Abstract
Schistosomes infect over 200 million people worldwide, but few studies have characterized the effects of Schistosoma mansoni infection and effective treatment on the lower gastrointestinal mucosa. In this prospective cohort study, we compared the clinical findings on sigmoidoscopy and laboratory measures in Tanzanian adults with and without S. mansoni infection at baseline and 6 months after praziquantel treatment. Grading of the endoscopic findings was done using the Mayo Scoring System for Assessment of Ulcerative Colitis Activity. Schistosome infection was confirmed by stool microscopy and serum circulating anodic antigen (CAA). Baseline comparisons were performed in Stata using Fisher’s exact and Wilcoxon rank-sum tests, and pre- and post-treatment comparisons using Wilcoxon matched-pairs signed-rank and McNemar’s tests.
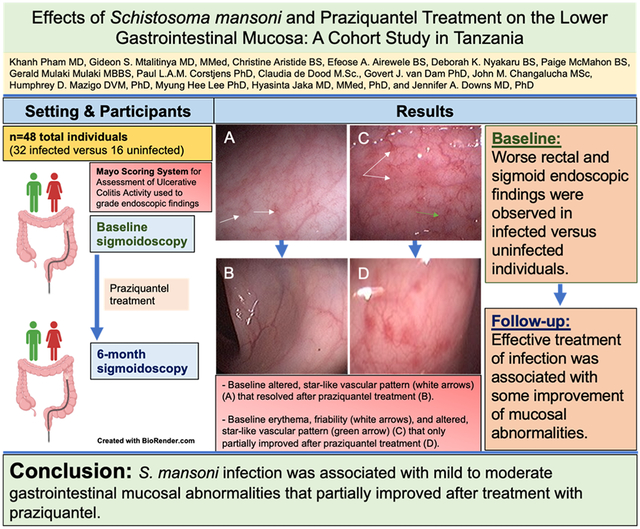
We investigated the clinical characteristics of 48 individuals: 32 with and 16 without S. mansoni infection. Infected individuals had greater severity of sigmoid and rectal mucosal abnormalities and higher Mayo scores and serum eosinophils (all p<0.05) than uninfected individuals at initial evaluation. At 6 months, 28 individuals completed repeat blood tests and sigmoidoscopy. Of these, 14 cleared their baseline infection (n=7) or experienced a greater than 7-fold decrease in serum CAA (n=7). Follow-up sigmoidoscopies revealed some improvements in sigmoid and rectal mucosal findings, although Mayo scores were not significantly lower. Both the median erythrocyte sedimentation rates (32.5→12.5 mm/hr) and percent of eosinophils (7.1→3.1%) decreased in this group from baseline to follow-up.
S. mansoni infection was associated with mild-to-moderate lower gastrointestinal mucosal abnormalities that were grossly visible during sigmoidoscopy, and these improved partially 6 months after effective treatment with praziquantel. Additional studies, of longer duration and focused on both clinical and mucosal immunologic effects of S. mansoni, could provide additional insight.
Keywords: Schistosoma mansoni, gastrointestinal tract, sigmoidoscopy, praziquantel
Graphical Abstract
1. Introduction
Schistosoma mansoni and other Schistosoma species are water-borne helminths endemic to many tropical and subtropical parts of the world that infect over 200 million people (1). Schistosome infection disproportionately affects the poor, particularly those with limited access to clean water. In many fishing communities near Lake Victoria in Tanzania, the prevalence of S. mansoni infection among adults commonly exceeds 50%, based on our prior experience (2,3). S. mansoni worms lay eggs in the host venules that migrate preferentially through host gastrointestinal tissue, causing immune dysregulation with granulomatous inflammation that can lead to significant morbidity and mortality. Intestinal and hepatosplenic manifestations of S. mansoni infection include abdominal pain, diarrhea, hepatosplenomegaly, portal hypertension, and variceal bleeding (4-7). Quantification of S. mansoni egg distribution in the gastrointestinal tract in 400 consecutive autopsies showed that tissue density of eggs was highest in the colon (847 eggs per gram of tissue), followed by the small intestine (461 per gram), and liver (150 per gram) (8). Within the colonic tissue, eggs were most concentrated distally in the rectum and decreased as more proximal sections of the colon were examined (9). Praziquantel, an anthelmintic agent, is the treatment of choice and is commonly used in mass drug administration programs in endemic countries (10).
Few studies have directly investigated the clinical effects of S. mansoni infection on the lower gastrointestinal mucosa before and after praziquantel treatment. A cohort study of 216 patients undergoing gastrointestinal endoscopies in Riyadh, Saudi Arabia, demonstrated that endoscopic colonic biopsy is more sensitive than stool microscopy at detecting S. mansoni ova and also documented frequencies of several characteristic colonic mucosal abnormalities in patients with intestinal S. mansoni infection (4). This group further reported histopathological findings from colonic mucosal biopsies in the same cohort, noting that the most common finding was S. mansoni ova surrounded by no or mild inflammatory cell infiltrates—a finding that correlated with normal or mildly inflamed endoscopic appearances in most patients (5). Of 98 patients who had abnormal mucosal appearances at initial evaluation and received praziquantel, only 28 (29%) had follow-up sigmoidoscopies 3-6 months later, and all of these revealed normal findings (5). Two case reports have also suggested that praziquantel treatment may promote mucosal healing (11,12). Notably, both the larger study and the case reports were hospital-based and evaluated patients seeking endoscopies. Neither included uninfected participants, had a community-based sampling strategy, or utilized a systematic grading scale to evaluate and monitor the gastrointestinal mucosa post-treatment.
These studies are complemented by several other studies that have utilized non-invasive measures, such as ultrasonography and surrogate markers of bowel inflammation, including fecal occult blood, fecal calprotectin and plasma endotoxin, to characterize the morbidity of intestinal S. mansoni infection (13-17). Several studies have found fecal occult and fecal calprotectin markers to correlate with intestinal morbidity (13-15), while studies of ultrasonography and plasma endotoxin levels have reported no correlation (16,17).
Our goal was to conduct a prospective cohort study to determine the association of S. mansoni infection with rectal and sigmoid mucosal abnormalities and to quantify the mucosal effects of praziquantel treatment using an established systematic grading scale, the Mayo Scoring System for Assessment of Ulcerative Colitis Activity. We additionally compared erythrocyte sedimentation rate (ESR) and hematologic, renal, and liver function tests between infected and uninfected individuals at baseline, as well as changes in infected individuals before and after praziquantel treatment. We hypothesized that prior to treatment, S. mansoni-infected individuals would have significantly more, and distinct, rectal and sigmoid mucosal abnormalities as compared to uninfected individuals. We also postulated that higher-intensity schistosome infections would be associated with worse mucosal disease and that after treatment, mucosal abnormalities would only partially improve as parasite eggs lodged in tissue and previous consequences of the granulomatous immune response to parasite eggs would not be completely eliminated.
2. Methods
2.1. Study Design
We conducted a prospective cohort study from June 2019 to February 2020 to document the effects of S. mansoni infection and its treatment on the lower gastrointestinal tract mucosa. This study was conducted in the fishing village of Kayenze near Mwanza, Tanzania, where S. mansoni infection is hyperendemic. This clinical study was part of a larger study investigating gastrointestinal mucosal immune effects of schistosome infections. Participants were healthy community members, defined as those who had no known or self-reported diagnoses of chronic schistosomiasis or other chronic diseases, who presented for screening for schistosomiasis at a community center during our visits to the village.
In order to study adults with confirmed active S. mansoni intestinal infection, we determined S. mansoni infection status using both serum circulating anodic antigen (CAA) and urine and stool microscopy results. We enrolled an S. mansoni-infected group that was positive for both CAA and stool ova indicative of S. mansoni egg morphology, and a schistosome-uninfected group that had a negative CAA and negative stool and urine microscopy for all schistosome eggs. We aimed to enroll those with and without S. mansoni infection in a 2:1 ratio to over-sample those with infection and allow visualization of a range of pathologies while still maintaining a comparison group. All enrolled participants were followed for 6 months. Participants with S. mansoni infection were treated with praziquantel at a dose of 40 milligrams per kilogram (mg/kg), in accordance with the World Health Organization (WHO) guidelines (10), after undergoing baseline laboratory measures and sigmoidoscopy.
Baseline sigmoidoscopy evaluations included photographic documentation of clinical findings and collection of biopsies for future studies. Participants provided peripheral blood for baseline laboratory measures that included a complete blood count (CBC), creatinine, aspartate/alanine aminotransferase (AST, ALT), and erythrocyte sedimentation rate (ESR) quantification. At 2- and 4-month follow-up visits, the serum CAA, stool, and urine tests were repeated. Any person who was either CAA- or S. mansoni egg-positive at any follow-up visit received an additional dose of praziquantel 40 mg/kg. All baseline tests, including sigmoidoscopy, were repeated at the 6-month follow-up.
2.2. Inclusion Criteria
The study’s inclusion criteria included adults aged 18-45 years who were able to give informed consent and demonstrate understanding of the study; had a reliable phone of their own or of a contact to schedule follow-up visits; and had plans to reside in the study village for at least 6 months. S. mansoni-infected participants had at least one S. mansoni egg seen during stool microscopy and a serum schistosome CAA ≥30 pg/mL, as well as negative stool and urine microscopy for S. haematobium. Uninfected participants were required to have a CAA < 30 pg/mL and no schistosome ova seen in the urine or stool. The age range of 18-45 years was chosen due to modeling studies that suggest stable levels of anti-schistosome immunity in this age group (18).
2.3. Exclusion Criteria
The study’s exclusion criteria included those with HIV infection (due to the effects of HIV infection on immune cell populations in the GI tract) or those who refused voluntary HIV counseling and testing (19,20); S. haematobium or other helminth infection detected during stool and urine microscopy; history of inflammatory bowel disease or other known bowel pathology due to the need to compare normal tissue that differs only by the presence or absence of S. mansoni infection; pregnancy due to the need for sigmoidoscopy; and acute diarrheal illness, defined as an increase in the frequency of stools that was different from the participant’s normal bowel habits, in the past month. We excluded those who had CAA ≥30 pg/mL but had no S. mansoni eggs seen in stool, as well as those who had S. mansoni eggs seen in stool but had CAA <30 pg/mL . This was done to ensure that those enrolled had active S. mansoni infection. This strategy was chosen to allow for accurate comparison of sigmoidoscopy findings between S. mansoni-infected versus uninfected individuals.
2.4. Study Procedures
Potential study participants met individually with a study nurse to provide written informed consent for screening. After consent was obtained, participants provided urine and stool for microscopy and peripheral blood for HIV testing and CAA quantification. The study nurse performed voluntary HIV counseling and testing in accordance with Tanzanian national guidelines. Any individual who tested positive for HIV on an initial point-of-care test (SD Bioline, Standard Diagnostics, Inc., Korea) underwent a confirmatory test using a second point-of-care test (Uni-gold, Trinity Biotech, Wicklow, Ireland). Individuals who were found to be HIV-infected were referred to the nearest HIV Care and Treatment Centre for free longitudinal care, and excluded from the study.
Participants were given a follow-up card with a date to return for results of schistosome testing 2 weeks after they were screened. At the follow-up visit, all participants received their results in a private setting. Those not eligible for cohort participation were counseled, given any indicated treatment for HIV or helminths other than S. mansoni, and thanked for their participation. Those who met study inclusion criteria were invited to enroll in the cohort portion of the study. In those willing to participate in the cohort, a second written informed consent was obtained for 2-, 4-, and 6-month follow-ups, additional collections of blood, urine, and stool, and flexible sigmoidoscopies. Six-month follow-up for repeat sigmoidoscopies was chosen as this was the time by which improvement had been noted in the prior hospital-based study of S. mansoni, though follow-up visits were limited (5). Participants also underwent a private interview with the study nurse regarding their medical and demographic history, including the presence of gastrointestinal symptoms.
Participants were scheduled for flexible sigmoidoscopies and instructed to eat a liquid meal the evening before sigmoidoscopy. Same-day lower bowel preparations were performed on the morning of the sigmoidoscopy. Bowel preparations were done with sodium phosphate enemas at baseline and with normal saline enemas to minimize any potential morphologic distal rectal changes caused by bowel preparation at follow-up (21). Blood was collected on the day of sigmoidoscopy for routine laboratory tests. The flexible sigmoidoscopy included inspection and digital photographic documentation with at least 10 pictures taken of the rectum and sigmoid colon. Any abnormal-appearing tissue suspicious for cancer was biopsied in both infected and uninfected individuals and sent for histopathology at Bugando Medical Centre (BMC) in Mwanza. If cancer, inflammatory bowel disease, or other pathology not related to intestinal schistosome infection was found on histopathology, a referral to BMC was made for further work-up and management.
After each sigmoidoscopy, the study gastroenterologist (GM), who was blinded to the infection status of the study participants, completed a standardized form to document and grade the findings with the goal of determining whether a systematic grading scale would be beneficial in diagnosing and characterizing schistosome infection and treatment. This form was based on the Mayo Scoring System for Assessment of Ulcerative Colitis (Mayo score), which scores overall severity on a scale ranging from 0 (no disease) to 12 (most severe) based on 4 components: the number of stools, frequency of blood seen in stools, findings during endoscopy, and the physician’s rating of disease severity (22). Each component is scored 0-3 points. Endoscopic findings that are considered “normal” include a branching pattern of vasculature. “Mild” disease is characterized by erythema, decreased vascular pattern, and mild friability. “Moderate” disease includes marked erythema, erosions, friability, and lack of vascular pattern. Spontaneous bleeding and ulcerations are seen in “severe” disease (23,24). The physician’s assessment of overall disease severity synthesizes endoscopic findings plus 3 other criteria: the patient’s daily recollection of abdominal discomfort and overall sense of well-being, and the patient’s performance status (24).
Participants with S. mansoni infection were given praziquantel 40 mg/kg and instructed to take it two days after the sigmoidoscopy, as some participants felt nauseated after the procedure. No adverse reactions to praziquantel were reported.
2.5. Field and Laboratory Testing Procedures
From a single stool sample, 5 slides were prepared and examined microscopically by a trained parasitologist from the National Institute for Medical Research (NIMR) laboratory in Mwanza using the Kato-Katz method. The slides were scanned in the field for hookworm eggs and re-examined the following day to count schistosome eggs. Five slides from a single sample have been shown to have a sensitivity comparable to obtaining stool samples on 3 separate days (25).
Ten milliliters of urine were filtered, and the filter was placed on a glass slide for microscopic evaluation for S. haematobium eggs in the field by a trained parasitologist.
Serum CAA was quantified using up-converting phosphor technology with the SCAA20 dry lateral flow assay at the NIMR lab in Mwanza, as previously described (26-28). CAA values ≥30 pg/mL were considered positive. CAA values above the limit of quantification were entered as 99,999 pg/mL. Clinical laboratory assays were performed at Manji’s Healthcare in Mwanza. Complete blood counts were performed using an automatic hematology analyzer (BC-5000, Mindray; Shenzhen, China). Creatinine, AST, and ALT were quantified using the cobas® c 111 analyzer (Roche Diagnostics; Indianapolis, IN). Peripheral blood for ESR was collected into sodium citrate and the sedimentation rate determined using an ESR fast stand (Kangjian, China). The laboratory performs calibrations with every new lot of reagents and performs internal quality assessments monthly.
2.6. Ethics
Written informed consent was obtained in a private setting in Kiswahili, the national language of Tanzania, by a trained study nurse. Before obtaining written consent, the nurse administered a verbal assessment quiz to verify participant understanding. A score of 90% or higher was used to document appropriate understanding of the study. All treatment for schistosome and other helminth infections was provided free of charge.
Permission for the conduct of this study was obtained from the joint research ethics committee of Bugando Medical Centre/the Catholic University for Health and Allied Sciences in Mwanza, Tanzania (CREC/273/2018), from the National Institute for Medical Research in Dar es Salaam (NIMR/HQ/R.8a/Vol.IX/2750) and from the Weill Cornell Medical College Institutional Review Board in New York (1108011873).
2.7. Data Management and Analysis
Study data were collected and managed using REDCap (Research Electronic Data Capture) software hosted at Weill Cornell (29,30). REDCap is a secure, web-based software platform designed to support data capture for research studies, providing 1) an interface for validated data capture; 2) audit trails for tracking data manipulation and export; 3) automated export procedures to common statistical packages; and 4) procedures for data integration and interoperability with external sources.
Analysis was performed in Stata IC/Version 15 (College Station, Texas). We used Fisher’s exact tests to determine differences in proportions, and Wilcoxon rank-sum tests to compare median values. Pre- and post-treatment data was compared using Wilcoxon matched-pairs signed-rank and McNemar’s tests. We used Firth multivariable logistic regression analysis to identify the factors independently associated with schistosome infection due to the presence of several factors that were significant and the small sample size (31).
From previous experience, we anticipated approximately 10% loss to follow-up and estimated that 10% of people would have persistent, recurrent, or incident S. mansoni infections during the six-month follow-up period, and we anticipated that these individuals would not be included in the primary analysis (32). Comparisons between those who followed up versus who did not follow up were performed. Given that we were working in a highly endemic village for schistosome infection among people who were frequently exposed to contaminated water, we further defined a priori that any person who experienced a >7-fold decrease in schistosome infection intensity, as quantified by CAA, would be classified as “effectively treated,” together with those whose infections were completely cleared. A >7-fold decrease was chosen after a scatterplot of CAA fold-changes indicated a clear divide between those with marked versus minor fold-changes. To understand the effects of this categorization, in separate sensitivity analyses we also examined those who completely cleared infections and those who had any fold-change decreases at all.
Based on prior pilot sigmoidoscopies performed by our team in preparation for this study, we predicted that those with S. mansoni infection would have a Mayo Score of 4 and that this would decrease to a score of 2 after treatment. Assuming an intra-person correlation of 0.4 and a standard deviation of 2, and allowing for 10% lost plus 10% with infection at the end of the study, we calculated that we would need to enroll 16 people to have 80% power to detect this difference in Mayo scores at study completion, allowing for the possibility of examining men and women separately. We further calculated that studying 32 infected people plus 16 uninfected people in a 2:1 ratio, analyzed separately by sex, would provide >80% power to detect a difference in Mayo score of 4 versus 1 at baseline.
3. Results
Between June and August 2019, 150 adults aged 18-45 years were screened for schistosome and HIV infections. Out of the initial 150 individuals screened, we excluded 54 people based on our exclusion criteria: 38 had discordant schistosome egg and serum CAA results; 11 were HIV-infected; 4 had hookworm infection detected on stool microscopy; and 1 was pregnant. No person had S. haematobium ova seen in urine. Twenty-four individuals who were initially screened did not return for their results. We sought to enroll individuals in a 2:1 ratio (S. mansoni : uninfected) and balanced by sex. As the study neared its target enrollment, 24 individuals were additionally not enrolled when a particular category of sex + infection status was already filled. We therefore enrolled a total of 48 individuals who provided written informed consent and completed all baseline study procedures for cohort study participation. At enrollment, 32 individuals (16 women and 16 men) were infected with S. mansoni and 16 individuals (10 women and 6 men) were uninfected (Figure 1).
Figure 1. Cohort Formation and Study Procedures.
3.1. Baseline Demographic and Clinical Findings
There were no significant differences in baseline characteristics between S. mansoni-infected and uninfected individuals (Table 1). Infected individuals were more likely to be employed as a farmer or fisherman when compared to uninfected individuals, though this did not reach statistical significance. Only one-fourth of all individuals reported receiving prior treatment for schistosome infection. Few differences in clinical findings were observed between the two groups, with S. mansoni-infected individuals having a significantly lower body mass index (BMI) (21.7 versus 24.3 kg/m2, p=0.019) and higher serum eosinophils (7.1% versus 3.1%, p=0.003) than those who were S. mansoni-uninfected. BMI and eosinophils did not remain significantly different on multivariate regression analysis that included age and sex (p=0.11 and 0.10, respectively). Additionally, no significant differences in baseline blood pressure, white blood cell count, hemoglobin, platelets, creatinine, AST, ALT, or ESR were observed.
Table 1.
Baseline Demographic and Clinical Findings in Schistosome-infected and Uninfected Adults.
| Patient characteristic | S. mansoni-infected (n=32) | S. mansoni-uninfected (n=16) |
|---|---|---|
| Number (%) or Median [IQR] | Number (%) or Median [IQR] | |
| Age (years) | 33.5 [29.5-37.5] | 34 [23.5-42.5] |
| Female gender | 16 (50%) | 10 (63%) |
| Marital status | ||
| Single | 1 (3.1%) | 3 (18.7%) |
| Married | 24 (75%) | 11 (68.8%) |
| Divorced | 4 (12.5%) | 2 (12.5%) |
| Other | 3 (9.4%) | 0 |
| Work | ||
| Farmer | 6 (18.7%) | 2 (12.5%) |
| Fisherman | 10 (31.2%) | 2 (12.5%) |
| Small shopkeeper | 2 (6.3%) | 2 (12.5%) |
| Self-employed | 4 (12.5%) | 5 (31.3%) |
| Student | 1 (3.1%) | 2 (12.5%) |
| Unemployed | 3 (9.4%) | 0 |
| Other | 6 (18.8%) | 3 (18.7%) |
| Years attended school | ||
| 0-5 | 11 (34.4%) | 4 (25%) |
| 6-10 | 18 (56.2%) | 8 (50%) |
| >10 | 2 (6.3%) | 3 (18.7%) |
| Did not answer | 1 (3.1%) | 1 (6.3%) |
| Reports ever receiving prior treatment for schistosomiasis | 9 (28.1%) | 3 (18.8%) |
| Days per year with diarrhea | 1.5 [1-2] | 1.5 [1-3] |
| Active tobacco use | 4 (12.5%) | 0 |
| Active alcohol use | 7 (21.8%) | 5 (31.3%) |
| BMI (kg/m2) | 21.7 [19.7-23.7]* | 24.3 [22.5-29]* |
| Systolic blood pressure (mmHg) | 115 [110-123.7] | 114.3 [101.8-123.7] |
| Diastolic blood pressure (mmHg) | 71.7 [68-78] | 74.5 [65.3-84.5] |
| White blood cell count (103/uL) | 4.3 [3.7-5.4] | 4.9 [4.5-5.3] |
| Eosinophils (%) | 7.1 [4.4-13.2]** | 3.1 [1-5.9]** |
| Absolute eosinophil count (103/uL) | 0.3 [0.2-0.7]** | 0.2 [0-0.3]** |
| Hemoglobin (g/dL) | 13.8 [13.1-14.7] | 13.2 [12.4-15.5] |
| Platelets (103/uL) | 169.5 [142-200] | 195 [143.5-224] |
| Creatinine (umol/L) | 62.6 [54.2-77.2] | 63.3 [50.3-77.5] |
| AST (U/L) | 32.6 [24.9-49] | 31.0 [26.5-39] |
| ALT (U/L) | 32.7 [27.5-59.8] | 32.4 [22.4-48.6] |
| ESR (mm/hr) | 30 [20-40] | 25 [15-35] |
| CAA (pg/mL)‡ | 11246 [1492-99999] | 0 [0-12] |
| S. mansoni stool eggs (eggs/gram)‡ | 52.8 [19.2-184.8] | 0 [0-0] |
p-value < 0.05 and
p-value < 0.01
Included in study definition and p-value not calculated.
3.2. Baseline Gastrointestinal and Sigmoidoscopy Findings
There were no significant differences in frequencies of reported gastrointestinal symptoms including abdominal pain, constipation, increased stool frequency, or blood in the stool (Table 2). At baseline, S. mansoni infection was associated with greater severity of rectal and sigmoid mucosal abnormalities on endoscopy (p<0.001 for both), higher severity of overall disease by physician rating (p<0.001), and higher Mayo scores (p<0.01). Specifically, infected individuals had significantly higher frequencies of patchy or mild erythema in the sigmoid and rectum (p<0.05 and p<0.01, respectively) and altered vascular pattern (p<0.01) in the sigmoid as compared to uninfected individuals. No markers of severe disease, such as spontaneous bleeding or ulcerations, were observed in any person. Only one person with S. mansoni infection had entirely normal mucosa, compared with 43.8% of those without infection.
Table 2.
Baseline Gastrointestinal Symptoms and Sigmoidoscopy Findings of Schistosome-infected and Uninfected Adults.
| Patient characteristic |
S. mansoni-infected (n=32) |
S. mansoni-uninfected (n=16) |
|---|---|---|
| Number (%) or Median [IQR] |
Number (%) or Median [IQR] |
|
| Abdominal pain in past week | ||
| No | 11 (34.4%) | 8 (50%) |
| Somewhat | 14 (43.8%) | 6 (37.5%) |
| Very much | 7 (21.8%) | 2 (12.5%) |
| Constipation in past week | ||
| No | 24 (75%) | 13 (81.2%) |
| Somewhat | 5 (15.6%) | 2 (12.5%) |
| Very much | 2 (6.3%) | 1 (6.3%) |
| Did not answer | 1 (3.1%) | 0 |
| Increased frequency of stool in past week | ||
| Normal | 28 (87.5%) | 13 (81.2%) |
| 1-2 times more than usual | 3 (9.4%) | 2 (12.5%) |
| 3-4 times more than usual | 1 (3.1%) | 1 (6.3%) |
| Blood in stool in past week | ||
| No | 24 (75%) | 14 (87.5%) |
| Somewhat (<50% of the time) | 5 (15.6%) | 0 |
| Very much (>50% of the time) | 2 (6.3%) | 2 (12.5%) |
| Did not answer | 1 (3.1%) | 0 |
| Overall endoscopy findings *** | ||
| Normal (0) | 1 (3.1%) | 7 (43.8%) |
| Mild disease (1) | 25 (78.1%) | 9 (56.2%) |
| Moderate disease (2) | 6 (18.8%) | 0 |
| Severe disease (3) | 0 | 0 |
| Physician’s Overall Rating of Disease Severity *** | ||
| Normal (0) | 1 (3.1%) | 7 (43.8%) |
| Mild (1) | 25 (78.1%) | 9 (56.2%) |
| Moderate (2) | 5 (15.6%) | 0 |
| Severe (3) | 1 (3.1%) | 0 |
| Mayo score ** | 2 [2-4] | 2 [0-2] |
| Sigmoid colon mucosal features | ||
| Mild disease | ||
| Patchy or mild erythema* | 8 (25%) | 0 |
| Altered vascular pattern** | 18 (56.2%) | 2 (12.5%) |
| Mild friability | 5 (15.6%) | 1 (6.3%) |
| Moderate disease | ||
| Marked erythema | 1 (3.1%) | 0 |
| Absent vascular markings | 2 (6.3%) | 0 |
| Moderate friability | 2 (6.3%) | 0 |
| Erosions | 1 (3.1%) | 0 |
| Overall sigmoid mucosal findings*** | 1 [0-1] | 0 [0-0] |
| (0=Normal, 1=Mild, 2=Moderate, 3=Severe) | ||
| Rectum mucosal features | ||
| Mild | ||
| Patchy or mild erythema** | 19 (59.4%) | 2 (12.5%) |
| Altered vascular pattern | 24 (75%) | 7 (43.8%) |
| Mild friability | 10 (31.2%) | 1 (6.3%) |
| Moderate | ||
| Marked erythema | 5 (15.6%) | 0 |
| Absent vascular markings | 3 (9.4%) | 0 |
| Moderate friability | 6 (18.8%) | 0 |
| Erosions | 2 (6.3%) | 0 |
| Overall rectal mucosal findings*** | 1 [1-1] | 1 [0-1] |
| (0=Normal, 1=Mild, 2=Moderate, 3=Severe) | ||
| Comparison of gastrointestinal sites *** | ||
| Mucosal abnormalities greater in rectum than sigmoid | 11 (34.4%) | 7 (43.8%) |
| Mucosal abnormalities equivalent between rectum and sigmoid | 20 (62.5%) | 2 (12.5%) |
| Normal mucosa at rectum and sigmoid | 1 (3.1%) | 7 (43.8%)# |
p-value < 0.05
p-value < 0.01
p-value < 0.001
Includes two women and three men who had normal rectal and sigmoid mucosa aside from abnormal findings isolated to the distal rectum.
Comparison of mucosal abnormalities by gastrointestinal site on sigmoidoscopy was significantly different throughout between infected and uninfected individuals (Table 2). Notably, rectal mucosal abnormalities were always greater than or equivalent in severity to sigmoid abnormalities. Only one person with S. mansoni infection had normal sigmoid and rectal mucosa, versus nearly half of uninfected individuals.
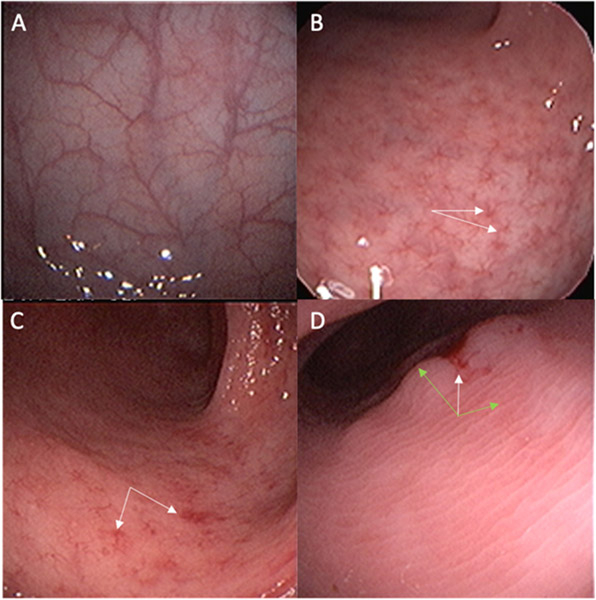
Representative endoscopic findings obtained from our study participants that illustrate the Mayo score of endoscopic severity of disease are shown (Figure 2). Findings included a range from normal mucosa to mild and moderate disease with specific abnormal characteristics indicated by white and green arrows in the figure. In addition, one patient had a polyp that was biopsied and sent for analysis to rule out malignancy; the biopsy showed S. mansoni eggs and no malignant changes.
Figure 2. Representative Endoscopic Findings Illustrating the Mayo Score of Endoscopic Severity of Disease.
Representative photos of sigmoidoscopic findings observed (same magnification):
A) Normal mucosa with intact vascular pattern
B) Mild disease with an altered, star-like vascular pattern (white arrows)
C) Mild disease with patchy areas of erythema and mild friability (white arrows)
D) Moderate disease with mucosal edema obscuring all vascular markings (green arrows), with some erythema and friability (white arrow)
3.3. Infection Status at 6-month Follow-up
Of the original 48 individuals, 28 returned for a 6-month follow-up sigmoidoscopy, of whom 19 were women and 9 were men. The schistosome infection status of these individuals at follow-up is summarized in Table 3. Notably, 7 individuals had completely cleared their infection, and 7 were still infected but had achieved a greater than 7-fold decrease in CAA (median decrease of −85.3-fold [IQR −29.6 to −296.1-fold]). These 14 individuals (12 women and 2 men) were classified as having had their infection effectively treated.
Table 3.
Schistosome Infection Status at 6-month Follow-up as Compared to Baseline Infection (n=28).
| Cleared infection |
Infected but had >7-fold decrease in CAA |
Infected and did not have at least 7-fold decrease in CAA |
Uninfected at baseline and stayed uninfected |
Uninfected at baseline and became infected at month 6 |
|
|---|---|---|---|---|---|
| No. of individuals | 7 | 7 | 5 | 6 | 3 |
| Female | 7 (100%) | 5 (71.4%) | 2 (40%) | 2 (33.3%) | 3 (100%) |
| Median [IQR] CAA fold change from baseline to month 6 | −641 [−23.7 to −5190] | −85.3 [−29.6 to −296.1] | −3.2 [−2.9 to −6.8] | n/a | +62 [+58 to +80] |
| Median [IQR] difference in eggs per gram of stool between baseline and month 6 | −52.8 [−24 to −81.6] *All eggs cleared at 6 months |
−21.6 [−4.8 to −72] *All but 2 cleared eggs at 6 months |
−33.6 [−9.6 to −52.8] *All eggs cleared at 6 months |
n/a | 0* |
| Median [IQR] 6-month Mayo score | 2 [0-2] | 2 [2-4] | 2 [2-3] | 2 [0-2] | 2 [0-2] |
| Median [IQR] difference in Mayo score between baseline and month 6 | − 2 [−1 to −3] | 0 [+2 to −0] | −0.5 [+1 to −1.5] | 0 [0 to −2] | 0 [0 to +2] |
All newly infected people were diagnosed by CAA only.
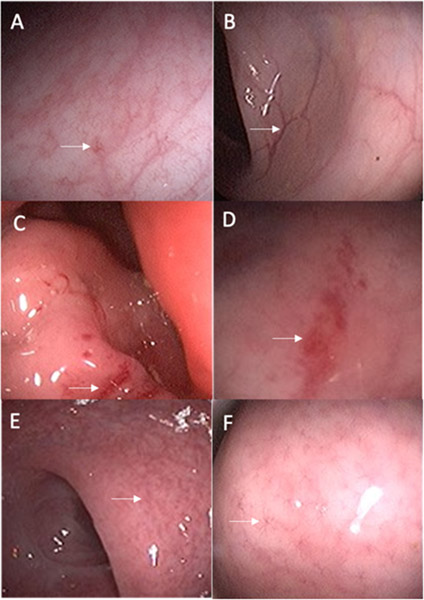
Representative pre-post gastrointestinal mucosal findings in three individuals are shown in Figure 3. These individuals demonstrated examples of mucosal changes that occurred in those with improvement, worsening, or no change in the severity of mucosal abnormalities.
Five individuals had decreases in serum CAA from baseline, but the decrease was not at least 7-fold and these were classified as persistently infected after inspection of distribution of fold-changes and before any statistical analyses. Additionally, 6 did not have baseline infection and remained uninfected at follow-up, and 3 did not have baseline infection but had become infected at 6 months.
3.4. Follow-up Findings in Individuals with Effective Treatment of Infection
The 14 people who were effectively treated, which included those who cleared infection or had a >7-fold decrease in CAA, experienced small but significant decreases in several laboratory measures from baseline to follow-up (Table 4), though notably these decreases in laboratory measures were changes within normal limits. While the overall sigmoid mucosal findings were significantly improved (p=0.0086), the overall endoscopy findings, physician’s rating of disease severity, and Mayo score did not significantly change in these individuals at 6 months. At follow-up, 6 of these individuals had no change or worsening of their Mayo scores and 8 had improvement. Among the 8 individuals, 6 had a baseline Mayo score of greater than or equal to 3 that decreased subsequently to 2 or less at 6 months.
Table 4.
Changes in Baseline Clinical Measurements After 6 Months of Follow-up in Individuals Who Were Effectively Treated for S. mansoni Infection.
| Characteristic | Baseline (Month 0) (n=14) |
Follow-up (Month 6) (n=14) |
|---|---|---|
| Number (%) or Median [IQR] |
Number (%) or Median [IQR] |
|
| White blood cell count (103/uL)* | 4.7 [3.6-5.5] | 3.6 [3.2-4.7] |
| Eosinophils (%)* | 5.7 [3.1-10.3] | 3.5 [1.7-6.3] |
| Absolute eosinophil count (103/uL)* | 0.3 [0.1-0.6] | 0.1 [0.1-0.3] |
| Hemoglobin (g/dL) | 13.7 [13.1-14.1] | 13.1 [12.6-14.1] |
| Platelets (cells/μL) | 179 [142-230] | 180.5 [139-213] |
| Creatinine (μmol/L)** | 55.3 [49.3-71.5] | 51 [44.5-61] |
| AST (U/L) | 30.1 [23.8-33.7] | 28.6 [25.5-31.7] |
| ALT (U/L)* | 30.7 [29.4-44.1] | 26.9 [20-33.3] |
| ESR (mm/hr)** | 32.5 [20-40] | 12.5 [10-30] |
| Systolic blood pressure (mmHg) | 110.7 [102.3-118] | 111.8 [98.3-122.7] |
| Diastolic blood pressure (mmHg) | 76.7 [71.7-78] | 72.7 [71.3-82] |
| Overall endoscopy findings | ||
| Normal (0) | 0 | 2 (14.3%) |
| Semi-normal (0.5) | 0 | 1 (7.1%) |
| Mild disease (1) | 12 (85.7%) | 10 (71.4%) |
| Moderate disease (2) | 2 (14.3%) | 1 (7.1%) |
| Physician’s Overall Rating of Disease Severity | ||
| Normal (0) | 0 | 2 (14.3%) |
| Mild (1) | 12 (85.7%) | 11 (78.6%) |
| Moderate (2) | 2 (14.3%) | 1 (7.1%) |
| Severe (3) | 0 | 0 |
| Mayo Score (0-12 score) | 2 [2-4] | 2 [2-2] |
| Sigmoid colon mucosal features | ||
| Mild disease | ||
| Patchy or mild erythema | 2 (14.3%) | 0 |
| Altered vascular pattern | 9 (64.3%) | 2 (14.3%) |
| Mild friability | 2 (14.3%) | 0 |
| Moderate disease | ||
| Marked erythema | 0 | 0 |
| Absent vascular markings | 0 | 0 |
| Moderate friability | 1 (7.1%) | 0 |
| Erosions | 0 | 0 |
| Overall sigmoid mucosal findings** | 1 [0-1] | 0 [0-0] |
| (0=Normal, 1=Mild, 2=Moderate, 3=Severe) | ||
| Rectum mucosal features | ||
| Mild | ||
| Patchy or mild erythema | 9 (64.3%) | 6 (42.9%) |
| Altered vascular pattern | 13 (92.9%) | 11 (78.6%) |
| Mild friability | 3 (21.4%) | 3 (21.4%) |
| Moderate | ||
| Marked erythema | 0 | 1 (7.1%) |
| Absent vascular markings | 1 (7.1%) | 1 (7.1%) |
| Moderate friability | 2 (14.3%) | 1 (7.1%) |
| Erosions | 0 | 0 |
| Overall rectal mucosal findings | 1 [1-1] | 1 [1-1] |
| (0=Normal, 1=Mild, 2=Moderate, 3=Severe) |
p-value < 0.05
p-value < 0.01
p-value < 0.001
3.5. Individuals Lost to Follow-up
A separate analysis was done comparing those who returned for follow-up sigmoidoscopy (n=28) versus those who were lost to follow-up (n=20) at 6 months. These two groups did not have significant differences in baseline demographics or clinical findings. Notably, there were no significant differences in S. mansoni egg excretion, serum CAA, or baseline Mayo score. The only significant difference was that those who did not attend their 6-month visit less frequently had altered vascular pattern in the rectum (45% versus 78.6%; p=0.031) on baseline sigmoidoscopy when compared to individuals who later attended their follow-up visit.
3.6. Sensitivity Analyses
Finally, we performed two sensitivity analyses to investigate the robustness of our findings when including only those individuals who completed cleared their baseline schistosome infections (n=7) and when including all individuals whose serum CAA decreased at all (n=19). The 7 people who completely cleared infection had significant decreases in the Mayo score (4 2; p=0.034), which was not observed when the additional 7 who had ≥ 7-fold decreases in CAA but still had CAA values ≥30 mg/dL were included (Table 4). The 7 who completely cleared infection also experienced significant changes (decreases within normal limits) in white blood cell count, creatinine, and ESR, as was observed in the 14 who were effectively treated for infection. Differences in eosinophils, ALT, and overall severity of sigmoid mucosal findings still trended in the same directions as those shown in Table 4 but were no longer significant. In addition, those who completely cleared their infection were noted to have a significant decrease in their systolic blood pressure (110.7 100.3 mm Hg; p=0.018).
In the second sensitivity analysis, we investigated pre- and post-treatment differences in the 19 people who had any improvement in baseline CAA. This group’s median Mayo score decreased from a score of 2.5 to 2 (p=0.059) at follow-up. Notably, these individuals had a significant improvement in the overall severity of rectal mucosal findings at 6 months. When compared to those who were effectively treated for infection, these individuals also had significant changes (decreases within normal limits) in white blood cell count, creatinine, ALT, ESR, and overall severity of sigmoid mucosal findings. A significant increase in hemoglobin was also seen. Changes in CAA throughout the study period are illustrated in Supplemental Figure 1.
Discussion
We provide, to the best of our knowledge, the first prospective report in which the mucosal effects of S. mansoni infection in the sigmoid colon and rectum were systematically quantified before and after anti-schistosome praziquantel treatment. In this community-based sample of adults, nearly all of those with S. mansoni (97%) had visible mucosal abnormalities in sigmoid and rectal mucosal tissue. We also demonstrated that some of these mucosal abnormalities, particularly those localized to the sigmoid, improved after effective treatment with praziquantel. Simultaneously, we report that even effective treatment with praziquantel did not completely eliminate macroscopic mucosal pathology, at least as observed in an endemic area and at 6 months. These clinical descriptions of S. mansoni-associated gastrointestinal mucosal disease and comparisons with uninfected individuals provide novel information that extends the data that was previously available from the few studies on this topic (4,5).
Notably, the visible mucosal lesions in the sigmoid and rectum of this community-based sample were observed in people who reported not to have current gastrointestinal symptoms. Our findings expand on those of the Riyadh study, which examined patients who were undergoing sigmoidoscopies for clinical reasons, of whom all were symptomatic and only 11% of whom were excreting S. mansoni ova in the stool (5). Among our participants who all had confirmed active egg excretion, we observed that the gross mucosal lesions in the sigmoid and rectum were only partially reversed six months after anti-schistosome treatment. Interestingly, we also found that mucosal disease was most severe in the rectum, which correlates with prior autopsy studies showing that schistosome eggs are most concentrated in the rectum (9). These mucosal pathologies observed among people who were not seeking medical care highlight the significant inflammation caused by S. mansoni in the gastrointestinal tract. Given the known broad effects of gastrointestinal immune cell populations both locally and systemically (33,34), the visible mucosal inflammation that we observed may contribute to a growing understanding of how even subclinical S. mansoni infection can alter host immunity to other pathogens (35-37).
Our use of the Mayo score provided objective quantification of mucosal pathology and was innovative in facilitating comparison of disease before and after treatment. Notably, the Mayo Score was developed for ulcerative colitis, and our participants’ median score was only 2 out of 12 possible, suggesting that the score is weighted towards capturing severer pathology than is typical for S. mansoni infection in community-based studies of adults. Further, mucosal disease did not correlate with intensity of infection and Mayo scores only decreased significantly post-treatment in participants whose schistosome infections were completely eradicated. We suggest that this scoring might be useful, possibly with modifications, for research purposes for schistosome infection.
Our results are concordant with one recent study, which utilized non-invasive ultrasonography to assess the morbidity of intestinal schistosome infection and found that intestinal ultrasound may not be a sensitive tool for detecting minor intestinal effects of S. mansoni (16). Other studies have found that the fecal markers occult blood and calprotectin were associated with morbidity related to intestinal schistosome infection (13-15). Despite differences in gastrointestinal mucosal abnormalities that were observed among infected versus uninfected individuals, our study did not demonstrate any differences in serum hemoglobin or participant report of blood in the stool between the 2 groups. Future studies investigating the effects of intestinal manifestations of S. mansoni may consider comparing findings between serum hemoglobin and fecal occult blood to further investigate morbidity, such as symptomatic anemia, that may be related to intestinal schistosomiasis.
Improvements in hematologic, kidney, and liver function tests have been reported in previous studies after treatment of schistosomiasis and surgical reversal of its chronic complications in humans and mice (38-45). We similarly documented minor but significant decreases in eosinophils, ESR, and other laboratory measures, though we note that such decreases represented changes within the normal limits of those tests.
Our study has several limitations. First, the higher than expected loss to follow-up limited our ability to fully detect post-treatment changes. High mobility in fishing communities due to occupation-related activities (46,47) may have partly accounted for the high loss to follow-up, together with the challenges of participants agreeing to two sigmoidoscopies within 6 months. Second, Kayenze is a highly endemic S. mansoni region and not every individual cleared his or her baseline infection. This may be due to high rates of re-infections in fishing villages (2,3) and repeated occupational, domestic, and recreational exposures to bodies of water that harbor the parasite. It is also possible that lack of directly observed treatment on the day of the sigmoidoscopy led to some participants not taking praziquantel two days later as instructed. We suspect that most people successfully took praziquantel based on very high levels of CAA seen in month 0 that decreased to lower levels in subsequent months (Supplemental Figure 1). It should be noted that for those who were infected at baseline, it is very difficult to distinguish between re-infection versus persistent infection in a highly endemic region. Third, our follow-up period was only 6 months, and a longer duration of follow-up may have allowed us to determine the more long-term effects of treatment for S. mansoni on the resolution of abnormalities in the lower gastrointestinal mucosa. Comparing our mucosal findings with histopathological diagnoses could have provided additional useful information.
Our study was also not powered to compare differences between men and women, which may be important as previous studies have shown that sex may play a substantial role in the immune response and clinical pathology due to schistosome infection. For instance, one previous study reported that female mice whose ear pinnae were injected with schistosome homogenates developed increased tissue swelling and erythema compared to male mice, underscoring sex differences in the inflammatory response to schistosomes (48). In another study, men were reported to excrete more schistosome eggs for a given worm burden when compared to women (49); egg deposition in host tissue is an important driver to the pathogenesis of schistosome infection. Multiple other studies have demonstrated significant clinical and immunologic differences between males and females with schistosome infection (40-56) and determining potential differential mucosal findings between men and women is an important future direction.
Overall, we believe that these novel data demonstrate how S. mansoni infection manifests clinically and provide endoscopic evidence that some of these abnormal manifestations may improve after praziquantel treatment. Improvements in gastrointestinal tissue mucosae were predominantly localized to the sigmoid. Additional studies, of longer duration and focused on both clinical and mucosal immunologic effects of S. mansoni, could provide additional insight.
Supplementary Material
Figure 3. Select Pre-Post Treatment Images of Gastrointestinal Mucosal Changes after 6 Months of Follow-up.
Improvement of mucosal changes: Pre-treatment image (A) showing star-like vascular pattern with mild erythema and post-treatment image (B) showing resolution of abnormalities and new normal mucosa in the same individual.
Worsening of mucosal changes: Pre-treatment image (C) showing patchy areas of erythema and friability and post-treatment image (D) showing worsening and more extensive erythema and friability in the same individual.
Stable mucosal findings: Pre-treatment image (E) showing decreased and star-like vascular pattern and post-treatment image (F) showing stable abnormalities in the same individual.
Acknowledgements
We thank Kumayl Hussein Pirbhai, MD and the team at Manji’s healthcare for the laboratory testing, our study nurses Jane Mlingi and Ndalloh Paul for their tireless work, and the gastroenterology team at the Ghana Family Polyclinic in Mwanza, Tanzania for their contributions to this research. We are grateful to the study participants for taking part in this study.
Funding
This research was supported by the Doris Duke Charitable Foundation (grant number 2017067). The REDCap database is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000457. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This study also received support from NewYork-Presbyterian Hospital (NYPH) and Weill Cornell Medical College (WCMC), including the Clinical and Translational Science Center (CTSC) (UL1TR000457) and Joint Clinical Trials Office (JCTO), as well as the Weill Cornell T32 training grant from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (T32AI007613 Research Training in Infectious Diseases).
Footnotes
Competing Interests
The authors have declared that no competing interests exist.
Data availability statement
Due to the small number of people involved in this study and the potential for study participants to be identified, this data is not being made publicly available. However, de-identified data may be available from the National Institute for Medical Research upon request to qualified researchers who meet the criteria for access to confidential data. Interested researchers may contact Dr. Lindsey Reif (lir2020@med.cornell.edu).
References
- 1.W H O. Schistosomiasis (Bilharzia) [Internet]. World Health Organization. 2017. [cited 2020 Aug 24]. Available from: https://www.who.int/health-topics/schistosomiasis#tab=tab_1 [Google Scholar]
- 2.Downs JA, de Dood CJ, Dee HE, McGeehan M, Khan H, Marenga A, et al. Schistosomiasis and human immunodeficiency virus in men in tanzania. Am J Trop Med Hyg. 2017. Apr;96(4):856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazigo HD, Dunne DW, Wilson S, Kinung’hi SM, Pinot de Moira A, Jones FM, et al. Co-infection with Schistosoma mansoni and Human Immunodeficiency Virus-1 (HIV-1) among residents of fishing villages of north-western Tanzania. Parasit Vectors. 2014. Dec 16;7:587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yasawy MI, El Shiekh Mohamed AR, Al Karawi MA. Comparison between stool examination, serology and large bowel biopsy in diagnosing Schistosoma mansoni. Trop Doct. 1989. Jul;19(3):132–4. [DOI] [PubMed] [Google Scholar]
- 5.Mohamed AR, al Karawi M, Yasawy MI. Schistosomal colonic disease. Gut. 1990. Apr;31(4):439–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Schistosomiasis [Internet]. 2020. [cited 2021 Jan 6]. Available from: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis
- 7.Schwartz C, Fallon PG. Schistosoma “Eggs-Iting” the Host: Granuloma Formation and Egg Excretion. Front Immunol. 2018. Oct 29;9:2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamel IA, Cheever AW, Elwi AM, Mosimann JE, Danner R. Schistosoma mansoni and S. haematobium infections in Egypt. I. Evaluation of techniques for recovery of worms and eggs at necropsy. Am J Trop Med Hyg. 1977. Jul;26(4):696–701. [DOI] [PubMed] [Google Scholar]
- 9.Cheever AW. A quantitative post-mortem study of Schistosomiasis mansoni in man. Am J Trop Med Hyg. 1968. Jan;17(1):38–64. [DOI] [PubMed] [Google Scholar]
- 10.WHO/Department of control of neglected tropical diseases. Preventive chemotherapy in human helminthiasis Coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers [Internet]. World Health Organization. 2006. [cited 2020 Aug 23]. Available from: https://www.who.int/neglected_diseases/resources/9241547103/en/ [Google Scholar]
- 11.Ngatchu T, Cash D, Langman G. Is indeterminate colitis really indeterminate? Gut. 2010. Sep;59(9):1177. [DOI] [PubMed] [Google Scholar]
- 12.Sharaiha R, Yu W, Swaminath A. An unusual case of diarrhea. Gastrointest Endosc. 2010. Aug;72(2):436–7; discussion 436. [DOI] [PubMed] [Google Scholar]
- 13.Kanzaria HK, Acosta LP, Langdon GC, Manalo DL, Olveda RM, McGarvey ST, et al. Schistosoma japonicum and occult blood loss in endemic villages in Leyte, the Philippines. Am J Trop Med Hyg. 2005. Feb;72(2):115–8. [PubMed] [Google Scholar]
- 14.Betson M, Sousa-Figueiredo JC, Rowell C, Kabatereine NB, Stothard JR. Intestinal schistosomiasis in mothers and young children in Uganda: investigation of field-applicable markers of bowel morbidity. Am J Trop Med Hyg. 2010. Nov;83(5):1048–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bustinduy AL, Sousa-Figueiredo JC, Adriko M, Betson M, Fenwick A, Kabatereine N, et al. Fecal occult blood and fecal calprotectin as point-of-care markers of intestinal morbidity in Ugandan children with Schistosoma mansoni infection. PLoS Negl Trop Dis. 2013. Nov 14;7(11):e2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamarozzi F, Buonfrate D, Badona Monteiro G, Richter J, Gobbi FG, Bisoffi Z. Ultrasound and intestinal lesions in Schistosoma mansoni infection: A case-control pilot study outside endemic areas. PLoS ONE. 2018. Dec 18;13(12):e0209333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klemperer KM, Reust MJ, Lee MH, Corstjens PLAM, van Dam GJ, Mazigo HD, et al. Plasma Endotoxin Levels Are Not Increased in Schistosoma mansoni-Infected Women without Signs or Symptoms of Hepatosplenic Disease. Am J Trop Med Hyg. 2020. Jun;102(6):1382–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell KM, Mutapi F, Woolhouse MEJ. The predicted impact of immunosuppression upon population age-intensity profiles for schistosomiasis. Parasite Immunol. 2008. Sep;30(9):462–70 [DOI] [PubMed] [Google Scholar]
- 19.Hatano H, Somsouk M, Sinclair E, Harvill K, Gilman L, Cohen M, et al. Comparison of HIV DNA and RNA in gut-associated lymphoid tissue of HIV-infected controllers and noncontrollers. AIDS. 2013. Sep 10;27(14):2255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaw JM, Hunt PW, Critchfield JW, McConnell DH, Garcia JC, Pollard RB, et al. Increased frequency of regulatory T cells accompanies increased immune activation in rectal mucosae of HIV-positive noncontrollers. J Virol. 2011. Nov;85(21):11422–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meisel JL, Bergman D, Graney D, Saunders DR, Rubin CE. Human rectal mucosa: proctoscopic and morphological changes caused by laxatives. Gastroenterology. 1977. Jun;72(6):1274–9. [PubMed] [Google Scholar]
- 22.Travis SPL, Schnell D, Krzeski P, Abreu MT, Altman DG, Colombel J-F, et al. Reliability and initial validation of the ulcerative colitis endoscopic index of severity. Gastroenterology. 2013. Nov;145(5):987–95. [DOI] [PubMed] [Google Scholar]
- 23.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987. Dec 24;317(26):1625–9. [DOI] [PubMed] [Google Scholar]
- 24.Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005. Dec 8;353(23):2462–76. [DOI] [PubMed] [Google Scholar]
- 25.Berhe N, Medhin G, Erko B, Smith T, Gedamu S, Bereded D, et al. Variations in helminth faecal egg counts in Kato-Katz thick smears and their implications in assessing infection status with Schistosoma mansoni. Acta Trop. 2004. Dec;92(3):205–12. [DOI] [PubMed] [Google Scholar]
- 26.Corstjens PLAM, van Lieshout L, Zuiderwijk M, Kornelis D, Tanke HJ, Deelder AM, et al. Up-converting phosphor technology-based lateral flow assay for detection of Schistosoma circulating anodic antigen in serum. J Clin Microbiol. 2008. Jan;46(1):171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Downs JA, van Dam GJ, Changalucha JM, Corstjens PLAM, Peck RN, de Dood CJ, et al. Association of Schistosomiasis and HIV infection in Tanzania. Am J Trop Med Hyg. 2012. Nov;87(5):868–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corstjens PLAM, De Dood CJ, Kornelis D, Fat EMTK, Wilson RA, Kariuki TM, et al. Tools for diagnosis, monitoring and screening of Schistosoma infections utilizing lateral-flow based assays and upconverting phosphor labels. Parasitology. 2014. Dec;141(14):1841–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009. Apr;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019. May 9;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Firth D Bias reduction of maximum likelihood estimates. Biometrika. 1993;80(1):27–38. [Google Scholar]
- 32.Mishra P, Colombe S, Paul N, Mlingi J, Tosiri I, Aristide C, et al. Insufficiency of annual praziquantel treatment to control Schistosoma mansoni infections in adult women: A longitudinal cohort study in rural Tanzania. PLoS Negl Trop Dis. 2019. Nov 21;13(11):e0007844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi N, Takahashi D, Takano S, Kimura S, Hase K. The roles of peyer’s patches and microfold cells in the gut immune system: relevance to autoimmune diseases. Front Immunol. 2019. Oct 9;10:2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spahn TW, Kucharzik T. Modulating the intestinal immune system: the role of lymphotoxin and GALT organs. Gut. 2004. Mar;53(3):456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reese TA, Wakeman BS, Choi HS, Hufford MM, Huang SC, Zhang X, et al. Helminth infection reactivates latent γ-herpesvirus via cytokine competition at a viral promoter. Science. 2014. Aug 1;345(6196):573–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bullington BW, Klemperer K, Mages K, Chalem A, Mazigo HD, Changalucha J, et al. Effects of schistosomes on host anti-viral immune response and the acquisition, virulence, and prevention of viral infections: A systematic review. PLoS Pathog. 2021. May 20;17(5):e1009555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McLaughlin TA, Nizam A, Hayara FO, Ouma GS, Campbell A, Khayumbi J, et al. Schistosoma mansoni Infection Is Associated With a Higher Probability of Tuberculosis Disease in HIV-Infected Adults in Kenya. J Acquir Immune Defic Syndr. 2021. Feb 1;86(2):157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopes Filho G de J, Haddad CM. Late clinical, biochemical, endoscopic and electroencephalographic evaluation of patients with schistosomal portal hypertension treated with distal splenorenal shunt. Int Surg. 1998. Mar;83(1):42–7. [PubMed] [Google Scholar]
- 39.Leite LAC, Pimenta Filho AA, Ferreira R de C dos S, da Fonseca CSM, dos Santos BS, Montenegro SML, et al. Splenectomy improves hemostatic and liver functions in hepatosplenic schistosomiasis mansoni. PLoS One. 2015. Aug 12;10(8):e0135370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo X, Zhu Y, Liu R, Song J, Zhang F, Zhang W, et al. Praziquantel treatment after Schistosoma japonicum infection maintains hepatic insulin sensitivity and improves glucose metabolism in mice. Parasit Vectors. 2017. Oct 2;10(1):453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang Y-J, Luo J, Yuan Q, Zheng D, Liu Y-P, Shi L, et al. New insight into the antifibrotic effects of praziquantel on mice in infection with Schistosoma japonicum. PLoS One. 2011. May 24;6(5):e20247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andrews P, Dycka J, Frank G. Effect of praziquantel on clinical-chemical parameters in healthy and schistosome-infected mice. Ann Trop Med Parasitol. 1980. Apr;74(2):167–77. [DOI] [PubMed] [Google Scholar]
- 43.Domingues AL, Coutinho AD. Reduction of morbidity in hepatosplenic schistosomiasis mansoni after treatment with praziquantel: a long term study. Rev Soc Bras Med Trop. 1990. Jun;23(2):101–7. [DOI] [PubMed] [Google Scholar]
- 44.Wambayi EJ, Kasili EG, Kimani G, Waruinge D. Effect of praziquantel on platelet levels in mice infected with S. mansoni. East Afr Med J. 1993. Oct;70(10):665–6. [PubMed] [Google Scholar]
- 45.Milesi M, Indovina C, Dino O, Di Bella F, Di Lorenzo F, Sanfilippo A, et al. Urinary schistosomiasis in migrant population: a case series from a single centre in southern Italy. Infection. 2019. Jun;47(3):395–8. [DOI] [PubMed] [Google Scholar]
- 46.Food and Agriculture Organization of the United Nations. Achieving Poverty Reduction Through Responsible Fisheries: Lessons From West and Central Africa (FAO Fisheries and Aquaculture Technical Papers). FAO; 2008. [Google Scholar]
- 47.Kwena Z, Nakamanya S, Nanyonjo G, Okello E, Fast P, Ssetaala A, et al. Understanding mobility and sexual risk behaviour among women in fishing communities of Lake Victoria in East Africa: a qualitative study. BMC Public Health. 2020. Jun 15;20(1):944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boissier J, Chlichlia K, Digon Y, Ruppel A, Moné H. Preliminary study on sex-related inflammatory reactions in mice infected with Schistosoma mansoni. Parasitol Res. 2003. Sep;91(2):144–50. [DOI] [PubMed] [Google Scholar]
- 49.Colombe S, Lee MH, Masikini PJ, van Lieshout L, de Dood CJ, Hoekstra PT, et al. Decreased Sensitivity of Schistosoma sp. Egg Microscopy in Women and HIV-Infected Individuals. Am J Trop Med Hyg. 2018. Feb 1;98(4):1159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Downs JA, Mguta C, Kaatano GM, Mitchell KB, Bang H, Simplice H, et al. Urogenital schistosomiasis in women of reproductive age in Tanzania’s Lake Victoria region. Am J Trop Med Hyg. 2011. Mar;84(3):364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dupnik KM, Reust MJ, Vick KM, Yao B, Miyaye D, Lyimo E, et al. Gene Expression Differences in Host Response to Schistosoma haematobium Infection. Infect Immun. 2019;87(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Booth M, Mwatha JK, Joseph S, Jones FM, Kadzo H, Ireri E, et al. Periportal fibrosis in human Schistosoma mansoni infection is associated with low IL-10, low IFN-gamma, high TNF-alpha, or low RANTES, depending on age and gender. J Immunol. 2004. Jan 15;172(2):1295–303. [DOI] [PubMed] [Google Scholar]
- 53.Mohamed-Ali Q, Elwali NE, Abdelhameed AA, Mergani A, Rahoud S, Elagib KE, et al. Susceptibility to periportal (Symmers) fibrosis in human schistosoma mansoni infections: evidence that intensity and duration of infection, gender, and inherited factors are critical in disease progression. J Infect Dis. 1999. Oct;180(4):1298–306. [DOI] [PubMed] [Google Scholar]
- 54.Berhe N, Myrvang B, Gundersen SG. Intensity of Schistosoma mansoni, hepatitis B, age, and sex predict levels of hepatic periportal thickening/fibrosis (PPT/F): a large-scale community-based study in Ethiopia. Am J Trop Med Hyg. 2007. Dec;77(6):1079–86. [PubMed] [Google Scholar]
- 55.Garba A, Pion S, Cournil A, Milet J, Schneider D, Campagne G, et al. Risk factors for Schistosoma haematobium infection and morbidity in two villages with different transmission patterns in Niger. Acta Trop. 2010. Aug;115(1–2):84–9. [DOI] [PubMed] [Google Scholar]
- 56.Nakazawa M, Fantappie MR, Freeman GL, Eloi-Santos S, Olsen NJ, Kovacs WJ, et al. Schistosoma mansoni: susceptibility differences between male and female mice can be mediated by testosterone during early infection. Exp Parasitol. 1997. Mar;85(3):233–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to the small number of people involved in this study and the potential for study participants to be identified, this data is not being made publicly available. However, de-identified data may be available from the National Institute for Medical Research upon request to qualified researchers who meet the criteria for access to confidential data. Interested researchers may contact Dr. Lindsey Reif (lir2020@med.cornell.edu).