Abstract
Background:
While fluctuations in healthy brain temperature have been investigated over time periods of weeks to months, dynamics over shorter time periods are less clear.
Purpose:
To identify physiological fluctuations in brain temperature in healthy volunteers over time scales of approximately 1 hour.
Study Type:
Prospective
Subjects:
30 healthy volunteers (15 female; 26±4 years old)
Sequence and Field Strength:
3T; T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE) and semi-localized by adiabatic selective refocusing (sLASER) single-voxel spectroscopy.
Assessments:
Brain temperature was calculated from the chemical shift difference between N-acetylaspartate and water. To evaluate within-scan repeatability of brain temperature and the brain-body temperature difference, 128 spectral transients were divided into two sets of 64-spectra. Between-scan repeatability was evaluated using two time periods, ~1–1.5 hours apart.
Statistical Tests:
A hierarchical linear mixed model was used to calculate within-scan and between-scan correlations (Rw and Rb, respectively). Significance was determined at p≤.05. Values are reported as the mean ± standard deviation.
Results:
A significant difference in brain temperature was observed between scans (−0.4 °C) but body temperature was stable (p=.59). Brain temperature (37.9±0.7 °C) was higher than body temperature (36.5±0.5 °C) for all but one subject. Within-scan correlation was high for brain temperature (Rw = 0.95) and brain-body temperature differences (Rw = 0.96). Between scans, variability was high for both brain temperature (Rb=0.30) and brain-body temperature differences (Rb=0.41).
Data Conclusion:
Significant changes in brain temperature over time scales of ~1 hour were observed. High short-term repeatability suggests temperature changes appear to be due to physiology rather than measurement error.
Keywords: MR thermometry, resting state brain, healthy brain temperature, brain-body temperature differences
INTRODUCTION
Brain temperature is a key marker of brain health, reflecting cerebral blood flow and volume, incoming arterial temperature, and metabolism (1). Fluctuations in brain temperature in both healthy and injured mammals have been observed (2–7). During illness or after trauma, increased brain temperature is recognized as a predictor for poor outcomes (7–11). In animal models, temperature changes ranging from 2 – 4 °C in response to visual and environmental stimuli have been observed (3,12). Brain temperature is generally higher than body temperature, though the direction and magnitude of the gradient can be influenced by a number of factors (e.g., environment, anesthesia) (4,12–14).
Most prior studies in humans have examined brain temperature fluctuations and the brain-body temperature gradient at single time points or over time scales of weeks to months (13,15,16). The presence and magnitude of short-term fluctuations in healthy humans are less clear. Given the reliance of brain temperature homeostasis on cerebral blood flow and oxygenated metabolism (1,17–21), combined with known fluctuations of these parameters over both short and long time scales (2,18,19,22), healthy human brain temperature may also fluctuate, even in resting conditions. MR-based thermometry has been used extensively in the research environment and is a viable tool for non-invasive and repeated measurements (5,16,23,24).
The aim of this study was to identify physiological fluctuations in brain temperature over time scales of approximately 1 hour by characterizing changes in body, MR-measured brain, and the brain-body temperature gradient using repeated measurements in healthy human volunteers.
METHODS
MR Data Acquisition
This prospective study was approved by the local Institutional Review Board and all subjects provided written informed consent prior to participation. MR data from 30 healthy volunteers (15 females; mean ± standard deviation (SD) age=26±4 years old; Table 1) were acquired on a 3T MR scanner (PrismaFit, Siemens, Erlangen, Germany) with a 32-channel phased-array receive head coil. Structural images were acquired with a T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE) sequence (TR/TI/TE=2300/900/3.39ms, flip angle=9°, field of view (FOV)=256×256mm2, matrix size=192×192, 160 slices, slice thickness=1mm) and used to place the MR spectroscopy (MRS) voxel. For chemical shift thermometry, single voxel MRS was acquired in the posterior cingulate cortex (Figure 1) twice on the same day, ~1–1.5 hours apart, using the semi localized by adiabatic selective refocusing (sLASER) sequence (TR/TE=2000/68 ms; flip angle=90°; averages=128; scan time=4 minutes and 40 s; spectral bandwidth=2000 Hz; complex data points=2048; voxel size=2×2×2 cm3; variable power radiofrequency pulses with optimized relaxation delays (VAPOR) water suppression (25) bandwidth=120 Hz; non-water suppressed spectra acquired using the same parameters but averages=8). The same MR operator (D.S., 4 years of experience) acquired all MRS data for consistency. Subjects were instructed to rest during the scan, provided with a blanket upon request, and acclimated to the temperature in the scanner for 22±1 minutes (range: 20 – 27 minutes) and 45±3 (range: 40 – 53 minutes) prior to the first and second MRS acquisitions, respectively. Between repeated acquisitions, subjects were removed from the scanner for 11±4 minutes (range: 3 – 19 minutes) and allowed to ambulate if desired. As single voxel MRS data was collected in the middle of a longer scan protocol for a larger study, most of the 1–1.5 hours between time periods, with the exception of the ~10 minute break where the subject was not in the scanner, was spent undergoing additional MR scans. Half of the subjects (n=15) were scanned in the morning and the other half (n=15) were scanned in the afternoon due to MR scanner availability. Body temperature was measured continuously during the scan using an MR-compatible, fiber optic axillary thermometer (OPG-MPK5, Opsens) and the mean values during MR thermometry acquisition are reported. Ambient temperature in the MR scanner suite was recorded before the start of the MR scan or during the break when the subject was removed from the scanner.
Table 1.
Subject characteristics including age (in years), biological sex, and self-reported race and ethnicity.
| Subject | Age | Sex | Race |
|---|---|---|---|
| 1 | 28 | M | Caucasian |
| 2 | 24 | F | Asian |
| 3 | 29 | M | Asian |
| 4 | 29 | M | Asian |
| 5 | 29 | M | Asian |
| 6 | 23 | F | Asian |
| 7 | 29 | M | Caucasian (E) |
| 8 | 27 | M | Black or African American |
| 9 | 29 | F | Caucasian |
| 10 | 22 | F | Asian |
| 11 | 26 | F | Asian |
| 12 | 21 | M | Caucasian |
| 13 | 20 | M | Black or African American |
| 14 | 23 | F | Black or African American |
| 15 | 32 | F | Caucasian |
| 16 | 26 | M | Asian |
| 17 | 26 | M | Caucasian |
| 18 | 36 | F | Caucasian (H) |
| 19 | 26 | M | Asian |
| 20 | 27 | F | Caucasian |
| 21 | 31 | M | Caucasian |
| 22 | 29 | F | Asian |
| 23 | 18 | F | Black or African American |
| 24 | 27 | F | Black or African American |
| 25 | 24 | F | Caucasian |
| 26 | 29 | M | Caucasian |
| 27 | 25 | F | Caucasian |
| 28 | 20 | F | Asian |
| 29 | 31 | M | Asian |
| 30 | 24 | M | Asian |
M: Male; F: Female; Caucasian (E): Egyptian; Caucasian (H): Hispanic
Figure 1.
Single-voxel position (yellow square) in the posterior cingulate cortex overlaid on the T1-weighted MPRAGE image for one subject acquired during the (a) first scan period and (b) second scan period. Single voxel spectroscopy was acquired using the sLASER sequence for MR thermometry.
MR Thermometry Analysis
Raw spectra (Siemens twix data format) from individual phased array coils were combined using OpTIMUS (optimized truncation to integrate multi-channel MRS data using rank-R singular value decomposition) (26). Temperature (°C) was calculated from the chemical shift difference (Δδ [ppm]) between N-acetylaspartate (NAA) (water suppressed spectrum) and water (non-water suppressed spectrum) as previously described (4,5,24). Briefly, the water suppressed and non-water suppressed spectra (attenuated by 95%) were combined, fit in LCModel (27), and the water and NAA chemical shifts were determined from the print file generated with LCModel. Temperature was then calculated using the relationship T = 37 + 100 (2.66 −Δδ)) (4,24,28). The second MRS scan for one subject was excluded as it could not be fit reliably with LCModel.
To evaluate within-scan repeatability, the first 64 and the last 64 transients from the 4 minutes and 40 s scan for each MRS acquisition were combined separately, and temperature was calculated for each spectrum. Thus, the average time between within-scan spectra was 2 minutes and 20 s. This was performed for both MRS acquisitions acquired in two separate scans.
To evaluate potential sources of error or artifacts that may contribute to temperature calculations, independent of physiological temperature changes, frequency drift and differences in gray and white matter fraction between repeated measurements were quantified. Transmission frequencies in Hz (labeled as ‘MRFrequency’) were extracted from Siemens rda header files. Frequency drift within paired non-water suppressed and water suppressed scans was evaluated at each time period (scan 1 and scan 2) for all subjects (transmission frequency of non-water suppressed scan – transmission frequency of water suppressed scan). The MRS voxel was co-registered to T1-weighted image space followed by automated tissue segmentation using SPM to determine the fraction of gray and white matter in each MRS voxel (29–31).
Statistical Analysis
Hierarchical linear mixed models (32) were used where brain temperature, body temperature, or brain-body temperature difference was the response variable, period (i.e., scan number) was a fixed effect, and a subject random effect and a subject-period random effect were included. The models contained three variance components: the subject-period random effect variance, ; the subject random effect variance, ; and the residual variance, . The within-scan correlation between the response variables and was then calculated as:
| Equation 1 |
Where i denotes subject, j denotes period, and the last index denotes the first 64 versus second 64 transients ( and , respectively). The between-scan correlation between the response variables and (acquired from subject i in period 1 and 2, respectively) does not include the subject-period random effect variance () in the numerator because the periods differ and was calculated as:
| Equation 2 |
Additionally, the intraclass correlation coefficients (ICCs) based on the mixed model formulation for brain temperature calculated using the first 64 versus last 64 transients in scan 1 and first 64 versus last 64 in scan 2. ICC was also calculated for the 128 transients in scan 1 versus 128 transients in scan 2 (not controlling for period). A mixed model with body temperature as the response, a fixed effect of period, and subject random effects was also created and the between-scan correlation was reported (32).
The gray matter/white matter ratio in scan 1 and scan 2 were compared using a Wilcoxon matched-pairs signed rank test. Lastly, brain temperature changes between time periods were compared for subjects scanned in the morning and the afternoon using a Mann-Whitney U test. The significance level was set at p≤.05 for all analyses. Values are reported as the mean ± SD unless otherwise noted.
RESULTS
Brain temperature ranged from 35.5 to 40.1 °C and mean ± SD temperature for all subjects was 38.1±0.6 °C (period 1) and 37.7±0.8 °C (period 2) (Figure 2a). Brain temperature fluctuations within period 1 and within period 2 ranged from −0.9 to 0.8 (−0.2±0.4) °C and −0.4 to 0.5 (0.1±0.2) °C, respectively. Mean ± SD time difference between these two time periods was 80±6 minutes (range: 71 – 96 minutes). Brain temperature was significantly different between time periods (−0.4 °C drop in second period), and the brain temperature in period 2 minus the brain temperature in period 1 ranged from −2.4 to 2.0 °C (calculated across pairs of repetitions). High repeatability of brain thermometry was observed within scans (Rw=0.95). When comparing brain temperatures at different periods (~1 hour apart), the between-scan repeatability was low (Rb=0.30). Similarly, the ICC between brain temperature calculated using the first 64 versus last 64 transients in scan 1 was 0.86; the ICC in scan 2 was 0.97; and the ICC between scans 1 and 2 was 0.18. Body temperature did not differ between time periods (period 2 change = −0.03 °C, P = .59), and the body temperature in period 2 minus the body temperature in period 1 ranged from −0.6 to 0.45 °C. Between scan repeatability of body temperature was high.
Figure 2.
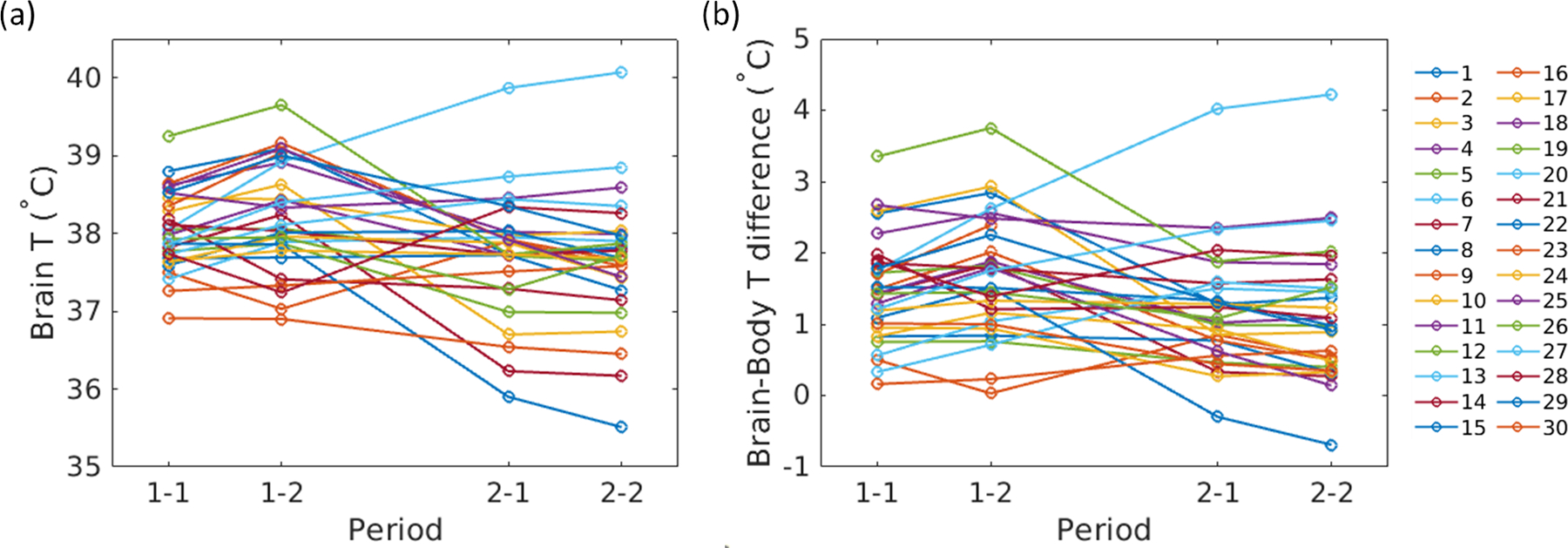
Within-scan and between-scan changes in (a) brain temperature and (b) the brain-body temperature gradient. Within-scan repeatability, on the order of minutes, was high with minimal changes, indicated by the first two pairs of data points (1–1 and 1–2) and the last two pairs of data points (2–1 and 2–2). Between time periods (~1 hour, between the second and third data points), fluctuations were observed in both brain temperature and brain-body temperature differences. Each line represents one subject.
Similar results were observed for brain-body temperature differences. The brain-body temperature gradient was positive for all but two within-scan measurements in one subject (mean ± SD for all subjects was 1.6±0.8 °C (period 1) and 1.2±0.8 °C (period 2)) (Figure 2b). The brain-body temperature gradient decreased between periods (−0.4 °C from the first to second period) and the brain-body difference in period 2 minus the brain-body difference in period ranged from −2.2 to 2.5 °C. Ten subjects had differences in the brain-body temperature gradient of >±1°C across periods. The within-scan repeatability and between-scan repeatability for brain-body temperature differences were Rw=0.96 and Rb=0.41, respectively.
Additional factors that could artifactually affect temperature calculations, for example, due to hardware or software, were not statistically significant, as follows. There was no significant difference in gray matter/white matter ratios for repeated measurements within subjects (4.09 ± 0.78 [period 1], 4.08 ± 0.80 [period 2], P = .91). Brain temperature changes ([period 2] − [period 1]) between subjects scanned in the morning compared to the afternoon were also not significantly different (−0.4 ± 0.94 [morning], −0.4 ± 0.8 [afternoon], P = .88). The approximate atmospheric temperature acquired by the axillary thermometer in MR scanner suite was 20.4±1.1 °C (range for all acquisitions: 18.6 – 23.8 °C) Lastly, frequency drift (transmission frequency of non-water suppressed scan – transmission frequency of water suppressed scan) was 0 Hz within pairs of non-water suppressed and water suppressed data for all scans and all subjects.
DISCUSSION
Over relatively short time periods (~1–1.5 hours), fluctuations in both brain temperature and the brain-body temperature gradient were observed. Our study design, which included repeated measurements over two time periods (within scan and between scans), enabled differentiation between measurement error (within scan) and physiological changes in temperature (between scans). The temperature change due to measurement error was not significant, as the repeatability assessed by within-scan correlations for both brain and brain-body temperature differences was very high but also voxel displacements between scans assessed by gray and white matter ratio were not significant. Thus, variations over ~1 hour are attributed, at least in part, to physiological effects on this time scale due to ambient temperature changes which affect both body and brain temperature, and to neural activity which affects brain temperature. In contrast, body temperature over ~ 1–1.5 hours was stable for all subjects. The temperature in the scanner suite was ~20 °C and often decreased over time. Participants were provided with blankets, which may have contributed to the observed decrease in brain temperature but not body temperature. Marshall et al. performed four ~10 minute multi-voxel MR thermometry scans in a single session, and voxel-wise standard deviation for repeated measurements was 1.2 °C (28). While their reported standard deviation is a combination of measurement error as well as physiological changes, reported theoretical precision of 0.3 °C suggests the presence of short time frame fluctuations that are due, in part, to physiology (28). Interestingly, Sharma et al. assessed reproducibility of MR thermometry by performing two acquisitions in healthy volunteers ~12 weeks apart. Mean brain temperature difference between the scans, across all voxels and subjects, was 0.4 °C, consistent with our results after ~1 hour (16).
Importantly, external hardware or software variations that could artificially result in temperature changes were well-controlled in our experiments. All scans were performed using identical hardware (i.e., same MR scanner and phased-array head coil) and acquired by a single MR operator, and repeated scans were acquired on the same day. MR chemical shift thermometry is insensitive to field drifts due to the internal reference. As non-water suppressed and water suppressed spectra, used to determine the water and NAA frequencies, respectively, were acquired as separate scans, frequency drift between these pairs of scans was evaluated. No frequency drift was observed between any scan pairs, suggesting this was not a contributor to observed temperature variations. While small differences in voxel position were present, there were no significant differences in the ratio of gray and white matter between scans, suggesting susceptibility or metabolic differences between tissue types was likely not a major contributor to brain temperature differences (13,33,34). We also did not observe significant brain temperature differences across subjects as a function of the time of day. These results further support the presence of brain temperature changes over a short time period driven primarily by physiology or environment rather than measurement error or other artifacts.
Global and mean brain and body temperatures have been shown to be closely associated in previous animal and human studies (1,19,35); however, effects of anesthesia or averaging across individuals over days or weeks confound some of the individual variation (4,16,36). A review by Mcilvoy of 15 studies showed higher brain temperatures compared to body temperatures across all studies with mean differences ranging from 0.4 to 2.5 °C (14). In healthy non-human primates under anesthesia, highly correlated brain and body temperature fluctuations have also been observed over several hours, with differences ranging from 0.1 – 1.2 °C (4).
Prior reports in healthy volunteers follow similar trends, though the magnitude of the brain-body temperature gradient varies. Childs et al. observed mean brain-body temperature differences of −0.8 to −0.1 °C in healthy volunteers, with body temperature calculated as the mean of oral, tympanic, and temporal artery temperatures (13). In comparison, Nybo et al. reported positive gradients of 0.2 °C or higher in healthy male subjects (37). Thrippleton et al. reported weak positive associations between brain and body temperature with differing association strength between oral and aural temperatures (36), suggesting some observed differences in the brain-body temperature gradient are likely due to the metric used for body temperature.
Neural activity, environment, and normal physiology can modulate brain temperature in healthy mammals as much as 1 – 2 °C (2). For example, previous work in humans has observed brain temperature changes in response to visual stimulation. Using a flashing visual stimulus, Rango et al. observed an average decrease in brain temperature (0.2 °C below the initial temperature) after four minutes of the stimulus, followed by an increase in brain temperature two minutes after the end of the stimulus (0.6 °C above the initial temperature) (38). Similarly, Yablonskiy et al. observed mean decreases of 0.2 °C during a four minute visual stimulus (21). Importantly, the direction and magnitude of temperature changes varied between individuals in both of these prior studies, and changes as high as ±1 °C were observed, on the same order as the changes we observed. Visual or other neural stimulation should affect brain temperature but not body temperature on short time scales, and as a result, could alter the brain-body temperature gradient. While the direction of brain temperature changes varied, we observed a mean decrease in brain temperature between time periods across all subjects. Subjects were not instructed to keep their eyes open, and the changes we observed could be due to a number of factors including visual stimulation or changes in ambient temperatures over time, among others.
Changes in blood flow can directly affect brain temperature (5,12,21). In a review by Wang et al., evidence from multiple studies supports the close relationship between brain temperature and blood flow mainly via convective heat exchange through cerebral circulation (12). This is supported by decreases in brain temperature during visual stimulation that are accompanied by increases in cerebral blood flow measured with blood oxygenation level-dependent (BOLD) imaging (21). Similar to resting state connectivity approximated from the BOLD signal, which is a surrogate for regional blood flow and oxygenated metabolism in the absence of a directed task, it may be reasonable to assume that brain temperature and the brain-body temperature gradient also maintains or exists in a resting state that is sensitive to individual brain structure and is altered during neural activity or other stimulation. As the BOLD signal varies in response to metabolism and blood flow, temperature would also be expected to vary for similar reasons. In the healthy human brain, these changes are relatively small; however, further characterization of the magnitude of these fluctuations may be warranted, particularly as brain thermal therapies (ablation, therapeutic cooling) are more widely implemented. An individual baseline for resting brain temperature, as well as the brain-body temperature gradient, may be important when implementing thermal therapies or for the development of brain temperature biomarkers after injury. It may also be feasible that in some prior studies of reproducibility, real physiological temperature changes contributed to some of the variation observed.
Limitations
We did not assess subject repositioning between scans or inter-observer variations in MR data acquisition or analysis, which may contribute to the temperature variation. We also did not control for sleep state between scans. Many subjects fell asleep during one or both scans, and effects of sleep cycle on brain temperature have been reported (39). It is also possible the scan itself, due to exposure to the magnetic field or the potential stress of undergoing an MR scan, contributed to some of the observed temperature changes. Lastly, whole-brain studies will be required to characterize regional effects, supported by prior work observing voxel-level differences in brain temperature (17,36).
Conclusions
Significant changes in healthy brain temperature and the brain-body temperature gradient were observed over a time scale of ~1 hour. Given high repeatability of single-voxel MR thermometry, demonstrated with repeated scans over a shorter time scale (~2 minutes) in a well-controlled environment, changes in brain temperature may reflect physiological fluctuations driven by body temperature and neural activity. These results emphasize the need for further characterization of healthy human brain temperature, particularly under controlled conditions to understand the extent of changes in brain temperature in health as an important baseline for the use of brain temperature as a metric of disease progression and treatment monitoring.
Acknowledgments:
Imaging studies were provided with the support of the Emory University Center for Systems Imaging Core.
Grant Support:
This study was supported, in part, by the US National Institutes of Health (Grant number: 1R21EB029622, CCF) and the Emory University Department of Radiology & Imaging Sciences Seed Grant (CCF). Portions of this manuscript were presented, with the support of an Educational Travel Stipend (KW), at the International Society for Magnetic Resonance in Medicine, May 2022, London, England.
REFERENCES
- 1.Hayward JN, Baker MA. A comparative study of the role of the cerebral arterial blood in the regulation of brain temperature in five mammals. Brain Res 1969;16(2):417–440. [DOI] [PubMed] [Google Scholar]
- 2.Kiyatkin EA, Brown PL, Wise RA. Brain temperature fluctuation: a reflection of functional neural activation. Eur J Neurosci 2002;16(1):164–168. [DOI] [PubMed] [Google Scholar]
- 3.Kiyatkin EA. Brain temperature and its role in physiology and pathophysiology: Lessons from 20 years of thermorecording. Temperature 2019;6(4):271–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dehkharghani S, Fleischer CC, Qiu D, Yepes M, Tong F. Cerebral temperature dysregulation: MR thermographic monitoring in a nonhuman primate study of acute ischemic stroke. Am J Neuroradiol 2017;38(4):712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleischer CC, Wu J, Qiu D, Park S-E, Nahab F, Dehkharghani S. The brain thermal response as a potential neuroimaging biomarker of cerebrovascular impairment. Am J Neuroradiol 2017;38(11):2044–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang JY, Lyeth BG, Clifton GL, Jenkins LW, Hamm RJ, Hayes RL. Relationship between body and brain temperature in traumatically brain-injured rodents. J Neurosurg 1991;74(3):492–496. [DOI] [PubMed] [Google Scholar]
- 7.Childs C Human brain temperature: regulation, measurement and relationship with cerebral trauma: part 1. Br J Neurosurg 2008;22(4):486–496. [DOI] [PubMed] [Google Scholar]
- 8.Jiang J-Y, Gao G-Y, Li W-P, Yu M-K, Zhu C Early indicators of prognosis in 846 cases of severe traumatic brain injury. J Neurotrauma 2002;19(7):869–874. [DOI] [PubMed] [Google Scholar]
- 9.Cairns CJ, Andrews PJ. Management of hyperthermia in traumatic brain injury. Curr Opin Crit Care 2002;8(2):106–110. [DOI] [PubMed] [Google Scholar]
- 10.Busto R, Dietrich WD, Globus M, Ginsberg MD. The importance of brain temperature in cerebral ischemic injury. Stroke 1989;20(8):1113–1114. [DOI] [PubMed] [Google Scholar]
- 11.Busto R, Dietrich WD, Globus MY-T, Valdés I, Scheinberg P, Ginsberg MD. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab 1987;7(6):729–738. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Wang B, Normoyle KP, et al. Brain temperature and its fundamental properties: a review for clinical neuroscientists. Front Neurosci 2014;8:307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Childs C, Hiltunen Y, Vidyasagar R, Kauppinen RA. Determination of regional brain temperature using proton magnetic resonance spectroscopy to assess brain–body temperature differences in healthy human subjects. Magn Reson Med 2007;57(1):59–66. [DOI] [PubMed] [Google Scholar]
- 14.Mcilvoy L Comparison of brain temperature to core temperature: a review of the literature. J Neurosci Nurs 2004;36(1):23–31. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Taub E, Mueller C, et al. Reproducibility of whole-brain temperature mapping and metabolite quantification using proton magnetic resonance spectroscopy. NMR Biomed 2020:e4313. [DOI] [PubMed]
- 16.Sharma AA, Nenert R, Mueller C, Maudsley AA, Younger JW, Szaflarski JP. Repeatability and reproducibility of in-vivo brain temperature measurements. Front Hum Neurosci 2020:573. [DOI] [PMC free article] [PubMed]
- 17.Sung D, Kottke PA, Risk BB, et al. Personalized predictions and non-invasive imaging of human brain temperature. Commun Phys 2021;4(1):1–10. [Google Scholar]
- 18.Hayward J, Baker M. Role of cerebral arterial blood in the regulation of brain temperature in the monkey. Am J Physiol 1968;215(2):389–403. [DOI] [PubMed] [Google Scholar]
- 19.Zhu M, Ackerman JJ, Yablonskiy DA. Body and brain temperature coupling: the critical role of cerebral blood flow. J Comp Physiol B 2009;179(6):701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michenfelder JD, Milde JH. The relationship among canine brain temperature, metabolism, and function during hypothermia. Anesthesiology 1991;75(1):130–136. [DOI] [PubMed] [Google Scholar]
- 21.Yablonskii DA, Ackerman JJ, Raichle ME. Coupling between changes in human brain temperature and oxidative metabolism during prolonged visual stimulation. Proc Natl Acad Sci USA 2000;97(13):7603–7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanier WL. Cerebral metabolic rate and hypothermia: their relationship with ischemic neurologic injury. J Neurosurg Anesthesiol 1995;7(3):216–221. [DOI] [PubMed] [Google Scholar]
- 23.Kuroda K Non-invasive MR thermography using the water proton chemical shift. Int J Hyperthermia 2005;21(6):547–560. [DOI] [PubMed] [Google Scholar]
- 24.Dehkharghani S, Mao H, Howell L, et al. Proton resonance frequency chemical shift thermometry: experimental design and validation toward high-resolution noninvasive temperature monitoring and in vivo experience in a nonhuman primate model of acute ischemic stroke. Am J Neuroradiol 2015;36(6):1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tkáč I, Starčuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 1999;41(4):649–656. [DOI] [PubMed] [Google Scholar]
- 26.Sung D, Risk BB, Owusu‐Ansah M, Zhong X, Mao H, Fleischer CC. Optimized truncation to integrate multi‐channel MRS data using rank‐R singular value decomposition. NMR Biomed 2020;33(7):e4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993;30(6):672–679. [DOI] [PubMed] [Google Scholar]
- 28.Marshall I, Karaszewski B, Wardlaw JM, et al. Measurement of regional brain temperature using proton spectroscopic imaging: validation and application to acute ischemic stroke. Magn Reson Imaging 2006;24(6):699–706. [DOI] [PubMed] [Google Scholar]
- 29.Collignon A, Maes F, Delaere D, Vandermeulen D, Suetens P, Marchal G. Automated multi-modality image registration based on information theory. Inf Process Med Imaging 1995;3(6):263–274. [Google Scholar]
- 30.Ashburner J, Friston KJ. Unified segmentation. Neuroimage 2005;26(3):839–851. [DOI] [PubMed] [Google Scholar]
- 31.Ashburner J, Friston KJ. Diffeomorphic registration using geodesic shooting and Gauss–Newton optimisation. Neuroimage 2011;55(3):954–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garson GD. Fundamentals of Hierarchical Linear and Multilevel Modeling. In: Hierarchical Linear Modeling: Guide and Applications SAGE Publications, Inc.; 2013:3–26. [Google Scholar]
- 33.Chadzynski GL, Bender B, Groeger A, Erb M, Klose U. Tissue specific resonance frequencies of water and metabolites within the human brain. J Magn Reson 2011;212(1):55–63. [DOI] [PubMed] [Google Scholar]
- 34.Maudsley AA, Goryawala MZ, Sheriff S. Effects of tissue susceptibility on brain temperature mapping. Neuroimage 2017;146:1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossi S, Zanier ER, Mauri I, Columbo A, Stocchetti N. Brain temperature, body core temperature, and intracranial pressure in acute cerebral damage. J Neurol Neurosurg Psychiatry 2001;71(4):448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thrippleton MJ, Parikh J, Harris BA, et al. Reliability of MRSI brain temperature mapping at 1.5 and 3 T. NMR Biomed 2014;27(2):183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nybo L, Secher NH, Nielsen B. Inadequate heat release from the human brain during prolonged exercise with hyperthermia. J Physiol 2002;545(2):697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rango M, Bonifati C, Bresolin N. Post-activation brain warming: a 1-H MRS thermometry study. PLoS One 2015;10(5):e0127314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Csernai M, Borbély S, Kocsis K, et al. Dynamics of sleep oscillations is coupled to brain temperature on multiple scales. The Journal of physiology 2019;597(15):4069–4086. [DOI] [PubMed] [Google Scholar]


