Abstract
Extracellular Arg-x- and Lys-x-specific cysteine proteinases are considered important virulence factors and pathogenic markers for Porphyromonas gingivalis, a bacterium implicated as a major etiological agent of chronic periodontitis. Three genes. rgpA, rgpB, and kgp, encode an Arg-x-specific proteinase and adhesins (RgpA), an Arg-x-specific proteinase (RgpB), and a Lys-x-specific proteinase and adhesins (Kgp), respectively. The contribution to pathogenicity of each of the proteinase genes of P. gingivalis W50 was investigated in a murine lesion model using isogenic mutants lacking RgpA, RgpB, and Kgp. Whole-cell Arg-x-specific proteolytic activity of both the RgpA− and RgpB− isogenic mutants was significantly reduced (3- to 4-fold) relative to that of the wild-type W50. However, for the Kgp− isogenic mutant, whole-cell Arg-x activity was similar to that of the wild-type strain. Whole-cell Lys-x proteolytic activity of the RgpA− and RgpB− mutants was not significantly different from that of wild-type W50, whereas the Kgp− mutant was devoid of Lys-x whole-cell proteolytic activity. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis using proteinase-specific antibodies of cell sonicates of the wild-type and mutant strains showed that the proteinase catalytic domain of each of the mutants was not expressed. This analysis further showed that RgpB appeared as 72- and 80-kDa bands, and the catalytic domains of RgpA and Kgp appeared as processed 45-kDa and 48-kDa bands, respectively. In the murine lesion model, mice were challenged with three doses of each mutant and wild-type strain. At the lower dose (3.0 × 109 viable-cells), no lesions were recorded for each of the mutants, whereas wild-type W50 induced large ulcerative lesions. At a dose of 6.0 × 109 viable-cells, all the mice challenged with the wild-type strain died, whereas mice challenged with the RgpA− and RgpB− isogenic mutants did not die but developed lesions. Mice challenged with the Kgp− isogenic mutant at this dose did not develop lesions. At a 1.2 × 1010 viable-cell dose, only 40% of mice challenged with the Kgp− mutant developed lesions, and these lesions were significantly smaller than lesions induced by the wild-type strain at the 3.0 × 109 viable-cell dose. All the mice challenged with the RgpA− mutant died at the 1.2 × 1010 viable-cell dose, whereas only 20% died when challenged with the RgpB− mutant at this dose. Wild-type phenotype was restored to the RgpB− mutant by complementation with plasmid pNJR12::rgpB containing the rgpB gene. There was no difference between the pNJR12::rgpB-complemented RgpB− mutant and the wild-type W50 strain in whole-cell Arg-x activity, protein profile, or virulence in the murine lesion model. These results show that the three proteinases, RgpA, RgpB, and Kgp, all contributed to virulence of P. gingivalis W50 in the murine lesion model and that the order in which they contributed was Kgp ≫ RgpB ≥ RgpA.
Porphyromonas gingivalis has been implicated as a major etiological agent in the onset and progression of chronic periodontitis, a destructive inflammatory disease of the supporting tissues of the teeth which affects between 10 and 15% of dentate adults (10, 20, 49). In a recent study, Griffen et al. (11) analyzed plaque samples from 311 subjects for the presence of heteroduplex types of P. gingivalis. Of the 11 different heteroduplex types detected, P. gingivalis W83/W50-like strains were found to be associated with periodontitis, whereas other strains, including 381-like strains, were not found to be associated with disease. This finding extends earlier animal studies in which strains W83 and W50 were classified as invasive based on their ability to cause ulcerative spreading lesions distant from the injection site, whereas strains 381 and ATCC 33277 were classified as noninvasive as they produced a localized abscess at the site of injection (29, 54). These results therefore suggest that W50 and related strains are more virulent in both animals and humans.
The pathogenicity of P. gingivalis has been attributed to a number of virulence factors, such as fimbriae (4), hemagglutinins (12, 13), lipopolysaccharide (LPS) (14), and the extracellular and cell-associated Arg-x- and Lys-x-specific cysteine proteinases and their associated adhesins (31, 33, 36, 45). Among these factors, the extracellular Arg-x- and Lys-x-specific cysteine proteinases are believed to play a major role in the pathogenesis of periodontal disease, as they are able to degrade a variety of host proteins and have the potential to dysregulate host defense (53).
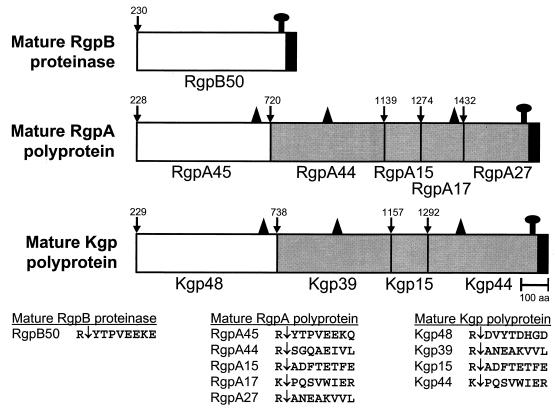
Three genes encode the major extracellular Arg-x- and Lys-x-specific cysteine proteinases of P. gingivalis, and these are designated rgpA, rgpB, and kgp (6). We have previously characterized the proteins encoded by rgpA and kgp of strain W50 as a cell-associated complex of noncovalently associated proteinases and adhesins, designated the RgpA-Kgp proteinase-adhesin complexes, formerly the PrtR-PrtK proteinase-adhesin complexes (3). The RgpA-Kgp complexes of P. gingivalis strain W50 are composed of a 45-kDa Arg-x-specific proteinase (RgpA45, formerly PrtR45) associated with four sequence-related adhesins, RgpA44, RgpA15, RgpA17, and RgpA27, all encoded by rgpA (Fig. 1). The RgpA-Kgp complexes are also characterized by a 48-kDa Lys-x-specific proteinase (Kgp48, formerly PrtK48) associated with three sequence-related adhesins, Kgp39, Kgp15, and Kgp44, all encoded by kgp (3, 46, 47) (Fig. 1).
FIG. 1.
Schematic representation of the processing of the RgpA and Kgp polyproteins and RgpB. The white areas indicate the catalytic domains of the proteinases, the shaded areas indicate the adhesins, and the filled C-terminal areas show the conserved C-terminal sequence proposed to be involved in secretion and cell attachment. ↑ marks the proposed outer membrane attachment to LPS. All processing sites are preceded by Arg or Lys residues (3, 46, 47). ▴ shows the location of an adhesin-binding motif proposed to be involved in binding of the RgpA45 and Kgp48 catalytic domains into large noncovalent complexes with the adhesins and in autoaggregation of the adhesins (46).
We have previously characterized the extracellular Arg-x-specific cysteine proteinase encoded by rgpB of strain W50 (46). This proteinase, designated RgpB, is not associated with adhesins, as the rgpB gene does not encode adhesins or an adhesin binding motif (Fig. 1) that is present in the RgpA and Kgp catalytic domains (46). This adhesin binding motif is also present in some of the adhesin domains of RgpA and Kgp (Fig. 1) and is proposed to be responsible for the incorporation of the RgpA and Kgp catalytic domains into noncovalently associated complexes with adhesins and for the autoaggregation of the adhesins into large complexes (46). The RgpB proteinase has been isolated as a 70- to 80-kDa membrane-associated protein and as a discrete 50-kDa protein from the culture supernatant (40, 46).
Spontaneous P. gingivalis mutants with reduced Arg-x and Lys-x proteinase activity and wild-type cells treated with a protease inhibitor (Nα-p-tosyl-l-lysine chloromethyl ketone [TLCK]) have been reported to be avirulent in animal models (16). Furthermore, a nonpigmented mutant of P. gingivalis W50/BE1, which has reduced Lys-x and Arg-x proteinase activity, is reported to be avirulent in animal models (26). However, this mutant also lacks gelatinase, collagenase, and dipeptidylaminopeptidase and shows reduced hemagglutinin activity, fimbrination, and extracellular vesicle production (5, 26, 43). Tokuda et al. (50, 51) have reported that two constructed isogenic mutants of P. gingivalis strain 381 lacking either RgpA or RgpB exhibited reduced aggregation, hemagglutination, and binding to matrix proteins relative to the wild-type 381 strain. Further, a triple mutant based on strain ATCC 33277 lacking RgpA, RgpB, and Kgp was reported not to agglutinate erythrocytes, bind to hemoglobin, or grow in defined medium containing bovine serum albumin (BSA) as the sole carbon and energy source (44). These studies suggest that the rgpA, rgpB, and kgp genes are important for the virulence of P. gingivalis.
The aim of this study therefore was to determine the virulence of isogenic mutants of the invasive W50 strain that lack the RgpA, RgpB, and Kgp proteinases in a murine lesion model.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacteria and plasmids used in this study are listed in Table 1. Lyophilized cultures of P. gingivalis W50 and mutant strains (501, D7, and K1A) were grown anaerobically at 37°C on lysed horse blood agar plates supplemented with 5 μg of hemin, 0.5 μg of cysteine (HB agar) and appropriate antibiotics (1.0 μg of tetracycline and/or 10 μg of erythromycin) (<10 passages) per ml. After 3 to 4 days, colonies were used to inoculate brain heart infusion medium containing 5 μg of hemin, 0.5 μg of cysteine (25), and appropriate antibiotics (0.5 μg of tetracycline and/or 5 μg of erythromycin) per ml. Batch cultures were grown anaerobically in an MK3 Anaerobic Workstation (Don Whitley Scientific Ltd., Adelaide, Australia). Cells were harvested during exponential growth phase by centrifugation (5,000 × g, 30 min, 4°C) and washed twice with PG buffer (50 mM Tris-HCl, 150 mM NaCl, 5 mM CaCl2, 5 mM cysteine-HCl, pH 8.0) in the anaerobic workstation for the whole-cell proteinase assays and the murine lesion model experiments. Growth of batch cultures was monitored at 650 nm using a spectrophotometer (model 295E; Perkin-Elmer). Culture purity was checked routinely by Gram stain, microscopic examination, and a variety of biochemical tests according to Slots (48).
TABLE 1.
Strains and plasmids
| Strain or plasmid | Description | Reference |
|---|---|---|
| P. gingivalis | ||
| W50 | Wild type | Slakeski et al. (46) |
| W501 | rgpA::erm | Rangarajan et al. (40) |
| D7 | rgpB::erm | Rangarajan et al. (40) |
| K1A | kgp::erm | Aduse-Opoku et al. (1) |
| Plasmids | ||
| pNJR12 | E. coli-Bacteroides shuttle containing a 2.5-kb SstI fragment of tetQ; Kanr (E. coli) | Maley et al. (21) |
| pNJR12::rgpB | pNJR12 containing rgpB; Kanr (E. coli), Tetr (P. gingivalis) | This paper |
Construction of pNJR12::rgpB-complemented RgpB− mutant.
A PCR-derived fragment containing the rgpB coding region and 5′ untranslated region was generated from the λGEM-12 P. gingivalis genomic clone (46) using Elongase (Life Technologies) according to the manufacturer's instructions on a PC-960 thermal sequencer (Corbett Research). The PCR was performed using the forward primer (5′-GCGCGCTCTAGAGGACAGTATCTGCAACCGTCG-3′) that consists of a six base-buffer, an XbaI site, and bases 495 to 515 of rgpB (previously prtRII; GenBank accession no. AF007124) and the reverse primer (5′-CCGAATGGATTCCTCGGC-3′) that consists of bases 3150 to 3167 of rgpB in an antisense orientation. The 2.7-kb PCR product was purified using the QIAquick PCR purification kit (Qiagen Pty Ltd.) and ligated into pGEM-T Easy (Promega Corporation), transformed into Escherichia coli JM109 (Promega Corporation), and selected on Luria-Bertani medium (LB) containing ampicillin (100 μg/ml) following standard procedures (42).
DNA purified from transformants containing recombinant pGEM-TEasy::rgpB was digested with XbaI and SalI (Promega Corporation), and the insert was purified and ligated into the E. coli-Bacteroides shuttle vector pNJR12 that had been digested with XbaI and SalI. The construct was used to transform E. coli JM109, which was then plated on LB agar containing kanamycin (50 μg/ml). The rgpB insert in the purified pNJR12::rgpB construct was verified by DNA sequencing. The verified construct was then used to transform electrocompetent P. gingivalis W50D7. The procedure for transformation and preparation of cells was essentially that of Fletcher et al. (9) except that transformed cells were grown to an optical density at 650 nm (OD650) of 0.5 and selected on HB agar containing 10 μg of erythromycin and 1 μg of tetracycline per ml after 7 to 10 days of incubation at 37°C under anaerobic conditions.
Arg-x-specific and Lys-x-specific whole-cell proteinase assays.
P. gingivalis cells (W50 and mutants 501, D7, and K1A) were harvested under anaerobic conditions at early exponential, mid-late exponential, and stationary growth phases (0.5, 0.9, and 1.25 O.D.650, respectively) by centrifugation (5,000 × g, 30 min, 4°C), washed, and resuspended in PG buffer (1 ml). Resuspended cells were analyzed immediately for Arg-x and Lys-x proteolytic activity using N-α-benzoyl-l-Arg-p-nitroanilide (Bz-Arg-pNA; Sigma) and benzyloxycarbonyl-l-Lys-p-nitroanilide (Bz-Lys-pNA; Sigma). These enzyme substrates were prepared as follows: 2 mM Bz-Arg-pNA or 2 mM Bz-Lys-pNA in 3 ml of isopropyl alcohol was subjected to sonication for 10 min, after which 7 ml of enzyme buffer (400 mM Tris-HCl, 100 mM NaCl, 20 mM cysteine, pH 8.0) was added. Amidolytic activity is expressed as units (micromoles of substrate converted per minute) at 37°C.
Resuspended cells (16 or 32 μl) were diluted in PG buffer (total volume, 360 μl), and 40 μl of fresh 100 mM cysteine-HCl (pH 8.0) was added. After incubation for 10 min at 37°C, 400 μl of Arg or Lys substrate buffer was added, and proteinase activity was measured every 10 s for 3 min at 37°C using a Hewlett Packard 8452A diode array spectrophotometer (Hewlett Packard, Melbourne, Australia) at a wavelength of 410 nm. Proteolytic activity data were statistically analyzed using a one-way classification analysis of variance with a post hoc Scheffe test.
SDS-PAGE and Western blot analysis of P. gingivalis W50 and mutant cell sonicates.
P. gingivalis strain W50 wild-type, mutants, and pNJR12::rgpB-complemented RgpB− mutant were grown in batch culture and harvested at late exponential phase by centrifugation (5,000 × g, 20 min, 4°C). Cells were washed once with 50 ml of TC buffer (20 mM Tris-HCl, 5 mM CaC12) containing 150 mM NaCl, pH 7.4, and sonicated at 4°C as previously described (3). The harvesting of cells and sonication were also performed with 10 mM TLCK in the buffer, with fresh 10 mM TLCK being added at every step. The sonicates were recentrifuged at 4°C for 10 min, and the collected supernatants were stored at −70°C. The protein concentration of each cell sonicate was determined by using the Bradford protein assay (Bio-Rad, North Ryde, New South Wales, Australia) with BSA as the standard. Each cell sonicate (10 μg of protein) was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in gels of 12.5% (wt/vol) acrylamide (1 mm) by the method of Laemmli (18) with a minigel system (Bio-Rad, North Ryde, New South Wales, Australia). Proteins were electrophoretically transferred onto a polyvinylidene difluoride (PVDF) membrane as described previously (8). After sectioning of the membrane, one section was stained with 0.1% (wt/vol) CBB R250, and the remaining section was blocked for 1 h at 20°C with 5% (wt/vol) nonfat skim milk powder in TN buffer (50 mM Tris-HCl [pH 7.4], 100 mM NaCl) and incubated with proteinase-specific antibody (32) diluted 1:25 with TN buffer.
The proteinase-specific antibody was raised in mice using a synthetic peptide conjugated to diphtheria toxoid (32). The synthetic peptide contained the putative active-site His and flanking amino acid sequence (HGSETAW) of RgpA/B and Kgp. After 5 h at 20°C, the section was washed with 4× TN buffer containing 0.05% (vol/vol) Tween 20 and then incubated for 1 h at 20°C with anti-mouse immunoglobulin G-horseradish peroxidase conjugate (Sigma-Aldrich, Sydney, New South Wales, Australia). After washing with 4× TN buffer containing 0.05% (vol/vol) Tween 20, bound antibody was detected with 0.05% (wt/vol) 4-chloro-1-naphthol in TN buffer containing 16.6% (vol/vol) methanol and 0.015% (vol/vol) H2O2. Color development was stopped by rinsing the membranes with water.
Murine lesion model.
The murine lesion model experiments were approved by the University of Melbourne Ethics Committee for Animal Experimentation and were conducted essentially as described previously (32). BALB/c mice 6 to 8 weeks old (10 animals per group) were challenged with either 3.0 × 109, 6.0 × 109, or 1.2 × 1010 viable cells of P. gingivalis strain W50 and mutants 501, D7, and K1A by subcutaneous injection (100 μl) in the abdomen, and lesion size and mortality were monitored over 14 days as described previously (32). Mice were also challenged with 3.0 × 109 and 6.0 × 109 viable cells of the RgpB− mutant D7 carrying the pNJR12 vector and the pNJR12::rgpB-complemented RgpB− mutant. The P. gingivalis inocula were prepared using PG buffer in the anaerobic workstation as described above. The number of viable cells in each inoculum was verified by enumeration on HB agar. The maximum sizes of the lesions developed were statistically analyzed using the Kruskal-Wallis test and Mann-Whitney U-Wilcoxon rank sum test with a Bonferroni correction for type 1 error (30).
RESULTS
Arg-x and Lys-x whole-cell proteinase activity of RgpA−, RgpB−, and Kgp− mutants of P. gingivalis W50.
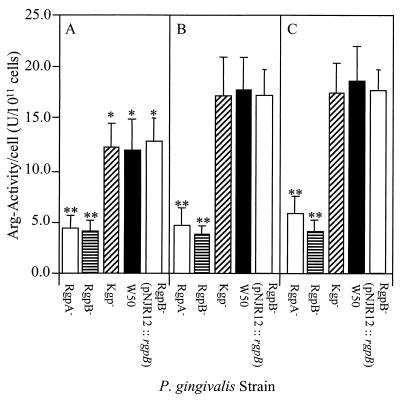
Whole-cell Arg-x and Lys-x proteinase activity was measured at the early exponential, mid-late exponential, and early stationary phases of growth for each of the isogenic mutants and wild-type strain (Fig. 2 and 3). The whole-cell Arg-x activity (in units per 1011 cells) (Fig. 2) of the Kgp− strain was found not to be significantly different at each growth phase measured compared with the wild-type strain. For both the Kgp− mutant and wild-type strain, the Arg-x activity at the early exponential phase of growth was significantly (P < 0.05) less (28%) than that at later growth phases. There was a significant (P < 0.001) difference in the Arg-x activity of both the RgpA− and RgpB− mutants compared with the wild-type strain. The Arg-x-specific whole-cell activity of both these mutants was consistently lower (3- to 4-fold) than that of the wild-type strain at each growth phase measured. However, the Arg-x activities of the RgpA− and RgpB− mutants were not significantly different from each other and did not increase with growth.
FIG. 2.
Whole-cell Arg-x proteolytic activity of P. gingivalis W50 and RgpA−, RgpB−, and Kgp− isogenic mutants and pNJR12::rgpB-complemented RgpB− mutant at different phases of growth. P. gingivalis cells were harvested anaerobically at early (A) and late (B) exponential and stationary (C) growth phases. Harvested cells were centrifuged, resuspended in PG buffer, and assayed for Arg-x proteolytic activity. Data presented represent the mean ± standard deviation (SD) of three separate experiments. ∗, groups significantly different (P < 0.05) from activity at later phases of growth. ∗∗, groups significantly different (P < 0.001) from activity of P. gingivalis wild-type W50 strain.
FIG. 3.
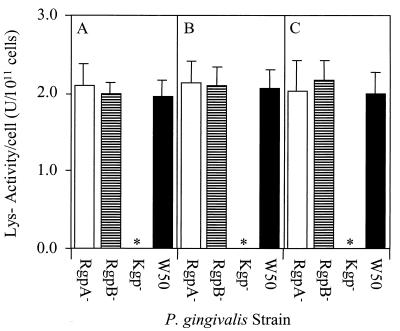
Whole-cell Lys-x proteolytic activity of P. gingivalis W50 and RgpA−, RgpB− and Kgp− isogenic mutants at different phases of growth. P. gingivalis cells were harvested anaerobically at early (A) and late (B) exponential and stationary (C) growth phases. Harvested cells were centrifuged, resuspended in PG buffer, and assayed for Lys-x proteolytic activity. Data presented represent the mean ± SD of three separate experiments. ∗, no whole-cell Lys-x proteolytic activity was detected for the Kgp− mutant.
Whole-cell Lys-x activity of RgpA−and RgpB− mutants and the wild type did not increase with growth as seen with the wild-type Arg-x activity, and there was no significant difference between strains (Fig. 3). No whole-cell Lys-x proteolytic activity was detected for the Kgp− mutant at any stage of growth.
SDS-PAGE and Western blot analysis of RgpA−, RgpB−, and Kgp− mutants of P. gingivalis W50.
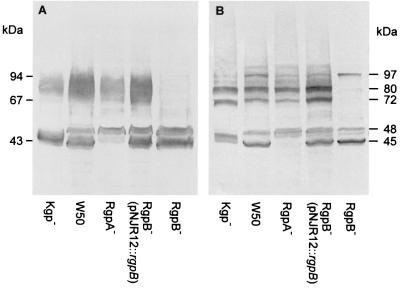
Cell sonicates of P. gingivalis W50 and RgpA−, RgpB−, and Kgp− mutants were subjected to SDS-PAGE and Western blot analysis using the proteinase-specific antibody (Fig. 4). The Western blot of the wild-type W50 sonicate prepared in the absence of TLCK (Fig. 4A) revealed diffuse immunoreactive bands at 45 kDa, 48 kDa, and 70 to 90 kDa. The same analysis of the proteinase isogenic mutants showed that the 48-kDa band in Kgp−, the 45-kDa band in RgpA− and the 70- to 90-kDa diffuse band in RgpB− were not detected, confirming the genotype of the three mutants and the previous assignment of these bands (3, 40). However, when the cell sonicates were prepared in the presence of 10 mM TLCK, the bands obtained on the Western blot were less diffuse, suggesting that TLCK was inhibiting proteolytic processing during preparation of the sample (Fig. 4B).
FIG. 4.
Western blot analysis of cell sonicates of isogenic mutants RgpA−, RgpB−, and Kgp−, pNJR12::rgpB-complemented RgpB− mutant, and wild-type strain using proteinase-specific sera. (A) Cell sonicate. (B) Cell sonicate prepared in the presence of 10 mM TLCK. The cell sonicates were subjected to SDS-PAGE and transferred onto a PVDF membrane. The membranes were probed with sera (1:25 in TN buffer) from mice immunized with a synthetic peptide which has the sequence HGSETAW that is common to each proteinase (32). Positions of molecular mass markers are indicated (in kilodaltons).
The Western blot of the wild-type W50 in the presence of 10 mM TLCK also showed the 45-kDa RgpA45 and 48-kDa Kgp48 processed catalytic domains. However, in the presence of TLCK, the diffuse 70- to 90-kDa band resolved into two major bands at 72 and 80 kDa. Western blot analysis of the isogenic mutants in the presence of 10 mM TLCK confirmed that the 45-kDa band was RgpA45 and that the 48-kDa band was Kgp48, although interestingly, the 48-kDa band resolved into two bands in the RgpA− and RgpB− mutants, suggesting a role for these Arg-x-specific proteinases in the processing of Kgp. Similarly, RgpA45 appeared at a slightly higher molecular weight in the Kgp− mutant, perhaps suggesting a role for the Lys-x-specific enzyme in processing of RgpA.
The Western blot of the RgpB− mutant in the presence of TLCK suggested that all the bands that appeared between 70 and 90 kDa, including the two major bands at 72 kDa and 80 kDa, were isoforms of RgpB. Interestingly, a band at 97 kDa was present in the Western blot of the wild-type W50 and RgpB− and RgpA− mutants in the presence of 10 mM TLCK which may be a partially processed form of Kgp. The Western blot analysis therefore confirmed the genotypes and the proteolytic enzyme profiles of the three isogenic mutants and suggested a role for each proteinase in the processing of the others. Western blot analysis of outer membranes of the wild-type W50 and isogenic mutants prepared in the presence and absence of 10 mM TLCK produced the same results as the cell sonicates shown in Fig. 4 (data not shown).
Characterization of pNJR12::rgpB-complemented RgpB− mutant.
A cell sonicate of the pNJR12::rgpB-complemented RgpB− mutant was subjected to SDS-PAGE and Western blot analysis using the proteinase-specific antibodies (Fig. 4). This analysis showed that the pNJR12::rgpB-complemented mutant had immunoreactive bands corresponding to the RgpA (45 kDa) and Kgp (48 kDa) catalytic domains as well as the 72- and 80-kDa immunoreactive protein bands corresponding to the RgpB proteinase. The intensity of the 72- and 80-kDa immunoreactive bands was similar to the same immunoreactive bands for the wild-type W50 strain. The bands, however, were absent in the Western blot of the RgpB− mutant D7 (Fig. 4).
Whole-cell Lys-x proteolytic activity of the pNJR12::rgpB-complemented mutant was not affected by the transformation, as there was no significant difference in the Lys-x activity compared with the wild-type W50 or the RgpB− mutant D7 at each growth phase measured (data not shown). The whole-cell Arg-x proteolytic activity of the pNJR12::rgpB-complemented mutant was found to be restored to a similar level to that of the wild-type W50 strain (Fig. 2), which was 3- to 4-fold higher than that of the RgpB− mutant D7 at each growth phase measured. There was also a significantly (P < 0.05) lower whole-cell Arg-x activity for the pNJR12::rgpB-complemented mutant in the early exponential growth phase compared with the same activity at later growth phases, as shown for the wild-type strain (Fig. 2).
Virulence of RgpA−, RgpB−, Kgp−, and pNJR12::rgpB-complemented RgpB− mutants in the murine lesion model.
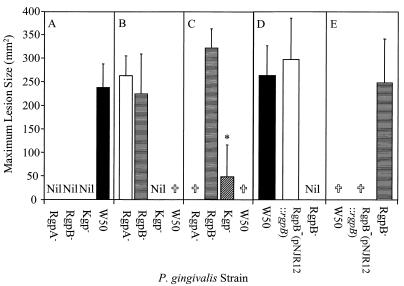
To evaluate the virulence of the isogenic mutants, BALB/c mice were challenged subcutaneously with the RgpA−, RgpB− and Kgp− mutants as well as the wild-type W50 strain at three doses, 3.0 × 109, 6.0 × 109 and 1.2 × 1010 viable cells. Lesions that developed were measured, and the maximum lesion size for each strain is shown in Fig. 5 A, B, and C. At the 3.0 × 109 viable-cell dose, only mice challenged with the wild-type W50 strain developed lesions. All the mice challenged with the wild-type W50 strain at the 6.0 × 109 viable-cell dose died 3 days after the inoculation. Both the RgpA− and RgpB− mutants induced lesions in all of the mice at the 6.0 × 109 viable-cell dose, and the lesion sizes were not significantly different from those induced by the wild-type W50 strain at the 3.0 × 109 viable-cell dose. However, no lesions were recorded for mice challenged with the Kgp− mutant at the 6.0 × 109 viable-cell dose. The 1.2 × 1010 viable-cell dose induced 100% mortality in mice inoculated with the RgpA− mutant and wild-type W50 strain. Challenge with the RgpB− mutant at the 1.2 × 1010 viable-cell dose resulted in only 20% mortality and an average increase of 35% in the lesion size compared with the lesions induced by the same mutant at the 6.0 × 109 viable-cell dose. In contrast, none of the mice challenged with the Kgp− mutant at the 1.2 × 1010 viable-cell dose died, and only 40% of the animals developed lesions. The mean lesion size produced by the Kgp− mutant at this dose was significantly (P < 0.001) smaller than that induced by the RgpB− mutant at the same dose and by the wild-type W50 strain at the 3.0 × 109 viable-cell dose.
FIG. 5.
Maximum lesion size of mice challenged with P. gingivalis W50, RgpA−, RgpB−, and Kgp− isogenic mutants, and pNJR12::rgpB-complemented RgpB− mutant. BALB/c mice were challenged subcutaneously with 3.0 × 109 (A), 6.0 × 109 (B), or 1.2 × 1010 (C) viable P. gingivalis W50 and isogenic mutant cells and with 3.0 × 109 (D) and 6.0 × 109 (E) viable P. gingivalis W50, RgpB− isogenic mutant, and pNJR12::rgpB-complemented RgpB− mutant cells. Animals were monitored over a 14-day period for mortality and lesion size. Data presented in panels A to E represent mean ± SD (n = 10) from separate experiments and were analyzed using the nonparametric Mann-Whitney U-Wilcoxon rank sum test. ∗, group significantly different (P < 0.001) from RgpB− at same dose and from wild-type W50 strain at the 3.0 × 109 viable-cell dose. †, 100% mortality. Nil, no lesions observed.
Mice were also challenged with 3.0 × 109 and 6.0 × 109 viable cells of the RgpB− mutant D7 carrying the pNJR12 vector, the pNJR12::rgpB-complemented RgpB− mutant, and the wild-type W50 strain (Fig. 5D and E). At the 3.0 × 109 viable-cell dose, the pNJR12::rgpB-complemented RgpB− mutant induced lesions in all of the mice, and the mean lesion size was not significantly different from that induced by the wild-type W50 strain at the same dose. Inoculation of mice with the pNJR12::rgpB-complemented RgpB− mutant at the 6.0 × 109 viable-cell dose induced 100% mortality within 3 days, which was consistent with the level of mortality induced by the wild-type W50 strain at the same dose. Challenge with the RgpB− mutant carrying the pNJR12 vector without the rgpB insert did not result in lesions at the 3.0 × 109 viable-cell dose and did not result in mortality at the 6.0 × 109 viable-cell dose.
DISCUSSION
In this study, the role of the Arg-x (RgpA and RgpB) and Lys-x (Kgp) proteinases in virulence of P. gingivalis was investigated in the murine lesion model using three isogenic mutants lacking the proteinases. Subjecting each of the isogenic mutants to SDS-PAGE and Western blot analysis with a proteinase-specific antibody showed that each of these proteinases was absent in the Western blot. The analysis of the form of the RgpA, RgpB, and Kgp proteinases of the wild type and the three isogenic mutants by SDS-PAGE and Western blot revealed that RgpB appeared as 72- and 80-kDa bands and the majority of RgpA and Kgp appeared as fully processed 45-kDa and 48-kDa catalytic domains, respectively. These results therefore are consistent with the previous characterization of these cell surface proteins (3, 46).
It has been proposed previously (3, 46) that the RgpA and Kgp polyproteins are secreted and then attached to the outer membrane, possibly through the conserved C-terminal segment (Fig. 1), as this segment is not found in the soluble RgpA-Kgp complexes released from the outer membrane (3, 36; unpublished data). As the N-terminal residue of the activated catalytic domains of RgpA, Kgp, and RgpB is a residue C-terminal to an Arg (3, 46), it is likely that the polyproteins are processed (activated) at these Arg residues to remove their profragments to produce mature 160-kDa (RgpA) and 163-kDa (Kgp) forms (Fig. 1). These mature RgpA and Kgp polyproteins must then undergo further processing at Arg and Lys residues (probably autolytic) to release the proteinase catalytic domains and several C-terminal sequence-related adhesin domains (Fig. 1) that have been characterized previously (3, 36, 47).
We have shown (46) that both the RgpA and Kgp catalytic domains, but not RgpB, contain a C-terminal adhesin-binding motif that is also found in the released adhesins (Fig. 1). Through this adhesin-binding motif, the proteinase catalytic domains may bind to their respective adhesins, which in turn may aggregate and bind to the putative anchored C-terminal adhesin, localizing RgpA and Kgp as noncovalently associated processed domains on the cell surface. The 50-kDa mature RgpB is presumably attached directly to the cell surface to form the membrane-associated 72- and 80-kDa forms (Fig. 1). This is supported by the work of Curtis et al. (7), who have shown that the 70- to 80-kDa membrane-associated form of RgpB, but not the 50-kDa isoform found in the culture supernatant, is immunoreactive with anti-LPS antibodies. In the presence of 10 mM TLCK, RgpB appeared as two major bands at 72 kDa and 80 kDa in the current study, which presumably represent two differently LPS-modified isoforms of the enzyme. The proposed C-terminal attachment of RgpB is also consistent with the work of Potempa et al. (39), who have shown that the 50-kDa isoform in the culture supernatant is C-terminally truncated by processing before the putative anchor sequence.
The processing of RgpA and Kgp, once secreted, to release the proteinase catalytic domains and the adhesin domains is thought to be autolytic, as the processing sites always involve Arg or Lys residues (Fig. 1). The slight change in molecular weight of the processed domains obtained in the Western blots of each of the isogenic mutants (Fig. 4) is consistent with a processing role for these enzymes. Furthermore, the 3- to 4-fold reduction in cell surface Arg-x-specific proteolytic activity upon inactivation of either rgpA or rgpB is also consistent with a processing role for both of these enzymes. It is interesting, however, that inactivating any one of the three proteinase genes did not abolish secretion and processing of the other two gene products. As multiple Arg and Lys residues exist between the processed domains of the polyproteins, it is very likely that any one of the proteinases can facilitate processing of the others.
The whole-cell Arg-x-specific proteolytic activity of both the Kgp− mutant and W50 wild-type strain was significantly less at the early exponential phase of growth and increased to a plateau at the mid-exponential phase. This increase in activity was not observed for the Lys-x activity of the RgpA− and RgpB− mutants or the wild-type W50 strain. In fact, the Lys-x-specific proteolytic activity of the wild-type and RgpA− and RgpB− mutants was similar at all phases of growth. The unchanged Lys-x proteolytic activity for the RgpA− and RgpB− mutants is in contrast to the findings of Tokuda et al. (52), who have reported that inactivation of the RgpA− gene in strain 381 resulted in downregulation of the kgp gene, as indicated by Northern blot analysis. However, whole-cell Arg-x and Lys-x proteolytic activities were not measured in that study. The data reported here suggest that Arg-x activity but not Lys-x activity may be influenced by growth phase or environmental factors. This is consistent with several earlier reports that have shown that environmental factors, such as hemin availability, pH, and temperature, can affect the Arg-x-specific proteolytic activity of P. gingivalis (22, 24, 35).
Inactivation of the rgpA, rgpB, and kgp genes, as well as reducing whole-cell proteolytic activity, also resulted in a measurable reduction in the pathogenicity of each mutant in the murine lesion model. No lesions were recorded for mice challenged with the 3.0 × 109 viable-cell dose for any of the mutants, but increasing the challenge dose resulted in differences in virulence for each of the isogenic mutants. Interestingly, although the Arg-x activity of the RgpA− and RgpB− mutants was similar, there was a significant difference in virulence at the 1.2 × 1010 viable-cell dose, with 100% mortality of mice challenged with the RgpA− mutant. The RgpB− mutant, however, at the same dose induced only 20% mortality.
The rgpA gene in W50 produces a noncovalently associated proteinase-adhesin complex (3). The rgpB gene in W50 produces two isoforms of RgpB that are not associated with adhesins, 72- and 80-kDa membrane-attached forms (40;this study) and a 50-kDa discrete proteinase in the culture supernatant (40, 46). The adhesins of the RgpA proteinase-adhesin complex have been shown to bind to host substrates, facilitating proteolysis (37). This targeting role of the adhesins has been speculated to increase the virulence of the proteinase. However, the results of this study suggest that RgpB may have a greater role in virulence. Recently, Tokuda et al. (50, 51) constructed RgpA− and RgpB− isogenic mutants of strain 381 and reported marked changes in surface and binding properties of the two mutants relative to the wild type. The authors noted differences between the two mutants, with the RgpB− mutant exhibiting a reduced ability to bind to epithelial cells. These and other studies (15, 28), showing pleiotropic effects of inactivation of the rgpA and rgpB genes, suggest that the Arg-x-specific proteolytic activity of P. gingivalis is involved in processing of not only the proteinases but also other surface proteins. Therefore, a greater role in pathogenicity for RgpB may reflect a greater role in processing for this enzyme.
The least virulent of the isogenic mutants in the murine lesion model was the Kgp− mutant, as only 40% of mice challenged with the 1.2 × 1010 viable-cell dose developed lesions and these were significantly smaller than the lesions induced by the wild-type strain at the 3.0 × 109 viable-cell dose. Kgp has been reported to be involved in hemoglobin binding and degradation and heme accumulation (17, 19, 34, 38). The nonpigmented phenotype of the Kgp− mutant (K1A) is consistent with the proposed role of this proteinase in heme accumulation. Heme has been reported to be essential for the growth and virulence of P. gingivalis (23), and thus the Kgp− mutant's reduced ability to accumulate heme may account for its reduced virulence in the murine lesion model.
In this study we also investigated the complementation of the RgpB− mutant with a plasmid containing the rgpB gene. Whole-cell Arg-x-specific proteolytic activity was fully restored by the complementation. The pNJR12::rgpB-complemented RgpB− mutant also displayed the same pattern of Arg-x activity/cell as the wild-type strain, with a lower activity at early exponential growth phase which increased in later phases of growth. Although pNJR12 is a low-copy-number plasmid, it may have been expected that the pNJR12::rgpB-complemented RgpB− mutant would have exhibited higher Arg-x activity than the wild-type W50 strain due to the presence of multiple copies of the plasmid and therefore of the rgpB gene. As an increase in Arg-x-specific activity was not observed, this suggests that the expression of the rgpB gene is regulated, which is again consistent with earlier reports of Arg-x proteolytic activity regulation (22, 24, 35).
The characteristic 72- and 80-kDa RgpB bands were also observed for the complemented RgpB− mutant, indicating that the plasmid-expressed RgpB was secreted and attached to the outer membrane in the same manner as the protein in the wild-type W50 strain. As well as restoring the enzymatic and protein profile, the pNJR12::rgpB complementation fully restored pathogenicity in the murine lesion model. Using techniques similar to those described here for mutant complementation, others have shown the importance of specific genes and their products for the virulence of Listeria monocytogenes, Proteus mirabilis, Vibrio anguillarum, and Yersinia enterocolitica (2, 27, 41, 55).
In conclusion, by characterizing the virulence of P. gingivalis isogenic mutants 501, D7, and K1A, lacking RgpA, RgpB, and Kgp, respectively, and the pNJR12::rgpB-complemented RgpB− mutant in the murine lesion model, we have shown that the three proteinases all contributed to virulence in this model and that the order of contribution was Kgp ≫ RgpB ≥ RgpA.
ACKNOWLEDGMENTS
We are grateful to Michael Curtis and Joseph Aduse-Opoku for supplying the P. gingivalis mutants 501, D7, and K1A.
This work was supported by the Australian National Health and Medical Research Council (Project No. 990199).
REFERENCES
- 1.Aduse-Opoku J, Davies N N, Gallagher A, Hashim A, Evans H E, Rangarajan M, Slaney J M, Curtis M A. Generation of Lys-gingipain protease activity in Porphyromonas gingivalis W50 is independent of Arg-gingipain protease activities. Microbiology. 2000;146:1933–1940. doi: 10.1099/00221287-146-8-1933. [DOI] [PubMed] [Google Scholar]
- 2.Bahrani F K, Massad G, Lockatell C V, Johnson D E, Russell R G, Warren J W, Mobley H L. Construction of an MR/P fimbrial mutant of Proteus mirabilis: role in virulence in a mouse model of ascending urinary tract infection. Infect Immun. 1994;62:3363–3371. doi: 10.1128/iai.62.8.3363-3371.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhogal P S, Slakeski N, Reynolds E C. A cell-associated protein complex of Porphyromonas gingivalis W50 composed of Arg- and Lys-specific cysteine proteinases and adhesins. Microbiology. 1997;143:2485–2495. doi: 10.1099/00221287-143-7-2485. [DOI] [PubMed] [Google Scholar]
- 4.Chandad F, Mouton C. Antigenic, structural, and functional relationships between fimbriae and the hemagglutinating adhesin HA-Ag2 of Porphyromonas gingivalis. Infect Immun. 1995;63:4755–4763. doi: 10.1128/iai.63.12.4755-4763.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collinson L M, Rangarajan M, Curtis M A. Altered expression and modification of proteases from an avirulent mutant of Porphyromonas gingivalis W50 (W50/BE1) Microbiology. 1998;144:2487–2496. doi: 10.1099/00221287-144-9-2487. [DOI] [PubMed] [Google Scholar]
- 6.Curtis M A, Kuramitsu H K, Lantz M, Macrina F L, Nakayama K, Potempa J, Reynolds E C, Aduse-Opoku J. Molecular genetics and nomenclature of proteases of Porphyromonas gingivalis. J Periodont Res. 1999;34:464–472. doi: 10.1111/j.1600-0765.1999.tb02282.x. [DOI] [PubMed] [Google Scholar]
- 7.Curtis M A, Thickett A, Slaney J M, Rangarajan M, Aduse-Opoku J, Shepherd P, Paramonov N, Hounsell E F. Variable carbohydrate modifications to the catalytic chains of the RgpA and RgpB proteases of Porphyromonas gingivalis W50. Infect Immun. 1999;67:3816–3823. doi: 10.1128/iai.67.8.3816-3823.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dashper S G, O'Brien-Simpson N M, Bhogal P S, Franzmann A D, Reynolds E C. Purification and characterization of a putative fimbrial protein/receptor of Porphyromonas gingivalis. Aust Dent J. 1998;43:99–104. [PubMed] [Google Scholar]
- 9.Fletcher H M, Schenkein H A, Morgan R M, Bailey K A, Berry C R, Macrina F L. Virulence of a Porphyromonas gingivalis W83 mutant defective in the prtH gene. Infect Immun. 1995;63:1521–1528. doi: 10.1128/iai.63.4.1521-1528.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox C H. New considerations in the prevalence of periodontal disease. Curr Opin Dent. 1992;2:5–11. [PubMed] [Google Scholar]
- 11.Griffen A L, Becker M R, Lyons S R, Moeschberger M L, Leys E J. Prevalence of Porphyromonas gingivalis and periodontal health status. J Clin Microbiol. 1999;36:3239–3242. doi: 10.1128/jcm.36.11.3239-3242.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han N, Whitlock J, Progulske Fox A. The hemagglutinin gene A (hagA) of Porphyromonas gingivalis 381 contains four large, contiguous, direct repeats. Infect Immun. 1996;64:4000–4007. doi: 10.1128/iai.64.10.4000-4007.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinode D, Masuda K, Yoshioka M, Watanabe K, Umemoto T, Grenier D, Mayrand D, Nakamura R. Immunological characterization and localization of a Porphyromonas gingivalis BApNA-hydrolyzing protease possessing hemagglutinating activity. FEMS Microbiol Lett. 1995;131:211–217. doi: 10.1111/j.1574-6968.1995.tb07779.x. [DOI] [PubMed] [Google Scholar]
- 14.Holt S C, Bramanti T E. Factors in virulence expression and their role in periodontal disease pathogenicity. Crit Rev Oral Biol Med. 1991;2:177–281. doi: 10.1177/10454411910020020301. [DOI] [PubMed] [Google Scholar]
- 15.Kadowaki T, Nakayama K, Yoshimura F, Okamoto K, Abe N, Yamamoto K. Arg-gingipain acts as a major processing enzyme for various cell surface proteins in Porphyromonas gingivalis. J Biol Chem. 1998;273:29072–29076. doi: 10.1074/jbc.273.44.29072. [DOI] [PubMed] [Google Scholar]
- 16.Kesavalu L, Holt S C, Ebersole J L. Porphyromonas gingivalis virulence in a murine lesion model: effects of immune alterations. Microb Pathog. 1997;23:317–326. doi: 10.1006/mpat.1997.0161. [DOI] [PubMed] [Google Scholar]
- 17.Kuboniwa M, Amano A, Shizukuishi S. Hemoglobin-binding protein purified from Porphyromonas gingivalis is identical to lysine-specific cysteine proteinase (Lys-gingipain) Biochem Biophys Res Commun. 1998;249:38–43. doi: 10.1006/bbrc.1998.8958. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Lewis J P, Dawson J A, Hannis J C, Muddiman D, Macrina F L. Hemoglobinase activity of the lysine gingipain protease (Kgp) of Porphyromonas gingivalis W83. J Bacteriol. 1999;181:4905–4913. doi: 10.1128/jb.181.16.4905-4913.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maiden M F J, Carman R J, Curtis M A, Gillett I R, Griffiths G S, Sterne J A C, A. W J M, Johnson N W. Detection of high-risk groups and individuals for periodontal diseases: laboratory markers based on the microbiological analysis of subgingival plaque. J Clin Periodontol. 1990;17:1–13. doi: 10.1111/j.1600-051x.1990.tb01040.x. [DOI] [PubMed] [Google Scholar]
- 21.Maley J, Shoemaker N B, Roberts I S. The introduction of colonic-Bacteroides shuttle plasmids into Porphyromonas gingivalis: identification of a putative P. gingivalis insertion-sequence element. FEMS Microbiol Lett. 1992;72:75–81. doi: 10.1016/0378-1097(92)90492-7. [DOI] [PubMed] [Google Scholar]
- 22.Marsh P D, McKee A S, McDermid A S. Effect of haemin on enzyme activity and cytotoxin production by Bacteroides gingivalis W50. FEMS Microbiol Lett. 1988;55:87–92. [Google Scholar]
- 23.Marsh P D, McDermid A S, McKee A S, Baskerville A. The effect of growth rate and haemin on the virulence and proteolytic activity of Porphyromonas gingivalis W50. Microbiology. 1994;140:861–865. doi: 10.1099/00221287-140-4-861. [DOI] [PubMed] [Google Scholar]
- 24.McDermid A S, McKee A S, Marsh P D. Effect of environmental pH on enzyme activity and growth of Bacteroides gingivalis W50. Infect Immun. 1988;56:1096–1100. doi: 10.1128/iai.56.5.1096-1100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKee A S, McDermid A S, Baskerville A, Dowsett A B, Ellwood D C, Marsh P D. Effect of hemin on the physiology and virulence of Bacteroides gingivalis W50. Infect Immun. 1986;52:349–355. doi: 10.1128/iai.52.2.349-355.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKee A S, McDermid A S, Wait R, Baskerville A. Isolation of colonial variants of Bacteroides gingivalis W50 with reduced virulence. J Med Microbiol. 1988;27:59–64. doi: 10.1099/00222615-27-1-59. [DOI] [PubMed] [Google Scholar]
- 27.Milton D L, R. O T, Horstedt P, Wolf-Watz H. Flagellin A is essential for the virulence of Vibrio anguillarum. J Bacteriol. 1996;178:1310–1319. doi: 10.1128/jb.178.5.1310-1319.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakayama K, Yoshimura F, Kadowaki T, Yamamoto K. Involvement of arginine-specific cysteine proteinase (Arg-gingipain) in fimbriation of Porphyromonas gingivalis. J Bacteriol. 1996;178:2818–2824. doi: 10.1128/jb.178.10.2818-2824.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neiders M E, Chen P B, Suido H, Reynolds H S, Zambon J J, Shlossman M, Genco R J. Heterogeneity of virulence of Bacteroides gingivalis. J Periodont Res. 1989;24:192–198. doi: 10.1111/j.1600-0765.1989.tb02005.x. [DOI] [PubMed] [Google Scholar]
- 30.Norusis M. SPSS for Windows: base system user's guide, release 6.0. Chicago, Ill: SPSS Inc.; 1993. [Google Scholar]
- 31.O'Brien-Simpson N M, Black C L, Bhogal P S, Cleal S M, Slakeski N, Higgins T J, Reynolds E C. Serum IgG and IgG subclass responses to the RgpA-Kgp proteinase-adhesin complex of P. gingivalis in adult periodontitis. Infect Immun. 2000;68:2704–2712. doi: 10.1128/iai.68.5.2704-2712.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Brien-Simpson N M, Paolini R, Reynolds E C. RgpA-Kgp peptide-based immunogens provide protection against Porphyromonas gingivalis challenge in a murine lesion model. Infect Immun. 2000;68:4055–4063. doi: 10.1128/iai.68.7.4055-4063.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okamoto K, Kadowaki T, Nakayama K, Yamamoto K. Cloning and sequencing of the gene encoding a novel lysine-specific cysteine protease (Lys-gingipain) in Porphyromonas gingivalis: structural relationship with arginine-specific cysteine protease (Arg-gingipain) J Biochem. 1996;120:398–406. doi: 10.1093/oxfordjournals.jbchem.a021426. [DOI] [PubMed] [Google Scholar]
- 34.Okamoto K, Nakayama K, Kadowaki T, Abe N, Ratnayake D B, Yamamoto K. Involvement of a lysine-specific cysteine proteinase in hemoglobin adsorption and heme accumulation by Porphyromonas gingivalis. J Biol Chem. 1998;273:21225–21231. doi: 10.1074/jbc.273.33.21225. [DOI] [PubMed] [Google Scholar]
- 35.Percival R S, Marsh P D, Devine D A, Rangarajan M, Aduse-Opoku J, Shepherd P, Curtis M A. Effect of temperature on growth, hemagglutination, and protease activity of Porphyromonas gingivalis. Infect Immun. 1999;67:1917–1921. doi: 10.1128/iai.67.4.1917-1921.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pike R, McGraw W, Potempa J, Travis J. Lysine- and arginine-specific proteinases from Porphyromonas gingivalis. J Biol Chem. 1994;269:406–411. [PubMed] [Google Scholar]
- 37.Pike R N, Potempa J, McGraw W, Coetzer T H T, Travis J. Characterization of the binding activities of proteinase-adhesin complexes from Porphyromonas gingivalis. J Bacteriol. 1996;178:2876–2882. doi: 10.1128/jb.178.10.2876-2882.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Potempa J, Pike R, Travis J. The multiple forms of trypsin-like activity present in various strains of Porphyromonas gingivalis are due to the presence of either Arg-gingipain or Lys-gingipain. Infect Immun. 1995;63:1176–1182. doi: 10.1128/iai.63.4.1176-1182.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Potempa J, Mikolajczyk-Pawlinska J, Brassell D, Nelson D, Thogersen I B, Enghild J J, Travis J. Comparative properties of two cysteine proteinases (gingipains R), the products of two related but individual genes of Porphyromonas gingivalis. J Biol Chem. 1998;273:21648–21657. doi: 10.1074/jbc.273.34.21648. [DOI] [PubMed] [Google Scholar]
- 40.Rangarajan M, Smith S J S U, Curtis M A. Biochemical characterization of the arginine-specific proteases of Porphyromonas gingivalis W50 suggests a common precursor. Biochem J. 1997;323:701–709. doi: 10.1042/bj3230701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Revell P A, Miller V L. A chromosomally encoded regulator is required for expression of the Yersinia enterocolitica inv gene and for virulence. Mol Microbiol. 2000;35:677–685. doi: 10.1046/j.1365-2958.2000.01740.x. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 43.Shah H, Gharbia S E, Kowlessur D, Wilkie E, Brocklehurst K. Isolation and characterization of gingivain, a cysteine proteinase from Porphyromonas gingivalis strain W83. Biochem Soc Trans. 1990;18:578–579. doi: 10.1042/bst0180578. [DOI] [PubMed] [Google Scholar]
- 44.Shi Y, Ratnayake D B, Okamoto K, Abe N, Yamamoto K, Nakayama K. Genetic analyses of proteolysis, hemoglobin binding, and hemagglutination of Porphyromonas gingivalis: construction of mutants with a combination of rgpA, rgpB, kgp, and hagA. J Biol Chem. 1999;274:17955–17960. doi: 10.1074/jbc.274.25.17955. [DOI] [PubMed] [Google Scholar]
- 45.Slakeski N, Cleal S M, Reynolds E C. Characterization of a Porphyromonas gingivalis gene prtR that encodes an arginine-specific thiol proteinase and multiple adhesins. Biochem Biophys Res Commun. 1996;224:605–610. doi: 10.1006/bbrc.1996.1073. [DOI] [PubMed] [Google Scholar]
- 46.Slakeski N, Bhogal P S, O'Brien-Simpson N M, Reynolds E C. Characterisation of a second cell-associated Arg-specific cysteine proteinase of Porphyromonas gingivalis and identification of an adhesin binding motif involved in association of the PrtR and PrtK proteinases and adhesins into large complexes. Microbiology. 1998;144:1583–1592. doi: 10.1099/00221287-144-6-1583. [DOI] [PubMed] [Google Scholar]
- 47.Slakeski N, Cleal S M, Bhogal P S, Reynolds E C. Characterization of a Porphyromonas gingivalis gene prtK that encodes a lysine-specific cysteine proteinase and three sequence-related adhesins. Oral Microbiol Immunol. 1999;14:92–97. doi: 10.1034/j.1399-302x.1999.140203.x. [DOI] [PubMed] [Google Scholar]
- 48.Slots J. Importance of black-pigmented Bacteriodes in human periodontal disease. In: Genco R J, Merganhagan S, editors. Host-parasite interaction in periodontal disease. Washington, D.C.: American Society for Microbiology; 1982. pp. 27–45. [Google Scholar]
- 49.Slots J, Bragd L, Wikström M, Dahlen G. The occurence of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Bacteroides intermedius in destructive periodontal disease in adults. J Clin Periodontol. 1986;13:570–577. doi: 10.1111/j.1600-051x.1986.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 50.Tokuda M, Duncan M, Cho M-I, Kuramitsu H K. Role of Porphyromonas gingivalis protease activity in colonization of oral surfaces. Infect Immun. 1996;64:4067–4073. doi: 10.1128/iai.64.10.4067-4073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tokuda M, Karunakaran T, Duncan M, Hamada N, Kuramitsu H. Role of Arg-gingipain A in virulence of Porphyromonas gingivalis. Infect Immun. 1998;66:1159–1166. doi: 10.1128/iai.66.3.1159-1166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tokuda M, Chen W, Karunakaran T, Kuramitsu H. Regulation of protease expression in Porphyromonas gingivalis. Infect Immun. 1998;66:5232–5237. doi: 10.1128/iai.66.11.5232-5237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Travis J, Pike R, Imamura T, Potempa J. Porphyromonas gingivalis proteinases as virulence factors in the development of periodontitis. J Periodont Res. 1997;32:120–125. doi: 10.1111/j.1600-0765.1997.tb01392.x. [DOI] [PubMed] [Google Scholar]
- 54.van Steenbergen T J, Kastelein P, Touw J J, de Graaff J. Virulence of black-pigmented Bacteroides strains from periodontal pockets and other sites in experimentally induced skin lesions in mice. J Periodont Res. 1982;17:41–49. doi: 10.1111/j.1600-0765.1982.tb01129.x. [DOI] [PubMed] [Google Scholar]
- 55.Vicente M F, Mengaud J, Chenevert J, Perez-Diaz J C, Geoffroy C, Baquero F, Cossart P, Berche P. Reacquisition of virulence of haemolysin-negative Listeria monocytogenes mutants by complementation with a plasmid carrying the hlyA gene. Acta Microbiol Hung. 1989;36:199–203. [PubMed] [Google Scholar]