Abstract
Excessive, high doses of ultraviolet B (UVB) UVB irradiation are known to cause skin cancer, aging, and immunosuppression. On the contrary, moderate low doses of UVB irradiation are shown to be essential and beneficial to human health, including a tumor-suppressive effect. However, the mechanism by which low levels of UVB suppress tumorigenesis remains unclear. Here, using tumor-bearing mouse models, we show that moderate low repetitive UVB irradiation increases the percentage of activated CD4+ and CD8+ T cells, and CD103+ conventional type 1 dendritic cells (cDC1s), while it decreases the number of immunosuppressive, M2-like macrophages in the tumors. Finally, in mice, deletion of Batf3, a transcription factor critical for the development of conventional dendritic cells, including the CD103+ cDC1s, showed increased tumor growth in both sham- and UVB-irradiated mice. Our findings demonstrate that moderate low UVB irradiation inhibits M2-like tumor-associated macrophages, increases CD103+ cDC1s, and promotes anti-tumor immunity in mice with an established tumor.
Keywords: ultraviolet B (UVB) irradiation, CD4+ T cells, CD8+ T cells, tumor-associated macrophages, dendritic cells, CD103+ cDC1s, Batf3 knockout
Graphical Abstract
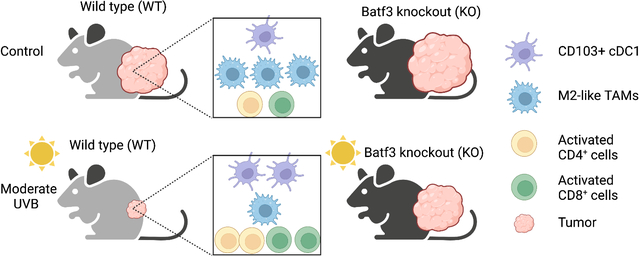
Using tumor-bearing mouse models, we show that moderate UVB irradiation increases the number of activated CD4+ and CD8+ T cells, CD103+ cDC1 dendritic cells, while it decreases the number of immunosuppressive, tumor-promoting M2-like tumor-associated macrophages (TAMs). Deletion of Batf3 increases tumor growth and abolishes the tumor-suppressive effect of moderate UVB irradiation. Our findings demonstrate that moderate UVB irradiation promotes anti-tumor immunity in tumor-bearing mice. (Created with BioRender.com).
INTRODUCTION
UVB irradiation in sunlight can have a pleiotropic effect on human health (1). On one hand, an excessive exposure to UVB irradiation can cause skin cancer, aging, and immunosuppression (2). On the other hand, moderately low doses of UVB irradiation is shown to be essential and beneficial for human health, by generating vitamin D, β-endorphin, melanin, antimicrobial peptides, and urocanic acid, as well as other biomolecules, and thus preventing cancer and other diseases and improving mood and memory (1, 3–7). The beneficial effect of moderate UV exposure for human health has been postulated to be associated with vitamin D synthesis, which can prevent carcinogenesis in mouse models and COVID-19 deaths (7–10). In humans, chronic but not intermittent sun exposure has been suggested to be associated with decreased risk of multiple cancers, including colorectal, prostate, breast cancer and hon-Hodgkin’s lymphoma (9). In addition, previous studies also showed that repetitive low doses of UVB light exposure triggered a type I interferon response and enhanced adaptive immunity (11, 12), which can suppress tumorigenesis (13). However, how moderate UVB irradiation modulates anti-tumor immune response remains unclear.
Anti-tumor immunity often depends on cytotoxic T cell activation (14). This anti-tumor T cell activation has shown to be suppressed by effects of other immune cells, including the enrichment of the immunosuppressive tumor-associated macrophages (TAMs) and the loss of function of dendritic cells (15, 16). Macrophages, which are one type of myeloid cells, are abundant in the tumor microenvironment. TAMs are a critical component of the complex tumor microenvironment in promoting tumorigenesis, tumor progression, immune evasion, and therapeutic resistance (15, 17). Based on activation signals and function, macrophages have been classified as M1 (activated) and M2 (alternatively activated) states from characterization in in vitro and in vivo models (15, 17, 18). Although TAMs often express both M1 and M2 macrophage markers, these macrophages can adopt a tumor-promoting M2-like phenotype by inhibiting dendric cell maturation, inducing the immunosuppressive Treg cells, and suppressing the cytotoxic T cells; there is presently extensive interest in developing cancer therapies targeting TAMs (15).
Another immune cell type critical for antitumor immunity is the dendritic cell. Dendritic cells (DCs) are the main antigen-presenting cells (APCs) that cross-prime cytotoxic T cells (16). Although dendritic cells are a rare immune cell population in tumors, they are crucial for the initiation of antigen-specific anti-tumor immune response (19). Therefore, many strategies have been developed by targeting dendritic cells to improve cancer therapy (16). Mouse conventional dendritic cells (cDCs) comprise two major subsets, the CD8α+ and CD103+ conventional type I dendritic cell (cDC1s) subset and the CD11b+ conventional type 2 dendritic cell (cDC2s) subset (20, 21). At the molecular level, the cDC1 development requires the transcription factor Batf3. Batf3 knockout mice are defective in the development of CD8α+ cDC1s within lymphoid tissues and CD103+ cDC1s in multiple tissues (22). These mice also show decreased priming of CD8 T cells following a pulmonary Sendai virus infection (22). In addition, tumor-residing CD103+ cDC1shave been shown to be required for the recruitment of effector T cells into the tumor microenvironment and are a main component of the development of the T cell-inflamed tumor microenvironment (23). However, how dendritic cells are regulated by external factors remains poorly understood.
In this study, using tumor-bearing mouse models, we show that moderate low UVB irradiation increases the number of activated CD4+ and CD8+ T cells, CD103+ cDC1s, while it decreases the number of immunosuppressive, tumor-promoting M2-like tumor-associated macrophages. Deletion of Batf3 increases tumor growth in both sham- and UVB-irradiated mice. Our findings demonstrate that moderate low UVB irradiation promotes anti-tumor immunity in tumor-bearing mice.
MATERIALS AND METHODS
Animal study.
All animal procedures have been approved by the University of Chicago Institutional Animal Care and Use Committee. Female C57BL/6 (6–7 weeks) were obtained from Harlan-Envigo. C56BL/6J wild-type (WT) mice (B6, Strain # 000664) and Batf3 knockout mice (Batf3 KO, B6.129S(C)-Batf3tm1Kmm/J, Strain #013755, donated by Dr. Kenneth Murphy) were obtained from the Jackson Laboratory. Mice were housed at the University of Chicago animal facility.
Tumor formation in mice for Mouse B16F10 melanoma and mouse MC38 colon cancer cells.
For the mouse syngeneic tumor model, B16F10 or MC38 cells (5 × 105 in 100 μl PBS) were inoculated subcutaneously into the right flanks of mice, as described previously (24). Tumor growth was monitored by measuring tumor diameters with caliper over a time course after tumor inoculation. The tumor volume was calculated as described previously (24).
UVB irradiation.
For UVB irradiation, mice were dorsally shaved using animal clippers at day 5 following tumor inoculation. Shaved mice were exposed to UVB (50 mJ/cm2 or 250 mJ/cm2, which do not cause sunburn) for five consecutive days from day 7–11 following tumor inoculation, as described previously (25–27). Non-irradiated shaved mice were used as sham controls.
Flow cytometric analysis of tumor-infiltrating immune cells and lymphocytes (TILs) from mouse tumor tissues.
Tumor tissue from mice with an established B16F10 tumor on day 16 following tumor inoculation was dissociated and filtered as described previously (24). Live/dead labeling was performed before the cell surface staining, using a Zombie NIR™ Fixable Viability Kit (Biolegend; catalog number 423106) diluted 1:1,000 in PBS for 10 min at room temperature in the dark. Cell surface staining was carried out on ice for 20 min. Intracellular cytokine staining was carried out as described previously (24). Flow cytometric analysis was performed on a Fortessa 4–15 (BD Biosciences) and Attune NxT (Thermo Fisher Scientific) with Flowjo V10.6.1 used for analysis. Antibodies used are listed in Supplementary Table S1.
Immunofluorescence analysis.
Immunofluorescence staining was carried out as described previously (24, 27, 28). Tissue slides were incubated at 4°C with primary anti-F4/80 (Bio-rad, F4/80_MCA497, 1:100), anti-CD206 (Proteintech, CD206_60143–1-Ig, 1:100), and anti-collagen alpha-1(I) (Col-1) (SouthernBiotech_Col-1_1310–01, 1:100) antibodies. Next slides were washed with PBS and then incubated at room temperature with Alexa Fluor® 488 AffiniPure Donkey Anti-Rat IgG (Jackson ImmunoResearch, 712–545-150, 1:100), Alexa Fluor® 594 Donkey Anti-Mouse IgG (abcam, ab150108, 1:100), or DyLight™ 405 AffiniPure Donkey Anti-Goat IgG (Jackson ImmunoResearch, 705–475-003, 1:50) for 1 h followed by washing with PBS and mounting. Stained slide samples were analyzed using a fluorescence microscope (Olympus IX71, Olympus Life Science, Japan). For statistical analysis by ImageJ, five areas (200 μm X 200 μm) were randomly selected from each sample and quantified.
Statistical analysis.
Statistical analysis was performed using GraphPad Prism software (GraphPad Software, Inc., CA, USA). All data were presented as mean with SEM unless otherwise indicated. Statistical differences among experimental groups were determined by using Student’s t-test. A P < 0.05 difference was considered statistically significant.
RESULTS
Moderate low dose of UVB irradiation reduces tumor growth in mice
To determine whether a moderately low UVB irradiation affects tumor growth, we assessed the effect of moderate, sub-erythemal UVB irradiation (50 mJ/cm2) on tumor growth in B6 mice daily for five consecutive days after tumor inoculation (Fig. 1A). Moderate low UVB irradiation reduced B16F10 melanoma tumor growth in mice (Fig. 1B). To determine whether this UVB effect is specific for a low dose at 50 mJ/cm2, we tested the effect of high dose UVB (250 mJ/cm2). Intriguingly, this dose of UVB irradiation reduced tumor growth, showing more tumor growth reduction than with the lower dose of 50 mJ/cm2 (Fig. 1C). The tumor-suppressive effect of moderate low UVB irradiation was also detected in the MC38 colon tumor model (Fig. 1D). Thus moderate low UVB irradiation reduced established tumor growth in mice.
Figure 1. Moderate low dose of UVB irradiation reduces tumor growth.
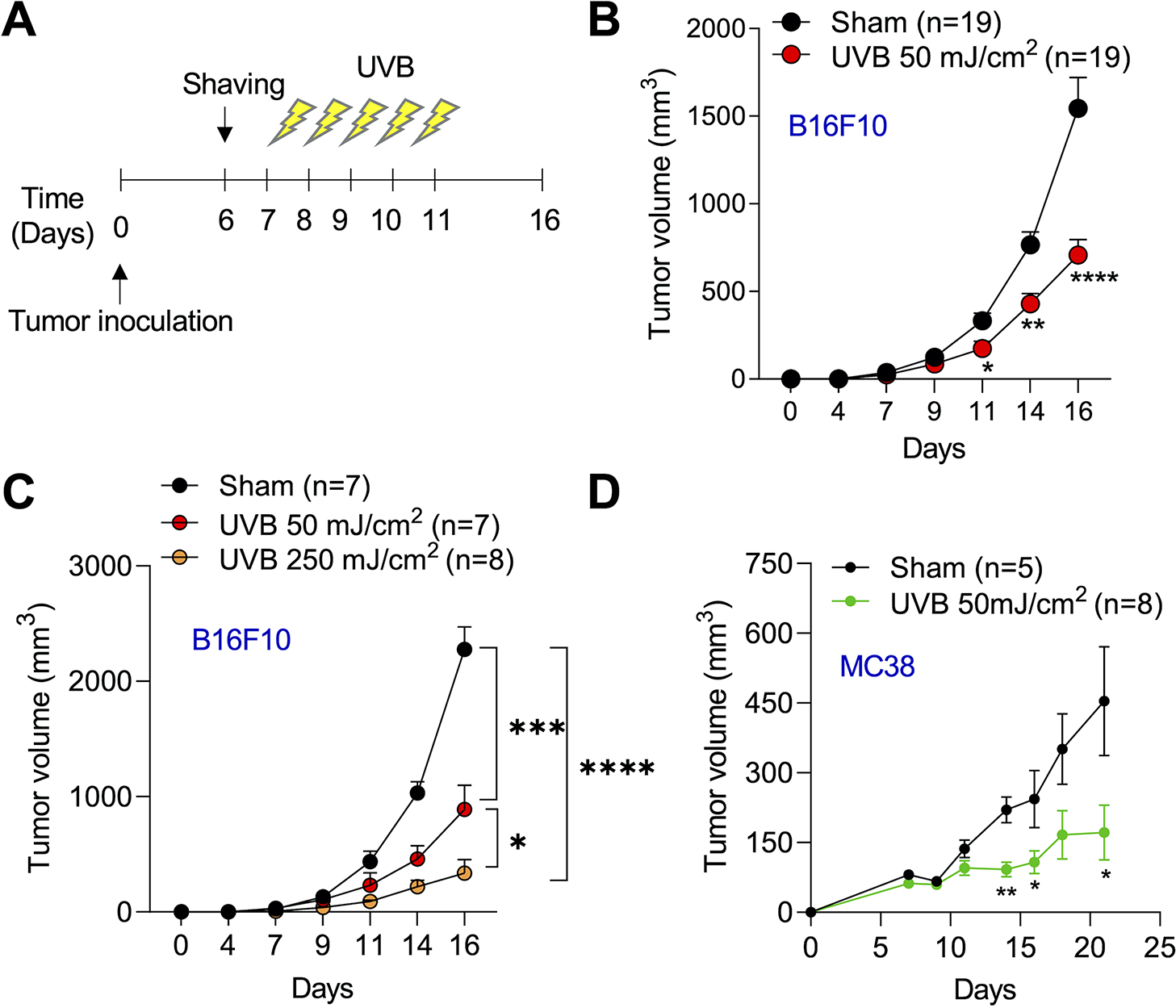
(a) Schematics of moderate low dose of UVB irradiation in tumor-bearing mice. Mice were shaved dorsally and the whole back was exposed to low dose of UVB irradiation (50 mJ/cm2) daily for 5 continuative days. (b) In vivo B16F10 tumor growth in mice (n=19) with or without UVB irradiation (50 mJ/cm2). (c) In vivo tumor growth in mice with or without different doses of UVB irradiation (50 or 250 mJ/cm2). (d) In vivo MC38 tumor growth in mice with or without UVB irradiation (50 mJ/cm2). Data are mean ± SEM. Data are representative of two or three independent experiments. *, P<0.05; **, P<0.01; ***, P<0.001; Student’s t-test.
Moderate low UVB irradiation increased tumor-infiltrating CD45+ cells
To determine whether moderate low UVB irradiation affects tumor microenvironment and anti-tumor immunity, we analyzed different lineages of tumor-infiltrating immune cells. We found that moderate low UVB irradiation increased the percentage of CD45+ lymphocytes infiltrated into tumors, while it did not affect the percentage of CD4+, CD8+ or Regulatory T cells (Tregs) (Fig. 2A and 2B).
Figure 2. Effect of moderate low UVB irradiation on tumor-infiltrating immune cells and lymphocytes (TILs).
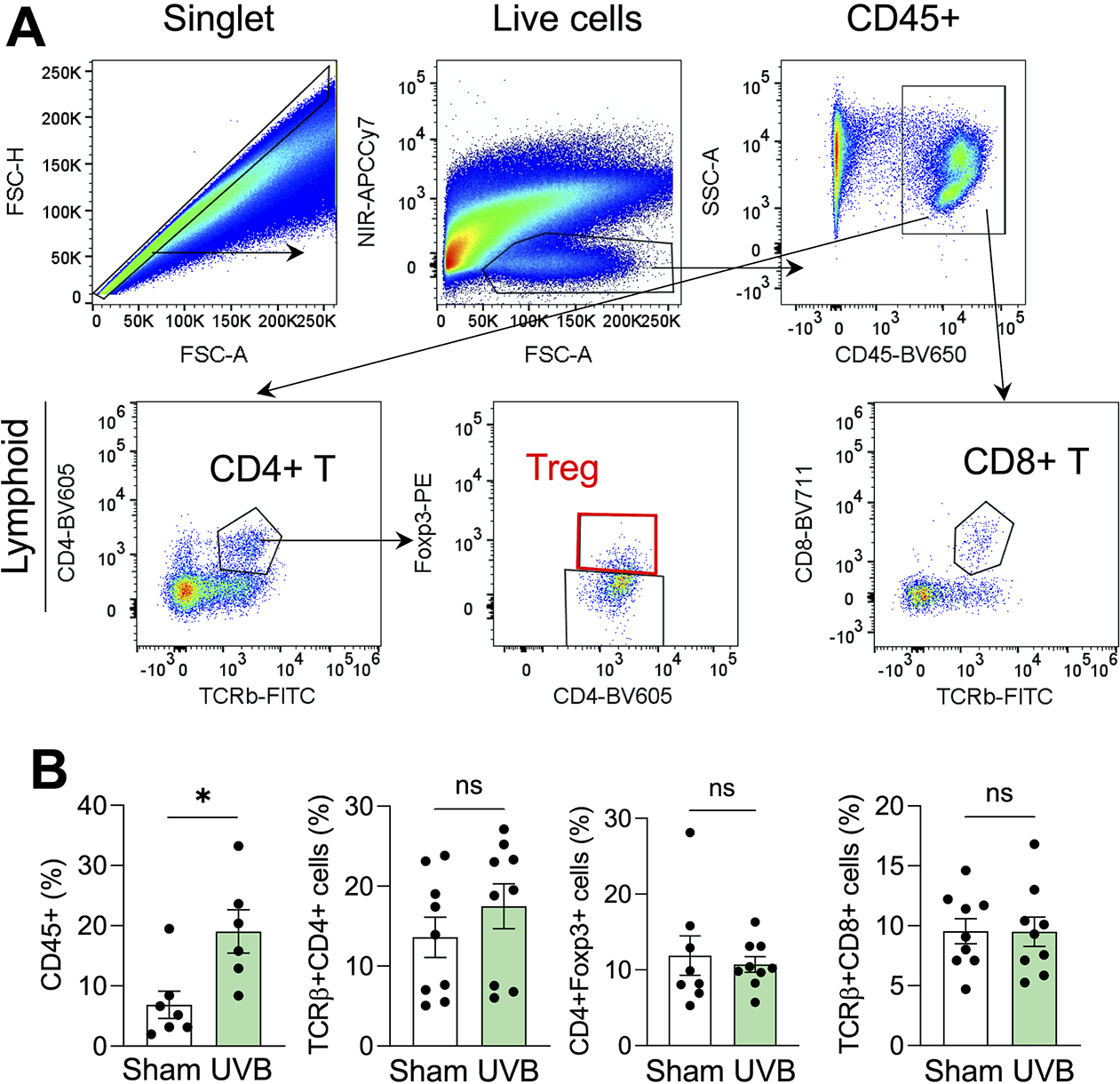
Mice were inoculated with B16F10 cells subcutneously and tumors were collected on day 16 for flow cytometric analysis. (A) Gating strategy of flow cytometric analysis of immune cells and lymphocytes from the tumors. (B) Percentage of CD45+ cells, and CD4+, Tregs (Foxp3+), and CD8+ T cells in the tumor in mice with or without UVB irradiation (50 mJ/cm2) on day 16 (n=6–8). *, P<0.05; Student’s t-test.
Moderate low UVB irradiation increased activated CD4+ and CD8+ tumor-infiltrating T lymphocytes (TILs)
To determine whether moderate low UVB irradiation modulates the activation of CD4+ or CD8+ T cells, we analyzed the effect of moderate low UVB irradiation on the activated state and cytokine production of T cells. Moderate low UVB irradiation increased the percentage of CD4+ T cells that expressed the CD69 receptor or Granzyme B, two inducible markers for T lymphocyte activation (Fig. 3A and 3B). Similarly, UVB irradiation also increased the percentage of CD8+ T cells that expressed CD69 or Granzyme B (Fig. 3C and 3D). However, UVB irradiation had no effect on CD4+ or CD8+ T cells expressing interferon γ (IFNγ) (Fig. 3E and 3F). These findings demonstrate that moderate low UVB irradiation increases the activation of CD4+ and CD8+ T cells.
Figure 3. Effect of moderate low UVB irradiation on tumor-infiltrating CD4+ and CD8+ T cells.
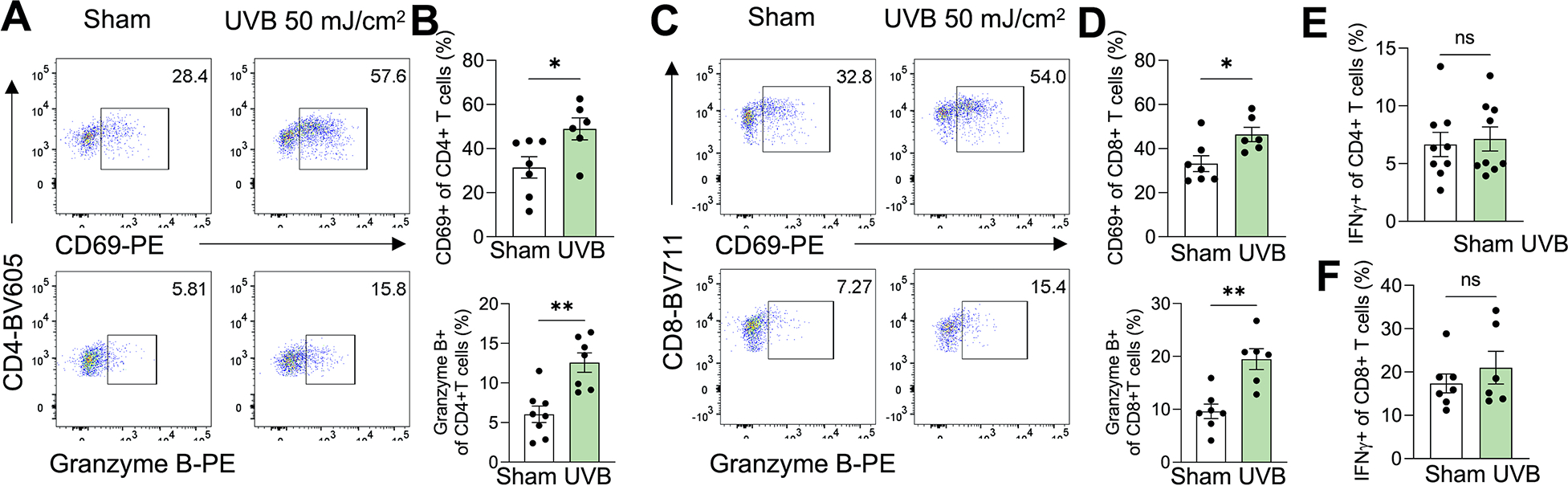
Mice were inoculated with B16F10 cells subcutneously and tumors were collected on day 16 for flow cytometric analysis. (A and B) Percentage of CD69+ and Granzyme B+ CD4+ T cells in TILs in the tumor in mice with or without UVB irradiation (50 mJ/cm2) on day 16, with representative flow cytometry plots (n=6–8) (A). (C and D) Percentage of CD69+ and Granzyme B+ CD8+ T cells in TILs in the tumor in mice with or without UVB irradiation on day 16, with representative flow cytometry plots (n=6–7) (C). (E and F) Percentage of interferon γ (IFNγ+) CD4+ (E) and CD8+ (F) T cells in the tumor in mice with or without UVB irradiation on day 16 (n=6–9). *, P<0.05; **, P<0.01; ns, not significant; Student’s t-test.
Moderate low UVB irradiation reduces the number of M2-like tumor-associated macrophages
To determine the potential mechanism by which moderate low UVB irradiation suppresses tumor growth and increases T cell activation, we assessed the effect of moderate low UVB irradiation on tumor-promoting macrophages. Using immunofluorescence analysis to assess the F4/80+ macrophage numbers and CD206+F4/80+ M2-like macrophage numbers, we found that moderate low UVB irradiation increased the number of tumor-associated macrophages (Fig. 4A and 4B), while it decreased the number of M2-like macrophages (Fig. 4C), suggesting a potential role of M2-like TAMs in the tumor-suppressive effect of UVB irradiation.
Figure 4. Effect of moderate low UVB irradiation on the number of tumor-associated macrophages (TAMs) and M2-like TAMs.
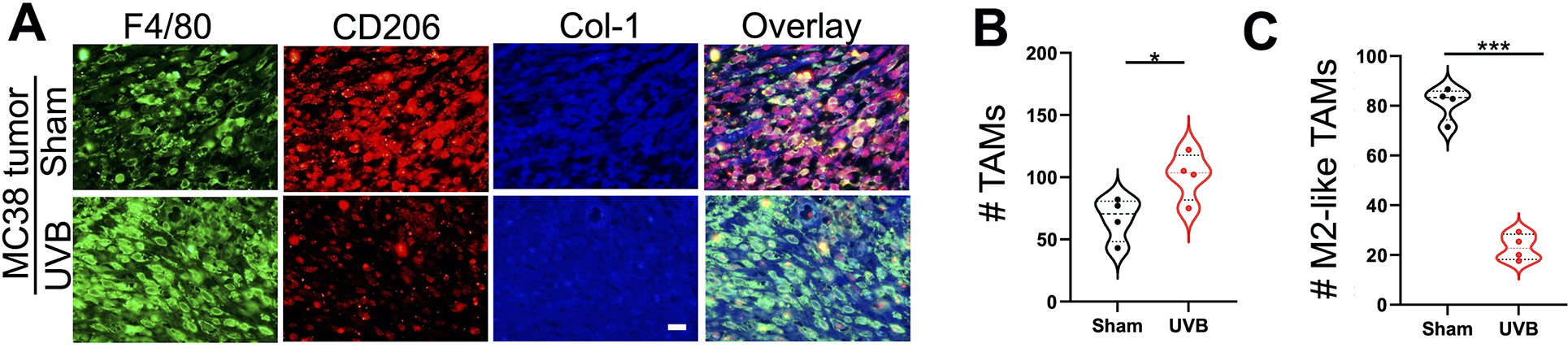
(A) Immunofluorescence analysis of F4/80+ (Green) TAMs and CD206+ (Red) TAMs (M2-like TAMs) from MC38 tumors in mice with or without moderate low UVB irradiation (50 mJ/cm2, n=4). Collagen-1 (Col-1) was used as a counterstain for tumor stroma (Blue). Scale bar, 20 μm. (B and C) Quantification of the number of F4/80+ TAMs (B) and F4/80+CD206+ M2-like TAMs (C). *, P<0.05; ***, P<0.001; Student’s t-test.
Moderate low UVB irradiation increased the tumor-infiltrating CD103+ cDC1s
To further determine the mechanism by which moderate low UVB irradiation suppresses tumor growth and increases T cell activation, we assessed the effect of UVB irradiation on dendritic cells. Flow cytometric analysis showed that UVB irradiation increased the percentage of dendritic cells (Fig. 5A–5C). Intriguingly, UVB irradiation increased the percentage of CD103+ cDC1s in the tumors, while it decreased the percentage of CD11b+ cDC2s (Fig. 5D and 5E). These finding indicate that moderate low UVB irradiation enriched the CD103+ cDC1s in the tumors, which may mediate tumor suppression via a crosstalk between CD103+ cDC1s and T cells.
Figure 5. DCs, specially CD103+ cDC1s, are enriched in the tumor by moderate low dose of UVB irradiation.
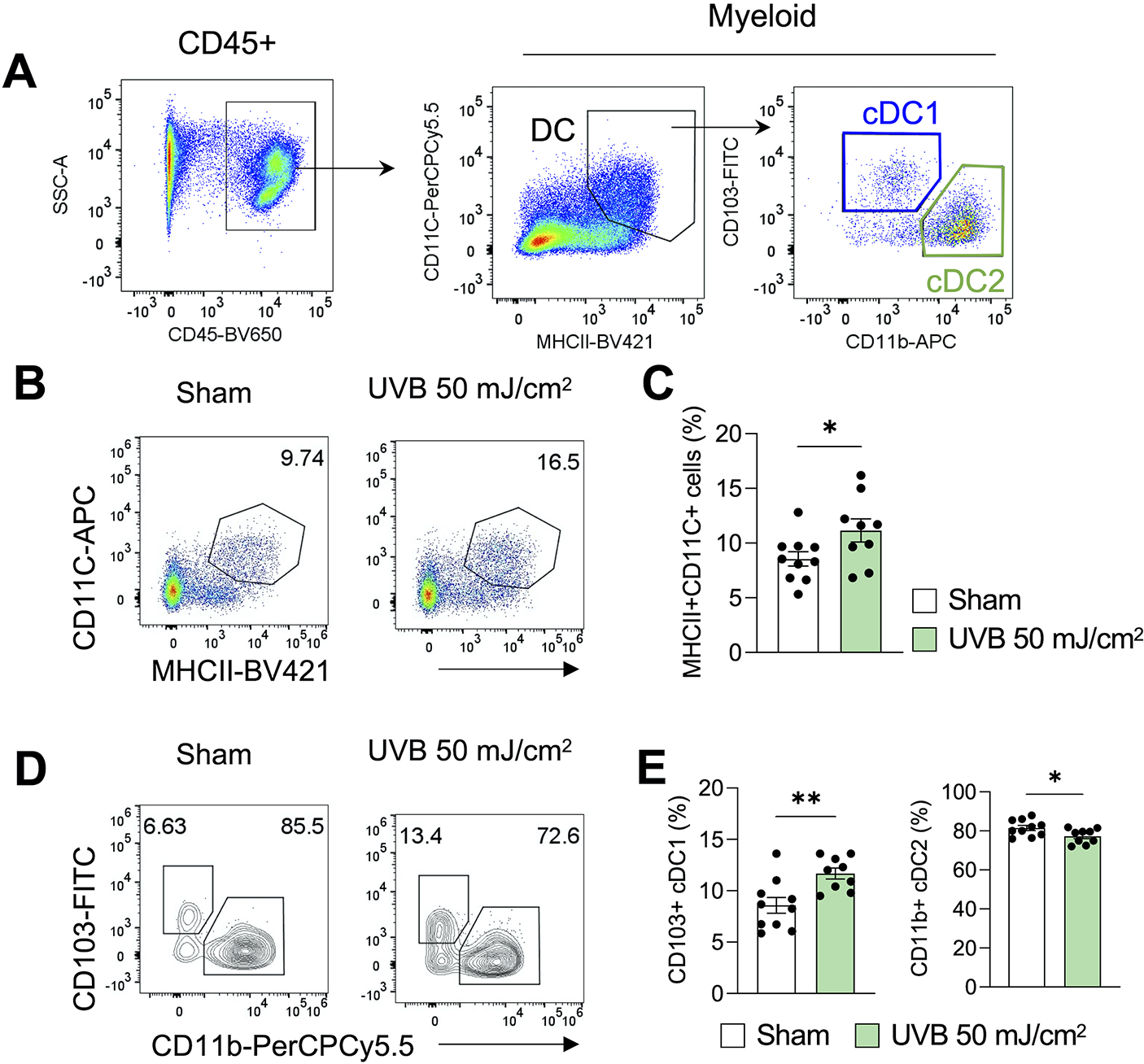
(A) Gating strategy of flow cytometric analysis of dendritic cells from the tumor. (B and C) Percentage of MHCII+CD11C+ cells in the tumor in mice with or without UVB irradiation (50 mJ/cm2) on day 16, with representative flow cytometry plots (n=9–10, B). (D-E) Percentage of CD103+ cDC1s and CD11b+ cDC2s among the CD45+MHCII+CD11C+ cells in in the tumor in mice with or without UVB irradiation on day 16, with representative contour plots (n=10, B, D). *, P<0.05; **, P<0.01; Student’s t-test.
Batf3 deletion increase tumor growth in both sham- and UVB-irradiated mice
Tumor-residing CD103+ cDC1s are necessary for CD8+ effector T cell recruitment and anti-tumor immunity (23). To determine the role of CD103+ cDC1 in the tumor-suppressive effect of moderate low UVB irradiation, we analyzed the difference in tumor growth between WT mice and Batf3 KO mice, a transcription factor required for CD103+ cDC1s development (22, 29). Batf3 deletion increased tumor growth in both sham- and UVB-irradiated mice (Fig. 6A and 6B). Notably, UVB-irradiated mice with Batf3 deletion showed similar tumor growth as in sham-irradiated wild-type mice (Fig. 6A and 6B). These studies indicate that Batf3-dependent CD103+ cDC1s are critical for the anti-tumor immunity in both sham-irradiated mice and mice treated with moderated low UVB irradiation.
Figure 6. Batf3 deletion increases tumor growth in both sham- and UVB-irradiated mice.
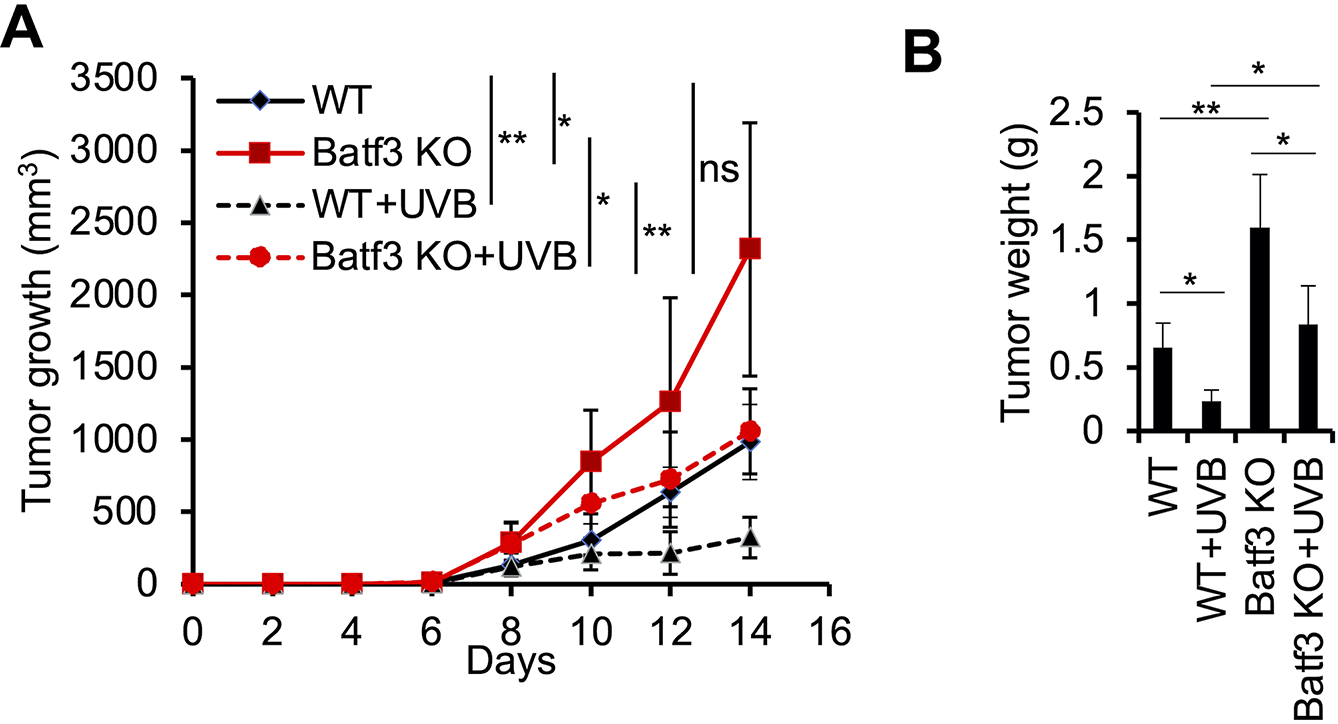
(A) In vivo tumor growth of B16F10 cells in wild-type (WT) and Batf3 knockout (KO) mice with or without moderate low UVB irradiation (50 mJ/cm2) as in Fig. 1A. (B) Tumor weight (g, gram) from A at the end of the study (day 14). Data are mean ± SD (n= 4). *, P<0.05; **, P<0.01; ns, not significant; Student’s t-test.
DISCUSSION
Moderate low UVB irradiation has been proposed to have multiple beneficial effect on human health, including reducing tumorigenesis. However, its role in anti-tumor immunity has been poorly understood. In this study, we report that moderate low UVB irradiation promotes the activation of effector CD4+ and CD8+ T cells and the enrichment of CD103+ cDC1s, reduces the tumor-promoting M2-like macrophages, and suppresses tumor growth. We further demonstrate that depletion of CD103+ cDC1s by Batf3 deletion increases tumor growth in both sham- and UVB-irradiated mice. Our findings have elucidated a new mechanism by which moderate low UVB irradiation enhances anti-tumor immunity in mice.
Our current findings suggest that the effect of UVB irradiation may depend on the type and dose of UVB irradiation and the mouse models used. Previously we and others have shown that in female hairless mice without tumors, tumorigenic high doses of UVB irradiation induce a tumor-promoting, immunosuppressive pro-inflammatory microenvironment, including the enrichment of CD4+ T cells and macrophages (26, 30). A recent study reported that in male hairless mice without tumors, low repetitive UVB irradiation (peak at 302 nm) increased CD4+ and CD8+ T cells in the mouse skin at 24 h post-UVB irradiation (12). However, in the present study using tumor-bearing B6 mice, we did not detect an effect of UVB irradiation (Peak at 312 nm) on the number of tumor-infiltrating CD4+ or CD8+ cells. Instead, we found that UVB increased CD4+ and CD8+ T cell activation, in parallel with decreased tumor-promoting M2-like macrophages and increased anti-tumor CD103+ cDC1s. These findings suggest that the effect of moderate UVB irradiation likely depends on the context, in addition to the UVB dose and mode of exposure.
We found that CD103+ cDC1s are critical for the anti-tumor immunity in mice treated with sham or UVB irradiation. It is possible that the decrease in the tumor-promoting M2-like macrophages mediated the effect of UVB on the increase in CD103+ cDC1s. Other immune cells may also play critical roles on the increase in CD103+ cDC1s, the decrease in M2-like TAMs, or the anti-tumor effect of moderate low UVB irradiation. UVB irradiation induces vitamin D synthesis; however, vitamin D seems to suppress dendritic cell generation (31). Future investigations are warranted to elucidate the molecular and cellular mechanism by which moderate low UVB irradiation enhances CD103+ cDC1s, reduces M2-like macrophages, and suppresses established tumors. In addition, other UV irradiation, such as UVA irradiation or the combination of UVB and UVA irradiation can be assessed to determine the difference of different types of UV irradiation on the immune response in tumor-bearing mice.
In summary, our findings demonstrate the beneficial effect of moderate low doses of UVB irradiation on the anti-tumor immune response and tumor suppression in tumor-bearing mice. Moderate low UVB irradiation activates several immune cell responses, including increases in CD103+ cDC1s and T cell activation, a decrease in tumor-promoting M2-like macrophages, and inhibition of tumor growth. While we showed that Batf3-dependent cDC1s play a critical role in the anti-tumor immunity in both sham- and UVB-irradiated mice, other immune cells in the tumor microenvironment may have important roles the tumor-suppressive effect of moderate low UVB irradiation, such as the decrease in M2-like TAMs. Our results can add new insights into the cellular basis of the beneficial impact of moderate low UVB exposure on anti-tumor immunity and may provide new opportunities to prevent cancer through promoting anti-tumor immunity by moderate UV exposure.
Supplementary Material
Table S1. List of antibodies used for flow cytometric analysis of immune cells.
ACKNOWLEDGEMENTS
We thank Dr. Ann Motten for a critical reading of the manuscript. This work was supported in part by NIH grants ES031534 (Y.Y.H), ES024373 (Y.Y.H.), ES030576 (Y.Y.H.), the CACHET (NIH ES027792), the University of Chicago Comprehensive Cancer Center (NIH CA014599), the CTSA (NIH UL1 TR000430), and the University of Chicago Friends of Dermatology Endowment Fund. Flow Cytometry was performed by the CAT Facility (RRID: SCR_017760) at the University of Chicago. Mouse tissue processing was performed by the Human Tissue Resource Center (RRID:SCR_019199) at the University of Chicago.
Footnotes
This article is part of a Special Issue celebrating the 50 th Anniversary of the American Society for Photobiology.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article:
REFERENCES
- 1.Hoel DG, Berwick M, de Gruijl FR and Holick MF (2016) The risks and benefits of sun exposure 2016. Dermatoendocrinol 8, e1248325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narayanan DL, Saladi RN and Fox JL (2010) Ultraviolet radiation and skin cancer. Int J Dermatol 49, 978–986. [DOI] [PubMed] [Google Scholar]
- 3.Fell GL, Robinson KC, Mao J, Woolf CJ and Fisher DE (2014) Skin beta-endorphin mediates addiction to UV light. Cell 157, 1527–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong SP, Kim MJ, Jung MY, Jeon H, Goo J, Ahn SK, Lee SH, Elias PM and Choi EH (2008) Biopositive effects of low-dose UVB on epidermis: coordinate upregulation of antimicrobial peptides and permeability barrier reinforcement. J Invest Dermatol 128, 2880–2887. [DOI] [PubMed] [Google Scholar]
- 5.Zhu H, Wang N, Yao L, Chen Q, Zhang R, Qian J, Hou Y, Guo W, Fan S, Liu S, Zhao Q, Du F, Zuo X, Guo Y, Xu Y, Li J, Xue T, Zhong K, Song X, Huang G and Xiong W (2018) Moderate UV Exposure Enhances Learning and Memory by Promoting a Novel Glutamate Biosynthetic Pathway in the Brain. Cell 173, 1716–1727 e1717. [DOI] [PubMed] [Google Scholar]
- 6.Lin SW, Wheeler DC, Park Y, Cahoon EK, Hollenbeck AR, Freedman DM and Abnet CC (2012) Prospective study of ultraviolet radiation exposure and risk of cancer in the United States. Int J Cancer 131, E1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rebel H, der Spek CD, Salvatori D, van Leeuwen JP, Robanus-Maandag EC and de Gruijl FR (2015) UV exposure inhibits intestinal tumor growth and progression to malignancy in intestine-specific Apc mutant mice kept on low vitamin D diet. Int J Cancer 136, 271–277. [DOI] [PubMed] [Google Scholar]
- 8.Grant WB (2009) How strong is the evidence that solar ultraviolet B and vitamin D reduce the risk of cancer?: An examination using Hill’s criteria for causality. Dermatoendocrinol 1, 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Rhee H, Coebergh JW and de Vries E (2013) Is prevention of cancer by sun exposure more than just the effect of vitamin D? A systematic review of epidemiological studies. Eur J Cancer 49, 1422–1436. [DOI] [PubMed] [Google Scholar]
- 10.Moozhipurath RK, Kraft L and Skiera B (2020) Evidence of protective role of Ultraviolet-B (UVB) radiation in reducing COVID-19 deaths. Sci Rep 10, 17705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sontheimer C, Liggitt D and Elkon KB (2017) Ultraviolet B Irradiation Causes Stimulator of Interferon Genes-Dependent Production of Protective Type I Interferon in Mouse Skin by Recruited Inflammatory Monocytes. Arthritis Rheumatol 69, 826–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cela EM, Gonzalez CD, Friedrich A, Ledo C, Paz ML, Leoni J, Gomez MI and Gonzalez Maglio DH (2018) Daily very low UV dose exposure enhances adaptive immunity, compared with a single high-dose exposure. Consequences for the control of a skin infection. Immunology 154, 510–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zitvogel L, Galluzzi L, Kepp O, Smyth MJ and Kroemer G (2015) Type I interferons in anticancer immunity. Nat Rev Immunol 15, 405–414. [DOI] [PubMed] [Google Scholar]
- 14.van der Leun AM, Thommen DS and Schumacher TN (2020) CD8(+) T cell states in human cancer: insights from single-cell analysis. Nat Rev Cancer 20, 218–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mantovani A, Marchesi F, Malesci A, Laghi L and Allavena P (2017) Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 14, 399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF and Sancho D (2020) Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol 20, 7–24. [DOI] [PubMed] [Google Scholar]
- 17.Noy R and Pollard JW (2014) Tumor-associated macrophages: from mechanisms to therapy. Immunity 41, 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mills CD (2012) M1 and M2 Macrophages: Oracles of Health and Disease. Crit Rev Immunol 32, 463–488. [DOI] [PubMed] [Google Scholar]
- 19.Steinman RM (2012) Decisions about dendritic cells: past, present, and future. Annu Rev Immunol 30, 1–22. [DOI] [PubMed] [Google Scholar]
- 20.Merad M, Sathe P, Helft J, Miller J and Mortha A (2013) The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol 31, 563–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mildner A and Jung S (2014) Development and function of dendritic cell subsets. Immunity 40, 642–656. [DOI] [PubMed] [Google Scholar]
- 22.Edelson BT, Kc W, Juang R, Kohyama M, Benoit LA, Klekotka PA, Moon C, Albring JC, Ise W, Michael DG, Bhattacharya D, Stappenbeck TS, Holtzman MJ, Sung SS, Murphy TL, Hildner K and Murphy KM (2010) Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med 207, 823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spranger S, Dai D, Horton B and Gajewski TF (2017) Tumor-Residing Batf3 Dendritic Cells Are Required for Effector T Cell Trafficking and Adoptive T Cell Therapy. Cancer Cell 31, 711–723 e714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang S, Wei J, Cui YH, Park G, Shah P, Deng Y, Aplin A, Lu Z, Hwang S, He C and He YY (2019) m6A mRNA demethylase FTO regulates melanoma tumorigenicity and response to anti-PD-1 blockade. Nat Commun 10, 2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ming M, Soltani K, Shea CR, Li X and He YY (2015) Dual role of SIRT1 in UVB-induced skin tumorigenesis. Oncogene 34, 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiang L, Sample A, Shea CR, Soltani K, Macleod KF and He YY (2017) Autophagy gene Atg7 regulates ultraviolet radiation-induced inflammation and skin tumorigenesis. Autophagy 13, 2086–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Z, Yang S, Cui YH, Wei J, Shah P, Park G, Cui X, He C and He YY (2021) METTL14 facilitates global genome repair and suppresses skin tumorigenesis. Proc Natl Acad Sci U S A 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui YH, Yang S, Wei J, Shea CR, Zhong W, Wang F, Shah P, Kibriya MG, Cui X, Ahsan H, He C and He YY (2021) Autophagy of the m(6)A mRNA demethylase FTO is impaired by low-level arsenic exposure to promote tumorigenesis. Nat Commun 12, 2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Theisen DJ, Ferris ST, Briseno CG, Kretzer N, Iwata A, Murphy KM and Murphy TL (2019) Batf3-Dependent Genes Control Tumor Rejection Induced by Dendritic Cells Independently of Cross-Presentation. Cancer Immunol Res 7, 29–39. [DOI] [PubMed] [Google Scholar]
- 30.Kim Y and He YY (2014) Ultraviolet radiation-induced non-melanoma skin cancer: Regulation of DNA damage repair and inflammation. Genes Dis 1, 188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barragan M, Good M and Kolls JK (2015) Regulation of Dendritic Cell Function by Vitamin D. Nutrients 7, 8127–8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of antibodies used for flow cytometric analysis of immune cells.


