Abstract
Dyslipidemia is an important risk factor for atherosclerotic cardiovascular disease (ASCVD). There are abundant and unequivocal data to indicate that low-density lipoproteins (LDL) are a cause of ASCVD. Reduction of plasma low-density lipoprotein cholesterol (LDL-C) by medical therapy such as statins, ezetimibe and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors have proven to significantly reduce the risk of cardiovascular events. However, for many reasons, many patients are not able to achieve LDL-C levels recommended by guidelines on currently available therapies. This has led to the development of new drugs lowering LDL-C, such as inclisiran, bempedoic acid, and evinacumab, in the hope of reducing cardiovascular (CV) risk. Drugs targeting lipoprotein (a) (Lp[a]) also have a role in the prevention of atherosclerosis, with genetic studies having established that 20%–30% of the human population inherits plasma Lp(a) levels in the atherogenic range. In this paper, we will review the recent progress made in the approaches to LDL-C and Lp(a) therapeutic modulation.
Keywords: Atherosclerosis, Low density lipoprotein cholesterol, Lipoprotein (a), Therapeutics, Cardiovascular diseases
INTRODUCTION
Atherosclerotic cardiovascular disease (ASCVD) is a leading cause of morbidity and mortality worldwide.1 A major cause of ASCVD is elevated apolipoprotein B (ApoB), which infiltrates the subendothelial space of arteries, leading to plaque growth and possible rupture.2 Therefore the reduction of ApoB lipoprotein, the level of which is approximated in clinical practice by low-density lipoprotein cholesterol (LDL-C) measurement, is a good target for therapeutic development. Medical therapy to lower LDL-C is mandatory for both primary and secondary ASCVD prevention, as current guidelines recommend.3,4 LDL-C lowering medications including statins, ezetimibe, and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors reduce cardiovascular (CV) risk proportionally to the reduction in LDL-C (23% per 1 mmol/L of LDL-C) regardless of the starting level, and without a threshold effect.5 Notably, the reduction in CV risk is independent of the class of agent.6
However, for many reasons, many patients are not able to achieve desired levels of LDL-C, which for secondary prevention are recommended by current guidelines as below 70 mg/dL or even below 55 mg/dL.7 This has led to the development of new drugs lowering LDL-C, such as inclisiran, bempedoic acid (BPA), and evinacumab in the hope of reducing CV risk. Drugs targeting lipoprotein (a) (Lp[a]) also have a role in the prevention of atherosclerosis, with genetic studies having established that across various ethnic groups 20%–30% of the human population inherits plasma Lp(a) levels in the atherogenic range.8,9 In this paper, we will review the recent progress made in the approaches to LDL-C and Lp(a) therapeutic modulation. Therapies targeting other mechanisms, such as triglyceride lowering, increasing high-density lipoprotein cholesterol (HDL-C), modulating inflammation, etc., are also being developed to reduce CV risk, which are covered in detail elsewhere.
LDL-C LOWERING AGENTS
As mentioned above, for more than three decades the cholesterol transported in LDL-C has been a major target for treatment. Randomized clinical trials of drugs reducing LDL-C as well as detailed studies of the human genetics of LDL-C and their relationship to ASCVD have beyond doubt established LDL-C as a cause for increased CV risk.10 Currently, three classes of drugs lowering LDL-C have shown to reduce outcomes related to ASCVD in randomized controlled trials. Statins have been demonstrated to significantly reduce CV risk through LDL-C lowering in numerous randomized clinical trials.5 The cholesterol absorption inhibitor ezetimibe has also been shown to further reduce CV risk as a combination to baseline statin therapy compared with statin monotherapy.11 PCSK9 inhibitors were first discovered through human genetic studies for LDL-C and were found to be related to LDL-C metabolism. Notably, these new LDL-lowering agents act through the reduction of hepatic cholesterol and the consequent upregulation of the transcriptional of the LDL receptor, which in turn leads to plasma LDL-C reduction. PCSK9 inhibitors have shown dramatic effect in the reduction of LDL-C promise to meet much of the unmet demand for LDL-C lowering and have been greeted with great expectations. Recent trials have confirmed that these novel agents reduce cardiovascular events, both in stable cardiovascular disease and acute coronary syndromes.12,13 However, cost issues and the need for frequent injections have hindered the widespread use of these drugs.
Despite the availability of LDL-C lowering medications including statins, an unmet need for LDL-C reduction has remained. The use of statins alone is often insufficient for reducing LDL-C to target levels, especially for patients with ASCVD for whom guidelines recommend lower LDL-C levels for primary or secondary prevention. In addition, some patients, especially those with genetic disorders such as familial hypercholesterolemia (FH) do not show sufficient response to statins.14 Furthermore, side effects prevent a significant number of patients from taking statins at the appropriate intensity or in some cases, exclude their use entirely.15 Statin intolerance rates have been reported at 7% to 29% in many registries and observational studies, with muscle-related side effects being predominant.16,17 Patients who cannot tolerate statins are less likely to attain adequate LDL-C levels, and as a result are at a higher risk for CV events. To reduce CV risk in these patients, additional options for lipid modulation are needed, and therefore the development of new LDL-C lowering therapies has been the focus of intense interest.
1. RNA interference targeting PCSK9: Inclisiran
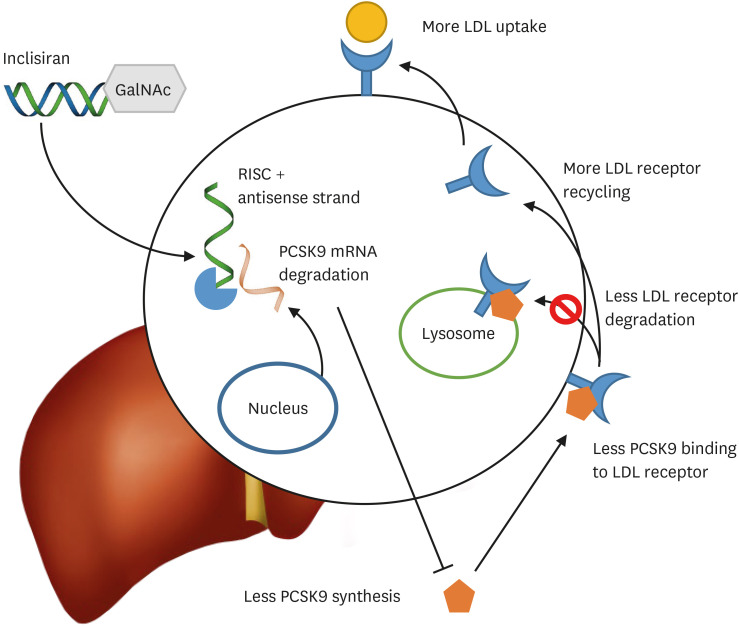
PCSK9 is an important regulator in the metabolism of LDL-C. After being synthesized and modified in the hepatocyte endoplasmic reticulum, PCSK9 promotes the degradation of cell surface LDL receptors through internalization and lysosomal degradation.18 Inclisiran is a modified small interfering RNA (siRNA), the effect of which is to inhibit the expression of the PCSK9 gene. Synthetic triantennary N-acetylgalactosamine (GalNAc) ensures rapid and specific hepatocyte delivery through the asialoglycoprotein receptor which is expressed on the surface of hepatocytes. In addition, the substitution by 2′-O-methylnucleotides or 2′-O-fluoronucleotides for molecules in the polynucleotide strands has led to greater drug stability (Fig. 1). In animal model preclinical studies, 1 mg/kg of inclisiran was associated with a 50% increase in PCSK9 inhibition, while the highest dose administration of over 3 mg/kg of led to PCSK9 activity inhibition by 85% and an LDL-C level decrease of approximately 60%.19 After preclinical studies showed promising results, the first phase I study on the safety of subcutaneously administered inclisiran was done on 24 healthy subjects who had an LDL-C level ≥100 mg/dL.20 The subjects were randomized to either a single injection of inclisiran, with varing doses from 25 up to 800 mg, or multiple administrations, with intervals of at least 1 week in between, of doses from 125 to 500 mg. A single dose of inclisiran of at least 300 mg or higher produced a significant decrease in the level of PCSK9. A single 100 mg injection of inclisiran was sufficient to achieve a significant reduction in LDL-C. After the administration of a single dose of 300 mg or higher of inclisiran, both PCSK9 and LDL-C levels remained significantly reduced for over 180 days. The maximal reduction in PCSK9 levels occurred in the subjects who were administered 500 mg of inclisiran twice a month for a total duration of 2 months. The maximal reduction in LDL-C levels was observed in the subjects who received a 300 mg dose injection twice a month for 2 months. In all the subjects who received inclisiran according to multiple dose schedules, LDL-C reduction continued to be significant at day 196 after the start of the first administration of inclisiran.
Fig. 1. Inclisiran is a siRNA. Having entered hepatocytes conjugated with GalNAc, the antisense strand then combines with RISC and targets PCSK9 mRNA for degradation. Less PCSK9 synthesis leads to less PCSK9-mediated LDL receptor degradation and more recycling of the LDL receptor, consequently resulting in more LDL uptake and lower plasma levels of LDL cholesterol.
siRNA, small interfering RNA; GalNAc, N-acetylgalactosamine; RISC, RNA-induced silencing complex; PCSK9, proprotein convertase subtilisin/kexin type 9; LDL, low-density lipoprotein.
The next steps to confirm the safety and efficacy of Inclisiran have been ongoing in the ORION program. The ORION I trial was a phase II trial to ascertain the efficacy of inclisiran on lipid-lowering.21 It was conducted on 501 high CV risk patients who had elevated serum LDL-C levels at baseline uncontrolled with background medical treatment even at maximally tolerated doses. The patients were randomized to either a single injection of inclisiran or placebo at doses of 200, 300, or 500 mg, or alternatively 2 injections (at day 1 and day 90) of inclisiran or placebo at doses of 100, 200, or 300 mg. One hundred eighty days after the first administration, the least-squares mean reductions in LDL-C were between 27.9% to 41.9% reduction after a single dose of inclisiran, and 35.5% to 52.6% after 2 administrations. Two injections of 300 mg of inclisiran produced the highest reduction in LDL-C. The reductions in LDL-C levels were largely consistent with those reported in other clinical trials for anti-PCSK9 monoclonal antibodies. However, the advantage of inclisiran versus available PCSK9 inhibitor antibodies is that the lipid-lowering effect of inclisiran persists significantly longer, meaning therapy can be achieved with fewer injections, such as a 300 mg subcutaneous injection given 3 months apart. Preliminary data from the ORION-10 and ORION-11 phase 3 trials, which randomized 3,174 patients to either placebo or 500 mg subcutaneous injections of inclisiran, revealed a decrease of 26% in the composite endpoint of CV death, non-fatal myocardial infarction, or stroke. Currently the ORION-4 study, a large multicenter clinical trial designed to access cardiovascular outcomes is underway.
2. ATP-citrate lyase (ACL) inhibitors: BPA
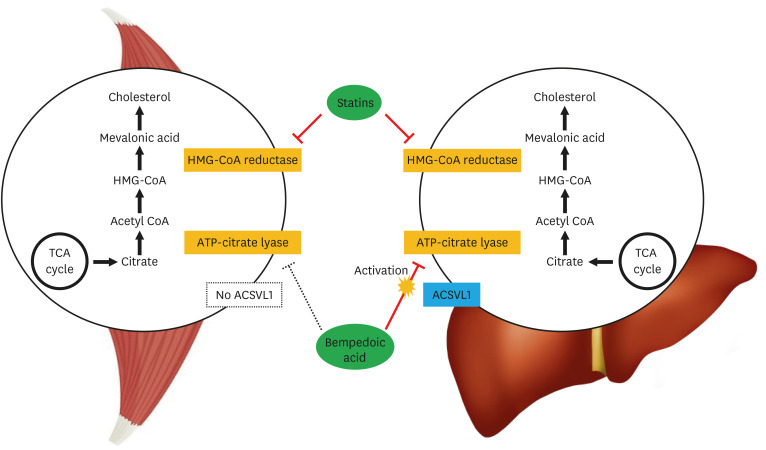
BPA is a small-molecule selective inhibitor of the enzyme ACL, a key component of the hepatic biosynthesis of cholesterol that works upstream of β-hydroxy β-methylglutaryl-coenzyme A (HMG-CoA) reductase.22,23 ACL inhibition results in decreased cholesterol synthesis, which results in LDL receptor upregulation, leading to increased uptake and clearance of circulating LDL particles in hepatocytes and as a result lowers LDL-C. BPA is a prodrug that is activated by very-long chain acyl-CoA synthetase-1 (ACSVL1), an enzyme expressed in hepatocytes but which is not present in skeletal myocytes (Fig. 2). Therefore, although BPA exerts its effect through the same pathway as statins, the liver-specific action of the drug may explain the absence of muscular adverse effects associated with statins, as has been reported.24
Fig. 2. Bempedoic acid is an inhibitor of ACL, a key step in the hepatic biosynthesis of cholesterol that works upstream of HMG-CoA reductase. Activation requires very-long chain ACSVL1, an enzyme expressed in the liver but not in skeletal muscle, a possible reason for the absence of muscular side effects associated with statins.
ACL, ATP-citrate lyase; HMG-CoA, β-hydroxy β-methylglutaryl-coenzyme A; ACSVL1, acyl-CoA synthetase-1; TCA, tricarboxylic acid.
The effectiveness and safety of BPA are undergoing testing in the Cholesterol Lowering via Bempedoic acid, an ACL-Inhibiting Regimen (CLEAR) clinical development program. The trials have proved the efficacy of BPA in lowering LDL-C either as monotherapy in statin resistant patients, in combination with ezetimibe, or alternatively in combination with statins. The phase 3 CLEAR serenity trial randomized 345 patients with dyslipidemia and statin intolerance to BPA or placebo. BPA treatment reduced LDL-C from baseline compared to placebo by 21.4% (p<0.001).25 Significant reductions were also observed in non-HDL-C (17.9%), total cholesterol (14.8%), ApoB (15.0%), and high-sensitivity C-reactive protein (hs-CRP) (24.3%). The phase 3 CLEAR Tranquility trial randomized 269 patients with a history of intolerance to more than low-intensity statin and an LDL-C ≥100 mg/dL even with maximal lipid-modifying therapy. The patients were randomized to BPA versus placebo added to ezetimibe. After 12 weeks, BPA added to background lipid-modifying therapy including ezetimibe reduced LDL-C by 28.5% compared to the placebo group.26 Significant reductions were also noted in the secondary endpoints of non-HDL-C (23.6%), total cholesterol (18.0%), ApoB (19.3%), and hs-CRP (31.0%) The CLEAR Wisdom trial assigned 779 patients with high CV risk due to preexisting ASCVD or multiple CVD risk factors, heterozygous FH, or both, to receive either BPA or placebo. BPA lowered LDL-C levels by −17.4% compared to placebo after 12 weeks.24 The large phase 3 CLEAR Harmony trial randomized 2,230 patients with ASCVD or heterozygous FH and an LDL-C level of at least 70 mg/dL while receiving maximally tolerated statin therapy, either with or without additional lipid-modifying treatment, to either BPA or placebo for 52 weeks. At the end of 12 weeks, BPA reduced the primary endpoint of LDL-C by 16.5% from baseline compared to placebo (p<0.001).27 The safety of BPA was also demonstrated in that the incidence of adverse events during 1 year did not significantly differ between the two groups. Based on these results, BPA has obtained FDA approval for the indication of lowering LDL-C. To access the efficacy of BPA in lowering CV outcomes, a large study (CLEAR Outcomes) is currently ongoing.
Lp(a)
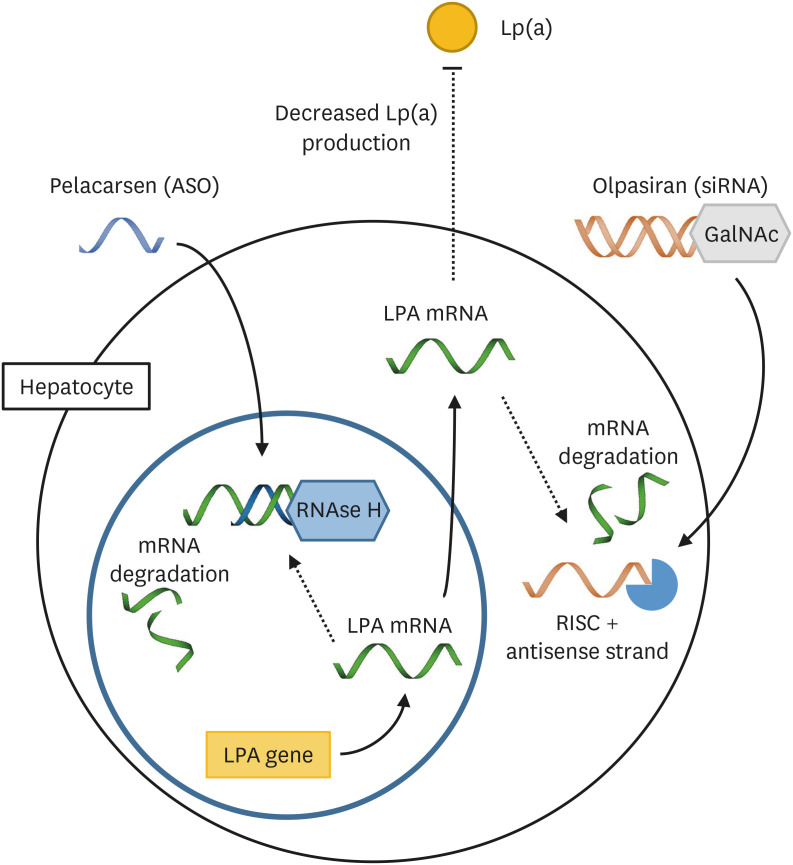
Lp(a) is lipoprotein, similar in structure to LDL, in which ApoB is linked by a covalent disulphide bond to Apo(a).28,29 The potential contribution to ASCVD of Lp(a) is through proinflammatory effects of its oxidized phospholipid content, proatherogenic effects of the LDL-like moiety, and prothrombotic effects through the plasminogen-like protease domain on Apo(a).30 Lp(a) is more potently atherothrombogenic than LDL, for not only it carries all the elements of LDL that dispose it to atherogenesis, but also oxidized phospholipids of Apo(a) that mediate thrombosis by pro-inflammatory and potential antifibrinolytic effects.31 LP(a) is an independent risk factor for ASCVD, with values above 30 to 50mg/dL generally accepted as being in the atherothrombotic range.32,33,34,35,36 As yet there are no FDA-approved pharmacologic therapies shown to specifically lower Lp(a) and so precise analysis of the contribution of Lp(a) to clinical ASCVD is not yet possible. Among current therapies for lipid modulation, statins do not reduce plasma Lp(a) levels, and PCSK9 antibodies only have a modest effect.37 Here we summarize the discovery and development of nucleic-acid-based drugs, such as olpasiran and pelacarsen, acting through inhibition of mRNA translation of the LPA gene in hepatocytes and effectively reducing the plasma concentration of Lp(a) (Fig. 3).
Fig. 3. Pelacarsen is an ASO targeting the mRNA of the LPA gene. Binding of the ASO results in mRNA degradation through RNAse H. Olpasiran is a siRNA. The antisense strand combines with RISC and targets LPA mRNA for degradation. The inhibition of LPA mRNA translation by pelacarsen and olpasiran results in decreased production of Apo(a) and Lp(a).
ASO, antisense oligonucleotide; siRNA, small interfering RNA; RISC, RNA-induced silencing complex; Apo, apolipoprotein; Lp(a), lipoprotein (a); GalNAc, N-acetylgalactosamine.
1. Pelacarsen (antisense oligonucleotide; ASO)
Pelacarsen is an ASO designed to inhibit the translation of mRNA of the LPA gene, responsible for production of Apo(a) in the hepatocyte.38 The IONIS-APO(a)Rx phase 2 trial was the first study to investigate a therapeutic intervention specifically targeting Lp(a) modulation in subjects with elevated Lp(a) concentrations and especially, the first randomized study of ASO therapy in such patients.39 The study demonstrated a significant reduction of 67%–72% from baseline compared to placebo in mean Lp(a) levels in patients receiving IONIS-APO(a)Rx, and also showed a significant reduction in the levels of LDL-C, ApoB, and Apo(a)-associated oxidized phospholipids. Finally, it also showed that lower levels of Lp(a) and associated oxidized phospholipids reduced monocyte activity which as the effect of the drug disappeared re-increased to baseline levels. The IONIS-APO[a]-LRX phase 1/2a trial demonstrated the potency of modified IONIS-APO(a)Rx conjugated with a GalNAc3 complex, which mediates hepatocyte delivery via the asialoglycoprotein receptor.24 IONIS-APO(a)-LRx was about 30 times more potent than the original ASO, enabling a more than tenfold lower dose and greatly improved tolerability. The highest dose in the trial, multi-day 40 mg subcutaneous injections of IONIS-APO(a)-LRx produced a 92.4% reduction in Lp(a) with no side-effects observed. This was also the first to demonstrate the efficacy of GalNAc3-conjugated ASO therapy, showing the feasibility of hepatocyte-specific therapeutic modalities. The AKCEA-APO(a)-LRx phase 2 trial randomized 286 patients with CV disease and Lp(a) levels of ≥60 mg/dL to APO(a)-LRx or placebo, and showed that treatment with APO(a)-LRx led to dose-dependent reductions in plasma Lp(a).30 These reductions were significant at all doses studied, and at the highest dose regimen of 20 mg weekly, patients attained a mean reduction in Lp(a) of 80%, with 98% of patients attaining a Lp(a) level of less than 50 mg/dL, a target value supported by current European and U.S. guidelines. APO(a)-LRx also reduced oxidized phospholipids on ApoB and Apo(a), both proinflammatory components associated with higher atherothrombotic risk. Finally, reductions in LDL-C and ApoB beyond those achieved with background aggressive lipid-lowering therapy were noted. APO(a)-LRx was well tolerated and no serious adverse events such as thrombocytopenia, decline in liver or renal function, or influenza-like symptoms were reported. Finally, Lp(a) HORIZON is a large phase 3 randomized controlled trial enrolling 8,323 patients with Lp(a) above 70 mg/dL randomized to pelacarsen or placebo, designed to investigate the primary outcome of expanded major adverse cardiovascular events. It has started in 2019 and is expected to run through 2024.
2. Olpasiran (siRNA)
Olpasiran is a GalNAc-conjugated synthetic siRNA designed to directly inhibit the translation of LPA mRNA, modified with 2′-fluoro and 2′-methoxy substitutions as well as terminal phosphorothioate internucleotide linkages for stabilization. In a preclinical study on Apo(a) transgenic mice, more than an 80% reduction in serum Lp(a) concentration was achieved after a single administration of 1 mg/kg of olpasiran. An independent study confirmed dose-dependent reduction in serum Lp(a) by administering increasing doses of olpasiran in Lp(a) transgenic mice. In a phase 1 clinical study, olpasiran also reduced Lp(a) levels dose-dependently, with a maximum percent change from baseline ranging from −71% to −97% in participants with an initial screening Lp(a) of 70–199 nM and from −76% to −91% in those with Lp(a) ≥200 nM.40 In the phase 2 OCEAN(a)-DOSE clinical study, 281 patients were randomized to olpasiran up to a dose of 225 mg every 12 weeks or placebo.41 Recently announced data demonstrated a reduction in Lp(a) of ≥90% from baseline at 36 weeks from the start of the first administration for the majority of doses. During this period, no new adverse effects or safety concerns were noted.
CONCLUSION
There exists unequivocal evidence that therapies lowering LDL-C reduce the risk for ASCVD in proportion to the achieved reduction in LDL-C levels. In recent years, several new therapeutic options have shown substantial promise to further reduce the morbidity and mortality from ASCVD. These new therapies have the potential to reduce LDL-C in unprecedented levels and with less frequent dosing compared to previous medications. In addition to the lowering of LDL-C, triglyceride-rich lipoprotein remnants and Lp(a) have been identified as new therapeutic targets, and much progress has been made in development of specific modalities. These developments are likely to culminate in the near future in novel therapies that will greatly help physicians reduce the burden of ASCVD.
Footnotes
Funding: None.
Conflict of Interest: The authors have no conflicts of interest to declare.
- Conceptualization: Kim KA, Park HJ.
- Methodology: Kim KA.
- Supervision: Park HJ.
- Writing - original draft: Kim KA.
- Writing - review & editing: Park HJ.
References
- 1.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 2.Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ Res. 2016;118:547–563. doi: 10.1161/CIRCRESAHA.115.306249. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168–3209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 5.Cholesterol Treatment Trialists’ (CTT) Collaboration. Fulcher J, O’Connell R, Voysey M, Emberson J, Blackwell L, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397–1405. doi: 10.1016/S0140-6736(14)61368-4. [DOI] [PubMed] [Google Scholar]
- 6.Khan SU, Khan MU, Valavoor S, Khan MS, Okunrintemi V, Mamas MA, et al. Association of lowering apolipoprotein B with cardiovascular outcomes across various lipid-lowering therapies: systematic review and meta-analysis of trials. Eur J Prev Cardiol. 2020;27:1255–1268. doi: 10.1177/2047487319871733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Presta V, Figliuzzi I, Miceli F, Coluccia R, Fogacci F, Cicero AFG, et al. Achievement of low density lipoprotein (LDL) cholesterol targets in primary and secondary prevention: Analysis of a large real practice database in Italy. Atherosclerosis. 2019;285:40–48. doi: 10.1016/j.atherosclerosis.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 8.Scheidt-Nave C, Du Y, Knopf H, Schienkiewitz A, Ziese T, Nowossadeck E, et al. Prevalence of dyslipidemia among adults in Germany: results of the German Health Interview and Examination Survey for Adults (DEGS 1) Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:661–667. doi: 10.1007/s00103-013-1670-0. [DOI] [PubMed] [Google Scholar]
- 9.Lippi G, Targher G, Franchini M, Guidi GC. Biochemical correlates of lipoprotein(a) in a general adult population. Possible implications for cardiovascular risk assessment. J Thromb Thrombolysis. 2009;27:44–47. doi: 10.1007/s11239-007-0171-0. [DOI] [PubMed] [Google Scholar]
- 10.Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459–2472. doi: 10.1093/eurheartj/ehx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 12.Giugliano RP, Pedersen TR, Park JG, De Ferrari GM, Gaciong ZA, Ceska R, et al. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet. 2017;390:1962–1971. doi: 10.1016/S0140-6736(17)32290-0. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM, Mora S, Rose L JUPITER Trial Study Group. Percent reduction in LDL cholesterol following high-intensity statin therapy: potential implications for guidelines and for the prescription of emerging lipid-lowering agents. Eur Heart J. 2016;37:1373–1379. doi: 10.1093/eurheartj/ehw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36:1012–1022. doi: 10.1093/eurheartj/ehv043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mancini GB, Baker S, Bergeron J, Fitchett D, Frohlich J, Genest J, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian Consensus Working Group Update (2016) Can J Cardiol. 2016;32:S35–S65. doi: 10.1016/j.cjca.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Yusuf S, Bosch J, Dagenais G, Zhu J, Xavier D, Liu L, et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med. 2016;374:2021–2031. doi: 10.1056/NEJMoa1600176. [DOI] [PubMed] [Google Scholar]
- 18.Urban D, Pöss J, Böhm M, Laufs U. Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis. J Am Coll Cardiol. 2013;62:1401–1408. doi: 10.1016/j.jacc.2013.07.056. [DOI] [PubMed] [Google Scholar]
- 19.Frank-Kamenetsky M, Grefhorst A, Anderson NN, Racie TS, Bramlage B, Akinc A, et al. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc Natl Acad Sci U S A. 2008;105:11915–11920. doi: 10.1073/pnas.0805434105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitzgerald K, White S, Borodovsky A, Bettencourt BR, Strahs A, Clausen V, et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N Engl J Med. 2017;376:41–51. doi: 10.1056/NEJMoa1609243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ray KK, Landmesser U, Leiter LA, Kallend D, Dufour R, Karakas M, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med. 2017;376:1430–1440. doi: 10.1056/NEJMoa1615758. [DOI] [PubMed] [Google Scholar]
- 22.Pinkosky SL, Newton RS, Day EA, Ford RJ, Lhotak S, Austin RC, et al. Liver-specific ATP-citrate lyase inhibition by bempedoic acid decreases LDL-C and attenuates atherosclerosis. Nat Commun. 2016;7:13457. doi: 10.1038/ncomms13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ference BA, Ray KK, Catapano AL, Ference TB, Burgess S, Neff DR, et al. Mendelian randomization study of ACLY and cardiovascular disease. N Engl J Med. 2019;380:1033–1042. doi: 10.1056/NEJMoa1806747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldberg AC, Leiter LA, Stroes ES, Baum SJ, Hanselman JC, Bloedon LT, et al. Effect of bempedoic acid vs placebo added to maximally tolerated statins on low-density lipoprotein cholesterol in patients at high risk for cardiovascular disease: the CLEAR Wisdom randomized clinical trial. JAMA. 2019;322:1780–1788. doi: 10.1001/jama.2019.16585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laufs U, Banach M, Mancini GB, Gaudet D, Bloedon LT, Sterling LR, et al. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc. 2019;8:e011662. doi: 10.1161/JAHA.118.011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ballantyne CM, Banach M, Mancini GB, Lepor NE, Hanselman JC, Zhao X, et al. Efficacy and safety of bempedoic acid added to ezetimibe in statin-intolerant patients with hypercholesterolemia: A randomized, placebo-controlled study. Atherosclerosis. 2018;277:195–203. doi: 10.1016/j.atherosclerosis.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Ray KK, Bays HE, Catapano AL, Lalwani ND, Bloedon LT, Sterling LR, et al. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med. 2019;380:1022–1032. doi: 10.1056/NEJMoa1803917. [DOI] [PubMed] [Google Scholar]
- 28.Berg K. A new serum type system in man--the Lp system. Acta Pathol Microbiol Scand. 1963;59:369–382. doi: 10.1111/j.1699-0463.1963.tb01808.x. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt K, Noureen A, Kronenberg F, Utermann G. Structure, function, and genetics of lipoprotein (a) J Lipid Res. 2016;57:1339–1359. doi: 10.1194/jlr.R067314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, Tardif JC, Baum SJ, Steinhagen-Thiessen E, et al. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med. 2020;382:244–255. doi: 10.1056/NEJMoa1905239. [DOI] [PubMed] [Google Scholar]
- 31.Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69:692–711. doi: 10.1016/j.jacc.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 32.Khera AV, Everett BM, Caulfield MP, Hantash FM, Wohlgemuth J, Ridker PM, et al. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) Circulation. 2014;129:635–642. doi: 10.1161/CIRCULATIONAHA.113.004406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nordestgaard BG, Chapman MJ, Ray K, Borén J, Andreotti F, Watts GF, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–2853. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varvel S, McConnell JP, Tsimikas S. Prevalence of elevated Lp(a) mass levels and patient thresholds in 532 359 patients in the United States. Arterioscler Thromb Vasc Biol. 2016;36:2239–2245. doi: 10.1161/ATVBAHA.116.308011. [DOI] [PubMed] [Google Scholar]
- 35.Capoulade R, Chan KL, Yeang C, Mathieu P, Bossé Y, Dumesnil JG, et al. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J Am Coll Cardiol. 2015;66:1236–1246. doi: 10.1016/j.jacc.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 36.van Buuren F, Horstkotte D, Knabbe C, Hinse D, Mellwig KP. Incidence of elevated lipoprotein (a) levels in a large cohort of patients with cardiovascular disease. Clin Res Cardiol Suppl. 2017;12:55–59. doi: 10.1007/s11789-017-0087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raal FJ, Honarpour N, Blom DJ, Hovingh GK, Xu F, Scott R, et al. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385:341–350. doi: 10.1016/S0140-6736(14)61374-X. [DOI] [PubMed] [Google Scholar]
- 38.Crooke ST, Witztum JL, Bennett CF, Baker BF. RNA-targeted therapeutics. Cell Metab. 2018;27:714–739. doi: 10.1016/j.cmet.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Viney NJ, van Capelleveen JC, Geary RS, Xia S, Tami JA, Yu RZ, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016;388:2239–2253. doi: 10.1016/S0140-6736(16)31009-1. [DOI] [PubMed] [Google Scholar]
- 40.Koren MJ, Moriarty PM, Baum SJ, Neutel J, Hernandez-Illas M, Weintraub HS, et al. Preclinical development and phase 1 trial of a novel siRNA targeting lipoprotein(a) Nat Med. 2022;28:96–103. doi: 10.1038/s41591-021-01634-w. [DOI] [PubMed] [Google Scholar]
- 41.O’Donoghue ML, G López JA, Knusel B, Gencer B, Wang H, Wu Y, et al. Study design and rationale for the Olpasiran trials of Cardiovascular Events And lipoproteiN(a) reduction-DOSE finding study (OCEAN(a)-DOSE) Am Heart J. 2022;251:61–69. doi: 10.1016/j.ahj.2022.05.004. [DOI] [PubMed] [Google Scholar]