Abstract
Background
Methicillin-resistant Staphylococcus aureus (MRSA) is a difficult to treat infection, particularly in residents of elderly care centers (ECCs). Despite the substantial burden of MRSA, an inadequate number of studies have analyzed MRSA prevalence in ECCs.
Objectives
We conducted a worldwide systematic review and meta-analysis on the prevalence and risk factors of MRSA in ECCs.
Methods
We searched MEDLINE/PubMed, EMBASE, Web of Science, and Scopus databases and the gray literature sources for all studies published between January 1980 and December 2022 on the prevalence of MRSA in ECCs. A random-effects model was utilized to estimate pooled prevalence rates at 95% confidence intervals (CI). Moreover, the data were analyzed based on World Health Organization-defined regions, income, and human development index levels.
Results
In total, 119 studies, including 164,717 participants from 29 countries, were found eligible for meta-analysis. The pooled global prevalence of MRSA was 14.69% (95% CI 12.39–17.15%; 16,793/164,717). Male gender [prevalence ratio (PR) = 1.55; 95% CI 1.47–1.64], previous MRSA infection (PR = 3.71; 95% CI 3.44–4.01), prior use of antibiotics (PR = 1.97; 95% CI 1.83–2.12), hospitalized within the previous year (PR = 1.32; 95% CI 1.20–1.45), have had any wound (PR = 2.38; 95% CI 2.23–2.55), have used urinary catheter (PR = 2.24; 95% CI 2.06–2.43), have used any medical device (PR = 1.78; 95% CI 1.66–1.91), and those with diabetes (PR = 1.55; CI 1.43–1.67) were more likely to be colonized by MRSA than other patients.
Conclusion
Screening programs and preventive measures should target MRSA in ECCs due to the high global prevalence rates.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13756-023-01210-6.
Keywords: Methicillin-resistant Staphylococcus aureus, Residential facilities, Nursing homes, Long-term care, Systematic review
Background
The improvement of lifestyle and medical care, and declining birth rates in recent decades, have led to a rapid increase in life expectancy and the mean age of the population, especially in developed countries [1]. According to the World Health Organization (WHO) report, by 2050, it is estimated that almost more than a quarter of the world's population will be over 60 years old [2]. The number of elderly is expected to reach 1.4 billion by 2030 and 2.1 billion by 2050 [3, 4]. It is evident that a considerable portion of seniors will need intensive care, while the majority of them merely need daycare facilities [5, 6]. Therefore, the exponential need for institutions providing long-term care facilities, nursing homes, and residential care homes (all defined as elderly care centers (ECCs) in this study) is anticipated [7].
Multidrug-resistant organisms (MDROs) are among the leading causes of morbidity and mortality in ECCs [8, 9]. Elderly at ECCs are prone to colonization/infection with MDROs mainly due to age-associated morbidities (i.e., cognitive disorders), perpetual living in a confined and crowded area, prolonged and recurrent use of broad-spectrum antibiotics, and frequent referral to and from acute-care hospitals [10–12]. One of the most prevalent MDROs in ECCs is methicillin-resistant Staphylococcus aureus (MRSA) [8, 13, 14]. MRSA is a global health-threatening organism in healthcare settings, as it is resistant to antibiotics making the treatment more complex [15]. MRSA infection could be responsible for fatal sepsis, pneumonia, and higher rates of myocardial infarction and heart failure in patients with bacteremia [16]. A national cohort study conducted in the United States indicated that MRSA colonization among community adults aged 40–85 is associated with a significantly increased mortality risk (hazard ratio, 1.75; 95% CI 1.12–2.73) [17]. Additionally, the attributable cost of MRSA among hospitalized individuals (≥ 65 years) is estimated to be 22,293 $ per patient (95% CI 19,101–24,485$) in the United States [18]. Another cohort study in Chinese nursing homes showed that MRSA colonization was an independent risk factor for 2 year infection-related mortality (hazard ratio, 1.96; 95% CI 1.01–3.78) [19].
Considering the significant toll of MRSA, monitoring the extent of colonization, and identifying the key risk factors of MRSA acquisition in ECCs is essential for controlling and reducing the burden of this disease in ECCs residents. In the past years, a growing body of epidemiological literature evaluated the prevalence rate of MRSA colonization in ECCs from various countries; nevertheless, there is no comprehensive study to estimate global and regional prevalence rates. To bridge this gap, we performed a systematic review and meta-analysis to evaluate the worldwide prevalence and identify potential determinants of MRSA colonization in the elderly living in ECCs.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines to perform and report this systematic review and meta-analysis [20] and registered it in PROSPERO (CRD42021291492).
Data source and searching strategies
Two authors (A.H.H and A.A.) independently searched MEDLINE/PubMed, EMBASE, Web of Science collection, and Scopus databases on December 20, 2021, for articles published since January 1, 1980. The search was updated for twice; first, on February 15, 2022, and second, on December 15, 2022. Moreover, grey literature was searched through manual inspections of bibliographies of retrieved studies and internet searches of Google and Google Scholar. We applied the following search terms: [(“Staphylococcus aureus” OR "methicillin resistant Staphylococcus aureus" OR "MRSA" OR "multidrug-resistant organisms" OR "methicillin resistance" OR “antibiotic-resistant infections” OR “antibiotic-resistant bacteria”) AND ("old age homes" OR "Nursing Homes" OR "homes for the aged" OR "residential facilities" OR “Residential Care Homes” OR "housing for the elderly" OR "Long term care facility") AND (“prevalence” OR “epidemiology” OR “incidence”)] (Additional file 1: Fig. S1). The search syntax was modified according to the properties of each database. We included studies in all languages, and those with languages unknown to the study team were translated into English using the “Google Translate” online tool. All articles were imported into EndNote reference manager software X8 (Thompson and Reuters, Philadelphia, USA), and duplicates were removed.
Inclusion and exclusion criteria
Two authors (A.H.H. and M.A.) independently screened titles and abstracts of retrieved citations to identify eligible studies that met the following inclusion criteria: (i) presented data on prevalence or incidence of MRSA in ECCs; (ii) reported data on the elderly (minimum age wasn’t specified, we accepted definition of the included studies for elderlies in ECCs); (iii) included at least 30 tested elderlies. Only the last report was included in multiple sequential articles that were generated from the same data set (e.g., cohort studies). In clinical trials, we only extracted baseline data. Articles were excluded if they were (i) performed only on MRSA patients; (ii) performed on elderly in community or hospitals; (iii) included only a specific group of elderly with a specific disease or situation; (iv) used datasets that overlapped with other articles; (v) studies following the MRSA outbreaks in ECCs; (vi) case series, case reports, and articles without original data such as reviews or systematic reviews, comments, editorials, and corresponding letters.
Data extraction and quality assessment
After screening the articles, the required data from each eligible study were extracted and imported into a standardized Microsoft Excel spreadsheet (version 2016; Microsoft Corporation, Redmond, USA). The following data were extracted from each study: name of the first author, year of publication, start and end year of study, country, type of ECCs, study design, body sampling sites, diagnostic methods for MRSA detection, age (mean and range) of tested elderlies, the total number of tested participants, the total number of participants tested positive for Staphylococcus aureus, methicillin-sensitive Staphylococcus aureus (MSSA), and MRSA. We stratified countries according to WHO-defined regions [17], World Bank's income category [21], gross national income per capita [22], and the human development index (HDI) [23]. Furthermore, to evaluate the main risk factors of MRSA prevalence, we extracted data (if available in individual studies) of gender, prior antibiotics use, prior MRSA infection, prior hospitalization, having any wound, urinary catheter, use of any medical device, diabetes, antacid use, and dementia. To conduct the quality assessment and the risk of bias in included studies, we used the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for studies that reported prevalence data [24]. The detailed items of JBI tools are presented in Additional file 1: Table S1.
Data synthesis and statistical analysis
Stata software version 16.0 (STATA Corp., College Station, Texas, USA) was used to perform the meta-analyses. Before pooling prevalence estimates, the variance of the raw prevalence from each included study was stabilized by using the Freeman-Tukey double arc-sine transformation [25]. The Cochran’s Q test and I2 index were used to calculate the between-studies heterogeneity [23, 26]. A P-value < 0.01 for the Cochran’s Q test and an I2 of > 75% are considered as significant and high heterogeneity, respectively [23, 26, 27]. DerSimonian and Laird random-effects model (REM) was used in case of high heterogeneity, to conservatively estimate the pooled prevalence of MRSA at 95% confidence intervals (CIs) [25, 28]. We estimated the prevalence in individual countries by synthesizing the prevalence rates of all studies from the same country. Further, we calculated the prevalence rates of MRSA for the WHO-defined regions by synthesizing the data for countries within the same region [29]. We did not assess publication bias, as in prevalence studies, there were no group comparisons or hypothesis tests of “treatment effect” [30].
We performed subgroup and meta-regression analysis to identify sources of heterogeneity, determine the risk factors and the effects of socio-demographic factors on prevalence rates. Subgroup analyses were undertaken according to WHO-defined regions, income and HDI levels, type of diagnostic methods, risk of bias, and key determinants. Corresponding prevalence ratios (PRs) were estimated for variables subjected to subgroup analysis. Meta-regressions were performed on publication year, sample size, income and HDI levels.
Results
Characteristics of the included studies
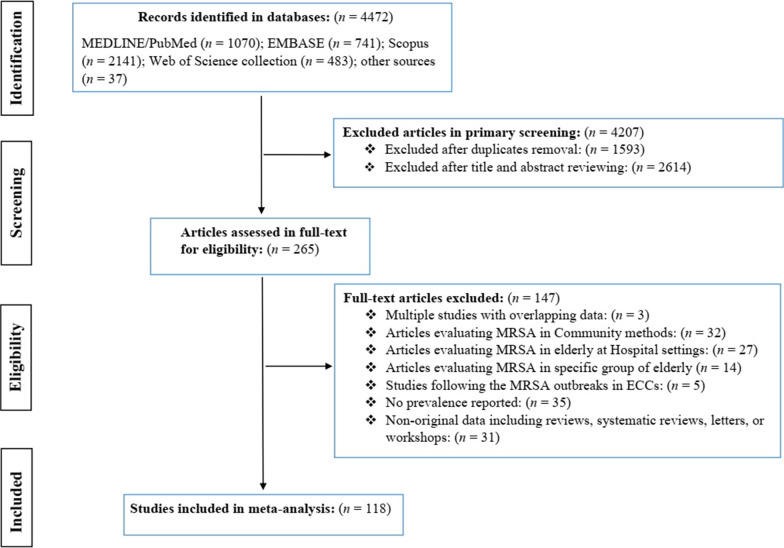
As outlined in Fig. 1, a total of 4472 articles were identified in our initial literature search, of these 118 articles (119 studies) involving 164,717 participants from 29 countries were included in the meta-analysis. The main characteristics of the included studies are shown in Additional file 1: Table S1. Studies included were published between 1990 and 2022. Twenty-six studies had data to determine the proportion of MRSA and MSSA. Overall, eligible studies were available for five WHO-defined regions; 65 studies were from European region, 34 from the region of the Americas, 18 studies from the Western Pacific region, and one study for each of the Eastern Mediterranean and African regions. Countries with the most eligible studies were the United States (31 studies), China (12 studies), Germany (10 studies), the United Kingdom (eight studies), and Italy (six studies). Regarding study designs, 88 studies were cross-sectional, 21 studies were prospective cohort, seven studies were randomized controlled trial (RCT), and three studies were case control. Regarding the location of studies, 71, 41, and 7 studies were performed in nursing homes, long-term care facilities, and residential care homes, respectively. Considering the risk of bias, 68 and 51 studies were determined as the low and moderate risk of biases. All studies used culture-based methods to determine the MRSA prevalence, and 54 performed further analyses using molecular methods. Additional information is presented in Tables 1 and 2.
Fig. 1.
The PRISMA diagram of the study selection
Table 1.
Global and regional pooled prevalence rates of MRSA among elderly living in ECCs; results from 119 studies performed in 30 countries
| WHO regions*/country | Number of datasets | Number of individuals screened (total) | Number of individuals with MRSA | Pooled prevalence % (95% CI) |
|---|---|---|---|---|
| Global | 119 | 164,717 | 16,793 | 14.69 (12.39–17.15) |
| Americas | 34 | 86,065 | 7508 | 22.27 (15.56–29.79) |
| United States | 31 | 21,457 | 5517 | 23.78 (19.12–28.77) |
| Brazil | 2 | 526 | 71 | 13.11 (10.34–16.14) |
| Canada | 1 | 64,082 | 1920 | 3.01 (2.87–3.13) |
| Western Pacific Region | 18 | 15,419 | 2936 | 16.57 (11.70–22.10) |
| China | 12 | 11,337 | 2101 | 18.07 (11.18–26.17) |
| Japan | 3 | 354 | 32 | 8.81 (5.99–12.08) |
| Singapore | 2 | 3613 | 785 | 21.72 (20.39–23.08) |
| Australia | 1 | 115 | 18 | 15.65 (9.55–23.60) |
| European region | 65 | 62,893 | 6319 | 10.93 (8.56–13.55) |
| Germany | 10 | 10,857 | 432 | 4.67 (2.59–7.29) |
| United Kingdom | 8 | 8633 | 2037 | 18.66 (12.07–26.28) |
| Belgium | 7 | 13,508 | 1403 | 8.97 (4.94–14.03) |
| Italy | 6 | 1710 | 246 | 16.34 (10.23–23.52) |
| Spain | 5 | 2700 | 460 | 15.45 (9.50–22.52) |
| Sweden | 4 | 1122 | 139 | 4.52 (0.01–33.07) |
| Netherlands | 3 | 5841 | 261 | 6.63 (0.01–35.64) |
| France | 3 | 1500 | 121 | 13.89 (3.70–29.01) |
| Israel | 3 | 545 | 68 | 14.82 (5.28–27.95) |
| Switzerland | 3 | 12,128 | 805 | 13.15 (7.08–20.70) |
| Finland | 1 | 213 | 2 | 0.94 (0.11–3.35) |
| Ireland | 2 | 818 | 82 | 9.46 (7.51–11.61) |
| Poland | 2 | 248 | 61 | 22.18 (17.16–27.62) |
| Slovenia | 2 | 209 | 22 | 10.50 (6.62–15.09) |
| Greece | 1 | 227 | 33 | 14.54 (10.22–19.81) |
| Croatia | 1 | 877 | 62 | 7.07 (5.46–8.97) |
| Georgia | 1 | 56 | 8 | 14.29 (6.38–26.22) |
| Austria | 1 | 500 | 0 | 0.01 (0.00–0.74) |
| Luxembourg | 1 | 954 | 69 | 7.23 (5.67–9.06) |
| Turkey | 1 | 247 | 8 | 3.24 (1.41–6.28) |
| African region | 1 | 152 | 13 | 8.55 (4.63–14.18) |
| South Africa | 1 | 152 | 13 | 8.55 (4.63–14.18) |
| Eastern Mediterranean | 1 | 188 | 17 | 9.04 (5.36–14.08) |
| Saudi Arabia | 1 | 188 | 17 | 9.04 (5.36–14.08) |
*The WHO regions are sorted based on prevalence rates, and countries are sorted based on the number of datasets
Table 2.
Prevalence estimates for the MRSA in the elderly, according to the study characteristics and socio-demographic factors
| Variable subgroup | Number of datasets | Number of elderlies screened (total) | Number of elderlies with MRSA | Pooled prevalence % (95% CI) |
|---|---|---|---|---|
| Income | ||||
| Upper middle | 16 | 12,071 | 2193 | 16.46 (10.79–23.05) |
| High | 103 | 152,646 | 14,600 | 14.42 (11.98–17.04) |
| HDI | ||||
| High | 15 | 12,015 | 2185 | 16.60 (10.74–23.42) |
| Very high | 104 | 152,702 | 14,608 | 14.42 (11.99–17.02) |
| Type of setting | ||||
| Long-term care facilities | 41 | 88,088 | 5069 | 16.29 (12.29–20.71) |
| Nursing homes | 71 | 69,652 | 10,730 | 14.35 (11.50–17.44) |
| Residential care homes | 7 | 6977 | 994 | 9.58 (3.62–17.93) |
| Study design | ||||
| Cross-sectional | 88 | 135,275 | 12,969 | 13.37 (10.68–16.30) |
| Prospective cohort | 21 | 23,022 | 2756 | 17.90 (12.66–23.82) |
| Case–control | 3 | 312 | 73 | 21.03 (3.67–46.99) |
| RCT | 7 | 6108 | 995 | 20.15 (12.55–29.01) |
| Publication year | ||||
| Before 2000 | 14 | 3505 | 441 | 12.84 (9.24–16.93) |
| 2001–2010 | 27 | 33,541 | 4365 | 16.12 (11.43–21.44) |
| 2011–2022 | 78 | 127,671 | 11,987 | 14.53 (11.58–17.75) |
| Risk of bias | ||||
| Low | 68 | 151,473 | 14,489 | 13.06 (10.26–16.15) |
| Moderate | 51 | 13,244 | 2304 | 17.11 (13.64–20.87) |
Prevalence of MRSA colonization in ECCs
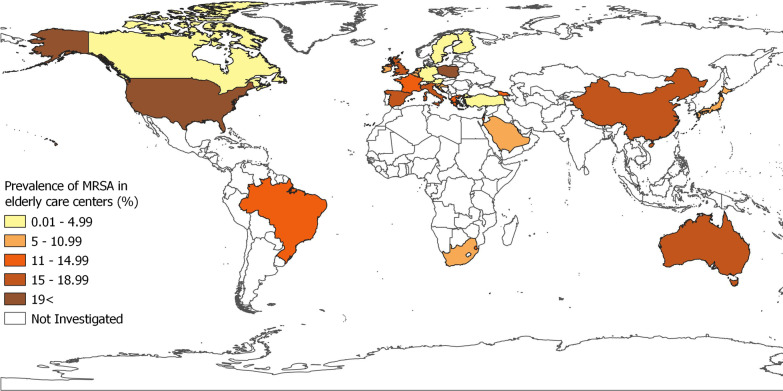
Table 2 presents the national and regional pooled prevalence of MRSA in residents of ECCs. The pooled global prevalence was 14.69% (95% CI 12.39–17.15%; 16,793/164,717) by using REM, with high heterogeneity across 119 studies (χ2 = 18,637.54, P < 0.001, I2 = 99.3%). According to WHO-defined regions, pooled prevalence rates (at 95% CI) were: 22.27% (15.56–29.79%) in the region of the Americas, 16.57% (11.70–22.10%) in the Western Pacific, 10.93% (8.56–13.55%) in Europe. Only one study was available for each of the Eastern Mediterranean and African regions, indicating prevalence rates of 8.55% (4.63–14.18%) and 9.04% (5.36–14.08%), respectively. For countries with two or more available studies, United States (23.78%), Singapore (22.72%), Poland (22.18%), United Kingdom (18.66%), China (18.07%), Italy (16.34%), Spain (15.45%), Israel (14.82%), France (13.89%) and Switzerland (13.15%) exhibited almost the highest prevalence rates (Fig. 2). Analyzing the data on the proportion of MRSA and MSSA in 26 studies using REM showed that 26% (18–36%) of all S. aureus isolates were MRSA (Additional file 1: Fig. S2A).
Fig. 2.
Worldwide distribution of MRSA colonization in ECCs
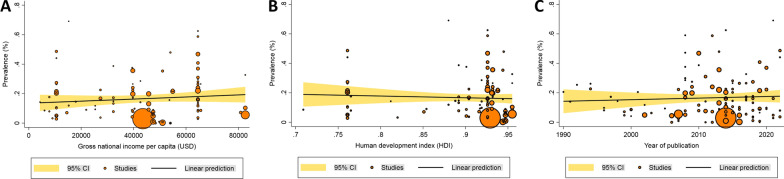
Subgroup analyses of income and HDI levels yielded relatively similar results; prevalence rate for countries with upper-middle income and high HDI levels was 16.5%, and for countries with high income and very high HDI levels was 14.4% (see Table 2). Meta-regression analyses indicated a non-significant increasing trend in prevalence with higher income [coefficient (C) = 0.000001; P = 0.259], and HDI values (C = -0.117; P = 0.573) (Fig. 3A, B). According to the type of ECCs, pooled prevalence rates of MRSA were 16.29% (12.29–20.71%), 14.35% (11.50–17.44%), and 9.85% (3.62–17.93%) for the elderly living in long-term care facilities, nursing homes, and residential care homes, respectively. Considering study designs, the lowest and highest prevalence rates were observed in studies with cross-sectional (13.37%, 10.68–16.30%) and RCT (20.15%, 12.55–29.01%) designs, respectively. Studies published after year of 2000 showed non-significant increasing prevalence rates (C = 0.001; P = 0.547) (Table 2 and Fig. 3C). Studies with low risk of bias (13.06%, 10.26–16.15%) showed lower prevalence rates than those with moderate risk of bias (17.11%, 13.64–20.87%) (Table 2).
Fig. 3.
Meta-regression analyses of MRSA prevalence among elderly living in ECCs concerning A Country's gross national income per capita, B HDI level and C Publication year
Risk factors associated with MRSA colonization in residents of ECCs
The analyses of key determinants of MRSA colonization in elderly living in ECCs showed that MRSA is more colonized in males than females (PR = 1.55; 95% CI 1.47–1.64). With regard to other risk factors, we found that elderly with a previous history of MRSA infection (PR = 3.71; 95% CI 3.44–4.01), prior use of antibiotics (PR = 1.97; 95% CI 1.83–2.12), history of hospitalization within the previous year (PR = 1.32; 95% CI 1.20–1.45), those with any wound (PR = 2.38; 95% CI 2.23–2.55), those who have used urinary catheter (PR = 2.24; 95% CI 2.06–2.43), those who have used any medical device (PR = 1.78; 95% CI 1.66–1.91), and those with diabetes (PR = 1.55; 95% CI 1.43–1.67) were more likely to be colonized by MRSA than other patients (Table 3). Other risk factors such as antacid use and dementia were not significantly associated with an increased risk of MRSA colonization (P-value > 0.05). More information is presented in Table 3.
Table 3.
Risk factors associated with MRSA colonization in elderly living in ECCs
| Variable subgroup | Number of datasets | Number of elderlies screened (total) | Number of elderlies with MRSA | Pooled prevalence % (95% CI) | Prevalence ratio (95% CI) |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 32 | 9149 | 2164 | 18.41 (13.14–24.30) | 1.55 (1.47–1.64) |
| Female | 32 | 15,572 | 2387 | 15.18 (10.81–20.12) | 1 |
| Prior antibiotics use | |||||
| Yes | 21 | 6746 | 1450 | 24.59 (17.82–32.03) | 1.97 (1.83–2.12) |
| No | 21 | 9492 | 1031 | 13.61 (9.67–18.09) | 1 |
| Prior MRSA infection | |||||
| Yes | 11 | 1430 | 669 | 49.86 (35.87–63.86) | 3.71 (3.44–4.01) |
| No | 11 | 10,044 | 1264 | 17.76 (11.25–25.37) | 1 |
| Hospitalization in past year | |||||
| Yes | 19 | 3149 | 559 | 21.34 (14.30–29.29) | 1.32 (1.20–1.45) |
| No | 18 | 6907 | 926 | 16.90 (10.99–23.76) | 1 |
| Any wound | |||||
| Yes | 24 | 4003 | 1056 | 27.03 (19.62–35.10) | 2.38 (2.23–2.55) |
| No | 24 | 15,439 | 1706 | 12.41 (9.01–16.27) | 1 |
| Urinary catheter | |||||
| Yes | 20 | 2949 | 661 | 19.59 (14.72–24.93) | 2.24 (2.06–2.43) |
| No | 20 | 15,656 | 1565 | 10.86 (7.44–14.83) | 1 |
| Any device | |||||
| Yes | 16 | 5413 | 1274 | 30.09 (19.43–41.85) | 1.78 (1.66–1.91) |
| No | 16 | 9715 | 1279 | 16.07 (10.97–21.91) | 1 |
| Diabetes | |||||
| Yes | 19 | 4990 | 870 | 17.11 (11.48–23.52) | 1.55 (1.43–1.67) |
| No | 19 | 13,734 | 1543 | 14.74 (9.69–20.60) | 1 |
| Antacid use | |||||
| Yes | 3 | 1364 | 72 | 8.95 (1.98–19.36) | 1.02 (0.76–1.38) |
| No | 3 | 1966 | 101 | 5.01 (4.06–6.03) | 1 |
| Dementia | |||||
| Yes | 5 | 1792 | 325 | 15.93 (7.93–25.97) | 1.04 (0.93–1.17) |
| No | 5 | 5243 | 994 | 15.08 (9.36–21.84) | 1 |
Discussion
MRSA infection continues to sustain as a major public health threat worldwide, especially in the elderly. In the present study, for the first time, we assembled data from all available studies (over 40 years) that had reported the prevalence of MRSA in ECCs. Among the key findings was the high pooled prevalence of MRSA colonization in the residents of ECCs (14.69%, 12.39–17.15%); which is over tenfold higher than the MRSA colonization rate among the general community (1.3%, 1.04–1.53%) [31]. Furthermore, the estimated colonization rate in our study is higher than those reported from other high-risk groups such as HIV + patients (7.0%, 5.0–9.0%) [32], hemodialysis patients (6.2%, 4.2–8.5%) [33], and patients admitted to intensive care units (7.0%, 5.8–8.3%) [34]. This highlights that elderly residents of ECCs are at very high risk for MRSA colonization, maybe due to cross-transmission between the elderlies in the crowded situation of ECCs or introduction of infection when admitting new elders from outside of ECCs (i.e., hospitals or community) [35]. Other possible explanations for this high prevalence of MRSA could be frailty, impaired immune system function, frequent hospitalization, and overuse of antibiotics [36, 37]. All the above-mentioned reasons underline the significance of recognizing contributing factors, and treating MRSA to reduce and prevent the spread of the disease.
Our results showed that the geographic distribution of MRSA colonization is heterogeneous, with the highest and lowest colonization rates reported in countries in regions of the Americas (22.27%) and Europe (10.93%), respectively. This finding is in accordance with previous meta-analysis studies in other high-risk populations [32–34]. Spatial variations of MRSA prevalence could be explained by differences in numerous demographic information among the countries studied and different ECCs in a country such as policies for prescription of antibiotics, various infection prevention programs, different education and training of staff and elderly for personal hygiene, different structure of health care systems, and facilities for MRSA diagnosis [32, 38–41]. Our study also highlighted a significant data gap in less developed countries, most strikingly in Latin America, Africa, Eastern Mediterranean, Central Asia, and South-East of Asia regions, where more data are required to obtain accurate estimates of the prevalence of MRSA colonization in elderly residents of ECCs. It should be noted that underdeveloped or developing countries may not have well-established elderly care systems or antibiotic stewardship programs. Due to the increasing number of ECCs in developing countries [42], these information gaps should be addressed through future representative epidemiological studies.
Our findings suggested different prevalence rates of MRSA colonization in nursing homes (14.35%), long-term care facilities (16.29%), and residential care homes (9.58%). The discrepancies in prevalence rates between different types of ECCs may stem from the differences in services rendered by each type of center. In nursing homes, patients usually receive daily or constant professional nursing care, and antibiotics can be prescribed with the consultation of a physician [43], therefore, antimicrobial overuse/abuse is prevalent [43]. In contrast, residential care centers are mostly restricted to personal care [44]. Despite monitoring schemes in hospitals for antibiotic use, records for the nursing homes environment are scarce [9]. Excessive use of antibiotics is one contributing factor to the increase in antibiotic resistance and the growth in the emergence of MDROs, which should be addressed in ECCs.
Our findings identified several risk factors for MRSA colonization in residents of ECCs, such as being male, prior antibiotics use, previous MRSA infection, hospitalization in the past 12 months, presence of any wound, urinary catheter, usage of any invasive medical device, and diabetes. Our findings are consistent with several studies about the MRSA prevalence in the community or high-risk populations such as HIV + and hemodialysis patients [2, 31–33, 45, 46]. Although there is no consensus on the role of gender in the prevalence of MRSA, it has been asserted that elderly males are more prone to the other predisposing factors of being infected with MRSA namely more complicated diabetes and wounds, frequent use of medical devices, and catheterization which may lead to a higher prevalence MRSA [47, 48]. Furthermore, frequent use of antibiotics is one of the main cause for the development of antimicrobial resistance in staphylococci and other bacteria [46, 49]. A previous MRSA infection could lead to re-infection secondary to a lack of proper eradication or stable colonization [45]. The constant presence of high-risk individuals in hospitals and routine antibiotics use justify the higher prevalence of MRSA in elders who were hospitalized in the last 12 months [34]. Medical devices make patients susceptible to MRSA through the mechanism of biofilm formation on the devices and subsequent detachment, which may contribute to bacteremia or sepsis [50]. A wide range of chronic diseases like diabetes renders the patients prone to MRSA given the state of immunosuppression and more complicated wounds [51, 52]. Similar to our findings, chronic illnesses, intravenous drug use, and contact with infected individuals are reported as risk factors for community-acquired MRSA [31]. These findings have implications for policymakers in identifying high-risk groups to reduce the disease burden by employing targeted interventions.
Our findings in this comprehensive systematic review and meta-analysis have implications for future research and clinical practices. However, this study has identified several shortcomings of the current data on MRSA colonization among the elderly in ECCs. First, our study contains reports from only 30 of ~ 200 countries globally; there were no country-level estimates for some countries, and no prevalence data were available from many countries. In addition, most of the available studies had a paucity of data on critical variables such as gender, age, ethnicity, and risk factors associated with MRSA colonization. Therefore, some non-significant results considering risk factors might be due to the low number of studies. For example, while among those who took antacids (as shown in Table 3) almost double MRSA (8.95%) has been estimated, the prevalence ratio was non-significant. Second, the studies included had different qualities and studies with lower qualities (17.11%) showed higher colonization rates than high quality studies (13.06%) which highlight the need for more robust surveys of MRSA colonization in residents of ECCs. Third, as expected in meta-analyses on prevalence studies [25, 29, 53–55], a substantial heterogeneity was found in our analyses that could not be explained by subgroup analyses. The sources of heterogeneity are likely related to study characteristics, including differences in geographical distribution, study design, sample size, diagnostic methods, leading to differences in the quality and performance of these methods. Moreover, this heterogeneity suggests that local risk factors and transmission routes of MRSA are varied in different geographical regions, countries, or even in individual ECCs.
Conclusion
In conclusion, this study found that the prevalence of MRSA colonization in residents of ECCs is high across the world and varies by gender and geographic location. Moreover, elders with previous MRSA infection, hospitalization, antibiotics and diabetes, and those that used medical devices are more prone to MRSA colonization. This sheds significant light on the essence of targeted interventions and screening programs to locate infected people, control risk factors, and reduce the transmission of MRSA and other MDROs to elderlies living in ECCs. Given the aging trend of the populations, there is an urgent need for studies, particularly in developing and underdeveloped countries to better estimate the risk of disease. We recommend several interventions to reduce the MRSA burden in ECCS. For instance, reducing the consumptions of antibiotics (especially fluoroquinolones and third generation cephalosporins), enhanced barrier precautions, chlorhexidine bathing, routine MDROs surveillance, and isolation of MRSA-infected patients in single rooms. Additionally, educating and training the elderly and health workers in personal hygiene should be promoted.
Supplementary Information
Additional file 1. Supplematary tables and figures.
Acknowledgements
The authors would like to thank to Health Research Institute of Babol University of Medical Sciences for their supports.
Author contributions
AHH and AR conceived the study. AHH, AR, MA, AA, AM and SRH conducted the searches, and collected data. AHH, MS, MA, AM and MJ analysed the data sets and interpreted the results. AHH, AM, AA, MB and AR drafted and edited the manuscript. All authors commented on, or edited, drafts and approved the final version of the manuscript.
Funding
No funding.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Suzman R, Beard JR, Boerma T, Chatterji S. Health in an ageing world—what do we know? Lancet. 2015;385(9967):484–486. doi: 10.1016/S0140-6736(14)61597-X. [DOI] [PubMed] [Google Scholar]
- 2.Wong JW, Ip M, Tang A, Wei VW, Wong SY, Riley S, Read JM, Kwok KO. Prevalence and risk factors of community-associated methicillin-resistant Staphylococcus aureus carriage in Asia-Pacific region from 2000 to 2016: a systematic review and meta-analysis. Clin Epidemiol. 2018;10:1489–1501. doi: 10.2147/CLEP.S160595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naja S, Din Makhlouf MME, Chehab MAH. An ageing world of the 21st century: a literature review. Int J Community Med Public Health. 2017;4(12):4363–4369. [Google Scholar]
- 4.Tarakcı E, Zenginler Y, Kaya Mutlu E. Chronic pain, depression symptoms and daily living independency level among geriatrics in nursing home. Agri. 2015;27(1):35–41. doi: 10.5505/agri.2015.14238. [DOI] [PubMed] [Google Scholar]
- 5.McClean P, Hughes C, Tunney M, Goossens H, Jans B, Group obotESoACNHP. Jans B, Stroobants R, Goossens H, Budimir A, et al. Antimicrobial prescribing in European nursing homes. J Antimicrob Chemother. 2011;66(7):1609–1616. doi: 10.1093/jac/dkr183. [DOI] [PubMed] [Google Scholar]
- 6.Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374(9696):1196–1208. doi: 10.1016/S0140-6736(09)61460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aschbacher R, Pagani E, Confalonieri M, Farina C, Fazii P, Luzzaro F, Montanera PG, Piazza A, Pagani L. Review on colonization of residents and staff in Italian long-term care facilities by multidrug-resistant bacteria compared with other European countries. Antimicrob Resist Infect Control. 2016;5:33. doi: 10.1186/s13756-016-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassone M, Mody L. Colonization with multidrug-resistant organisms in nursing homes: scope, importance, and management. Curr Geriatr Rep. 2015;4(1):87–95. doi: 10.1007/s13670-015-0120-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tinelli M, Tiseo G, Falcone M. Prevention of the spread of multidrug-resistant organisms in nursing homes. Aging Clin Exp Res. 2021;33(3):679–687. doi: 10.1007/s40520-020-01746-2. [DOI] [PubMed] [Google Scholar]
- 10.Gruber I, Heudorf U, Werner G, Pfeifer Y, Imirzalioglu C, Ackermann H, Brandt C, Besier S, Wichelhaus TA. Multidrug-resistant bacteria in geriatric clinics, nursing homes, and ambulant care–prevalence and risk factors. Int J Med Microbiol. 2013;303(8):405–409. doi: 10.1016/j.ijmm.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Lim CJ, Cheng AC, Kennon J, Spelman D, Hale D, Melican G, Sidjabat HE, Paterson DL, Kong DC, Peleg AY. Prevalence of multidrug-resistant organisms and risk factors for carriage in long-term care facilities: a nested case–control study. J Antimicrob Chemother. 2014;69(7):1972–1980. doi: 10.1093/jac/dku077. [DOI] [PubMed] [Google Scholar]
- 12.Dyar OJ, Pagani L, Pulcini C. Strategies and challenges of antimicrobial stewardship in long-term care facilities. Clin Microbiol Infect. 2015;21(1):10–19. doi: 10.1016/j.cmi.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Budimir A, Pal MP, Bošnjak Z, Mareković I, Vuković D, Križan IR, Milas J, Plečko V, Kalenić S. Prevalence and molecular characteristics of methicillin-resistant Staphylococcus aureus strains isolated in a multicenter study of nursing home residents in Croatia. Am J Infect Control. 2014;42(11):1197–1202. doi: 10.1016/j.ajic.2014.07.032. [DOI] [PubMed] [Google Scholar]
- 14.Lasseter G, Charlett A, Lewis D, Donald I, Howell-Jones R, McNulty CAM. Staphylococcus aureus carriage in care homes: identification of risk factors, including the role of dementia. Epidemiol Infect. 2010;138(5):686–696. doi: 10.1017/S0950268810000233. [DOI] [PubMed] [Google Scholar]
- 15.Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 16.Inagaki K, Lucar J, Blackshear C, Hobbs CV. Methicillin-susceptible and methicillin-resistant Staphylococcus aureus bacteremia: nationwide estimates of 30-day readmission, in-hospital mortality, length of stay, and cost in the United States. Clin Infect Dis. 2019;69(12):2112–2118. doi: 10.1093/cid/ciz123. [DOI] [PubMed] [Google Scholar]
- 17.Mainous AG, Rooks BJ, Carek PJ. Methicillin-resistant Staphylococcus aureus colonization and mortality risk among community adults aged 40–85. J Am Board Fam Med. 2021;34(2):439–441. doi: 10.3122/jabfm.2021.02.200394. [DOI] [PubMed] [Google Scholar]
- 18.Nelson RE, Hyun D, Jezek A, Samore MH. Mortality, length of stay, and healthcare costs associated with multidrug-resistant bacterial infections among elderly hospitalized patients in the United States. Clin Infect Dis. 2021;74(6):1070–1080. doi: 10.1093/cid/ciab696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan TC, Cheng VCC, Hung IFN, Chan FHW, Ng WC, Yuen KY. The association between methicillin resistant Staphylococcus aureus colonization and mortality in Chinese nursing home older adults: a 2-year prospective cohort. J Am Med Dir Assoc. 2015;9(16):796–797. doi: 10.1016/j.jamda.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. 2014;3(3):123–128. doi: 10.15171/ijhpm.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13(3):147–153. doi: 10.1097/XEB.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 25.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Pae MJ, Welch VA. Cochrane handbook for systematic reviews of interventions. New York: John Wiley & Sons; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45:139–145. doi: 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rostami A, Sepidarkish M, Leeflang MM, Riahi SM, Shiadeh MN, Esfandyari S, Mokdad AH, Hotez PJ, Gasser RB. SARS-CoV-2 seroprevalence worldwide: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27(3):331–340. doi: 10.1016/j.cmi.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunter JP, Saratzis A, Sutton AJ, Boucher RH, Sayers RD, Bown MJ. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol. 2014;67(8):897–903. doi: 10.1016/j.jclinepi.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin Infect Dis. 2003;36(2):131–139. doi: 10.1086/345436. [DOI] [PubMed] [Google Scholar]
- 32.Sabbagh P, Riahi SM, Gamble HR, Rostami A. The global and regional prevalence, burden, and risk factors for methicillin-resistant Staphylococcus aureus colonization in HIV-infected people: a systematic review and meta-analysis. Am J Infect Control. 2019;47(3):323–333. doi: 10.1016/j.ajic.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 33.Zacharioudakis IM, Zervou FN, Ziakas PD, Mylonakis E. Meta-analysis of methicillin-resistant Staphylococcus aureus colonization and risk of infection in dialysis patients. J Am Soc Nephrol. 2014;25(9):2131–2141. doi: 10.1681/ASN.2013091028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zervou FN, Zacharioudakis IM, Ziakas PD, Rich JD, Mylonakis E. Prevalence of and risk factors for methicillin-resistant Staphylococcus aureus colonization in HIV infection: a meta-analysis. Clin Infect Dis. 2014;59(9):1302–1311. doi: 10.1093/cid/ciu559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sasahara T, Ae R, Yoshimura A, Kosami K, Sasaki K, Kimura Y, Akine D, Ogawa M, Hamabata K, Hatakeyama S, et al. Association between length of residence and prevalence of MRSA colonization among residents in geriatric long-term care facilities. BMC Geriatr. 2020;20(1):481. doi: 10.1186/s12877-020-01885-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denkinger CM, Grant AD, Denkinger M, Gautam S, D'Agata EM. Increased multi-drug resistance among the elderly on admission to the hospital–a 12-year surveillance study. Arch Gerontol Geriatr. 2013;56(1):227–230. doi: 10.1016/j.archger.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Yoshikawa TT. Epidemiology and unique aspects of aging and infectious diseases. Clin Infect Dis. 2000;30(6):931–933. doi: 10.1086/313792. [DOI] [PubMed] [Google Scholar]
- 38.Kavanagh KT. Control of MSSA and MRSA in the United States: protocols, policies, risk adjustment and excuses. Antimicrob Resist Infect Control. 2019;8(1):103. doi: 10.1186/s13756-019-0550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piper Jenks N, Pardos de la Gandara M, D’Orazio BM, Correa da rosa J, Kost RG, Khalida C, Vasquez KS, Coffran C, Pastagia M, Evering TH, et al. Differences in prevalence of community-associated MRSA and MSSA among U.S. and non-U.S. born populations in six New York Community Health Centers. Travel Med Infect Dis. 2016;14(6):551–560. doi: 10.1016/j.tmaid.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borg MA, Camilleri L, Waisfisz B. Understanding the epidemiology of MRSA in Europe: Do we need to think outside the box? J Hosp Infect. 2012;81(4):251–256. doi: 10.1016/j.jhin.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Al-Orphaly M, Hadi HA, Eltayeb FK, Al-Hail H, Samuel BG, Sultan AA, Skariah S. Epidemiology of multidrug-resistant pseudomonas aeruginosa in the Middle East and North Africa Region. mSphere. 2021;6(3):e00202–00221. doi: 10.1128/mSphere.00202-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L, Qi N, Zhou Y, Zhang H. Prevention and infection control of COVID-19 in nursing homes: experience from China. Oxford: Oxford University Press; 2020. pp. 894–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnold SH, Jensen JN, Bjerrum L, Siersma V, Bang CW, Kousgaard MB, Holm A. Effectiveness of a tailored intervention to reduce antibiotics for urinary tract infections in nursing home residents: a cluster, randomised controlled trial. Lancet Infect Dis. 2021;21(11):1549–1556. doi: 10.1016/S1473-3099(21)00001-3. [DOI] [PubMed] [Google Scholar]
- 44.Netten A, Darton R, Bebbington A, Brown P. Residential or nursing home care? The appropriateness of placement decisions. Ageing Soc. 2001;21(1):3–23. [Google Scholar]
- 45.Huang SS, Platt R. Risk of methicillin-resistant Staphylococcus aureus infection after previous infection or colonization. Clin Infect Dis. 2003;36(3):281–285. doi: 10.1086/345955. [DOI] [PubMed] [Google Scholar]
- 46.Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Invest. 2003;111(9):1265–1273. doi: 10.1172/JCI18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kupfer M, Jatzwauk L, Monecke S, Möbius J, Weusten A. MRSA in a large German University Hospital: Male gender is a significant risk factor for MRSA acquisition. GMS Krankenhhyg Interdiszip 2010;5(2). [DOI] [PMC free article] [PubMed]
- 48.Walker JN, Flores-Mireles AL, Pinkner CL, Schreiber HLT, Joens MS, Park AM, Potretzke AM, Bauman TM, Pinkner JS, Fitzpatrick JAJ, et al. Catheterization alters bladder ecology to potentiate Staphylococcus aureus infection of the urinary tract. Proc Natl Acad Sci U S A. 2017;114(41):E8721–E8730. doi: 10.1073/pnas.1707572114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tenover FC. Mechanisms of antimicrobial resistance in bacteria. Am J Med. 2006;119(6):S3–S10. doi: 10.1016/j.amjmed.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 50.Zheng Y, He L, Asiamah TK, Otto M. Colonization of medical devices by staphylococci. Environ Microbiol. 2018;20(9):3141–3153. doi: 10.1111/1462-2920.14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R. Type 2 diabetes and its impact on the immune system. Curr Diabetes Rev. 2020;16(5):442–449. doi: 10.2174/1573399815666191024085838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stacey HJ, Clements CS, Welburn SC, Jones JD. The prevalence of methicillin-resistant Staphylococcus aureus among diabetic patients: a meta-analysis. Acta Diabetol. 2019;56(8):907–921. doi: 10.1007/s00592-019-01301-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwatra G, Cunnington MC, Merrall E, Adrian PV, Ip M, Klugman KP, Tam WH, Madhi SA. Prevalence of maternal colonisation with group B streptococcus: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16(9):1076–1084. doi: 10.1016/S1473-3099(16)30055-X. [DOI] [PubMed] [Google Scholar]
- 54.Rostami A, Riahi S, Gamble H, Fakhri Y, Shiadeh MN, Danesh M, Behniafar H, Paktinat S, Foroutan M, Mokdad A. Global prevalence of latent toxoplasmosis in pregnant women: a systematic review and meta-analysis. Clin Microbiol Infect. 2020;26(6):673–683. doi: 10.1016/j.cmi.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 55.Rostami A, Sepidarkish M, Fazlzadeh A, Mokdad AH, Sattarnezhad A, Esfandyari S, Riahi SM, Mollalo A, Dooki ME, Bayani M. Update on SARS-CoV-2 seroprevalence: regional and worldwide. Clin Microbiol Infect. 2021;27(12):1762–1771. doi: 10.1016/j.cmi.2021.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplematary tables and figures.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.