Abstract
Organophosphate esters (OPEs) are developmental toxicants in experimental studies of animals, but limited evidence is available in humans. We included 340 mother-infant pairs in the Health Outcomes and Measures of the Environment (HOME) Study (Cincinnati, Ohio, USA) for the analysis. We evaluated gestational exposure to OPEs with gestation age at birth and newborn anthropometric measures. We quantified four OPE urinary metabolites at 16 weeks and 26 weeks of gestation. We extracted gestational age at birth, newborn weight, length, and head circumference from the chart review. We calculated z-scores for these anthropometric measures and the ponderal index. We used multiple informant models to examine the associations between repeated OPE measurements and the outcomes. We used modified Poisson regression to estimate the association of gestational exposure to OPEs with preterm birth. We also explored effect modification by infant sex and the potential mediation effect by the highest maternal blood pressure and glucose levels. We found that bis(2-chloroethyl) phosphate (BCEP) at 16 weeks and diphenyl phosphate at 26 weeks of pregnancy were positively associated with gestational age and inversely associated with preterm birth. In female newborns, BCEP at 16 weeks was inversely related to birth weight and length z-scores. In male newborns, we observed negative associations of 26-week di-n-butyl phosphate with the ponderal index at birth. No mediation by the highest maternal blood pressure or glucose levels during pregnancy was identified. In this cohort, gestational exposure to some OPEs was associated with gestational age, preterm birth, and neonatal anthropometric measures. Certain associations tended to be window- and infant sex-specific.
Keywords: Organophosphate esters, Pregnancy outcomes, Gestational age, Preterm birth
1. Introduction
US manufacturers voluntarily phased out polybrominated diphenyl ethers (PBDEs) in the mid-2000s because of their deleterious effects on ecosystems and human health. Organophosphate esters (OPEs) replaced PBDE in various consumer products to meet fire safety requirements (Hou et al., 2021). Tri(2-chloroethyl) phosphate (TCEP), tri(1-chloro-2-propyl) phosphate (TCIPP), tri(1,3-dichloro-2-propyl) phosphate (TDCIPP), tri-n-butyl phosphate (TNBP), and triphenyl phosphate (TPHP) are some commonly used OPE alternatives to PBDEs (Stapleton et al., 2012; van der Veen and de Boer, 2012; Wei et al., 2015). Phosphorus flame retardants have been used for over 150 years (Andrae and others, 2008). TDCIPP and tris(2,3-dibromopropyl) phosphate were added to sleepwear in the 1970s but banned later because of carcinogenicity, indicating the long existence of OPEs in the market (Blum et al., 1978; Gold et al., 1978). Similar to their PBDE predecessors, OPEs do not covalently bind to the products that they are used in, so OPEs can enter the environment over time by volatilization, abrasion, and leaching (Hou et al., 2021; Wei et al., 2015). A growing number of studies have reported detectable OPEs in different environmental matrices, including indoor dust, air, biota, water, sediment, and food (Hoffman et al., 2015; Hou et al., 2021; Li et al., 2019; Sundkvist et al., 2010; Zhang et al., 2022).
Multiple exposure routes to OPEs have been reported, including inhalation, ingestion, skin contact, and diet (Cequier et al., 2015; Ding et al., 2019; Hu et al., 2021; Li et al., 2019; Phillips et al., 2018). Unlike PBDEs, most OPEs have relatively short biological half-lives (Hou et al., 2021; Luo et al., 2021). After exposure, OPEs are metabolized by the liver and eliminated via urine excretion (Hoffman et al., 2018a; Van den Eede et al., 2013, 2016; Wang et al., 2020). Biomonitoring research has revealed the increasing exposure burden both in the general population (Hoffman et al., 2017a) and in vulnerable populations, such as pregnant women and children (Butt et al., 2014; Carignan et al., 2017; Chen et al., 2018; Hoffman et al., 2017b; Percy et al., 2020).
The detection of OPEs in human chorionic villi, deciduae, and placenta tissue has suggested OPEs can transfer from the mother to the fetus in pregnancy, raising concerns of the potential adverse impacts on the growing fetus (Ding et al., 2016; Zhao et al., 2017). Experimental studies have provided evidence on the developmental toxicity and endocrine-disrupting properties of OPEs (Crump et al., 2012; Farhat et al., 2013; Fu et al., 2013; Li et al., 2017; McGee et al., 2012; Patisaul et al., 2013; Ren et al., 2019; Sun et al., 2016; Wang et al., 2013; Yu et al., 2017; Yuan et al., 2018; Zhu et al., 2015), suggesting their potential to impact pregnancy outcomes via pathways related to oxidative stress, epigenetic regulation, and potentially other mechanisms, such as the disruption of peroxisome proliferator-activated receptor-γ (PPARγ) signaling pathways and the dysregulation of hypothalamic-pituitary-thyroid (HPT) axis, hypothalamic-pituitary-gonadal (HPG) axis, hypothalamic-pituitary-adrenal (HPA) axis, as well as growth hormone/insulin-like growth factor (GH/IGF) axis and placental function (Yan et al., 2021).
Maternal exposure to OPEs has been related to adverse pregnancy outcomes in human studies, such as reduced proportion of successful pregnancy and live births, increased risk of spontaneous abortion, and fetal chromosome abnormality (Carignan et al., 2017; Li et al., 2021; Zhao et al., 2021). Limited epidemiological studies have investigated the associations of maternal exposure to OPEs with gestational age and neonatal anthropometrics, and have had mixed findings (Bommarito et al., 2021a; Crawford et al., 2020; Feng et al., 2016; Hoffman et al., 2018a; Kuiper et al., 2020; D. Luo et al., 2020; Luo et al., 2021). These studies did not report trimester-specific associations between maternal OPE metabolites and fetal growth except one (Luo et al., 2021).
In the present study, using a well-established pregnancy and birth cohort with multiple measurements of maternal urinary OPE metabolites at 16 and 26 weeks of gestation, we aimed to 1) examine the associations of maternal urinary OPE metabolites with gestational age, newborn anthropometric measures, and preterm birth; 2) test whether the associations vary by infant sex as previous studies have suggested that the associations of OPEs with pregnancy outcomes may be fetal sex-dependent (Hoffman et al., 2018b; R. Yang et al., 2022). Given the reported associations of OPE exposure with blood pressure or glucose levels (Hu et al., 2022; Y. Li et al., 2020; K. Luo et al., 2020; W. Yang et al., 2022) and the associations of maternal blood pressure or glucose during pregnancy and birth outcomes (Johns et al., 2018; Lei et al., 2018), we further explored if maternal blood pressure or glucose could mediate the effects of OPEs on pregnancy outcomes.
2. Materials and methods
2.1. Study population
The Health Outcomes and Measures of the Environment (HOME) Study is a prospective pregnancy and birth cohort in the Greater Cincinnati Metropolitan Area, Ohio, USA, designed to evaluate associations between early life exposures to environmental toxicants and children’s health (Braun et al., 2017). Pregnant women were recruited between March 2003 and January 2006 if they met the following inclusion criteria: 1) age >18 years, 2) 13–19 weeks pregnancy, 3) residing in a home built in or before 1978, 5) fluent in English, 6) planning to live in the study area for the next year, 7) planning to continue prenatal care and deliver at the participating hospitals. Women were excluded if they were living in a mobile or trailer home, were on medications for thyroid or seizure disorders, or diagnosed with bipolar disorder, schizophrenia, diabetes, or cancer requiring radiation or chemotherapy. Detailed information on the cohort and follow-up visits has been published elsewhere (Braun et al., 2020, 2017). Enrolled pregnant women understood this study before committing to participate and signed informed consent forms.
The present study included 340 participants with a live-born singleton infant without congenital malformation, who provided at least one spot urine sample during pregnancy for quantification of OPE metabolites and had neonatal anthropometry abstracted from medical records. The institutional review boards (IRB) at Cincinnati Children’s Hospital Medical Center (CCHMC) and other participating institutions approved this study. The Centers for Disease Control and Prevention (CDC) deferred to the CCHMC IRB as the IRB of record.
2.2. Maternal urinary OPE metabolites
Maternal urine samples were collected in polypropylene specimen cups at an average of 16±2 and 26±3 weeks of gestation. Samples were stored at or below −20 °C until further analysis. Samples were shipped overnight on dry ice to the CDC's National Center for Environmental Health for quantification of OPEs.
Urinary OPE metabolites are used as biomarkers of OPE exposure in epidemiological studies because, rapidly after exposure, OPEs metabolize and eliminate via urine (Blum et al., 2019; Kosarac et al., 2016; Van den Eede et al., 2013). We quantified four metabolites: bis(2-chloroethyl) phosphate (BCEP), bis(1,3-dichloro-2-propyl) phosphate (BDCIPP), di-n-butyl phosphate (DNBP), and diphenyl phosphate (DPHP) following a published analytical approach (Jayatilaka et al., 2019, 2017). In short, urinary OPE metabolite conjugates underwent enzymatic deconjugation, preconcentration with automated off-line solid-phase extraction, separation with high-performance liquid chromatography, and quantification with isotope dilution tandem mass spectrometry. The limit of detection (LOD) for each metabolite was 0.1 μg/L. Details of analytical and quality control methods for urine samples in the HOME Study have been published previously (Percy et al., 2020).
We measured specific gravity at room temperature with an Atago model PAL-10S handheld refractometer (ATAGO CO., Tokyo, Japan) at CCHMC Schubert Research Clinic after the OPE measurements. Then, we calculated specific gravity standardized concentrations to account for hydration status during pregnancy with the following formula (MacPherson et al., 2018):
where OPE metabolite SGstd represents the specific gravity standardized urinary OPE metabolite concentration, OPEi is the measured OPE metabolite concentration, SGi is the actual specific gravity of the sample, and SGm is the median specific gravity of the cohort at each time point.
2.3. Pregnancy outcomes
HOME Study staff extracted gestational age and infant anthropometric parameters at birth from medical charts. Gestational age in weeks was estimated by last menstrual period for 330 participants and by ultrasound (n=7) or Ballard scores (n=2) for very limited cases (Kalloo et al., 2020); one participant had missing information on the methods to determine gestational age. Neonatal anthropometric parameters included birth weight (g), length (cm), and head circumference (cm). We calculated sex and gestational age standardized weight z-score, length z-score, and head circumference z-score at birth using values from the 2010 Olsen growth charts (Olsen et al., 2010). The ponderal index, a measure of fetal growth, was calculated as 100 × weight(g)/length(cm)3 (Miller and Hassanein, 1971). Preterm birth was defined as birth prior to 37 completed weeks of gestation (Goldenberg et al., 2008; Hviid et al., 2022).
2.4. Covariates
The covariates included in the final models were based on their potential associations with both gestational OPE metabolite concentrations and gestational age or anthropometric parameters at birth using directed acyclic graphs (DAGs) (Supplementary Material Figure S1), which included maternal age, maternal race/ethnicity, household income, maternal education, marital status, infant sex, parity, pre-pregnancy BMI categories (underweight and normal-weight if lower than 25 kg/m2, overweight if between 25 and 29.9 kg/m2, and obesity if at or more than 30.0 kg/m2) (Rasmussen et al., 2009), maternal serum cotinine and blood lead concentrations at 16 weeks of gestation. We did not have a large proportion of missing covariates except for self-reported pre-pregnancy weight data (25% missing), which were imputed using a two-step machine learning process published previously (Romano et al., 2021). Other missing covariates were not imputed.
2.5. Statistical analysis
For analytic purposes, when the percentage of OPE metabolite concentrations below LOD was lower than 10%, the concentrations were replaced by LOD/√2 (Hornung and Reed, 1990); when the percentage was higher than 10%, we applied multiple imputations using a truncated normal distribution (Lubin et al., 2004; Uh et al., 2008). In the multiple imputation models, birth weight was the dependent variable to indicate the outcomes, and the auxiliary variables included maternal age, maternal race/ethnicity, maternal education, household income, parity, infant sex, marital status, pre-pregnancy BMI, and maternal serum cotinine and blood lead concentrations at 16 weeks of pregnancy. We generated 20 imputed datasets and used Rubin’s rule to combine the estimates from regression models (Hippel, 2018; Rubin, 1987). After standardization by specific gravity, OPE metabolite concentrations were log10-transformed to achieve approximate normal distributions.
We summarized descriptive statistics for demographic characteristics and distributions of specific gravity standardized urinary OPE metabolite concentrations. We also calculated intra-class correlation coefficients (ICCs) using a linear mixed-effects model to assess the reproducibility and temporal variability of unstandardized and specific gravity standardized OPE metabolite concentrations across the two-time points. The ICC was defined as the inter − subject variation / the total variation, ranging from 0 (poor reproducibility) to 1 (perfect reproducibility) (Luo et al., 2021; Rosner, 2015).
Since urinary OPE metabolites were quantified twice during pregnancy, we applied multiple informant models to jointly evaluate the window-specific relationships between urinary OPE metabolite concentrations and gestational age as well as newborn size at birth. Briefly, we treated the two exposure windows (16 weeks and 26 weeks of gestation) as informants using non-standard generalized estimating equations (GEE) to examine whether OPE concentrations in different windows was related to gestational age and neonatal anthropometric parameters at birth and identify potential critical windows of susceptibility (Luo et al., 2021; Sánchez et al., 2011; Zhang et al., 2018). To test the robustness of the results for gestational age, we excluded three participants with very preterm births (gestational age <32 weeks). We also conducted two complete case analyses: one excluding participants with imputed concentrations for a specific metabolite and pre-pregnancy BMI, and the other one only excluding participants with imputed concentrations for a specific metabolite. We used correction methods developed by Benjamini and Yekutieli accounting for multiple comparisons (Benjamini and Yekutieli, 2001).
We further examined period-specific risk ratios (RR) of individual OPE metabolites with preterm birth using a modified Poisson regression with robust error variance combined with multiple informant models (Zou, 2004). As a sensitivity analysis, we used e-value methodology to assess the robustness of the RRs to unmeasured confounding (VanderWeele and Ding, 2017). Additionally, the urinary OPE metabolite concentrations were modelled in tertiles of their distributions, with the 1st tertile as the reference. We assessed the linear trend by assigning the median value in each tertile as a continuous variable in the regression models (Greenland, 1995; Zhang et al., 2018). Since previous research has reported effect modifications by infant sex (Hoffman et al., 2018a), we tested the interaction between OPE metabolites and infant sex in the full models (with interaction term p<0.1 to be statistically significant) and stratified the regression models by infant sex. For complete case analyses, since the sample size dropped, we only reported the overall estimates.
In exploratory analyses, we used Cox proportional hazards regression analyses with gestational age as the underlying time scale to estimate hazard ratios (HRs) assessing the occurrence of preterm birth according to OPE metabolite concentrations at 16 weeks of gestation, 26 weeks of gestation, and their average, calculated as (concentration at 16 weeks + concentration at 26 weeks)/2 before log10 transformation (Hu et al., 2020; Mitchell et al., 2015). With a counterfactual framework, we also explored whether the associations between maternal OPE metabolite concentrations and gestational age and neonatal anthropometric parameters at birth were mediated through maternal highest blood pressure after 20 weeks of pregnancy and glucose levels from glucose challenge test in mid-pregnancy (Lamm and Zhang, 2018). To maintain temporality, we only examined the mediating effects for OPE metabolite concentration at 16 weeks of gestation.
We performed data analysis using SAS (Version 9.4; SAS Institute Inc., Cary, NC, USA) and used the CAUSALMED Procedure for the exploratory mediation analysis (Lamm and Zhang, 2018). We used R packages (mice and qgcomp) for left-truncated multiple imputation (Buuren and Groothuis-Oudshoorn, 2011; Keil, 2021; R Core Team, 2021).
3. Results
Of 340 mother-infant dyads in the analysis, more than half of the mothers were non- Hispanic white and had an annual household income of more than $40,000. Most mothers were younger than 35 years old at the time of birth (83.0%) and had at least some college education (76.8%). Nearly half of the mothers had a pre-pregnancy BMI above the normal range (48.2%) and 44.1% were nulliparous. Just over half of the infants were females (54.4%). Mean gestational age, weight-z-score, length z-score, head circumference z-score, and the ponderal index at birth were 39±2 weeks, 0.20±1.00, 0.35±0.90, 0.08±0.98, and 2.54±0.28 g/cm3, respectively (Table 1). There were 30 preterm births (8.8%), and three newborns were very preterm births (0.9%) in the cohort. Maternal characteristics of the analytic sample (n=340) at baseline were comparable to the original study (n=389 singleton live births, Supplementary Material Table S1).
Table 1.
Distribution of maternal specific gravity standardized urinary OPE metabolite concentrations at 16 weeks (μg/L), gestational age at birth (weeks), and anthropometric parameters at birth a, HOME Study (2003-2006)
| Categorical characteristics | N | BCEP (GM [GSD]) |
BDCIPP (GM [GSD]) |
DNBP (GM [GSD]) |
DPHP (GM [GSD]) |
Gestational age (mean±SD) |
Weight z-score (mean±SD) |
Length z-score (mean±SD) |
Head circumference z-score (mean±SD) |
Ponderal index (mean±SD) |
|---|---|---|---|---|---|---|---|---|---|---|
| All participants | 340 | 0.60 (3.16) | 0.80 (2.52) | 0.26 (2.05) | 1.82 (2.58) | 39.0±1.7 | 0.20±1.00 | 0.35±0.90 | 0.08±0.98 | 2.54±0.28 |
| Race/ethnicity | ||||||||||
| Non-Hispanic White | 223 | 0.51 (3.12) b | 0.77 (2.56) | 0.26 (2.12) | 1.77 (2.67) | 39.2±1.6 b | 0.37±1.01 b | 0.49±0.90 b | 0.26±0.96 b | 2.56±0.28 |
| Non-Hispanic Black and others | 117 | 0.79 (3.18) b | 0.86 (2.45) | 0.25 (1.94) | 1.91 (2.44) | 38.6±1.9 b | −0.13±0.89 b | 0.08±0.82 b | −0.24±0.94 b | 2.52±0.29 |
| Marital status | ||||||||||
| Married/living with partner | 271 | 0.55 (3.16) b | 0.76 (2.51) b | 0.25 (2.10) | 1.78 (2.64) | 39.1±1.6 | 0.30±1.00 b | 0.44±0.86 b | 0.16±0.96 b | 2.55±0.27 |
| Not married, living alone | 69 | 0.78 (3.24) b | 0.86 (2.45) b | 0.26 (1.92) | 1.96 (2.35) | 38.6±2.0 | −0.22±0.86 b | −0.03±0.93 b | −0.20±1.02 b | 2.53±0.32 |
| Child Sex | ||||||||||
| Male | 155 | 0.56 (3.41) | 0.72 (2.51) b | 0.26 (2.17) | 1.82 (2.68) | 39.0±1.8 | 0.31±1.08 | 0.40±0.92 | 0.10±0.99 | 2.53±0.24 |
| Female | 185 | 0.63 (3.02) | 0.88 (2.52) b | 0.25 (1.97) | 1.81 (2.51) | 39.0±1.7 | 0.10±0.92 | 0.30±0.88 | 0.07±0.98 | 2.55±0.31 |
| Maternal Age, years | ||||||||||
| <25 | 73 | 0.88 (3.99) c | 0.98 (2.46) | 0.25 (2.04) | 2.21 (2.31) | 38.6±2.1 c | −0.29±0.76 c | −0.09±0.82 c | −0.26±1.00 c | 2.52±0.29 |
| 25-34 | 209 | 0.54 (3.08) c | 0.78 (2.44) | 0.25 (2.12) | 1.76 (2.60) | 39.2±1.5 c | 0.33±1.00 c | 0.44±0.86 c | 0.13±0.95 c | 2.56±0.28 |
| ≥35 | 58 | 0.51 (3.58) c | 0.70 (2.87) | 0.27 (1.89) | 1.57 (2.80) | 38.8±1.7 c | 0.34±1.08 c | 0.55±0.94 c | 0.38±0.95 c | 2.52±0.27 |
| Maternal Education | ||||||||||
| High school or less | 79 | 0.71 (2.97) | 1.00 (2.58) | 0.28 (2.01) | 2.00 (2.69) | 38.9±1.6 | −0.14±0.92 c | −0.12±0.89 c | −0.18±0.98 c | 2.58±0.30 |
| Some college/2 yr degree | 80 | 0.69 (3.28) | 0.81 (2.42) | 0.25 (2.10) | 1.78 (2.31) | 38.8±1.8 | 0.10±0.89 c | 0.34±0.88 c | −0.06±1.00 c | 2.52±0.30 |
| Bachelor's | 107 | 0.51 (3.52) | 0.74 (2.61) | 0.25 (1.99) | 1.73 (2.72) | 39.2±1.6 | 0.43±1.02 c | 0.57±0.83 c | 0.28±0.96 c | 2.56±0.27 |
| Graduate or professional | 74 | 0.52 (2.80) | 0.70 (2.39) | 0.23 (2.17) | 1.79 (2.60) | 39.1±1.8 | 0.33±1.06 c | 0.52±0.84 c | 0.24±0.93 c | 2.50±0.26 |
| Family Income | ||||||||||
| <$40,000 | 125 | 0.75 (2.86) c | 0.90 (2.58) | 0.26 (2.03) | 2.09 (2.49) | 38.7±1.8 c | −0.13±0.88 c | 0.02±0.87 c | −0.24±1.01 c | 2.55±0.29 |
| $40,000-$79,999 | 118 | 0.54 (3.76) c | 0.79 (2.35) | 0.26 (2.03) | 1.61 (2.63) | 39.1±1.7 c | 0.49±1.06 c | 0.56±0.96 c | 0.32±0.91 c | 2.58±0.30 |
| ≥$80,000 | 97 | 0.49 (2.83) c | 0.70 (2.64) | 0.24 (2.14) | 1.75 (2.62) | 39.3±1.6 c | 0.26±0.95 c | 0.52±0.71 c | 0.22±0.93 c | 2.50±0.24 |
| Maternal pre-pregnancy BMI (kg/m2) | ||||||||||
| <25 | 176 | 0.52 (3.18) c | 0.76 (2.53) c | 0.24 (2.07) c | 1.78 (2.69) | 39.1±1.6 | 0.06±0.94 c | 0.26±0.84 | 0.01±0.95 | 2.52±0.26 |
| 25-29 | 94 | 0.55 (3.31) c | 0.72 (2.57) c | 0.24 (2.06) c | 1.76 (2.68) | 38.9±1.9 | 0.30±1.01 c | 0.43±0.92 | 0.20±0.93 | 2.56±0.28 |
| ≥30 | 70 | 0.93 (2.78) c | 1.06 (2.34) c | 0.30 (1.99) c | 1.99 (2.22) | 38.8±1.7 | 0.40±1.08 c | 0.46±0.99 | 0.12±1.13 | 2.58±0.32 |
| Parity | ||||||||||
| 0 | 150 | 0.58 (3.17) | 0.74 (2.47) | 0.23 (2.10) c | 1.66 (2.65) | 39.3±1.6 c | 0.03±0.98 c | 0.29±0.88 | 0.01±1.02 | 2.50±0.29 c |
| 1 | 108 | 0.58 (3.28) | 0.80 (2.34) | 0.29 (2.07) c | 1.93 (2.43) | 39.0±1.4 c | 0.28±0.92 c | 0.38±0.84 | 0.15±0.92 | 2.57±0.24 c |
| 2+ | 82 | 0.62 (3.17) | 0.93 (2.85) | 0.26 (1.94) c | 1.97 (2.66) | 38.4±2.0 c | 0.39±1.09 c | 0.41±1.00 | 0.14±1.00 | 2.59±0.30 c |
Anthropometric parameters at birth included weight/length/head circumference z-scores and the ponderal index (g/cm3).
P-value < 0.05 (two-sided p-values using t-test).
P-value < 0.05 (two-sided p-values using analysis of variance).
Abbreviations: Bis(2-chloroethyl) phosphate (BCEP), bis(1,3-dichloro-2-propyl) phosphate (BDCIPP), di-n-butyl phosphate (DNBP), and diphenyl phosphate (DPHP)
For mothers with pre-pregnancy BMI ≥30 kg/m2, urinary concentrations of BCEP, BDCIPP, and DNBP were higher, and their newborns had higher mean birth weight z-scores. Mothers who did not married or were living alone had higher BCEP and BDCIPP concentrations, but their newborns had lower mean z-scores of weight, length, and circumference at birth. Mothers who were non-White, younger than 25 years old at the time of giving births, and with an annual household income <$40,000 during pregnancy had higher urinary BCEP concentrations, and their newborns had lower gestational age at birth and lower weight, length, and head circumference z-scores at birth (Table 1).
The detection frequency and distribution of specific gravity standardized urinary OPE metabolites concentrations were similar at 16- and 26-week gestation (Table 2). DPHP was the most frequently detected metabolite in all urine samples, followed by BDCIPP, BCEP, and DNBP. For metabolites with a percentage of <LOD higher than 10%, maternal race, age, education, household income, pre-pregnancy BMI, and parity were associated with the detection of the metabolites (Supplementary Material Tables S2-S3). Generally, OPE metabolites had higher geometric mean concentrations at 16 weeks of gestation than at 26 weeks of gestation, with DPHP having the highest concentrations, followed by BDCIPP, BCEP, and DNBP (Table 2). The ICCs using specific gravity standardized OPE metabolite concentrations ranged from 0.17 (DNBP) to 0.43 (BCEP), lower than those calculated with unstandardized values, with the range from 0.24 (DPHP) to 0.51 (BCEP), which suggested poor to fair reproducibility.
Table 2.
Specific gravity (SG) standardized maternal urinary OPE metabolite concentrations (μg/L) during gestation, HOME Study (2003-2006)
| OPE metabolite | N | N<LOD (%) | GM (GSD) | Percentiles |
||
|---|---|---|---|---|---|---|
| 25th | 50th | 75th | ||||
| 16-week | ||||||
| BCEP | 339 | 40 (11.9%) | 0.60 (3.16) | 0.32 | 0.59 | 1.06 |
| BDCIPP | 335 | 14 (4.2%) | 0.80 (2.52) | 0.40 | 0.76 | 1.49 |
| DNBP | 339 | 54 (16.0%) | 0.26 (2.05) | 0.16 | 0.25 | 0.37 |
| DPHP | 339 | 4 (1.2%) | 1.82 (2.58) | 0.97 | 1.61 | 3.19 |
| 26-week | ||||||
| BCEP | 329 | 54 (16.5%) | 0.51 (4.33) | 0.22 | 0.49 | 1.08 |
| BDCIPP | 329 | 35 (10.7%) | 0.60 (3.29) | 0.27 | 0.60 | 1.12 |
| DNBP | 329 | 81 (25.2%) | 0.20 (2.29) | 0.12 | 0.20 | 0.30 |
| DPHP | 328 | 8 (2.4%) | 1.24 (2.55) | 0.68 | 1.23 | 2.07 |
Abbreviations: Bis(2-chloroethyl) phosphate (BCEP), bis(1,3-dichloro-2-propyl) phosphate (BDCIPP), di-n-butyl phosphate (DNBP), and diphenyl phosphate (DPHP)
GM, geometric mean; GSD, geometric standard deviation. LOD, limit of detection. LOD was 0.1 μg/L for all OPE metabolites.
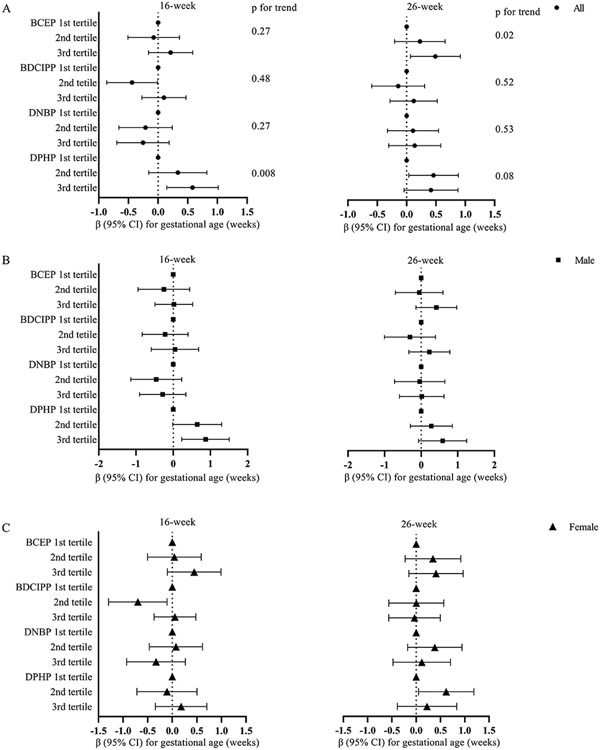
We found positive associations between certain maternal OPE metabolite concentrations with gestational age at birth (Table 3). In the adjusted model, every 10-fold increase unit increase in 26-week BCEP concentrations was associated with a 0.33-week increase in gestational age (95% CI: 0.06-0.61), but this association was not observed after stratifying by infant sex. Both 16- and 26-week DPHP concentrations were positively associated with gestational age, but only observed in male infants. The associations between DNBP and gestational age were different across the two time points (p (OPE*period) = 0.03), but the window-specific estimates did not reach statistical significance.
Table 3.
Adjusted window-specific regression estimates (in weeks) and 95% confidence intervals (CIs) between log10-transformed maternal specific gravity standardized urinary OPE metabolites (μg/L) and gestational age at birth
| OPE metabolite | All a | Male b | Female b | p (OPE*sex) |
|---|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | ||
| BCEP c | ||||
| 16-week | 0.13 (−0.16, 0.42) | 0.09 (−0.32, 0.51) | 0.10 (−0.32, 0.52) | 0.90 |
| 26-week | 0.33 (0.06, 0.61) * | 0.30 (−0.03, 0.62) | 0.23 (−0.14, 0.60) | 0.82 |
| p (OPE*window) | 0.18 | 0.31 | 0.45 | |
| BDCIPP d | ||||
| 16-week | 0.13 (−0.29, 0.54) | −0.14 (−0.82, 0.55) | 0.29 (−0.29, 0.87) | 0.29 |
| 26-week | 0.17 (−0.14, 0.48) | 0.18 (−0.24, 0.61) | 0.03 (−0.44, 0.49) | 0.48 |
| p (OPE*window) | 0.67 | 0.45 | 0.18 | |
| DNBP e | ||||
| 16-week | −0.33 (−0.87, 0.21) | −0.28 (−0.91, 0.36) | −0.58 (−1.50, 0.33) | 0.89 |
| 26-week | 0.34 (−0.09, 0.77) | −0.10 (−0.64, 0.44) | 0.39 (−0.18, 0.97) | 0.05 |
| p (OPE*window) | 0.03 | 0.52 | 0.04 | |
| DPHP f | ||||
| 16-week | 0.44 (0.08, 0.81) * | 0.61 (0.11, 1.11) * | 0.17 (−0.31, 0.65) | 0.28 |
| 26-week | 0.41 (−0.01, 0.83) | 0.92 (0.25, 1.60) * | 0.02 (−0.48, 0.53) | 0.07 |
| p (OPE*window) | 0.98 | 0.34 | 0.64 |
Adjusted for maternal age at delivery, race, household income, education, marital status, infant sex, parity, pre-pregnancy BMI, maternal serum cotinine concentrations at 16 weeks of gestation, and maternal blood lead levels at 16 weeks of gestation.
Adjusted for maternal age at delivery, race, household income, education, marital status, parity, pre-pregnancy BMI, maternal serum cotinine concentrations at 16 weeks of gestation, and maternal blood lead levels at 16 weeks of gestation.
Number of observations used: n = 339 (male: 155, female: 184) for BCEP at 16 weeks, n = 329 (male: 152, female: 177) for BCEP at 26 weeks.
Number of observations used: n = 335 (male: 153, female: 182) for BDCIPP at 16 weeks, n = 329 (male: 152, female: 177) for BDCIPP at 26 weeks.
Number of observations used: n = 339 (male: 155, female: 184) for DNBP at 16 weeks, n = 329 (male: 152, female: 177) for DNBP at 26 weeks.
Number of observations used: n = 339 (male: 155, female: 184) for DPHP at 16 weeks, n = 328 (male: 151, female: 177) for DPHP at 26 weeks.
indicates statistical significance.
Abbreviations: Bis(2-chloroethyl) phosphate (BCEP), bis(1,3-dichloro-2-propyl) phosphate (BDCIPP), di-n-butyl phosphate (DNBP), and diphenyl phosphate (DPHP)
Maternal concentrations of BCEP were negatively associated with weight and length z-scores at birth among female newborns (Table 4). Specifically, every log10-transformed unit increase in BCEP concentrations at 16 weeks of gestation was related to a decrease of 0.25 in birth weight z-score (95% CI: −0.46, −0.04) and a decrease of 0.31 (95% CI: −0.56, −0.07) in birth length z-score; also, BCEP concentration at 26 weeks of gestation was negatively associated with length z-score at birth for females (β= −0.18 [95% CI: −0.35, −0.02]). The associations between DNBP concentrations and the ponderal index at birth varied at the two time points (p (OPE* period) = 0.02) but only among male infants (for 16-week DNBP, β=0.06 [95% CI: −0.05, 0.17], p (OPE*sex) = 0.61; for 26-week DNBP, β= −0.13 [95% CI: −0.23, −0.03], p (OPE*sex) = 0.02; Supplementary Material Table S4). No other associations were observed for BCEP, BDCIPP and DPHP with the ponderal index. But after adjustment for multiple comparisons, the associations became not statistically significant.
Table 4.
Adjusted window-specific regression estimates and 95% confidence intervals (CIs) between log10-transformed maternal specific gravity standardized urinary OPE metabolites (μg/L) during gestation and anthropometric parameters at birth
| OPE metabolite |
Weight z-score | Length z-score | Head circumference z-score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All a | Male b | Female b | p (OPE*sex) | All a | Male b | Female b | p (OPE*sex) | All a | Male b | Female b | p (OPE* sex) | ||
| β (95% CI) |
β (95% CI) |
β (95% CI) |
β (95% CI) |
β (95% CI) |
β (95% CI) |
β (95% CI) |
β (95% CI) |
β (95% CI) |
|||||
| BCEP c | |||||||||||||
| 16-week | −0.11 (−0.31, 0.09) | 0.03 (−0.29, 0.35) | −0.25 (−0.46, −0.04)* | 0.26 | −0.17 (−0.36, 0.02) | −0.05 (−0.32, 0.22) | −0.31 (−0.56, −0.07)* | 0.28 | 0.02 (−0.20, 0.24) | 0.03 (−0.26, 0.31) | 0.11 (−0.22, 0.44) | 0.98 | |
| 26-week | 0.07 (−0.11, 0.26) | 0.20 (−0.14, 0.54) | −0.11 (−0.26, 0.04) | 0.14 | −0.06 (−0.20, 0.08) | 0.01 (−0.21, 0.23) | −0.18 (−0.35, −0.02)* | 0.21 | −0.02 (−0.18, 0.14) | 0.01 (−0.23, 0.25) | 0.01 (−0.19, 0.21) | 0.93 | |
| p (OPE* window) | 0.07 | 0.34 | 0.25 | 0.27 | 0.69 | 0.43 | 0.95 | 0.98 | 0.60 | ||||
| BDCIPP d | |||||||||||||
| 16-week | −0.14 (−0.39, 0.11) | −0.22 (−0.60, 0.16) | −0.08 (−0.37, 0.21) | 0.41 | −0.08 (−0.30, 0.15) | −0.11 (−0.44, 0.21) | −0.12 (−0.42, 0.17) | 0.90 | −0.22 (−0.46, 0.03) | −0.18 (−0.56, 0.21) | −0.25 (−0.58, 0.09) | 0.69 | |
| 26-week | 0.02 (−0.16, 0.20) | 0.02 (−0.23, 0.27) | −0.05 (−0.28, 0.18) | 0.80 | −0.06 (−0.22, 0.11) | 0 (−0.22, 0.22) | −0.14 (−0.38, 0.10) | 0.36 | −0.15 (−0.34, 0.04) | −0.15(−0.40, 0.10) | −0.15 (−0.42, 0.12) | 0.81 | |
| p (OPE* window) | 0.15 | 0.13 | 0.84 | 0.92 | 0.32 | 0.75 | 0.57 | 0.84 | 0.53 | ||||
| DNBP e | |||||||||||||
| 16-week | −0.02 (−0.40, 0.36) | −0.06 (−0.64, 0.52) | 0.03 (−0.42, 0.48) | 0.57 | −0.07 (−0.40, 0.26) | −0.22 (−0.72, 0.29) | 0.08 (−0.37, 0.54) | 0.20 | −0.10 (−0.49, 0.29) | −0.06 (−0.62, 0.50) | −0.14 (−0.64, 0.36) | 0.82 | |
| 26-week | −0.20 (−0.50, 0.11) | −0.49 (−1.00, 0.01) | 0 (−0.37, 0.38) | 0.004 | −0.11 (−0.39, 0.17) | −0.20 (−0.68, 0.28) | −0.06 (−0.39, 0.27) | 0.27 | −0.08 (−0.38, 0.23) | −0.46(−0.96, 0.04) | 0.19 (−0.17, 0.56) | 0.008 | |
| p (OPE*window) | 0.33 | 0.16 | 0.97 | 0.48 | 0.80 | 0.31 | 0.96 | 0.25 | 0.32 | ||||
| DPHP f | |||||||||||||
| 16-week | 0.14 (−0.10, 0.37) | 0.29 (−0.09, 0.66) | 0.02 (−0.24, 0.28) | 0.36 | 0.13 (−0.08, 0.34) | 0.30 (−0.02, 0.62) | 0.02 (−0.24, 0.28) | 0.33 | 0.13 (−0.10, 0.36) | 0.32 (−0.02, 0.66) | 0.03 (−0.24, 0.30) | 0.24 | |
| 26-week | 0 (−0.24, 0.25) | −0.09 (−0.58, 0.39) | 0.04 (−0.21, 0.29) | 0.48 | 0.05 (−0.18, 0.28) | 0.13 (−0.27, 0.53) | −0.01 (−0.27, 0.24) | 0.75 | −0.10 (−0.34, 0.14) | −0.08 (−0.47, 0.31) | −0.05 (−0.32, 0.22) | 0.99 | |
| p (OPE*window) | 0.37 | 0.12 | 0.91 | 0.59 | 0.39 | 0.87 | 0.15 | 0.13 | 0.63 | ||||
Adjusted for maternal age at delivery, race, household income, education, marital status, infant sex, parity, pre-pregnancy BMI, maternal serum cotinine concentrations at 16 weeks of gestation, and maternal blood lead levels at 16 weeks of gestation.
Adjusted for maternal age at delivery, race, household income, education, marital status, parity, pre-pregnancy BMI, maternal serum cotinine concentrations at 16 weeks of gestation, and maternal blood lead levels at 16 weeks of gestation.
The range of sample size: n = 330-339 (males: 148-155, females: 182-184) for BCEP at 16 weeks, n = 320-329 (males: 145-152, females: 175-177) for BCEP at 26 weeks.
The range of sample size: n = 326-335 (males: 146-153, females: 180-182) for BDCIPP at 16 weeks, n = 320-329 (males: 145-152, females: 175-177) for BDCIPP at 26 weeks.
The range of sample size: n = 330-339 (males: 148-155, females: 182-184) for DNBP at 16 weeks, n = 320-329 (males: 145-152, females: 175-177) for DNBP at 26 weeks.
The range of sample size: n = 330-339 (males: 148-155, females: 182-184) for DPHP at 16 weeks, n = 319-328 (males: 144-151, females: 175-177) for DPHP at 26 weeks.
indicates statistical significance.
Abbreviations: Bis(2-chloroethyl) phosphate (BCEP), bis(1,3-dichloro-2-propyl) phosphate (BDCIPP), di-n-butyl phosphate (DNBP), and diphenyl phosphate (DPHP).
We found that concentrations (per log10 increment) of both BCEP and DPHP at 26 weeks of gestation were inversely associated with preterm birth (for BCEP, RR=0.48 [95% CI: 0.27, 0.83]; for DPHP, RR=0.32 [95% CI: 0.11, 0.92]; Supplementary Material Table S5), consistent with previous positive findings of gestational age. The E-values for the point estimates and upper confidence bound were 3.61 and 1.69 for BCEP, and 5.68 and 1.39 for DPHP. In sex-stratified analyses, the association only existed for DPHP at 26 weeks of gestation among males (RR=0.14 [95% CI: 0.04, 0.48], p (OPE* sex)=0.41). The results from the Cox proportional hazard models indicated that 26-week and the average concentrations of BCEP and DPHP were inversely associated with preterm birth (Supplementary Material Table S6).
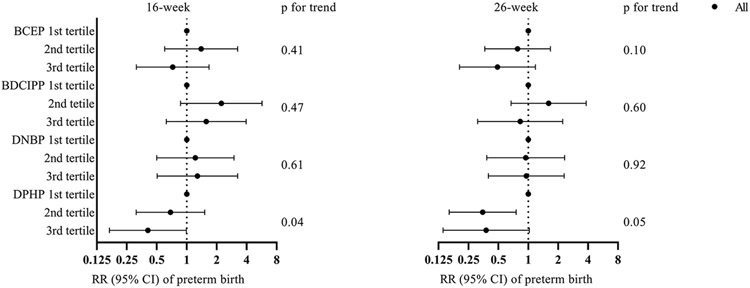
We identified similar associations between the tertiles of maternal urinary OPE metabolite concentrations and gestational age (Figure 1). Maternal urinary BCEP concentrations at 26 weeks had a positive trend with gestational age (p for trend=0.02). 16-week and 26-week DPHP concentrations also tended to be positively associated with gestational age at delivery (p for trend=0.008 and 0.08, respectively). When compared to the 1st tertile of urinary DPHP concentrations, the 3rd tertile of 16-week concentrations were inversely associated with preterm birth (RR=0.41, 95% CI [0.17, 0.99], p for trend=0.04, Figure 2). The inverse associations were also observed for the 2nd and 3rd tertiles of DPHP at 26 weeks of gestation (RR=0.35, 95% CI [0.16, 0.76]; RR=0.38, 95% CI [0.14, 1.03], respectively; p for trend=0.05). We did not find any linear trends for neonatal anthropometric measurement z-scores and the ponderal index (Supplementary Material Figures S2-S5).
Figure 1.
Adjusted window-specific regression estimates (β and its 95% CI) between tertiles of maternal specific gravity standardized urinary OPE metabolites (μg/L) and gestational age (weeks) at birth. A. Overall associations: models adjusted for maternal age at delivery, race, household income, education, marital status, infant sex, parity, pre-pregnancy BMI, maternal serum cotinine concentrations at 16 weeks of gestation, and maternal blood lead levels at 16 weeks of gestation. P for trend tests the linear trend by assigning the median of each tertile of each OPE metabolite concentration. B (male) & C (female). Infant sex-specific associations: models adjusted for maternal age at delivery, race, household income, education, marital status, parity, pre-pregnancy BMI, maternal serum cotinine concentrations at 16 weeks of gestation, and maternal blood lead levels at 16 weeks of gestation.
Cut-off points of the tertiles at 16 weeks were: 0.40 and 0.88 μg/L for BCEP, 0.51 and 1.13 μg/L for BDCIPP, 0.18 and 0.32 μg/L for DNBP, and 1.13 and 2.36 μg/L for DPHP; Cut-off points of the tertiles at 26 weeks were: 0.28 and 0.77 μg/L for BCEP, 0.34 and 0.82 μg/L for BDCIPP, 0.14 and 0.26 μg/L for DNBP, and 0.86 and 1.67 μg/L for DPHP.
Abbreviations: Bis(2-chloroethyl) phosphate (BCEP), bis(1,3-dichloro-2-propyl) phosphate (BDCIPP), di-n-butyl phosphate (DNBP), and diphenyl phosphate (DPHP).
Figure 2.
Adjusted window-specific relative risks (RRs) and 95% confidence intervals for preterm birth across tertiles of maternal specific gravity standardized urinary OPE metabolite concentrations (μg/L). Models adjusted for maternal age at delivery, race, household income, education, marital status, infant sex, parity, pre-pregnancy BMI, maternal serum cotinine concentrations at 16 weeks of gestation, and maternal blood lead levels at 16 weeks of gestation.
Cut-off points of the tertiles at 16 weeks were: 0.40 and 0.88 μg/L for BCEP, 0.51 and 1.13 μg/L for BDCIPP, 0.18 and 0.32 μg/L for DNBP, and 1.13 and 2.36 μg/L for DPHP; Cut-off points of the tertiles at 26 weeks were: 0.28 and 0.77 μg/L for BCEP, 0.34 and 0.82 μg/L for BDCIPP, 0.14 and 0.26 μg/L for DNBP, and 0.86 and 1.67 μg/L for DPHP.
Abbreviations: Bis(2-chloroethyl) phosphate (BCEP), bis(1,3-dichloro-2-propyl) phosphate (BDCIPP), di-n-butyl phosphate (DNBP), and diphenyl phosphate (DPHP).
Additionally, we explored whether the associations between maternal urinary OPE metabolite concentrations at 16 weeks and pregnancy outcomes were mediated through the highest maternal blood pressure or maternal glucose levels. The average of the highest maternal blood pressure was 117.39 ± 13.56 mmHg and 71.85 ± 9.07 mmHg; the average of maternal glucose was 102.22 ± 29.31 mg/dL, indicating the measurements were within normal limits for most participants, but we identified 27 cases of physician-diagnosed gestational hypertension or pre-eclampsia, and 11 cases of physician-diagnosed diabetes. The results showed negligible natural indirect effects, indicating neither the highest maternal blood pressure nor maternal glucose levels acted as the mediators on the pathway between maternal OPE exposure and gestational age as well as neonatal anthropometry (Supplementary Material Tables S7-S11).
To test the robustness of the estimates from multiple informant models, we excluded the three very preterm births (gestation length <32 weeks) to eliminate the potential influence of extreme values. The results did not change substantially compared with those in the primary analysis (Supplementary Material Tables S12-S14). But the associations between both BDCIPP and DNBP concentrations at 26 weeks of pregnancy and gestational age at delivery became positive (β=0.27 weeks [95% CI: 0.01, 0.53] for BDCIPP; β=0.39 weeks [95% CI: 0.00, 0.77] for DNBP). In complete case analyses, the results stayed similar when only excluding participants with imputed concentrations for a specific metabolite but certain associations became stronger when further excluding participants with imputed pre-pregnancy BMI (Supplementary Material Table S15).
4. Discussion
In the present study, we used data from mother-newborn pairs enrolled in a prospective pregnancy and birth cohort to examine the window-specific associations between four OPE metabolites (quantified in maternal urine collected at 16 and 26 weeks of gestation) and pregnancy outcomes, including gestational age, weight z-score, length z-score, head circumference z-score, and the ponderal index at birth. Our results showed that BCEP and DPHP concentrations in maternal urine at 16 weeks or 26 weeks of pregnancy were positively associated with gestational age and inversely with preterm birth. BCEP was negatively associated with birth weight and length z-scores in female newborns. Negative associations between DNBP concentrations at 26 weeks of gestation and the ponderal index at birth were only observed in males. No mediation by the highest maternal blood pressure or glucose levels during pregnancy were identified.
Accumulating evidence from experimental studies suggests that OPE exposure can impact embryonic development. Chronic exposure to TPHP caused developmental and reproductive toxicities in Daphnia magna (Yuan et al., 2018). A more recent study reported chronic exposure to TCEP promoted the growth of Daphnia magna, which might be related to the change of cytosolic DNA-sensing pathway after TCEP exposure (W. Li et al., 2020). But decreased body length was observed after exposure to TCEP and TPHP in Japanese medaka (Sun et al., 2016). Parental exposure to TDCIPP has been shown to reduce offspring growth in Daphnia magna (Li et al., 2017, 2015) and zebrafish (Ren et al., 2019; Yu et al., 2017; Zhu et al., 2015). Further transcriptional analysis showed that TDCIPP-induced developmental toxicities might be related to disturbed pathways of protein synthesis and metabolism and endocytosis in Daphnia magna (Li et al., 2015) while down-regulation of genes in the growth hormone/insulin-like growth factor axis might be responsible for transgenerational toxicity in zebrafish (Yu et al., 2017). Another recent study reported that exposure to TCEP decreased body length and delayed hatching in zebrafish, which could be related to altered thyroid hormones and gene expression in hypothalamic–pituitary–thyroid (HPT) axis (Hu et al. (2021); such findings may help explain the associations of BCEP with increased gestational age and decreased infant size z-scores at birth. Although the exact mechanisms of developmental toxicities of OPE chemicals have not been elucidated, their potential endocrine-disrupting properties, especially their interference with insulin, glucocorticoid, estrogenic, and thyroid nuclear receptors, may cause disturbance on the health status of both the mother and the fetus (Kojima et al., 2013; Street and Bernasconi, 2020). Nevertheless, given the mixed findings from experimental studies, it is difficult to conclude the impacts of OPEs exposure on offspring growth in animals.
OPEs have been widely detected among pregnant individuals (Hoffman et al., 2017b; Kosarac et al., 2016; Kuiper et al., 2020; Luo et al., 2021; Percy et al., 2020; Romano et al., 2017). The exposure levels in our cohort were generally comparable to those reported in the 2013-2014 National Health and Nutrition Examination Survey (NHANES) for females aging 20-59 years old and other cohorts in the U.S. with some exceptions (Percy et al., 2020). Only three published epidemiological studies investigated the association between maternal urinary OPE metabolites and gestational age at birth, with conflicting results (Crawford et al., 2020; Hoffman et al., 2018a; Kuiper et al., 2020). Hoffman et al. measured OPE metabolites in one spot urine sample collected at the late 2nd trimester or early 3rd trimester in the Pregnancy Infection and Nutrition Study (PIN) with 349 mother-infant dyads in North Carolina; maternal BDCIPP concentrations were associated with shorter gestation among female infants, and DPHP was not associated with gestation in either sex (Hoffman et al., 2018a). Kuiper et al. quantified five maternal urinary OPE metabolites at up to three visits during the 2nd and 3rd trimesters for 76 pregnant women in Maryland and they did not identify any metabolites concentrations associated with gestational age at birth (Kuiper et al., 2020). Another study with a relatively small sample size (N=56) in Rhode Island also did not report any associations between three metabolites and gestational age at birth (Crawford et al., 2020). In our study, we found that BCEP and DPHP were associated with longer gestation, but its clinical significance may need further research. These discrepant findings may relate to differences in concentrations of OPE metabolites quantified and analytical methods used for quantification, study period, timing of urine collection, sample size, and study population. Our findings on preterm birth should be interpreted with caution because we only had 30 preterm births in the dataset, and a larger study will be needed to examine preterm birth as a binary outcome.
To the best of our knowledge, associations between gestational OPE exposure and fetal growth measures were examined in seven studies, with inconsistent results (Bommarito et al., 2021b; Crawford et al., 2020; Feng et al., 2016; Hoffman et al., 2018a; Kuiper et al., 2020; D. Luo et al., 2020; Luo et al., 2021). Different outcomes have been assessed, including birth weight (Crawford et al., 2020; Feng et al., 2016; Hoffman et al., 2018a; Luo et al., 2021), birth weight z-score (Hoffman et al., 2018a; Kuiper et al., 2020), birth length (Crawford et al., 2020; Kuiper et al., 2020; Luo et al., 2021), head circumference (Crawford et al., 2020), abdominal circumference (Crawford et al., 2020), the ponderal index (Kuiper et al., 2020), low birth weight (D. Luo et al., 2020), small- or large-for-gestational-age (Bommarito et al., 2021b), respectively. Feng et al. did not find any correlations between DPHP or BDCIPP and birth weight among pregnant women (Feng et al., 2016). Null associations of maternal OPE metabolites with birth weight z-score and birth length were also reported by Kuiper et al. (Kuiper et al., 2020). Similarly, Crawford et al. also found null associations of BCEP, BDCPP, and DPHP with weight, length, head circumference, abdominal circumference at birth (Crawford et al., 2020). But Kuiper found positive associations of BDCIPP with the ponderal index (0.06 g/cm3 [95 % CI: 0, 0.12] per standard deviation increase) (Kuiper et al., 2020). Luo et al. found that increased maternal urinary DPHP concentrations were associated with low birth weight in a nested case-control study (D. Luo et al., 2020). Bommarito et al. reported that gestational exposure to OPE mixtures was associated with lower odds of LGA births (OR: 0.49, [95% CI: 0.27, 0.89]) (Bommarito et al., 2021b). The inconsistent findings might be explained by the different outcomes examined in each study (for example, using birth weight adjusting for gestational age vs. using birth weight z-score), the target OPE metabolites, study period (before vs. after the wide application of OPEs) and regions, as well as sampling timing.
Identifying windows of susceptibility to environmental chemicals during pregnancy can improve maternal and child health (Luo et al., 2021; Sánchez et al., 2011). Recently, Luo et al. reported trimester-specific associations between maternal OPE exposure biomarkers and birth size: they identified the 3rd trimester as a potential critical exposure window for maternal BDCIPP and bis(2-butoxyethyl) phosphate to adversely affect birth size(Luo et al., 2021). In our study, although we found that both BCEP and DPHP were associated with newborn size measures, the estimates were not heterogeneous across the two time points. The relationship between DNBP and the ponderal index varied at 16 and 26 weeks of gestation but this association was only noted in males, suggesting that the influence of DNBP on body mass might be sex-specific and time-sensitive. Future research with repeated measures of OPE exposure at each trimester plus an ultrasound assessment or biomarkers of fetal growth may help interpret and validate the findings.
The study has several strengths. Repeated measurements of maternal urinary OPE concentrations during pregnancy helped us examine windows of susceptibility. To account for the repeated exposure measurements, we used a non-standardized GEE modeling approach with formal testing of difference in estimates across a priori defined windows (Sánchez et al., 2011). Also, detailed information on covariates has been collected to control for potential confounders.
Our findings still need to be interpreted with caution due to several limitations. First, we did not collect maternal urine samples during the 1st trimester, a critical window for fetal development and growth; we also did not consider that the time of sample collection (e.g. morning vs. afternoon) may be related to the exposure level. Second, our cohort was established between 2003 and 2006, the period before the wider application of OPEs. Still, the concentrations of urinary metabolites (e.g., DPHP, BCEP, and BDCIPP) were not largely different from those measured in U.S. birth cohorts established later (Hoffman et al., 2014; Romano et al., 2017). Also, we only evaluated associations between four OPE metabolites and pregnancy outcomes, so we may have overlooked other unmeasured OPEs (e.g., isopropylphenyl phenyl phosphate, bis(2-butoxyethyl) phosphate). We did not assess fetal growth during the in utero period or biomarkers of metabolism among newborns to provide better interpretation of our birth outcome findings. Additionally, we cannot rule out the possibility of residual confounding (such as maternal diet and physical activity). Based on the E-values for the upper confidence limit, comparatively weaker confounder associations might explain away the observed associations between BCEP and DPHP at 26 weeks of pregnancy and preterm birth. Moreover, we did not consider exposure to other environmental toxicants, which may also impact fetal development. Admittedly, the associations were not statistically significant after adjustment for multiple comparisons. But in the scenario of environmental health, we assume that the cost of a false negative is higher than a false positive. Considering the limitations of this study, future research conducted in different populations would be useful to confirm the findings. Also, given the changes in exposure to chemicals at different time periods, it will be meaningful to conduct age-period-cohort analyses in larger cohorts with longer enrollment period to decompose statistics into age, period, and cohort effects, which may help illustrate the effect of the time-varying exposure on pregnancy outcomes.
5. Conclusion
In our cohort, increased BCEP and DPHP concentrations in maternal urine at 16 weeks or 26 weeks of gestation were associated with longer gestation and reduced risk of preterm birth. BCEP was also negatively associated with weight and length z-scores at birth in females. Negative associations between 26-week DNBP and the ponderal index at birth were only observed in male infants. Given the ubiquitous OPE exposure and potential impacts on life-long health, other studies can help investigating these associations with exposure assessment in the first and third trimesters.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Environmental Health Sciences and the US Environmental Protection Agency (NIEHS P01 ES11261, R01 ES014575, R01 ES020349, R01 ES027224, R01 ES028277, P30 ES006096; EPA P01 R829389).
The study protocol was approved by the Institutional Review Board (IRB) at the Cincinnati Children’s Hospital Medical Center (CCHMC). The Centers for Disease Control and Prevention (CDC) deferred to the CCHMC IRB as the IRB of record.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
Reference:
- Andrae NJ, others, 2008. Durable and environmentally friendly flame retardants for synthetics. [Google Scholar]
- Benjamini Y, Yekutieli D, 2001. The control of the false discovery rate in multiple testing under dependency. Ann. Statist 29. 10.1214/aos/1013699998 [DOI] [Google Scholar]
- Blum A, Behl M, Birnbaum LS, Diamond ML, Phillips A, Singla V, Sipes NS, Stapleton HM, Venier M, 2019. Organophosphate Ester Flame Retardants: Are They a Regrettable Substitution for Polybrominated Diphenyl Ethers? Environ. Sci. Technol. Lett 6, 638–649. 10.1021/acs.estlett.9b00582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum A, Gold MD, Ames BN, Kenyon C, Jones FR, Hett EA, Dougherty RC, Horning EC, Dzidic I, Carroll DI, Stillwell RN, Thenot J-P, 1978. Children Absorb Tris-BP Flame Retardant from Sleepwear: Urine Contains the Mutagenic Metabolite, 2,3-Dibromopropanol. Science 201, 1020–1023. 10.1126/science.684422 [DOI] [PubMed] [Google Scholar]
- Bommarito PA, Welch BM, Keil AP, Baker GP, Cantonwine DE, McElrath TF, Ferguson KK, 2021a. Prenatal exposure to consumer product chemical mixtures and size for gestational age at delivery. Environ Health 20, 68. 10.1186/s12940-021-00724-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bommarito PA, Welch BM, Keil AP, Baker GP, Cantonwine DE, McElrath TF, Ferguson KK, 2021b. Prenatal exposure to consumer product chemical mixtures and size for gestational age at delivery. Environ Health 20, 68. 10.1186/s12940-021-00724-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Buckley JP, Cecil KM, Chen A, Kalkwarf HJ, Lanphear BP, Xu Y, Woeste A, Yolton K, 2020. Adolescent follow-up in the Health Outcomes and Measures of the Environment (HOME) Study: Cohort profile. BMJ Open 10. 10.1136/bmjopen-2019-034838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalloo G, Chen A, Dietrich KN, Liddy-Hicks S, Morgan S, Xu Y, Yolton K, Lanphear BP, 2017. Cohort Profile: The Health Outcomes and Measures of the Environment (HOME) study. Int J Epidemiol 46, 24. 10.1093/ije/dyw006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt CM, Congleton J, Hoffman K, Fang M, Stapleton HM, 2014. Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers. Environ Sci Technol 48, 10432–10438. 10.1021/es5025299 [DOI] [PubMed] [Google Scholar]
- van Buuren S, Groothuis-Oudshoorn K, 2011. mice: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software 45, 1–67. [Google Scholar]
- Carignan CC, Mínguez-Alarcón L, Butt CM, Williams PL, Meeker JD, Stapleton HM, Toth TL, Ford JB, Hauser R, for the EARTH Study Team, 2017. Urinary Concentrations of Organophosphate Flame Retardant Metabolites and Pregnancy Outcomes among Women Undergoing in Vitro Fertilization. Environ Health Perspect 125, 087018. 10.1289/EHP1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cequier E, Sakhi AK, Marcé RM, Becher G, Thomsen C, 2015. Human exposure pathways to organophosphate triesters — A biomonitoring study of mother–child pairs. Environment International 75, 159–165. 10.1016/j.envint.2014.11.009 [DOI] [PubMed] [Google Scholar]
- Chen Y, Fang J, Ren L, Fan R, Zhang J, Liu G, Zhou L, Chen D, Yu Y, Lu S, 2018. Urinary metabolites of organophosphate esters in children in South China: Concentrations, profiles and estimated daily intake. Environmental Pollution 235, 358–364. 10.1016/j.envpol.2017.12.092 [DOI] [PubMed] [Google Scholar]
- Crawford KA, Hawley N, Calafat AM, Jayatilaka NK, Froehlich RJ, Has P, Gallagher LG, Savitz DA, Braun JM, Werner EF, Romano ME, 2020. Maternal urinary concentrations of organophosphate ester metabolites: associations with gestational weight gain, early life anthropometry, and infant eating behaviors among mothers-infant pairs in Rhode Island. Environ Health 19, 97. 10.1186/s12940-020-00648-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump D, Chiu S, Kennedy SW, 2012. Effects of Tris(1,3-dichloro-2-propyl) phosphate and Tris(1-chloropropyl) phosphate on Cytotoxicity and mRNA Expression in Primary Cultures of Avian Hepatocytes and Neuronal Cells. Toxicological Sciences 126, 140–148. 10.1093/toxsci/kfs015 [DOI] [PubMed] [Google Scholar]
- Ding J, Deng T, Ye X, Covaci A, Liu J, Yang F, 2019. Urinary metabolites of organophosphate esters and implications for exposure pathways in adolescents from Eastern China. Science of The Total Environment 695, 133894. 10.1016/j.scitotenv.2019.133894 [DOI] [PubMed] [Google Scholar]
- Ding J, Xu Z, Huang W, Feng L, Yang F, 2016. Organophosphate ester flame retardants and plasticizers in human placenta in Eastern China. Science of The Total Environment 554–555, 211–217. 10.1016/j.scitotenv.2016.02.171 [DOI] [PubMed] [Google Scholar]
- Farhat A, Crump D, Chiu S, Williams KL, Letcher RJ, Gauthier LT, Kennedy SW, 2013. In Ovo Effects of Two Organophosphate Flame Retardants—TCPP and TDCPP—on Pipping Success, Development, mRNA Expression, and Thyroid Hormone Levels in Chicken Embryos. Toxicological Sciences 134, 92–102. 10.1093/toxsci/kft100 [DOI] [PubMed] [Google Scholar]
- Feng L, Ouyang F, Liu L, Wang Xu, Wang Xia, Li Y-J, Murtha A, Shen H, Zhang J, Zhang JJ, 2016. Levels of Urinary Metabolites of Organophosphate Flame Retardants, TDCIPP, and TPHP, in Pregnant Women in Shanghai. J Environ Public Health 2016, 9416054. 10.1155/2016/9416054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Han J, Zhou B, Gong Z, Santos EM, Huo X, Zheng W, Liu H, Yu H, Liu C, 2013. Toxicogenomic Responses of Zebrafish Embryos/Larvae to Tris(1,3-dichloro-2-propyl) Phosphate (TDCPP) Reveal Possible Molecular Mechanisms of Developmental Toxicity. Environ. Sci. Technol 47, 10574–10582. 10.1021/es401265q [DOI] [PubMed] [Google Scholar]
- Gold MD, Blum A, Ames BN, 1978. Another Flame Retardant, Tris-(1,3-Dichloro-2-Propyl)-Phosphate, and Its Expected Metabolites Are Mutagens. Science 200, 785–787. 10.1126/science.347576 [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R, 2008. Epidemiology and causes of preterm birth. The Lancet 371, 75–84. 10.1016/S0140-6736(08)60074-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S, 1995. Avoiding Power Loss Associated with Categorization and Ordinal Scores in Dose-Response and Trend Analysis: Epidemiology 6, 450–454. 10.1097/00001648-199507000-00025 [DOI] [PubMed] [Google Scholar]
- Hippel PT von, 2018. How many imputations do you need? A two-stage calculation using a quadratic rule. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Butt CM, Webster TF, Preston EV, Hammel SC, Makey C, Lorenzo AM, Cooper EM, Carignan C, Meeker JD, Hauser R, Soubry A, Murphy SK, Price TM, Hoyo C, Mendelsohn E, Congleton J, Daniels JL, Stapleton HM, 2017a. Temporal Trends in Exposure to Organophosphate Flame Retardants in the United States. Environ. Sci. Technol. Lett 4, 112–118. 10.1021/acs.estlett.6b00475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Daniels JL, Stapleton HM, 2014. Urinary metabolites of organophosphate flame retardants and their variability in pregnant women. Environment International 63, 169–172. 10.1016/j.envint.2013.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Garantziotis S, Birnbaum LS, Stapleton HM, 2015. Monitoring Indoor Exposure to Organophosphate Flame Retardants: Hand Wipes and House Dust. Environmental Health Perspectives 123, 160–165. 10.1289/ehp.1408669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Lorenzo A, Butt CM, Adair L, Herring AH, Stapleton HM, Daniels JL, 2017b. Predictors of urinary flame retardant concentration among pregnant women. Environ. Int 98, 96–101. 10.1016/j.envint.2016.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Stapleton HM, Lorenzo A, Butt CM, Adair L, Herring AH, Daniels JL, 2018a. Prenatal exposure to organophosphates and associations with birthweight and gestational length. Environment International 116, 248–254. 10.1016/j.envint.2018.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Stapleton HM, Lorenzo A, Butt CM, Adair L, Herring AH, Daniels JL, 2018b. Prenatal exposure to organophosphates and associations with birthweight and gestational length. Environ Int 116, 248–254. 10.1016/j.envint.2018.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, Reed LD, 1990. Estimation of Average Concentration in the Presence of Nondetectable Values. Applied Occupational and Environmental Hygiene 5, 46–51. 10.1080/1047322X.1990.10389587 [DOI] [Google Scholar]
- Hou M, Shi Y, Na G, Cai Y, 2021. A review of organophosphate esters in indoor dust, air, hand wipes and silicone wristbands: Implications for human exposure. Environment International 146, 106261. 10.1016/j.envint.2020.106261 [DOI] [PubMed] [Google Scholar]
- Hu JMY, Arbuckle TE, Janssen P, Lanphear BP, Braun JM, Platt RW, Chen A, Fraser WD, McCandless LC, 2020. Associations of prenatal urinary phthalate exposure with preterm birth: the Maternal-Infant Research on Environmental Chemicals (MIREC) Study. Can J Public Health 111, 333–341. 10.17269/s41997-020-00322-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Yu M, Li Y, Liu L, Li X, Song L, Wang Y, Mei S, 2022. Association of exposure to organophosphate esters with increased blood pressure in children and adolescents. Environmental Pollution 295, 118685. 10.1016/j.envpol.2021.118685 [DOI] [PubMed] [Google Scholar]
- Hu Z, Yin L, Wen X, Jiang C, Long Y, Zhang J, Liu R, 2021. Organophosphate Esters in China: Fate, Occurrence, and Human Exposure. Toxics 9, 310. 10.3390/toxics9110310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hviid A, Laksafoss A, Hedley P, Lausten-Thomsen U, Hjalgrim H, Christiansen M, Olsen SF, 2022. Assessment of Seasonality and Extremely Preterm Birth in Denmark. JAMA Netw Open 5, e2145800. 10.1001/jamanetworkopen.2021.45800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayatilaka NK, Restrepo P, Davis Z, Vidal M, Calafat AM, Ospina M, 2019. Quantification of 16 urinary biomarkers of exposure to flame retardants, plasticizers, and organophosphate insecticides for biomonitoring studies. Chemosphere 235, 481–491. 10.1016/j.chemosphere.2019.06.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayatilaka NK, Restrepo P, Williams L, Ospina M, Valentin-Blasini L, Calafat AM, 2017. Quantification of three chlorinated dialkyl phosphates, diphenyl phosphate, 2,3,4,5-tetrabromobenzoic acid, and four other organophosphates in human urine by solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 409, 1323–1332. 10.1007/s00216-016-0061-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns EC, Denison FC, Norman JE, Reynolds RM, 2018. Gestational Diabetes Mellitus: Mechanisms, Treatment, and Complications. Trends in Endocrinology & Metabolism 29, 743–754. 10.1016/j.tem.2018.09.004 [DOI] [PubMed] [Google Scholar]
- Kalloo G, Wellenius GA, McCandless L, Calafat AM, Sjodin A, Romano ME, Karagas MR, Chen A, Yolton K, Lanphear BP, Braun JM, 2020. Exposures to chemical mixtures during pregnancy and neonatal outcomes: The HOME study. Environment International 134, 105219. 10.1016/j.envint.2019.105219 [DOI] [PubMed] [Google Scholar]
- Keil A, 2021. qgcomp: Quantile G-Computation. [Google Scholar]
- Kojima H, Takeuchi S, Itoh T, Iida M, Kobayashi S, Yoshida T, 2013. In vitro endocrine disruption potential of organophosphate flame retardants via human nuclear receptors. Toxicology 314, 76–83. 10.1016/j.tox.2013.09.004 [DOI] [PubMed] [Google Scholar]
- Kosarac I, Kubwabo C, Foster WG, 2016. Quantitative determination of nine urinary metabolites of organophosphate flame retardants using solid phase extraction and ultra performance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS). Journal of Chromatography B 1014, 24–30. 10.1016/j.jchromb.2016.01.035 [DOI] [PubMed] [Google Scholar]
- Kuiper JR, Stapleton HM, Wills-Karp M, Wang X, Burd I, Buckley JP, 2020. Predictors and reproducibility of urinary organophosphate ester metabolite concentrations during pregnancy and associations with birth outcomes in an urban population. Environ Health 19, 55. 10.1186/s12940-020-00610-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm M, Zhang W, 2018. Causal Mediation Analysis with the CAUSALMED Procedure. [Google Scholar]
- Lei F, Liu D, Shen Y, Zhang L, Li S, Liu X, Shi G, Li J, Zhao Y, Kang Y, Dang S, Yan H, 2018. Study on the influence of pregnancy-induced hypertension on neonatal birth weight. J Investig Med 66, 1008–1014. 10.1136/jim-2017-000626 [DOI] [PubMed] [Google Scholar]
- Li H, Su G, Zou M, Yu L, Letcher RJ, Yu H, Giesy JP, Zhou B, Liu C, 2015. Effects of Tris(1,3-dichloro-2-propyl) Phosphate on Growth, Reproduction, and Gene Transcription of Daphnia magna at Environmentally Relevant Concentrations. Environ. Sci. Technol 49, 12975–12983. 10.1021/acs.est.5b03294 [DOI] [PubMed] [Google Scholar]
- Li H, Yuan S, Su G, Li M, Wang Q, Zhu G, Letcher RJ, Li Y, Han Z, Liu C, 2017. Whole-Life-Stage Characterization in the Basic Biology of Daphnia magna and Effects of TDCIPP on Growth, Reproduction, Survival, and Transcription of Genes. Environ. Sci. Technol 51, 13967–13975. 10.1021/acs.est.7b04569 [DOI] [PubMed] [Google Scholar]
- Li J, Zhao L, Letcher RJ, Zhang Y, Jian K, Zhang J, Su G, 2019. A review on organophosphate Ester (OPE) flame retardants and plasticizers in foodstuffs: Levels, distribution, human dietary exposure, and future directions. Environment International 127, 35–51. 10.1016/j.envint.2019.03.009 [DOI] [PubMed] [Google Scholar]
- Li L, Lv L, Zhang G, Zhang H, 2021. Associations between the exposure to organophosphate flame retardants during early pregnancy and the risk of spontaneous abortion based on metabolomics combined with tandem mass spectrometry. Ann. Transl. Med 9. 10.21037/atm-21-3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Yuan S, Sun Q, Liu C, 2020. Toxicity of tris(2-chloroethyl) phosphate in Daphnia magna after lifetime exposure: Changes in growth, reproduction, survival and gene transcription. Ecotoxicology and Environmental Safety 200, 110769. 10.1016/j.ecoenv.2020.110769 [DOI] [PubMed] [Google Scholar]
- Li Y, Li D, Chen J, Zhang S, Fu Y, Wang N, Liu Y, Zhang B, 2020. Presence of organophosphate esters in plasma of patients with hypertension in Hubei Province, China. Environ Sci Pollut Res 27, 24059–24069. 10.1007/s11356-020-08563-0 [DOI] [PubMed] [Google Scholar]
- Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, Bernstein L, Hartge P, 2004. Epidemiologic Evaluation of Measurement Data in the Presence of Detection Limits. Environmental Health Perspectives 112, 1691–1696. 10.1289/ehp.7199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D, Liu W, Tao Y, Wang L, Yu M, Hu L, Zhou A, Covaci A, Xia W, Li Y, Xu S, Mei S, 2020. Prenatal Exposure to Organophosphate Flame Retardants and the Risk of Low Birth Weight: A Nested Case-Control Study in China. Environ Sci Technol 54, 3375–3385. 10.1021/acs.est.9b06026 [DOI] [PubMed] [Google Scholar]
- Luo D, Liu W, Wu W, Tao Y, Hu L, Wang L, Yu M, Zhou A, Covaci A, Xia W, Xu S, Li Y, Mei S, 2021. Trimester-specific effects of maternal exposure to organophosphate flame retardants on offspring size at birth: A prospective cohort study in China. Journal of Hazardous Materials 406, 124754. 10.1016/j.jhazmat.2020.124754 [DOI] [PubMed] [Google Scholar]
- Luo K, Aimuzi R, Wang Y, Nian M, Zhang J, 2020. Urinary organophosphate esters metabolites, glucose homeostasis and prediabetes in adolescents. Environ Pollut 267, 115607. 10.1016/j.envpol.2020.115607 [DOI] [PubMed] [Google Scholar]
- MacPherson S, Arbuckle TE, Fisher M, 2018. Adjusting urinary chemical biomarkers for hydration status during pregnancy. J Expo Sci Environ Epidemiol 28, 481–493. 10.1038/s41370-018-0043-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee SP, Cooper EM, Stapleton HM, Volz DC, 2012. Early Zebrafish Embryogenesis Is Susceptible to Developmental TDCPP Exposure. Environmental Health Perspectives 120, 1585–1591. 10.1289/ehp.1205316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller HC, Hassanein K, 1971. DIAGNOSIS OF IMPAIRED FETAL GROWTH IN NEWBORN INFANTS. Pediatrics 48, 511–522. 10.1542/peds.48.4.511 [DOI] [PubMed] [Google Scholar]
- Mitchell EM, Hinkle SN, Schisterman EF, 2015. It’s About Time: A Survival Approach to Gestational Weight Gain and Preterm Delivery. Epidemiology 1. 10.1097/EDE.0000000000000413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS, 2010. New Intrauterine Growth Curves Based on United States Data. Pediatrics 125, e214–e224. 10.1542/peds.2009-0913 [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Roberts SC, Mabrey N, McCaffrey KA, Gear RB, Braun J, Belcher SM, Stapleton HM, 2013. Accumulation and Endocrine Disrupting Effects of the Flame Retardant Mixture Firemaster ® 550 in Rats: An Exploratory Assessment: FM550 IS A CANDIDATE ENDOCRINE DISRUPTOR. J Biochem Mol Toxicol 27, 124–136. 10.1002/jbt.21439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy Z, Vuong AM, Ospina M, Calafat AM, La Guardia MJ, Xu Y, Hale RC, Dietrich KN, Xie C, Lanphear BP, Braun JM, Cecil KM, Yolton K, Chen A, 2020. Organophosphate esters in a cohort of pregnant women: Variability and predictors of exposure. Environ Res 184, 109255. 10.1016/j.envres.2020.109255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AL, Hammel SC, Hoffman K, Lorenzo AM, Chen A, Webster TF, Stapleton HM, 2018. Children’s residential exposure to organophosphate ester flame retardants and plasticizers: Investigating exposure pathways in the TESIE study. Environment International 116, 176–185. 10.1016/j.envint.2018.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2021. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Rasmussen KM, Catalano PM, Yaktine AL, 2009. New guidelines for weight gain during pregnancy: what obstetrician/gynecologists should know. Curr Opin Obstet Gynecol 21, 521–526. 10.1097/GCO.0b013e328332d24e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Wang W, Zhao X, Ren B, Chang L, 2019. Parental exposure to tris(1,3-dichloro-2-propyl) phosphate results in thyroid endocrine disruption and inhibition of growth in zebrafish offspring. Aquatic Toxicology 209, 132–141. 10.1016/j.aquatox.2019.02.004 [DOI] [PubMed] [Google Scholar]
- Romano ME, Gallagher LG, Eliot MN, Calafat AM, Chen A, Yolton K, Lanphear B, Braun JM, 2021. Per- and polyfluoroalkyl substance mixtures and gestational weight gain among mothers in the Health Outcomes and Measures of the Environment study. International Journal of Hygiene and Environmental Health 231, 113660. 10.1016/j.ijheh.2020.113660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano ME, Hawley NL, Eliot M, Calafat AM, Jayatilaka NK, Kelsey K, McGarvey S, Phipps MG, Savitz DA, Werner EF, Braun JM, 2017. Variability and predictors of urinary concentrations of organophosphate flame retardant metabolites among pregnant women in Rhode Island. Environ Health 16, 40. 10.1186/s12940-017-0247-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner B, 2015. Fundamentals of biostatistics. Cengage learning. [Google Scholar]
- Rubin DB, 1987. Multiple imputation for nonresponse in surveys. [Google Scholar]
- Sánchez BN, Hu H, Litman HJ, Téllez-Rojo MM, 2011. Statistical Methods to Study Timing of Vulnerability with Sparsely Sampled Data on Environmental Toxicants. Environmental Health Perspectives 119, 409–415. 10.1289/ehp.1002453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, Blum A, 2012. Novel and High Volume Use Flame Retardants in US Couches Reflective of the 2005 PentaBDE Phase Out. Environ. Sci. Technol 46, 13432–13439. 10.1021/es303471d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street ME, Bernasconi S, 2020. Endocrine-Disrupting Chemicals in Human Fetal Growth. IJMS 21, 1430. 10.3390/ijms21041430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Tan H, Peng T, Wang S, Xu W, Qian H, Jin Y, Fu Z, 2016. Developmental neurotoxicity of organophosphate flame retardants in early life stages of Japanese medaka ( Oryzias latipes ): Neurotoxicity of organophosphate flame retardants in medaka. Environ Toxicol Chem 35, 2931–2940. 10.1002/etc.3477 [DOI] [PubMed] [Google Scholar]
- Sundkvist AM, Olofsson U, Haglund P, 2010. Organophosphorus flame retardants and plasticizers in marine and fresh water biota and in human milk. J Environ Monit 12, 943–951. 10.1039/b921910b [DOI] [PubMed] [Google Scholar]
- Uh H-W, Hartgers FC, Yazdanbakhsh M, Houwing-Duistermaat JJ, 2008. Evaluation of regression methods when immunological measurements are constrained by detection limits. BMC Immunol 9, 59. 10.1186/1471-2172-9-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Eede N, Maho W, Erratico C, Neels H, Covaci A, 2013. First insights in the metabolism of phosphate flame retardants and plasticizers using human liver fractions. Toxicology Letters 223, 9–15. 10.1016/j.toxlet.2013.08.012 [DOI] [PubMed] [Google Scholar]
- Van den Eede N, Tomy G, Tao F, Halldorson T, Harrad S, Neels H, Covaci A, 2016. Kinetics of tris (1-chloro-2-propyl) phosphate (TCIPP) metabolism in human liver microsomes and serum. Chemosphere 144, 1299–1305. 10.1016/j.chemosphere.2015.09.049 [DOI] [PubMed] [Google Scholar]
- van der Veen I, de Boer J, 2012. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 88, 1119–1153. 10.1016/j.chemosphere.2012.03.067 [DOI] [PubMed] [Google Scholar]
- VanderWeele TJ, Ding P, 2017. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med 167, 268. 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- Wang Q, Liang K, Liu J, Yang L, Guo Y, Liu C, Zhou B, 2013. Exposure of zebrafish embryos/larvae to TDCPP alters concentrations of thyroid hormones and transcriptions of genes involved in the hypothalamic–pituitary–thyroid axis. Aquatic Toxicology 126, 207–213. 10.1016/j.aquatox.2012.11.009 [DOI] [PubMed] [Google Scholar]
- Wang X, Liu Q, Zhong W, Yang L, Yang J, Covaci A, Zhu L, 2020. Estimating renal and hepatic clearance rates of organophosphate esters in humans: Impacts of intrinsic metabolism and binding affinity with plasma proteins. Environment International 134, 105321. 10.1016/j.envint.2019.105321 [DOI] [PubMed] [Google Scholar]
- Wei G-L, Li D-Q, Zhuo M-N, Liao Y-S, Xie Z-Y, Guo T-L, Li J-J, Zhang S-Y, Liang Z-Q, 2015. Organophosphorus flame retardants and plasticizers: Sources, occurrence, toxicity and human exposure. Environmental Pollution 196, 29–46. 10.1016/j.envpol.2014.09.012 [DOI] [PubMed] [Google Scholar]
- Yan Z, Jin X, Liu D, Hong Y, Liao W, Feng C, Bai Y, 2021. The potential connections of adverse outcome pathways with the hazard identifications of typical organophosphate esters based on toxicity mechanisms. Chemosphere 266, 128989. 10.1016/j.chemosphere.2020.128989 [DOI] [PubMed] [Google Scholar]
- Yang R, Wang X, Wang J, Chen P, Liu Q, Zhong W, Zhu L, 2022. Insights into the sex-dependent reproductive toxicity of 2-ethylhexyl diphenyl phosphate on zebrafish (Danio rerio). Environment International 158, 106928. 10.1016/j.envint.2021.106928 [DOI] [PubMed] [Google Scholar]
- Yang W, Braun JM, Vuong AM, Percy Z, Xu Y, Xie C, Deka R, Calafat AM, Ospina M, Werner E, Yolton K, Cecil KM, Lanphear BP, Chen A, 2022. Maternal urinary OPE metabolite concentrations and blood pressure during pregnancy: The HOME study. Environmental Research 207, 112220. 10.1016/j.envres.2021.112220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Jia Y, Su G, Sun Y, Letcher RJ, Giesy JP, Yu H, Han Z, Liu C, 2017. Parental transfer of tris(1,3-dichloro-2-propyl) phosphate and transgenerational inhibition of growth of zebrafish exposed to environmentally relevant concentrations. Environmental Pollution 220, 196–203. 10.1016/j.envpol.2016.09.039 [DOI] [PubMed] [Google Scholar]
- Yuan S, Li H, Dang Y, Liu C, 2018. Effects of triphenyl phosphate on growth, reproduction and transcription of genes of Daphnia magna. Aquatic Toxicology 195, 58–66. 10.1016/j.aquatox.2017.12.009 [DOI] [PubMed] [Google Scholar]
- Zhang H, Yolton K, Webster GM, Ye X, Calafat AM, Dietrich KN, Xu Y, Xie C, Braun JM, Lanphear BP, Chen A, 2018. Prenatal and childhood perfluoroalkyl substances exposures and children’s reading skills at ages 5 and 8 years. Environment International 111, 224–231. 10.1016/j.envint.2017.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Wang Y, Zhang C, Yao Y, Wang L, Sun H, 2022. A review of organophosphate esters in soil: Implications for the potential source, transfer, and transformation mechanism. Environmental Research 204, 112122. 10.1016/j.envres.2021.112122 [DOI] [PubMed] [Google Scholar]
- Zhao F, Chen M, Gao F, Shen H, Hu J, 2017. Organophosphorus Flame Retardants in Pregnant Women and Their Transfer to Chorionic Villi. Environ. Sci. Technol 51, 6489–6497. 10.1021/acs.est.7b01122 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Ding J, Lv L, Zhang H, 2021. Exposure to organophosphate flame esters during early pregnancy and risk of spontaneous abortion: A case-control study. Chemosphere 268, 129375. 10.1016/j.chemosphere.2020.129375 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Ma X, Su G, Yu L, Letcher RJ, Hou J, Yu H, Giesy JP, Liu C, 2015. Environmentally Relevant Concentrations of the Flame Retardant Tris(1,3-dichloro-2-propyl) Phosphate Inhibit Growth of Female Zebrafish and Decrease Fecundity. Environ. Sci. Technol 49, 14579–14587. 10.1021/acs.est.5b03849 [DOI] [PubMed] [Google Scholar]
- Zou G, 2004. A Modified Poisson Regression Approach to Prospective Studies with Binary Data. American Journal of Epidemiology 159, 702–706. 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.