Abstract
Objective
Endoscopic third ventriculostomy (ETV) is an option for treatment of hydrocephalus, including for patients who have a history of previous treatment with CSF shunt insertion (CSF shunt). The purpose of this study was to report the success of postshunt ETV by using data from a multicenter, prospective registry.
Methods
Prospectively collected data in the Hydrocephalus Clinical Research Network (HCRN) Core Data Project (i.e., HCRN Registry) were reviewed. Children who underwent ETV between 2008 and 2019 and had a history of previous treatment with a CSF shunt were included. A Kaplan-Meier survival curve was created for the primary outcome: time from postshunt ETV to subsequent CSF shunt placement or revision. Univariable Cox proportional hazards models were created to evaluate for an association between clinical and demographic variables and subsequent shunt surgery. Postshunt ETV complications were also identified and categorized.
Results
A total of 203 children were included: 57% male, 43% female; 74% White, 23% Black, and 4% other race. The most common hydrocephalus etiologies were postintraventricular hemorrhage secondary to prematurity (56, 28%) and aqueductal stenosis (42, 21%). The ETV Success Score ranged from 10 to 80. The median patient age was 4.1 years. The overall success of postshunt ETV at 6 months was 41%. Only the surgeon’s report of a clear view of the basilar artery was associated with a lower likelihood of postshunt ETV failure (HR 0.43, 95% CI 0.23–0.82, p = 0.009). None of the following variables were associated with postshunt ETV success: age at the time of postshunt ETV, etiology of hydrocephalus, sex, race, ventricle size, number of previous shunt operations, ETV performed at time of shunt infection, and use of external ventricular drainage. Overall complications were reported in 22% of patients, with CSF leak (8.6%) the most common complication.
Conclusions
Postshunt ETV was successful in treating hydrocephalus, without subsequent need for a CSF shunt, in 41% of patients, with a clear view of the basilar artery the only variable significantly associated with success. Complications occurred in 22% of patients. ETV is an option for treatment of hydrocephalus in children who have previously undergone shunt placement, but with a lower than expected likelihood of success.
Keywords: ventriculoperitoneal shunt, endoscopic third ventriculostomy, ETV revision, Hydrocephalus Clinical Research Network
In Brief
Researchers from the Hydrocephalus Clinical Research Network (HCRN) queried the HCRN core data set for children who had undergone endoscopic third ventriculostomy (ETV) after previously undergoing shunt placement. The overall success rate of ETV in patients who previously received a shunt was 41%. The only variable to show significant association with ETV success was a surgeon’s report of a clear view of the basilar artery. The observed rate of success was lower than predicted by the ETV Success Score.
Cerebrospinal fluid shunting remains the most common treatment for pediatric hydrocephalus. Endoscopic third ventriculostomy (ETV) is an alternative to CSF shunting that is potentially favorable due to the lack of implanted hardware. Most previous studies of ETV have focused on its role as an initial treatment for hydrocephalus and on comparing ETV to CSF shunting. However, there are some children who have previously been treated with a CSF shunt who may be candidates for ETV. This clinical scenario—ETV performed in children with a history of CSF shunting—has not been as rigorously studied as primary ETV and is the focus of this study.
The ETV Success Score (ETVSS) is the most widely used scale to estimate the likelihood of ETV success for a given patient.1 Although the age of the patient is the most important component of the ETVSS, history of previous treatment with a shunt is also included in the model. According to the ETVSS, previous shunting reduces the likelihood of ETV success by approximately 10%.1,2 There have been numerous previous studies of ETV in children with a history of a CSF shunt (postshunt ETV), reporting success rates from 50% to 80%.3-18 Two review articles also covered this topic, one of which reported ETV success by meta-analysis in 68% of 519 pooled patients.19,20 However, nearly all of these reports were single-center retrospective reviews, the largest of which included 88 patients. Most previous studies included fewer than 50 patients. The primary purpose of this study was to report the effectiveness of ETV in children who had previously been treated with a CSF shunt at one of the institutions in the multicenter Hydrocephalus Clinical Research Network (HCRN).
Because children with a history of a CSF shunt may have smaller ventricles than patients whose hydrocephalus has not been treated, endoscopic treatments like ETV may be more challenging. Thus, the risk of complication may be higher with postshunt ETV than with primary ETV. A secondary aim of this study was to evaluate the safety of postshunt ETV. Finally, we examined common clinical and demographic factors for association with postshunt ETV success.
Methods
Study Design
This study included all children with a history of treatment with a CSF shunt who underwent ETV between April 2008 and December 2019 at one of 13 HCRN centers (Children’s Hospital of Alabama, Birmingham, AL; Primary Children’s Hospital, Salt Lake City, UT; Seattle Children’s Hospital, Seattle, WA; Children’s Hospital of Pittsburgh, PA; St. Louis Children’s Hospital, St. Louis, MO; Texas Children’s Hospital, Houston, TX; SickKids Hospital, Toronto, Canada; Monroe Carell Jr. Children’s Hospital at Vanderbilt, Nashville, TN; British Columbia Children’s Hospital, Vancouver, BC; Alberta Children’s Hospital, Calgary, AB; Children’s Hospital of Los Angeles, CA; Children’s Hospital Colorado, Aurora, CO; and Nationwide Children’s Hospital, Columbus, OH). Data were collected prospectively into the observational HCRN Core Data Project (i.e., HCRN Registry), tracking all hydrocephalus surgeries at each HCRN center from date of joining the HCRN to the present. Informed consent is not required at most centers, allowing for comprehensive enrollment and construction of a representative sample. IRB approval was obtained from each clinical site as well as the data-coordinating center.21
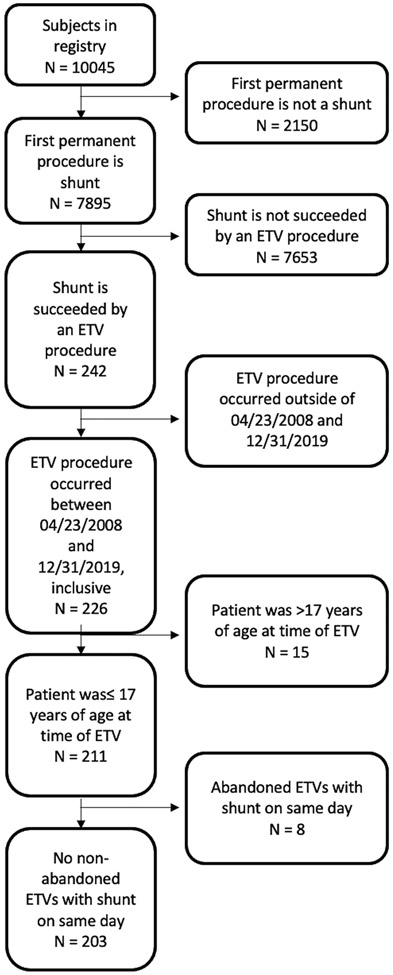
The HCRN Registry database was reviewed for all patients who had placement of a shunt as the first permanent treatment of hydrocephalus. Among these children, those who had a subsequent ETV procedure while younger than 17 years within the specified time frame were identified. Children who had a record of a CSF shunt on the same day as ETV were excluded, presuming that these represented attempted but unsuccessful ETV or ETV with immediate failure.
Outcomes
The primary analysis was an estimate of the time to CSF shunt surgery (placement or revision) after a postshunt ETV. Time to shunt surgery was defined as placement of a subsequent CSF shunt following the ETV or revision of CSF shunt. Shunt surgery was selected as the primary outcome, rather than ETV failure, because the goal for most postshunt ETV procedures is to render the patient shunt-free. In addition, postprocedural (during the hospital stay) complications after a postshunt ETV were identified and defined as follows: any new neurological deficit; CSF leak (defined as any episode of CSF leak, regardless of treatment); wound infection; diabetes insipidus; or other complications (hyponatremia, urinary tract infection, sepsis, intracranial fluid collection, meningitis, seizure, pseudomeningocele, hemorrhage). Finally, the ETVSS was calculated for each patient.
Statistical Analysis
Patient characteristics and postprocedural complications are presented as counts and percentages for categorical variables and as the median, first quartile, and third quartile for continuous variables. A Kaplan-Meier curve of time to shunt surgery was created for the primary analysis. Patients were censored at the time of most recent follow-up, relocation away from HCRN-participating sites, or death.
After verifying that a proportional hazards assumption was plausible, univariable Cox proportional hazards models were created to evaluate for association between clinical and demographic variables and time to shunt surgery after a postshunt ETV. Candidate predictors included the following: corrected age at postshunt ETV procedure; sex; race; etiology of hydrocephalus (e.g., post–intraventricular hemorrhage [IVH] secondary to prematurity, myelomeningocele, aqueductal stenosis, and other etiology); frontal/occipital horn ratio (FOR); performance of a septostomy; choroid plexus cauterization (CPC); presence of bleeding during the procedure (mild = not totally obstructing the view; moderate = view totally obstructed, but clearing within 2–3 minutes; severe = more than 5 minutes required to return to clear working conditions); method of dilation of the ETV site; characterization of the view of the basilar artery (BA); and whether the postshunt ETV was performed at the time of shunt infection. The p values were reported based on a 2-sided alternative and are considered significant where p < 0.05. A multivariable Cox proportional hazard model was created including age at the time of postshunt ETV, etiology of hydrocephalus, and any variable with p < 0.1 on the univariable analysis. Patients with missing data were only excluded from analyses involving the missing data point. Results are reported as hazard ratios and 95% confidence intervals. All analyses were conducted using SAS 9.4 (SAS Institute).
Results
A total of 203 patients were included in the study cohort. Figure 1 shows the composition of the study cohort from the broader HCRN Registry. There were 115 (57%) male and 88 (43%) female children. The majority of children were White (121, 74%), with Black and other race constituting 23% and 4%, respectively. Primary medical insurance coverage was mixed between private (38%); public (Medicare, Medicaid, Canadian provincial health insurance) (43%); and military (18%). The most common hydrocephalus etiologies were post-IVH secondary to prematurity, myelomeningocele, and aqueductal stenosis. ETVSS ranged from 10 to 80, with 65% of patients having an ETVSS of 60 or 70. Across the entire sample, the mean ETVSS was 60.1 (SD 15.5). Details of the makeup of the study sample are shown in Table 1.
FIG. 1.
CONSORT flow diagram.
TABLE 1.
Characteristics of patients who received postshunt ETV
| Overall, n = 203 | |
|---|---|
| Male | 115 (56.7%) |
| Race* | |
| White | 121 (73.8%) |
| Black or African American | 37 (22.6%) |
| Other | 6 (3.7%) |
| Gestational age at birth in wks, median (IQR)† | 36.0 (28.0–39.0) |
| Primary insurance classification‡ | |
| Public—Medicaid, Medicare | 87 (43.3%) |
| Private | 77 (38.3%) |
| Other—i.e., military | 37 (18.4%) |
| Etiology of hydrocephalus | |
| Post-IVH secondary to prematurity | 56 (27.6%) |
| Myelomeningocele | 30 (14.8%) |
| Aqueductal stenosis | 42 (20.7%) |
| Other etiology | 75 (36.9%) |
| ETVSS | |
| 10 | 4 (2.0%) |
| 20 | 2 (1.0%) |
| 30 | 11 (5.4%) |
| 40 | 21 (10.3%) |
| 50 | 11 (5.4%) |
| 60 | 59 (29.1%) |
| 70 | 73 (36.0%) |
| 80 | 22 (10.8%) |
| Average ETVSS | 60.1 |
Missing in 39 patients.
Missing in 42 patients.
Missing in 2 patients.
The median age at the time of postshunt ETV was 4.1 years (interquartile range [IQR] 1.0–10.2). The median FOHR was 0.48 (IQR 0.42–0.58). In 50 cases (25%) postshunt ETV was performed in the setting of a shunt infection. CPC was not performed during most postshunt ETV procedures (106, 67%). Bleeding during the procedure occurred in 86 (42%), most often classified as mild (75 mild bleeding events, 37% of the total sample). Many different methods were used for dilation of the ETV, including forceps, balloons, and the endoscope. Details are shown in Table 2. A clear view of the BA after performance of ETV was reported in 170 cases (93%). After surgery, the median length of stay was 5 days (IQR 2.0–10.0). External ventricular drains (EVDs) were used in 107 cases (58%).
TABLE 2.
Postshunt ETV procedure summary
| Overall, n = 203 | |
|---|---|
| Age at time of ETV in yrs, median (IQR) | 4.1 (1.0–10.2) |
| FOR, median (IQR)* | 0.48 (0.42–0.58) |
| Septostomy performed† | 23 (17.3%) |
| CPC done‡ | |
| None | 106 (67.1%) |
| Unilat— partial | 7 (4.4%) |
| Unilat— complete | 4 (2.5%) |
| Bilat— partial | 13 (8.2%) |
| Bilat— complete | 28 (17.7%) |
| Bleeding during procedure | |
| None | 117 (57.6%) |
| Mild | 75 (36.9%) |
| Moderate | 9 (4.4%) |
| Severe | 2 (1.0%) |
| Dilation method§ | |
| Forceps | 28 (15.6%) |
| Fogarty catheter | 51 (28.3%) |
| Neuroballoon | 46 (25.6%) |
| Spreader | 8 (4.4%) |
| Endoscope | 32 (17.8%) |
| Multiple | 15 (8.3%) |
| BA clear view¶ | 170 (92.9%) |
| EVD placement during ETV procedure** | 107 (57.5%) |
| Length of stay after ETV in days, median (IQR)†† | 5.0 (2.0–10.0) |
| ETV was part of a shunt infection treatment | 50 (24.6%) |
Missing in 31 patients.
Missing in 70 patients.
Missing in 45 patients.
Missing in 23 patients.
Missing in 20 patients.
Missing in 17 patients.
Missing in 4 patients.
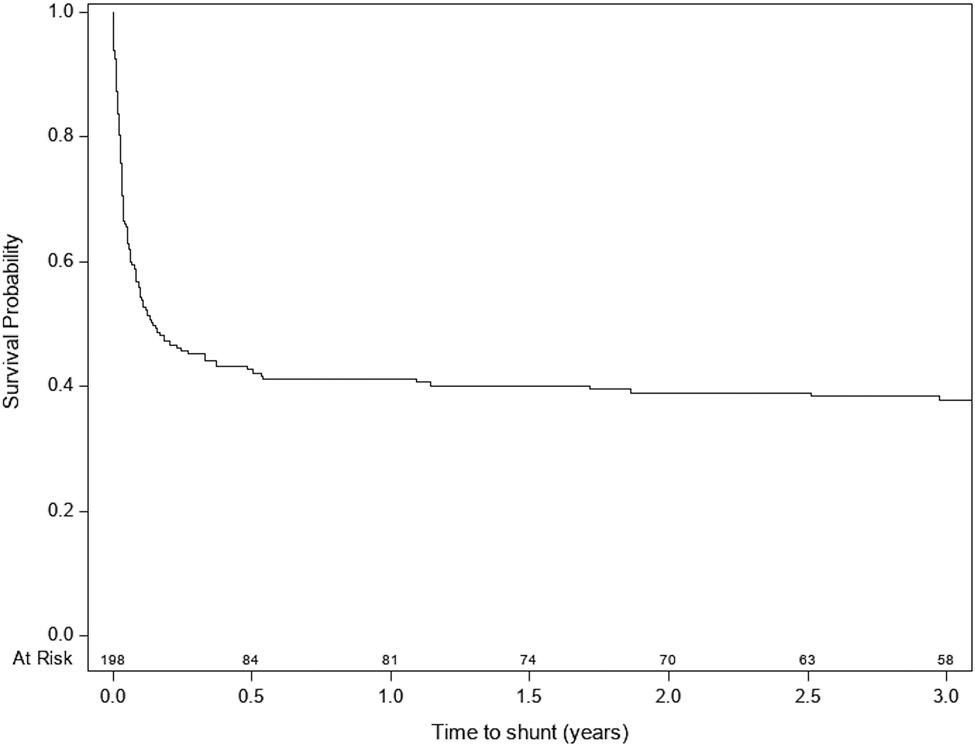
Figure 2 shows the Kaplan-Meier survival plot for shunt surgery after a postshunt ETV. The overall success rate at 6 months was 41% (83 patients). Univariable Cox proportional hazard analysis of factors associated with time to shunt surgery is shown in Table 3. A surgeon’s report of a clear view of the BA in the prepontine cistern was associated with lower HR for shunt surgery (HR 0.43, 95% CI 0.23–0.82, p = 0.009). In addition, severe bleeding during the procedure was associated with higher HR compared to no bleeding (HR 4.83, 95% CI 1.17–19.96). A multivariable model, including age, hydrocephalus etiology, clear view of the BA, and bleeding during the procedure showed only a clear BA view (HR 0.45, 95% CI 0.23–0.86, p = 0.016) to be significantly associated with remaining shunt free after a postshunt ETV (Table 4). Older age was very nearly significant (HR 0.96, 95% CI 0.92–1.0, p = 0.05).
FIG. 2.
Kaplan-Meier curve of time to shunt surgery after a postshunt ETV.
TABLE 3.
Univariable associations with time to shunt surgery in postshunt ETV revisions
| [Au: Deleted head for consistency w/ Table 4] | ||
|---|---|---|
| HR (95% CI) | p Value | |
| Age at time of PSETV in yrs | 0.98 (0.94–1.01) | 0.184 |
| Sex | 0.383 | |
| Female | Reference | |
| Male | 0.85 (0.60, 1.22) | |
| Race | 0.221 | |
| White | Reference | |
| Black or African American | 0.63 (0.37, 1.07) | |
| Other | 1.04 (0.38, 2.86) | |
| Etiology of hydrocephalus | 0.840 | |
| Post-IVH secondary to prematurity | Reference | |
| Myelomeningocele | 0.98 (0.56, 1.73) | |
| Aqueductal stenosis | 0.81 (0.48, 1.36) | |
| Other etiology | 0.87 (0.56, 1.36) | |
| FOR | 0.96 (0.83, 1.11) | 0.617 |
| Septostomy performed | 1.34 (0.76, 2.36) | 0.313 |
| CPC done | 0.811 | |
| No | Reference | |
| Yes | 1.05 (0.68, 1.62) | |
| Bleeding during procedure | 0.098 | |
| None | Reference | |
| Mild | 1.32 (0.91, 1.91) | |
| Moderate | 1.07 (0.43, 2.65) | |
| Severe | 4.83 (1.17, 19.96) | |
| Dilation method | 0.984 | |
| Forceps | 1.13 (0.57, 2.27) | |
| Fogarty catheter | 1.20 (0.66, 2.20) | |
| Neuroballoon | 1.09 (0.58, 2.03) | |
| Spreader | 1.39 (0.51, 3.78) | |
| Endoscope | Reference | |
| Multiple | 1.23 (0.56, 2.70) | |
| BA clear view | 0.43 (0.23, 0.82) | 0.009 |
| ETV was part of a shunt infection treatment approach | 1.04 (0.69, 1.54) | 0.864 |
| Gestational age at birth, wks | 1.00 (0.96, 1.03) | 0.813 |
| Primary insurance classification | 0.927 | |
| Public—Medicaid, Medicare | Reference | |
| Private | 1.07 (0.72, 1.59) | |
| Other—i.e., military | 1.08 (0.65, 1.80) | |
| EVD placement during PSETV procedure | 0.140 | |
| No | Reference | |
| Yes | 1.34 (0.91, 1.98) | |
PSETV = postshunt ETV.
TABLE 4.
Multivariable
| HR (95% CI) | p Value | |
|---|---|---|
| Age at time of PSETV, in yrs | 0.96 (0.92–1.00) | 0.050 |
| Etiology of hydrocephalus | 0.937 | |
| Post-IVH secondary to prematurity | Reference | |
| Myelomeningocele | 0.85 (0.44, 1.62) | |
| Aqueductal stenosis | 0.85 (0.49, 1.49) | |
| Other etiology | 0.89 (0.54, 1.46) | |
| BA clear view | 0.45 (0.23, 0.86) | 0.016 |
| Bleeding during procedure | 0.123 | |
| No | Reference | |
| Yes | 1.36 (0.92, 2.01) |
There was a subset of 76 patients in whom their entire shunt history from initial insertion to postshunt ETV, including all intervening shunt revisions, was available in the HCRN Registry. Within this subset, the median number of shunt revisions prior to ETV was 1 (IQR 0–2). A Cox regression model determined that there was no association between the number of previous shunt revisions and time to shunt after a postshunt ETV (HR 0.97, 95% CI 0.82–1.15, p = 0.695).
Immediate postprocedural complications were reported in 38 of 175 cases for which data were available (22%). Table 5 contains details about complications.
TABLE 5.
Complications after PSETV procedure
| Postprocedural Complications, n = 175 |
|
|---|---|
| New neurological deficit | 4 (2.3%) |
| CSF leak | 15 (8.6%) |
| Wound infection | 2 (1.1%) |
| Diabetes insipidus | 0 (0%) |
| Hyponatremia | 3 (1.7%) |
| Urinary tract infection | 3 (1.7%) |
| Sepsis | 3 (1.7%) |
| Intracranial fluid collection | 3 (1.7%) |
| Overdrainage/underdrainage Sxs | 3 (1.7%) |
| Documented bacterial meningitis | 3 (1.9%) |
| Seizure | 6 (3.4%) |
| Pseudomeningocele | |
| Minor | 7 (4.0%) |
| Major | 1 (0.6%) |
| Postop hemorrhage | |
| IVH | 3 (1.7%) |
| ICH | 1 (0.6%) |
| EDH | 0 (0%) |
| SDH | 0 (0%) |
EDH = epidural hemorrhage; ICH = intracerebral hemorrhage; SDH = subdural hemorrhage; Sxs = symptoms.
Total of 57 complications in 38 patients.
Discussion
In this study, we have examined children with shunted hydrocephalus who underwent ETV. Although several previous studies have investigated this question, the present sample is the largest single study and represents prospectively collected data from multiple centers. We found an overall success rate for ETV in previously shunt-treated patients of 41% at 6 months. This success rate is lower than most reports from the literature. The median age in the present study was 4.1 years (IQR 1.0–10.2). In much of the published literature, patients included in studies of ETV are older, and it is well established that older age is associated with higher likelihood of ETV success. For example, in a systematic review of 15 studies, including 519 patients, the ETV success rate was 68.2%, but the mean age of included patients was 9.8 years.19 In addition, the ETVSS for the present sample predicts likelihood of ETV success to be 60%, higher than the 41% observed. This suggests that the ETVSS may overestimate the likelihood of ETV success in children with an existing CSF shunt.
Of all variables examined, only the presence of a clear view of the BA was associated with a lower hazard ratio for ETV failure. A clear view of the BA is an indication that there is minimal arachnoid scarring present in the prepontine cistern. In earlier HCRN studies, this variable showed significant association with ETV success in a sample of children undergoing first-time ETV without CPC, but no significant association when CPC was included.22,23 Other studies have also reported on the importance of minimal cisternal scarring.24 This variable is somewhat subject to the judgement of the operating surgeon. While there is no specific study of “clear view of BA,” the assessment of scarring in the prepontine cistern has been shown to have moderate agreement in an interrater reliability study.25 Our present findings reinforce the importance of an open, unscarred cistern for ETV success, as judged by a clear view of the BA. In addition, based on these results, a surgeon could decide to proceed with immediate shunt placement if a scarred or closed cistern is encountered at the time of postshunt ETV.
Other variables, such as age and hydrocephalus etiology, did not show significant association with success of postshunt ETV. Some previous studies have shown a significant effect of age,5 whereas others have shown no significant effect of age on outcome.11,18 Similarly, the etiology of hydrocephalus has had a significant effect on outcome in some studies,15,17 but not in others.11 One possible explanation for the lack of significance in the present study is the lack of variation in the sample for these two components of the ETVSS. Most of the included patients were between 1 and 10 years of age. All of these patients would be assigned the same age score using the ETVSS.1 Similarly, the etiology of hydrocephalus for 63% of patients in the current study was either post-IVH, myelomeningocele, or aqueductal stenosis. Using the ETVSS, these would be assigned a very similar etiology score. Thus, although there is a wide range of pediatric patients represented in this sample, it represents a narrower range of ETV success likelihood.
Previous studies have also shown an association between a history of multiple shunt revisions and lower likelihood of success of postshunt ETV.13,15,16 Our analysis of this risk factor was limited to a subset of the overall sample: those patients for whom the details of all previous shunt surgeries were known. However, in that subset we saw no association between number of shunt revisions and postshunt ETV success. Therefore, postshunt ETV might be considered as a treatment option regardless of the number of previous shunt surgeries.
Examination of the survival curve for postshunt ETV shows that nearly all ETV failures occur within the first 6 months, and many occur within the first month. Existing literature demonstrates a similar pattern. Among studies that report time to postshunt ETV failure, 6 state that 95% or more of the failures occur within 1 month.4,5,8,9,11,26 The preponderance of failures early after ETV suggests that cautious optimism might be appropriate for physicians and patients if a postshunt ETV continues to show signs of success after 6 months.
Complications have been reported to occur after a postshunt ETV in 0%–30% of cases.3,4,12,13 The complication rate in the present study was 22%, most commonly CSF leaks. Wound infection, diabetes insipidus, and neurological deficit were rare. A systematic review of reports of complications after ETV shows an overall complication rate of 8.5%.27 The observed complication rate of this series is higher. This may be related to the presence of smaller ventricles when performing an ETV in a patient who has a CSF shunt, although new neurological deficit or diabetes insipidus (the complications that might be more likely in a patient with small ventricles) were rare (2.3% and 0%, respectively). In addition, previous studies comparing neuroendoscopy in patients with small ventricles to those with large ventricles showed no additional risk with smaller ventricles.28 Another possibility is that the reporting of complications is robust in this prospective sample. For example, any small amount of hemorrhage or subcutaneous fluid on postoperative imaging studies would be considered a hemorrhage or a minor pseudomeningocele. The most common complication was a CSF leak (8.6%). Post hoc analysis of endoscope type revealed equal CSF leak rates using flexible versus rigid endoscopes. Given the low rate of success overall, this is likely to be a common way for postshunt ETV to show failure: persistent elevation of intracranial pressure would lead to increased risk of CSF leak. Surgeons should be aware of the risk of these complications when deciding whether to offer postshunt ETV.
Limitations
This study is based on data from a registry of patients treated surgically for hydrocephalus. The analysis was performed retrospectively. However, all data were collected prospectively, with established protocols for data fidelity, validation, and quality control. The registry includes only centers in North America, so generalization of these data to other parts of the world may not be appropriate. Surgeons selected patients for postshunt ETV based on their own, nonstandardized clinical criteria. This represents a potential source of selection bias.
Shunt surgery was used as the primary outcome for this analysis, rather than any hydrocephalus treatment. Therefore, in a patient who had a postshunt ETV followed by a temporary EVD or a redo ETV, that would not be counted as a failed ETV. This was intentional given that the goal for most postshunt ETV procedures is to render the patient shunt-free. We performed the analysis again (data not shown) with ETV failure (defined as any hydrocephalus procedure) as the outcome, and there were no differences in any parameter from the analyses shown here. Of note, 7 patients had redo ETV as treatment for presumed failure of the postshunt ETV. Of these 7 redo procedures, 4 were successful, with no record of additional treatment. The other 3 patients later had shunt placement.
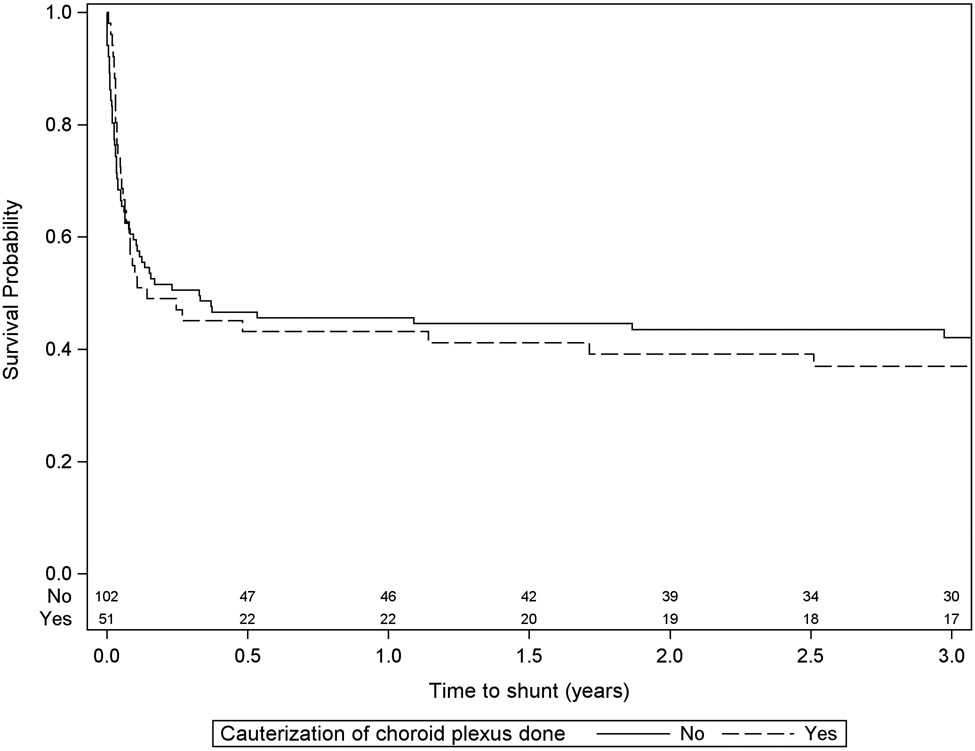
Some data points were missing for some patients, and these individuals were excluded only from the involved analyses. This could be a source of bias if the missing data were not random. No effort was made to control for this. However, the amount of missing data in the variables that were significant in our model was small, and therefore we estimate that the risk of this bias is small. This analysis considers children who underwent ETV and ETV+CPC together, even though ETV+CPC is usually only performed in very young children. The primary analysis (Table 3) showed no significant effect of including CPC. We constructed post hoc Kaplan-Meier survival curves to assess for a difference between ETV and ETV+CPC, and these show no difference in the rate of shunt surgery depending on CPC (Fig. 3). Finally, we found no relationship between EVD use and ETV success. However, no information was available about how the drain was used (clamped, open, duration of use, etc.). It is possible that with more granular detail, a relationship might be uncovered. Similarly, different types of infections (different organisms) might have led to different failure rates even though no overall effect of infection was observed.
FIG. 3.
Kaplan-Meier curve of time to shunt surgery after a postshunt ETV, with versus without CPC.
Rocque et al.
Conclusions
The 6-month success of ETV in children who had previously been treated with shunts was 41%. A clear surgical view of the BA in the prepontine cistern was associated with a higher likelihood of postshunt ETV success. Success observed in this study was lower than predicted by the ETVSS. Complications occurred in more than 20% of patients, which was higher than published series of ETV, indicating that postshunt ETV may be more challenging than ETV as initial treatment for hydrocephalus.
Acknowledgments
We thank our colleagues for their past and ongoing support of HCRN: D Brockmeyer, M Walker, R Bollo, S Cheshier, J Blount, J Johnston, B Rocque, L Acakpo-Satchivi, WJ Oakes, P Dirks, J Rutka, M Taylor, D Curry, G Aldave, R Dauser, A Jea, S Lam, H Weiner, T Luerssen, R Ellenbogen, J Ojemann, A Lee, A Avellino, S Greene, E Tyler-Kabara, T Abel, TS Park, J Strahle, S McEvoy, M Smyth, N Tulipan, A Singhal, P Steinbok, D Cochrane, W Hader, C Gallagher, M Benour, E Kiehna, JG McComb, P Chiarelli, A Robison, A Alexander, M Handler, B O’Neill, C Wilkinson, L Governale, A Drapeau, J Leonard, E Sribnick, A Shaikhouni, E Ahn, A Cohen, M Groves, S Robinson, CM Bonfield, and C Shannon.
In addition, our work would not be possible without the outstanding support of the dedicated personnel at each clinical site and the data coordinating center. Special thanks go to: L Holman, J Clawson, P Martello, N Tattersall, T Bach (Salt Lake City); T Caudill, P Komarova, A Arynchyna, A Bey (Birmingham); H Ashrafpour, M Lamberti-Pasculli, L O’Connor (Toronto); E Santisbon, E Sanchez, S Martinez, S Ryan (Houston); K Hall, C Gangan, J Klein, A Anderson, G Bowen (Seattle); S Thambireddy, K Diamond, A Luther (Pittsburgh); A Morgan, H Botteron, D Morales, M Gabir, D Berger, D Mercer (St. Louis); M Stone, A Wiseman, J Stoll, D Dawson, S Gannon (Nashville); A Cheong, R Hengel (Vancouver, British Columbia); R Rashid, S Ahmed (Calgary); J Yea, A Loudermilk (Baltimore); N Chapman, N Rea, C Cook (Los Angeles); S Staulcup (Colorado); S Saraswat, A Sheline (Columbus); and N Nunn, M Langley, V Wall, D Austin, B Conley, V Freimann, L Herrera, B Miller (Utah Data Coordinating Center).
Disclosures
Dr. Limbrick received support for a non–study-related research or clinical effort that he oversaw from Medtronic, Inc., and Microbot Medical, Inc. Dr. Hauptman is a consultant for Medtronic and BK Medical. The HCRN is thankful for the following sources of funding: National Institute of Neurological Disorders and Stroke (NINDS grant no. 1RC1NS068943-01 Challenge); NINDS grant no. 1U01NS107486-01A1 ESTHI; Patient Centered Outcome Research Institute (PCORI grant no. CER-1403-13857); The Gerber Foundation (reference no. 1692-3638); private philanthropy; and the Hydrocephalus Association.
Abbreviations
- BA
basilar artery
- CPC
choroid plexus cauterization
- ETV
endoscopic third ventriculostomy
- ETVSS
ETV Success Score
- EVD
external ventricular drain
- FOR
frontal/occipital horn ratio
- HCRN
Hydrocephalus Clinical Research Network
- IQR
interquartile range
- IVH
intraventricular hemorrhage
References
- 1.Kulkarni AV, Drake JM, Kestle JR, Mallucci CL, Sgouros S, Constantini S. Predicting who will benefit from endoscopic third ventriculostomy compared with shunt insertion in childhood hydrocephalus using the ETV Success Score. J Neurosurg Pediatr. 2010;6(4):310–315. [DOI] [PubMed] [Google Scholar]
- 2.Kulkarni AV, Riva-Cambrin J, Browd SR. Use of the ETV Success Score to explain the variation in reported endoscopic third ventriculostomy success rates among published case series of childhood hydrocephalus. J Neurosurg Pediatr. 2011;7(2):143–146. [DOI] [PubMed] [Google Scholar]
- 3.Furtado LMF, da Costa Val Filho JA, Holliday JB, et al. Endoscopic third ventriculostomy in patients with myelomeningocele after shunt failure. Childs Nerv Syst. 2020;36(12):3047–3052. [DOI] [PubMed] [Google Scholar]
- 4.Heshmati B, Habibi Z, Golpayegani M, Salari F, Anbarlouei M, Nejat F. Endoscopic third ventriculostomy in children with failed ventriculoperitoneal shunt. Asian J Neurosurg. 2019;14(2):399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irrinki RNNS, Bawa M, Hegde S, Chhabra R, Gupta V, Gupta SK. Functional and radiological parameters to assess outcome of endoscopic third ventriculostomy in shunt failure patients. J Pediatr Neurosci. 2019;14(2):65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaikh S, Deopujari CE, Karmarkar V, Muley K, Mohanty C. Role of secondary endoscopic third ventriculostomy in children: review of an institutional experience. Pediatr Neurosurg. 2019;54(3):188–195. [DOI] [PubMed] [Google Scholar]
- 7.Lee SH, Kong DS, Seol HJ, Shin HJ. Endoscopic third ventriculostomy in patients with shunt malfunction. J Korean Neurosurg Soc. 2011;49(4):217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bilginer B, Oguz KK, Akalan N. Endoscopic third ventriculostomy for malfunction in previously shunted infants. Childs Nerv Syst. 2009;25(6):683–688. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien DF, Javadpour M, Collins DR, Spennato P, Mallucci CL. Endoscopic third ventriculostomy: an outcome analysis of primary cases and procedures performed after ventriculoperitoneal shunt malfunction. J Neurosurg. 2005;103(5)(suppl):393–400. [DOI] [PubMed] [Google Scholar]
- 10.Brichtova E, Chlachula M, Hrbac T, Lipina R. Endoscopic third ventriculostomy in previously shunted children. Minim Invasive Surg. 2013;2013:584567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marton E, Feletti A, Basaldella L, Longatti P. Endoscopic third ventriculostomy in previously shunted children: a retrospective study. Childs Nerv Syst. 2010;26(7):937–943. [DOI] [PubMed] [Google Scholar]
- 12.Takeshige N, Uchikado H, Nakashima D, et al. Endoscopic third ventriculostomy for myelomeningocele-related hydrocephalus after shunt failure: long-term outcome in a series of 8 patients. Clin Neurol Neurosurg. 2021;201:106406. [DOI] [PubMed] [Google Scholar]
- 13.Hader WJ, Walker RL, Myles ST, Hamilton M. Complications of endoscopic third ventriculostomy in previously shunted patients. Neurosurgery. 2008;63(1)(suppl 1):ONS168–ONS175. [DOI] [PubMed] [Google Scholar]
- 14.Iglesias S, Ros B, Ibáñez G, Delgado A, Ros Á, Arráez MÁ. Shunt independence in paediatric hydrocephalus: our 16-year experience and review. Childs Nerv Syst. 2019;35(9):1547–1555. [DOI] [PubMed] [Google Scholar]
- 15.Choudhary A, Sobti S, Zambre S, Bhaskar S. Endoscopic third ventriculostomy in failed ventriculoperitoneal shunt in pediatric population. Asian J Neurosurg. 2020;15(4):937–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talamonti G, Nichelatti M, Picano M, Marcati E, D’Aliberti G, Cenzato M. Endoscopic third ventriculostomy in cases of ventriculoperitoneal shunt malfunction: does shunt duration play a role? World Neurosurg. 2019;127:e799–e808. [DOI] [PubMed] [Google Scholar]
- 17.Zhao R, Shi W, Yang H, Li H. Endoscopic third ventriculostomy instead of shunt revision in children younger than 3 years of age. World Neurosurg. 2016;88:92–96. [DOI] [PubMed] [Google Scholar]
- 18.Chhun V, Sacko O, Boetto S, Roux FE. Third ventriculocisternostomy for shunt failure. World Neurosurg. 2015;83(6):970–975. [DOI] [PubMed] [Google Scholar]
- 19.Waqar M, Ellenbogen JR, Mallucci C. Endoscopic third ventriculostomy for shunt malfunction in children: a review. J Clin Neurosci. 2018;51:6–11. [DOI] [PubMed] [Google Scholar]
- 20.Boschert J, Hellwig D, Krauss JK. Endoscopic third ventriculostomy for shunt dysfunction in occlusive hydrocephalus: long-term follow up and review. J Neurosurg. 2003;98(5):1032–1039. [DOI] [PubMed] [Google Scholar]
- 21.Tamber MS, Kestle JRW, Reeder RW, et al. Temporal trends in surgical procedures for pediatric hydrocephalus: an analysis of the Hydrocephalus Clinical Research Network Core Data Project. J Neurosurg Pediatr. 2020;27(3):269–276. [DOI] [PubMed] [Google Scholar]
- 22.Kulkarni AV, Riva-Cambrin J, Holubkov R, et al. Endoscopic third ventriculostomy in children: prospective, multicenter results from the Hydrocephalus Clinical Research Network. J Neurosurg Pediatr. 2016;18(4):423–429. [DOI] [PubMed] [Google Scholar]
- 23.Riva-Cambrin J, Kestle JRW, Rozzelle CJ, et al. Predictors of success for combined endoscopic third ventriculostomy and choroid plexus cauterization in a North American setting: a Hydrocephalus Clinical Research Network study. J Neurosurg Pediatr. 2019;24(2):128–138. [DOI] [PubMed] [Google Scholar]
- 24.Greenfield JP, Hoffman C, Kuo E, Christos PJ, Souweidane MM. Intraoperative assessment of endoscopic third ventriculostomy success. J Neurosurg Pediatr. 2008;2(5):298–303. [DOI] [PubMed] [Google Scholar]
- 25.He L, Gannon S, Shannon CN, Rocque BG, Riva-Cambrin J, Naftel RP. Surgeon interrater reliability in the endoscopic assessment of cistern scarring and aqueduct patency. J Neurosurg Pediatr. 2016;18(3):320–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buxton N, Macarthur D, Robertson I, Punt J. Neuroendoscopic third ventriculostomy for failed shunts. Surg Neurol. 2003;60(3):201–204. [DOI] [PubMed] [Google Scholar]
- 27.Bouras T, Sgouros S. Complications of endoscopic third ventriculostomy. J Neurosurg Pediatr. 2011;7(6):643–649. [DOI] [PubMed] [Google Scholar]
- 28.Naftel RP, Shannon CN, Reed GT, et al. Small-ventricle neuroendoscopy for pediatric brain tumor management. J Neurosurg Pediatr. 2011;7(1):104–110. [DOI] [PubMed] [Google Scholar]