Abstract
Low-density lipoprotein cholesterol (LDL-C)-lowering therapy that increases LDL receptor expression in several ways robustly reduces the risk of atherosclerotic cardiovascular disease (CVD). However, a substantial risk of CVD still remains after intensive LDL-C reduction, which requires new treatment modalities for dyslipidemia and cardiovascular risk management. Triglycerides (TGs) and triglyceride-rich lipoproteins (TRLs) have received attention as indicators of residual cardiovascular risk and as direct causal factors for atherosclerosis and CVDs. Advances in understanding TG and TRL metabolism and their association with clinically evident CVDs have led to the development of novel therapeutic targets, including apolipoprotein C-III (apoC-III) and angiopoietin-like protein 3 (ANGPTL3). Genetic association studies have indicated that both apoC-III and ANGPTL3 play a causal role in the development of atherosclerotic CVD. Both molecules contribute to lipid dysregulation and atherosclerosis primarily by inhibiting lipoprotein lipase; however, recent evidence has shown that novel pathways exist in relation to their lipid-modifying activities. Notably, recent progress in therapeutic approaches, such as monoclonal antibodies or antisense oligonucleotides, has led to several novel therapeutics targeting apoC-III and ANGPTL3. This review summarized the recent updates and discussions related to apoC-III and ANGPTL3 expression.
Keywords: Lipid metabolism, Triglycerides, Lipoprotein lipase, Apolipoprotein C-III, Angiopoietin-like protein 3
ATHEROSCLEROTIC CARDIOVASCULAR DISEASE AND RESIDUAL RISK
In modern society, cardiovascular disease (CVD) is, undoubtedly, one of the most serious health problems. The prevalence of CVD was estimated to be 523 million, and CVD deaths reached 18.6 million in 2019 worldwide.1 Dyslipidemia, a dysregulated lipid and lipoprotein metabolism, is one of the leading causes of the development of atherosclerotic CVD.2 A substantial number of epidemiologic studies showed that an increase in circulating low-density lipoprotein cholesterol (LDL-C) concentration is associated with the occurrence of myocardial infarction and ischemic stroke,3,4 and mechanistic studies confirmed that LDL particles and their oxidized form have a causal role in atheroma formation within the vasculature.5,6
LDL-C lowering therapies that increase LDL receptor (LDLR) expression, such as statins and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, robustly lowered the risk of atherosclerotic CVDs in patients with or at risk of CVD.7,8,9 The question of how low the LDL-C level should be to prevent CVD is still under debate; however, most experts agree on the concept that the lower the LDL-C level, the better for cardiovascular health.10 It is possible to reach very low LDL-C levels through treatment with ezetimibe and PCSK9 inhibitors together with statins.11,12,13 The Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) trial showed that PCSK9 inhibitor evolocumab treatment lowered LDL-C to 30 mg/dL, with a 15% reduction in the major adverse cardiovascular events (MACE) incidence compared to placebo.13 Nevertheless, the residual risk of cardiovascular disease still exists. In the FOURIER trial, the incidence of major cardiovascular events in the evolocumab group at 3 years was 12.6%. Therefore, the reduction of residual risk following adequate LDL-C-lowering therapy is one of the most important topics in the field of dyslipidemia treatment.14
TRIGLYCERIDE, TRIGLYCERIDE-RICH LIPOPROTEIN, AND RESIDUAL RISK
In the blood, the triglycerides (TGs) are contained and transported in a variety of lipoproteins, most of which are contained in TG-rich lipoproteins (TRLs), such as chylomicron, very low-density lipoprotein (VLDL), and intermediate-density lipoprotein (IDL).15 Therefore, the measurement of blood TG levels approximately indicates the total amount of TGs contained in the TRLs, and the measurement of fasting TG level refers to the amount of TGs in the VLDL and IDL.
Hypertriglyceridemia, which is frequently accompanied by low high-density lipoprotein cholesterol (HDL-C), is a characteristic lipid abnormality in people with insulin resistance.16 Impaired insulin action increases free fatty acid release in the adipose tissue, leading to increased hepatic VLDL production and elevated blood TG levels. The TG-rich VLDL also transports excess TGs to HDL and LDL particles through the cholesteryl ester transfer protein (CETP) and accepts cholesteryl esters to produce TG-rich HDL and TG-rich LDL. Subsequently, the lipoprotein lipase (LPL) and hepatic lipase (HL) hydrolyze TGs, leading to small, dense HDL and LDL.17 In the Framingham Heart Study, diabetic patients had similar circulating LDL-C concentrations compared to those without diabetes; instead, they showed higher TG and lower HDL-C levels, which indicated dysregulated lipoprotein metabolism associated with insulin resistance.18 The association of circulating TG levels with the risk of CVD has been supported by epidemiological evidence. A pooled analysis of 29 epidemiological studies, which included 262,535 people, found that subjects with TG levels in the upper tertile had an approximately 72% increased risk of coronary heart disease compared to those in the lower tertile. These results were not affected by fasting status or adjustment for HDL-C level.19
Beyond the role of hypertriglyceridemia as a risk indicator of atherosclerotic CVD, various studies have been conducted to determine whether TG or TRL has a causal role in the development of atherosclerosis and CVD. It has been suggested that VLDL2, which is a relatively small VLDL particle, and IDL are able to penetrate the intima and are subsequently ingested by macrophages to form foam cells.20,21 Chylomicron remnants and TRLs ranging in size from 55 to 150 nm were retained within the arterial intima, and they showed prolonged residence time in the vascular space.22,23 A genetic study in humans convincingly suggested the causality of TRL for atherosclerotic CVD. A genome-wide association study (GWAS), The Global Lipids Genetics Consortium (GLGC) Metabochip study, indicated that the effect size of single nucleotide polymorphisms (SNPs) associated with TG elevation on ischemic heart disease was similar to that of SNPs associated with LDL-C elevation.24 On the other hand, SNPs associated with HDL-C reduction had no significant effect on the occurrence of ischemic heart disease. A Mendelian randomization study involving 73,513 people reported that the risk of ischemic heart disease increased as the number of genetic variant alleles associated with remnant cholesterol increased.25 Meanwhile, another Mendelian randomization study showed that TG-lowering variants in the LPL gene had similar odds of coronary heart disease compared to LDL-C-lowering variants in the LDLR gene.26
Taken together, TG and TRL are responsible for the development of atherosclerotic CVD; this is supported by epidemiological, genetic, and biological studies. Nevertheless, clinical evidence on whether TG-lowering therapy reduces the risk of atherosclerotic CVD is unclear. The cardiovascular protective role of fibrate, a peroxisome proliferator-activated receptor (PPAR) alpha agonist, is limited.27,28 Omega-3 fatty acids appear to have a protective effect on atherosclerotic CVD at high doses of eicosapentaenoic acid (EPA) only. In the Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial (REDUCE-IT), 4 g of EPA per day significantly reduced the incidence of MACE in high-risk patients treated with statins; however, TG lowering by EPA supplementation did not fully explain the favorable outcomes of REDUCE-IT.29,30 Given this, how should we interpret the conflicting results of TG/TRL’s causal role in atherosclerotic CVD and the clinical impact of TG-lowering therapy? One possibility is that the TG level was not sufficiently reduced with the currently available drugs. Additionally, other strategies to reduce circulating TG/TRL levels may be necessary. Therefore, advances in the understanding of TG metabolism centered on LPL and recent discussions on novel target molecules are noteworthy. Apolipoprotein C-III (apoC-III) and angiopoietin-like protein 3 (ANGPTL3) are currently the centers of this review, and novel therapies targeting these molecules are presented in Table 1 and Fig. 1. We would like to describe them more in detail.
Table 1. Major clinical trials of novel lipid-lowering agents targeting apoC-III and ANGPTL3.
| Target | Drug name | Mechanism of action | Major trials | Study participants | Main results* |
|---|---|---|---|---|---|
| ApoC-III | Volanesorsen | ASO | Phase 1 trial31 | 33 healthy adults | TG ↓ (44%) |
| Phase 2 trial32 | 15 Patients with T2D and hypertriglyceridemia | TG ↓ (69%), HDL-C ↑ (42%) | |||
| Phase 3 trial33 | 66 patients with familial chylomicronemia syndrome | TG ↓ (77%) | |||
| Phase 3 trial34 | 114 patients with severe hypertriglyceridemia or familial chylomicronemia syndrome | TG ↓ (71%) | |||
| Olezarsen | GALNAc3-modified ASO | Phase 1/2 trial35 | 40 (single ascending dose study) and 10 (multiple ascending dose study) healthy adults | TG ↓ (77%) | |
| ANGPTL3 | Evinacumab | Monoclonal antibody | Phase 1 trial36 | 83 healthy adults | TG ↓ (76%), LDL-C ↓ (23%), HDL-C ↓ (18%) |
| Phase 1 trial37 | 83 (single ascending dose study) and 56 (multiple ascending dose study) healthy adults | TG ↓ (83%) | |||
| Phase 2 trial38 | 272 patients with refractory hypercholesterolemia | LDL-C ↓ (50%) | |||
| Phase 2 trial39 | 51 patients with severe hypertriglyceridemia | TG ↓ (57%) | |||
| Phase 3 trial40 | 65 patients with homozygous familial hypercholesterolemia | LDL-C ↓ (47%) | |||
| Vupanorsen | GALNAc3-modified ASO | Phase 1 trial41 | 44 healthy adults | TG ↓ (63%), LDL-C ↓ (33%) | |
| Phase 2 trial42 | 105 patients with TG >150 mg/dL, T2D, and hepatic steatosis | TG ↓ (53%), HDL-C ↓ (18%) |
ApoC-III, apolipoprotein C-III; ANGPTL3, angiopoietin-like protein 3; ASO, antisense oligonucleotide; GALNAc3, N-acetyl galactosamine; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; T2D, type 2 diabetes; TG, triglycerides.
*Lipid-lowering effects were dose-dependent, and the maximal percent change in plasma concentrations from baseline is presented here.
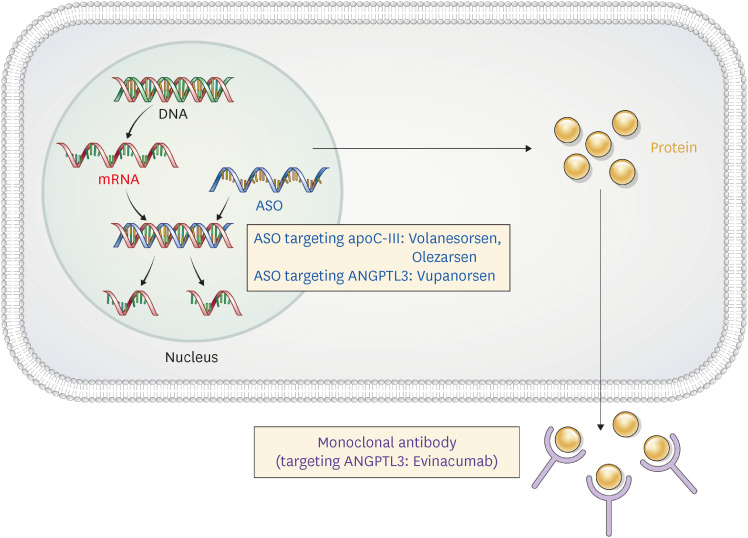
Fig. 1. Therapeutic approaches targeting apoC-III and ANGPTL3.
ASO for the inactivation of apoC-III (volanesorsen and olezarsen) and ANGPTL3 (vupanorsen), and a monoclonal antibody (evinacumab) to ANGPTL3 have been developed.
ASO, antisense oligonucleotide; apoC-III, apolipoprotein C-III; ANGPTL3, angiopoietin-like protein 3.
ApoC-III
1. The role of apoC-III in lipid metabolism
ApoC-III, an apolipoprotein encoded by APOC3, is a 79-amino acid glycoprotein synthesized by hepatocytes and enterocytes. It is carried on almost all types of lipoproteins in varying proportions; TRLs, specifically VLDL and chylomicrons, are major lipoproteins carrying apoC-III.43
ApoC-III exerts diverse effects on lipoprotein metabolism (Fig. 2). It inhibits lipolysis of TRLs by interfering with the interaction of TRLs with LPL and HL, which results in elevated circulating levels of TRLs and TG.44 It also reduces hepatic uptake of the TRL remnants by interrupting the binding of apoB or apoE to LDLR and low-density lipoprotein-related protein 1 (LRP1).45 Aside from its effects on delayed clearance of TRLs, evidence indicates that apoC-III also increases the production and secretion of VLDL.46 In addition, the apoC-III contributes to vascular inflammation and atherogenesis via multiple pathways, including modulation of LDL affinity to the arterial wall, formation of apoC-III-HDL, and upregulation of proinflammatory mediators.47,48,49
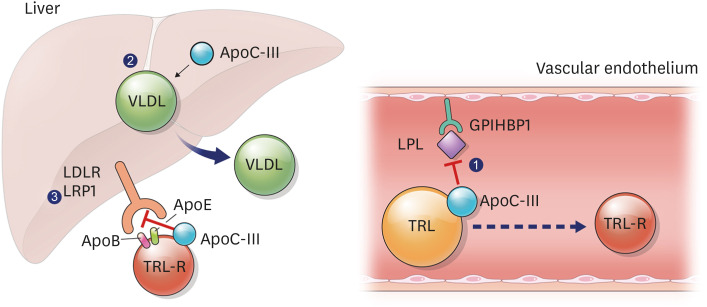
Fig. 2. The role of apoC-III in triglyceride-rich lipoprotein metabolism.
ApoC-III ① inhibits the interaction between LPL and TRLs, resulting in reduced lipolysis of TRLs, ② increases the synthesis and secretion of hepatic VLDL, ③ decreases hepatic clearance of TRL remnants via LDLR or LRP1 by preventing the binding of apoB and apoE to those receptors.
ApoC-III, apolipoprotein C-III; LPL, lipoprotein lipase; TRL, triglyceride-rich lipoprotein; VLDL, very low-density lipoprotein; LDLR, low-density lipoprotein receptor; LRP1, LDLR-related protein 1; apoB, apolipoprotein B; apoE, apolipoprotein E; GPIHBP1, glycosylphosphatidylinositol anchored high density lipoprotein binding protein 1; TRL-R, triglyceride-rich lipoprotein remnants.
2. Genetic association of APOC3 with lipoprotein metabolism and cardiovascular disease
The relationship between apoC-III and CVD has been reported for several decades. A large-scale case-control study of 800 people found an association between the apoC-III gene polymorphism and angiographically documented coronary artery disease.50 This study identified three polymorphisms in the apoC-III gene, among which the homozygosity for the T-445C variant was found in 18.6% of coronary artery disease patients, which was more than double that of 9.2% in the coronary artery disease-free group. Even after adjusting for other cardiovascular risk factors, the T-445C variant was found to be an independent genetic susceptibility factor for coronary artery disease with an odds ratio of 2.18.
A GWAS published in 2008 identified the relationship between the APOC3 null mutation and lipid profile and CVD.51 Approximately 5% of Lancaster Amish were heterozygous carriers of a null mutation (R19X) in APOC3. They had significantly lower fasting and post-high-fat challenge TG levels than non-carriers and showed significantly lower LDL-C, non-HDL-C, and significantly higher HDL-C levels. In addition, these mutation carriers showed significantly lower Framingham 10-year coronary heart disease risk and coronary artery calcium (CAC) scores than non-carriers, suggesting that the APOC3 null mutation is associated with clinical CVD beyond lipid modification.
Two large prospective studies done on the Danish general population (Copenhagen City Heart Study and Copenhagen General Population Study), including 75,725 participants, validated the relationship between the loss-of-function mutations in APOC3 and CVD.52 There was a significantly positive association between nonfasting plasma TG levels and ischemic vascular disease/ischemic heart disease. Three APOC3 mutations (R19X, IVS2+1G→A, and A43T) were assessed. Heterozygote carriers for any of these mutations showed a 44% decrease in non-fasting TG levels, 41% lower risk of ischemic vascular disease, and 36% lower risk of ischemic heart disease than non-carriers.
These genetic studies showed that apoC-III has a causal relationship with the occurrence of clinically evident CVD beyond its effect on plasma TG levels, suggesting that apoC-III targeting therapy is a promising approach in reducing CVD risk.
3. Therapeutic approaches targeting apoC-III
Antisense oligonucleotides (ASOs) are single-stranded oligonucleotides that bind to target pre-mRNA or mRNA to regulate the expression of target molecules via degradation, isoform switching, and translation inhibition (Fig. 1).53 The antisense inhibition of apoC-III induced an effective reduction of apoC-III levels and TG/TRL levels as shown in preclinical and clinical studies. In a preclinical model and phase 1 clinical trial, apoC-III ASO ISIS 304801 (volanesorsen) demonstrated a dose-dependent decrease in apoC-III and TG levels.31 In healthy human volunteers, treatment with the maximal dose of ISIS 304801 reduced apoC-III levels by more than 77.5% and TG levels by 43.8%. In a randomized placebo-controlled trial for type 2 diabetes patients with hypertriglyceridemia, a 300 mg subcutaneous dose of volanesorsen once a week was used.32 Volanesorsen decreased plasma apoC-III levels by 88%, TG levels by 69%, and increased HDL-C levels by 42% compared to placebo. Notably, the whole-body insulin sensitivity measured by the hyperinsulinemic-euglycemic clamp in the volanesorsen-treated group increased by 57%, which was significantly correlated with the decrease in plasma apoC-III and TG levels.
A phase-3, double-blind, randomized 52-week trial was conducted to evaluate the TG lowering effect of volanesorsen in patients with familial chylomicronemia (APPROACH trial).33 In this study, 66 patients with familial chylomicronemia syndrome were stratified into the volanesorsen (300 mg subcutaneously once a week) group or the placebo group. At baseline, the mean TG level was 2,209 mg/dL, and 76% of the participants had a history of pancreatitis. The fasting TG level measured at three months decreased by 76.5% and the HDL-C level increased by 46.1% in the volanesorsen group. The treatment effects persisted for 52 weeks in individuals who received the drug without any dose reduction. This study showed that a new effective treatment is possible for patients with a rare genetic lipoprotein disorder, familial chylomicronemia syndrome, for which there has been no effective treatment before. At the same time, it showed that apoC-III inhibition effectively lowered TG levels through an LPL-independent pathway. A few safety concerns associated with volanesorsen treatment, including thrombocytopenia, have been noted. A platelet count of less than 140,000/µL was observed in 76% of patients in the volanesorsen treatment group.
The COMPASS study is a multinational, phase 3, randomized controlled trial in patients with severe hypertriglyceridemia (>500 mg/dL) or familial chylomicronemia syndrome.34 This is the largest study of volanesorsen, wherein 114 patients were assigned subcutaneous volanesorsen 300 mg once a week or placebo for 26 weeks. Volanesorsen reduced circulating TG, chylomicron TG, VLDL cholesterol (VLDL-C), apolipoprotein B48, and apoC-III levels by >70% at three months. Non-HDL-C levels were also reduced by 27.3%. Pancreatitis occurred in three of the 38 patients in the placebo group, whereas none of the 75 patients in the volanesorsen developed pancreatitis. Thrombocytopenia with a platelet count of less than 100,000/µL occurred in nine patients in the volanesorsen group.
In summary, apoC-III targeting ASO volanesorsen effectively reduced TG levels and induced a significant decrease in non-HDL-C levels, which raises expectations for the reduction of cardiovascular risk. However, evidence for the application of volanesorsen therapy to a broader patient population and for favorable cardiovascular outcomes is still limited. In addition, it is necessary to consider whether the side effects of thrombocytopenia are acceptable in clinical situations. In 2019, the European Medicines Agency approved volanesorsen as a treatment for familial chylomicronemia syndrome54; however, the U.S. Food and Drug Administration (FDA) did not because of several concerns, including thrombocytopenia.
A new ASO approach to minimize the side effects of volanesorsen and lower TG more effectively was proposed. This was an N-acetyl galactosamine (GalNAc3)-conjugated ASO targeting apoC-III, olezarsen (formerly known as AKCEA-APOCIII-LRx), which has hepatocyte-specific action and can minimize systemic exposure.55 Olezarsen was evaluated to be at least 15 times more potent than volanesorsen. In a phase 1/2a study involving healthy volunteers, olezarsen decreased apoC-III and TG levels in a dose-dependent manner.35 At a maximal dose (120 mg subcutaneously once a week), a TG reduction of 77% was observed. In addition, significant reductions in non-HDL-C, VLDL-C, and apoB levels were observed, and no significant adverse events, including thrombocytopenia, were observed.
Another therapeutic approach targeting apoC-III, including apoC-III monoclonal antibody56 and RNA interference targeting apoC-III57 has been introduced.
ANGPTL3
1. The role of ANGPTL3 in lipid metabolism
ANGPTL3, a member of the angiopoietin-like protein family (ANGPTL1-8), is primarily synthesized and secreted by the liver.58,59 It inhibits the activity of LPL, a key enzyme involved in the clearance of TRLs, such as chylomicrons and VLDL (Fig. 3A).60 Thus, ANGPTL3 increases serum TG levels, which has been proved in animal experiments. Overexpression of ANGPTL3 or injection of recombinant ANGPTL3 protein in hypolipidemic KK/San mice resulted in high levels of total cholesterol, TG, and free fatty acids.61 The function of ANGPTL3 is enhanced by ANGPTL8.62 ANGPTL8 increases the ability of ANGPTL3 to bind LPL, and the ANGPTL3/8 complex showed enhanced inhibition of LPL compared to ANGPTL3 alone.63 ANGPTL3 also inhibits the activity of endothelial lipase (EL) which hydrolyzes HDL phospholipid (Fig. 3B).64 Consequently, ANGPTL3 increases plasma HDL levels. ANGPTL3-deficient mice showed low plasma HDL-C levels, which were increased by adenovirus vectors containing ANGPTL3.65
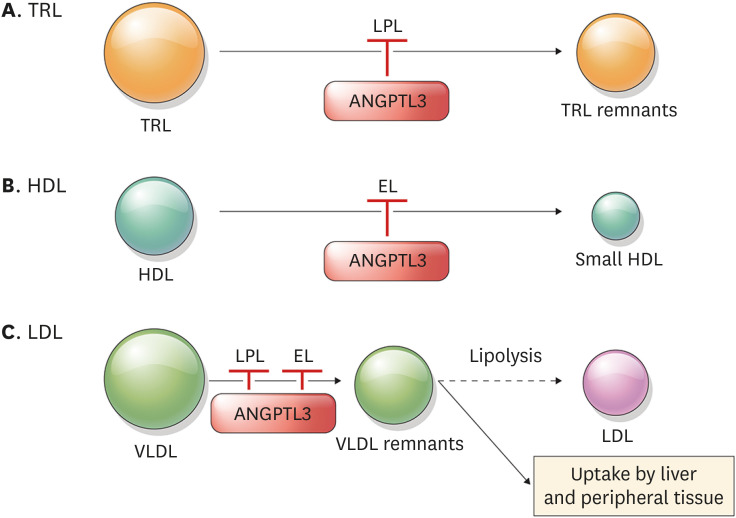
Fig. 3. The role of ANGPTL3 in the metabolism of TRL (A), HDL (B), and LDL (C).
ANGPTL3 inhibits LPL and EL, which are key enzymes involved in the clearance of TRL and HDL, respectively. These enzymes are also related to VLDL clearance and production of LDL, as the VLDL remnants are the precursors of LDL. By inhibiting ANGPTL3, the process drawn in a solid line is facilitated, while the process drawn in a dotted line is decreased.
ANGPTL3, angiopoietin-like protein 3; TRL, triglyceride-rich proteins; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LPL, lipoprotein lipase; EL, endothelial lipase; VLDL, very low-density lipoproteins.
The mechanism by which ANGPTL3 regulates LDL metabolism is not yet clear; however, it is evident that inhibition of ANGPTL3 results in low LDL-C levels. Previous studies showed that mice deficient in LDLR (Ldlr−/−) and humans with absent LDLR activity have reduced LDL-C levels by ANGPTL3 inhibition, indicating that inhibition of ANGPTL3 lowered LDL-C independently of LDLR.40 , 66 In contrast, mice deficient in both EL and LDLR (Lipg−/− Ldlr−/−) showed no reduction in LDL-C levels after ANGPTL3 inhibition, suggesting that EL is critical in LDL regulation by ANGPTL3.66 Given that EL promotes VLDL catabolism, and VLDL clearance is delayed in Lipg−/− mice, one possible explanation is that when ANGPTL3 is inhibited, hydrolysis of VLDL and VLDL remnant clearance is promoted. This consequently leads to the depletion of the LDL precursor pool and reduced production of LDL (Fig. 3C).
2. Genetic association of ANGPTL3 with lipoprotein metabolism and CVD
Human genetic studies have revealed that ANGPTL3 is closely associated with circulating lipid levels. Loss-of-function (LOF) variants of ANGPTL3 are associated with decreased plasma levels of TG, LDL-C, and HDL-C. Whole exome sequencing of two patients with familial combined hypolipidemia, an inherited disorder of lipid metabolism with extremely low levels of LDL-C, HDL-C, and TG, revealed two nonsense alleles (S17X and E129X), leading to complete deficiency of ANGPTL3.67
Exome sequencing of ANGPTL3 in 58,335 individuals in the DiscovEHR human genetics study also showed that people with heterozygous LOF variants in ANGPTL3 have lower serum LDL-C, HDL-C, and TG levels and ANGPTL3 concentrations.36 In another study with 20,092 people in the Myocardial Infarction Genetics Consortium studies, heterozygous carriers of ANGPTL3 LOF mutation had an 11.8% and 17% reduction in LDL-C and TG, respectively, compared with non-carriers.68 HDL-C levels were 5.2% lower in LOF carriers without statistical significance.
Associations between ANGPTL3 deficiency and the risk of coronary artery disease have been evaluated. Three patients with complete ANGPTL3 deficiency and their first-degree relatives without ANGPTL3 deficiency were examined for coronary atherosclerotic plaque burden by computed tomography angiography.68 All three patients with complete ANGPTL3 deficiency showed no evidence of coronary plaques. In contrast, 2 of the 3 matched control participants had positive coronary calcium scores. Similarly, carriers of the LOF mutation in ANGPTL3 showed a lower risk of atherosclerotic CVD in several cohort studies. A cohort-based meta-analysis of 19 studies comprising 21,980 patients with coronary artery disease and 158,200 controls, demonstrated that carriers of ANGPTL3 LOF mutation had a 34% lower risk of coronary artery disease than non-carriers.68 In the analysis of 13,102 patients with coronary artery disease and 40,430 controls, ANGPTL3 LOF variants were associated with 41% lower odds of coronary artery disease.36 In a meta-analysis of five independent population studies, carriers of ANGPTL3 LOF mutations showed a 39% lower risk of coronary artery disease than non-carriers.36 The cardioprotective effects of ANGPTL3 LOF mutations have led to further studies evaluating the association between the plasma ANGPTL3 concentrations and myocardial infarction risk. Plasma ANGPTL3 concentrations were measured in 1,493 patients with myocardial infarction and 3,231 controls.68 Individuals in the lowest tertile of circulating ANGPTL3 concentrations had a 35% lower risk of myocardial infarction than those in the highest tertile.
3. Therapeutic approaches targeting ANGPTL3 – monoclonal antibodies
Based on the results of biological and genetic studies, ANGPTL3 has been suggested as a promising therapeutic target for dyslipidemia. Two different strategies for the inactivation of ANGPTL3 have been developed: a monoclonal antibody and an ASO (Fig. 1). Evinacumab is a fully humanized anti-ANGPTL3 antibody binding ANGPTL3 with high affinity, which can reverse ANGPTL3-induced inhibition of LPL activity in vitro and in vivo.69 A phase 1 study was performed among 83 healthy adults with fasting TG levels of 150 to 450 mg/dL and LDL-C levels of 100 mg/dL or greater.36 Lipid-lowering effects of evinacumab were dose-dependent, with reductions in plasma concentrations up to 23% in LDL-C, 18% in HDL-C, and 76% in TG. No serious adverse events leading to discontinuation of the drug were observed.
Evinacumab has been evaluated for the treatment of homozygous familial hypercholesterolemia (HoFH). In the phase 3 clinical trial, 65 patients with HoFH receiving lipid-lowering therapy at the maximally tolerated doses were randomly assigned to the intravenous infusion of evinacumab (15 mg/kg every 4 weeks) group or the placebo group.40 The mean baseline LDL-C concentration was 255.1 mg/dL, and the mean non-HDL-C concentration was 277.8 mg/dL. At week 24, a 47.1% reduction in LDL-C level from the baseline was observed in the evinacumab group, while a 1.9% increase was observed in the placebo group. The LDL-C-lowering effects of evinacumab were comparable between the patients with null-null variants and those with non-null variants. Influenza-like illness was reported in 5 of 44 patients receiving evinacumab; however, other adverse events did not significantly increase with evinacumab treatment. Antidrug antibodies were not developed during the trial. Evinacumab has recently been approved for HoFH treatment by the U.S. FDA (EVKEEZA™).70
Evinacumab also effectively reduces LDL-C levels in patients with refractory hypercholesterolemia. In the phase 2 trial, 272 patients with or without heterozygous familial hypercholesterolemia who had refractory hypercholesterolemia were randomly assigned evinacumab or placebo.38 Refractory hypercholesterolemia was defined as having an LDL-C level ≥70 mg/dL with atherosclerosis or LDL-C ≥100 mg/dL without atherosclerosis, on treatment with lipid-lowering therapies at the maximally tolerated doses. At baseline, 99% of the participants received PCSK9 inhibitors, 70% received statins, and 33% were treated with ezetimibe. Evinacumab was administered subcutaneously (SC) or intravenously (IV) at doses of 450 mg SC weekly, 300 mg SC weekly, 300 mg SC every 2 weeks, 15 mg/kg IV every 4 weeks, or 5 mg/kg IV every 4 weeks. The baseline LDL-C levels of the participants were 150 mg/dL in the SC group and 145 mg/dL in the IV group. The LDL-C reduction by evinacumab was dose-dependent, with up to 49.9% reduction from baseline at the maximum dose.
Evinacumab has also been tested for the treatment of hypertriglyceridemia. A phase 1 trial involving subjects with TG levels >150 and ≤450 mg/dL showed a robust TG-lowering effect of evinacumab.37 The trial enrolled 83 subjects for a single ascending dose study, and 56 for a multiple ascending dose study. TGs were reduced in a dose-dependent manner, with a maximum reduction of approximately 80% in both studies. No serious adverse events were reported; however, mild elevations in alanine aminotransferase, aspartate aminotransferase, and creatinine phosphokinase levels were observed. In the phase 2 trial (NCT03452228), 51 patients with severe hypertriglyceridemia with TG ≥500 mg/dL who had prior hospitalization for acute pancreatitis were randomly assigned to receive evinacumab 15 mg/kg IV every 4 weeks or placebo for 12 weeks.39 At week 12, the patients receiving evinacumab showed a median of 805.8 mg/dL reduction in TGs (−56.6% from baseline) as compared with an increase of 50.7 mg/dL (+1.8% from baseline) in the placebo group. However, the treatment response varied according to genotype, requiring further elucidation. A phase 2b trial investigating the efficacy of evinacumab in the prevention of recurrent acute pancreatitis (NCT04863014) is ongoing.
4. Therapeutic approaches targeting ANGPTL3 – ASO
ASO targeting ANGPTL3 mRNA is another way to inactivate ANGPTL3. GalNAc3-modified ASO is a novel technology to selectively target RNAs expressed by hepatocytes through binding of the asialoglycoprotein receptors, enabling them to have an efficacy similar to unconjugated ASOs with a 20- to 30-fold lowered dose.71 Vupanorsen (previously known as IONIS-ANGPTL3-LRx) is a second-generation GalNAc3-modified ASO targeting hepatic ANGPTL3 mRNA. In a phase 1 trial with 44 healthy participants, vupanorsen reduced the plasma levels of ANGPTL3 (47%–85% from baseline), TG (33%–63%), LDL-C (1%–33%), VLDL-C (28%–60%), and non-HDL-C (10%–37%) after six weeks of treatment.41 No serious adverse events were reported.
A phase 2 trial was conducted among 105 patients with type 2 diabetes and hepatic steatosis with TG levels higher than 150 mg/dL.42 The participants received either placebo or vupanorsen 40 mg every 4 weeks, 80 mg every 4 weeks, or 20 mg every week, subcutaneously for 6 months. The median baseline TG level was 252 mg/dL. TG levels were reduced by 36%, 53%, and 47% in patients receiving 40 mg of vupanorsen every 4 weeks, 80 mg every 4 weeks, and 20 mg every week, respectively. Vupanorsen 80 mg every four weeks reduced ANGPTL3 levels by 59%, LDL-C levels by 7%, and HDL-C levels by 18%. The most frequent adverse events were injection-site pruritus (14%) and erythema (12%). Vupanorsen was not associated with thrombocytopenia; however, 2 patients receiving vupanorsen showed an increase in alanine aminotransferase and aspartate aminotransferase levels.
CONCLUSION
In this review, we summarized the recent updates and discussions regarding apoC-III and ANGPTL3. Genetic variants of apoC-III and ANGPTL3 are closely associated with lipid dysregulation and predispose CVDs. Pharmacological inhibition of apoC-III and ANGPTL3 profoundly reduced circulating TG, LDL-C, and non-HDL-C levels in multiple clinical studies, suggesting their promising role in cardiovascular risk reduction. Further studies are expected to elucidate the clinical impact these strategies in the near future.
Footnotes
Funding: This research was supported by a grant of Korea University Anam Hospital, Seoul, Republic of Korea (No. O2207631).
Conflict of Interest: Nam Hoon Kim is Deputy Editor, and Ji Yoon Kim is editor of Journal of Lipid and Atherosclerosis. However, they were not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
- Conceptualization: Kim NH.
- Writing - original draft: Kim JY, Kim NH.
- Writing - review & editing: Kim JY, Kim NH.
References
- 1.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pol T, Held C, Westerbergh J, Lindbäck J, Alexander JH, Alings M, et al. Dyslipidemia and risk of cardiovascular events in patients with atrial fibrillation treated with oral anticoagulation therapy: insights from the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial. J Am Heart Assoc. 2018;7:e007444. doi: 10.1161/JAHA.117.007444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharrett AR, Ballantyne CM, Coady SA, Heiss G, Sorlie PD, Catellier D, et al. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2001;104:1108–1113. doi: 10.1161/hc3501.095214. [DOI] [PubMed] [Google Scholar]
- 4.Mortensen MB, Nordestgaard BG. Elevated LDL cholesterol and increased risk of myocardial infarction and atherosclerotic cardiovascular disease in individuals aged 70-100 years: a contemporary primary prevention cohort. Lancet. 2020;396:1644–1652. doi: 10.1016/S0140-6736(20)32233-9. [DOI] [PubMed] [Google Scholar]
- 5.Steinbrecher UP, Parthasarathy S, Leake DS, Witztum JL, Steinberg D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci U S A. 1984;81:3883–3887. doi: 10.1073/pnas.81.12.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol. 2009;29:431–438. doi: 10.1161/ATVBAHA.108.179564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross SD, Allen IE, Connelly JE, Korenblat BM, Smith ME, Bishop D, et al. Clinical outcomes in statin treatment trials: a meta-analysis. Arch Intern Med. 1999;159:1793–1802. doi: 10.1001/archinte.159.15.1793. [DOI] [PubMed] [Google Scholar]
- 9.Karatasakis A, Danek BA, Karacsonyi J, Rangan BV, Roesle MK, Knickelbine T, et al. Effect of PCSK9 inhibitors on clinical outcomes in patients with hypercholesterolemia: a meta-analysis of 35 randomized controlled trials. J Am Heart Assoc. 2017;6:e006910. doi: 10.1161/JAHA.117.006910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289–1297. doi: 10.1001/jama.2016.13985. [DOI] [PubMed] [Google Scholar]
- 11.Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 13.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 14.Fruchart JC, Sacks F, Hermans MP, Assmann G, Brown WV, Ceska R, et al. The Residual Risk Reduction Initiative: a call to action to reduce residual vascular risk in patients with dyslipidemia. Am J Cardiol. 2008;102:1K–34K. doi: 10.1016/S0002-9149(08)01833-X. [DOI] [PubMed] [Google Scholar]
- 15.Kwiterovich PO., Jr The metabolic pathways of high-density lipoprotein, low-density lipoprotein, and triglycerides: a current review. Am J Cardiol. 2000;86:5L–10L. doi: 10.1016/s0002-9149(00)01461-2. [DOI] [PubMed] [Google Scholar]
- 16.Grundy SM. Hypertriglyceridemia, atherogenic dyslipidemia, and the metabolic syndrome. Am J Cardiol. 1998;81:18B–25B. doi: 10.1016/s0002-9149(98)00033-2. [DOI] [PubMed] [Google Scholar]
- 17.Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab. 2009;5:150–159. doi: 10.1038/ncpendmet1066. [DOI] [PubMed] [Google Scholar]
- 18.Kannel WB. Lipids, diabetes, and coronary heart disease: insights from the Framingham Study. Am Heart J. 1985;110:1100–1107. doi: 10.1016/0002-8703(85)90224-8. [DOI] [PubMed] [Google Scholar]
- 19.Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115:450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 20.Packard CJ, Boren J, Taskinen MR. Causes and consequences of hypertriglyceridemia. Front Endocrinol (Lausanne) 2020;11:252. doi: 10.3389/fendo.2020.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626–635. doi: 10.1016/S0140-6736(14)61177-6. [DOI] [PubMed] [Google Scholar]
- 22.Proctor SD, Vine DF, Mamo JC. Arterial permeability and efflux of apolipoprotein B-containing lipoproteins assessed by in situ perfusion and three-dimensional quantitative confocal microscopy. Arterioscler Thromb Vasc Biol. 2004;24:2162–2167. doi: 10.1161/01.ATV.0000143859.75035.5a. [DOI] [PubMed] [Google Scholar]
- 23.Proctor SD, Vine DF, Mamo JC. Arterial retention of apolipoprotein B(48)- and B(100)-containing lipoproteins in atherogenesis. Curr Opin Lipidol. 2002;13:461–470. doi: 10.1097/00041433-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Do R, Willer CJ, Schmidt EM, Sengupta S, Gao C, Peloso GM, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet. 2013;45:1345–1352. doi: 10.1038/ng.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varbo A, Benn M, Tybjærg-Hansen A, Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013;61:427–436. doi: 10.1016/j.jacc.2012.08.1026. [DOI] [PubMed] [Google Scholar]
- 26.Ference BA, Kastelein JJ, Ray KK, Ginsberg HN, Chapman MJ, Packard CJ, et al. Association of triglyceride-lowering LPL variants and LDL-C-lowering LDLR variants with risk of coronary heart disease. JAMA. 2019;321:364–373. doi: 10.1001/jama.2018.20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 28.ACCORD Study Group. Ginsberg HN, Elam MB, Lovato LC, Crouse JR, 3rd, Leiter LA, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 30.Sheikh O, Vande Hei AG, Battisha A, Hammad T, Pham S, Chilton R. Cardiovascular, electrophysiologic, and hematologic effects of omega-3 fatty acids beyond reducing hypertriglyceridemia: as it pertains to the recently published REDUCE-IT trial. Cardiovasc Diabetol. 2019;18:84. doi: 10.1186/s12933-019-0887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graham MJ, Lee RG, Bell TA, 3rd, Fu W, Mullick AE, Alexander VJ, et al. Antisense oligonucleotide inhibition of apolipoprotein C-III reduces plasma triglycerides in rodents, nonhuman primates, and humans. Circ Res. 2013;112:1479–1490. doi: 10.1161/CIRCRESAHA.111.300367. [DOI] [PubMed] [Google Scholar]
- 32.Digenio A, Dunbar RL, Alexander VJ, Hompesch M, Morrow L, Lee RG, et al. Antisense-mediated lowering of plasma apolipoprotein C-III by volanesorsen improves dyslipidemia and insulin sensitivity in type 2 diabetes. Diabetes Care. 2016;39:1408–1415. doi: 10.2337/dc16-0126. [DOI] [PubMed] [Google Scholar]
- 33.Witztum JL, Gaudet D, Freedman SD, Alexander VJ, Digenio A, Williams KR, et al. Volanesorsen and triglyceride levels in familial chylomicronemia syndrome. N Engl J Med. 2019;381:531–542. doi: 10.1056/NEJMoa1715944. [DOI] [PubMed] [Google Scholar]
- 34.Gouni-Berthold I, Alexander VJ, Yang Q, Hurh E, Steinhagen-Thiessen E, Moriarty PM, et al. Efficacy and safety of volanesorsen in patients with multifactorial chylomicronaemia (COMPASS): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2021;9:264–275. doi: 10.1016/S2213-8587(21)00046-2. [DOI] [PubMed] [Google Scholar]
- 35.Alexander VJ, Xia S, Hurh E, Hughes SG, O’Dea L, Geary RS, et al. N-acetyl galactosamine-conjugated antisense drug to APOC3 mRNA, triglycerides and atherogenic lipoprotein levels. Eur Heart J. 2019;40:2785–2796. doi: 10.1093/eurheartj/ehz209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dewey FE, Gusarova V, Dunbar RL, O’Dushlaine C, Schurmann C, Gottesman O, et al. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N Engl J Med. 2017;377:211–221. doi: 10.1056/NEJMoa1612790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmad Z, Banerjee P, Hamon S, Chan KC, Bouzelmat A, Sasiela WJ, et al. Inhibition of angiopoietin-like protein 3 with a monoclonal antibody reduces triglycerides in hypertriglyceridemia. Circulation. 2019;140:470–486. doi: 10.1161/CIRCULATIONAHA.118.039107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenson RS, Burgess LJ, Ebenbichler CF, Baum SJ, Stroes ES, Ali S, et al. Evinacumab in patients with refractory hypercholesterolemia. N Engl J Med. 2020;383:2307–2319. doi: 10.1056/NEJMoa2031049. [DOI] [PubMed] [Google Scholar]
- 39.Rosenson RS, Gaudet D, Ballantyne CM, Baum SJ, Bergeron J, Kershaw EE, et al. A phase 2 trial of the efficacy and safety of evinacumab in patients with severe hypertriglyceridemia. Atherosclerosis. 2021;331:e293 [Google Scholar]
- 40.Raal FJ, Rosenson RS, Reeskamp LF, Hovingh GK, Kastelein JJP, Rubba P, et al. Evinacumab for homozygous familial hypercholesterolemia. N Engl J Med. 2020;383:711–720. doi: 10.1056/NEJMoa2004215. [DOI] [PubMed] [Google Scholar]
- 41.Graham MJ, Lee RG, Brandt TA, Tai LJ, Fu W, Peralta R, et al. Cardiovascular and metabolic effects of ANGPTL3 antisense oligonucleotides. N Engl J Med. 2017;377:222–232. doi: 10.1056/NEJMoa1701329. [DOI] [PubMed] [Google Scholar]
- 42.Gaudet D, Karwatowska-Prokopczuk E, Baum SJ, Hurh E, Kingsbury J, Bartlett VJ, et al. Vupanorsen, an N-acetyl galactosamine-conjugated antisense drug to ANGPTL3 mRNA, lowers triglycerides and atherogenic lipoproteins in patients with diabetes, hepatic steatosis, and hypertriglyceridaemia. Eur Heart J. 2020;41:3936–3945. doi: 10.1093/eurheartj/ehaa689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kohan AB. Apolipoprotein C-III: a potent modulator of hypertriglyceridemia and cardiovascular disease. Curr Opin Endocrinol Diabetes Obes. 2015;22:119–125. doi: 10.1097/MED.0000000000000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ginsberg HN, Le NA, Goldberg IJ, Gibson JC, Rubinstein A, Wang-Iverson P, et al. Apolipoprotein B metabolism in subjects with deficiency of apolipoproteins CIII and AI. Evidence that apolipoprotein CIII inhibits catabolism of triglyceride-rich lipoproteins by lipoprotein lipase in vivo. J Clin Invest. 1986;78:1287–1295. doi: 10.1172/JCI112713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordts PL, Nock R, Son NH, Ramms B, Lew I, Gonzales JC, et al. ApoC-III inhibits clearance of triglyceride-rich lipoproteins through LDL family receptors. J Clin Invest. 2016;126:2855–2866. doi: 10.1172/JCI86610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sundaram M, Zhong S, Bou Khalil M, Links PH, Zhao Y, Iqbal J, et al. Expression of apolipoprotein C-III in McA-RH7777 cells enhances VLDL assembly and secretion under lipid-rich conditions. J Lipid Res. 2010;51:150–161. doi: 10.1194/jlr.M900346-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mendoza S, Trenchevska O, King SM, Nelson RW, Nedelkov D, Krauss RM, et al. Changes in low-density lipoprotein size phenotypes associate with changes in apolipoprotein C-III glycoforms after dietary interventions. J Clin Lipidol. 2017;11:224–233.e2. doi: 10.1016/j.jacl.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zvintzou E, Lhomme M, Chasapi S, Filou S, Theodoropoulos V, Xapapadaki E, et al. Pleiotropic effects of apolipoprotein C3 on HDL functionality and adipose tissue metabolic activity. J Lipid Res. 2017;58:1869–1883. doi: 10.1194/jlr.M077925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawakami A, Aikawa M, Nitta N, Yoshida M, Libby P, Sacks FM. Apolipoprotein CIII-induced THP-1 cell adhesion to endothelial cells involves pertussis toxin-sensitive G protein- and protein kinase C alpha-mediated nuclear factor-kappaB activation. Arterioscler Thromb Vasc Biol. 2007;27:219–225. doi: 10.1161/01.ATV.0000249620.68705.0d. [DOI] [PubMed] [Google Scholar]
- 50.Olivieri O, Stranieri C, Bassi A, Zaia B, Girelli D, Pizzolo F, et al. ApoC-III gene polymorphisms and risk of coronary artery disease. J Lipid Res. 2002;43:1450–1457. doi: 10.1194/jlr.m200145-jlr200. [DOI] [PubMed] [Google Scholar]
- 51.Pollin TI, Damcott CM, Shen H, Ott SH, Shelton J, Horenstein RB, et al. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 2008;322:1702–1705. doi: 10.1126/science.1161524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014;371:32–41. doi: 10.1056/NEJMoa1308027. [DOI] [PubMed] [Google Scholar]
- 53.Dias N, Stein CA. Antisense oligonucleotides: basic concepts and mechanisms. Mol Cancer Ther. 2002;1:347–355. [PubMed] [Google Scholar]
- 54.Paik J, Duggan S. Volanesorsen: first global approval. Drugs. 2019;79:1349–1354. doi: 10.1007/s40265-019-01168-z. [DOI] [PubMed] [Google Scholar]
- 55.Lüscher TF. Frontiers in lipid research: lipoprotein(a), apolipoprotein C-III and E, and PCSK9 and inflammation. Eur Heart J. 2019;40:2741–2744. doi: 10.1093/eurheartj/ehz633. [DOI] [PubMed] [Google Scholar]
- 56.Khetarpal SA, Zeng X, Millar JS, Vitali C, Somasundara AV, Zanoni P, et al. A human APOC3 missense variant and monoclonal antibody accelerate apoC-III clearance and lower triglyceride-rich lipoprotein levels. Nat Med. 2017;23:1086–1094. doi: 10.1038/nm.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kettunen S, Ruotsalainen AK, Ylä-Herttuala S. RNA interference-based therapies for the control of atherosclerosis risk factors. Curr Opin Cardiol. 2022;37:364–371. doi: 10.1097/HCO.0000000000000972. [DOI] [PubMed] [Google Scholar]
- 58.Conklin D, Gilbertson D, Taft DW, Maurer MF, Whitmore TE, Smith DL, et al. Identification of a mammalian angiopoietin-related protein expressed specifically in liver. Genomics. 1999;62:477–482. doi: 10.1006/geno.1999.6041. [DOI] [PubMed] [Google Scholar]
- 59.Kersten S. Angiopoietin-like 3 in lipoprotein metabolism. Nat Rev Endocrinol. 2017;13:731–739. doi: 10.1038/nrendo.2017.119. [DOI] [PubMed] [Google Scholar]
- 60.Shimizugawa T, Ono M, Shimamura M, Yoshida K, Ando Y, Koishi R, et al. ANGPTL3 decreases very low density lipoprotein triglyceride clearance by inhibition of lipoprotein lipase. J Biol Chem. 2002;277:33742–33748. doi: 10.1074/jbc.M203215200. [DOI] [PubMed] [Google Scholar]
- 61.Koishi R, Ando Y, Ono M, Shimamura M, Yasumo H, Fujiwara T, et al. Angptl3 regulates lipid metabolism in mice. Nat Genet. 2002;30:151–157. doi: 10.1038/ng814. [DOI] [PubMed] [Google Scholar]
- 62.Quagliarini F, Wang Y, Kozlitina J, Grishin NV, Hyde R, Boerwinkle E, et al. Atypical angiopoietin-like protein that regulates ANGPTL3. Proc Natl Acad Sci U S A. 2012;109:19751–19756. doi: 10.1073/pnas.1217552109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chi X, Britt EC, Shows HW, Hjelmaas AJ, Shetty SK, Cushing EM, et al. ANGPTL8 promotes the ability of ANGPTL3 to bind and inhibit lipoprotein lipase. Mol Metab. 2017;6:1137–1149. doi: 10.1016/j.molmet.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jaye M, Lynch KJ, Krawiec J, Marchadier D, Maugeais C, Doan K, et al. A novel endothelial-derived lipase that modulates HDL metabolism. Nat Genet. 1999;21:424–428. doi: 10.1038/7766. [DOI] [PubMed] [Google Scholar]
- 65.Shimamura M, Matsuda M, Yasumo H, Okazaki M, Fujimoto K, Kono K, et al. Angiopoietin-like protein3 regulates plasma HDL cholesterol through suppression of endothelial lipase. Arterioscler Thromb Vasc Biol. 2007;27:366–372. doi: 10.1161/01.ATV.0000252827.51626.89. [DOI] [PubMed] [Google Scholar]
- 66.Adam RC, Mintah IJ, Alexa-Braun CA, Shihanian LM, Lee JS, Banerjee P, et al. Angiopoietin-like protein 3 governs LDL-cholesterol levels through endothelial lipase-dependent VLDL clearance. J Lipid Res. 2020;61:1271–1286. doi: 10.1194/jlr.RA120000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Musunuru K, Pirruccello JP, Do R, Peloso GM, Guiducci C, Sougnez C, et al. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N Engl J Med. 2010;363:2220–2227. doi: 10.1056/NEJMoa1002926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stitziel NO, Khera AV, Wang X, Bierhals AJ, Vourakis AC, Sperry AE, et al. ANGPTL3 deficiency and protection against coronary artery disease. J Am Coll Cardiol. 2017;69:2054–2063. doi: 10.1016/j.jacc.2017.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gusarova V, Alexa CA, Wang Y, Rafique A, Kim JH, Buckler D, et al. ANGPTL3 blockade with a human monoclonal antibody reduces plasma lipids in dyslipidemic mice and monkeys. J Lipid Res. 2015;56:1308–1317. doi: 10.1194/jlr.M054890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Markham A. Evinacumab: first approval. Drugs. 2021;81:1101–1105. doi: 10.1007/s40265-021-01516-y. [DOI] [PubMed] [Google Scholar]
- 71.Crooke ST, Baker BF, Xia S, Yu RZ, Viney NJ, Wang Y, et al. Integrated assessment of the clinical performance of GalNAc3-conjugated 2′-O-methoxyethyl chimeric antisense oligonucleotides: I. Human volunteer experience. Nucleic Acid Ther. 2019;29:16–32. doi: 10.1089/nat.2018.0753. [DOI] [PMC free article] [PubMed] [Google Scholar]