Abstract
Yersinia enterocolitica, Yersinia pseudotuberculosis, and Yersinia pestis deliver different Yop (Yersinia outer proteins) effector proteins into mammalian cells by a type III secretion mechanism. Recently, it was shown that Yersinia producing YopT leads to disruption of the actin cytoskeleton of HeLa cells (M. Iriarte and G. R. Cornelis, Mol. Microbiol. 29:915–929, 1998). To analyze the molecular mechanism of YopT, we cloned and expressed YopT as a glutathione S-transferase fusion protein. Recombinant YopT caused rounding up of embryonic bovine lung cells and redistribution of the actin cytoskeleton rapidly after microinjection. The Escherichia coli cytotoxic necrotizing factor (CNF1), which constitutively activates Rho proteins, was not able to inhibit or revert YopT-induced cell rounding. YopT caused release of RhoA from embryonic bovine lung membranes and released recombinant isoprenylated RhoA from artificial PE or PE/PIP2 vesicles. Incubation of lysate or cytosol with YopT caused inhibition of the RhoA-rhotekin interaction but led to increased RhoA-RhoGDI interaction. It is suggested that inhibition of the interaction between RhoA and effectors is the underlying mechanism of the YopT action on the cytoskeleton.
The genus Yersina includes the pathogenic species Yersinia enterocolitica, Yersinia pseudotuberculosis, and Yersinia pestis, which are able to resist the nonspecific immune defense of the host. The pathogenicity of Yersinia is dependent on the presence of a 70-kb virulence plasmid pYV in Y. enterocolitica. The plasmid encodes proteins which form a complex type III secretion machinery (Ysc) as well as Yop proteins (Yersinia outer proteins), which can be divided into two groups, translocator and effector Yop proteins (6). After binding of Yersinia to its eukaryotic host cell, effector Yop proteins are translocated into the cytoplasm by type III secretion (6). Once inside the host cell, the effector Yop proteins engage in signal transduction pathways with the aim to subvert the host cell defense. To date, six effector Yop proteins of Y. enterocolitica are known, including YopM, YopH, YopO (YpkA in Y. pseudotuberculosis), YopP (YopJ in Y. pseudotuberculosis), YopE, and YopT (5, 7, 19). Up to now, no cellular phenotype for YopM has been reported. YopH is a tyrosine phosphatase which leads to disruption of peripheral focal complexes in HeLa cells by dephosphorylating p130Cas and FAK (20). YopO/YpkA, which shares significant homology with eukaryotic Ser/Thr protein kinases, was reported to interact with the small GTPases RhoA and Rac1 (2, 9). YopP/YopJ is involved in inducing apoptosis probably by inhibiting NF-κB activation (23). YopE, which exhibits GTPase-activating protein (GAP) activity, inactivates the small GTPases RhoA, Rac-1, and Cdc42, leading to depolymerization of actin stress fibers in eukaryotic cells (27). Recently, another Yop (YopT) was reported to affect the actin cytoskeleton of the host cell. It was shown that infection of HeLa cells with wild-type Y. enterocolitica and a yopE mutant, but not with a yopT mutant, caused an increase in electrophoretic mobility of RhoA. YopT-dependent modification also revealed an acidic shift of RhoA as tested by isoelectric focusing (28). Furthermore, in cells infected with Y. enterocolitica, YopT leads to redistribution of membrane-bound RhoA towards the cytosol, suggesting that YopT acts on the actin cytoskeleton by inactivating RhoA (14).
Small GTPases of the Rho family are involved in the organization of the actin cytoskeleton and act as molecular switches in various signaling processes. Rho GTPases cycle between an inactive GDP-bound state and an activated GTP-bound state. Activation occurs by GDP and GTP exchange induced by guanine nucleotide exchange factors (GEFs) and inactivation by hydrolysis of the bound GTP, a process that is facilitated by GAPs. Finally, activated Rho proteins can interact with a large spectrum of effector proteins to mediate downstream signaling (12, 22).
The cycling between the two nucleotide-bound states of Rho is accompanied by its cycling between the cytosol and the cell membranes. In this subcellular cycling, guanine nucleotide dissociation inhibitors (GDIs) are involved. The GDIs bind to posttranslationally modified (isoprenylated) Rho proteins in the cytosol, keep them in the GDP-bound form, and inhibit nucleotide exchange (11).
A large variety of bacterial toxins and exotoxins target small GTPases of the Rho family, thereby disrupting the redistribution of the actin cytoskeleton. C3-like exoenzymes (e.g., Clostridium botulinum exoenzym C3 and Clostridium limosum transferase) ADP-ribosylate RhoA, RhoB, and RhoC at asparagine 41, thereby inhibiting the biological functions of the GTPases (3, 4, 15, 26). All members of the Rho subfamily are glucosylated by large clostridial cytotoxins (e.g., Clostridium difficile toxins A and B and Clostridium sordellii LT) (1, 16, 17). In addition, Rho is activated by another class of toxins, including the cytotoxic necrotizing factors (CNF1 and -2) of Escherichia coli and the CNF1-related dermonecrotic toxin (DNT) from Bordetella species (24). CNF and DNT deamidate and/or transglutaminate glutamine 63 of Rho (glutamine 61 of Rac and Cdc42), resulting in a constitutively activated form of the GTPases (10, 25). Here, we cloned, expressed, and purified YopT for the first time and studied the effects of the recombinant protein on Rho GTPases.
MATERIALS AND METHODS
Cloning and purification of YopT.
The YopT gene with flanking BamHI and EcoRI sites was generated by PCR (sense, 5′-GGATCCATGGACAGTATTCACGGACACTACC-3′; antisense, 5′-GAATTCTTAAACCTCCTTGGAGTCAAATGTTAAC-3′) from the virulence plasmid pYV from Y. enterocolitica JB580v (18) and cloned in-frame into the expression vector pGEX2TGL. The proper construct was checked by DNA sequencing. Expression of the glutathione S-transferase fusion protein in E. coli TG1 cells growing at 37°C was induced by adding 0.2 mM isopropyl-β-d-thiogalactopyranoside (final concentration) at an optical density of 0.6. Four hours after induction, cells were collected and lysed by sonication in lysis buffer (20 mM Tris-HCl [pH 7.4], 10 mM NaCl, 1% Triton, 1 mM phenylmethylsulfonylfluoride [PMSF], and 5 mM dithiotreitol) and purified by affinity chromatography with glutathione-Sepharose (Amersham Pharmacia Biotech). Loaded beads were washed once with washing buffer (50 mM Tris-HCl [pH 7.4] and 150 mM NaCl) and subsequently five times with lysis buffer (without PMSF) at 4°C. YopT was eluted from the beads by thrombin digestion (200 μg of thrombin/ml, 150 mM NaCl, 50 mM triethanolamine hydrochloride [pH 7.5], and 2.5 mM CaCl2) for 45 min at room temperature. Thrombin was removed by incubation with benzamidine-Sepharose beads. The GST-YopT fusion protein was eluted from the beads by glutathione (10 mM glutathione and 50 mM Tris-HCl [pH 7.4]) twice for 10 min at room temperature.
Microinjection.
For microinjection, embryonic bovine lung (EBL) cells were seeded subconfluently on glass coverslips (CELLocate; Eppendorf) and cultivated for 24 h in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum in humidified 5% CO2 at 37°C. GST-YopT (100 ng/μl) in 50 mM Tris-HCl (pH 7.4) was microinjected into EBL cells with an Eppendorf 5242 microinjector. For the identification of injected cells, an unspecific rabbit immunoglobulin G (IgG) (500 ng/μl) was coinjected with GST-YopT and as a control rabbit IgG alone. After the indicated times of incubation at 37°C, cells were fixed with 4% formaldehyde and 0.1% Tween 20 in phosphate-buffered saline (PBS) at room temperature for 10 min. Formaldehyde-fixed cells were washed with PBS. Then, the cells were incubated with rhodamine-conjugated phalloidin (1 U/ coverslip) together with an Alexa-labeled anti-rabbit IgG antibody at room temperature for 1 h, washed again, and applied for fluorescence microscopy (as bleaching preservative Kaiser's glycerol gelatin [Merck] was used).
Lysis and fractionation of cells.
Confluent cell cultures of 10-cm-diameter dishes were rinsed with ice-cold PBS and scraped off into 250 μl of lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 0.5 mM phenylmethylsulfonylfluoride, 2.5 mM dithiothreitol, 40 μg of aprotinin/ml, and 80 μg of benzamidine/ml). The cells were disrupted mechanically by sonication (five times on ice), followed by centrifugation for 10 min at 1,000 × g to remove the nuclear fraction and intact cells. The supernatant was used as cell lysate.
Lysates were centrifuged at 100,000 × g for 1 h to prepare cytosolic and total particular fractions. The high-speed pellet, which consists of the heavy and the light membrane fractions, was washed with mammalian lysis buffer. This membrane preparation was used for the membrane release assay.
Preparation of rat brain membranes.
Collected rat brains were homogenized in an appropriate volume of homogenization buffer (100 mM NaCl, 50 mM HEPES [pH 7.2] containing 10 μg of leupeptin/ml, 80 μg of benzamidine/ml, 40 μg of aprotinin/ml, and 0.5 mM PMSF).
The homogenate was centrifuged for 10 min at 1,000 × g to remove nuclei and connecting tissue. The supernatant was centrifuged for 60 min at 30,000 × g and subsequently separated in cytosolic and membrane fractions by ultracentrifugation for 60 min at 100,000 × g. Membranes were washed once with homogenization buffer and stored at −20°C.
Before using in the membrane release assay, rat brain membranes were washed three times in lysis buffer. All steps were carried out at 4°C.
Preparation of artificial vesicles and loading with isoprenylated RhoA.
For preparation of lipid vesicles, 100 μM phosphatidylethanolamine (PE) or 100 μM PE and 17 μM phosphatidyl-inositol-bisphosphate (PIP2) was vacuum dried to remove organic solvent. Lipids were dissolved in lipid buffer (50 mM HEPES, 2 mM desoxycholate, and 150 mM NaCl, pH 7.0), and vesicles were generated by sonication.
Isoprenylated RhoA was expressed in Spodoptera frugiperda insect cells (Sf9 cells) and purified from a Triton X-100-soluble fraction by affinity chromatography. Glutathione S-transferase (GST)-fusion protein was cleaved with thrombin. Purified isoprenylated RhoA was incubated with the PE or PE/PIP2 vesicles for 30 min at room temperature by head-over-head rotation. The samples were washed three times with lysis buffer before incubation with YopT.
Membrane release assay.
Washed membranes or artificially RhoA-loaded vesicles were washed in lysis buffer and incubated with different concentrations of YopT or GST-YopT for 30 min at 37°C or as indicated. After incubation, membranes and supernatants were isolated by ultracentrifugation (60 min at 100,000 × g), separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and analyzed by immunoblotting or ADP ribosylation as previously described (22). For quantification of the marked RhoA bands, the ImageQuant software (Amersham Pharmacia Biotech) was used.
Immunoblot analysis.
Proteins were separated on SDS–12.5% polyacrylamide gels and transferred onto a polyvinylidene difluoride (PVDF) membrane, followed by blocking with 5% (wt/vol) nonfat dried milk for 1 h. Blots were incubated overnight at 4°C with the monoclonal antibody raised against RhoA (26C4; Santa Cruz) and then for 1 h with a horseradish peroxidase-conjugated secondary antibody.
Rhotekin pulldown.
The Rho-binding region, encoding the N-terminal 90 amino acids of rhotekin, was expressed as a GST-fusion protein in E. coli BL21. Overnight cultures were diluted 1:100 and then grown for 1 h at 37°C. Then, 0.1 mM isopropyl-β-d-thiogalactopyranoside (final concentration) was added. Two hours after induction, cells were collected and lysed by sonication in rhotekin lysis buffer (20% sucrose, 10% glycerol, 50 mM Tris-HCl [pH 8.0], 0.2 mM sodium bisulfite, 2 mM MgCl2, 0.5 mM PMSF, 2 mM dithiothreitol, 40 μg of aprotinin/ml, and 80 μg of benzamidine/ml) and purified by affinity chromatography with glutathione-Sepharose (Amersham Pharmacia Biotech). Loaded beads were washed three times with rhotekin lysis buffer and once with buffer A (10% glycerol, 50 mM Tris-HCl [pH 7.4], 100 mM NaCl, 1% Igepal, 2 mM MgCl2, and 0.5 mM PMSF) and then incubated with lysates or cytosolic fractions of HeLa cells for 1 h at 4°C by head-over-head rotation. After washing once with buffer A, SDS sample buffer was added. The samples were boiled and separated by SDS-PAGE. RhoA was analyzed by immunoblotting.
GDI pulldown.
GDI (GDI-1) was expressed and purified as a GST-fusion protein. Loaded beads were washed three times with lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 0.5 mM PMSF, 2.5 mM dithiothreitol, 40 μg of aprotinin/ml, and 80 μg of benzamidine/ml) and then incubated with lysates or cytosolic fractions of HeLa cells for 1 h at room temperature by head-over-head rotation. After washing once with lysis buffer, SDS sample buffer was added and the samples were boiled and separated by SDS-PAGE. RhoA was analyzed by immunoblotting.
RESULTS
To analyze the molecular mechanism of Yersinia YopT, we amplified the gene from the virulence plasmid pYV from Y. enterocolitica JB580v (18) by PCR, introducing BamHI and EcoRI sites for subcloning into the expression vector pGEX2TGL. The proper construct was checked by DNA sequencing. Sequencing revealed some differences in the YopT sequence in comparison to the sequence published by Iriarte and Cornelis (14). Repeated PCR yielded the same results, indicating that the differing sequence which is shown in Fig. 1 is not due to mistakes of the polymerase. YopT1 is an isoform of YopT.
FIG. 1.
Sequence of gene encoding YopT and SDS-PAGE of purified recombinant YopT. (A) Sequence alignment between YopT (accession no. AF102990) and YopT1. The YopT1 gene was amplified from the plasmid pYV from Y. enterocolitica JB580v (18). Differences are indicated by black boxes. (B) SDS-PAGE (12.5% polyacrylamide) of recombinant GST-YopT and YopT. The protein was expressed from pGEX-2TGL in E. coli TG-1 cells, purified by affinity chromatography (lane 1), and cleaved with thrombin (lane 2).
Biological activity of recombinant YopT.
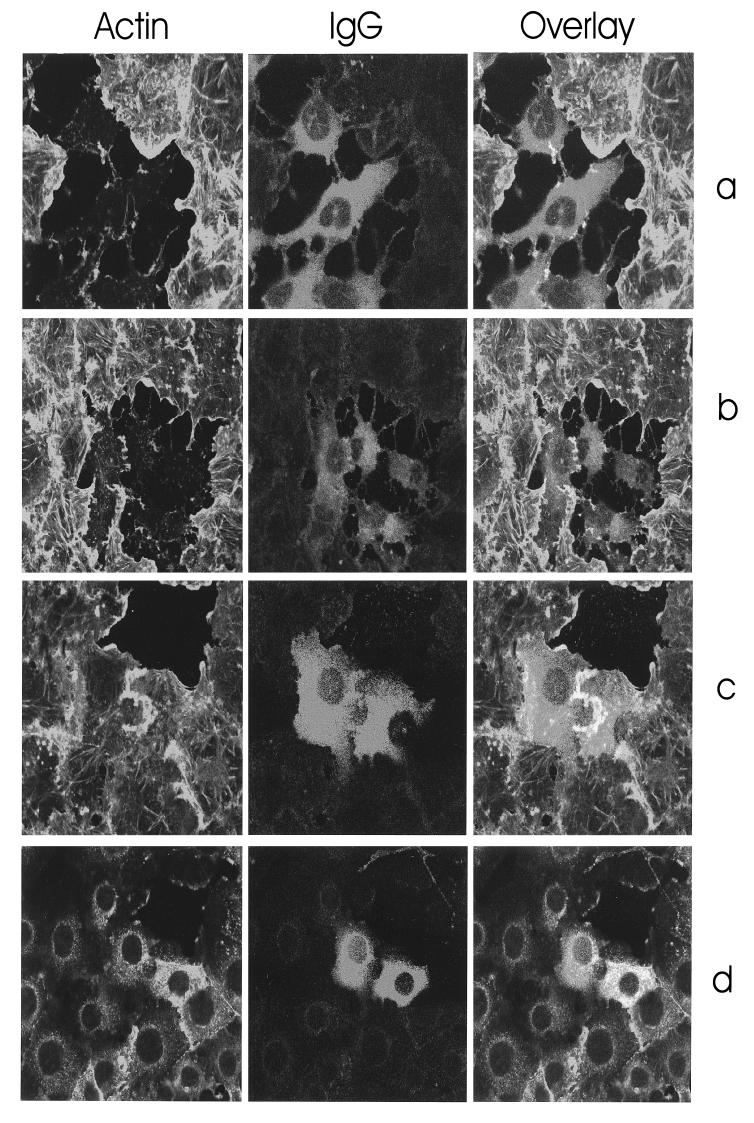
To analyze the biological activity of recombinant YopT, we expressed the toxin, purified it as a GST-YopT protein by affinity chromatography (Fig. 1b), and injected it into EBL cells. After 10 to 15 min, GST-YopT- and YopT-injected cells (data not shown) started to retract and rounded up after 30 to 60 min. IgG-injected cells did not round up, indicating that the recombinant YopT was biologically active. In a second experiment, we intended to identify the injected cells after actin staining. Therefore, we coinjected unspecific rabbit IgG (500 ng/μl) together with GST-YopT (100 ng/μl) (Fig. 2a and b) and, as a control, rabbit IgG alone (500 ng/μl; Fig. 2c and d), fixed the cells after a 15-min incubation at 37°C, and performed double staining with rhodamine-phalloidin and Alexa-labeled anti-rabbit IgG antibody. As shown in Fig. 2a to d, rhodamine-phalloidin-staining of IgG-injected control cells was the same as for uninjected cells, whereas GST-YopT-injected cells exhibited a complete redistribution of actin filaments.
FIG. 2.
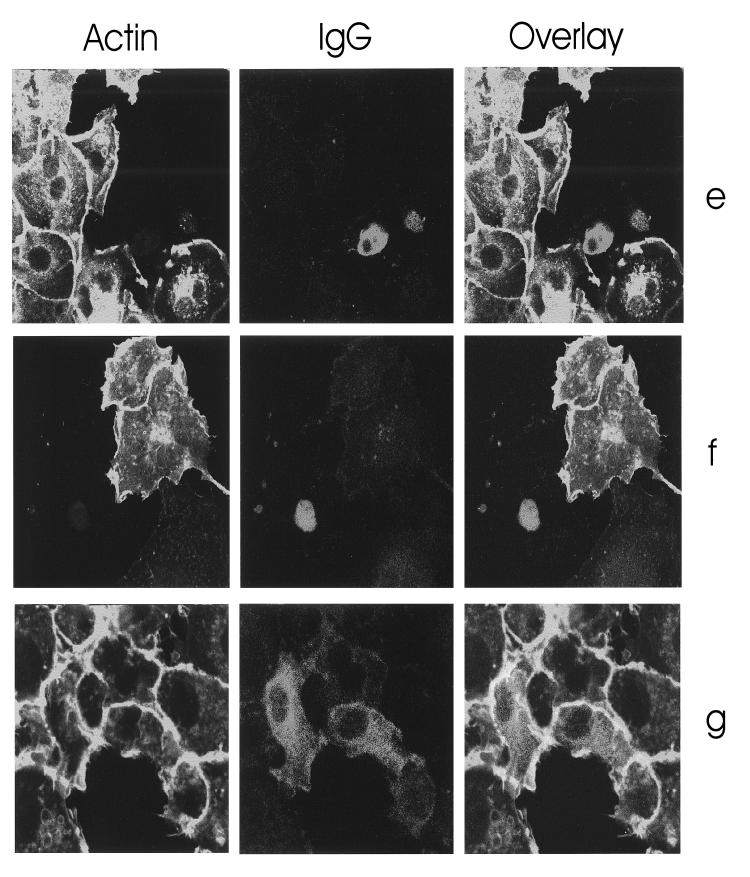
Morphological effects in EBL cells induced by microinjection of YopT. (a to d) EBL cells were comicroinjected with GST-YopT (100 ng/μl) and rabbit IgG (500 ng/μl) (a and b) or microinjected with rabbit IgG alone (500 ng/μl) (c and d). The cells were fixed after 15 min and stained for F actin with rhodamine-phalloidin and for rabbit IgG with Alexa-labeled anti-rabbit IgG antibodies. Original magnifications: a and c, ×80; b and d, ×40. (e) EBL cells were comicroinjected with GST-YopT (100 ng/μl) and rabbit IgG (500 ng/μl) and, directly after injection, were incubated for 2 h with 1 μg of CNF1/ml. The cells were then fixed and stained for F actin with rhodamine-phalloidin. To identify the injected cells, immunostaining was performed as described above. Note that the uninjected cells show the typical CNF1-induced accumulation of cortical actin, indicating that CNF1 was active. (f and g) Cells were incubated for 2 h with CNF1 before comicroinjection with GST-YopT and rabbit IgG (f) or microinjection of IgG without the toxin (g). The cells were fixed after further incubation for 2 h and stained. Original magnification (e to g): ×80.
YopT leads to release of RhoA from cell membranes and from artificial vesicles.
Recently, Zumbhil et al. suggested that YopT leads to inactivation of the small GTP-binding protein RhoA by a direct modification of the GTPase (28). They reported that YopT leads to cytosolic localization of RhoA after incubation of mammalian cells with YopT-producing Yersinia.
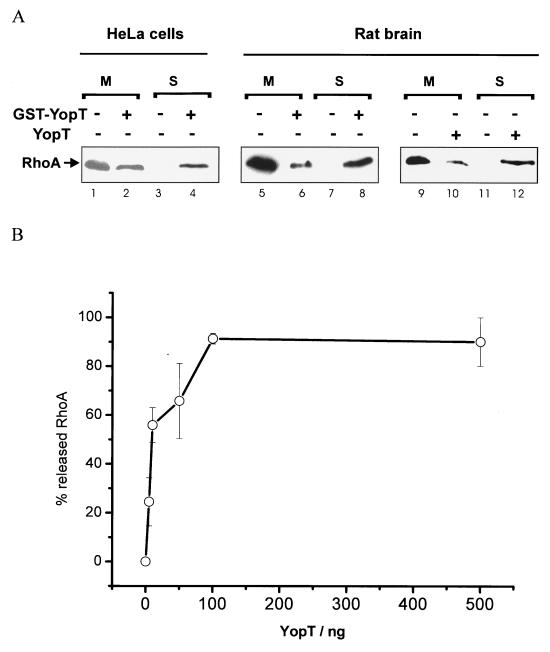
To test whether recombinant YopT is able to release RhoA from isolated cell membranes, we prepared membranes from HeLa cells as well as rat brain membranes as described in the Materials and Methods section and incubated the isolated, washed membranes for 30 min at 37°C in the absence and presence of GST-YopT or YopT as indicated. Thereafter, YopT-treated membranes were then again separated into the membrane and the supernatant fractions by ultracentrifugation and tested for released RhoA by SDS-PAGE and subsequent Western blotting. As shown in Fig. 3A, incubation of isolated HeLa cell membranes with GST-YopT led to release of the GTPase into the supernatant (Fig. 3A, lanes 1 to 4). Heat inactivation of YopT blocked this release, excluding a buffer effect (not shown). The same result as shown for HeLa membranes was found when purified rat brain membranes were used (Fig. 3A, lanes 5 to 8, with GST-YopT, and lanes 9 to 12, with YopT). Rat brain membranes were used to study the YopT concentration-dependent release of RhoA because a large amount of material was needed for this assay. Washed membranes were incubated with different concentrations of YopT (5 to 500 ng/sample) or without the toxin for 30 min at 37°C. To study whether RhoA is released by YopT from the membranes, the samples were again separated into membrane and supernatant fractions as described above. The amount of released RhoA was quantified by ADP ribosylation with C3 transferase in the presence of [32P]NAD, subsequent SDS-PAGE, and phosphorimaging (maximal release was set at 100%). As shown in Fig. 3B, 100 ng of YopT released the maximal amount of RhoA from isolated rat brain membranes. The release of RhoA from rat brain membranes (Fig. 3B) and the cell rounding after microinjection (not shown) occurred with about 1 to 10 ng of purified recombinant GST-YopT/μl. The rather low concentration suggests that YopT acts catalytically.
FIG. 3.
Membrane release of RhoA by GST-YopT. (A) Isolated and washed HeLa cell membranes (lanes 1 to 4) or isolated rat brain membranes (lanes 5 to 12) were incubated with or without GST-YopT (5 μg/sample) as indicated for 30 min at 37°C. Afterwards, the samples were again separated into membrane (M) and supernatant (S) fractions by ultracentrifugation and tested for RhoA by SDS-PAGE and subsequent Western blotting. Shown is a typical result of more than three independent experiments. (B) Concentration-dependent release of RhoA from cell membranes by YopT. For this assay, isolated rat brain membranes instead of HeLa membranes were used, because a lot of material was needed. Washed membranes were incubated with different concentrations of YopT (5 to 500 ng/sample) or without the toxin for 30 min at 37°C. To study whether RhoA is released by YopT from the membranes, the samples were again separated into membrane and supernatant fractions by ultracentrifugation and tested for RhoA by ADP ribosylation with C3 transferase and [32P]NAD. After SDS-PAGE, the amount of released RhoA was quantified by phosphor-imaging (maximal release was set as 100%). Shown is the mean of three independent experiments ± standard error of the mean.
To analyze the effect of YopT on RhoA, which was bound to artificial membranes, PE/PIP2 vesicles were generated as described in Materials and Methods and loaded with recombinant, isoprenylated RhoA. After washing in lysis buffer, vesicles were treated with GST-YopT, heat-inactivated GST-YopT, or buffer. After incubation, the samples were separated into membranes and supernatants by ultracentrifugation and then tested for RhoA by SDS-PAGE and subsequent Western blotting. As shown in Fig. 4, RhoA was removed from the vesicles into the supernatants after incubation with GST-YopT but not with heat-inactivated GST-YopT or buffer. Release of RhoA from the vesicles with YopT also occurred when vesicles that consisted only of PE were used.
FIG. 4.
Release of RhoA from artificial vesicles. Artificial PE/PIP2 (lanes 1 to 6) or PE vesicles (lanes 7 to 12) were incubated with recombinant, isoprenylated RhoA for 60 min at room temperature. Then, the RhoA-containing vesicles were separated by ultracentrifugation (100,000 × g; 60 min; 4°C) and washed three times with lysis buffer. Thereafter, vesicles were resuspended in lysis buffer and incubated without (−) and with (+) native GST-YopT and heat-inactivated (hi) GST-YopT for 30 min at 37°C. The samples were separated into supernatant and vesicles by ultracentrifugation (100,000 × g; 60 min; 4°C), incubated with SDS sample buffer for 5 min at 95°C, and tested for RhoA by SDS-PAGE and subsequent Western blotting. Shown is a typical result of three independent experiments.
CNF1 is not able to prevent or revert the YopT phenotype.
The E. coli cytotoxic necrotizing factor (CNF) directly activates Rho proteins by deamidation of glutamine 63/61, thereby blocking intrinsic and GAP-stimulated GTPase activity of Rho. To analyze whether the direct activation of RhoA by CNF1 counteracts the effect of YopT, we microinjected GST-YopT into EBL cells and incubated them afterwards for 2 h with 1 μg of CNF1/ml to allow modification of Rho, fixed the cells, and stained filamentous actin with rhodamine-phalloidin. To identify the injected cells, we performed coinjection with rabbit IgG and immunostaining as described (Fig. 2e). Microinjection of GST-YopT leads to complete rounding of the cells even in the presence of CNF1. In contrast to the cells shown in Fig. 2a to d, the cells in this experiment were incubated for 2 h after microinjection before fixation and staining. In uninjected CNF-treated EBL cells, the actin cytokeleton shows a typical accumulation of cortical actin, indicating that CNF1 was active during the 2-h incubation period. In a second experiment, we studied whether CNF1 is able to prevent cell rounding induced by YopT. Therefore, we incubated the cells for 2 h with CNF1 before microinjection with GST-YopT/IgG or IgG without toxin and fixed the cells after further incubation for 2 h. As can be seen in Fig. 2f, CNF1-intoxicated cells rounded up after microinjection of GST-YopT. Initial cell retraction occurred already after 15 min, as observed for not-intoxicated cells (not shown). In both experiments, CNF1 was not able to counteract the YopT-induced disruption of the actin cytoskeleton.
RhoA from YopT-treated cytosol does not bind to rhotekin but still interacts with RhoGDI.
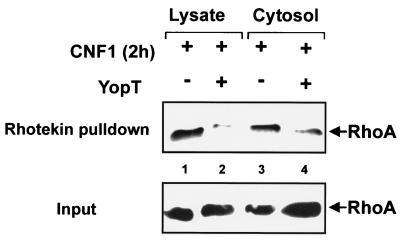
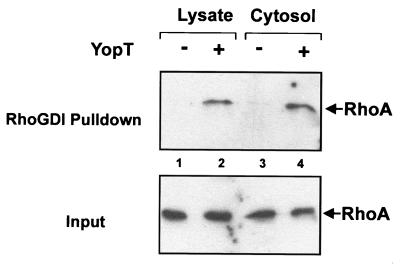
To test the interaction of modified RhoA or control RhoA with the Rho effector rhotekin, we performed a rhotekin pulldown assay. In intact HeLa cells, RhoA was activated by incubation with CNF1 for 2 h. Cells were then lysed in buffer A. Cell lysate or cytosol (generated by centrifugation at 100,000 × g for 60 min) was incubated with YopT or buffer for 30 min at 37°C. Thereafter, 1/15 of the volume was taken as input control shown in the lower panel of Fig. 5. The rest of the YopT-treated lysates and cytosols were incubated with beads loaded with GST-rhotekin as described in Materials and Methods. Washed beads were resuspended in SDS sample buffer. After boiling, bound proteins were supplied to SDS–12.5% PAGE. Rhotekin-bound RhoA (upper panel) as well as RhoA from the input control was detected by Western blotting with RhoA-specific antibody. As shown in Fig. 5, incubation of lysate and cytosol with YopT led to loss of binding of RhoA to its effector rhotekin. From this result, we concluded that RhoA is also modified by YopT in the presence of cytosol but in the absence of cell membranes. Next, we studied whether treatment of RhoA from cell lysate or cytosol with YopT affects binding to RhoGDI. Therefore, cell lysate or cytosol was incubated with buffer or YopT. Then, recombinant GST-RhoGDI coupled to beads was added. After further incubation, RhoA was precipitated by centrifugation. As shown in Fig. 6 (upper panel), a larger amount of RhoA was precipitated with RhoGDI-beads from samples treated with YopT than from controls. The same results as shown in Fig. 5 and 6 with YopT were found with GST-YopT (not shown).
FIG. 5.
Rhotekin pulldown assay. Lysates and cytosols of CNF1-treated (400 ng of CNF1/ml, 3 h) HeLa cells were incubated with and without YopT (5 μg/sample) for 30 min at 37°C. After incubation with YopT, 1/15 of the volume was taken as input control shown in the lower panel. The rest of the lysates and cytosols were incubated with GST-rhotekin beads for 60 min at 4°C. After washing, rhotekin-bound RhoA and the input control were separated by SDS-PAGE and detected by Western blotting with a RhoA-specific antibody. After blotting, it was checked by Ponceau staining that equal amounts of beads have been used for precipitation. Shown is a typical result of three independent experiments.
FIG. 6.
RhoGDI pulldown assay. HeLa cell lysates (lanes 1 and 2) and cytosols (lanes 3 and 4) were incubated with and without YopT (5 μg/sample) for 30 min at 37°C. Then, cytosols were incubated with GST-RhoGDI beads for 60 min at 4°C. Lysates were separated in cytosolic and membrane fraction by ultracentrifugation after incubation with YopT and then also incubated with GST-RhoGDI beads. Before loading to the beads, 1/15 of the volume was taken as input control shown in the lower panel. After incubation of the cytosols with the beads, loaded beads were washed three times with lysis buffer and incubated with SDS sample buffer for 5 min at 95°C. Then, the supernatants as well as the input controls were separated by SDS-PAGE, transferred onto a PVDF membrane, and analyzed by Western blotting using a specific RhoA antibody. After blotting, it was checked by Ponceau staining that equal amounts of beads have been used for precipitation. Shown is a result typical for three independent experiments.
DISCUSSION
Recently, it was shown that YopT from Yersinia, which is delivered by type III secretion into mammalian cells, leads to morphological changes and cytotoxic effects in HeLa cells and macrophages, respectively (14). In order to further analyze the molecular mechanism of YopT, we generated the plasmid pGEX-2T-GL-YopT by amplification of the YopT gene from the virulence plasmid pYV and subsequent subcloning into the expression vector pGEX-2T-GL. Sequencing revealed slight differences in the sequence of the YopT gene amplified from the pYV plasmid used in this study from the YopT gene published recently (14). These differences are probably caused by using different Y. enterocolitica strains. GST-YopT was expressed in E. coli TG1 cells and purified by affinity chromatography. Microinjection studies revealed that the recombinant GST-fusion protein and the thrombin-cleaved YopT were fully active and caused a complete degradation of the actin cytoskeleton and rounding up within a short time (15 min). By contrast, cells injected with rabbit IgG alone did not induce changes of the actin cytoskeleton. Similar rounding up of cells and disruption of the actin cytoskeleton was observed by Iriarte and Cornelis (14) with HeLa cells infected with Yersinia, producing YopT as the only Yop protein.
It has been shown that inhibition of RhoA with C3-transferase of C. botulinum as well as treatment of cells with YopT of Y. enterocolitica leads to disruption of the filamentous actin network, but does not disrupt cell-cell contacts or peripheral actin in human umbilical vein endothelial cell (HUVEC) cells (28). The authors described that bacterially translocated YopT leads to an enlarged spread-out phenotype in confluent HUVEC cells associated with the loss of stress fibers. In our experiments, YopT was microinjected into subconfluent EBL cells with only a few or even no cell contacts, which lead to the loss of stress fibers and subsequent cell rounding. The two YopT-induced morphologies observed may be explained by differences in the confluence of the cells studied rather than by the use of different cell lines.
Recently, it was shown that YopT-producing Yersinia leads to the translocation of RhoA from membranes to the cytosol in intact cells (28). Therefore, we studied whether recombinant YopT has the same activity. As found for YopT-producing Yersinia, the recombinant YopT caused release of RhoA from membranes after incubation in vitro. This effect was clearly concentration dependent. To identify, whether any additional cellular factor was necessary for the translocation, we studied the action of YopT with artificial membranes. For this purpose, recombinant isoprenylated RhoA obtained from a baculovirus-system was used and loaded onto artifical lipid vesicles. Similar to observations with cell-derived membranes, YopT released RhoA from the artificial membranes, indicating that no additional cofactors were necessary. Moreover, these studies also showed that Rho-GDI, which keeps GDP-bound Rho in the cytosol, is not necessary for this effect.
It was reported recently that cell rounding induced by the Yersinia effector protein YopE, which is a GAP protein specific for Rho GTPases, can be reverted by the E. coli CNF (27). CNF1 deamidates Rho-GTPases at glutamine 63/61. This modification leads to a complete block of the intrinsic and GAP-stimulated GTPase activity of Rho proteins and their constitutive activation. To test whether CNF1 reverses or prevents YopT-induced cell rounding, EBL cells were incubated with CNF1 before or after microinjection of YopT. Then, the cells were fixed and stained for F actin. Neither preincubation with CNF nor CNF treatment after microinjection inhibited the YopT-induced cell rounding. Staining of the actin cytokeleton in control cells showed a typical accumulation of cortical actin in CNF-treated EBL cells. Moreover, control lysates of CNF1-treated EBL cells (400 ng/ml, 2 h) showed a full shift of RhoA in SDS-PAGE (not shown), indicating that CNF1 was active. Thus, activation of Rho by CNF is not able to revert or prevent the YopT-induced effects on the actin cytoskeleton. On the other hand, we observed that YopT-treated Rho was still able to bind GTP. Therefore, we suggested that YopT affects the Rho-effector interaction. To test this hypothesis, we performed pulldown assays with the RhoA-binding domain of its effector rhotekin. The binding domain of rhotekin only interacts with activated GTP-bound Rho and can therefore be used to identify Rho activation and Rho-effector coupling (21). To activate Rho, CNF was used which largely increased the Rho-rhotekin interaction in controls. Although only a small amount of activated RhoA (∼5% of input RhoA) bound to the rhotekin beads, it is obvious that treatment of lysates and of cytosolic fractions with YopT caused nearly complete inhibition of the binding of CNF-activated RhoA to rhotekin. From the input controls, it is also evident that RhoA is not degraded by the action of YopT. Thus, these findings indicate that YopT interferes with the Rho-effector coupling.
Recently, it was shown that phosphorylation of Ser188 of RhoA by cAMP dependent protein kinase A impairs its biological activity, leads to release of the GTPase from membranes and causes morphological changes of cells (8). Moreover, phosphorylation of RhoA at the C terminus blocks RhoA-effector interaction. So far, no evidence exists that YopT possesses kinase activity. The finding that YopT effects (e.g., release of RhoA) are observed with artificial membranes in the absence of additional factors largely exclude a typical kinase reaction. Moreover, the fact that inhibition of the RhoA-effector interaction occurs not only in lysates but also in cytosol separated from membranes indicates that the presence of membranes is not necessary for the YopT effect. Therefore, we conclude that the release of RhoA from membranes is not causative for cell rounding. It was speculated that changes in the C terminus of RhoA, like demethylation of Cys 190 or cleavage of the geranyl-geranyl moiety, are the reasons for membrane release and RhoA inactivation (28). Because isoprenylation is known to be necessary for Rho-RhoGDI interaction, we analyzed the interaction of RhoA with RhoGDI. Only GDP-bound Rho that is isoprenylated interacts with GDI (11, 13). In contrast to the finding with rhotekin, RhoGDI precipitated RhoA after treatment with YopT. The amount of precipitated RhoA was even higher after YopT treatment than without treatment. Moreover, YopT-treated RhoA was still able to bind nucleotide (G. Schmidt, unpublished data). The findings indicate that YopT still interacts with RhoGDI and suggest that the geranyl-geranyl moiety of RhoA is not cleaved by YopT.
Taken together, our studies show that recombinant YopT is able to induce morphological changes, redistribution of the actin cytoskeleton and Rho release from membranes similarly as observed after infection of cells with Yersinia producing YopT. The effect of YopT does not depend on additional cytosolic or membrane-located factors and affect the Rho-effector interaction.
ACKNOWLEDGMENT
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 388) and by the Fonds of the Chemische Industrie.
REFERENCES
- 1.Aktories K. Bacterial protein toxins as tools in cell biology and pharmacology. In: Cossart P, Boquet P, Normark S, Rappuoli R, editors. Cellular Microbiology. Washington, D.C.: ASM Press; 2000. pp. 221–237. [Google Scholar]
- 2.Barz C, Abahji T N, Trülzsch K, Heesemann J. The Yersinia Ser/Thr protein kinase YpkA/YopO directly interacts with the small GTPases RhoA and Rac-1. FEBS Lett. 2000;482:139–143. doi: 10.1016/s0014-5793(00)02045-7. [DOI] [PubMed] [Google Scholar]
- 3.Braun U, Habermann B, Just I, Aktories K, Vandekerckhove J. Purification of the 22 kDa protein substrate of botulinum ADP-ribosyltransferase C3 from porcine brain cytosol and its characterization as a GTP-binding protein highly homologous to the rho gene product. FEBS Lett. 1989;243:70–76. doi: 10.1016/0014-5793(89)81220-7. [DOI] [PubMed] [Google Scholar]
- 4.Chardin P, Boquet P, Madaule P, Popoff M R, Rubin E J, Gill D M. The mammalian G protein rho C is ADP - ribosylated by Clostridium botulinum exoenzyme C3 and affects actin microfilament in Vero cells. EMBO J. 1989;8:1087–1092. doi: 10.1002/j.1460-2075.1989.tb03477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornelis G R. Type III secretion: a bacterial device for close combat with cells of their eukaryotic host. Philos Trans R Soc Lond B Biol Sci. 2000;355:681–693. doi: 10.1098/rstb.2000.0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornelis G R, Boland A, Boyd A P, Geuijen C, Iriarte M, Neyt C, Sory M-P, Stainier I. The virulence plasmid of Yersinia, an antihost genome. Microbiol Mol Biol Rev. 1998;62:1315–1352. doi: 10.1128/mmbr.62.4.1315-1352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denecker G, Declercq W, Geuijen C A W, Boland A, Benabdillah R, van Gurp M, Sory M-P, Vandenabeele P, Cornelis G R. Yersinia enterocolitica YopP-induced apoptosis of macrophages involves the apoptotic signaling cascade upstream of bid. J Biol Chem. 2001;276:19706–19714. doi: 10.1074/jbc.M101573200. [DOI] [PubMed] [Google Scholar]
- 8.Dong J-M, Leung T, Manser E, Lim L. cAMP-induced morphological changes are counteracted by the activated RhoA small GTPase and the Rho kinase ROKα. J Biol Chem. 1998;273:22554–22562. doi: 10.1074/jbc.273.35.22554. [DOI] [PubMed] [Google Scholar]
- 9.Dukuzumuremyi J-M, Rosqvist R, Hallberg B, Akerström B, Wolf-Watz H, Schesser K. The Yersinia protein kinase A is a host factor inducible RhoA/Rac-binding virulence factor. J Biol Chem. 2000;275:35281–35290. doi: 10.1074/jbc.M003009200. [DOI] [PubMed] [Google Scholar]
- 10.Flatau G, Lemichez E, Gauthier M, Chardin P, Paris S, Fiorentini C, Boquet P. Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature. 1997;387:729–733. doi: 10.1038/42743. [DOI] [PubMed] [Google Scholar]
- 11.Gosser Y Q, Nomanbhoy T K, Aghazadeh B, Manor D, Combs C, Cerione R A, Rosen M K. C-terminal binding domain of Rho GDP-dissociation directs N-terminal inhibitory peptide to GTPases. Nature. 1997;387:814–815. doi: 10.1038/42961. [DOI] [PubMed] [Google Scholar]
- 12.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman G R, Nassar N, Cerione R A. Structure of the Rho family GTP-binding protein Cdc42 in complex with the multifunctional regulator RhoGDI. Cell. 2000;100:345–356. doi: 10.1016/s0092-8674(00)80670-4. [DOI] [PubMed] [Google Scholar]
- 14.Iriarte M, Cornelis G R. YopT, a new Yersinia Yop effector protein, affects the cytoskeleton of host cells. Mol Microbiol. 1998;29:915–929. doi: 10.1046/j.1365-2958.1998.00992.x. [DOI] [PubMed] [Google Scholar]
- 15.Just I, Mohr C, Schallehn G, Menard L, Didsbury J R, Vandekerckhove J, van Damme J, Aktories K. Purification and characterization of an ADP-ribosyltransferase produced by Clostridium limosum. J Biol Chem. 1992;267:10274–10280. [PubMed] [Google Scholar]
- 16.Just I, Selzer J, Hofmann F, Green G A, Aktories K. Inactivation of Ras by Clostridium sordellii lethal toxin-catalyzed glucosylation. J Biol Chem. 1996;271:10149–10153. doi: 10.1074/jbc.271.17.10149. [DOI] [PubMed] [Google Scholar]
- 17.Just I, Selzer J, Wilm M, Von Eichel-Streiber C, Mann M, Aktories K. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature. 1995;375:500–503. doi: 10.1038/375500a0. [DOI] [PubMed] [Google Scholar]
- 18.Kinder S A, Badger J L, Bryant G O, Pepe J C, Miller V L. Cloning of the YenI restriction endonuclease and methyltransferase from Yersinia enterolitica serotype O8 and construction of a transformable R-M+ mutant. Gene. 1993;136:271–275. doi: 10.1016/0378-1119(93)90478-l. [DOI] [PubMed] [Google Scholar]
- 19.Palmer L E, Hobbie S, Galán J E, Bliska J B. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNF-α production and downregulation of the MAP kinases p38 and JNK. Mol Microbiol. 1998;27:953–965. doi: 10.1046/j.1365-2958.1998.00740.x. [DOI] [PubMed] [Google Scholar]
- 20.Persson C, Carballeira N, Wolf-Watz H, Fällman M. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130Cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. EMBO J. 1997;16:2307–2318. doi: 10.1093/emboj/16.9.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reid T, Furayashiki T, Ishizaki T, Watanabe G, Watanabe N, Fujisawa K, Morii N, Madaule P, Narumiya S. Rhotekin, a new putative target for Rho bearing homology to a serine/threonine kinase, PKN, and rhophilin in the Rho-binding domain. J Biol Chem. 1996;271:13556–13560. doi: 10.1074/jbc.271.23.13556. [DOI] [PubMed] [Google Scholar]
- 22.Ridley A. Rho. In: Hall A, editor. GTPases. Oxford, United Kingdom: Oxford University Press; 2000. pp. 89–136. [Google Scholar]
- 23.Ruckdeschel K, Machold J, Roggenkamp A, Schubert S, Pierre J, Zumbihl R, Liautard J-P, Heesemann J, Rouot B. Yersinia enterolitica promotes deactivation of macrophage mitogen-activated protein kinases extracellular signal-regulated kinase-1/2, p38, and c-Jun NH2-terminal kinase. J Biol Chem. 1997;272:15920–15927. doi: 10.1074/jbc.272.25.15920. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt G, Aktories K. Rho GTPase-activating toxins: cytotoxic necrotizing factors and dermonecrotic toxin. In: Balch W E, Der C J, Hall A, editors. Regulators and effectors of small GTPases. San Diego, Calif: Academic Press; 2000. pp. 125–136. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt G, Sehr P, Wilm M, Selzer J, Mann M, Aktories K. Gln63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor 1. Nature. 1997;387:725–729. doi: 10.1038/42735. [DOI] [PubMed] [Google Scholar]
- 26.Sekine A, Fujiwara M, Narumiya S. Asparagine residue in the rho gene product is the modification site for botulinum ADP-ribosyltransferase. J Biol Chem. 1989;264:8602–8605. [PubMed] [Google Scholar]
- 27.von Pawel-Rammingen U, Telepnev M V, Schmidt G, Aktories K, Wolf-Watz H, Rosqvist R. GAP activity of the Yersinia YopE cytotoxin specifically targets the Rho pathway: a mechanism for disruption of actin microfilament structure. Mol Microbiol. 2000;36:737–748. doi: 10.1046/j.1365-2958.2000.01898.x. [DOI] [PubMed] [Google Scholar]
- 28.Zumbihl R, Aepfelbacher M, Andor A, Jacobi C A, Ruckdeschel K, Rouot B, Heesemann J. The cytotoxin YopT of Yersinia enterocolitica induces modification and cellular redistribution of the small GTP-binding protein RhoA. J Biol Chem. 1999;274:29289–29293. doi: 10.1074/jbc.274.41.29289. [DOI] [PubMed] [Google Scholar]