Abstract
Diabetic cardiomyopathy was originally described as the presence of ventricular dysfunction in the absence of coronary artery disease and/or hypertension. It is characterized by diastolic dysfunction and is more prevalent in people with diabetes than originally realized, leading to the suggestion in the field that it simply be referred to as diabetic heart disease. While there are currently no approved therapies for diabetic heart disease, a multitude of studies clearly demonstrate that it is characterized by several disturbances in myocardial energy metabolism. One of the most prominent changes in myocardial energy metabolism in diabetes is a robust impairment in glucose oxidation. Herein we will describe the mechanisms responsible for the diabetes-induced decline in myocardial glucose oxidation, and the pharmacological approaches that have been pursued to correct this metabolic disorder. With surmounting evidence that stimulating myocardial glucose oxidation can alleviate diastolic dysfunction and other pathologies associated with diabetic heart disease, this may also represent a novel strategy for decreasing the prevalence of heart failure with preserved ejection fraction in the diabetic population.
Keywords: Diabetic cardiomyopathies, Diabetic heart disease, Diastolic dysfunction, Cardiac energetics, Pyruvate dehydrogenase, Glucose oxidation
INTRODUCTION
People living with diabetes, both type 1 and type 2 (T1D/T2D), are at increased risk of cardiovascular disease, many of whom will ultimately die from either myocardial infarction (MI) or heart failure.1 Diabetes has proven to be such a powerful risk factor for cardiovascular disease that it is now viewed as a “cardiovascular risk equivalent,” whereby subjects with diabetes but without coronary heart disease have similar coronary mortality rates to nondiabetic subjects who had a previous coronary event.2 Because of this increased cardiovascular risk, there has been a significant effort invested towards elucidating the mechanisms by which diabetes contributes to cardiovascular disease, as well as understanding the cardiovascular safety profiles of glucose-lowering medications. Excitingly, the completion of numerous cardiovascular outcomes trials from 2 of the most recently approved therapies for T2D, the glucagon-like peptide-1 receptor (GLP-1R) agonists and sodium-glucose cotransporter-2 inhibitors, has demonstrated that these agents decrease death rates from cardiovascular causes in subjects with T2D.3,4
Of clinical relevance, people during the early stages of T2D are likely to have diastolic dysfunction, a defining feature of diabetic cardiomyopathy. Diabetic cardiomyopathy was first observed by Rubler et al.5 in 4 subjects (3 females/1 male) who had diabetes of at least 3 years duration with gross cardiomegaly and congestive heart failure, culminating in its original description of the presence of ventricular dysfunction in the absence of coronary artery disease and/or hypertension in a person with diabetes. Based on this description in the 1970s, diabetic cardiomyopathy was a pathological condition thought to not be highly prevalent in people with diabetes. However, the increasing recognition that diastolic dysfunction is often present in early stage T2D is shifting the consensus view in the field, whereby support for diabetic cardiomyopathy being more prevalent in people with diabetes is gaining traction. Furthermore, this diastolic dysfunction is often asymptomatic and unfortunately undiagnosed in diabetic subjects, since routine cardiovascular screening is often ignored in people with T2D until notable cardiovascular decline becomes evident.3,6 Therefore, it has been proposed that due to the significant advancements made in the 21st century regarding the pathology of diabetic cardiomyopathy, it may be time to perhaps change its definition, or re-term the condition, with the suggestion it now be referred to as “diabetic heart disease.”6 Indeed, we share that viewpoint, especially with diastolic dysfunction and diabetic cardiomyopathy being key risk factors contributing to the progression of heart failure with preserved ejection fraction (HFpEF). Because HFpEF is enriched in people with diabetes, developing therapies that can reverse the pathology of diabetic cardiomyopathy, which we will herein refer to as diabetic heart disease, may represent a potential long-term strategy to decrease HFpEF prevalence.
With improved characterization of multiple animal models of diabetes,7 our knowledge of the various mechanisms that contribute to the pathology of diabetic heart disease has greatly increased.6,8 Some of the most extensively interrogated mechanisms include oxidative stress, endoplasmic reticulum stress, microvascular dysfunction, cardiomyocyte apoptosis, cardiac lipotoxicity, and alterations in cardiac energetics that may be secondary to insulin resistance and/or mitochondrial dysfunction.6,8,9 With regards to the latter, the defining metabolic features of the myocardium in T2D include an elevation in fatty acid oxidation rates, concomitant with a decline in glucose oxidation rates (Fig. 1).6,9,10 The latter will be the primary emphasis of this review, where we will interrogate the mechanisms responsible for the decline in myocardial glucose oxidation in diabetes, and whether this may represent a pharmacological target to alleviate diabetic heart disease.
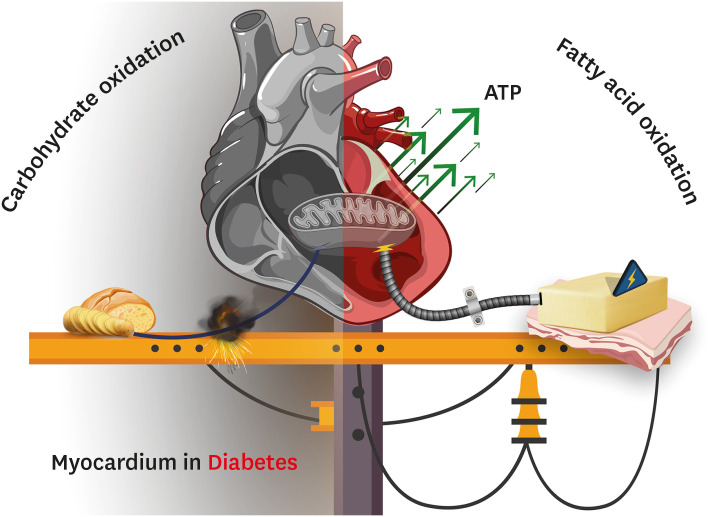
Fig. 1. Myocardial energy metabolism in diabetic heart disease. The heart in diabetes (both type 1 and type 2) experiences several perturbations in intermediary energy metabolism. In particular, the heart in an individual with diabetes produces the majority of its energy (ATP) from the mitochondrial oxidation of fatty acids (right half of the figure), which comes at the expense of a severe impairment in the mitochondrial oxidation of carbohydrates (i.e., glucose) (left half of the figure). This figure was created with BioRender.com.
MYOCARDIAL GLUCOSE OXIDATION & PYRUVATE DEHYDROGENASE (PDH) IN DIABETES
While fatty acids are the predominant fuel source of the heart, carbohydrates (i.e., glucose) are also a major fuel source for the heart, especially following nutrient ingestion and largely driven by increased insulin secretion.11 The 2 primary transporters mediating glucose uptake in cardiomyocytes are glucose transporter 1 and 4, the latter of which is insulin sensitive. Once taken up into the cardiomyocyte, glucose is rapidly phosphorylated to glucose-6-phosphate by hexokinase, which has a very high affinity for glucose. The ensuing glucose-6-phosphate can either metabolically proceed towards glycogen synthesis (energy storage), glycolysis and glucose oxidation (energy production), or the pentose phosphate pathway, though metabolism through the latter is negligible in the heart.12
Numerous preclinical models of diabetes, both T1D and T2D, result in marked declines in myocardial glucose oxidation rates. A single administration of 55 mg/kg streptozotocin (STZ) in male Sprague Dawley rats (up to 230–250 g) to induce T1D, leads to a robust decline in glucose oxidation rates assessed during aerobic isolated working heart perfusions, with glucose oxidation accounting for less than 5% of overall oxidative energy production.13 Likewise, both leptin deficient male ob/ob mice and leptin receptor deficient male db/db mice at 4-weeks and 15-weeks of age, exhibit marked declines in glucose oxidation rates assessed during aerobic isolated working heart perfusions.14 Similarly, aerobic perfusion of isolated working hearts from male C57BL/6J mice subjected to experimental obesity via provision of a high-fat diet (60% kcal from lard) for 8-weeks also revealed a marked impairment of glucose oxidation in the absence or presence of insulin.15 Finally, experimental T2D resulting from high-fat diet provision plus a single low-dose injection of STZ (25 mg/kg in rats or 75 mg/kg in mice) also produces a robust decline in myocardial glucose oxidation rates as assessed using either 13C hyperpolarized nuclear magnetic resonance (NMR) (in rats),16 or perfusion with [U-14C]glucose in isolated working hearts (in mice).17,18 13C hyperpolarized NMR studies have also identified reduced myocardial glucose oxidation rates in a mouse model of diabetes resulting from inducible expression of a Kir6.2-V59M transgene in islet β-cells that does not cause obesity or dyslipidemia.19 Conversely, saponin permeabilized myocardial biopsy samples from subjects with T2D and coronary artery disease displayed similar ADP-stimulated respiration rates with pyruvate as a substrate, when compared to subjects with coronary artery disease without T2D.20 Although reasons for these discrepancies remain unresolved, respiration rates in permeabilized fibers are not indicative of actual in vivo flux through glucose oxidation, and coronary artery disease itself already impairs glucose oxidation.21 Unfortunately, due to limitations in methodologies to assess in vivo metabolic flux in humans,22 there have been minimal studies to date exploring whether myocardial glucose oxidation rates are decreased in human subjects with diabetes. Studies using positron emission tomography imaging with 18F-fluourodeoxyglucose (FDG) have demonstrated decreased myocardial glucose uptake in obese, prediabetic women, but this does not necessarily reflect a decrease in glucose oxidation since 18F-FDG is not metabolized further once phosphorylated by hexokinase.22 However, the advancements in sensitivity afforded by 13C hyperpolarized NMR have been successfully used to measure myocardial pyruvate metabolism in human subjects,23 and provide an opportunity to confirm that myocardial glucose oxidation is impaired in diabetes. Indeed, recent studies in 13 subjects with T2D and 12 control participants fasted for at least 9 hours prior to receiving an oral glucose tolerance test (75 g glucose), observed marked reductions in myocardial glucose oxidation using 13C hyperpolarized NMR.24
Mechanisms responsible for the diabetes-mediated decline in myocardial glucose oxidation can be secondary to insulin resistance, which would result in decreased myocardial glucose uptake for subsequent energy production.25 Furthermore, the glucose-fatty acid cycle attributed to findings from Philip Randle and colleagues26 (though first reported by Shipp et al.27), whereby glucose and fatty acids compete for oxidative metabolism in the heart, represents another mechanism for the decline in glucose oxidation, as myocardial fatty acid oxidation is increased in both T1D and T2D.6,8,9,10
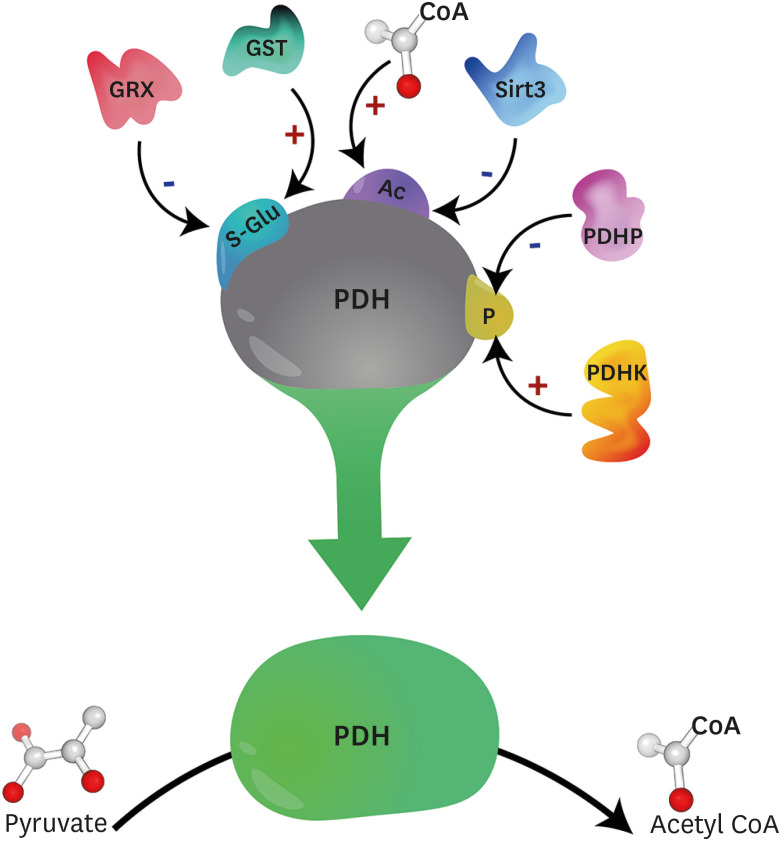
Molecular perturbations also contribute to the impaired myocardial glucose oxidation observed in diabetes, and primarily reflect decreased activity of the PDH complex, the rate-limiting enzyme of glucose oxidation, which is responsible for decarboxylating pyruvate into acetyl coenzyme A.28,29 The mammalian PDH complex is a multienzyme complex comprised of multiple copies of 6 components, which includes 3 catalytic enzymes; PDH (designated as E1), dihydrolipoamide acetyltransferase (designated as E2), and dihydrolipoamide dehydrogenase (designated as E3). The PDH complex also contains a binding protein, E3-binding protein, and 2 regulatory enzymes that maintain the phosphorylation status of PDH, PDH kinase (PDHK) and PDH phosphatase (PDHP). Our focus herein will be on the specific role of PDH (E1) in the control of myocardial glucose oxidation in diabetic heart disease, and we refer the reader to more extensive reviews dealing with the multifaceted regulation of the PDH complex.28,30 With regards to phosphorylation, PDH is primarily regulated via phosphorylation mediated inactivation by 4 isoforms of PDHK (PDHK1–4), or dephosphorylation mediated activation by 2 isoforms of PDHP (PDHP1–2) (Fig. 2).28,29 However, it is now becoming increasingly recognized that PDH is also subject to regulation via other post-translational modifications, including acetylation, which has been proposed to decrease PDH activity (Fig. 2).31 Recent studies have also demonstrated that PDH can be S-glutathionylated, which can also decrease PDH activity and impair glucose oxidation (Fig. 2).32 In addition to post-translational modifications, PDH can also be regulated via mitochondrial calcium levels similar to the dehydrogenase enzymes of the Krebs Cycle,28,29 whereby calcium increases the affinity of PDHP for phosphorylated PDH to relieve its inhibition.33 It has been observed in mice with experimental T2D via high-fat diet provision plus low-dose STZ (75 mg/kg), that protein expression of the mitochondrial calcium uniporter complex inhibitory subunit (MCUb) increases, thereby decreasing mitochondrial matrix calcium levels and reducing myocardial PDH activity.34
Fig. 2. Post-translational modifications impacting PDH activity. PDH activity is subject to numerous post-translational modifications, including phosphorylation, which is the best characterized. PDHK mediated phosphorylation inhibits PDH activity, whereas PDHP mediated dephosphorylation stimulates PDH activity. PDH may also be inactivated by acetylation, with recent studies suggesting that increases in mitochondrial acetyl CoA increase mitochondrial protein acetylation nonenzymatically, whereas Sirt3 has been demonstrated to increase PDH activity via deacetylation. Finally, PDH is also subject to S-glutathionylation mediated inactivation, though the role of GST and GRX in catalyzing and removing S-glutathionylation modifications of PDH, respectively, remains to be determined.
PDH, pyruvate dehydrogenase; PDHK, pyruvate dehydrogenase kinase; PDHP, pyruvate dehydrogenase phosphatase; CoA, coenzyme A; Sirt, sirtuin; GST, glutathione-S-transferases; GRX, glutaredoxins.
Supporting the notion that impaired myocardial PDH activity and subsequent reductions in glucose oxidation contribute to the pathology of diabetic heart disease, cardiac-specific deletion of PDH (PDHCardiac−/−) in mice produces a diabetic heart disease-like phenotype.35 Male PDHCardiac−/− mice exhibit a near complete abolishment of glucose oxidation during isolated working heart perfusion studies, and these mice also display signs of diastolic dysfunction following ultrasound echocardiography analysis (reduced E/A ratio). In addition, a robust cardiac hypertrophy is also present in male PDHCardiac−/− mice. Accordingly, it will be important for future studies to understand how these various post-translational modifications of PDH integrate during the pathology of diabetic heart disease, and which are most relevant to the decline in myocardial glucose oxidation. Acquiring such knowledge will play an essential role in identifying the most effective strategies to stimulate PDH activity, which can then be leveraged to guide drug development aimed at promoting glucose oxidation.
STIMULATING PDH ACTIVITY TO INCREASE MYOCARDIAL GLUCOSE OXIDATION AND ALLEVIATE DIABETIC HEART DISEASE
A simple yet effective approach to increase myocardial glucose oxidation is to use small molecules to activate PDH. This can be achieved by inhibiting PDHK to prevent the phosphorylation mediated inactivation of PDH, which is the molecular target of dichloroacetate (DCA).36,37 In a rat model of T2D involving high-fat diet supplementation for 7-weeks with a single dose of STZ (25 mg/kg) administered at day 12 of the protocol, treatment with DCA (dissolved in the drinking water at 1 mM) during the final 4-weeks produced salutary actions.16 13C hyperpolarized NMR was utilized to confirm that DCA increased myocardial glucose oxidation rates in male rats with T2D, which was associated with an alleviation of diastolic dysfunction as determined by a decreased E/e' ratio.
Another strategy to promote myocardial glucose oxidation is to target the transcriptional expression of the 4 Pdk isoforms, which are the genes that encode for PDHK1–4. Pdk isoforms are transcriptionally regulated by several transcription factors including peroxisome proliferator activated receptor α, estrogen related receptor α, hypoxia inducible factor 1α, and forkhead box O1 (FoxO1).38,39,40,41 Studies have demonstrated that FoxO1 is a key transcription factor regulating Pdk4 (but not Pdk1, Pdk2, or Pdk3) expression in the heart.39 Furthermore, both experimental obesity in male C57BL/6J mice, or genetic obesity in male ob/ob and db/db mice result in increased myocardial FoxO1 protein expression and activity.42 Targeting this FoxO1-PDHK4 axis has been shown to alleviate diastolic dysfunction in male C57BL/6J mice subjected to experimental T2D via high-fat diet provision for 10-weeks with STZ (75 mg/kg) administered at week 4 of the protocol.18 Indeed, treatment with the FoxO1 antagonist, AS1842856 (100 mg/kg), twice daily during the final 2-weeks of the 10-week protocol increased PDH activity and glucose oxidation rates assessed during isolated working heart perfusions. An alleviation of diastolic dysfunction was also observed during ultrasound echocardiography experiments, reflected by an increase in the mitral E/A ratio, an increase in the Tissue Doppler e'/a' ratio, and a decrease in the E/e' ratio. Confirming that the increase in myocardial glucose oxidation was required for the FoxO1 inhibition-induced protection against diabetic heart disease, AS1842856 treatment failed to alleviate diastolic dysfunction in PDHCardiac−/− mice subjected to experimental T2D. Similarly, inhibiting FoxO1 to promote PDH activity and glucose oxidation also appears beneficial in preclinical models of T1D. Male Sprague Dawley rats (up to 200–250 g) subjected to experimental T1D via administration of STZ (65 mg/kg) treated with AS1842856 (50 mg/kg twice daily) for 1-week demonstrated improved parameters of cardiac function during invasive hemodynamics with pressure-volume conductance catheters.43 Cardiomyocytes isolated from these rats demonstrated decreased PDH phosphorylation (indicative of increased PDH activity28,29), as well as an increased oxygen consumption rate with either glucose or pyruvate as a substrate using a Seahorse XF24 analyzer.
As previously mentioned, GLP-1R agonists have been shown to improve cardiovascular outcomes in subjects with T2D, though much of this improvement appears to be attributed to actions that mitigate atherothrombosis and decreased rates of MI.4,44 Nonetheless, insulin is a potent stimulator of myocardial glucose oxidation,25 and as GLP-1R agonists augment glucose-stimulated insulin secretion, this class of glucose-lowering medication may also have utility against diabetic heart disease. In support of this statement, treatment with the GLP-1R agonist liraglutide (30 ug/kg once daily) during the final 2-weeks in male C57BL/6J mice subjected to experimental T2D via high-fat diet provision for 10-weeks with STZ (75 mg/kg) administered at week 4 of the protocol, mitigated diabetic heart disease.17 This was reflected by an increased mitral E/A ratio and decreased E/e' ratio during ultrasound echocardiography studies. Of interest, an increase in glucose oxidation rates was only observed in the isolated working heart, if hearts were extracted from mice treated systemically with liraglutide, but not if the working heart was directly treated with liraglutide added to the perfusate. Such observations are entirely consistent with GLP-1R expression being absent in cardiomyocytes,4,45 and that GLP-1R agonist-induced increases in myocardial glucose oxidation are dependent on its canonical actions on the islet β-cell GLP-1R to promote insulin secretion.
In support of increases in the MCUb inactivating PDH activity in T2D, it has been shown that gene therapy using an adeno-associated viral vector to express a dominant negative MCUb transgene restored myocardial glucose oxidation in mice with experimental T2D due to high-fat diet provision plus low-dose STZ (75 mg/kg) administration.34 However, parameters of diastolic function were not assessed in this study. Thus, it cannot be concluded whether the authors strategy to promote glucose oxidation alleviated diabetic heart disease, though isolated Langendorff perfusion studies demonstrated a restored responsivity to adrenergic stimulation.
MECHANISMS EXPLAINING HOW INCREASED MYOCARDIAL GLUCOSE OXIDATION ALLEVIATES DIABETIC HEART DISEASE AND POTENTIAL CONCERNS
The predominant view in the field as to why increases in myocardial glucose oxidation impart protection against diabetic heart disease centers on an improvement in cardiac efficiency. Cardiac mechanical efficiency refers to the relationship of work performed by the myocardium and the energy it consumes (i.e., oxygen consumption) throughout the course of cardiac contraction, a concept first introduced by Bing et al.46 in the late 1940s. As the oxidation of glucose produces more ATP per mole of oxygen consumed than the oxidation of a fatty acid, glucose is the more efficient fuel to support cardiac work, and numerous studies have demonstrated that increasing glucose oxidation does indeed improve cardiac mechanical efficiency in the diseased myocardium.47,48,49 However, whether this does explain how increasing myocardial glucose oxidation alleviates diabetic heart disease remains to be conclusively determined. Furthermore, as diabetic heart disease is characterized by diastolic dysfunction, it will be imperative for future studies to investigate the mechanisms by which increasing glucose oxidation may facilitate improved ventricular relaxation. Of relevance, myocardial relaxation during diastole is also an energy dependent process,50 thus it may be possible that an improvement in cardiac efficiency is also mechanistically involved. It is also likely that increased myocardial glucose oxidation mediated protection against diabetic heart disease is multifactorial, as increases in myocardial PDH activity have been shown to alleviate cardiomyocyte apoptosis, and may even lead to decreased cardiac fibrosis.18,51 The latter would lead to decreased ventricular stiffness and may also explain how increases in glucose oxidation specifically alleviate diastolic dysfunction.
While the aforementioned studies support that promoting myocardial glucose oxidation has salutary actions against diabetic heart disease, there are potential concerns with such a strategy that need to be considered. The most prominent concern stems from Randle’s glucose-fatty acid cycle,26 since increases in glucose oxidation in the myocardium often result in a corresponding decline in fatty acid oxidation, keeping overall energy production constant with energy demand. A decline in myocardial fatty acid oxidation may increase steatosis and lipid accumulation, thereby promoting cardiac lipotoxicity. Nonetheless, the majority of studies that have focused on stimulating myocardial glucose oxidation to treat diabetic heart disease have not reported any notable cardiac lipotoxicity that exacerbated the disease pathology. Moreover, increases in myocardial steatosis and triacylglycerol accumulation secondary to a reduction in fatty acid oxidation may not necessarily be harmful, as such increases may buffer from potential increases in other lipid intermediates that can be toxic to cardiomyocytes, including diacylglycerols and ceramides.9
FINAL SUMMARY AND THE FUTURE SURROUNDING PDH-FOCUSED DRUG DEVELOPMENT
Advancements in models of diabetic heart disease, imaging technologies to assess in vivo cardiac function, and methods to assess intermediary metabolism have clearly demonstrated that myocardial energy metabolism is severely impacted by diabetes. One of the most notable defects is a marked impairment in glucose oxidation. It is likely that this reduction in myocardial glucose oxidation directly contributes to the pathology of diabetic heart disease, as numerous preclinical studies support that promoting myocardial glucose oxidation can alleviate diastolic dysfunction and improve cardiovascular outcomes in experimental models of both T1D and T2D.
Pharmacological stimulation of PDH activity is thus an attractive therapeutic target for diabetic heart disease. However, the most extensively characterized agent that can promote PDH activity, DCA, is limited by poor pharmacokinetics (i.e., very short half-life37) that would not be ideal in the setting of diabetic heart disease, where chronic therapy lasting decades may be required. Inhibitors with improved specificity against PDHK isoforms relative to DCA are in development.52 This includes 2-[(2,4-dihydroxyphenyl)sulfonyl]isoindoline-4,6-diol, which has been demonstrated to increase myocardial PDH activity and glucose oxidation in obese mice,53 though whether it can alleviate the pathology of diabetic heart disease remains unknown. Antagonism of FoxO1 to prevent the transcription of Pdk4 is another promising approach to potentially stimulate PDH activity in diabetic heart disease. On the contrary, as a transcription factor regulating the expression of numerous genes that influence multiple aspects of cell biology, there may also be unforeseen concerns relating to toxicity with chronic FoxO1 inhibition.
GLP-1R agonists may represent the most feasible approach to stimulate myocardial glucose oxidation in diabetic heart disease, as this glucose-lowering drug class used in the management of T2D has also been demonstrated to improve cardiovascular outcomes,4 illustrating the translational relevance of this approach. However, GLP-1R agonists are not used for the treatment of T1D, and not all patients with T2D are amendable to therapy with GLP-1R agonists, especially those with waning β-cell function. Taken together, the specific development of new pharmacological agents that can either inhibit PDHK or directly stimulate PDH, represent an exciting approach for the specific treatment of diabetic heart disease.
Footnotes
Funding: This study was supported by a Project Grant from the Canadian Institutes of Health Research (CIHR) to John R. Ussher. John R. Ussher is a Tier 2 Canada Research Chair (Pharmacotherapy of Energy Metabolism in Obesity). Amanda A. Greenwell is supported by a Vanier Canada Graduate Scholarship from CIHR.
Conflict of Interest: The authors have no conflicts of interest to declare.
- Writing - original draft: Tabatabaei Dakhili SA, Greenwell AA, Ussher JR.
- Writing - review & editing: Tabatabaei Dakhili SA, Greenwell AA, Ussher JR.
References
- 1.Scherer PE, Hill JA. Obesity, diabetes, and cardiovascular diseases: a compendium. Circ Res. 2016;118:1703–1705. doi: 10.1161/CIRCRESAHA.116.308999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 3.Gopal K, Chahade JJ, Kim R, Ussher JR. The impact of antidiabetic therapies on diastolic dysfunction and diabetic cardiomyopathy. Front Physiol. 2020;11:603247. doi: 10.3389/fphys.2020.603247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ussher JR, Greenwell AA, Nguyen MA, Mulvihill EE. Cardiovascular effects of incretin-based therapies: integrating mechanisms with cardiovascular outcome trials. Diabetes. 2022;71:173–183. doi: 10.2337/dbi20-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30:595–602. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- 6.Ritchie RH, Abel ED. Basic mechanisms of diabetic heart disease. Circ Res. 2020;126:1501–1525. doi: 10.1161/CIRCRESAHA.120.315913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heather LC, Hafstad AD, Halade GV, Harmancey R, Mellor KM, Mishra PK, et al. Guidelines on models of diabetic heart disease. Am J Physiol Heart Circ Physiol. 2022;323:H176–H200. doi: 10.1152/ajpheart.00058.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018;122:624–638. doi: 10.1161/CIRCRESAHA.117.311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zlobine I, Gopal K, Ussher JR. Lipotoxicity in obesity and diabetes-related cardiac dysfunction. Biochim Biophys Acta. 2016;1861:1555–1568. doi: 10.1016/j.bbalip.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Lopaschuk GD, Folmes CD, Stanley WC. Cardiac energy metabolism in obesity. Circ Res. 2007;101:335–347. doi: 10.1161/CIRCRESAHA.107.150417. [DOI] [PubMed] [Google Scholar]
- 11.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 12.Ussher JR, Jaswal JS, Lopaschuk GD. Pyridine nucleotide regulation of cardiac intermediary metabolism. Circ Res. 2012;111:628–641. doi: 10.1161/CIRCRESAHA.111.246371. [DOI] [PubMed] [Google Scholar]
- 13.Sakamoto J, Barr RL, Kavanagh KM, Lopaschuk GD. Contribution of malonyl-CoA decarboxylase to the high fatty acid oxidation rates seen in the diabetic heart. Am J Physiol Heart Circ Physiol. 2000;278:H1196–H1204. doi: 10.1152/ajpheart.2000.278.4.H1196. [DOI] [PubMed] [Google Scholar]
- 14.Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun UJ, et al. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology. 2005;146:5341–5349. doi: 10.1210/en.2005-0938. [DOI] [PubMed] [Google Scholar]
- 15.Ussher JR, Koves TR, Jaswal JS, Zhang L, Ilkayeva O, Dyck JR, et al. Insulin-stimulated cardiac glucose oxidation is increased in high-fat diet-induced obese mice lacking malonyl CoA decarboxylase. Diabetes. 2009;58:1766–1775. doi: 10.2337/db09-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Page LM, Rider OJ, Lewis AJ, Ball V, Clarke K, Johansson E, et al. Increasing pyruvate dehydrogenase flux as a treatment for diabetic cardiomyopathy: a combined 13C hyperpolarized magnetic resonance and echocardiography study. Diabetes. 2015;64:2735–2743. doi: 10.2337/db14-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almutairi M, Gopal K, Greenwell AA, Young A, Gill R, Aburasayn H, et al. The GLP-1 receptor agonist liraglutide increases myocardial glucose oxidation rates via indirect mechanisms and mitigates experimental diabetic cardiomyopathy. Can J Cardiol. 2021;37:140–150. doi: 10.1016/j.cjca.2020.02.098. [DOI] [PubMed] [Google Scholar]
- 18.Gopal K, Al Batran R, Altamimi TR, Greenwell AA, Saed CT, Tabatabaei Dakhili SA, et al. FoxO1 inhibition alleviates type 2 diabetes-related diastolic dysfunction by increasing myocardial pyruvate dehydrogenase activity. Cell Reports. 2021;35:108935. doi: 10.1016/j.celrep.2021.108935. [DOI] [PubMed] [Google Scholar]
- 19.Rohm M, Savic D, Ball V, Curtis MK, Bonham S, Fischer R, et al. Cardiac dysfunction and metabolic inflexibility in a mouse model of diabetes without dyslipidemia. Diabetes. 2018;67:1057–1067. doi: 10.2337/db17-1195. [DOI] [PubMed] [Google Scholar]
- 20.Ljubkovic M, Gressette M, Bulat C, Cavar M, Bakovic D, Fabijanic D, et al. Disturbed fatty acid oxidation, endoplasmic reticulum stress, and apoptosis in left ventricle of patients with type 2 diabetes. Diabetes. 2019;68:1924–1933. doi: 10.2337/db19-0423. [DOI] [PubMed] [Google Scholar]
- 21.Jaswal JS, Keung W, Wang W, Ussher JR, Lopaschuk GD. Targeting fatty acid and carbohydrate oxidation--a novel therapeutic intervention in the ischemic and failing heart. Biochim Biophys Acta. 2011;1813:1333–1350. doi: 10.1016/j.bbamcr.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 22.Taegtmeyer H, Young ME, Lopaschuk GD, Abel ED, Brunengraber H, Darley-Usmar V, et al. Assessing cardiac metabolism: a scientific statement from the American Heart Association. Circ Res. 2016;118:1659–1701. doi: 10.1161/RES.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cunningham CH, Lau JY, Chen AP, Geraghty BJ, Perks WJ, Roifman I, et al. Hyperpolarized 13C metabolic MRI of the human heart: initial experience. Circ Res. 2016;119:1177–1182. doi: 10.1161/CIRCRESAHA.116.309769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rider OJ, Apps A, Miller JJ, Lau JY, Lewis AJ, Peterzan MA, et al. Noninvasive in vivo assessment of cardiac metabolism in the healthy and diabetic human heart using hyperpolarized 13C MRI. Circ Res. 2020;126:725–736. doi: 10.1161/CIRCRESAHA.119.316260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brownsey RW, Boone AN, Allard MF. Actions of insulin on the mammalian heart: metabolism, pathology and biochemical mechanisms. Cardiovasc Res. 1997;34:3–24. doi: 10.1016/s0008-6363(97)00051-5. [DOI] [PubMed] [Google Scholar]
- 26.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 27.Shipp JC, Opie LH, Challoner D. Fatty acid and glucose metabolism in the perfused heart. Nature. 1961;189:1018–1019. [Google Scholar]
- 28.Patel MS, Korotchkina LG. Regulation of the pyruvate dehydrogenase complex. Biochem Soc Trans. 2006;34:217–222. doi: 10.1042/BST20060217. [DOI] [PubMed] [Google Scholar]
- 29.Saed CT, Tabatabaei Dakhili SA, Ussher JR. Pyruvate dehydrogenase as a therapeutic target for nonalcoholic fatty liver disease. ACS Pharmacol Transl Sci. 2021;4:582–588. doi: 10.1021/acsptsci.0c00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel MS, Nemeria NS, Furey W, Jordan F. The pyruvate dehydrogenase complexes: structure-based function and regulation. J Biol Chem. 2014;289:16615–16623. doi: 10.1074/jbc.R114.563148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jing E, O’Neill BT, Rardin MJ, Kleinridders A, Ilkeyeva OR, Ussar S, et al. Sirt3 regulates metabolic flexibility of skeletal muscle through reversible enzymatic deacetylation. Diabetes. 2013;62:3404–3417. doi: 10.2337/db12-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Brien M, Chalker J, Slade L, Gardiner D, Mailloux RJ. Protein S-glutathionylation alters superoxide/hydrogen peroxide emission from pyruvate dehydrogenase complex. Free Radic Biol Med. 2017;106:302–314. doi: 10.1016/j.freeradbiomed.2017.02.046. [DOI] [PubMed] [Google Scholar]
- 33.Pettit FH, Roche TE, Reed LJ. Function of calcium ions in pyruvate dehydrogenase phosphatase activity. Biochem Biophys Res Commun. 1972;49:563–571. doi: 10.1016/0006-291x(72)90448-2. [DOI] [PubMed] [Google Scholar]
- 34.Cividini F, Scott BT, Suarez J, Casteel DE, Heinz S, Dai A, et al. Ncor2/PPARα-dependent upregulation of MCUb in the type 2 diabetic heart impacts cardiac metabolic flexibility and function. Diabetes. 2021;70:665–679. doi: 10.2337/db20-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gopal K, Almutairi M, Al Batran R, Eaton F, Gandhi M, Ussher JR. Cardiac-specific deletion of pyruvate dehydrogenase impairs glucose oxidation rates and induces diastolic dysfunction. Front Cardiovasc Med. 2018;5:17. doi: 10.3389/fcvm.2018.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stacpoole PW, Henderson GN, Yan Z, Cornett R, James MO. Pharmacokinetics, metabolism and toxicology of dichloroacetate. Drug Metab Rev. 1998;30:499–539. doi: 10.3109/03602539808996323. [DOI] [PubMed] [Google Scholar]
- 37.Stacpoole PW, Henderson GN, Yan Z, James MO. Clinical pharmacology and toxicology of dichloroacetate. Environ Health Perspect. 1998;106(Suppl 4):989–994. doi: 10.1289/ehp.98106s4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, et al. The cardiac phenotype induced by PPARα overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121–130. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gopal K, Saleme B, Al Batran R, Aburasayn H, Eshreif A, Ho KL, et al. FoxO1 regulates myocardial glucose oxidation rates via transcriptional control of pyruvate dehydrogenase kinase 4 expression. Am J Physiol Heart Circ Physiol. 2017;313:H479–H490. doi: 10.1152/ajpheart.00191.2017. [DOI] [PubMed] [Google Scholar]
- 40.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Ma K, Sadana P, Chowdhury F, Gaillard S, Wang F, et al. Estrogen-related receptors stimulate pyruvate dehydrogenase kinase isoform 4 gene expression. J Biol Chem. 2006;281:39897–39906. doi: 10.1074/jbc.M608657200. [DOI] [PubMed] [Google Scholar]
- 42.Battiprolu PK, Hojayev B, Jiang N, Wang ZV, Luo X, Iglewski M, et al. Metabolic stress-induced activation of FoxO1 triggers diabetic cardiomyopathy in mice. J Clin Invest. 2012;122:1109–1118. doi: 10.1172/JCI60329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan D, Cai Y, Luo J, Liu J, Li X, Ying F, et al. FOXO1 contributes to diabetic cardiomyopathy via inducing imbalanced oxidative metabolism in type 1 diabetes. J Cell Mol Med. 2020;24:7850–7861. doi: 10.1111/jcmm.15418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation. 2017;136:849–870. doi: 10.1161/CIRCULATIONAHA.117.028136. [DOI] [PubMed] [Google Scholar]
- 45.Ussher JR, Baggio LL, Campbell JE, Mulvihill EE, Kim M, Kabir MG, et al. Inactivation of the cardiomyocyte glucagon-like peptide-1 receptor (GLP-1R) unmasks cardiomyocyte-independent GLP-1R-mediated cardioprotection. Mol Metab. 2014;3:507–517. doi: 10.1016/j.molmet.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bing RJ, Hammond MM, Handelsman JC, Powers SR, Spencer FC, Eckenhoff JE, et al. The measurement of coronary blood flow, oxygen consumption, and efficiency of the left ventricle in man. Am Heart J. 1949;38:1–24. doi: 10.1016/0002-8703(49)90788-7. [DOI] [PubMed] [Google Scholar]
- 47.Liu Q, Docherty JC, Rendell JC, Clanachan AS, Lopaschuk GD. High levels of fatty acids delay the recovery of intracellular pH and cardiac efficiency in post-ischemic hearts by inhibiting glucose oxidation. J Am Coll Cardiol. 2002;39:718–725. doi: 10.1016/s0735-1097(01)01803-4. [DOI] [PubMed] [Google Scholar]
- 48.Taniguchi M, Wilson C, Hunter CA, Pehowich DJ, Clanachan AS, Lopaschuk GD. Dichloroacetate improves cardiac efficiency after ischemia independent of changes in mitochondrial proton leak. Am J Physiol Heart Circ Physiol. 2001;280:H1762–H1769. doi: 10.1152/ajpheart.2001.280.4.H1762. [DOI] [PubMed] [Google Scholar]
- 49.Ussher JR, Wang W, Gandhi M, Keung W, Samokhvalov V, Oka T, et al. Stimulation of glucose oxidation protects against acute myocardial infarction and reperfusion injury. Cardiovasc Res. 2012;94:359–369. doi: 10.1093/cvr/cvs129. [DOI] [PubMed] [Google Scholar]
- 50.Pouleur H. Diastolic dysfunction and myocardial energetics. Eur Heart J. 1990;11(Suppl C):30–34. doi: 10.1093/eurheartj/11.suppl_c.30. [DOI] [PubMed] [Google Scholar]
- 51.Kato T, Niizuma S, Inuzuka Y, Kawashima T, Okuda J, Tamaki Y, et al. Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Circ Heart Fail. 2010;3:420–430. doi: 10.1161/CIRCHEARTFAILURE.109.888479. [DOI] [PubMed] [Google Scholar]
- 52.Tso SC, Qi X, Gui WJ, Wu CY, Chuang JL, Wernstedt-Asterholm I, et al. Structure-guided development of specific pyruvate dehydrogenase kinase inhibitors targeting the ATP-binding pocket. J Biol Chem. 2014;289:4432–4443. doi: 10.1074/jbc.M113.533885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu CY, Satapati S, Gui W, Wynn RM, Sharma G, Lou M, et al. A novel inhibitor of pyruvate dehydrogenase kinase stimulates myocardial carbohydrate oxidation in diet-induced obesity. J Biol Chem. 2018;293:9604–9613. doi: 10.1074/jbc.RA118.002838. [DOI] [PMC free article] [PubMed] [Google Scholar]