.G. guided the chemical synthesis; D.S. and C.R. performed in vitro parasitology assays; A.F. and J.K. performed in vivo efficacy, B.P., T.A., T.T., and A.O. guided the medicinal chemistry design, B.P., C.R., I.R., S.C., C.K., O.Y., R.Y., Y.A., and N.W. coordinated the Booster project at the respective partners; C.M., B.P., G.P., N.W., A.N., and M.S. conceived and implemented the Booster project; and T.T., T.A., and B.P. prepared the manuscript.
This work and the NTD drug discovery booster were funded by Global Health Innovation Technology Fund (GHIT) grants H2014-201, H2016-203, and H2017-001. The work was benefitted from generous in-kind contributions of resource from Takeda Pharmaceutical Company Limited, Eisai Co. Ltd., Shionogi & Co. Ltd., AstraZeneca plc, and Celgene Corporation.
The authors declare no competing financial interest.
Supplementary Material
References
- Field M. C.; Horn D.; Fairlamb A. H.; Ferguson M. A. J.; Gray D. W.; Read K. D.; de Rycker M.; Torrie L. S.; Wyatt P. G.; Wyllie S.; Gilbert I. H. Anti-Trypanosomatid Drug Discovery: An Ongoing Challenge and a Continuing Need. Nat. Rev. Microbiol. 2017, 15, 217–231. 10.1038/nrmicro.2016.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . Investing to Overcome the Global Impact of Neglected Tropical Diseases; WHO, 2016. [Google Scholar]
- McCall L. I.; McKerrow J. H. Determinants of Disease Phenotype in Trypanosomatid Parasites. Trends Parasitol. 2014, 30, 342–349. 10.1016/j.pt.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Irish A.; Whitman J. D.; Clark E. H.; Marcus R.; Bern C. Updated Estimates and Mapping for Prevalence of Chagas Disease among Adults, United States. Emerg Infect Dis. 2022, 28, 1313–1320. 10.3201/eid2807.212221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira M. S.; Borges A. S. Some Aspects of Protozoan Infections in Immunocompromised Patients - A Review. Mem. Inst. Oswaldo Cruz 2002, 97, 443–457. 10.1590/S0074-02762002000400001. [DOI] [PubMed] [Google Scholar]
- da Mata J. R.; da Mata F. R.; do Nascimento G. N. L.; Ferreira T. A. A. Pequeno Comprometimento de Encéfalo Em Ratos Holtzman Imunossuprimidos e Infectados Por Trypanosoma cruzi. Acta Sci. Health Sci. 2009, 31, 45–50. 10.4025/actascihealthsci.v31i1.831. [DOI] [Google Scholar]
- Gascon J.; Bern C.; Pinazo M. J. Chagas Disease in Spain, the United States and Other Non-Endemic Countries. Acta Trop. 2010, 115, 22–27. 10.1016/j.actatropica.2009.07.019. [DOI] [PubMed] [Google Scholar]
- WHO . Chagas disease. https://www.who.int/chagas/home_more/en/ (accessed June 12, 2020).
- Briceño-León R.; Galván J. M. The Social Determinants of Chagas Disease and the Transformations of Latin America. Mem. Inst. Oswaldo Cruz 2007, 102, 109–112. 10.1590/S0074-02762007005000095. [DOI] [PubMed] [Google Scholar]
- Pérez-Molina J. A.; Norman F.; López-Vélez R. Chagas Disease in Non-Endemic Countries: Epidemiology, Clinical Presentation and Treatment. Curr. Infect. Dis. Rep. 2012, 14, 263–274. 10.1007/s11908-012-0259-3. [DOI] [PubMed] [Google Scholar]
- Abad-Franch F.; Lima M. M.; Sarquis O.; Gurgel-Gonçalves R.; Sánchez-Martín M.; Calzada J.; Saldaña A.; Monteiro F. A.; Palomeque F. S.; Santos W. S.; Angulo V. M.; Esteban L.; Dias F. B. S.; Diotaiuti L.; Bar M. E.; Gottdenker N. L. On Palms, Bugs, and Chagas Disease in the Americas. Acta Trop. 2015, 151, 126–141. 10.1016/j.actatropica.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Aguilar H. M.; Abad-Franch F.; Dias J. C. P.; Junqueira A. C. V.; Coura J. R. Chagas Disease in the Amazon Region. Mem. Inst. Oswaldo Cruz 2007, 102, 47–55. 10.1590/s0074-02762007005000098. [DOI] [PubMed] [Google Scholar]
- Coura J. R.; Vĩas P. A. Chagas Disease: A New Worldwide Challenge. Nature 2010, 465, S6–S7. 10.1038/nature09221. [DOI] [PubMed] [Google Scholar]
- de Rycker M.; Baragaña B.; Duce S. L.; Gilbert I. H. Challenges and Recent Progress in Drug Discovery for Tropical Diseases. Nature 2018, 559, 498–506. 10.1038/s41586-018-0327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabarin-Lima A.; González-Vázquez M. C.; Rodríguez-Morales O.; Baylón-Pacheco L.; Rosales-Encina J. L.; Reyes-López P. A.; Arce-Fonseca M. Chagas Disease (American Trypanosomiasis) in Mexico: An Update. Acta Trop. 2013, 127, 126–135. 10.1016/j.actatropica.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Scarim C. B.; Jornada D. H.; Chelucci R. C.; de Almeida L.; dos Santos J. L.; Chung M. C. Current Advances in Drug Discovery for Chagas Disease. Eur. J. Med. Chem. 2018, 155, 824–838. 10.1016/j.ejmech.2018.06.040. [DOI] [PubMed] [Google Scholar]
- Díaz S.; Villavicencio B.; Correia N.; Costa J.; Haag K. L. Triatomine Bugs, Their Microbiota and Trypanosoma cruzi: Asymmetric Responses of Bacteria to an Infected Blood Meal. Parasites Vectors 2016, 9, 636. 10.1186/s13071-016-1926-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. C.; Ward A.; Olmo F.; Jayawardhana S.; Francisco A. F.; Lewis M. D.; Kelly J. M. Intracellular DNA Replication and Differentiation of Trypanosoma cruzi Is Asynchronous within Individual Host Cells in Vivo at All Stages of Infection. PLoS Neglected Trop. Dis. 2020, 14, e0008007 10.1371/journal.pntd.0008007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . Chagas Disease - Biology. https://www.cdc.gov/parasites/chagas/biologyhtml (accessed Nov 20, 2020).
- Prata A. Clinical and Epidemiological Aspects of Chagas Disease. Lancet Infect. Dis. 2001, 1, 92–100. 10.1016/S1473-3099(01)00065-2. [DOI] [PubMed] [Google Scholar]
- Carvalho A. B.; Goldenberg R. C. D. S.; Campos de Carvalho A. C. Cell Therapies for Chagas Disease. Cytotherapy 2017, 19, 1339–1349. 10.1016/j.jcyt.2017.07.014. [DOI] [PubMed] [Google Scholar]
- Pecoul B.; Batista C.; Stobbaerts E.; Ribeiro I.; Vilasanjuan R.; Gascon J.; Pinazo M. J.; Moriana S.; Gold S.; Pereiro A.; Navarro M.; Torrico F.; Bottazzi M. E.; Hotez P. J. The BENEFIT Trial: Where Do We Go from Here?. PLoS Neglected Trop. Dis. 2016, 10, e0004343 10.1371/journal.pntd.0004343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bern C. Chagas’ Disease. N. Engl. J. Med. 2015, 373, 456–466. 10.1056/NEJMra1410150. [DOI] [PubMed] [Google Scholar]
- Molina I.; Gómez i Prat J.; Salvador F.; Treviño B.; Sulleiro E.; Serre N.; Pou D.; Roure S.; Cabezos J.; Valerio L.; Blanco-Grau A.; Sánchez-Montalvá A.; Vidal X.; Pahissa A. Randomized Trial of Posaconazole and Benznidazole for Chronic Chagas’ Disease. N. Engl. J. Med. 2014, 370, 1899–1908. 10.1056/nejmoa1313122. [DOI] [PubMed] [Google Scholar]
- Chatelain E. Chagas Disease Drug Discovery: Toward a New Era. SLAS Discovery 2015, 20, 22–35. 10.1177/1087057114550585. [DOI] [PubMed] [Google Scholar]
- Urbina J. A.; Docampo R. Specific Chemotherapy of Chagas Disease: Controversies and Advances. Trends Parasitol. 2003, 19, 495–501. 10.1016/j.pt.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Coura J. R.; De Castro S. L. A Critical Review on Chagas Disease Chemotherapy. Mem. Inst. Oswaldo Cruz 2002, 97, 3–24. 10.1590/S0074-02762002000100001. [DOI] [PubMed] [Google Scholar]
- Sykes M. L.; Avery V. M. 3-Pyridyl Inhibitors with Novel Activity against Trypanosoma cruzi Reveal in Vitro Profiles Can Aid Prediction of Putative Cytochrome P450 Inhibition. Sci. Rep. 2018, 8, 4901 10.1038/s41598-018-22043-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morillo C. A.; Waskin H.; et al. Benznidazole and Posaconazole in Eliminating Parasites in Asymptomatic T. cruzi Carriers: The STOP-CHAGAS Trial. J. Am. Coll. Cardiol. 2017, 69, 939–947. 10.1016/j.jacc.2016.12.023. [DOI] [PubMed] [Google Scholar]
- Morillo C. A.; Marin-Neto J. A.; Avezum A.; Sosa-Estani S.; Rassi A.; Rosas F.; Villena E.; Quiroz R.; Bonilla R.; Britto C.; Guhl F.; Velazquez E.; Bonilla L.; Meeks B.; Rao-Melacini P.; Pogue J.; Mattos A.; Lazdins J.; Rassi A.; Connolly S. J.; Yusuf S. Randomized Trial of Benznidazole for Chronic Chagas’ Cardiomyopathy. N. Engl. J. Med. 2015, 373, 1295–1306. 10.1056/NEJMoa1507574. [DOI] [PubMed] [Google Scholar]
- Torrico F.; Gascon J.; Ortiz L.; Alonso-Vega C.; Pinazo M.-J.; Schijman A.; Almeida I. C.; Alves F.; et al. Treatment of Adult Chronic Indeterminate Chagas Disease with Benznidazole and Three E1224 Dosing Regimens: A Proof-of-Concept, Randomised, Placebo-Controlled Trial. Lancet Infect. Dis. 2018, 18, 419–430. 10.1016/S1473-3099(17)30538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco C. H.; Warhurst D. C.; Bhattacharyya T.; Au H. Y. A.; Le H.; Giardini M. A.; Pascoalino B. S.; Torrecilhas A. C.; Romera L. M. D.; Madeira R. P.; Schenkman S.; Freitas-Junior L. H.; Chatelain E.; Miles M. A.; Moraes C. B. Novel Structural CYP51 Mutation in Trypanosoma cruzi Associated with Multidrug Resistance to CYP51 Inhibitors and Reduced Infectivity. Int. J. Parasitol.: Drugs Drug Resist. 2020, 13, 107–120. 10.1016/j.ijpddr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle A. S.; Khare S.; Kumar A. B.; Supek F.; Buchynskyy A.; Mathison C. J. N.; Chennamaneni N. K.; Pendem N.; Buckner F. S.; Gelb M. H.; Molteni V. Recent Developments in Drug Discovery for Leishmaniasis and Human African Trypanosomiasis. Chem. Rev. 2014, 114, 11305–11347. 10.1021/cr500365f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare S.; Nagle A. S.; Biggart A.; Lai Y. H.; Liang F.; Davis L. C.; Barnes S. W.; Mathison C. J. N.; Myburgh E.; Gao M. Y.; Gillespie J. R.; Liu X.; Tan J. L.; Stinson M.; Rivera I. C.; Ballard J.; Yeh V.; Groessl T.; Federe G.; Koh H. X. Y.; Venable J. D.; Bursulaya B.; Shapiro M.; Mishra P. K.; Spraggon G.; Brock A.; Mottram J. C.; Buckner F. S.; Rao S. P. S.; Wen B. G.; Walker J. R.; Tuntland T.; Molteni V.; Glynne R. J.; Supek F. Proteasome Inhibition for Treatment of Leishmaniasis, Chagas Disease and Sleeping Sickness. Nature 2016, 537, 229–233. 10.1038/nature19339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese S.; Rahmani R.; Russell S.; Deora G. S.; Ferrins L.; Toynton A.; Jones A.; Sykes M.; Kessler A.; Eufrásio A.; Cordeiro A. T.; Sherman J.; Rodriguez A.; Avery V. M.; Piggott M. J.; Baell J. B. Discovery of Potent N -Ethylurea Pyrazole Derivatives as Dual Inhibitors of Trypanosoma brucei and Trypanosoma cruzi. ACS Med. Chem. Lett. 2020, 11, 278–285. 10.1021/acsmedchemlett.9b00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalta F.; Rachakonda G. Advances in Preclinical Approaches to Chagas Disease Drug Discovery. Expert Opin. Drug Discovery 2019, 14, 1161–1174. 10.1080/17460441.2019.1652593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug D. M.; Diaz-Gonzalez R.; DeLano T. J.; Mavrogiannaki E. M.; Buskes M. J.; Dalton R. M.; Fisher J. K.; Schneider K. M.; Hilborne V.; Fritsche M. G.; Simpson Q. J.; Tear W. F.; Devine W. G.; Pérez-Moreno G.; Ceballos-Pérez G.; García-Hernández R.; Bosch-Navarrete C.; Ruiz-Pérez L. M.; Gamarro F.; González-Pacanowska D.; Martinez-Martinez M. S.; Manzano-Chinchon P.; Navarro M.; Pollastri M. P.; Ferrins L. Structure–Property Studies of an Imidazoquinoline Chemotype with Antitrypanosomal Activity. RSC Med. Chem. 2020, 11, 950–959. 10.1039/D0MD00103A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sear C. E.; Pieper P.; Amaral M.; Romanelli M. M.; Costa-Silva T. A.; Haugland M. M.; Tate J. A.; Lago J. H. G.; Tempone A. G.; Anderson E. A. Synthesis and Structure–Activity Relationship of Dehydrodieugenol B Neolignans against Trypanosoma cruzi. ACS Infect. Dis. 2020, 6, 2872–2878. 10.1021/acsinfecdis.0c00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima M. L.; Tulloch L. B.; Corpas-Lopez V.; Carvalho S.; Wall R. J.; Milne R.; Rico E.; Patterson S.; Gilbert I. H.; Moniz S.; MacLean L.; Torrie L. S.; Morgillo C.; Horn D.; Zuccotto F.; Wyllie S. Identification of a Proteasome-Targeting Arylsulfonamide with Potential for the Treatment of Chagas’ Disease. Antimicrob. Agents Chemother. 2022, 66, e01535-21 10.1128/AAC.01535-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rycker M.; Wyllie S.; Horn D.; Read K. D.; Gilbert I. H. Anti-Trypanosomatid Drug Discovery: Progress and Challenges. Nat. Rev. Microbiol. 2023, 21, 35–50. 10.1038/s41579-022-00777-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Screening Chagas disease | DNDi. https://dndi.org/research-development/portfolio/screening-chagas/ (accessed March 31, 2021).
- NTD Drug Discovery Booster Hit-to-lead | DNDi. https://dndi.org/research-development/portfolio/drug-discovery-booster (accessed Feb 03, 2021).
- Akao Y.; Canan S.; Cao Y.; Condroski K.; Engkvist O.; Itono S.; Kaki R.; Kimura C.; Kogej T.; Nagaoka K.; Naito A.; Nakai H.; Pairaudeau G.; Radu C.; Roberts I.; Shimada M.; Shum D.; Watanabe N.; Xie H.; Yonezawa S.; Yoshida O.; Yoshida R.; Mowbray C.; Perry B. Collaborative Virtual Screening to Elaborate an Imidazo[1,2- a]Pyridine Hit Series for Visceral Leishmaniasis. RSC Med. Chem. 2021, 12, 384–393. 10.1039/d0md00353k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. P.; Rees S. S.; Kalindjian S. B.; Philpott K. L. Principles of Early Drug Discovery. Br. J. Pharmacol. 2011, 162, 1239–1249. 10.1111/j.1476-5381.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripphausen P.; Nisius B.; Bajorath J. State-of-the-Art in Ligand-Based Virtual Screening. Drug Discovery Today 2011, 16, 372–376. 10.1016/j.drudis.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Kogej T.; Blomberg N.; Greasley P. J.; Mundt S.; Vainio M. J.; Schamberger J.; Schmidt G.; Hüser J. Big Pharma Screening Collections: More of the Same or Unique Libraries? The AstraZeneca-Bayer Pharma AG Case. Drug Discovery Today 2013, 18, 1014–1024. 10.1016/j.drudis.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Orhan I.; Şener B.; Kaiser M.; Brun R.; Tasdemir D. Inhibitory Activity of Marine Sponge-Derived Natural Products against Parasitic Protozoa. Mar. Drugs 2010, 8, 47–58. 10.3390/md8010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SciFinder Discovery Platform | CAS. https://www.cas.org/scifinder-discovery-platform (accessed March 31, 2021).
- Phan T.-N.; Baek K.-H.; Lee N.; Byun S. Y.; Shum D.; No J. H. In Vitro and in Vivo Activity of MTOR Kinase and PI3K Inhibitors Against Leishmania Donovani and Trypanosoma Brucei. Molecules 2020, 25, 1980. 10.3390/molecules25081980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Gonzalez R.; Kuhlmann F. M.; Galan-Rodriguez C.; da Silva L. M.; Saldivia M.; Karver C. E.; Rodriguez A.; Beverley S. M.; Navarro M.; Pollastri M. P. The Susceptibility of Trypanosomatid Pathogens to PI3/MTOR Kinase Inhibitors Affords a New Opportunity for Drug Repurposing. PLoS Neglected Trop. Dis. 2011, 5, e1297 10.1371/journal.pntd.0001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baell J. B.; Holloway G. A. New Substructure Filters for Removal of Pan Assay Interference Compounds (PAINS) from Screening Libraries and for Their Exclusion in Bioassays. J. Med. Chem. 2010, 53, 2719–2740. 10.1021/jm901137j. [DOI] [PubMed] [Google Scholar]
- Daina A.; Michielin O.; Zoete V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly D. J.; Guiry P. J. A Facile and Versatile Route to 2-Substituted-4(3H)-Quinazolinones and Quinazolines. Synlett 2001, 2001, 1707–1710. 10.1055/s-2001-18080. [DOI] [Google Scholar]
- Riley J.; Brand S.; Voice M.; Caballero I.; Calvo D.; Read K. D. Development of a Fluorescence-Based Trypanosoma cruzi CYP51 Inhibition Assay for Effective Compound Triaging in Drug Discovery Programmes for Chagas Disease. PLoS Neglected Trop. Dis. 2015, 9, e0004014 10.1371/journal.pntd.0004014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller E.; Chalfin M.; Bohmaker K. Serotonin 5-HT1a Receptor-Mediated Hypothermia in Mice: Absence of Spare Receptors and Rapid Induction of Tolerance. Pharmacol., Biochem. Behav. 1992, 43, 405–411. 10.1016/0091-3057(92)90169-G. [DOI] [PubMed] [Google Scholar]
- Watanabe A.; Mayumi K.; Nishimura K.; Osaki H. In Vivo Use of the CYP Inhibitor 1-Aminobenzotriazole to Increase Long-Term Exposure in Mice. Biopharm. Drug Dispos. 2016, 37, 373–378. 10.1002/bdd.2020. [DOI] [PubMed] [Google Scholar]
- Lewis M. D.; Fortes Francisco A.; Taylor M. C.; Burrell-Saward H.; Mclatchie A. P.; Miles M. A.; Kelly J. M. Bioluminescence Imaging of Chronic Trypanosoma cruzi Infections Reveals Tissue-Specific Parasite Dynamics and Heart Disease in the Absence of Locally Persistent Infection. Cell. Microbiol. 2014, 16, 1285–1300. 10.1111/cmi.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa F. C.; Francisco A. F.; Jayawardhana S.; Calderano S. G.; Lewis M. D.; Olmo F.; Beneke T.; Gluenz E.; Sunter J.; Dean S.; Kelly J. M.; Taylor M. C. Expanding the Toolbox for Trypanosoma cruzi: A Parasite Line Incorporating a Bioluminescence-Fluorescence Dual Reporter and Streamlined CRISPR/Cas9 Functionality for Rapid in Vivo Localisation and Phenotyping. PLoS Neglected Trop. Dis. 2018, 12, e0006388 10.1371/journal.pntd.0006388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- daylight. https://www.daylight.com/.
- Chemaxon. http://www.chemaxon.com/.
- Openeye. https://www.eyesopen.com/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Full data set for all compounds explored in this series are available online as sdf file at https://dndi.org/research-development/portfolio/drug-discovery-booster/.