Abstract
Our body keeps separating the toxic chemicals in the blood from the brain. A significant number of drugs do not enter the central nervous system (CNS) due to the blood-brain barrier (BBB). Certain diseases, such as tumor growth and stroke, are known to increase the permeability of the BBB. However, the heterogeneity of this permeation makes it difficult and unpredictable to transport drugs to the brain. In recent years, research has been directed toward increasing drug penetration inside the brain, and nanomedicine has emerged as a promising approach. Active targeting requires one or more specific ligands on the surface of nanoparticles (NPs), which brain endothelial cells (ECs) recognize, allowing controlled drug delivery compared to conventional targeting strategies. This review highlights the mechanistic insights about different cell types contributing to the development and maintenance of the BBB and summarizes the recent advancement in brain-specific NPs for different pathological conditions. Furthermore, fundamental properties of brain-targeted NPs will be discussed, and the standard lesion features classified by neurological pathology are summarized.
Keywords: Brain cancer, Blood-brain barrier, Nanomedicine, Medulloblastoma, Glioblastoma
1. Introduction
Neurons require a specific environment for proper function, and the blood-brain barrier (BBB) is an important immunological feature of the central nervous system (CNS) [1]. Therefore, BBB restricts most drugs from entering the brain and poses a challenge for treating brain tumors and other CNS diseases [2]. The optimal CNS therapeutic drug is typically 400 Da, has high lipophilicity of log P 2.5, is nonpolar, unionized, has low hydrogen bond capacity, is not a substrate for efflux transporters, and has low protein binding [3]. However, finding all these properties in drug molecules is a Herculean task, and indeed most drugs fall away from these properties [4]. Additionally, macromolecular pharmaceuticals including peptides, proteins, antibodies, and oligonucleotides cannot pass the BBB [5]. Tremendous efforts have been made to enhance drug transport into the brain parenchyma, including chemical modification of drugs, chemically or osmotically opening of tight junctions (TJs), physical disruption of the BBB layer, and the use of specific carriers/transporters. Each method has its advantages and limitations. Chemical modification of a drug requires a tremendous effort and needs to go through the Investigational New Drug (IND) Applications [6]. Furthermore, the prodrug approach may interact with drug distribution and its target binding properties, resulting in lower efficacy or toxicity in other organs. Disruption of the BBB by injecting a hyperosmolar substance such as mannitol or physically by ultrasound enables paracellular transport of blood toxins that may cause toxicity to neurons and glia cells [7]. Furthermore, this technique is limited mainly to small molecules. Nanocarriers are also being explored to increase drug penetration across the BBB. Nanoparticles (NPs) modulate biodistribution, prolong blood circulation, decrease toxicity, and improve the safety profile of drugs [8]. Several NPs properties are desired for effective BBB transport of cargo such as their particle size, zeta potential, polydispersity, composition, and surface modifications. For example, NPs with larger diameters (>100 nm) are non-permeable to the brain endothelium and are hydrophobic in nature readily taken up by the cells of the reticuloendothelial system (RES) [9]. Furthermore, the surface properties of NPs may interfere with their interaction with brain capillary cells and binding plasma proteins [10]. For proper function and to keep the CNS in equilibrium, the brain needs critical nutrients and ions., numerous unique endogenous transport pathways are located at the BBB, which provide these nutrients and serve as possible routes for drug delivery [11]. Using receptor-mediated transcytosis (RMT), engaging receptors/proteins expressed at the BBB is one solution for decreasing the nonspecific interaction of the drug/carrier in the body and brain-specific uptake. However, designing NPs decorated with ligands targeting the BBB receptor/protein (e.g., LRP1, TfR1, GLUT1, and SLC7A5) has been reported with modest success due to the lack of brain-specific expression of the target proteins [12]. For example, LRP1/TfR1, GLUT1, and SLC7A5 receptors are expressed in other organs such as lung, kidney, and intestine epithelium/endothelium, respectively. Furthermore, RMT is limited by endogenous substrates, which usually compete with drug carriers for target binding under physiological conditions [13]. Some of these targeting approaches gained attention due to the successful completion of the initial phases of clinical trials, such as ANG1005, to treat leptomeningeal disease from breast cancer [14]. Even after crossing the BBB, some therapeutics, especially macromolecules, have issues crossing the cell membrane. Therefore, a dual-targeting approach, BBB transport, and cell-penetrating peptides are also being developed [15]. This article will explore current techniques for brain drug delivery and platforms for future brain medication development that combine BBB drug delivery technology with brain drug discovery.
2. Blood-brain barrier physiology
The interface between blood and the brain tissue is through a microvasculature network extending from the arterioles to capillaries and the venules. The integrity and functionality of the BBB are essential for normal hemostasis. Expression of junctional adhesion molecules (JAMs) and tight junction proteins (TJPs), which block transcellular diffusion pathways, (2) suppression of pinocytic vesicles and fenestrae, which hinder the paracellular diffusion of substances, and (3) expression of efflux pumps, which actively prevent substances from passing through brain endothelial cell membranes, (4) along with expression of enzymes that metabolize compounds before they reach the brain [16]. This section will discuss the various structural and physiological aspects of BBB.
2.1. Structural components of BBB
The BBB primarily protects the brain parenchyma from pathogens and toxic substances. The selectively permeable membrane allows the diffusion of low molecular weight lipophilic molecules, such as alcohol and anesthetics. Proteins are majorly transported by TJ solute carriers using electrochemical gradients or concentration gradients, while small structures such as amino acids, ketones, glucose, nucleotides, and ions can be taken up by RMT. Complex cellular and noncellular components work together to keep the BBB functioning. Brain microvascular endothelial cells (BMECs), pericytes, and astrocytic end feet all contribute to the formation of the BBB (Fig. 1).
Fig. 1.
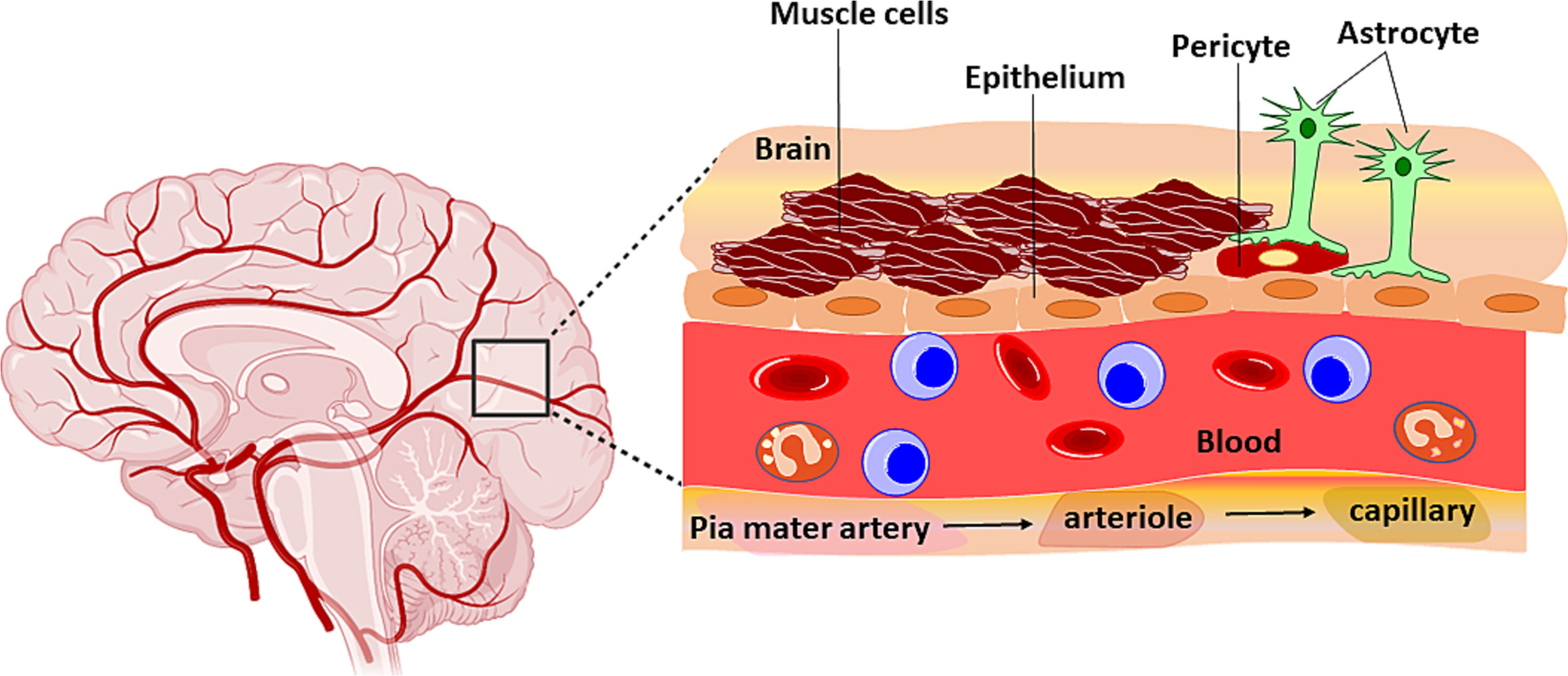
Structural components of blood brain barrier.
2.1.1. Endothelial cells
The core of the BBB is composed of BMECs, which differ from other ECs in that they have more mitochondria and do not have fenestration. They express TJPs, which impede the entry of most molecules, except for minor and lipophilic molecules. In addition to passive penetration, efflux and RMT are common material exchange mechanisms across the BBB. BMECs exhibit efflux transporters such as the P-glycoprotein-related multiple drug resistance 1 (MDR1) and the breast cancer resistance protein (BCRP) (P-gp) responsible for active transport of molecules across the BBB [17,18]. Transporting proteins on BMECs recognize and transport essential substances from the blood, such as glucose, amino acids, purine bases, and nucleosides, to the brain via RMT.
2.1.2. Pericytes
Pericytes encase the ECs lining the capillaries. These cells play an essential role in BBB metabolism by allowing the exchange of ions and metabolites between ECs [19]. Both pericytes and ECs are enveloped by a continuous basal membrane between the two cell types required for the TJs. The functional impermeability of BBB is not exclusively the property of ECs, enhanced by abiding cells, including pericytes. Pericytes aid in the maintenance of TJPs expression on ECs (e.g., Cldn-5, Ocln, and ZO1) and therefore promote the integrity and normal functionality of the BBB [20]. In one study, Armulik et al. showed that adult viable pericyte-deficient mice had significantly high BBB permeability to water and other small or large molecule tracers [21]. Pericytes do that by releasing chemicals, affecting the TJPs of EC, and polarizing the astrocyte end feet. Pericytes also repair the cerebral blood vessels and promote angiogenesis due to stem cell-like properties [19]. Pericyte networks propagate signals along the capillary to control blood flow through the capillaries.
2.1.3. Astrocytes
Astrocytes provide metabolic support to ECs and secrete factors that induce the BBB phenotype in ECs. Astrocytes support the integrity of the BBB by secreting stimulants such as sonic hedgehog (Shh), vascular endothelial growth factor (VEGF), angiopoietins-1 (Ang-1), angiotensin-converting enzyme-1 (ACE-1), and Apolipoprotein E (ApoE) [22].
2.1.4. Microglia in BBB
Macrophages in the CNS are known as microglia cells, which are classified into cytotoxic (M1) and neuroprotective (M2) based on inflammatory conditions [23,24], According to a recent study by Haruwaka et al., microglia relocate toward and accumulate around brain arteries in response to inflammation before any observable alteration in BBB permeability. Surprisingly, the initial microglial contact with cerebral blood vessels protects BBB integrity. However, chronic inflammation causes a more active microglial phenotype to predominate, resulting in astrocytic end-feet phagocytosis and BBB integrity loss. They used Cx3cr1-GFP mice, which express green fluorescent protein (GFP) specifically in microglia cells [25]. When lipopolysaccharides (LPS) were administered, the microglial contact with the arteries significantly increased the permeability of dextran (10 kDa), which is normally impermeable [24]. This study sheds light on the strategies that may be utilized to manipulate the BBB integrity by targeting microglial cells.
2.1.5. Basement membrane
Extracellular matrix (ECM) is a dynamic component of the BBB that influences cell-cell and cell-matrix interactions to control its form and function [26]. Between the blood and the brain, there are two types of basement membranes (BMs): endothelial BM and parenchymal BM. Collagen IV, fibronectin, and laminins comprise the endothelium BM that coats the capillaries [27]. The primary cells found embedded within the endothelium BM are pericytes. Whereas ECM, laminin, astrocyte, and neuron end foot processes constitute the mass of the parenchymal BM. By coating the perivascular space, the BMs restrict leukocytes from invading the CNS under inflammatory events [28].
2.1.6. Blood CSF barrier at the choroid plexus
The choroid plexus (CP) is a highly vascularized epithelial tissue that forms the lateral, third, and fourth ventricles [29]. CP is mainly responsible for cerebrospinal fluid (CSF) release. The blood-cerebrospinal fluid barrier (BCSFB), which maintains blood away from CSF and brain tissue, is formed by the CP epithelial cells. Like the BBB, the BCSFB functions to prevent the passage of pathogens and toxins into the brain; however, the BCSFB has structural differences that allow the transport of diverse substances [30]. The choroidal capillaries are highly permeable and, unlike the BBB cells, are arranged as a single layer and are not encased by the astrocytes and pericytes. A unique component of the BCSFB in the fenestrated ECs facilitates the transportation of water, lipophilic molecules, and gases. Microvilli on the surface of cells facing the CSF contain Na+/K+ ATPase channels, creating an electrochemical gradient allowing Na+ entry into the CSF [31]. The surface of the CP tissue is covered with villi. The microvilli also increase the surface area and facilitate fluid secretion. The stroma lining CP contains collagen bundles and is bordered by leptomeningeal cells [32]. Depending on the age and disease conditions, the stroma of the CP could be changed, and collagen fibers may form spheres or be calcified [33]. The ECs comprising CP still form the TJs, however, the electrical resistance observed is much lower than the BBB. Consequently, the CP membrane is relatively leaky than the BBB. Due to its leaky nature, CP is also the main site of pathogen infiltration in the brain [34]. Therefore, CP is an attractive gateway from the drug delivery perspective to the brain.
2.1.7. Circumventricular organ barrier
Fenestrated and vascularized blood channels called circumventricular organs (CVOs) enable neurons to detect hormones in the blood and transmit this information to other areas of the brain [35]. CVOs perform two main functions in the body: sensory and secretory. There are three sensory CVOs named the organum vasculosum of the lamina terminalis, the subfornical organ, and the region postrema. Furthermore, the intermediate lobe of the pituitary gland, pineal gland, the median eminence (ME) and the neurohypophysis are examples of four types of secretory CVOs [36]. Ependymal cells bordering CVOs make the lining of the ventricles as well as the parenchyma side of the brain, which is flanked by astroglia cells. The epithelium of the mouse brain’s ventricles expresses all of TJPs of the ECs. The fenestrae-rich capillaries of ME allows liberal exchange of material of from the blood vessels into the CVOs. According to some reports larger molecules such as leptin may be transported between CSF and blood by ependymal cells at the ventral interface.
2.2. Physiological functions of BBB
2.2.1. Maintenance of ionic and nutrition homeostasis
Through various specialized ion channels and transporters, the BBB maintains a regulated milieu that is optimum for neuronal and synaptic signaling processes [37]. The potassium levels in CSF and ISF, for example, are kept between 2.5 and 2.9 mM, whereas its plasma concentration is typically 4.5 mM. Homeostasis of Ca2+ and K+ is required for proper neuronal excitability and critical for macrophage displacement across BBB. The BBB also regulates other ions, including calcium and magnesium. The BBB’s specific ion channels and transporters provide the optimum synaptic and neuronal functions environment [38]. The abluminal sodium pump (Na+, K+ ATPase) transfers Na + into the brain and K+ out to maintain their correct ratio in brain ISF. The luminal Na+, K+ Cl− cotransporter, on the other hand, enhances the transport of Na+, K+, and Cl− from the blood to the endothelium. Ion transport across the BBB is regulated by calcium transporters (Na+ – Ca2+ exchangers) and voltage-gated K+ channels. Passive permeation allows critical water-soluble nutrients to permeate across the BBB. Specific transport pathways are present for some nutrients that cannot pass through passively.
2.2.2. Regulation of neurotransmitter levels
The CNS and peripheral nerve systems share a number of neurotransmitters, and fluctuations in plasma levels could result in severe consequences, therefore, BBB protect the brain by maintaining a barrier [39]. For example, the neuroexcitatory amino acid glutamate fluctuates in blood plasma after meals [40]. Without the BBB, a high glutamate concentration can cause neuronal injury. This is evident by the observation that after neurotoxic/neuroexcitatory damage to brain tissue caused by glutamate overproduction from hypoxic neurons during ischemic stroke. Similarly, some neurotransmitters are transported from the brain to the bloodstream via Na+ coupled and Na+ independent amino acid transporters. These transporters are essential for regulating brain homeostasis.
2.2.3. Inhibition of leakage of plasma proteins into the brain
The CP is a filter unit to eliminate unwanted plasma proteins for CSF production. This mechanism regulates CSF’s protein concentration, resulting in lower protein levels in CSF than in plasma. Plasma proteins such albumin, prothrombin, and plasminogen that leak could cause seizures, glial activation, cell proliferation, scarring, and cell death [41]. This is because various activators for these proteins may be found throughout the CNS. For example, Factor Xa protein transforms prothrombin to thrombin, and tissue plasminogen activator converts plasminogen to plasmin [42,43]. The active form of these proteins may start injury cascades upon binding to receptors in brain tissue.
2.2.4. Protection of the brain against neurotoxins
Fully differentiated neurons in the adult CNS have limited proliferation. The presence of excessive neurotoxin in the brain could lead to apoptosis of the neurons. Blood contains many endogenous and exogenous neurotoxins. Therefore, BBB protects the brain from blood-borne neurotoxins [44]. Multiple active ABC energy-dependent efflux transporters eliminate many inadvertently absorbed toxins on the abluminal side of BBB. Proinflammatory cytokines, reactive oxygen species, growth factors, metalloproteases, miRNAs, and several other signaling molecules are a few examples of such biomolecules.
2.3. BBB permeation regulation
Many signaling factors have been reported to regulate the structural components of the BBB to affect permeability [45]. Further, molecules such as isoflurane, a volatile anesthetic, can increase BBB permeability to some extent [46].
2.3.1. Tight junctions
TJs are composed of transmembrane adhesion proteins, including claudins (Cldn), occluding (Ocln), and junction adhesion molecules. Cldn serve as the primary regulators of TJ permeability. Cldn-1, -3, -5, and -12 are the primary constituents of endothelial TJs and create selective hydrophilic paracellular pores [47]. The principle Cldn in the BBB is Cldn-5, which has two primary functions: regulation of BBB permeability (allows small molecules <800 Da) and regulation of cell motility. Modifying TJ protein expression affects the BBB permeability functions. For instance, the expression of Cldn-5 is known to decrease within the first hours of ischemia [48]. Ocln, on the other hand, acts as a redox sensor of the cell. Under hypoxic or ischemic reducing conditions, Ocln is re-distributed from the TJs to the cytosol, acting as an oxidase to counterbalance the altered redox status of the cells [49]. Cldn proteins have two extracellular loops 1 and 2 (ECL1 and ECL2), an intracellular loop, and cytosolic N- and C-termini. Their paracellular sealing action is mediated by the extracellular domains. It was shown that peptides derived from the Cldn ECL1 loop can be used to improve the paracellular transport of drugs [50]. For instance, the transit of impermeable small molecules through the BBB in rats was boosted by the peptide C1C2, which was generated from the Cldn-1 sequence. It was discovered that C1C2 essentially modifies the structure of the TJ-strand, suppresses the expression of Cldn-1, and redistributes Cldn from junctions to the cytosol. Dithmer et al. designed a peptide C5C2 from the ECL1 of murine Cldn-5 with a composition of D-aa [50]. C5C2 peptide reversibly reduced the TEER of bEND.3 cells by decreasing membranous Cldn-5 and increasing its cytosolic accumulation. Parallel to this, the C5C2 treatment considerably increased the permeability for both small (457 Da) and large (10 kDa) molecules than control. In addition, the peptides increased the permeability of doxorubicin (580 Da) by factors of 5.
2.3.2. Signaling molecules
Inflammation in the brain leads to the production of cytokines by stimulating leukocytes, astrocytes, and microglial cells. Increased cytokines (IL-1, IL-6, and TNF) in the brain are strongly linked to altered TJ function and BBB permeability [51]. For instance, Quagliarello et al. observed that rats were more susceptible to IL-1-induced BBB breakdown resulting in meningitis. Further, Zhen et al. showed that IL-1 increased BBB permeability by decreasing astrocyte production of sonic hedgehog, ultimately leading to the downregulation of TJPs [52]. Additionally, cytokines such as IL-6 and TNF-α are known to increase paracellular transport in BMECs primarily by affecting TJPs and increasing the production of reactive oxygen species (ROS). TNF-α was also discovered to affect the BBB permeability by inducing COX2 and prostaglandin (PG) in BMECs [53]. According to Haruwaka et al., microglia stimulated by LPS may emit TNF-α, resulting in BBB disruption [24]. TJP expression and BBB integrity were enhanced by HPI201 and minocycline, which inhibited TNF-α, IL-1, and IL-6 upregulation [54]. Inflammatory mediators such as eicosanoids, in addition to cytokines, are involved in CNS inflammation. PGs and leukotrienes (LTs) are eicosanoids produced from arachidonic acid. Inflammation causes COX1 and COX2 to be secreted. PGE2 has been linked to BBB disruption. Free radical overproduction has also been linked to the pathogenesis of various neurological disorders. For example, LPS-induced BBB impairment is mediated by microglia-generated ROS and reactive nitrogen species (RNS). In ischemia-reperfusion, ROS production may lead to the BBB leakage. ROS cause breakdown of BM and by triggering matrix metalloproteinases (MMPs) while inhibiting tissue inhibitor of matrix metalloproteinase 1 (TIMPs) and increase phosphorylation of tyrosine residue of TJPs. Furthermore, gp120, the HIV-1 envelope protein, increased MMP-2/9 expression through gp120-mediated ROS. The mitochondria-rich astrocytes that surround ECs are the principal source of ROS [55].
2.3.3. Transporters on BBB
The brain cannot synthesize most of its required nutrients for growth and functioning and is thus supplied from circulation. For this purpose, the EC expresses more than 40 different transporter proteins. These transporters are either concentration- or energy-dependent. Glucose transporter 1 (GLUT1), monocarboxylic acid transporter 1 (MCT1), and amino acid transporters are a few examples of non-energy-dependent transporters on BBB. While P-gp, BCRP, and MDR are some examples of energy-dependent ATP-binding cassette (ABC) proteins on the BBB.
2.3.3.1. Energy-independent transporter proteins.
The functions of organic anion transporting polypeptide (OATP) 1A4 has been extensively investigated in murine models. In vivo microdialysis studies have shown that OATPS transport amphipathic organic anions such as estradiol 17-glucuronide (E17G) [56,57]. Mammalian OATPS are present in a wide range of issues, particularly on barrier cells. For instance, rat retinal capillary ECs exhibit a high expression level of OATPS transporters, including OATP1A4 (OATP2/SLCO1A4) and OATP1C1 (OATP14/SLCO1C1) [58]. These transporters have variable substrate selectivity, as the alpha-4 (OATP1A4) and 1C1 subunits of the Na+/K+ -transporting ATPase have an affinity for amphipathic molecules in general, while OATP1A4 are responsible for transporting the cardiac glycosides such as digoxin [59]. The localizations of OATP1A4 vary in different organs. In rat brains, for example, OATP1A4 and 1C1 transporters are expressed on both the luminal and abluminal sides of the BBB. On the other hand, in the EC’s CP are present only on the basolateral side. Furthermore, in the retina, OATP1A4 has been shown to localize at the apical membrane of the retinal capillary ECs. Either change in the expression of OATP1A4 or the presence of its inhibitors may affect the distribution of their substrates, such as [D-penicillamine] enkephalin [60]. Hydrophobic cationic medicines, such as pyrilamine, oxycodone, and tramadol, have also been postulated to traverse the BBB via OATPS [61]. Studies also found that several D2 dopamine receptor antagonists, such as olanzapine, haloperidol, and risperidone, had higher receptor occupancy in the cortex than in the human pituitary by some undefined mechanism(s) [62]. These non-energy-dependent carriers are saturable; thus, expression protein is the rate-limiting step in drug transport.
2.3.3.2. ATP-binding cassette (ABC) proteins.
Several ABC are energy-coupled efflux pumps that bind and hydrolyze ATP. These large plasma membrane proteins expel xenobiotics and endogenous metabolites across the cell membrane. Several pathological conditions can influence the expression levels of active transporters of BBB. For example, it is known that the ABC efflux pumps regulate the quantity of beta-amyloid peptides (Aβ) in the brain. These transporters’ expression may change, or their functioning may decline, which might increase the buildup of peptide Ab in the brain and increase the risk of Alzheimer’s disease (AD). Conversely, ABC overexpression on blood tumor barrier (BTB)can pump drugs out of the brain and induce resistance mechanisms. P-gp, BCRP, and MRPs are the three main efflux proteins accountable for the MDR phenotype. The BBB’s luminal membrane is densely packed with P-gp receptors, which are responsible for the efflux of drugs back into the blood. P-gp can efflux a wide range of structurally and functionally diverse compounds from the brain, including vinca alkaloids, paclitaxel, etoposide, verapamil, quinidine, and dexamethasone, among others [63]. Human tissues such as the brain, liver, kidney, intestine, testis, mammary gland, and placenta express the BCRP. BCRP is important in the context of drug uptake by the brain. For instance, the antifolate drug methotrexate (MTX), a routinely used treatment for primary CNS cancer, has only 5% distribution in the brain. A BCRP knockout model confirmed that BCRP plays a direct role in poor MTX penetration across the BBB [64]. Similar to this, a tyrosine kinase inhibitor (TKI) imatinib mesylate is frequently used to treat several peripheral tumors. However, its the brain concentration is limited due to BCRP. When [14C] labeled imatinib mesylate was injected into P-gp and BCRP knockout mice, its clearance was reduced by 1.25 times, respectively, compared to wild-type control animals [65]. Similarly, dasatinib; a second generation TKI is a substrate of BCRP or P-gp Further, show 10 folds higher concentration in the brain of P-gp/BCRP knockout mice compared to FVB wild-type mice [66].
MRP1 and MRP2 are two main isoforms of the multidrug resistance-associated protein (MRP). These transporters may be found on the luminal or abluminal sides of the BBB. Organic anions like glutathione, sulfate, or glucuronide-conjugated substances, as well as nucleoside analogs, are transported by MRP-1, a prominent ABC efflux transporter. MRP2 (ABCC2) has recently been found at the luminal surface of the brain capillary endothelium [66]. Phenytoin, a common antiepileptic medication, has been discovered to be an MRP2 substrate. In rats, the MRP2 inhibitor probenecid dramatically boosted its extracellular brain concentration. MRP2 prefers water-soluble conjugates as a substrate.
2.4. BBB disruption, disease, injury, and BTB
As discussed earlier, tight regulation of capillary permeability of the BBB is instrumental for neuronal functioning. However, certain disease conditions can cause a dysfunction of the BBB. For instance, following an ischemic stroke, the decrease in cerebral blood flow causes hypoxia and the glucose molecules needed by the ECs to produce ATP. This leads to the inactivation of Glutamate ATP-dependent channels and increased activity of other transporters, such as the Na+/H+ channel. Increased intracellular Na+ levels cause an increase in isosmotic volume, causing edema. The increased Na+ also leads to disruption of Na+/Ca2+ antiporters, causing an efflux of Ca2+. High Ca2+ signals glutamate release from astrocytes, which binds to receptors. Sustained activation of glutamate receptors continues to elevate Ca2+ levels, which promote the release of ROS that can further disrupt the BBB.
2.4.1. Inflammation
It has been proven that peripheral inflammation directly contributes to the development and progression of CNS disorders like AD, Parkinson’s disease (PD), multiple sclerosis (MS), and stroke. The brain’s neuroimmune system lymphocytes are primarily not present during homeostasis. CNS ECs express fewer leukocyte adhesion molecules than peripheral ECs. Peripheral inflammation in AD patients is known to elevate the Ab levels in the brain [67]. The LPS injection increases BBB permeability in amyloid precursor protein (APP) transgenic mice, enabling inflammatory cytokines including IL6 and TNF to enter and accelerate disease development [68,69]. Further, elevated levels of cytokines, including IL-1β, IL-2, and TNF-α, in the periphery and T lymphocytes (CD4+ and CD8+ in CSF are a potential risk factor for PD) [70–72]. Similarly, infiltration of autoreactive CD4+ T cells is one of the most important causes of disease progression of MS. Likewise, the severity of experimental autoimmune encephalomyelitis (EAE) sickness closely correlates with the level of BBB permeability [73].
2.4.2. Brain injury
Acute and secondary brain injuries are linked to immune cell infiltration. Before immune cells infiltrate the brain require the development of a peripheral acute cytokine response (ACR), which equips leukocytes for transmigration to the brain injury site. Inflammatory cytokine IL-1 has a significant role in this process via controlling the inflammatory response to brain injury and supports the recruitment of Ly6b+ leukocytes at the damage site [74]. Dickens et al. demonstrated that the molecular cargo transported by brain astrocytes stimulates leukocytes adequately.
2.4.3. Brain tumors
An imbalance between pro- and anti-angiogenic signals and molecules, such as soluble vascular endothelial growth factor receptor-1 (sVEGFR-1), thrombospondins, and semaphorins, occurs in cases of brain tumors disrupting normal angiogenesis. Due to this imbalance, the arteries enlarge and develop tortuous and hyperpermeable basement membranes. This BBB type is often called the BTB [75]. In BTB, adherents and TJPs are frequently downregulated, resulting in increased paracellular permeability and macromolecule access to the interstitial component of the tumor [76]. Increased numbers of activated astrocytes, VEGF-induced decrease in the expression of TJ proteins, and the disintegration of the basement membrane. Further, the tumor cells intervene with the ECs and astrocytic end-feet interaction characteristics of BTB. Further, pericytes in the BTB express low levels of platelet-derived growth factor receptors (PDGFR) and increased desmin expression. Therefore, BTB is considered leakier with heterogeneous permeability than the normal BBB.
A key pathologic feature of Glioblastoma multiform (GBM) is its microvascular proliferation. GBM displays rapid and robust angiogenesis by expressing VEGF and other angiogenic factors, including fibroblast growth factor (FGF). VEGF causes degradation of the vascular membrane resulting in leaky, enlarged vessels. The increased permeability, along with displacement of astrocytes, causes cerebral edema. Additionally, VEGF mobilizes blood monocytes and neutrophils, which can further produce VEGF and other angiogenic factors [77]. Vessel normalization, using antiangiogenic treatment such as bevacizumab, have improved tumor oxygenation and response to radiation therapy.
Nonetheless, the failure of several clinical studies suggests that the extent and homogeneity of BTB leakage are inadequate to allow the buildup of effective drug concentrations in the brain. Some studies had shown a direct relationship between tumor size and BBB permeability when the tumor’s vascular permeability was investigated using fluorescent tracers. However, other research, such as in the case of the breast cancer model of brain metastasis, found no such correlation. Further, the BTB permeability has been demonstrated to differ dramatically between tumors type and even between different tumor regions in the same animal. For instance, recent research discovered that the accumulation of fluorescent tracers and small drugs like paclitaxel, doxorubicin, and lapatinib in various tumor regions varied by a magnitude of up to 100. Thus, heterogeneous areas in BTB correlate to and are attributable to treatment failure patterns. Additionally, studies employing patient-derived xenograft (PDX) models of GBM, and brain metastases and transporter-knockout mice have shown that the BBB, BTB, and active efflux restrict the efficacy of systemic administration of therapies. A minor increase in BTB permeability may perhaps benefit small amounts of tracer, but not clinically meaningful drug dose.
2.4.4. Stroke
Ischemic strokes occur when a clot within a blood vessel supplying the brain is obstructed, interrupting the crucial supply of oxygen and nutrients. Without oxygen and glucose, the brain cannot form ATP, which is utilized to maintain the plasma membrane potential and gradients of intracellular ions via the Na+-K+-ATPase and Ca2+-ATPase pumps. Under steady-state conditions, intracellular fluid has increased K+ and lower levels of Na+ and Ca2+; the ATPase pumps prevent intracellular accumulation of Na+ and water. In cases of ischemia, decreased activity of the Na+-K+-ATPase pump leads to increased intracellular Na+ followed by Cl− and water, causing cerebral edema. The decrease in membrane potential additionally allows the activation of voltage-dependent Ca2+ channels and a rise in intracellular calcium, causing a toxic release of glutamate. Experimental models and clinical observations have shown that ischemic and hemorrhagic stroke also results in BBB disruption [78,79]. Following cerebral ischemia, the adaptive immune system is activated, and peripheral immune cells such as T and B cells rapidly invade the brain. The release of proinflammatory cytokines from these cells potentially damages the BBB [80]. Furthermore, immunosuppression increases the risk of infections like pneumonia following a stroke [81,82]. Therefore, stroke impacts both the cellular and noncellular components of the BBB, resulting in BBB disruption. The zinc-containing endopeptidases known as MMPs can digest the ECM proteins. Specifically, MMP-2 and MMP-9 are known to degrade TJPs, Cldn-5, and Ocln to open the BBB. The increased expression of these MMPs may contribute to the biphasic opening of the BBB during ischemic stroke. One study found an increase of MMP-2 during an initial reversible opening of the BBB, and MMP-9 is additionally implicated in increased BBB permeability following stroke [83].
3. In vitro methods used for evaluation of BBB drug penetration
Penetration of drugs through BBB is critical for the treatment of CNS illness, but the fact is that many drugs cannot make it to the brain. Further, testing drug permeability directly in animals is time-consuming and costly. This has generated a great deal of interest and forced the creation of in vitro models that can mimic the functionalities of in vivo BBB.
3.1. In vitro 2D BBB model
Immortalized mammalian cell lines are widely used to generate the basic in vitro BBB models. These models are simple, affordable, and well-suited for high throughput screening of drugs. However, these cells do not possess all the characteristics and complexity of in vivo model and thus show poor correlation. As a result, primary cells that retain many qualities of in vivo cells (e.g., TJ, solute carriers, receptors, efflux transporter) have emerged as the preferred cell type. They have high trans epithelial electrical resistance (TEER) with limited permeability. Using a Transwells system, developing a polarized EC layer to distinguish luminal and abluminal compartments is also quite feasible. Additionally, it allows co-culture with other cell types of BBB, such as astrocytes and pericytes. The coculture system has been shown to retain some of the in vivo features of the BECs. Primary cells derived from mouse, rat, porcine, or bovine brains are used in the most thoroughly investigated models [84]. These BBB models are static and do not replicate the shear stress that is often produced in vivo by blood flow. However, due to the simplicity of their creation, static BBB models are valuable tools for initial permeability research [85].
3.2. In vitro 3D BBB models
Due to the complicated in vivo physiology, 2D BBB models frequently fail to predict human drug responses, making it extremely difficult to undertake mechanistic experiments. These limitations highlight the need to develop in vitro systems reflecting the human BBB’s prime physiological structure and functions. However, establishing how other cells of BBB, such as astrocytes, contribute to the stability and disruption of the BBB into in-vitro models is still challenging. Some evidence suggests that Lipocalin-2 (LCN2) and Serpin Family A Member 3 (Serpina3) are two genes that are found altered by reactive astrocytes. Therefore, in vitro BBB models incorporating healthy and reactive astrocytes to enhance model usefulness for CNS disease modeling were generated [86]. The tight endothelial barrier function has been attempted to be replicated by an established in vitro human BBB-on-chip models using a variety of platforms, including monolayer of brain ECs and co-culture with astrocytes and pericytes in a 3D milieu. Recent studies on the BBB have used a 3D culture of the endothelium, pericytes, and astrocytes to recreate direct cellular interactions, leading to barrier function with reduced permeability than in earlier monoculture or coculture studies. Choi et al. created a 3D model of the BBB on a microfluidic device using this model. They demonstrated that the BBB prevents dye leakage and migration of immune cells toward chemoattractant. Additionally, they confirmed that TJPs, including ZO-1 and VE-Cadherin, were located at endothelial cell interfaces. They further investigated the BBB’s disruption by neuro-inflammatory mediators and ischemia circumstances, measuring the protective effect of antioxidant and ROCK-inhibitor therapies [87]. To understand the transmigration/trafficking of immune cells more precisely, a new model with hollow fibers and a vascular micro aperture of 2–4 m was developed. However, this BBB model is very complex and has several drawbacks. For instance, we cannot directly visualize the endothelial morphology on the luminal side, it requires a large number of cells (>1 × 106), and the steady-state TEER establishment takes a longer time (9–12 days) compared to a simple co-culture model (3–4 days). Further, it cannot be used on large-scale screens due to these drawbacks. However, this model aids in the validation and optimization of novel drugs [88]. Other than the BBB integrity, the presence of functional efflux pumps governs the drug penetration into the brain. Without these efflux mechanisms, the in vitro BBB does not reflect the exact in vivo functional features. It was shown that Madin-Darby Canine Kidney (MDCK) model could be used as a surrogate for BBB (tight, adherent, and active P-gp). This model was tested in situ rat brain perfusion study and was found very useful in predicting the CNS-penetration potential of drugs. Furthermore, Sherman et al. combined the Corning® HTS Transwell®–96 tissue culture system and Corning 96-well spheroid microplates to generate a 3D BBB model for evaluating label-free transport and brain tumor cytotoxicity assays. This simple 3D high-throughput assay allows for a more thorough experiment and may be customized to cell types and screening applications [89]. Some of the 3D BBB models are also available as commercial kits (e.g., RBT-24H, BBB Kit), to speed up the BBB transport studies.
3.3. Dynamic in vitro (DIV) model—Microfluidic vascular network
As mentioned, shear forces generated by blood flow dynamics in the capillaries also affect the BBB functionality. Therefore, a dynamic in vitro (DIV) model of the BBB was established using a synthetic capillary with flowing media. A fibrous gas-permeable silicone tubing was used for framing for co-culturing cells and making channels. The luminal side was lined by ECs, while the outer surrounding was lined by supporting cells (pericytes and astrocytes). The coculture self-assemble into a flat, optically accessible shape resembling the circular vasculature of that of capillaries. The media was perfused by a peristaltic pump mimicking the appropriate in vivo physiologically. Thus, the DIV model provided both the shear force resulting from fluid flow and the biological interactions of ECs with supporting cells. Both of these fundamental stimuli could be independently attributed to maintaining the barrier phenotype. By combining three different human cell types (iPSC-EC mixed with primary brain pericytes and astrocytes). Campisi et al. generated a 3D cocultured in a cast made up via a soft lithography process [90]. The cast was composed of polydimethylsiloxane (PDMS; Sylgard) and produced the first capillary-like vascular architectures. The geometric architecture of the micro-vasculature networks was characterized using confocal imaging in terms of a) lateral and transverse vessel diameters, b) a percentage of image area containing vessels, and c) total branch length. In contrast to iPSC-ECs in monoculture, contact with pericytes or the conditioned media generated smaller, rounder, and heavily branching vasculature. Tri-culture with astrocytes further increased this interconnected and branched architecture. The lateral vessel diameters were smaller in tri-culture (42 μm) compared to iPSC-ECs in monoculture (108 μm) and co-culture (64 μm), indicating a greater circularity in tri-culture compared to more elliptical vessel shapes in monoculture. Accordingly, the average branch length was reduced in triculture (136 ± 24 μm) from monoculture (266 ± 40 um) and coculture (179 ± 31 μm). Vascular networks resembled in vitro vessel morphology more because they were more linked, thinner, and covered less space. The immunostaining and mRNA expressions of ECM proteins (laminin and collagen IV) and junction proteins (ZO-1, Ocln, and Cldn-5) were found elevated in triculture, indicating robust barrier functions. Dextran tracers (40 kDa) with fluorescent tags were introduced, and permeability coefficients were determined using confocal imaging. Dextran’s permeability coefficient was gradually reduced in tri-culture, 0.89 × 107 cm s−1 compared to 6.6; and 2.5; × 107 cm s−1 in mono and co-culture, respectively. This study confirmed that the permeability of the tri-culture model was lower than other previously studied microfluidic models. Applications for these microfluidic models range from investigating vascular interactions with inflammatory cells in various diseases, extravasation of metastatic cancer cells, and BBB permeability assays of fluorescently labeled nanocarriers. 3D printing has advanced the development of complex DIV models resembling that in vivo. However, fluidic methods are not well established and not suitable for high throughput studies.
3.4. Organoid culture
Organoids are the structural units generated by growing stem cells in 3D to form organs or tissue that partially resemble the organ’s structure and function. The main concept is to promote the development of pluripotent stem cells (PSCs) or adult stem cells (AdSCs) into the appropriate tissue or organ [91]. Since emerging BBB organoids may provide much information equivalent to in vivo experiments. Organoid in vitro models, highlighting BBB sensitivity to neurological disease and drug delivery, might be used to study the complexities of the neurovascular unit and its physiological restrictions in more detail [92,93]. Moreover, organoids simulate endothelium permeability and model the direct physical association of cells and drug interaction to the brain cells. Although we cannot measure the TEER value or drug permeability across the organoids simply because they are clusters of cells. We can quickly assess the kinetics of drug penetration using techniques like fluorescent drug labeling or MALDI-MSI. There is also a potential to incorporate fluid flow into spheroids with ease of transwells, which are readily available.
Several organoids have been created from human forebrain or cerebral cells to represent GBM and Medulloblastoma (MB). Bian et al. created neoplastic cerebral organoid, a 3D in vitro model (neoCOR) [94] to simulate the development of brain tumors. They produced neoplasms that resembled GBM and CNS primitive neuroectodermal tumor (CNS-PNET) by introducing oncogenic mutations utilizing molecular biology tools like CRISPR-Cas9. These organoids were suitable for studying tumor biologics such as invasiveness and drug efficacy. Furthermore, Ogawa et al. developed a GBM organoid to study tumor initiation and continuous microscopic observations. They disrupted the tumor suppressor gene TP53 and introduced oncogene HRas(G12V)-IRES-tdTomato construct using CRISPR/Cas9 [95]. These transformed cells were not only formed highly aggressive spheroids but also invaded nearby organoids. Furthermore, these organoids maintained mesenchymal phenotype with invasiveness when orthotopically implanted into immunodeficient NSG mice. Generating MB organoids using cerebellar cells is a slightly difficult. Notably, Ballabio et al. generated MB organoids using human iPSC. They observed that only cells which were electroporated with Gfi1 + MYC-c (GM) and Otx2 + MYC-c (OM) genes were able to produce organoids. Gfi1 is a Zink finger transcription factor that stabilize and increase cMYC protein, whereas Otx2 and MYC cooperatively enhance the gene expression levels in MB. Consequently, GM and OM organoids showed proliferating cell nuclear antigen (PCNA) and Sox9-positive markers with a fewer β3-tubulin-positive cells compared with control cells. These results indicate that both GM and OM genes initiate the proliferation of human cerebellar progenitors and reduce their differentiation potential [96]. These examples clearly indicate that not organoid forming cells could be induced to do so by manipulating their genetic signature.
4. Opportunities for drug delivery to cross the BBB
Depending upon the physicochemical and structural properties, a molecule can cross the BBB by either transcellular or paracellular pathways. Lipophilic molecules can easily interact with plasma membrane and generally acquire the transcellular pathways to cross the BBB. Conversely, hydrophilic molecules cannot penetrate through the cell membrane and penetrate via paracellular pathways or through carrier proteins at the TJs. However, carrier mediated pathways are saturable and structure specific. In some studies, it was also shown that using chemicals or oligonucleotides the TJ can be reversibly opened allowing paracellular uptake of molecules [97]. As previously mentioned, the BBB contains a wide range of transporters, including influx transporters like the sodium-coupled glucose transporters (SGLTs), monocarboxylate lactate transporter 1 (MCT1), cationic amino acid transporter 1 (CAT1), choline transporter (ChT), and l-type amino acid transporter 1 (LAT1). Efflux inhibition or using drug itself as an influx substrate can be utilized for improved drug distribution in the brain. The transporter-mediated uptake is roughly ten times faster than transmembrane diffusion. Efflux inhibitors such as sildenafil could avoid the exit of drug already penetrated in the brain thus help in the concentration build up. Sildenafil is a BCRP inhibitor and was tested for its ability to enhance anticancer drugs docetaxel and topotecan into the brain. It was found that BCRP and P-gp KO mice showed higher brain penetration without sildenafil. Interestingly, sildenafil concentration was also found higher in BCRP and P-gp KO mice showing it is a substrate of these transporter proteins.
RMT also represents a promising method of transcytosis. Several receptor transporters that help supply brain the useful biomolecules from peripheral blood could be used for transport of drug or carrier across the BBB.
For example, the insulin receptor (IR) transports the required quantity of insulin in the brain despite it is not produced there. Similarly, iron is transported from the blood across the BBB through the transferrin receptor (TfR), and apo-transferrin is transported from the brain to the peripheral circulation. Further, numerous other receptors including the leptin receptor (LepR), neonatal Fc receptor (FcRn), and low-density lipoprotein receptor (LDLR) have also been identified on the brain epithelium, which may transport substrate across the BBB. This section will discuss various approaches to enhance drug transport through BBB (Fig. 2).
Fig. 2.
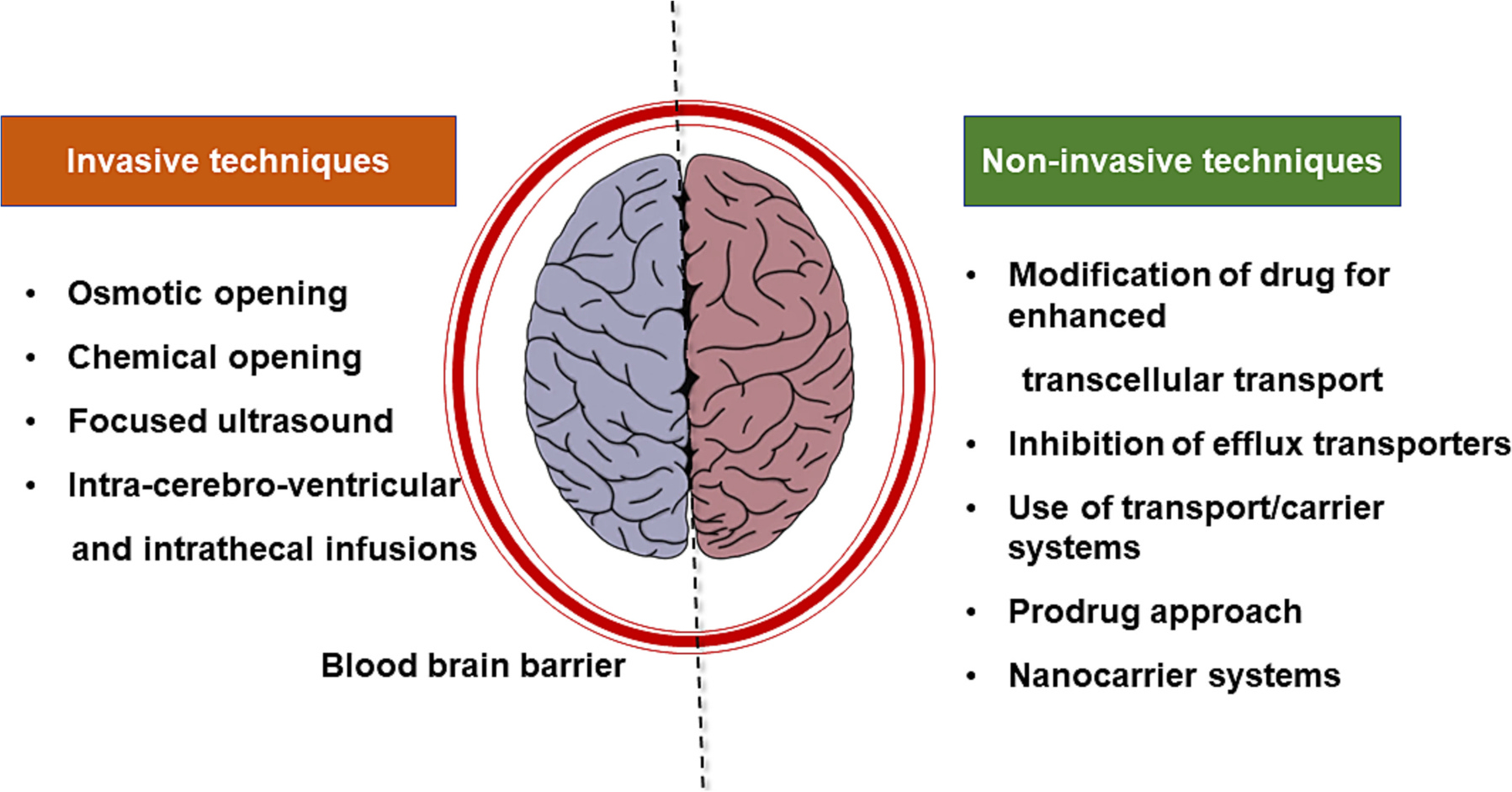
Strategies to improve therapeutics across the blood brain barrier.
4.1. Design of BBB penetrating drug molecules
Different approaches used for designing small molecules for enhanced BBB penetration will be discussed in this section. The majority of CNS-active drugs are small molecules. By enhancing diffusion, reducing efflux, and activating carrier transporters, small molecule structures may be modified to cross the BBB (Table 1). Strategies for increasing BBB penetrability of a drug may include structural changes that modulate passive diffusion or active transport. Lipinski’s rule of five (RO5), are widely implemented for calculating permeability based on physicochemical factors. Later, various techniques were employed to refine these rules to improve the potential druggability of substances.
Table 1.
Structural attributes of drug molecules for BBB penetration.
A multiparameter optimization (MPO) approach, for instance, was created by Wager et al. by combining six physicochemical parameters [104]. The CNS MPO technique is advantageous for extending the design space for CNS medications and considering drug absorption, distribution, metabolism, and excretion (ADME) properties along with BBB penetrability since it can balance numerous factors while avoiding rigid cutoffs.
Using high-throughput screening, Brand et al. developed a lead compound 3 (DDD85646) (Fig. 3) [102]. The molecule DDD85646 demonstrated significant activity against N-myristoyl transferase (NMT). The inhibition of NMT is a possible therapeutic target for the parasite protozoa Trypanosoma brucei, which is known to be responsible for causing Human African Trypanosomiasis (HAT). However, it was not BBB penetrant (Kp < 0.1). Wyatt and co-workers recently sought to design and discover a series of derivatives of compound 3 with improved BBB penetrability. The study reported that the low brain exposure was attributed to high topological polar surface area (tPSA) (89 Å2) and low lipophilicity (cLogP = 1.79). Based on this consideration, compound 5 was synthesized. Compound 5 showed remarkable inhibitory activity, good microsomal stability, and significant levels of brain penetration.
Fig. 3.
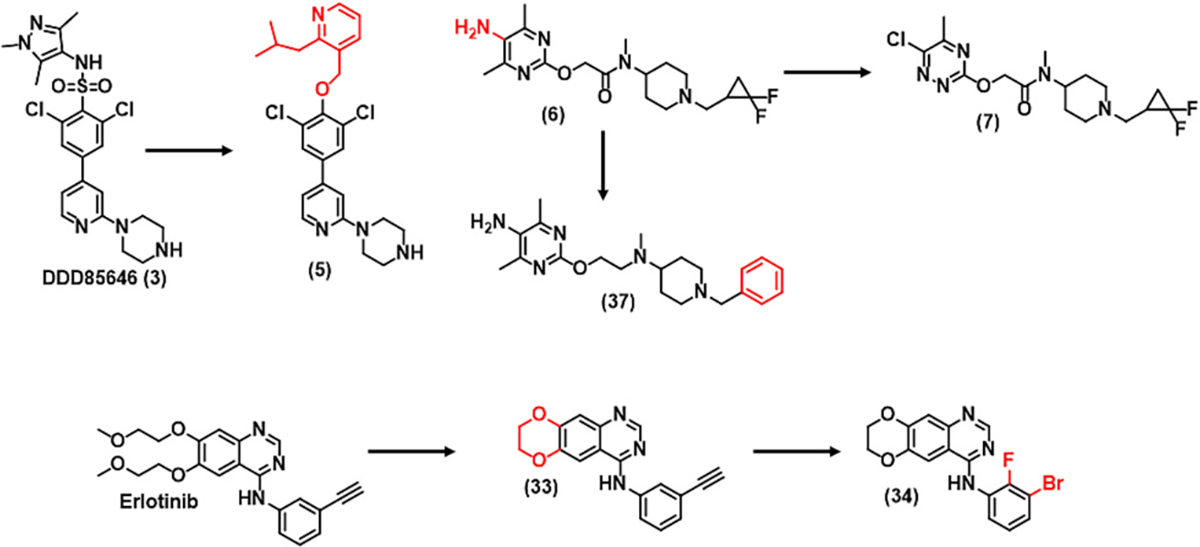
Drug molecules designed to penetrate the blood brain barrier.
RO5 defines the hydrogen bond donors (HBD) of desirable drug candidates as <5, while CNS drugs usually have fewer HBDs (<3). In recent research, reducing HBD capacity has been one of the most frequently utilized drug design strategies for improving BBB penetration. For example, Sakai et al. replaced the 5-aminopyrimidine of 6 with 3-chloropyridazine to gain a fibroblast growth factor (FGF) receptor modulator 7 with improved brain exposure [total brain-to-plasma partition ratio (Kp) = AUCb/AUCp] and reduced risk of phospholipidosis [100]. Modulator 7 may have the potential to imitate the biological activities of basic FGF, such as neuroprotective and cell proliferative activities, important for neurodegenerative diseases.
Fushimi et al. explored the removal of hydrogen bond acceptors (HBAs) and HBDs of compound 8 that did not form essential interactions with the anaplastic lymphoma kinase (ALK) active site-removing one of the HBDs to obtain compound 10 significantly inhibited MDR1 efflux, indicating a significant improvement in brain exposure. When a methyl group shielded the oxygen atom on the morpholine ring, the MDR1 efflux ratio further decreased [101].
The functional groups on the molecule can greatly impact the lipophilicity and permeability. For example, fluorine is known to enhance molecule lipophilicity and also improve metabolic stability by occupying the site of oxidative metabolism. Furthermore, fluorine can be easily introduced into a molecule without significantly altering the steric environment because of its small size. For instance, Pettersson et al. found that a high MW due to the introduction of fluorine does not lead to increased MDR-ER liability. Crizotinib is an anti-cancer drug with low CNS penetration that is ineffective against metastatic tumors in the brain. Radaram et al. improved crizotinib’s brain permeability by adding fluoroethyl moiety. At 5 min after injection, the [18F] fluoroethyl crizotinib ([18F]2) showed accumulation of 6.6% ID/cc, compared to only 0.2% ID [99].
Even some of lipophilic drugs are ineffective across the BBB due to the efflux by P-gp and BCRP receptors. These proteins are significantly expressed at the luminal side of the BBB. Efflux ratio (ER), an indicator of efflux occurrence is calculated as Papp (B − A)/Papp (A − B), when ER > 2.5, the experimental results imply a high possibility of efflux [98]. Cells expressing high levels of these receptors such as MDCK-MDR1 and MDCK-BCRP are quite useful in evaluating transporter-mediated efflux mechanism [101,105]. pKa is known to affect the efflux mechanism where drugs with higher pKa are substrate of efflux pumps. Therefore, efflux could be obstructed by reducing the drug pKa value. For example, initially, the ER of original lead compound 37 (Fig. 3) was 6.3, while with slight modification molecule 6 was found not a substrate for P-gp (ER = 1.1). These observations underline that the reduction in basicity of compound 6 avoided its recognition by P-gp receptors.
Erlotinib is a potent t TKI used in several cancer types. However, erlotinib due to its poor BBB penetration is not suitable for treating brain tumors such as GBM. It was found that the flexible alkyl ether tails, a large non-rigid bonding (NRB) (10) and high tPSA (75 Å2) in erlotinib structure were responsible for its poor brain penetration [103]. When the flexible alkoxy chains were closed to form a 1,4-dioxane ring fused to the quinazoline scaffold resulted in compound 33, with a reduced NRB (10 → 2) and tPSA (75 → 56 Å2). These structural modifications increased the BBB penetration of compound 33 close to 10 folds compared with parent compound. Furthermore, with introduction of haloalkyl substitution resulted in compound 34 (JCN037) with further increased BBB penetration (Kp, uu =1.30). When tested in cell culture and orthotopic xenografts model of brain tumor compound 34 displayed high potency. Although a better BBB penetration is resulted with higher lipid solubility. However, it may also enhance oxidative metabolism and unfavorable drug distribution. Therefore, increasing lipophilicity must be carefully balanced with membrane permeability and metabolism.
4.2. Modulation of BBB tight junction
TJs of BBB prevent potential neurotoxins in the peripheral blood from attacking neurons. Therefore, several strategies, including chemically, radiation, osmotic agents, and surgical procedures, have been tried to improve drug delivery through BBB. Some preclinical and clinical examples are discussed in this section.
4.2.1. Chemical disruption
Disturbance of TJs in the BBB by modifying the surface of NPs is one possible strategy for improving drug penetration into the brain. Some peptides, such as FD7 peptide (FDFWITP) and cyclic (CD) peptide (CDTPPVC; abbreviated as CCD peptide), are known to disrupt the BBB TJs resulting in increased drug penetration. FD7 peptide binds to the Cldn proteins and disturbs the TJs of the BBB. On the other hand, the CCD peptide interacts with the EC1 domain of E-cadherin proteins found on cell-cell adhesion junctions and prevents vascular ECs from forming the BBB. Based on these facts, Lo et al. prepared Afatinib-loaded lipid nanoparticles (LNPs) coated with FD7 and CCD peptides to enhance delivery across the in vitro BBB model [106]. Afatinib is an EGFR-TKI with low BBB penetration ability. LPN coated with TJ-modulating FD7 or CCD enhanced Afatinib’s cytotoxicity on PC9 non-small cell lung cancer (NSCLC) cells across the BBB [106].
Bradykinin (BK) is a vasodilator and increases vascular permeability in general. BK has also been shown to enhance BTB permeability through enhancing caveolin protein concentration and ramping up the transcellular pathways. For example, Liu et al. showed that caveolin-1 and caveolin-2 levels increased within 5 min post-BK treatment in the C6 rat brain glioma model. This effect lasted for around 15 min, and the BTB permeability increased significantly during this period. Further, other studies show that BK also increases the number of pinocytotic vesicles in ECs [107].
Neurotransmitters (NTs) are endogenous chemicals released at the neuron junction and enable neurotransmission. NTs are mainly of two types: small molecules, i.e., dopamine and glutamate, and neuropeptides such as insulin and oxytocin. Small-molecule NT acts directly on neighboring cells, while the neuropeptides control the communication at the synapse. Researchers have identified more than 60 distinct types of NTs in the human brain, and some of these NTs were even shown to cross the BBB. Based on these BBB crossing properties of NTs, Ma et al. used NTs-derived synthetic lipids for delivering cargo into the brain. They synthesized several lipids conjugated NTs termed “NT-lipidoids” by simply reacting the alkyl acrylate in the lipids with a primary amine in the NT. From the various NTs, the tryptamine-conjugated lipidoids (NT1-lipidoids) could penetrate the BBB efficiently. Lipid NPs (NT1-LNPs) incorporating the NT1-lipidoid also crossed the BBB. The NTs tryptamine, phenethylamine, and phenylethanolamine lipidoids were synthesized in the initial comparison. In an aqueous solution, the resulting NT-lipidoids were amphiphilic and self-assembled into micelles of the average hydrodynamic size of 180 ± 10 nm. The formulation NT1-O12B, where tryptamine conjugated with a lipid chain of C12 carbon, was found most suitable for brain delivery. When the BBB-impermeable non-targeted lipid NPs (NT-LNPs) were mixed with a 10% (w/w) of NT1-O12B, significantly increased transport of IR fluorescent dye across the BBB within 1 h of intravenous injection. They were able to deliver a variety of molecules across the BBB using NT1-LNPs, which are otherwise impermeable, including the drug amphotericin B (AmB), an antisense oligonucleotide (Tau-ASOs), and an mRNA of green fluorescent protein (GFP)–Cre. This study demonstrated that these NT-1 LNPs facilitated the delivery of functional cargo across the BBB and protected it from degradation [108].
4.2.2. Focused ultrasound (FU)
Ultrasound waves can be directed intensely into BBB tissue without harm and temporarily disrupt it when combined with circulating microbubbles (MB). The mechanical interaction between low-intensity focused ultrasound (FUS), and microbubbles disrupt the TJ and enhance delivery in small animals’ brain parenchyma. Further, this technique has also been quite effective in rhesus macaques, as shown by McDannold et al. [109]. In their study, the BBB disruption was assessed by T1-weighted images using a BBB impermeable MRI contrast agent gadolinium diethylenetriaminepentaacetic acid (Gd-DPTA). Any tissue damage which might have occurred as determined by comparing T2*-weighted images taken before and after treatment. It was well tolerated in all animals, and complete recovery was obtained quickly. More importantly, even after multiple injections, animals did not lose their ability to conduct complex visual acuity tasks indicating that the membrane disruption was reversible.
RNA interference (RNAi) and CRISPR-based therapeutics could revolutionize tumor therapy. The short half-life in vivo due to esterase, poor brain absorption due to large molecular size, low cellular uptake due to negatively charged backbone, rapid clearance by the kidney, and high endosomal degradation make it difficult to administer these therapies effectively [110]. Guo et al. recently formulated cationic NPs for siRNA to increase retention time and improve cellular uptake. To enhance RNA-loaded NPs in brain tumors, they used MB-FUS and successfully delivered a siRhoB, a fluorescently tagged siRNA, and Smoothened (SMO) siRNA for treatment of glioma and medulloblastoma mouse models [111].
4.2.3. Radiotherapy (RT)
The integrity of the BBB may be affected by a clinical dose of radiation. Cao et al. used MRI to investigate the impact of RT on BBB/BTB status throughout high-grade gliomas. Gd-DTPA based contrast agent was administered to sixteen patients with grade 3 or 4 supratentorial malignant glioma before, during, and after conformal RT. After roughly 10 Gy (P.01), there was a significant increase in permeability of Gd-DTPA in the tumor region, which maximized after receiving approximately 30 Gy. On the other hand, the healthy brain revealed trivial alterations during and after irradiation. These findings suggest that during RT, BTB permeability is increased in the tumor region but not in the healthy brain [112].
4.2.4. Convection-enhanced drug delivery
Convection-enhanced delivery (CED) is a potential method for delivering medication to the brain. The drug is delivered throughout the tumor via convection, creating a local positive pressure gradient. This approach has two key advantages: medicine may spread in a wider tissue section owing to convection forces, and it provides a consistent rate of drug infusion inside the tissue. In this technique, a catheter is stereotaxically inserted into a brain tumor to deliver the drug directly. Upadhyayula et al. co-infused Gd to monitor the real-time drug disposition amid CED. This method can be easily adapted for multiple disease types, repeated injections, and drug combination regimens [113].
4.2.5. Hyperosmotic agent
Intra-arterial (IA) injections of hyperosmotic agents such as mannitol, arabinose, and hypertonic urea have permeabilized the BBB. These substances absorb water from ECs and transport it into blood arteries, causing them to contract and disrupt TJs [113]. However, due to high variability, the reproducibility of this method, especially in repeated therapy, is low. Further, restoration of normal TJ functional can vary, and prolonged disruption can cause severe side effects such as transient aphasia, hemiparesis, or edema-induced intracranial herniation. Chu et al. reduced this variability under the guidance of interventional MRI. The approach must induce blockage of the middle cerebral artery in small animals. This is an essential technique for studying the effects of BBB on drug delivery [114].
4.2.6. Polymer wafers
Gliadel wafers (GW) are made up of biodegradable polymer containing the drug carmustine. These are implanted in the brain at the site of GBM resection, and they provide a source of local drug delivery to the brain. However, their effectiveness in GBM therapy is controversial. While some studies show their effectiveness, others highlight their questionable survival benefit and potential side effects. A systematic survey by Bregy et al. concluded that in newly diagnosed GBM patients, the use of GW led to overall survival (OS) of 16.2 months vs. 14 months in the control group that underwent surgery and adjuvant chemoradiotherapy. The adverse event ratio in the GW group, on the other hand, was 42.7%, demonstrating that GW has a significant risk of side effects [115].
4.3. Receptor-mediated transcytosis (RMT)
Several proteins/receptors are expressed on the BBB as well as in meningeal blood arteries and chimeric polymersomes (CP). These receptors transport essential nutrition and signaling molecules from blood to the brain. One approach is to take advantage of these receptors to increase drug or nanoparticle interaction with BBB ECs and enhance uptake. Several receptors include Trf, low-density lipoproteins (LDLs), rabies virus glycoproteins (RVG), and many more. The most significant advantage of this approach is that it may be applied to a wide variety of targeting agents such as antibodies, peptides, neurotransmitters, and aptamers. Further, it is a noninvasive method; therefore, it could be used repeatedly. In this section, we will discuss studies that have utilized RMT to enhance drug penetration through the BBB. LRP and LRP-related proteins 1 (LRP1 and LRP2) mediate the transport of several proteins, including lactoferrin and melanotransferrin, tissue-type plasminogen activator, aprotinin, bikunin, and secreted β-amyloid precursor protein (APP) across the BBB. LRP1 is essential in the internalization of different molecules and is involved in several other signaling pathways [116]. Some reports also suggest that LRP1 affects the TJs and thus regulates the BBB permeability. Proteins lacking the Kunitz protease inhibitor (KPI) domain is a poor substrate for LRP. Based on these, Regina et al. identified a brain penetrating peptide Angiopep-2 (TFFYGGCRGKRNNFKTEEY) containing Kunitz domain for drug delivery [117]. Paclitaxel (PTX) is a cornerstone of several peripheral cancers; however, its use in CNS tumors is limited due to being a P-gp substrate. Therefore, Demeule et al. prepared an Angiopep-2 conjugate containing three PTX molecules to enhance its brain delivery. The conjugate increased PTX concentration (>50-fold) in brain metastases studies [14]. LRP1 is also expressed on some tumor cells in CNS and systemic metastases. Therefore, ANG1005, after gaining entry via LRP1, released PTX upon cleavage by lysosomal esterase. ANG1005 was also shown to be effective in individuals with leptomeningeal carcinomatosis and recurrent brain metastases of breast cancer [14].
Further, to understand the structural activity relationship (SAR) and increase target specificity, several modifications of Angiopep were evaluated. Replacing the cysteine at position 7 with serine to prevent peptide disulfide bonds with serum proteins, Angiopep-2 was synthesized. Angiopep-5 was created by placing the arginin residue at position 10 of Angiopep-2, and further Angiopep-7 was created by placing the arginine at position 15 of Angiopep-5. Each of these peptide analogs had a net charge value of +2. The cellular uptake study showed that only Angiopep-2 retains its binding efficacy and parenchymal transport [118]. Notably, the transportation of Angiopeps in the brain was unaffected by the P-gp efflux pump.
MMPs are the metzincin-containing endopeptidases with the function of degrading ECM proteins such as collagen. MMPs are implicated in the progression and metastases of various tumors [119]. In humans, twenty-three different MMPs have been identified. MMP-9, also known as gelatinase, is expressed in the nervous system, and its levels are elevated in traumatic brain damage and other neurological illnesses [120]. To downregulate MMP-9 activity, Islam et al. used NPs loaded with its selective inhibitor peptide (CTTHWGFTLC) to improve brain targeting and decrease other side effects [121]. They designed an amphiphilic hybrid peptide by conjugating brain-targeting ligands (HAIYPRH or CKAPETALC) on the C terminal and cholesterol at the N-terminus of an MMP-9 inhibitory peptide (Chol-GGGCTTHWGFTLCHAIYPRH). The peptide was self-assembled with an average particle size of 200 nm. In an in vitro model, the amphiphilic peptide readily crossed the BBB.
The BBB capillary ECs also express ligand-gated ion channels called nicotine acetylcholine receptors (nAChRs). Ligand binding to nAChRs could be utilized for BBB cargo transport. A 16-amino acid peptide LCDX (FKESWREARGTRIERG) was designed to bind to nAChRs and transport micelles through the BBB [122]. To further improve its stability against proteases, a retro-inverse isomer of LCDX was designed termed DCDX. Experimentally, it was confirmed that both DCDX and LCDX functioned as a competitive antagonist of nAChRs in a dose-dependent manner. The docking analysis showed that both peptides bind to receptors by electrostatic, cation–π, and hydrophobic interactions. DCDX peptides were conjugated to Rhodamine B-loaded liposomes for brain-targeted delivery. Lysosomal and serum stability of D-peptide with higher transcytosis efficiency than parent L-peptide in vitro BBB monolayer. Further, DCDX decorated liposomes improved the therapeutic efficacy of doxorubicin in xenograft nude mouse models of human GBM.
5. Nanomedicine for brain delivery
NPs are an intriguing tool potentially able to enhance drug transport across the BBB/BTB. NPs could be customized for functionalities and for carrying diverse payloads. Nanocarriers avoid drug efflux transport, shield from metabolism, and bypass TJs of encapsulated drugs. The multifunctionality of NPs for brain medicine may make it easier to target the BBB while also improving permeability. The drug permeability across the BBB, when encapsulated in NPs is determined by the physicochemical and biological properties of the NPs rather than the chemical structure of the drug. Biodistribution of NPs is significantly influenced by their physical characteristics, including particle size, shape, surface charge, and functional groups. For improving the transport of NPs across the BBB and enhancing cellular uptake the brain tumor cells by decorating NPs with targeting ligands such as antibodies, peptides, small compounds, and aptamers have been investigated (Table 2). The use of peptides as targeting ligands is emphasized since these molecules are less expensive, less immunogenic, and have more chemical diversity than significant proteins like Trojan horse antibodies. Various mechanisms by which small molecules and NPs penetrate the BBB are shown in Fig. 4.
Table 2.
Different nanomedicine for improving BBB transport.
| SN | Targeting ligand | model/Species | Disease | Outcome | Reference |
|---|---|---|---|---|---|
|
| |||||
| 1 | LRP | mice | MB | Improved efficacy | [123] |
| 2 | Angiopeps | In vitro, mice, human | Normal | High permeability | [118] |
| 3 | nAChRs | Nude mice | GBM | Improved efficacy | [112] |
| 4 | TfR | In vitro and mice | GBM | Increase in survival | [124] |
| 5 | Chlorotoxin | Mice | Inflammation | Imaging, efficacy | [125] |
| 6 | MiniAp-4 | BBB model, mice | Normal | Increased permeation | [126] |
Fig. 4.
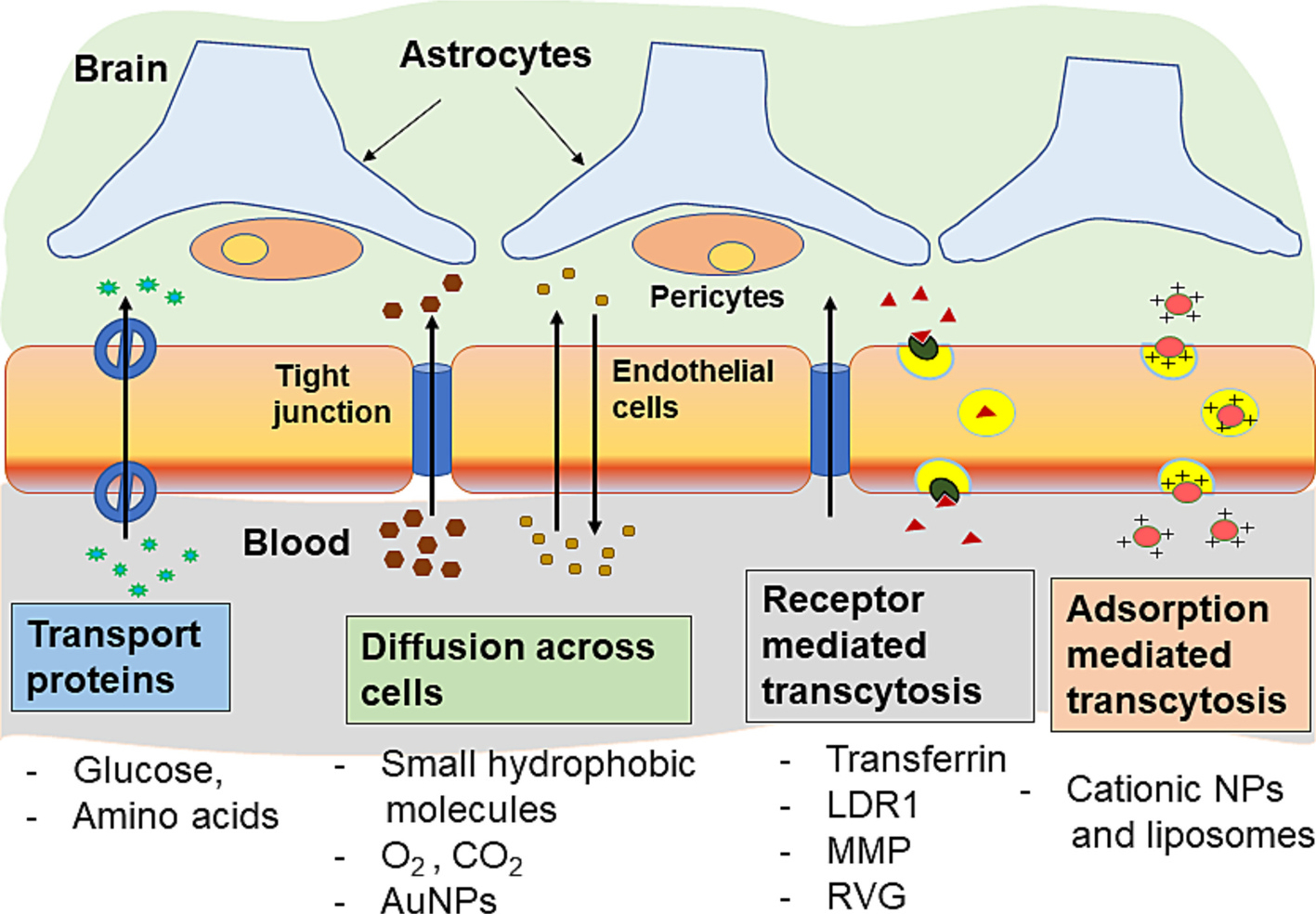
NPs uptake mechanisms of different molecules and carrier particles across the blood brain barrier.
5.1. Ideal characteristics of NPs for brain delivery
5.1.1. Composition of NPs
The composition of NPs governs its inherent properties such as size, shape, drug loading, stability, and interaction with biological membranes. Lipid and polymer-based nanocarriers (liposomes, micelles, and NPs) could impact their interaction with complement systems and accumulation at a specific site. Further, types of decoration of NPs surfaces may also influence complement activation.
5.1.2. Particle size
The particle size of NPs is a critical factor in its penetration to the brain. Generally, a smaller size favors BBB penetration. The reason is that smaller NPs may penetrate the brain parenchyma, while the larger particles are more likely to stay within the arteriole. For example, 24 nm NPs have been demonstrated to cross the glia limitans but not 100 nm NPs. Further, with the influence of the convective flow of the interstitial fluid (ISF), small NPs may leave the brain, while larger NPs release their payload in the basement membrane due to MMP-9 enzymatic breakdown. Consequently, the payload might be transported to the brain parenchyma through convective CSF flow. Pedersen et al. used dextran-coated NPs of various diameters (250 and 600 nm) and human IgM antibodies conjugated to dextran to study the role of NP curvature in complement activation. Interestingly, 250 nm-sized NPs were shown to be more powerful activators of an antibody-mediated complement than 600 nm particles [127].
5.1.3. Surface charge
The interaction of NPs with BBB ECs and complement system activation have both been demonstrated to be influenced by the surface charge. For instance, negative and positively charged NPs activate the complement system more efficiently than their neutral counterparts. This observation has led to the development of nanomaterials such as polypropylene sulfide NPs, ionizable lipid nano-capsules, NPs based on polycations and cyclodextrin, as well as polystyrene nanospheres. In a porcine model, Szebeni and colleagues discovered that negatively charged NPs cause complement activation-related pseudo allergy (CARPA) [128]. However, employing polymer NPs, Tran et al. demonstrated that positive surface charge also triggered complement activation [129]. Misra et al. investigated the effects of several NP properties on utilizing carbon spherical NPs [130]. It has been reported that methoxy polyethylene glycol grafted liposomes cause acute non-IgE mediated hypersensitivity responses. The anionic phosphate‑oxygen moiety of the PEGylated phospholipid is assumed responsible for complement activation. It is noteworthy that complement activation by PEGylated liposomes was inhibited by methylation of this phosphate oxygen moiety [76].
5.1.4. Polydispersity
Complement activation can rapidly eliminate NPs from circulation. It was observed that complement activation is induced more by NP aggregates than individual particles. The effects of particle size and surface charge of NPs on hemocompatibility were examined by Mayer et al. [131]. A positive surface charge induces NP aggregates and leads to complement activation. Further, Fülöp et al. recently explored the roles of iron core composition and particle surface coating on CARPA [132]. They discovered that SPIONs coated with dextran (Sinerem®) and carboxymethyldextran (Resosvist®) significantly increased complement activation, whereas the SPIONs coated with citric acid, phosphatidylcholine, starch, and chitosan had no such impact. Further, Sinerem® was found more potent complement activator due to its multimodal size distribution with a large percentage of aggregates [67,133].
5.1.5. Surface characteristics/modifications
Decorating the particle surface with hydrophilic molecules, including PEG and dextran, is a popular strategy to minimize NP clearance from circulation by the reticuloendothelial system (RES). This method has been helpful in clinical pharmacology for more prolonged systemic circulation. However, published findings indicate a slight danger that this mechanism can activate complement, resulting in an anaphylactic response.
5.1.6. Cytotoxicity, hemolysis, and immunogenicity
It is critical for brain administration that NPs be safe for long-term usage and do not cause cytotoxicity or immunogenicity in neurons. Therefore, in vitro assays of CNS targeting NPs might be done using neuronal cells. Studies to test the effects of NPs on neuronal viability or activities, change in cell metabolism, and gene expression may be used to ensure the safety [134].
5.2. Non-targeted NPs
Some NPs have been reported to cross the BBB without any targeting ligand. Gold and silica NPs, for example, have been found to enter the brain and concentrate in neurons even without any functionalization, via a method like adsorption-mediated transcytosis or under the influence of an external driving force (i.e., magnetic).
5.2.1. Non-magnetic NPs
Hwang et al. prepared polyoxazoline (Pox) triblock polymer to deliver hedgehog (Hh) pathway inhibitor vismodegib across the BBB. Vismodegib downregulates the hyperactivated Hh by binding to smoothened and was approved by the FDA to treat basal cell carcinoma (BCC). MB patients also show Hh activation, but vismodegib has various limitations, such as low potency, low water solubility, and bone toxicity. To overcome these hurdles, the author used polyoxazoline polymer to encapsulate vismodegib. Polyoxazoline-vismodegib micelles were formulated by film hydration. NPs were stable upon storage at 4 °C and during lyophilization. A genetically modified mouse model of medulloblastoma SmoM2 mice, was used to test the effectiveness of vismodegib-loaded poly(2-oxazoline) (POx-vis) NPs. Smo mice develop MB by 8–10 weeks and, if untreated, die of tumor progression. The findings revealed that non-targeted NP formulations have the potential to enhance systemic brain tumor treatment in general and vismodegib therapy for SHH-driven malignancies. These non-targeting NPs have advantages as these NPs will not bind to any specific cancer receptors and keep circulating into the bloodstream without invading the CNS. These NPs have a dense hydrophilic outer layer compared to PEG [135].
5.2.2. Magnetic field-derived NPs
Magnetic NPs (MNPs) are now employed in various biological applications, including as an MRI contrast agent, a hyperthermic cancer therapy, cell labeling and sorting, and magnetoreception. However, magnetically induced displacement force may cause tissue damage, and FDA guidelines direct that magnetic field intensity should be about 30–50 cm working distance from the patient. As a result, the utility of MNPs in the brain has been relatively limited (8 T for adults, 4 T for children).
MNPs do not penetrate the BBB without surface modification with a targeting ligand or the influence of an external magnetic field. Rousseau et al. observed that superparamagnetic iron oxide nanoparticle (SPIONs) could only infiltrate the BBB when it was impaired by intracarotid injection of mannitol. Therefore, nowadays, three basic strategies are applied for MNPs: one, attachment of functional ligands; second, an external magnetic field and third, MNPs are subjected to a controlled radio frequency (RF) field in order to generate heat and disturb the BBB. These strategies are used alone or in combination to achieve the optimum BBB permeation of MNPs. PEGylated iron oxide NPs (IONPs) were synthesized to eliminate isolated cancer cells due to generating hyperthermia by magnetic fluid. It was found that multicore IONPs were more cytotoxic than single core ones due to different mode of interaction with U87 GBM cells [136].
Furthermore, utilizing PEGylated rhodamine-labeled phospholipids, magnetic-fluid (magnemite nanocrystals)-loaded liposomes (MFLs) with a hydrodynamic diameter of 212 ± 29 nm were created. MFLs were used by two main methods: selective magnetic ablation of brain malignancies and non-invasive MRI monitoring. Accordingly, an in vivo 7-T MRI and ex vivo electron spin resonance study revealed that 4 h exposure to a magnetic field gradient effectively focused MFLs into human GBM cells U87 implanted in the brain of mice. The magneto-liposomes were then maintained for 24 h, as determined by MRI monitoring. Under the magnetic field, MLF explicitly distributed in the tumor, with minimum concentration determined in other parts of the brain [137]. Significant macrophage infiltration in GBM margins is a characteristic feature and often results in poor prognoses. To enhance macrophage absorption via endocytosis, the researchers designed fluorescent silica-coated iron oxide NPs (NF-SIONs) with 37 nm-diameter. Both in vivo fluorescence imaging and in vitro magnetic imaging of living cells were carried out using NF-SIONs to identify tumor-associated macrophage (TAM) populations. NF-SIONs were able to traverse the BBB and accurately define the tumor in orthotopic GBM xenograft models. During the surgery of orthotopic xenografts, IONPs coated with near-infrared fluorescent silica facilitated in the detection of TAMs [138].
Safer delivery of antiretroviral therapies to the brain to minimize human immunodeficiency virus (HIV) infection and neurotoxicity may benefit the decrease of neurocognitive impairments in HIV patients. TIMP-1 is known for the neuroprotective effect in HIV-infected cells by reducing viral levels and oxidative stress. However, the delivery of TIMP-1 in the brain is very challenging. Therefore, Atluri et al. used TIMP-1 adsorbed on the surface of MNPs (particle size of 10 nm) and guided NPs to the brain. NPs penetrated the human in vitro BBB model in the presence of a static magnetic field without leading in any neuronal injury [139].
Recently, radiotherapy was also combined with IONPs to improve cancer therapy [140]. In this trial, six GBM patients pre-treated with intracavitary thermotherapy received cavity wall coating of SPIONs (“NanoPaste” technique) post resection. Hyperthermia in tumor tissues was induced by SPIONs subjected to an alternating magnetic field (AMF). The radio beam cause aggregation of SPIONs resulting in local necrosis. After thermotherapy a strong upregulation of HSP70 and Caspase-3 was observed surrounding SPIONs. Higher, HSP70 is linked to innate and adaptive immunity through the upregulation of MHC class I molecules on cancer cells. Consequently, a significant increase in CD3+, CD8+ T-cells and CD68+ macrophages were observed in the surrounding tumor tissue post thermotherapy.
In a separate study, potency of radiotherapy for glioma was enhanced by repolarizing myeloid-derived suppressor cells (MDSCs) using magnetic NPs. MNPs were based on Zn0.4Fe2.6O4 platform slightly modified with di-mercapto succinic acid (DMSA) and the cationic polymer polyethyleneimine (PEI). Two glioma preclinical models (CT-2A, U87) when 25 μg NPs and exposed to 2 Gy of radiation prolonged median survival of CT-2A model to 38 days. In U-87 MG xenograft model combination of radiotherapy and NPs increase median survival to 45-day compared with the PBS group. NPs with radiation while functioned as a local antitumor modality, their combination also stimulated the MDSCs to attack tumor cells, and, therefore, showed a synergistic antitumor effect [141].
5.3. Targeted nanocarriers
Functionalized NPs are being tested for medication transport over the BBB endothelium. For this mechanism, specific receptors or proteins are required on the luminal side of ECs for increasing interactions with NPs. Some of the proteins that pass-through ECs and reach the brain include insulin, transferrin, apolipoproteins, and 2-macroglobulin. NP delivery is a good match for this strategy since existing cellular mechanisms for delivering macromolecular payloads may also be used to transport functionalized NPs.
5.3.1. Apolipoprotein’s shuttle
When a nanocarrier enters the bloodstream, many plasma proteins interact and get adsorbed on their surface. Most of these proteins are either inert or have unknown functions. In contrast, specific corona proteins were shown to mediate targeting by maintaining the function of target recognition. Lipid transport is regulated by apolipoproteins such as ApoA, ApoC, and ApoE, which are present in the lymphatic and circulatory systems. Apolipoproteins (ApoA1 and ApoE) after accumulation on NP corona may help the BBB penetrate with the aid of LDLR and the LRP RMT (LDLR-related protein, LRP). However, this requires their presence in abundance and the correct orientation to make accessibility to receptor-binding pockets.
Several groups have reported that increasing ApoE proteins on the NP surface significantly increases brain penetration. Koffie et al. prepared polysorbate 80 coated NPs (~200 nm diameter) made up of poly (n-butyl cyanoacrylate) (PBCA) [142]. The BBB permeability of PBCA NPs were investigated after loading with molecular imaging contrast agents (Hoechst dye, Texas Red, and Trypan Blue) or biologics (Alexa-488–conjugated anti-A antibody; 6E10) in the mouse brain for in vivo multiphoton microscopy and MRI. It was revealed that PBCA NPs do not disrupt the BBB, but they do absorb ApoE proteins from the blood on their surface and bind to LDLRs of BBB to induce RMT. Polysorbate 80 induces covalent coupling with ApoE, A-I, or B-100 proteins, thus enhancing the uptake of NPs by the brain capillary ECs. The progressive accumulation of Aβ peptides is a pivotal event in AD. Clearance of Aβ plaques is achieved by trans-BBB efflux into peripheral blood circulation mediated by ApoE, ApoJ, and ApoA1 chaperones. Apolipoproteins form complexes with Aβ and traverse the BBB. A short peptide Aβ35–32 retains the properties of Aβ and has been well studied for its neurodegenerative disease by c-Jun N-terminal kinase (JNK) induced apoptosis of neurons. A modified Aβ35–32 (NH2-CGSNKGAIIGLM-CONH2) peptide with the amide form of the C terminal methionine (SP) is less neurotoxic and was suitable for ApoE absorption. Zhang et al. developed doxorubicin-loaded SP-modified liposomes (SP-sLip/Dox) to effectively control the content and functions of the resulting protein corona. When SP-sLip was pre-incubated with mouse plasma for 1 h, it absorbed 8.4-fold rhLRP1 compared to non-targeted sLip. When mice with orthotopic tumors established by intracranial injection of U87 cells were treated with 10 mg/kg of SP-sLip/DOX, their median survival time was dramatically increased compared to that of mice treated with non-targeted sLip/DOX [143].
However, the deposition of corona proteins is not specific and may be affected by several biological factors. Therefore, ApoE protein (34kD) was covalently linked to NPs to target the brain in vivo. Although antibodies and other large proteins are specific for their targets, their manufacture is expensive, protein decorating on NPs is complex, and frequent use may trigger immunological reactions [144]. Therefore, numerous ApoE-derived peptides that preserve critical capabilities with LDLR have been discovered recently for penetrating the BBB. An ApoE mimetic peptide COG133 (LRVRLASHLRKLRKRLL) was derived from the critical binding region of amino acids 133–149 [145]. We recently studied the anticancer effect of BRD4 inhibitor JQ1 encapsulated in PEG-PBC polymeric NPs decorated COG-133 peptide to preferentially target MB cells (Fig. 5A) [146]. Further, we encapsulated a dual BRD4 and PI3K inhibitor SF2523 and novel hedgehog pathway inhibitor MDB5 into COG-PEG-PBC NPs (Fig. 5B). There was a significantly higher uptake of COG-133-NPs than non-targeted NPs by both HD-MB03 (G3 MB) and DAOY (SHH-MB) cells (Fig. 5B-i and B-ii). The in vivo study showed that COG-133-NPs enhanced drug delivery to the cerebellum 6 and 24 h following i.v. injection into mice bearing orthotopic SHH-MB tumors (Fig. 5B-iii and B-iv). Also, treatment with COG-133-NPs loaded with SF2523 and MDB5 decreased the tumor burden in orthotopic MB-bearing NSG mice compared to treatment with non-targeted drug-loaded NPs, without having caused any liver toxicity [123].
Fig. 5.
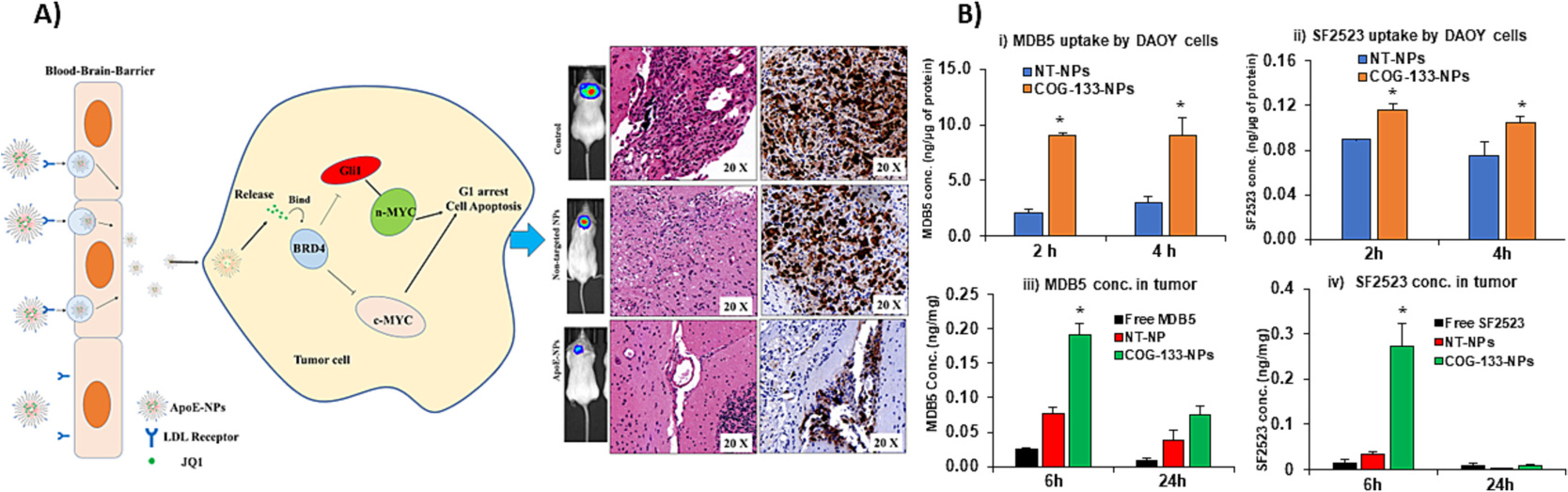
ApoE mimetic peptide (COG-133) enhances the therapeutic efficacy of drug loaded nanoparticles (NPs) in orthotopic medulloblastoma bearing NSG mice. A) COG-133-NPs loaded with BRD4 inhibitor JQ1 decreased MB tumor burden. B) COG-133 decoration increased drug uptake and tumor biodistribution in orthotopic MB mice. The uptake of i) MDB5 and SF2523 loaded COG-133-NPs were increased in DAOY cells measured at 2 and 4 h time points. COG-133 decoration on NPs enhanced iii) MDB5 and iv) SF2523 in intracranial MB tumors compared to free drug and non-targeted NPs.
Jiang et al. prepared ApoE mimetic peptide (LRKLRKRLL)2 decorated PEG-b-poly (dithiolane trimethylene carbonate-co-trimethylene carbonate)-b-polyethyleneimine triblock copolymer [PEG–P (DTC–TMC)–PEI] based chimeric polymersomes. ApoE-chimeric polymersomes (CP) loaded with SaporinS6 protein (SAP) showed enhanced BBB penetration and GBM-targeting ability, effectively inhibiting intracranial U-87 MG human GBM xenografts in nude mice [147].
Another synthetic peptide, K16ApoE (KKKK-KKKK-KKKK-KKKK-LRVR-LASH-LRKL-RKRL-LRDA), was recently explored to carry cargos into the mouse brain through the LDLR mediated RMT. In NOD/SCID mice, advanced MRI techniques were utilized to determine the treatment timeframe following K16ApoE BBB permeabilization. After 10 min of injection with a 200 mg dose of K16ApoEA, a contrast agent Omniscan was observed in the brain tissue. Interestingly, it was found that after K16ApoE injection, the BBB permeability was higher for up to 1 h and allowed permeation of 18F-albumin (~67 kDa) and 18F-IgG (~150 kDa) as observed by PET/CT. Further, K16-ApoE enhanced the delivery of BRAF inhibitor dabrafenib to treat preclinical brain metastases. The peptide increased uptake by clathrin- and dynamin mediated endocytosis at lower concentrations (30.89 to 86.18 mg/mL) and cell lysis and BBB disruption at higher concentrations [148].
5.3.2. Transferrin receptors
TfR mediates iron distribution to the brain and is highly expressed on the BBB. Therefore, TfR can be explored as a gateway to the CNS for drug delivery. Johnsen et al. employed oxaliplatin-loaded liposomes and TfR binding antibody conjugated gold nanoparticles (AuNPs) [149]. They decorated NPs with different densities of antibody (~ 0.15, 0.3, and 0.6 × 103 antibodies/μm2) and evaluated their BBB penetration in vitro and in vivo. There was a direct correlation between antibody density and transport of AuNPs across the BBB. However, the percentage of dose transferred across the capillary was capacities was still relatively poor.
Likewise, Lam et al. developed TfR and Folate (Fol) functionalized and CY5.5 dye conjugated NPs to deliver combination of temozolomide and JQ1 in two orthotopic models of GBM. Post intravenous administration, the intravital imaging to revealed that TfR-NPs could penetrate the intact BBB in mice and directly bind to tumors. Among the targeted NPs, TfR-NPs showed higher uptake of 1.7%, than Fol-NPs (0.9%). Consequently, TfR-NPs loaded with temozolomide and JQ1 reduced tumor burden (1.5- to 2-fold) in mice and an increased in survival compared to other treatment groups. Additionally, TfR-NPs also showed very low systemic drug toxicity [124], showing targeted NPS can avoid unwanted side effects of drugs.
Recently, a 12-amino acid peptide THR (H-THRPPMWSPVWP-NH2) has been shown to be specifically bound to TfR. In their study, Prades et al. found that THR peptide could shuttle AuNPs over the BBB [150]. THR peptide conjugated AuNPs coupled with another peptide CLPFFD helped reducing β-amyloid build up in the brain. LPFFD is a peptide that recognizes and breaks the Aβ toxic protein aggregates. However, like many other peptides, the half-life of THR peptide was only 30 min, and therefore the full therapeutic benefit is not achieved.
To overcome this limitation, Prades et al. N-methylated the positions on the peptide backbone as H-T (NMe)H(NMe)RPPM(NMe)WSPVWP-NH2. In addition, they synthesized both the enantio (THRPPMNWSPVWP) and retro-enantio (H-PWVPSWMPPRHT-NH2) analog of the peptide. These modifications significantly increased the half-life of 12 h for N-methylated and above 24 h for the enantio and retro-enantio versions. The increased stability was because proteases do not recognize d-amino acids. The cell internalization assays with a carboxyfluorescein-labeled version of peptide in BMECs, and astrocytes showed complete peptides uptake after 3 h of incubation. These modified peptides were tested in in vitro BBB model composed of bovine brain ECs and rat astrocytes in a transwells system.
The retro-enantio variant demonstrated nearly a 2-fold increase in permeability compared to the original peptide. In contrast, the retro peptide retained the same permeability as the parent peptide. Furthermore, using a chitosan (CS)-g-poly (methyl methacrylate) (PMMA) copolymer decorated with the retro-enantio peptide H-PWVPSWMPPRHT-NH2, Bukchin et al. created self-assembling NPs (THRre). Polyanionic poly (acrylic acid) (PAAc) residues placed to the pendant hydrophobic blocks boost the copolymer’s ability to form micelles in water. The system encapsulated and delivered a topoisomerase inhibitor SN-38 for the treatment of diffuse intrinsic pontine glioma (DIPG). NPs decorated with the retro-enantio peptide increased their accumulation in the mouse brain after i.v. injection compared to the unmodified counterparts [151]. In early studies, rodent TfR binding antibodies were shown to enhance brain exposure compared to control antibodies, suggesting TfR mediated BBB transcytosis. In spite of the fact that these antibodies were primarily stuck in the brain endothelium due to their high affinity for the target.
Recent findings have shown that monovalent and low-affinity antibodies to human TfR improved brain permeability in nonhuman primates and mice. For example, the brain absorption of bispecific antibodies targeting TfR and -secretase 1 (BACE1) was boosted compared to BACE1 only targeting antibody. BACE1 binds with Aβ and helps in its clearance [152]. Similarly, compared to the original antibody, an anti-Aβ antibody fused to an anti-TfR single-chain Fv (scFv) significantly reduced brain Aβ plaque formation in an AD mouse model [153,154].
Additional research by Kariolis and colleagues has shown that BACE1 is a therapeutic target that may be exploited to assess target engagement in the brain and CSF. Combining BBB transport vehicles with anti-BACE1 antibody Fabs has shown that ATVs cross the BBB and have a direct pharmacological effect on CNS amyloid formation in nonhuman primates and human TfR-engineered mice. A bispecific transport vehicle was created by fusing two distinct antibody Fab arms, which may be employed for an enzyme Fc fusion due to modulating BBB transport vehicles.
Immunotherapy makes use of the immune system to get rid of cancer cells in the body. Checkpoint inhibitor antibodies/molecules targeting programmed cell death-1 (PD-1) and CTLA-4 are a major component of cancer immunotherapy [155]. Immunotherapy is a powerful approach in clinical oncology with a high success rate in many cancers. However, the penetration of antibodies in the brain is minimal, making this approach non-fit for CNS malignancies. A poly (-L-malic acid) (PMLA) based drug carrier was employed by Anna et al., to transfer nano-immunoconjugate (NIC) medicine across the BBB to treat GBM. The NIC system included PEG-PMLA backbone conjugated with anti-mouse TfR antibody (msTfR) for BBB penetration, trileucine (LLL) to stabilize PMLA against hydrolytic degradation, and covalently linked PD-1 IgG or CTLA-4 IgG2b. PMLA cross BBB by using TfR-mediated transcytosis. Using PMLA, they delivered CTLA-4 and PD-1 antibodies (a-CTLA-4 and a-PD-1) to tumor cells in the brain of orthotopic GBM-bearing animals. When mice with intracranial GL261 GBM are treated with NIC, CD8+ T cells, NK cells, and macrophages rise while regulatory T cells (Tregs) decrease in the vicinity of the brain tumor. GBM-bearing mice treated with NIC combination inhibitors had considerably higher survival than a single checkpoint inhibitor-containing NIC or a free agent. Further, Angiopep-2 (AP-2) was also employed in this study to increase the BBB penetration of nanotherapeutics [156].
However, the primary disadvantage of TfR-mediated BBB trafficking is that resident macrophages express these receptors in the spleen and liver. Therefore, both of these organs have higher chance for Tf-NPs accumulation than brain.
5.3.3. Rabies virus glycoprotein peptide
Rabies virus glycoprotein (RVG) is a 505 amino acid glycoprotein of the rabies virus capsule, which facilitates the virus with neurotropism [157]. Due to bulky ligands unsuitable for drug delivery, a BBB interacting sequence of 29 amino acids contains cell penetrating domain of RVG. Through nAChR-mediated cellular transduction, RVG peptide can pass through the BBB and enter brain cells. It can effectively make nanomedicine penetrate the BBB when coupled with them. As a result, numerous nanocarriers, including liposomes, micelles, polymers, exosomes, dendrimers, and proteins, have been reported that have been modified with RVG peptides.
VGF nerve growth factor inducible (VGF) is a polypeptide that is very important for learning and memory, ramping up synaptic activity. Further, replenishment of VGF levels in the brain could prevent AD development. However, the clinical use of VGF protein (~67 kDa) is inhibited by its poor transport across the BBB and cell membrane. The preclinical model demonstrated the effectiveness of intracerebroventricular administration of VGF-derived 21 amino acid peptides. Arora et al. prepared a liposomal formulation of VGF expressing plasmid with chitosan and then encapsulated it inside the liposomes to solve this issue. For efficient transport of gene delivery to the brain, liposomes were decorated with mannose (MAN), a specific substrate for GLUT-1. To further improve the penetration of the carrier through the cell membrane, the surface of the liposome was additionally modified with cell-penetrating peptides (CPPs) such as chimeric rabies virus glycoprotein fragment (RVG9R) (YTIWMPENPRPGTPCDIFTNSRGKRASNGGGGRRRRRRRRR), rabies virus-derived peptide (RDP) (KSVRTWNEIIPSKGCLRVGGRCHPHVNGGGRRRRRRRRR), penetration (Pen) peptide (RQIKIWFQNRRMKWKKGG), or CGN (CGNHPHLAKYNGT) peptide. Within 4 h of incubation, liposomes modified with Pen and PenMAN exhibited 75–80% cellular absorption by different cells including murine bEnd.3 cells and primary astrocytes, hCMEC/D3, human astrocytes (HA), and SHSY5Y cells. Furthermore, these bio-functionalized liposomes higher transfection and increased VGF levels (~ 2-fold) in the mice brain [158].
5.3.4. Glucose receptor
GLUT1 receptors are found abundant at the BBB to supply glucose to the brain. Nanocarriers decorated with N-palmitoyl glucosamine directed to GLUT1 receptors have been used to deliver peptides and small molecules. Woods et al. demonstrated that targeted nanocarriers Non-ionic surfactant vesicles (NISVs) composed of mono-palmitoyl glycerol (MPG) showed higher brain penetration potential [159]. First, these NISVs showed enhanced transcytosis of encapsulated Hoechst 33342 dye across BBB in vitro and brain penetration in vivo. NISV was then used to encapsulate a rabbit IgG-FITC for BBB penetration in vitro. Finally, they demonstrated that NISV formulations loaded with antibody Hu1A3B-7 improved survival and reduced tissue virus VEEV-infected mice.
The transport of glutamine (g)NISV across the BBB was discovered to be somewhat energy-dependent and to require both dynamin-dependent and independent processes. Transport was found not receptor-mediated as gNISV was not found in the clathrin pit. Further, gNISV did not colocalize with EEA1, a marker of early endosomes. The authors hypothesize that glucosamine decoration of NISV may increase its ‘dwell time’ at the BBB due to interaction with GLUT1 and increased clathrin-independent carriers (CLIC) pathway endocytosis.
5.3.5. EGFR targeting peptide
Epidermal growth factor receptor (EGFR) is overexpressed in a variety of epithelial malignancies. Therefore, EGFR targeting peptide GE11 (YHWYGYTPQNVI) and modified GE11R peptide (YRWYGYTPQNVI) have been discovered for selective delivery of the NPs loaded with drugs. However, the hydrophobic nature of these peptides results in the conjugate aggregation. Therefore, two shorter peptides, D4 (LARLLT) and AEYLR were discovered. Among these, the AEYLR peptide shows high EGFR selectivity. Goddard et al. used an AEYLR peptide modified with a lysine residue at the C-terminus for PEG conjugation (HS-PEG-COOH) and a FITC-Ala at the N-terminus to form the peptide FITC-AAEYLRK. The selectivity of the modified peptide was confirmed in EGFR overexpressing A549 cells [160]. FITC-βAAEYLRK peptide was conjugated to. 4 nm gold NPs (AuNPs) and PEG as a linker. The AuNPs also carried a photosensitizing agent, zinc phthalocyanine (C11Pc). At nanomolar doses, selective phototoxicity was observed in A549 cells with negligible dark toxicity. To precisely deliver the photosensitizer Pc4 to glioma cells, GE11 targeted PEGylated AuNPs were used [161]. It could be assumed that βAAEYLRK targeted C11Pc- AuNPs could be a better option, although they need to be assessed experimentally.
5.3.6. Quorum sensing peptides
Bacteria communicate by releasing specialized chemicals and peptides recognized by other bacteria, and the process is called ‘quorum sensing’. These quorum sensing peptides (QSPs) also interact with mammalian cells and generate critical physiological changes. Quorum-sensing molecules could cross the BBB since some neurological disorders are associated with altered microbiota compositions. Wynendaele et al. selected three quorum sensing peptides with different chemical compositions, BIP-2 (GLWEDLLYNINRYAHYIT), PhrANTH2 (SKDYN), and PhrCACET1 (SYPGWSW), from the Quorumpeps database to study their BBB permeation ability [162]. These peptides were radiolabeled (Iodine-125) and injected into ICR-CD-1 mice via jugular vein, where dermorphin was used as a positive control, and BSA protein was used as a negative control. At various time points, blood was drawn from the mouse carotid artery and decapitated. Radioactivity levels were measured in the brain. The peptide PhrCACET1 was most efficient in brain penetration, followed by BIP-2 and dermorphin, whereas peptides PhrANTH2 and BSA did not penetrate the brain. Furthermore, PhrCACET1 peptide was found in the brain parenchyma (77%–85%) with low capillary retention (15%–23%), indicating effective transfer into the brain. It was concluded that brain influx of these peptides was based on their lipophilicity (PhrANTH2 < BIP-2 < PhrCACET1) and metabolic stability (PhrCACET1 lowest and BIP-2 highest). Based on these results, it would be interesting to study if these peptides can deliver cargo into the brain.
5.3.7. Cell-penetrating peptides
Cell-penetrating peptides (CPPs) are non-specific cationic peptides that facilitate drug transport across the cell membrane and the BBB. Among various CPPs, trans-activator of transcription (TAT) peptide has been successfully used to deliver a large variety of cargoes. TATp(47–57) (YGRKKRRQRRR) is a derivative of TAT of the (HIV. TAT peptide contains positively charged amino acids, arginine, and lysine, which increase its interaction with the negatively charged cell membrane. In an intracranial GBM model using nude mice, Gupta et al. investigated the efficacy of TATp-modified liposomes (TATp-lipoplexes) to enhance the transport of the model gene, a plasmid encoding GFP (pEGFP-N1) to human brain tumor U-87 cells in vitro. TATp-lipoplexes showed more efficient pEGFP-N1 delivery to tumor cells in the intracranial brain tumor model than nontargeted plasmid-loaded lipoplexes. Notably, GFP transfection was limited to tumor cells because there was no fluorescence in the nearby normal brain [163]. Various types of nanocarriers such as micelles, polymeric NPs, liposomes, and MNPs have been utilized for BBB delivery (Fig. 6A) and CPPs have been utilized with each carrier type. The major limitation of these peptides which they bear a cationic charge making them interacting with any cell type and non-specific for BBB.
Fig. 6.
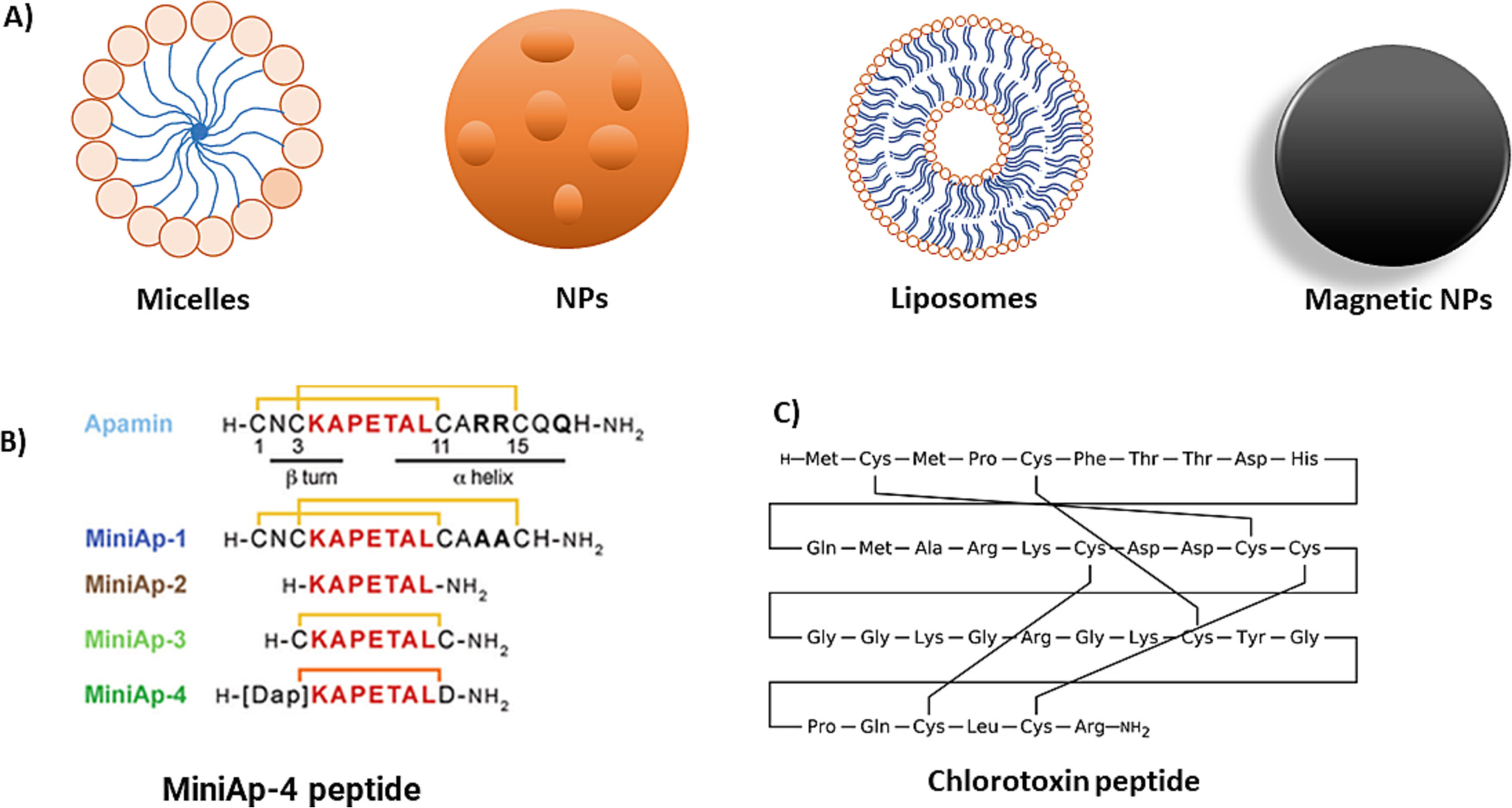
Types of brain-targeted nanomedicine and BBB-targeting peptides derived from venom. A) Commonly reported nanomedicines for brain delivery. B and C) Peptides designed for BBB penetration.
5.3.8. Venom-derived peptides
Several venom peptides are natural cyclic peptides with CNS targeting ability, such as peptide apamin from bee venom and chlorotoxin (CTX) from scorpion venom. These peptides have been investigated as a targeting ligand for delivering different payloads to the brain. However, natural peptides are unstable in vivo and quickly lose their affinity to target proteins. Further, the high toxicity associated with these further hinders their clinical development. For instance, the bicyclic bee venom peptide named apamin (CNCKAPETALCARRCQQH with disulfide bridges between Cys1-Cys11 and Cys3-Cys15) inhibits the calcium-mediated potassium channels and cross BBB efficiently [125]. However, it is highly toxic and immunogenic, and its structure is relatively complex for large-scale production. Several modification attempts have been made to improve apamin usability in vivo. It was found that amino acids Cys1, Lys4, Arg13, Arg14, and His18 are mainly responsible for toxicity [164]. It was suggested that substituting Arg13:14 with Orn13:14 resulted in a non-toxic apamin analog without affecting BBB penetration ability [165]. However, its in-vivo stability was still not improved. Oller-Salvia et al. minimized apamin structure using the all-L-protease resistant analogs. One of the analogs named MiniAP-4 (H-DAP-KAPETALD-NH2) was discovered with reduced toxicity and immunogenicity [126].
Analogs of apamin without any toxic residue were synthesized to simplify the structure. The analog MiniAp-1 showed 60% higher permeability than the original peptide in an in vitro BBB model. It was found that MiniAp-1 was transcytoses through active mechanisms. MiniAp-1 transported small molecules including dopamine, carboxyfluorescein (cF), sulforhodamine B, as well as the heavy molecule of amyloid b-sheet breaker peptide.
Further, the loop between Cys-3 and Cys-11 was also used to shorten the native peptide structure. MiniAp-2, the linear version of MiniAp-1 was less efficient in transporting due to its fast degradation by serum proteases. MiniAp-3, a monocyclic analog bridging Cys-3 and Cys-11, retained permeability equivalent to MiniAp-1 but was found resistant to serum proteases. Further, the peptide MiniAp-4 was produced by replacing the disulfide with a lactam bridge, which showed 50% more permeation than MiniAp-3. MiniAp-4 was found resistant to serum proteases with a t1/2 of 24 h, which was attributed to its better performance. The MiniAp-4 transported proteins and NPs across the in vitro BBB model and into the mouse brain parenchyma (Fig. 6B).
While testing the venom composition of the deathstalker scorpion, Leiurus quinquestriatus hebraeus, a chloride channel blocking peptide CTX was discovered. CTX has 36 residues with four disulfide bonds within its structure and possesses high-affinity chloride channel-blocking properties (Fig. 6C). CTX was also found to bind to glioma, medulloblastoma, prostate cancer, sarcoma, and intestinal cancer via MMP-2 and annexin A2 receptors [166,167]. Therefore, CTX has been tested both as an CNS delivery agent for the radiopharmaceuticals and an in vivo diagnostic tool. For Instance, CTX conjugated 131I-labeled (TM-601) probe was evaluated in clinical trials for recurrent high-grade glioma in 2003 and for malignant melanoma in 2008 [168,169]. Phase 1 and 2 trial results confirmed well tolerance of I131-TM-601 in glioma patients when administered intracavitary. Further, 131I-TM-601 was found corresponding to the tumor area for a long-time with the minimal off-target distribution. Upon treatment over 180 days, four of the patients showed stable disease, and one had a partial response.
Identifying tumor tissue from the normal brain is one of the primary challenges in the surgical procedure. This problem is exacerbated further in metastatic tumors. Several image-guided surgeries-based techniques such as preoperative MRI scans, intra-operative MRI (iMRI), intra-operative ultrasound, time-resolved fluorescence spectroscopy, and Raman spectroscopy are currently being applied in the field. However, these techniques are not sensitive and require complicated hardware that is extremely difficult to deploy in the procedure rooms. This problem was solved by attaching a fluorescent dye Cy5.5 to the CTX peptide called Tozuleristide, which is an imaging agent for cancer cells. Tozuleristide was successfully used for image-guided surgery in the operating room using IR glasses. Additionally, tozuleristide was found in preclinical investigations to be 500 times more sensitive than MRI techniques at seeing tumors with as few as 2000 cancer cells. Similarly, a near-infrared dye indocyanine green (ICG) was covalently linked to CTX peptide and called BLZ-100. In preclinical studies, BLZ-100 was found safe and showed behavioral or neurologic abnormalities in treated animals. For the intraoperative imaging of human malignancies, BLZ-100 is now undergoing clinical testing [170].
CTX peptide was also conjugated with nanoprobes (NPCP-CTX-Cy5.5) for selective accumulation in brain tumors. The nanoprobe comprises a targeting ligand, CTX, a Cy.5.5 dye, and iron oxide NPs decorated with a PEGylated chitosan–branched copolymer. Nanoprobes were equipped with dual contrast agents appropriate for both MRI and bioimaging methods, thanks to the Cy.5.5 dye and iron oxide core. The amphiphilic PEG with high lipid solubility was responsible for increasing the nanoprobe’s endothelial permeability and thus facilitating its BBB passage [171].
6. Conclusions and future perspective
There is no point denying that delivering pharmaceuticals through the BBB is a difficult task. However, there are several opportunities for drug delivery scientists to fill this gap. Studies have shown that BBB is not uniform and has several loose components that can be explored. For instance, drug delivery from the blood into the CSF via the choroid plexus is comparatively simpler, providing a considerably leakier barrier than the BBB. In an invasive procedure, the drug is injected directly into the brain through a burr hole in the skull. As a result, drug distribution is primarily limited to the site of injection and is constrained within the brain. Combination microbubble and ultrasonic strategies necessitate intravenous administration of microbubbles to enhance BBB opening, followed by a second intravenous injection of the chemotherapeutic drug. The short half-life of both microbubbles and chemotherapeutic drugs, however, may restrict the usefulness of this method.
The BTB exhibits significant structural and functional heterogeneity within the same lesion’s microenvironment and between cancer subtypes. Emerging research has revealed the cellular, molecular, and tumor subtype-specific properties of the BTB’s and may lead to improved treatment strategies. Thus, a combination of strategies penetrating both permeable and normal BBB might have to be considered. Effective improvements in the exposure of the brain to drugs through the BBB can be achieved via structural modification of small molecule compounds. Studies report a direct relationship between structure and BBB penetration and could provide modification strategies for CNS drugs.
FDA approval for safer and more effective treatments for brain illnesses will necessitate a concerted effort to develop new BBB drug delivery technology platforms. Delivering diverse cargos into the CNS via crossing the BBB using different nanomedicines such as liposomes, cationic polymers, inorganic NPs, and chitosan has shown potential. Although the emphasis of such technology is invariably on endogenous CMT systems, intricate alterations are constantly required to guarantee that the particles created are BBB-permeable. The development of BBB medication delivery necessitates efforts comparable to those currently put out in CNS drug discovery. Improved barrier functions are expected by applying in vitro systems such as integrating dynamic flow-through microfluidic devices.
Acknowledgments
The National Institutes of Health (R01NS116037, R01NS128336 and R01CA266759 to RIM), and Pediatric Cancer Research Group of the University of Nebraska Medical Center and Children’s Hospital (LB805) are duly acknowledged for providing financial support for this work.
Abbreviations:
- ABC
ATP-binding cassette
- AD
Alzheimer’s disease
- ApoE
Apolipoprotein E
- Aβ
Beta-amyloid
- BBB
Blood brain barrier
- BCSFB
Blood-cerebrospinal fluid barrier
- BMECs
Brain microvascular endothelial cells
- BMs
Basement membranes
- BTB
Blood tumor barrier
- CPPs
Cell-penetrating peptides
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- CTX
Chlorotoxin
- LDLs
Low-density lipoproteins
- LNPs
Lipid nanoparticles
- MB
Medulloblastoma
- nAChRs
Nicotine acetylcholine receptors
- P-gp
P-glycoprotein
- RDP
Rabies virus-derived peptide
- RMT
Receptor-mediated transcytosis
- TEER
Trans epithelial electrical resistance
- TfR
Transferrin receptor
- TJPs
Tight junction proteins
- TKI
Tyrosine kinase inhibitor
- VGF
VGF nerve growth factor inducible
Footnotes
Declaration of Competing Interest
The authors declare no conflict of interest.
References
- [1].Power EA, Rechberger JS, Gupta S, Schwartz JD, Daniels DJ, Khatua S, Drug delivery across the blood-brain barrier for the treatment of pediatric brain tumors - An update, Adv. Drug Deliv. Rev. 185 (2022), 114303. [DOI] [PubMed] [Google Scholar]
- [2].Elizabeth Nance SHP, Rajiv Saigal, Drew L. Sellers, Drug delivery to the central nervous system, Nature Rev. Mater. 7 (2022) 314–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Banks WA, Greig NH, Small molecules as central nervous system therapeutics: old challenges, new directions, and a philosophic divide, Future Med. Chem. 11 (2019) 489–493. [DOI] [PubMed] [Google Scholar]
- [4].Mikitsh JL, Chacko AM, Pathways for small molecule delivery to the central nervous system across the blood-brain barrier, Perspect Medicin Chem. 6 (2014) 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wolak DJ, Thorne RG, Diffusion of macromolecules in the brain: implications for drug delivery, Mol. Pharm. 10 (2013) 1492–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wu KM, Farrelly JG, Regulatory perspectives of type II prodrug development and time-dependent toxicity management: nonclinical pharm/Tox analysis and the role of comparative toxicology, Toxicology 236 (2007) 1–6. [DOI] [PubMed] [Google Scholar]
- [7].Gust J, Hay KA, Hanafi LA, Li D, Myerson D, Gonzalez-Cuyar LF, Yeung C, Liles WC, Wurfel M, Lopez JA, Chen J, Chung D, Harju-Baker S, Ozpolat T, Fink KR, Riddell SR, Maloney DG, Turtle CJ, Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells, Cancer Discov. 7 (2017) 1404–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kumar V, Kumar V, McGuire T, Coulter DW, Sharp JG, Mahato RI, Challenges and recent advances in Medulloblastoma therapy, Trends Pharmacol. Sci. 38 (2017) 1061–1084. [DOI] [PubMed] [Google Scholar]
- [9].Ohta S, Kikuchi E, Ishijima A, Azuma T, Sakuma I, Ito T, Investigating the optimum size of nanoparticles for their delivery into the brain assisted by focused ultrasound-induced blood-brain barrier opening, Sci. Rep. 10 (2020) 18220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ou H, Cheng T, Zhang Y, Liu J, Ding Y, Zhen J, Shen W, Xu Y, Yang W, Niu P, Liu J, An Y, Liu Y, Shi L, Surface-adaptive zwitterionic nanoparticles for prolonged blood circulation time and enhanced cellular uptake in tumor cells, Acta Biomater. 65 (2018) 339–348. [DOI] [PubMed] [Google Scholar]
- [11].Zhang W, Liu QY, Haqqani AS, Leclerc S, Liu Z, Fauteux F, Baumann E, Delaney CE, Ly D, Star AT, Brunette E, Sodja C, Hewitt M, Sandhu JK, Stanimirovic DB, Differential expression of receptors mediating receptor-mediated transcytosis (RMT) in brain microvessels, brain parenchyma and peripheral tissues of the mouse and the human, Fluids Barriers CNS 17 (2020) 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gonzalez-Carter D, Liu X, Tockary TA, Dirisala A, Toh K, Anraku Y, Kataoka K, Targeting nanoparticles to the brain by exploiting the blood-brain barrier impermeability to selectively label the brain endothelium, Proc. Natl. Acad. Sci. U. S. A. 117 (2020) 19141–19150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Prades R, Oller-Salvia B, Schwarzmaier SM, Selva J, Moros M, Balbi M, Grazu V, de La Fuente JM, Egea G, Plesnila N, Teixido M, Giralt E, Applying the retro-enantio approach to obtain a peptide capable of overcoming the blood-brain barrier, Angew. Chem. Int. Ed. Eng. 54 (2015) 3967–3972. [DOI] [PubMed] [Google Scholar]
- [14].Kumthekar P, Tang SC, Brenner AJ, Kesari S, Piccioni DE, Anders C, Carrillo J, Chalasani P, Kabos P, Puhalla S, Tkaczuk K, Garcia AA, Ahluwalia MS, Wefel JS, Lakhani N, Ibrahim N, ANG1005, a brain-penetrating peptide-drug conjugate, shows activity in patients with breast cancer with Leptomeningeal Carcinomatosis and recurrent brain metastases, Clin. Cancer Res. 26 (2020) 2789–2799. [DOI] [PubMed] [Google Scholar]
- [15].Cui Y, Sun J, Hao W, Chen M, Wang Y, Xu F, Gao C, Dual-target peptide-modified erythrocyte membrane-enveloped PLGA nanoparticles for the treatment of Glioma, Front. Oncol. 10 (2020), 563938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pulgar VM, Transcytosis to cross the blood brain barrier, new advancements and challenges, Front. Neurosci. 12 (2018) 1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mahringer A, Fricker G, BCRP at the blood-brain barrier: genomic regulation by 17beta-estradiol, Mol. Pharm. 7 (2010) 1835–1847. [DOI] [PubMed] [Google Scholar]
- [18].Marchi N, Hallene KL, Kight KM, Cucullo L, Moddel G, Bingaman W, Dini G, Vezzani A, Janigro D, Significance of MDR1 and multiple drug resistance in refractory human epileptic brain, BMC Med. 2 (2004) 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Brown LS, Foster CG, Courtney JM, King NE, Howells DW, Sutherland BA, Pericytes and neurovascular function in the healthy and diseased brain, Front. Cell. Neurosci. 13 (2019) 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sweeney MD, Ayyadurai S, Zlokovic BV, Pericytes of the neurovascular unit: key functions and signaling pathways, Nat. Neurosci. 19 (2016) 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C, Pericytes regulate the blood-brain barrier, Nature 468 (2010) 557–561. [DOI] [PubMed] [Google Scholar]
- [22].Sonar SA, Lal G, Blood-brain barrier and its function during inflammation and autoimmunity, J. Leukoc. Biol 103 (2018) 839–853. [DOI] [PubMed] [Google Scholar]
- [23].Ronaldson PT, Davis TP, Regulation of blood-brain barrier integrity by microglia in health and disease: a therapeutic opportunity, J. Cereb. Blood Flow Metab. 40 (2020) S6–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Haruwaka K, Ikegami A, Tachibana Y, Ohno N, Konishi H, Hashimoto A, Matsumoto M, Kato D, Ono R, Kiyama H, Moorhouse AJ, Nabekura J, Wake H, Dual microglia effects on blood brain barrier permeability induced by systemic inflammation, Nat. Commun. 10 (2019) 5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Garcia JA, Cardona SM, Cardona AE, Analyses of microglia effector function using CX3CR1-GFP knock-in mice, Methods Mol. Biol. 1041 (2013) 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Reed MJ, Damodarasamy M, Banks WA, The extracellular matrix of the blood-brain barrier: structural and functional roles in health, aging, and Alzheimer’s disease, Tissue Barriers 7 (2019) 1651157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hawkins RA, O’Kane RL, Simpson IA, Vina JR, Structure of the blood-brain barrier and its role in the transport of amino acids, J. Nutr. 136 (2006) 218S–226S. [DOI] [PubMed] [Google Scholar]
- [28].van Horssen J, Bo L, Vos CM, Virtanen I, de Vries HE, Basement membrane proteins in multiple sclerosis-associated inflammatory cuffs: potential role in influx and transport of leukocytes, J. Neuropathol. Exp. Neurol. 64 (2005) 722–729. [DOI] [PubMed] [Google Scholar]
- [29].Javed K, Reddy V, Lui F, Neuroanatomy, Choroid Plexus, in: StatPearls, Treasure Island (FL), 2022. [PubMed] [Google Scholar]
- [30].Engelhardt B, Sorokin L, The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction, Semin. Immunopathol. 31 (2009) 497–511. [DOI] [PubMed] [Google Scholar]
- [31].Redzic Z, Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: similarities and differences, Fluids Barriers CNS 8 (2011) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fernandez-Sevilla LM, Valencia J, Flores-Villalobos MA, Gonzalez-Murillo A, Sacedon R, Jimenez E, Ramirez M, Varas A, Vicente A, The choroid plexus stroma constitutes a sanctuary for paediatric B-cell precursor acute lymphoblastic leukaemia in the central nervous system, J. Pathol. 252 (2020) 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Islam Y, Leach AG, Smith J, Pluchino S, Coxon CR, Sivakumaran M, Downing J, Fatokun AA, Teixido M, Ehtezazi T, Physiological and pathological factors affecting drug delivery to the brain by nanoparticles, Adv. Sci. (Weinh) 8 (2021), e2002085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Figueiredo CA, Steffen J, Morton L, Arumugam S, Liesenfeld O, Deli MA, Kroger A, Schuler T, Dunay IR, Immune response and pathogen invasion at the choroid plexus in the onset of cerebral toxoplasmosis, J. Neuroinflammation 19 (2022) 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Brian MJM, Oldfield J, Circumventricular organs, in: The Rat Nervous System (Fourth Edition), Academic Press, 2015, pp. 315–333. [Google Scholar]
- [36].Lechan RM, Neuroendocrinology, in: Williams Textbook of Endocrinology, Elsevier, 2020, pp. 114–183.e117. [Google Scholar]
- [37].Segarra M, Aburto MR, Acker-Palmer A, Blood-brain barrier dynamics to maintain brain homeostasis, Trends Neurosci. 44 (2021) 393–405. [DOI] [PubMed] [Google Scholar]
- [38].O’Donnell ME, Blood-brain barrier Na transporters in ischemic stroke, Adv. Pharmacol. 71 (2014) 113–146. [DOI] [PubMed] [Google Scholar]
- [39].Boonstra E, de Kleijn R, Colzato LS, Alkemade A, Forstmann BU, Nieuwenhuis S, Neurotransmitters as food supplements: the effects of GABA on brain and behavior, Front. Psychol. 6 (2015) 1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hawkins RA, The blood-brain barrier and glutamate, Am. J. Clin. Nutr. 90 (2009) 867S–874S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pappolla MA, Andorn AC, Serum protein leakage in aged human brain and inhibition of ligand binding at alpha 2-adrenergic and cholinergic binding sites, Synapse 1 (1987) 82–89. [DOI] [PubMed] [Google Scholar]
- [42].Billy D, Briede J, Heemskerk JW, Hemker HC, Lindhout T, Prothrombin conversion under flow conditions by prothrombinase assembled on adherent platelets, Blood Coagul. Fibrinolysis 8 (1997) 168–174. [DOI] [PubMed] [Google Scholar]
- [43].Ismail AA, Shaker BT, Bajou K, The plasminogen-activator plasmin system in physiological and pathophysiological angiogenesis, Int. J. Mol. Sci. 23 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fu BM, Transport across the blood-brain barrier, Adv. Exp. Med. Biol. 1097 (2018) 235–259. [DOI] [PubMed] [Google Scholar]
- [45].Pun PB, Lu J, Moochhala S, Involvement of ROS in BBB dysfunction, Free Radic. Res. 43 (2009) 348–364. [DOI] [PubMed] [Google Scholar]
- [46].Shi C, Yi D, Li Z, Zhou Y, Cao Y, Sun Y, Chui D, Guo X, Anti-RAGE antibody attenuates isoflurane-induced cognitive dysfunction in aged rats, Behav. Brain Res. 322 (2017) 167–176. [DOI] [PubMed] [Google Scholar]
- [47].Sasson E, Anzi S, Bell B, Yakovian O, Zorsky M, Deutsch U, Engelhardt B, Sherman E, Vatine G, Dzikowski R, Ben-Zvi A, Nano-scale architecture of blood-brain barrier tight-junctions, Elife 10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Berndt P, Winkler L, Cording J, Breitkreuz-Korff O, Rex A, Dithmer S, Rausch V, Blasig R, Richter M, Sporbert A, Wolburg H, Blasig IE, Haseloff RF, Tight junction proteins at the blood-brain barrier: far more than claudin-5, Cell. Mol. Life Sci. 76 (2019) 1987–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Castro V, Bertrand L, Luethen M, Dabrowski S, Lombardi J, Morgan L, Sharova N, Stevenson M, Blasig IE, Toborek M, Occludin controls HIV transcription in brain pericytes via regulation of SIRT-1 activation, FASEB J. 30 (2016) 1234–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Dithmer S, Staat C, Muller C, Ku MC, Pohlmann A, Niendorf T, Gehne N, Fallier-Becker P, Kittel A, Walter FR, Veszelka S, Deli MA, Blasig R, Haseloff RF, Blasig IE, Winkler L, Claudin peptidomimetics modulate tissue barriers for enhanced drug delivery, Ann. N. Y. Acad. Sci. 1397 (2017) 169–184. [DOI] [PubMed] [Google Scholar]
- [51].Mantle JL, Lee KH, A differentiating neural stem cell-derived astrocytic population mitigates the inflammatory effects of TNF-alpha and IL-6 in an iPSC-based blood-brain barrier model, Neurobiol. Dis. 119 (2018) 113–120. [DOI] [PubMed] [Google Scholar]
- [52].Zhen H, Zhao L, Ling Z, Kuo L, Xue X, Feng J, Wip1 regulates blood-brain barrier function and neuro-inflammation induced by lipopolysaccharide via the sonic hedgehog signaling signaling pathway, Mol. Immunol. 93 (2018) 31–37. [DOI] [PubMed] [Google Scholar]
- [53].Candelario-Jalil E, Taheri S, Yang Y, Sood R, Grossetete M, Estrada EY, Fiebich BL, Rosenberg GA, Cyclooxygenase inhibition limits blood-brain barrier disruption following intracerebral injection of tumor necrosis factor-alpha in the rat, J. Pharmacol. Exp. Ther. 323 (2007) 488–498. [DOI] [PubMed] [Google Scholar]
- [54].Almutairi MM, Gong C, Xu YG, Chang Y, Shi H, Factors controlling permeability of the blood-brain barrier, Cell. Mol. Life Sci. 73 (2016) 57–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kim Y, Cho AY, Kim HC, Ryu D, Jo SA, Jung YS, Effects of natural polyphenols on oxidative stress-mediated blood-brain barrier dysfunction, Antioxidants (Basel) 11 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kanai N, Lu R, Bao Y, Wolkoff AW, Vore M, Schuster VL, Estradiol 17 beta-D-glucuronide is a high-affinity substrate for oatp organic anion transporter, Am. J. Phys. 270 (1996) F326–F331. [DOI] [PubMed] [Google Scholar]
- [57].Watanabe M, Watanabe T, Yabuki M, Tamai I, Dehydroepiandrosterone sulfate, a useful endogenous probe for evaluation of drug-drug interaction on hepatic organic anion transporting polypeptide (OATP) in cynomolgus monkeys, Drug Metab Pharmacokinet 30 (2015) 198–204. [DOI] [PubMed] [Google Scholar]
- [58].Akanuma S, Hirose S, Tachikawa M, Hosoya K , Localization of organic anion transporting polypeptide (Oatp) 1a4 and Oatp1c1 at the rat blood-retinal barrier, Fluids Barriers CNS 10 (2013) 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Takano J, Maeda K, Kusuhara H, Sugiyama Y, Organic anion transporting polypeptide 1a4 is responsible for the hepatic uptake of cardiac glycosides in mice, Drug Metab. Dispos. 46 (2018) 652–657. [DOI] [PubMed] [Google Scholar]
- [60].Ronaldson PT, Finch JD, Demarco KM, Quigley CE, Davis TP, Inflammatory pain signals an increase in functional expression of organic anion transporting polypeptide 1a4 at the blood-brain barrier, J. Pharmacol. Exp. Ther. 336 (2011) 827–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Higuchi K, Kitamura A, Okura T, Deguchi Y, Memantine transport by a proton-coupled organic cation antiporter in hCMEC/D3 cells, an in vitro human blood-brain barrier model, Drug Metab Pharmacokinet 30 (2015) 182–187. [DOI] [PubMed] [Google Scholar]
- [62].Arakawa R, Okumura M, Ito H, Takano A, Takahashi H, Takano H, Maeda J, Okubo Y, Suhara T, Positron emission tomography measurement of dopamine D (2) receptor occupancy in the pituitary and cerebral cortex: relation to antipsychotic-induced hyperprolactinemia, J. Clin. Psychiatry 71 (2010) 1131–1137. [DOI] [PubMed] [Google Scholar]
- [63].van Assema DM, Lubberink M, Boellaard R, Schuit RC, Windhorst AD, Scheltens P, Lammertsma AA, van Berckel BN, P-glycoprotein function at the blood-brain barrier: effects of age and gender, Mol. Imaging Biol. 14 (2012) 771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Iorio AL, Ros M, Fantappie O, Lucchesi M, Facchini L, Stival A, Becciani S, Guidi M, Favre C, Martino M, Genitori L, Sardi I, Blood-brain barrier and breast cancer resistance protein: a limit to the therapy of CNS tumors and neurodegenerative diseases, Anti Cancer Agents Med. Chem. 16 (2016) 810–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Breedveld P, Pluim D, Cipriani G, Wielinga P, van Tellingen O, Schinkel AH, Schellens JH, The effect of Bcrp1 (Abcg2) on the in vivo pharmacokinetics and brain penetration of imatinib mesylate (Gleevec): implications for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patients, Cancer Res. 65 (2005) 2577–2582. [DOI] [PubMed] [Google Scholar]
- [66].Chen Y, Agarwal S, Shaik NM, Chen C, Yang Z, Elmquist WF, P-glycoprotein and breast cancer resistance protein influence brain distribution of dasatinib, J. Pharmacol. Exp. Ther. 330 (2009) 956–963. [DOI] [PubMed] [Google Scholar]
- [67].Le Page A, Dupuis G, Frost EH, Larbi A, Pawelec G, Witkowski JM, Fulop T, Role of the peripheral innate immune system in the development of Alzheimer’s disease, Exp. Gerontol. 107 (2018) 59–66. [DOI] [PubMed] [Google Scholar]
- [68].Jaeger LB, Dohgu S, Sultana R, Lynch JL, Owen JB, Erickson MA, Shah GN, Price TO, Fleegal-Demotta MA, Butterfield DA, Banks WA, Lipopolysaccharide alters the blood-brain barrier transport of amyloid beta protein: a mechanism for inflammation in the progression of Alzheimer’s disease, Brain Behav. Immun. 23 (2009) 507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Takeda S, Sato N, Ikimura K, Nishino H, Rakugi H, Morishita R, Increased blood-brain barrier vulnerability to systemic inflammation in an Alzheimer disease mouse model, Neurobiol. Aging 34 (2013) 2064–2070. [DOI] [PubMed] [Google Scholar]
- [70].Garcia-Dominguez I, Vesela K, Garcia-Revilla J, Carrillo-Jimenez A, Roca-Ceballos MA, Santiago M, de Pablos RM, Venero JL, Peripheral inflammation enhances microglia response and Nigral dopaminergic cell death in an in vivo MPTP model of Parkinson’s disease, Front. Cell. Neurosci. 12 (2018) 398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Liu Y, Zhang S, Li X, Liu E, Wang X, Zhou Q, Ye J, Wang JZ, Peripheral inflammation promotes brain tau transmission via disrupting blood-brain barrier, Biosci. Rep. 40 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Mei M, Zhou Y, Liu M, Zhao F, Wang C, Ding J, Lu M, Hu G, Antioxidant and anti-inflammatory effects of dexrazoxane on dopaminergic neuron degeneration in rodent models of Parkinson’s disease, Neuropharmacology 160 (2019), 107758. [DOI] [PubMed] [Google Scholar]
- [73].Fabis MJ, Scott GS, Kean RB, Koprowski H, Hooper DC, Loss of blood-brain barrier integrity in the spinal cord is common to experimental allergic encephalomyelitis in knockout mouse models, Proc. Natl. Acad. Sci. U. S. A. 104 (2007) 5656–5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Dickens AM, Tovar YRLB, Yoo SW, Trout AL, Bae M, Kanmogne M, Megra B, Williams DW, Witwer KW, Gacias M, Tabatadze N, Cole RN, Casaccia P, Berman JW, Anthony DC, Haughey NJ, Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions, Sci. Signal. 10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Lockman PR, Mittapalli RK, Taskar KS, Rudraraju V, Gril B, Bohn KA, Adkins CE, Roberts A, Thorsheim HR, Gaasch JA, Huang S, Palmieri D, Steeg PS, Smith QR, Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer, Clin. Cancer Res. 16 (2010) 5664–5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Anchordoquy TJ, Barenholz Y, Boraschi D, Chorny M, Decuzzi P, Dobrovolskaia MA, Farhangrazi ZS, Farrell D, Gabizon A, Ghandehari H, Godin B, La-Beck NM, Ljubimova J, Moghimi SM, Pagliaro L, Park JH, Peer D, Ruoslahti E, Serkova NJ, Simberg D, Mechanisms and barriers in cancer Nanomedicine: addressing challenges, looking for solutions, ACS Nano 11 (2017) 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Hambardzumyan D, Bergers G, Glioblastoma: defining tumor niches, trends, Cancer 1 (2015) 252–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Li Y, Zhu ZY, Huang TT, Zhou YX, Wang X, Yang LQ, Chen ZA, Yu WF, Li PY, The peripheral immune response after stroke-a double edge sword for blood-brain barrier integrity, CNS Neurosci. Ther. 24 (2018) 1115–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Saand AR, Yu F, Chen J, Chou SH, Systemic inflammation in hemorrhagic strokes - a novel neurological sign and therapeutic target? J. Cereb. Blood Flow Metab. 39 (2019) 959–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Ao LY, Yan YY, Zhou L, Li CY, Li WT, Fang WR, Li YM, Immune cells after ischemic stroke onset: roles, migration, and target intervention, J. Mol. Neurosci. 66 (2018) 342–355. [DOI] [PubMed] [Google Scholar]
- [81].Jin R, Liu S, Wang M, Zhong W, Li G, Inhibition of CD147 attenuates stroke-associated pneumonia through modulating lung immune response in mice, Front. Neurol. 10 (2019) 853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Prass K, Meisel C, Hoflich C, Braun J, Halle E, Wolf T, Ruscher K, Victorov IV, Priller J, Dirnagl U, Volk HD, Meisel A, Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation, J. Exp. Med. 198 (2003) 725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Jia W, Lu R, Martin TA, Jiang WG, The role of claudin-5 in blood-brain barrier (BBB) and brain metastases (review), Mol. Med. Rep. 9 (2014) 779–785. [DOI] [PubMed] [Google Scholar]
- [84].Thomsen MS, Humle N, Hede E, Moos T, Burkhart A, Thomsen LB, The blood-brain barrier studied in vitro across species, PLoS One 16 (2021), e0236770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Kumar V, Sethi B, Yanez E, Leung DH, Ghanwatkar YY, Cheong J, Tso J, Narang AS, Nagapudi K, Mahato RI, Effect of magnesium stearate surface coating method on the aerosol performance and permeability of micronized fluticasone propionate, Int. J. Pharm. 615 (2022), 121470. [DOI] [PubMed] [Google Scholar]
- [86].Ahn SI, Sei YJ, Park HJ, Kim J, Ryu Y, Choi JJ, Sung HJ, MacDonald TJ, Levey AI, Kim Y, Microengineered human blood-brain barrier platform for understanding nanoparticle transport mechanisms, Nat. Commun. 11 (2020) 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Cho H, Seo JH, Wong KH, Terasaki Y, Park J, Bong K, Arai K, Lo EH, Irimia D, Three-dimensional blood-brain barrier model for in vitro studies of neurovascular pathology, Sci. Rep. 5 (2015) 15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Cucullo L, Marchi N, Hossain M, Janigro D, A dynamic in vitro BBB model for the study of immune cell trafficking into the central nervous system, J. Cereb. Blood Flow Metab. 31 (2011) 767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Sherman H, Rossi AE, A novel three-dimensional Glioma blood-brain barrier model for high-throughput testing of Tumoricidal capability, Front. Oncol. 9 (2019) 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Campisi M, Shin Y, Osaki T, Hajal C, Chiono V, Kamm RD, 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes, Biomaterials 180 (2018) 117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Clevers H, Modeling development and disease with Organoids, Cell 165 (2016) 1586–1597. [DOI] [PubMed] [Google Scholar]
- [92].Williams-Medina A, Deblock M, Janigro D, In vitro models of the blood-brain barrier: tools in translational medicine, Front. Med. Technol. 2 (2020), 623950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Nzou G, Wicks RT, Wicks EE, Seale SA, Sane CH, Chen A, Murphy SV, Jackson JD, Atala AJ, Human cortex spheroid with a functional blood brain barrier for high-throughput neurotoxicity screening and disease modeling, Sci. Rep. 8 (2018) 7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Bian S, Repic M, Guo Z, Kavirayani A, Burkard T, Bagley JA, Krauditsch C, Knoblich JA, Genetically engineered cerebral organoids model brain tumor formation, Nat. Methods 15 (2018) 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Ogawa J, Pao GM, Shokhirev MN, Verma IM, Glioblastoma Model Using Human Cerebral Organoids, Cell Rep. 23 (2018) 1220–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Ballabio C, Anderle M, Gianesello M, Lago C, Miele E, Cardano M, Aiello G, Piazza S, Caron D, Gianno F, Ciolfi A, Pedace L, Mastronuzzi A, Tartaglia M, Locatelli F, Ferretti E, Giangaspero F, Tiberi L, Modeling medulloblastoma in vivo and with human cerebellar organoids, Nat. Commun. 11 (2020) 583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Lin F, Hoogendijk L, Buil L, Beijnen JH, van Tellingen O, Sildenafil is not a useful modulator of ABCB1 and ABCG2 mediated drug resistance in vivo, Eur. J. Cancer 49 (2013) 2059–2064. [DOI] [PubMed] [Google Scholar]
- [98].Gross G, Strategies for enhancing oral bioavailability and brain penetration, in: The Practice of Medicinal Chemistry (Fourth Edition), Elsevier Ltd, 2015, pp. 631–655. [Google Scholar]
- [99].Radaram B, Pisaneschi F, Rao Y, Yang P, Piwnica-Worms D, Alauddin MM, Novel derivatives of anaplastic lymphoma kinase inhibitors: synthesis, radiolabeling, and preliminary biological studies of fluoroethyl analogues of crizotinib, alectinib, and ceritinib, Eur. J. Med. Chem. 182 (2019), 111571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Sakai H, Inoue H, Murata K, Toba T, Shimmyo Y, Narii N, Ueno SY, Igawa Y, Takemoto N, Fibroblast growth factor receptor modulators employing diamines with reduced phospholipidosis-inducing potential, Bioorg. Med. Chem. 28 (2020), 115562. [DOI] [PubMed] [Google Scholar]
- [101].Fushimi M, Fujimori I, Wakabayashi T, Hasui T, Kawakita Y, Imamura K, Kato T, Murakami M, Ishii T, Kikko Y, Kasahara M, Nakatani A, Hiura Y, Miyamoto M, Saikatendu K, Zou H, Lane SW, Lawson JD, Imoto H, Discovery of potent, selective, and brain-penetrant 1 H-Pyrazol-5-yl-1 H-pyrrolo[2,3-b] pyridines as anaplastic lymphoma kinase (ALK) inhibitors, J. Med. Chem. 62 (2019) 4915–4935. [DOI] [PubMed] [Google Scholar]
- [102].Frearson JA, Brand S, McElroy SP, Cleghorn LA, Smid O, Stojanovski L, Price HP, Guther ML, Torrie LS, Robinson DA, Hallyburton I, Mpamhanga CP, Brannigan JA, Wilkinson AJ, Hodgkinson M, Hui R, Qiu W, Raimi OG, van Aalten DM, Brenk R, Gilbert IH, Read KD, Fairlamb AH, Ferguson MA, Smith DF, Wyatt PG, N-myristoyltransferase inhibitors as new leads to treat sleeping sickness, Nature 464 (2010) 728–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Tsang JE, Urner LM, Kim G, Chow K, Baufeld L, Faull K, Cloughesy TF, Clark PM, Jung ME, Nathanson DA, Development of a potent brain-penetrant EGFR tyrosine kinase inhibitor against malignant brain tumors, ACS Med. Chem. Lett. 11 (2020) 1799–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Wager TT, Hou X, Verhoest PR, Villalobos A, Moving beyond rules: the development of a central nervous system multiparameter optimization (CNS MPO) approach to enable alignment of druglike properties, ACS Chem. Neurosci. 1 (2010) 435–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Feng B, West M, Patel NC, Wager T, Hou X, Johnson J, Tremaine L, Liras J, Validation of human MDR1-MDCK and BCRP-MDCK cell lines to improve the prediction of brain penetration, J. Pharm. Sci. 108 (2019) 2476–2483. [DOI] [PubMed] [Google Scholar]
- [106].Yu-Li Lo H-CL, Shu-Ting Hong, Chih-Hsien Chang, Chen-Shen Wang, Anya Maan-Yuh Lin, Lipid polymeric nanoparticles modified with tight junction-modulating peptides promote afatinib delivery across a blood–brain barrier model, Cancer Nanotechnol. 12 (2021). [Google Scholar]
- [107].Liu LB, Xue YX, Liu YH, Bradykinin increases the permeability of the blood-tumor barrier by the caveolae-mediated transcellular pathway, J. Neuro-Oncol. 99 (2010) 187–194. [DOI] [PubMed] [Google Scholar]
- [108].Ma F, Yang L, Sun Z, Chen J, Rui X, Glass Z, Xu Q, Neurotransmitter-derived lipidoids (NT-lipidoids) for enhanced brain delivery through intravenous injection, Sci. Adv. 6 (2020), eabb4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].McDannold N, Vykhodtseva N, Hynynen K, Effects of acoustic parameters and ultrasound contrast agent dose on focused-ultrasound induced blood-brain barrier disruption, Ultrasound Med. Biol. 34 (2008) 930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Kumar V, Kumar V, Luo J, Mahato RI, Therapeutic potential of OMe-PS-miR-29b1 for treating liver fibrosis, Mol.Ther. 26 (12) (2018) 2798–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Guo Y, Lee H, Fang Z, Velalopoulou A, Kim J, Thomas MB, Liu J, Abramowitz RG, Kim Y, Coskun AF, Krummel DP, Sengupta S, MacDonald TJ, Arvanitis C, Single-cell analysis reveals effective siRNA delivery in brain tumors with microbubble-enhanced ultrasound and cationic nanoparticles, Sci. Adv. 7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Cao Y, Tsien CI, Shen Z, Tatro DS, Ten Haken R, Kessler ML, Chenevert TL, Lawrence TS, Use of magnetic resonance imaging to assess blood-brain/blood-glioma barrier opening during conformal radiotherapy, J. Clin. Oncol. 23 (2005) 4127–4136. [DOI] [PubMed] [Google Scholar]
- [113].Upadhyayula PS, Spinazzi EF, Argenziano MG, Canoll P, Bruce JN, Convection enhanced delivery of Topotecan for Gliomas: a single-center experience, Pharmaceutics 13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Chu C, Jablonska A, Gao Y, Lan X, Lesniak WG, Liang Y, Liu G, Li S, Magnus T, Pearl M, Janowski M, Walczak P, Hyperosmolar blood-brain barrier opening using intra-arterial injection of hyperosmotic mannitol in mice under real-time MRI guidance, Nat. Protoc. 17 (2022) 76–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Bregy A, Shah AH, Diaz MV, Pierce HE, Ames PL, Diaz D, Komotar RJ, The role of Gliadel wafers in the treatment of high-grade gliomas, Expert. Rev. Anticancer. Ther. 13 (2013) 1453–1461. [DOI] [PubMed] [Google Scholar]
- [116].Potere N, Del Buono MG, Mauro AG, Abbate A, Toldo S, Low density lipoprotein receptor-related Protein-1 in cardiac inflammation and infarct healing, Front. Cardiovasc. Med. 6 (2019) 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Demeule M, Currie JC, Bertrand Y, Che C, Nguyen T, Regina A, Gabathuler R, Castaigne JP, Beliveau R, Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector angiopep-2, J. Neurochem. 106 (2008) 1534–1544. [DOI] [PubMed] [Google Scholar]
- [118].Demeule M, Regina A, Che C, Poirier J, Nguyen T, Gabathuler R, Castaigne JP, Beliveau R, Identification and design of peptides as a new drug delivery system for the brain, J. Pharmacol. Exp. Ther. 324 (2008) 1064–1072. [DOI] [PubMed] [Google Scholar]
- [119].Piskor BM, Przylipiak A, Dabrowska E, Niczyporuk M, Lawicki S, Matrilysins and Stromelysins in pathogenesis and diagnostics of cancers, Cancer Manag. Res. 12 (2020) 10949–10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Inzitari D, Giusti B, Nencini P, Gori AM, Nesi M, Palumbo V, Piccardi B, Armillis A, Pracucci G, Bono G, Bovi P, Consoli D, Guidotti M, Nucera A, Massaro F, Micieli G, Orlandi G, Perini F, Tassi R, Tola MR, Sessa M, Toni D, Abbate R, Group MS, MMP9 variation after thrombolysis is associated with hemorrhagic transformation of lesion and death, Stroke 44 (2013) 2901–2903. [DOI] [PubMed] [Google Scholar]
- [121].Islam Y, Khalid A, Pluchino S, Sivakumaran M, Teixido M, Leach A, Fatokun AA, Downing J, Coxon C, Ehtezazi T, Development of brain targeting peptide based MMP-9 inhibiting nanoparticles for the treatment of brain diseases with elevated MMP-9 activity, J. Pharm. Sci. 109 (2020) 3134–3144. [DOI] [PubMed] [Google Scholar]
- [122].Zhan C, Li B, Hu L, Wei X, Feng L, Fu W, Lu W, Micelle-based brain-targeted drug delivery enabled by a nicotine acetylcholine receptor ligand, Angew. Chem. Int. Ed. Eng. 50 (2011) 5482–5485. [DOI] [PubMed] [Google Scholar]
- [123].Kumar V, Wang Q, Sethi B, Lin F, Kumar V, Coulter DW, Dong Y, Mahato RI, Polymeric nanomedicine for overcoming resistance mechanisms in hedgehog and Myc-amplified medulloblastoma, Biomaterials 278 (2021), 121138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Lam FC, Morton SW, Wyckoff J, Vu Han TL, Hwang MK, Maffa A, Balkanska-Sinclair E, Yaffe MB, Floyd SR, Hammond PT, Enhanced efficacy of combined temozolomide and bromodomain inhibitor therapy for gliomas using targeted nanoparticles, Nat. Commun. 9 (2018) 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Wu J, Jiang H, Bi Q, Luo Q, Li J, Zhang Y, Chen Z, Li C, Apamin-mediated actively targeted drug delivery for treatment of spinal cord injury: more than just a concept, Mol. Pharm. 11 (2014) 3210–3222. [DOI] [PubMed] [Google Scholar]
- [126].Oller-Salvia B, Sanchez-Navarro M, Ciudad S, Guiu M, Arranz-Gibert P, Garcia C, Gomis RR, Cecchelli R, Garcia J, Giralt E, Teixido M, MiniAp-4: a venom-inspired Peptidomimetic for brain delivery, Angew. Chem. Int. Ed. Eng. 55 (2016) 572–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Pedersen MB, Zhou X, Larsen EK, Sorensen US, Kjems J, Nygaard JV, Nyengaard JR, Meyer RL, Boesen T, Vorup-Jensen T, Curvature of synthetic and natural surfaces is an important target feature in classical pathway complement activation, J. Immunol. 184 (2010) 1931–1945. [DOI] [PubMed] [Google Scholar]
- [128].Szebeni J, Complement activation-related pseudoallergy: a stress reaction in blood triggered by nanomedicines and biologicals, Mol. Immunol. 61 (2014) 163–173. [DOI] [PubMed] [Google Scholar]
- [129].Tran AQ, Kaulen C, Simon U, Offenhausser A, Mayer D, Surface coupling strength of gold nanoparticles affects cytotoxicity towards neurons, Biomater. Sci. 5 (2017) 1051–1060. [DOI] [PubMed] [Google Scholar]
- [130].Misra SK, Chang HH, Mukherjee P, Tiwari S, Ohoka A, Pan D, Regulating biocompatibility of carbon spheres via defined Nanoscale chemistry and a careful selection of surface functionalities, Sci. Rep. 5 (2015) 14986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Mayer A, Vadon M, Rinner B, Novak A, Wintersteiger R, Frohlich E, The role of nanoparticle size in hemocompatibility, Toxicology 258 (2009) 139–147. [DOI] [PubMed] [Google Scholar]
- [132].Fulop T, Nemes R, Meszaros T, Urbanics R, Kok RJ, Jackman JA, Cho NJ, Storm G, Szebeni J, Complement activation in vitro and reactogenicity of low-molecular weight dextran-coated SPIONs in the pig CARPA model: correlation with physicochemical features and clinical information, J. Control. Release 270 (2018) 268–274. [DOI] [PubMed] [Google Scholar]
- [133].Lassenberger A, Scheberl A, Stadlbauer A, Stiglbauer A, Helbich T, Reimhult E, Individually stabilized, Superparamagnetic nanoparticles with controlled Shell and size leading to exceptional stealth properties and high Relaxivities, ACS Appl. Mater. Interfaces 9 (2017) 3343–3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Hawkins SJ, Crompton LA, Sood A, Saunders M, Boyle NT, Buckley A, Minogue AM, McComish SF, Jimenez-Moreno N, Cordero-Llana O, Stathakos P, Gilmore CE, Kelly S, Lane JD, Case CP, Caldwell MA, Nanoparticle-induced neuronal toxicity across placental barriers is mediated by autophagy and dependent on astrocytes, Nat. Nanotechnol. 13 (2018) 427–433. [DOI] [PubMed] [Google Scholar]
- [135].Hwang D, Dismuke T, Tikunov A, Rosen EP, Kagel JR, Ramsey JD, Lim C, Zamboni W, Kabanov AV, Gershon TR, Sokolsky-Papkov DM, Poly(2-oxazoline) nanoparticle delivery enhances the therapeutic potential of vismodegib for medulloblastoma by improving CNS pharmacokinetics and reducing systemic toxicity, Nanomedicine 32 (2021), 102345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Hemery G, Genevois C, Couillaud F, Lacomme S, Gontier E, Ibarboure E, Lecommandoux S, Garanger E, Sandre O, Monocore vs. multicore magnetic iron oxide nanoparticles: uptake by glioblastoma cells and efficiency for magnetic hyperthermia, Molecular Sys. Design Eng. 2 (2017) 629–639. [Google Scholar]
- [137].Marie H, Lemaire L, Franconi F, Lajnef S, Frapart Y-M, Nicolas V, Frébourg G, Trichet M, Ménager C, Lesieur S, Superparamagnetic liposomes for MRI monitoring and external magnetic field-induced selective targeting of malignant brain tumors, Adv. Funct. Mater. 25 (2015) 1258–1269. [Google Scholar]
- [138].Lee C, Kim GR, Yoon J, Kim SE, Yoo JS, Piao Y, In vivo delineation of glioblastoma by targeting tumor-associated macrophages with near-infrared fluorescent silica coated iron oxide nanoparticles in orthotopic xenografts for surgical guidance, Sci. Rep. 8 (2018) 11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Atluri VS, Jayant RD, Pilakka-Kanthikeel S, Garcia G, Samikkannu T, Yndart A, Kaushik A, Nair M, Development of TIMP1 magnetic nanoformulation for regulation of synaptic plasticity in HIV-1 infection, Int. J. Nanomedicine 11 (2016) 4287–4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Grauer O, Jaber M, Hess K, Weckesser M, Schwindt W, Maring S, Wolfer J, Stummer W, Combined intracavitary thermotherapy with iron oxide nanoparticles and radiotherapy as local treatment modality in recurrent glioblastoma patients, J. Neuro-Oncol. 141 (2019) 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Wu C, Muroski ME, Miska J, Lee-Chang C, Shen Y, Rashidi A, Zhang P, Xiao T, Han Y, Lopez-Rosas A, Cheng Y, Lesniak MS, Repolarization of myeloid derived suppressor cells via magnetic nanoparticles to promote radiotherapy for glioma treatment, Nanomedicine 16 (2019) 126–137. [DOI] [PubMed] [Google Scholar]
- [142].Koffie RM, Farrar CT, Saidi LJ, William CM, Hyman BT, Spires-Jones TL, Nanoparticles enhance brain delivery of blood-brain barrier-impermeable probes for in vivo optical and magnetic resonance imaging, Proc. Natl. Acad. Sci. U. S. A. 108 (2011) 18837–18842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Zhang Z, Guan J, Jiang Z, Yang Y, Liu J, Hua W, Mao Y, Li C, Lu W, Qian J, Zhan C, Brain-targeted drug delivery by manipulating protein corona functions, Nat. Commun. 10 (2019) 3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Oller-Salvia B, Sanchez-Navarro M, Giralt E, Teixido M, Blood-brain barrier shuttle peptides: an emerging paradigm for brain delivery, Chem. Soc. Rev. 45 (2016) 4690–4707. [DOI] [PubMed] [Google Scholar]
- [145].van Rooy I, Mastrobattista E, Storm G, Hennink WE, Schiffelers RM, Comparison of five different targeting ligands to enhance accumulation of liposomes into the brain, J. Control. Release 150 (2011) 30–36. [DOI] [PubMed] [Google Scholar]
- [146].Wang Q, Kumar V, Lin F, Sethi B, Coulter DW, McGuire TR, Mahato RI, ApoE mimetic peptide targeted nanoparticles carrying a BRD4 inhibitor for treating Medulloblastoma in mice, J. Control. Release 323 (2020) 463–474. [DOI] [PubMed] [Google Scholar]
- [147].Jiang Y, Zhang J, Meng F, Zhong Z, Apolipoprotein E peptide-directed chimeric Polymersomes mediate an ultrahigh-efficiency targeted protein therapy for Glioblastoma, ACS Nano 12 (2018) 11070–11079. [DOI] [PubMed] [Google Scholar]
- [148].Aasen SN, Espedal H, Holte CF, Keunen O, Karlsen TV, Tenstad O, Maherally Z, Miletic H, Hoang T, Eikeland AV, Baghirov H, Olberg DE, Pilkington GJ, Sarkar G, Jenkins RB, Sundstrom T, Bjerkvig R, Thorsen F, Improved drug delivery to brain metastases by peptide-mediated Permeabilization of the blood-brain barrier, Mol. Cancer Ther. 18 (2019) 2171–2181. [DOI] [PubMed] [Google Scholar]
- [149].Johnsen KB, Bak M, Kempen PJ, Melander F, Burkhart A, Thomsen MS, Nielsen MS, Moos T, Andresen TL, Antibody affinity and valency impact brain uptake of transferrin receptor-targeted gold nanoparticles, Theranostics 8 (2018) 3416–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Prades R, Guerrero S, Araya E, Molina C, Salas E, Zurita E, Selva J, Egea G, Lopez-Iglesias C, Teixido M, Kogan MJ, Giralt E, Delivery of gold nanoparticles to the brain by conjugation with a peptide that recognizes the transferrin receptor, Biomaterials 33 (2012) 7194–7205. [DOI] [PubMed] [Google Scholar]
- [151].Alexandra Bukchin MS-N, Carrera Adam, Resa-Pares Claudia, B.-L L. Castillo-Ecija Helena, Teixidó Meritxell, Nagore G. Olaciregui, Ernest Giralt, Angel AAS, Carcaboso M, Amphiphilic Polymeric Nanoparticles Modified with a Protease-Resistant Peptide Shuttle for the Delivery of SN-38 in Diffuse Intrinsic Pontine Glioma, ACS Appl. Nano Mater. 4 (2021) 1314–1329. [Google Scholar]
- [152].Kariolis MS, Wells RC, Getz JA, Kwan W, Mahon CS, Tong R, Kim DJ, Srivastava A, Bedard C, Henne KR, Giese T, Assimon VA, Chen X, Zhang Y, Solanoy H, Jenkins K, Sanchez PE, Kane L, Miyamoto T, Chew KS, Pizzo ME, Liang N, Calvert MEK, DeVos SL, Baskaran S, Hall S, Sweeney ZK, Thorne RG, Watts RJ, Dennis MS, Silverman AP, Zuchero YJY, Brain delivery of therapeutic proteins using an Fc fragment blood-brain barrier transport vehicle in mice and monkeys, Sci. Transl. Med. 12 (2020). [DOI] [PubMed] [Google Scholar]
- [153].Boado RJ, Zhou QH, Lu JZ, Hui EK, Pardridge WM, Pharmacokinetics and brain uptake of a genetically engineered bifunctional fusion antibody targeting the mouse transferrin receptor, Mol. Pharm. 7 (2010) 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Weber F, Bohrmann B, Niewoehner J, Fischer JAA, Rueger P, Tiefenthaler G, Moelleken J, Bujotzek A, Brady K, Singer T, Ebeling M, Iglesias A, Freskgard PO, Brain shuttle antibody for Alzheimer’s disease with attenuated peripheral effector function due to an inverted binding mode, Cell Rep. 22 (2018) 149–162. [DOI] [PubMed] [Google Scholar]
- [155].Sun Y, Jiang L, Wen T, Guo X, Shao X, Qu H, Chen X, Song Y, Wang F, Qu X, Li Z, Trends in the research into immune checkpoint blockade by anti-PD1/PDL1 antibodies in cancer immunotherapy: a Bibliometric study, Front. Pharmacol. 12 (2021), 670900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Galstyan A, Markman JL, Shatalova ES, Chiechi A, Korman AJ, Patil R, Klymyshyn D, Tourtellotte WG, Israel LL, Braubach O, Ljubimov VA, Mashouf LA, Ramesh A, Grodzinski ZB, Penichet ML, Black KL, Holler E, Sun T, Ding H, Ljubimov AV, Ljubimova JY, Blood-brain barrier permeable nano immunoconjugates induce local immune responses for glioma therapy, Nat. Commun. 10 (2019) 3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Wang Q, Cheng S, Qin F, Fu A, Fu C, Application progress of RVG peptides to facilitate the delivery of therapeutic agents into the central nervous system, RSC Adv. 11 (2021) 8505–8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Arora S, Singh J, In vitro and in vivo optimization of liposomal nanoparticles based brain targeted vgf gene therapy, Int. J. Pharm. 608 (2021), 121095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Woods S, O’Brien LM, Butcher W, Preston JE, Georgian AR, Williamson ED, Salguero FJ, Modino F, Abbott NJ, Roberts CW, D’Elia RV, Glucosamine-NISV delivers antibody across the blood-brain barrier: optimization for treatment of encephalitic viruses, J. Control. Release 324 (2020) 644–656. [DOI] [PubMed] [Google Scholar]
- [160].Goddard ZR, Beekman AM, Cominetti MMD, O’Connell MA, Chambrier I, Cook MJ, Marin MJ, Russell DA, Searcey M, Peptide directed phthalocyanine-gold nanoparticles for selective photodynamic therapy of EGFR overexpressing cancers, RSC Med. Chem. 12 (2020) 288–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Cheng Y, Meyers JD, Agnes RS, Doane TL, Kenney ME, Broome AM, Burda C, Basilion JP, Addressing brain tumors with targeted gold nanoparticles: a new gold standard for hydrophobic drug delivery? Small 7 (2011) 2301–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Wynendaele E, Verbeke F, Stalmans S, Gevaert B, Janssens Y, Van De Wiele C, Peremans K, Burvenich C, De Spiegeleer B, Quorum sensing peptides selectively penetrate the blood-brain barrier, PLoS One 10 (2015), e0142071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Gupta B, Levchenko TS, Torchilin VP, TAT peptide-modified liposomes provide enhanced gene delivery to intracranial human brain tumor xenografts in nude mice, Oncol. Res. 16 (2007) 351–359. [DOI] [PubMed] [Google Scholar]
- [164].Vincent JP, Schweitz H, Lazdunski M, Structure-function relationships and site of action of apamin, a neurotoxic polypeptide of bee venom with an action on the central nervous system, Biochemistry 14 (1975) 2521–2525. [DOI] [PubMed] [Google Scholar]
- [165].Oller-Salvia B, Teixido M, Giralt E, From venoms to BBB shuttles: synthesis and blood-brain barrier transport assessment of apamin and a nontoxic analog, Biopolymers 100 (2013) 675–686. [DOI] [PubMed] [Google Scholar]
- [166].David AM, Kittle S, Julia E Parrish-Novak, Stacey Hansen, Rameshwar Patil, Pallavi R. Gangalum, Julia Ljubimova, Keith L. Black, Pramod Butte, Fluorescence-guided tumor visualization using the tumor paint BLZ-100, Cureus 100 (2014). [Google Scholar]
- [167].Kittle DS, Mamelak A, Julia E Parrish-Novak, Fluorescence-guided tumor visualization using the tumor paint BLZ-100, Cureus 6 (9) (2014), e210, 10.7759/cureus.210. [DOI] [Google Scholar]
- [168].NCT00040573, Safety and Tolerability Study of 131I-TM-601 to Treat Adult Patients With Recurrent Glioma, TransMolecular, 2003. [Google Scholar]
- [169].NCT00733798, A Safety and Efficacy Study of Intravenous 131I-TM601 in Adult Patients With Malignant Melanoma, in, ClinicalTrials.gov, 2008. [Google Scholar]
- [170].Yamada M, Miller DM, Lowe M, Rowe C, Wood D, Soyer HP, Byrnes-Blake K, Parrish-Novak J, Ishak L, Olson JM, Brandt G, Griffin P, Spelman L, Prow TW, A first-in-human study of BLZ-100 (tozuleristide) demonstrates tolerability and safety in skin cancer patients, Contemp. Clin. Trials Commun. 23 (2021), 100830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Veiseh O, Sun C, Fang C, Bhattarai N, Gunn J, Kievit F, Du K, Pullar B, Lee D, Ellenbogen RG, Olson J, Zhang M, Specific targeting of brain tumors with an optical/magnetic resonance imaging nanoprobe across the blood-brain barrier, Cancer Res. 69 (2009) 6200–6207. [DOI] [PMC free article] [PubMed] [Google Scholar]


