Abstract
Patients receiving anti-CD20 antibodies showed limited efficacy of a booster dose of BNT162b2. Patients with lymphomas combine such immunotherapies with cytotoxic chemotherapies that could result in an even greater alteration of the immune response to vaccination. We report here the impact of a third vaccine dose on T cell specific responses in a small cohort of patients treated in our center by anti-CD20 therapies and cytotoxic chemotherapies for lymphoid malignancies. Our results showed that a third dose in these severely immune suppressed patients could improve the expansion on CD4+Th1+T cell responses while the effect CD8 + T cell responses was marginal.
Keywords: Third dose, COVID-19, Immunocompromised, Vaccine, T cell responses, Hematological malignancies
1. To the editor
Patients with hematological malignancies are at higher risks of severe COVID-19[1] and are not fully protected by vaccination against SARS-CoV-2[2] and a third dose of mRNA vaccine is recommended in these populations. We reported earlier[3], alongside others[4], that a third dose of vaccine could significantly improve its immunogenicity amongst patients with plasma cell disorders, including patients who were treated by anti-CD38 monoclonal antibodies and who displayed lower IgG anti-S concentrations after two vaccine doses. On the other hand, patients affected by B cell malignancies did not benefit from a third vaccine injection, as it did not significantly improve neither seroconversion rates nor IgG anti-S concentrations. This mainly resulted from the broad usage of anti-CD20 monoclonal antibodies in this population, which significantly impairs humoral responses after two[2], [5] or three[3] vaccine injections. This population is therefore expected to be more vulnerable to severe forms of the disease[6] or have prolonged infections from which can arise new viral variants[7].
Nonetheless, resolutive infections have been documented amongst patients treated with B-cell depleting therapeutics[8], revealing a major role of the T cell responses in tampering the severity of SARS-CoV-2 infection and promoting its resolution. Indeed, recent research have unraveled the protective effect of an early T cell response, especially early CD4 + T cells[9], [10], as well as the devastating effects of a completely depleted T cell response, even with a proper humoral response[8]. Available vaccines have been shown to induce rapid and coordinated CD4+ and CD8+ T cell responses, including against recent variant of concerns[9], [11] and in B-depleted patients with cancer[12], [13], [14].
Considering the uncertain benefit of a third vaccination in patients affected by B cell malignancies receiving B-cell depleting therapies, we aimed to assess the T cell responses induced by such vaccinal strategy.
We selected in our center patients affected by B cell malignancies and treated with anti-CD20 monoclonal antibodies in the last year among a previously described cohort[3]. Patients were proposed to receive a third dose of mRNA vaccine in accordance of French guidelines since April 2021. Blood samples for cellular analysis were collected before the third vaccination (either during a previous routine visit, or on the same day as the injection), and 21 days later.
SARS-CoV-2 specific T cells functionality was assessed by intracellular staining as previously described[15]. Briefly, PBMCs were stimulated in vitro overnight with a set of 2 pools of 15-mer peptides, overlapping by 11 amino acids, covering the whole spike protein of the SARS-CoV-2 virus from reference strain Human 2019-nCoV HKU-SZ-005b (JPT Peptide Technologies, Berlin, Germany): S1 (168 peptides), S2 (144 peptides). Total S-specific responses producing cytokines (IL-2 / IFN-γ / TNF-α) were determined by summing S1 and S2 responses. Unstimulated cells were used as negative control. The flow cytometry panel included a viability marker, CD3, CD4, and CD8 to determine T cell lineage, and IFN-γ, IL-2 and TNF antibodies. Data were acquired on a LSRII Fortessa 4-laser (488, 640, 561 and 405 nm) cytometer (BD Biosciences), analyzed using FlowJo software version 9.9.6 (Tree Star inc.). Gating strategy used to determine cytokine-positive T cells is shown in supplementary Figure 2. Patients gave their written consent to the study, which was approved by the ethical committee.
We included 14 patients in the analysis. Briefly, the cohort was composed of 8 patients affected by diffuse large B cell lymphomas, 4 by follicular lymphomas and 2 by mantle cell lymphomas. The median time from last anti-CD20 monoclonal antibodies administration was 31 days (IQR [14.3; 97.3], See Table 1 ). In association to anti-CD20 antibodies, 9 patients were treated with CHOP or DHAC chemotherapy, 2 with bendamustin, 1 with lenalidomide and 2 patients received maintenance therapy (the end of chemotherapy being 19 and 7 months before the booster injection).
Table 1.
Patient characteristics.
| Clinical characteristics (n = 14) | |
|---|---|
| Age (years) | 69.5 [57.5; 73.4] |
| Male gender (%) | 6 (43%) |
| Malignancy type (%) | Diffuse Large B Cell Lymphomas 8 (57%)Follicular Lymphomas 4 (29%) Mantle Cell Lymphomas 2 (14%) |
|
Anti CD20 type (%) Rituximab Obinutuzumab |
11 (79%) 3 (21%) |
|
Associated chemo CHOP/DHAC Bendamustine Revlimid Maintenance (>6 months after chemotherapy) |
9 (64%) 2 (14%) 1 (7%) 2 (14%) |
| Disease response status (%) | |
| Partial/complete responses (PR/CR) | 9 (64%) |
| Stable disease | 1 (7%) |
|
Progressive disease NA |
0 (0%) 4 (29%) |
| Number of prior lines of therapy (n) | 1 [1; 1] |
| Time from last anti-CD20 administration (days) | 31.5 [14.3; 97.3] |
| Time between second and third vaccine injection (days) | 113 [85.8; 124.5] |
| Time from third vaccine injection to T cell responses analysis (days) | 21 [15.5; 21] |
IgG-anti S were undetectable in all patients after two vaccine injections, except in one case (measured at 44 BAU/mL).
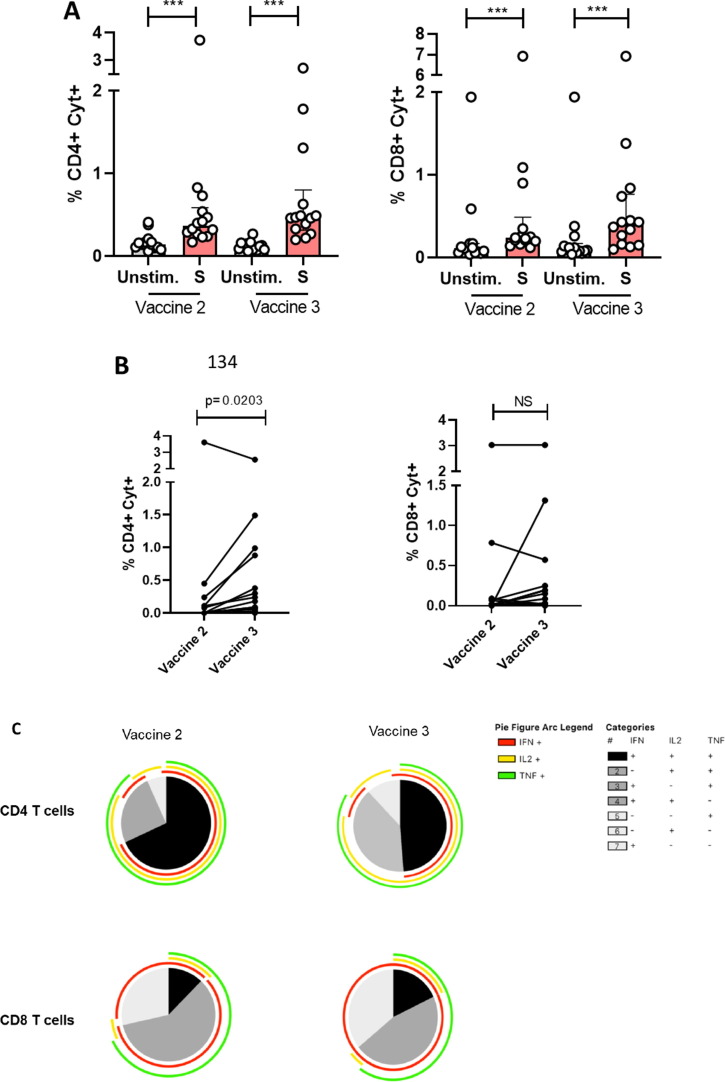
In contrast, specific CD4+ and CD8+ T cell responses were detectable in all patients after 2 vaccine injections. Following stimulation with SARS-CoV-2 Spike-protein peptide pools (Figure, panel A), median frequencies [IQR] of S-specific CD4 and CD8 T cells (IFN-γ +/- IL-2+/- TNFα) were 0.36% [0.26–0.59] and 0.23% [0.19–0.49], respectively (P = 0.0001 for all comparisons to unstimulated condition). After a third vaccine injection, they were 0.48% [0.32–1.19] and 0.4% [0.16–0.77], respectively (P = 0.0001 for all comparisons to unstimulated condition). The third dose increased the frequency of S-specific CD4 + T cells (P = 0.02) but not CD8 + T cells (Figure, panel B). The increase in S-specific CD4 + T cells was not correlated to the elapsed time since the last administration of hematological treatment (Supplementary Figure 1). Spike-specific CD4+ and CD8+ T cells were polyfunctional after the second injection since a large majority of these cells produced up to three cytokines simultaneously. The functionality of S-specific T cells was maintained after the third injection (Figure, panel C; individual pie charts, Supplementary Figure 3).
Fig. 1.
Specific T cell responses in patients after vaccination. S-specific CD4 T cell and CD8 T cell responses (total cytokines: IFN-γ +/- IL-2+/- TNFα) of patients after the second (Vaccine 2) and third (Vaccine 3) vaccine injection (n = 14) after overnight SARS-CoV-2-specific stimulation (S peptide pools) in vitro as compared to unstimulated condition (A). Individual S-specific CD4 and CD8 T cell responses after the second (Vaccine 2) and third (Vaccine 3) vaccine injection (n = 14). Results are represented background subtracted (B). Median values ± IQR are shown, and Wilcoxon test was used for comparisons. Functional composition of SARS-CoV2-specific CD4+ and CD8+ T cell responses in vaccinated patients after the second (Vaccine 2) and third (Vaccine 3) vaccine injection. Responses are color coded according to the combinations of cytokines produced. The arcs identify cytokine-producing subsets (IFN-γ, IL-2, and TNF-α) within the CD4 + and CD8 + T cell populations (C).
Data have recently emerged on response to anti-SARS-CoV-2 mRNA vaccine in patients treated by anti CD20 antibodies for autoimmune and rheumatismal diseases[16], suggesting that a third vaccine dose could be efficient in this population. A recent report suggested that patients with B-cell lymphoma receiving a maintenance treatment with anti-CD20 antibodies developed specific T cell responses while the humoral response was deeply impaired[17]. However, in contrary to patients with autoimmune disease, patients suffering from lymphomas frequently received chemotherapy in addition to anti-CD20 antibodies, which could worsen cellular response to vaccination. Our study described cellular response after BNT162b2 vaccine in patients mostly treated with the combination of chemotherapy and anti-CD20. We identified in all patients a cellular response with the presence of S specific polyfunctional CD4+ and CD8+ T cells after two vaccine injections, with a modest increase in the CD4+ response after third dose injection, and an unchanged proportion of CD8+ T cells. The marginal effect of the third vaccine dose on CD8+ T cell responses could be explained by multiple mechanisms: lymphocyte anergy or exhaustion and/or co-administration of other cytotoxic drugs with anti-CD20 therapies. However, we showed that a third dose of mRNA vaccine improves the expansion of CD4+ Th1+ T cell responses in severe immune suppressed patients treated by anti-CD20 therapies in combination to chemotherapy. A major finding of our study is the persistent polyfunctionality of the expended CD4+ T cells. Indeed, polyfunctional T cells appear to be crucial to coordinate adaptative immune responses to overcome infection[18]. Therefore, a benefit of a vaccine booster limited to CD4+ T cells might still be valuable. Unfortunately, due to the limited number of patients (n = 14), we do not have the power to study the impact of chemotherapy type, for example bendamustin versus CHOP, on vaccine response. The number of patients also prevented us from generalizing the observed data to a wider range of hematological patients, and our study should support larger prospective studies. This is especially true as variants of concern continue to emerge, prompting for continuous adaptation of current vaccination guidelines that need to be supported by robust data.
While these results are limited by the low number of patients, our study underscores the need to demonstrate in real life how these immunogenicity data translate to disease protection. Pending that, there is a need to implement alternative prophylactic strategies in these patients.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: F. Lemonnier received research funding from Institut Roche and travel grant from Gilead, G. Melica received non-financial support from MSD and research grants and non-financial support from Pfizer.
Acknowledgments
We thank Lydia Guillaumat and Corinne Krief for biobanking.
SB. Gressens, A. Wiedemann, M. Déchenaud, J. Dupuis, G. Melica, C. Haioun performed the research.
F. Lemonnier and Y. Levy designed the research study.
SB. Gressens, A. Wiedemann, S. Gallien, G. Melica, F. Lemonnier, Y. Levy analysed the data.
SB. Gressens, A. Wiedemann, F. Lemonnier, Y. Levy wrote the paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2023.01.064.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Liebers N, Speer C, Benning L, et al. Humoral and cellular responses after COVID-19 vaccination in anti-CD20 treated lymphoma patients. Blood. 2021;(blood.2021013445). doi: https://doi.org/10.1182/blood.2021013445. [DOI] [PMC free article] [PubMed]
- 2.Maneikis K, Šablauskas K, Ringelevičiūtė U, et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. The Lancet Haematology. 2021;8(8):e583-e592. doi: https://doi.org/10.1016/S2352-3026(21)00169-1. [DOI] [PMC free article] [PubMed]
- 3.Gressens SB, Fourati S, Le Bouter A, et al. Anti-SARS-CoV-2 antibody response after 2 and 3 doses of BNT162b2 mRNA vaccine in patients with lymphoid malignancies. Clinical Microbiology and Infection. Published online March 2022:S1198743X22001069. doi: https://doi.org/10.1016/j.cmi.2022.02.029. [DOI] [PMC free article] [PubMed]
- 4.Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Anti-spike antibody response to SARS-CoV-2 booster vaccination in patients with B cell-derived hematologic malignancies. Cancer Cell. Published online September 2021:S1535610821004906. doi: https://doi.org/10.1016/j.ccell.2021.09.001. [DOI] [PMC free article] [PubMed]
- 5.Greenberger L.M., Saltzman L.A., Senefeld J.W., Johnson P.W., DeGennaro L.J., Nichols G.L. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell. 2021;39(8):1031–1033. doi: 10.1016/j.ccell.2021.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duléry R., Lamure S., Delord M., et al. Prolonged in-hospital stay and higher mortality after Covid-19 among patients with non-Hodgkin lymphoma treated with B-cell depleting immunotherapy. American J Hematol. 2021;96(8):934–944. doi: 10.1002/ajh.26209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corey L, Beyrer C, Cohen MS, Michael NL, Bedford T, Rolland M. SARS-CoV-2 Variants in Patients with Immunosuppression. The new england journal of medicine. Published online 2021:5. [DOI] [PMC free article] [PubMed]
- 8.Bange E.M., Han N.A., Wileyto P., et al. CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat Med. 2021;27(7):1280–1289. doi: 10.1038/s41591-021-01386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Painter M.M., Mathew D., Goel R.R., et al. Rapid induction of antigen-specific CD4+ T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity. 2021;54(9):2133–2142.e3. doi: 10.1016/j.immuni.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan A.T., Linster M., Tan C.W., et al. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep. 2021;34(6) doi: 10.1016/j.celrep.2021.108728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarke A., Sidney J., Methot N., et al. Impact of SARS-CoV-2 variants on the total CD4+ and CD8+ T cell reactivity in infected or vaccinated individuals. Cell Reports Medicine. 2021;2(7) doi: 10.1016/j.xcrm.2021.100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehmsen S., Asmussen A., Jeppesen S.S., et al. Antibody and T cell immune responses following mRNA COVID-19 vaccination in patients with cancer. Cancer Cell. 2021;39(8):1034–1036. doi: 10.1016/j.ccell.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh B.L.Z., Tan N., de Alwis R., et al. Enhanced BNT162b2 vaccine-induced cellular immunity in anti-CD19 CAR T cell–treated patients. Blood. 2022;140(2):156–160. doi: 10.1182/blood.2022016166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atanackovic D., Luetkens T., Omili D., et al. Vaccine-induced T-cell responses against SARS-CoV-2 and its Omicron variant in patients with B cell–depleted lymphoma after CART therapy. Blood. 2022;140(2):152–156. doi: 10.1182/blood.2022016175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiedemann A., Foucat E., Hocini H., et al. Long-lasting severe immune dysfunction in Ebola virus disease survivors. Nat Commun. 2020;11(1):3730. doi: 10.1038/s41467-020-17489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felten R, Gallais F, Schleiss C, et al. Cellular and humoral immunity after the third dose of SARS-CoV-2 vaccine in patients treated with rituximab. The Lancet Rheumatology. 2021;4(1):S2665991321003519. doi: https://doi.org/10.1016/S2665-9913(21)00351-9. [DOI] [PMC free article] [PubMed]
- 17.Candon S, Lemee V, Leveque E, et al. Dissociated humoral and cellular immune responses after a three-dose schema of BNT162b2 vaccine in patients receiving anti-CD20 monoclonal antibody maintenance treatment for B-cell lymphomas. haematol. Published online December 2, 2021. doi: https://doi.org/10.3324/haematol.2021.280139. [DOI] [PMC free article] [PubMed]
- 18.Rydyznski Moderbacher C., Ramirez S.I., Dan J.M., et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell. 2020;183(4):996–1012.e19. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.