Abstract
Hyperhidrosis (HH) is a central nervous dysfunction characterized by abnormally increased sweating due to a central dysregulation of sweat secretion. HH significantly affects the quality of life of patients in their private, social and professional environments. Physiologically, sweating is a mechanism that regulates body temperature, but it may also be triggered by emotional or gustatory stimuli. There are two main types of sweat glands: eccrine and apocrine glands. The central nervous system controls sweat secretion through the release of neurotransmitters into the autonomous nervous system (ANS) that activate the sweat glands. The hypothalamus has two separate neuronal pathways, one for thermoregulation and one for emotions. HH may thus be due to either a neuronal dysfunction of ANS regulation leading to a hyperactivity of the sympathetic nervous system, or to abnormal central processing of emotions. Crucially, there is no dysfunction of the sweat glands themselves. Various pathogenic mechanisms have been proposed to be involved in pathological sweat secretion in HH, ranging from structural changes within the ANS to increased expression of aquaporin 5 and upregulation of activin A receptor type 1 in eccrine sweat glands. Although a genetic predisposition has been demonstrated, it remains unclear exactly which genes are involved. To identify new, potential therapeutic targets and to improve treatment options, a good understanding of the signaling pathways involved, the underlying mechanisms, and the genetic components is essential. In this review we discuss the various aspects of sweat physiology and function that are necessary to explain pathological sweating. Our aim is to raise awareness of the complexity of HH to promote a better understanding of the disorder.
Keywords: Hyperhidrosis, Sweat secretion, Nervous dysfunction
Key Summary Points
| Physiological sweating is a bodily function that is primarily aimed at thermoregulation of the body and is triggered by different types of sweat glands. |
| Emotional and gustatory sweating can also occur. |
| Hyperhidrosis (HH) is a pathological dysregulation of the central nervous system that can be triggered by thermal stimuli, physical activity, or emotional stress. |
| there is no dysfunction of or pathological changes in the sweat glands in HH. |
| The clinical symptom of HH is not necessarily increased sweating, but rather an arbitrary, pathological dysregulation of sweating due to low exposure to a trigger. |
| HH has a large negative effect on patients' quality of life. |
Introduction
In the literature, hyperhidrosis (HH) is usually defined as a condition characterized by abnormally increased sweating beyond that required to regulate body temperature [1]. While excessive sweating is the hallmark of HH, in this review we discuss why this definition can be misleading. The term ‘excessive’ is usually based on descriptions of symptoms recorded from patients’ medical histories and can therefore lead to the impression that HH can be reduced to a mere problem of sweat amount [2]. However, HH is a complex neuronal dysregulation involving an alteration in the control mechanisms of sweating that results in a mismatch between the required and actual sweat production [2, 3].
The disproportionate sweat production that characterizes HH results in a disabling medical condition with profound effects on the patient’s quality of life. HH affects work productivity, daily routine activities, emotional well-being, and personal relationships [4]. The age of onset is usually before 25 years, meaning that the disease affects patients’ lives very early in their life [2, 5].
A distinction can be made between primary (idiopathic) and secondary (defined etiology) HH. In secondary HH, the pathological mechanisms are largely determined by the underlying condition and may differ from person to person [3]. The focus of this review is on primary HH.
In this review, we discuss various aspects of sweat physiology and function that are necessary for understanding pathological sweating. As HH is an underestimated disorder, we aim to highlight and draw attention to the complexity of the underlying mechanisms to promote a better understanding of the condition [6].
This review article does not contain any new studies with human participants or animals performed by any of the authors.
Physiological Significance of Sweating
Reasons for Sweating
Sweat evaporation from the skin is crucial for thermoregulation, i.e., maintaining body temperature [7], but sweat production is also a response to emotional stress [8]. Sweat on the palms of hands or soles of feet helps with activities that require good grip by increasing friction; this physiological response is likely to have developed during evolution, for example, to improve flight reactions [9, 10]. Sweat may be important for skin hydration and microbial defense, although more research is needed in this area [7]. Finally, it is also believed that waste products are released to the skin surface in sweat, but these effects appear to be minor compared to other functions [7, 8].
Types of Sweat
There are different types of sweat. Thermal sweating, in the context of thermoregulation, is considered to be the primary physiological function of sweat. In response to heat generated by exercise or environmental factors, the body may release heat from the body in the form of water on the skin surface, which evaporates to restore normal body temperature. This release of water (sweat) occurs over the entire body surface. In a pathological condition, there is a mismatch between the required and actual sweat production [7, 10, 11].
Emotional sweating, on the other hand, is triggered by stress, anxiety, pain, and fear. Sexual arousal can also cause sweat secretion. Emotional sweating occurs all over the body, but is most prominent on hands, feet, face, and the axillary region [10]. These areas also correspond to those most commonly affected in HH [5, 8, 10]. In contrast to thermal sweating, emotional sweating is thought to decrease during sleep and relaxation [10, 12].
Gustatory sweating is sweating triggered by food and drink. It can be caused by an increase in metabolism, leading to an elevated body temperature and thermal sweating, but also by hot and spicy food. Gustatory sweating is usually confined to the face, scalp, and neck. Pathologically, the most common form of gustatory sweating is Frey’s syndrome following surgery of the parotid gland or injury to the auriculotemporal nerve, which can lead to a misdirected regeneration of parasympathetic fibers that normally stimulate the salivary glands but now switch to a sympathetic response and stimulate the sweat glands [8, 10].
Sweat Glands
Only a few mammals can cool the body by evaporating water on the surface of the skin. In humans, this mechanism is highly effective due to the small amount of body hair in comparison to the high density of sweat glands [13]. There are two main types of sweat glands, namely, eccrine and apocrine glands, with each type producing a different kind of sweat; eccrine glands are involved in thermoregulation. A third type of sweat gland, the apoeccrine sweat gland, has also been described, but its existence remains controversial [8, 14].
Eccrine Glands
Eccrine glands are active from birth. They are found on the entire body surface with the exception of the lips and glans penis; they are the only sweat glands found on the palms of the hands and soles of the feet. Anatomically, the eccrine glands consist of a spiral formed by a single layer of secretory cells, classified as clear, dark, and myoepithelial cells. The excretory duct is directly connected to the skin surface. The watery secretion of the eccrine glands is crucial for thermoregulation [7, 10, 15–17]. Eccrine glands are stimulated by sympathetic nerve fibers, and the major neurotransmitter is acetylcholine (ACh) [18]. These glands respond to thermal and emotional stimuli [7, 16].
Apocrine Glands
Apocrine glands are present form birth but not active until puberty. They are located in the axillae, breast, scalp, and perineum, but not on the palms of the hands and soles of the feet. The secretory part of the gland consists of a single layer of epithelial cells surrounded by myoepithelial cells. Apocrine glands are larger than their eccrine counterparts, and their excretory duct releases sweat into the hair follicle. The sweat from these glands is a viscous liquid that elicits is detectable as a body odor. However, the function of the apocrine glands remains unclear [7, 10, 15]. The innervation of the apocrine glands is poorly understood, but most likely regulation is through sympathetic nervous control of a peripheral mechanism involving an adrenergic pathway [19], although apocrine glands have also been found to respond to cholinergic stimuli [7]. The major neurotransmitters are catecholamines [18]. Apocrine glands respond to emotional stimuli [10].
Apoeccrine Glands
Apoeccrine glands were first described by Sato et al. in 1987 [14]. However, their existence remains controversial as no evidence of their presence was detected in other studies [20]. Apoeccrine glands are thought to evolve from eccrine glands during puberty. They are reported to be found in the hairy areas of the body, such as the axilla, mammary, perineal, and genital regions. Anatomically, their excretory duct is directly connected to the skin surface and produces a watery secretion similar to eccrine sweat, while the secretory coil is similar to apocrine glands. The true functional importance of apoeccrine glands is not known, but it is unlikely that they are important for thermoregulation [7, 10, 14, 15]. Their innervation is also still poorly understood, but in vitro models suggest that they are more sensitive to cholinergic than adrenergic stimuli [7].
Mechanisms of Sweat Regulation and Secretion
Sweat secretion is controlled by the transduction of signals from the central nervous system (CNS) to the peripheral autonomic nervous system (ANS) [8].
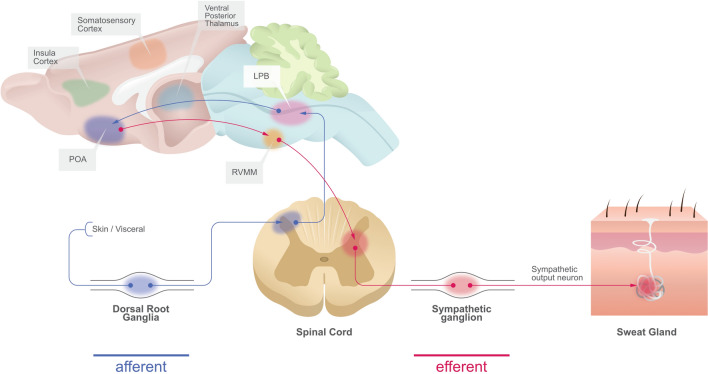
The hypothalamus is the part of the CNS which regulates body temperature. Two innervation pathways connect the hypothalamus to several areas of the nervous system and the rest of the body. The efferent sympathetic sudomotor pathway for thermoregulation runs from the cerebral cortex to the hypothalamus and from there to the medulla oblongata. The nerve fibers then cross within the medulla and project to the lateral horn of the spinal cord and intermediolateral cell nuclei, from where they connect to the paravertebral sympathetic ganglia. Unmyelinated postganglionic sympathetic nerve fibers eventually stimulate receptors on the eccrine sweat glands [5, 8, 21, 22]. The CNS can also respond to emotional changes. Emotional sweating is regulated by the limbic system, including the amygdala, cingulate cortex, and hypothalamus, via efferent fibers that connect to preganglionic sympathetic neurons in the nucleus inter-medio lateralis (Fig. 1) [5, 8, 23, 24].
Fig. 1.
Schematic illustration of the afferent and efferent route of innervation cascades to regulate the activity of sweat gland secretion (modified according to Tan et al. [24]). LPB Lateral parabrachial nucleus, POA preoptic area, RVMM rostral ventromedial medulla
The ANS receives signals from the CNS and secretes neurotransmitters and peptides to control sweat gland activation. ACh regulates the eccrine glands and is the primary neurotransmitter of thermal sweating. Catecholamines (e.g., noradrenaline) control the eccrine and apocrine glands in emotional stress-induced sweating [8, 25, 26].
Pathological Sweating
Sweating is pathological when it occurs disproportionally to the need of temperature compensation. This may be evident by the formation of droplets on the skin; dripping sweat is not useful for cooling.
Primary HH can be induced by thermal triggers, physical activity, and emotional stress [2]. While both eccrine and apocrine glands respond to emotional stimuli, findings from Bovell et al. [27] suggest that the eccrine glands are the main source of fluid transport in HH rather than apocrine or apoeccrine glands.
HH is not necessarily an increase in the absolute amount of sweat, but a change in sweat regulation. Increased sweating does not occur permanently, but small triggers lead to disproportionate sweating [2, 3]. Because patients can barely control sweating, it leads to stress and significant limitations in their private and professional lives, as can be assessed using the Dermatology Life Quality Index [28].
In a recent review, Kristensen et al. concluded that HH is still an underestimated and understudied chronic disorder [6]. The prevalence of HH is estimated at 1.6% in the UK and between 1.0 and 4.8% in the USA [29–31]. However, data on prevalence vary depending on data collection methods and factors such as ethnicity and age. Other sources report a prevalence as high as 16.7% in Poland and 12.3% in Canada (Vancouver region) [32, 33]. Additionally, there is likely a large number of cases that go undiagnosed due to shame or lack of knowledge [34]. Although the condition is widespread, it is often not part of the training of physicians and caregivers. Accordingly, the level of knowledge about HH is low, even in a clinical context [9].
Differences Between Primary and Secondary HH
Hyperhidrosis can be focal or generalized. Focal HH refers to excessive sweating that is limited to specific parts of the body, whereas generalized HH implicates increased sweating over the entire body. Additionally, HH can be primary or secondary [1].
In the majority of patients, primary HH is an idiopathic disorder. Primary focal HH is usually a bilateral symmetrical disorder affecting the palms of the hands, soles of the feet, axillae, or forehead, whereas sweating in primary generalized HH usually affects the head and trunk, or even extremities, groin and glute [5, 9]. In contrast, secondary HH occurs as a result of an underlying disease, such as endocrine disorders, infections, or neurologic diseases [1, 5]. Accordingly, the pathological mechanisms of secondary HH are largely determined by the underlying condition [3]. In the generalized form of HH, it can be difficult to differentiate between primary and secondary HH, and further screening may be required. Secondary focal HH involves regional or asymmetric sweating, or may be compensatory; for example, loss of sweating in one area leads to increased sweating in another [5, 9].
Sudomotor Dysfunction
Sudomotor function refers to perspiration and the release of sweat to the skin surface to cool the body [35]. Because sweat is regulated by the ANS, sudomotor function can be affected by ANS dysfunction.
Given the innervation pathways of the hypothalamus, primary HH can be understood to be a neuronal regulation disorder of the ANS involving pathologic hyperactivity of the sympathetic system that results in hyperstimulation of otherwise normal sweat glands [2, 5, 36]. Alternatively, the etiology of primary HH may be an abnormal central control of emotions, with the sweat center in the hypothalamus being controlled by the influence of the cortex without input from thermoregulatory components [5]. For example, patients with primary palmar HH show an altered perception of warmth sensation and exaggerated sudomotor responses [37]. The lowered thresholds may be explained either by a regulatory dysfunction in the autonomic centers of the brainstem that are responsible for inhibiting sensory perception and peripheral autonomic activity or, alternatively, by dysfunction at the cortical level, associated with impaired control of emotional sweating.
A variety of tools are available for analyzing sweat excretion. The most important diagnostic tool is the patient history, including the onset of symptoms, sweating patterns, and familial predisposition, as measured, for example, by the Hyperhidrosis Disease Severity Scale [38]. However, when the cause of excessive sweating is unclear, laboratory tools that more objectively assess the different responses and sudomotor pathways may provide a more sensitive and quantifiable assessment. These tools can range from quantitative sudomotor axon reflex testing to thermoregulatory sweat testing to sympathetic skin responses [35, 39]. For example, Hirakawa et al. [40] used a mental arithmetic problem in a study to induce stress and measure emotional sweating. The choice of test depends on the clinical condition and should be preceded by careful evaluation of the medical history and a neurologic examination of other collateral deficits. Therefore, understanding the test methods and autonomic neurology is critical to interpreting the results [35, 41].
Abnormal Sweat Secretion
If HH is understood as a regulatory disorder involving the ANS, an explanation for the abnormal sweat secretion in processes and pathways proximally to the sweat gland level is required. For example, a metabolic explanation for the hyperactivity of sympathetic neurons is offered by data showing a higher expression of ACh and alpha-7 neuronal nicotinic receptor subunits in the sympathetic ganglia [3, 36, 42, 43]. Other structural changes in patients with HH include a larger number of ganglion cells in the ganglia, thicker myelin sheaths of the affected axons, and larger diameter of the thoracic sympathetic chain ganglia [36, 43].
Crucially, the increased sweat secretion in patients with HH is not due to abnormalities in the sweat glands per se, but to regulatory processes that affect the sweat production of the glands. There is no dysfunction in the sweat glands of these patients, nor changes in their size, number, or histologic appearance [2, 5, 44]. However, while Du et al. [44] found no significant differences in the morphological characteristics or the number of sweat glands between axillary HH patients and healthy subjects, the authors did detect a significantly higher number of secretory granules in patients who exhibited hypersecretion of axillary sweat glands. This could be explained by a higher expression of aquaporin 5 (AQP5), a selective water-selective channel protein that increases water permeability, in epithelial cells of patients [44].
Lin et al. [45] found that activin A receptor type 1 (ACVR1) is upregulated in the sweat glands of patients with primary axillary HH compared to control subjects. Overexpression of ACVR1 boosted the expression of AQP5 and Na–K–Cl cotransporter 1 (NKCC1), and thus may affect sweat secretion by regulating water and ion channels [45]. In addition, upregulation of the cholinergic receptor nicotinic alpha-1 subunit (CHRNA1) is a typical feature of sweat glands in patients with primary focal HH. CHRNA1 regulates the binding and gating of ACh neurotransmitters. Silencing of CHRNA1 decreased sweat secretion and the number of sweat secretory granules in a mouse model of HH [46]. It also decreased the expression of serum ACh, AQP5, and calcium voltage-gated channel subunit alpha-1 C (CACNA1C) in the sweat glands. Thus, upregulation of CHRNA1 is a potential biomarker for primary focal HH. Respectively, its downregulation may be a potential treatment target with the aim of inactivating the sympathetic system [46].
Genetic Predisposition
Several studies suggest that primary HH has a genetic component as demonstrated by the high frequency of positive family histories for individuals with primary axillar or palmoplantar HH [47]. While familial predisposition is more or less established, there is still uncertainty about the specific genes involved.
Most studies report autosomal dominant or recessive inheritance [47–52]. Results from family genetic studies have identified various loci for primary HH, but the results are inconsistent. For example, a study by Higashimoto et al. [49] indicated a linkage to loci on chromosome 14q11.2-q13, but this association could not be confirmed using a microsatellite method [50, 51]. Chen et al. [51] identified candidate genes on chromosome 2q22.1–q31.1, and Schote et al. [52], using genome-wide whole-exome sequencing, subsequently found four significant loci which overlap with the locus reported by Chen et al. [51]. In addition, a genetic polymorphism analysis suggested an association between –116A and K-variants on the BCHE gene in patients with HH, although this association was non-significant [53].
Various pathogenic mechanisms have been proposed to explain primary HH, such as the pronounced presence of AQP5 in the epithelial cells of the sweat glands in patients with primary HH, as mentioned earlier [44]. This possible involvement of the AQP5 gene is also supported by evidence in patients with hypohidrosis, i.e., insufficient secretion of sweat, as a symptom of Sjogren’s syndrome, in whom AQP5 expression is reduced [47]. However, family genetic studies have not identified loci on the AQP5 gene [49, 51]. Other candidate genes are the PLB1, PPP1CB, NDR2, and ABC11 genes [47]. In addition, there are several hereditary disorders associated with HH that refer to different chromosomes, such as nail-patella syndrome with a locus on chromosome 9 [47].
It should be noted, however, that analysis of genetics data is difficult: studies differ in their methods for the classification of HH, qualitative and quantitative measurements, review of medical records, and the surveys and interviews used [47]. Moreover, data can also be susceptible to recall bias as data are often collected from interviews [50].
Overall, findings in genetics suggest considerable heterogeneity in the disorder, and it is likely that HH is a multifactorial disorder. Data are still limited, and further genetics studies on large patient cohorts are needed.
Treatment
While numerous therapeutic strategies exist, there remains an unmet medical need and, in addition, lack of knowledge about the disorder may hinder appropriate treatment. Treatment options depend on the localization of HH. Current therapeutic approaches include topical treatments (e.g., aluminum chloride or topical anticholinergics) as first-line treatments for focal HH. When topical treatments are insufficient or not applicable, local intradermal injections of botulinum toxin, iontophoresis, or microwave thermolysis are indicated. Systemic oral medication, such as anticholinergics, may be considered; however, the use of such medications are limited by systemic anticholinergic side effects. In some countries, endoscopic thoracic sympathectomy is used as last option, which, although rare, may be associated with serious side effects [3, 9, 22, 26, 54]. For focal axillary HH a variety of local surgical procedures have been described, ranging from curettage, laser-assisted ablation to partial and radical excision techniques [54–57]. To identify new potential therapeutic targets and improve treatment options, a good understanding of the genetic components, the signaling pathways involved, and possible explanations of abnormal sweat secretion is necessary. This review article does not contain any new studies with human participants or animals performed by any of the authors.
Conclusions
The intention of this review was to show that HH is an underestimated chronic disorder based on the dysregulation of the CNS and peripheral ANS. The disorder is associated with significant impairment in the quality of life of patients who suffer from focal or generalized HH. HH is still an under-researched condition, and progress in the diagnostics, etiology, and treatment of HH is limited [6]. Because it is a neurologic disorder of the ANS, knowledge on the physiology, anatomic pathways, and diagnostic procedures is necessary to optimize treatment for patients. Therefore, it is crucial for physicians to understand the complex relationship between thermoregulation and pathophysiology [2].
There are several difficulties in studying HH that also highlight the need for greater awareness of the condition. HH is often unreported due to its socially distressing aspects [47]. Further problems result from the fact that sweating is influenced by various factors. Thus, distinguishing between different forms of HH and, for example, social stress and anxiety, is difficult [47]. Studies often include more severe forms of HH, which also may bias the reported results [4, 47, 48].
The overall aim of this review was to draw attention to HH and its complexity to promote a better understanding of the disorder, which is absolutely necessary for meaningful research, targeted treatment approaches, and the provision of appropriate treatment.
Acknowledgements
Funding
Preparation of the manuscript was financed in part by Dr. August Wolff GmbH & Co. KG, Bielefeld, Germany, and performed independently without influence of the sponsor. The Rapid Service Fee and the costs for Medical Writer were funded by Dr. August Wolff GmbH & Co. KG, Bielefeld, Germany.
Medical Writing
Medical writing support was provided by Tatjana Lux (co.medical, Berlin, Germany).
Author Contribution
Johannes Wohlrab: concept and design, co-drafting manuscript, corresponding author, editing manuscript. Falk Bechara: concept and design, editing manuscript. Christoph Schick: editing manuscript. Markus Naumann: concept and design, editing manuscript.
Disclosures
Johannes Wohlrab declares that in the last 5 years he has received honoraria for consulting and/or presentations and/or sponsoring for scientific projects and/or clinical studies from the following relevant companies: Bayer, Beiersdorf, Dermapharm, Galderma, Jenapharm, Leo, L’Oréal, Mavena, Mibe, Riemser, Skinomics, and Dr. Wolff. Falk Bechara declares that in the last 5 years he has received honoraria for consulting and/or presentations and/or sponsoring for scientific projects and/or clinical studies from the following relevant companies: Beiersdorf and Dr. Wolff. Christoph Schick declares that in the last 5 years he has received honoraria for consulting and/or presentations and/or sponsoring for scientific projects and/or clinical studies from the following relevant companies: Dr. Wolff. Markus Naumann:declares that in the last 5 years he has received honoraria for consulting and/or presentations and/or sponsoring for scientific projects and/or clinical studies from the following relevant companies: Abbvie, Merz and Biogen.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
References
- 1.Oshima Y, Tamada Y. Classification of systemic and localized sweating disorders. In: Yokozeki H, Murota H, Katayama I, editors. Perspiration research. Current problems in dermatology, vol 51. Basel: Karger; 2016. p. 7–10. [DOI] [PubMed]
- 2.Schick CH. Pathophysiology of hyperhidrosis. Thorac Surg Clin. 2016;26(4):389–393. doi: 10.1016/j.thorsurg.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Wohlrab J, Kreft B. Hyperhidrosis—aetiopathogenesis, diagnosis, clinical symptoms and treatment. Hautarzt. 2018;69(10):857–869. doi: 10.1007/s00105-018-4265-8. [DOI] [PubMed] [Google Scholar]
- 4.Hamm H, Naumann MK, Kowalski JW, Kutt S, Kozma C, Teale C. Primary focal hyperhidrosis: disease characteristics and functional impairment. Dermatology. 2006;212(4):343–353. doi: 10.1159/000092285. [DOI] [PubMed] [Google Scholar]
- 5.Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: a comprehensive review. Part I. Etiology and clinical work-up. J Am Acad Dermatol. 2019;81(3):657–666. doi: 10.1016/j.jaad.2018.12.071. [DOI] [PubMed] [Google Scholar]
- 6.Kristensen JK, Nielsen C. Progress and lack of progress in hyperhidrosis research 2015–2020. A concise systematic review. Int J Dermatol. 2021;61(2):148–157. doi: 10.1111/ijd.15654. [DOI] [PubMed] [Google Scholar]
- 7.Baker LB. Physiology of sweat gland function: the roles of sweating and sweat composition in human health. Temperature (Austin) 2019;6(3):211–259. doi: 10.1080/23328940.2019.1632145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu Y, Converse C, Lyons MC, Hsu WH. Neural control of sweat secretion: a review. Br J Dermatol. 2018;178(6):1246–1256. doi: 10.1111/bjd.15808. [DOI] [PubMed] [Google Scholar]
- 9.Rystedt A, Brismar K, Aquilonius S-M, Naver H, Swartling C. Hyperhidrosis—an unknown widespread “silent” disorder. J Neurol Neuromed. 2016;1(4):25–33. doi: 10.29245/2572.942X/2016/4.1037. [DOI] [Google Scholar]
- 10.Wilke K, Martin A, Terstegen L, Biel SS. A short history of sweat gland biology. Int J Cosmet Sci. 2007;29:169–179. doi: 10.1111/j.1467-2494.2007.00387.x. [DOI] [PubMed] [Google Scholar]
- 11.Hölzle E. Pathophysiology of sweating. In: Kreyden OP, Böni R, Burg E, editors. Hyperhydrosis of botulinum toxin in dermatology. Basel: Karger; 2002. [Google Scholar]
- 12.Sato K, Kang WH, Saga K, Sato KT. Biology of sweat glands and their disorders. II. Disorders of sweat gland function. J Am Acad Dermatol. 1989;20(5):713–726. doi: 10.1016/S0190-9622(89)70081-5. [DOI] [PubMed] [Google Scholar]
- 13.Kamberov YG, Karlsson EK, Kamberova GL, et al. A genetic basis of variation in eccrine sweat gland and hair follicle density. Proc Natl Acad Sci USA. 2015;112(32):9932–9937. doi: 10.1073/pnas.1511680112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato K, Leidal R, Sato F. Morphology and development of an apoeccrine sweat gland in human axillae. Am J Physiol. 1987;252(1):R166–R180. doi: 10.1152/ajpregu.1987.252.1.R166. [DOI] [PubMed] [Google Scholar]
- 15.Lonsdale-Eccles A, Leonard N, Lawrence C. Axillary hyperhidrosis: eccrine or apocrine? Clin Exp Dermatol. 2003;28:2–7. doi: 10.1046/j.1365-2230.2003.01162.x. [DOI] [PubMed] [Google Scholar]
- 16.Kreyden OP, Scheidegger EP. Anatomy of the sweat glands, pharmacology of botulinum toxin, and distinctive syndromes associated with hyperhidrosis. Clin Dermatol. 2004;22(1):40–44. doi: 10.1016/j.clindermatol.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 17.Sato K, Kang WH, Saga K, Sato KT. Biology of sweat glands and their disorders. I. Normal sweat gland function. J Am Acad Dermatol. 1989;20(4):537–563. doi: 10.1016/S0190-9622(89)70063-3. [DOI] [PubMed] [Google Scholar]
- 18.Groscurth P. Anatomy of sweat glands. In: Kreyden OP, Böni R, Burg E, editors. Hyperhydrosis of botulinum toxin in dermatology. Basel: Karger; 2002. [Google Scholar]
- 19.Lindsay SL, Holmes S, Corbett AD, Harker M, Bovell DL. Innervation and receptor profiles of the human apocrine (epitrichial) sweat gland: routes for intervention in bromhidrosis. Br J Dermatol. 2008;159(3):653–660. doi: 10.1111/j.1365-2133.2008.08740.x. [DOI] [PubMed] [Google Scholar]
- 20.Bovell DL, Corbett AD, Holmes S, MacDonald A, Harker M. The absence of apoeccrine glands in the human axilla has disease pathogenetic implications, including axillary hyperhidrosis. Br J Dermatol. 2007;156(6):1278–1286. doi: 10.1111/j.1365-2133.2007.07917.x. [DOI] [PubMed] [Google Scholar]
- 21.Cheshire WP, Low PA. Disorders of sweating and thermoregulation. CONTINUUM Lifelong Learn Neurol. 2007;13(6):143–164. doi: 10.1212/01.CON.0000299969.51137.ad. [DOI] [Google Scholar]
- 22.Lakraj AA, Moghimi N, Jabbari B. Hyperhidrosis: anatomy, pathophysiology and treatment with emphasis on the role of botulinum toxins. Toxins (Basel) 2013;5(4):821–840. doi: 10.3390/toxins5040821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bechara FG. Surgical treatment of focal hyperhidrosis. Dtsch Arztebl Int. 2009;106(26):448. doi: 10.3238/arztebl.2009.0448a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan CL, Knight ZA. Regulation of body temperature by the nervous system. Neuron. 2018;98(1):31–48. doi: 10.1016/j.neuron.2018.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui CY, Schlessinger D. Eccrine sweat gland development and sweat secretion. Exp Dermatol. 2015;24(9):644–650. doi: 10.1111/exd.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlereth T, Dieterich M, Birklein F. Hyperhidrosis—causes and treatment of enhanced sweating. Dtsch Arztebl Int. 2009;106(3):32–37. doi: 10.3238/arztebl.2009.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bovell DL, MacDonald A, Meyer BA, et al. The secretory clear cell of the eccrine sweat gland as the probable source of excess sweat production in hyperhidrosis. Exp Dermatol. 2011;20(12):1017–1020. doi: 10.1111/j.1600-0625.2011.01361.x. [DOI] [PubMed] [Google Scholar]
- 28.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 29.Strutton DR, Kowalski JW, Pharm D, Glaser DA, Stang PE. US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: results from a national survey. J Am Acad Dermatol. 2004;51(2):241–248. doi: 10.1016/j.jaad.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 30.Ricchetti-Masterson K, Symons JM, Aldridge M, et al. Epidemiology of hyperhidrosis in 2 population-based health care databases. J Am Acad Dermatol. 2018;78(2):358–362. doi: 10.1016/j.jaad.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Doolittle J, Walker P, Mills T, Thurston J. Hyperhidrosis: an update on prevalence and severity in the United States. Arch Dermatol Res. 2016;308(10):743–749. doi: 10.1007/s00403-016-1697-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stefaniak T, Tomaszewski KA, Proczko-Markuszewska M, Idestal A, Royton A, Abi-Khalil C. Is subjective hyperhidrosis assessment sufficient enough? Prevalence of hyperhidrosis among young Polish adults. J Dermatol. 2013;40(10):819–823. doi: 10.1111/1346-8138.12238. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Bahar R, Kalia S, et al. Hyperhidrosis prevalence and demographical characteristics in dermatology outpatients in Shanghai and Vancouver. PLoS One. 2016;11(4):e0153719. doi: 10.1371/journal.pone.0153719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shayesteh A, Gerdsdorff F, Persson M, Brulin C, Nylander E. Navigating in the fog. Facing delays, rejection and ignorance when seeking help for primary hyperhidrosis. Int J Qual Stud Health Well-being. 2021;16(1):1930642. doi: 10.1080/17482631.2021.1930642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheshire WP. Sudomotor dysfunction. Semin Neurol. 2020;40(5):560–568. doi: 10.1055/s-0040-1713847. [DOI] [PubMed] [Google Scholar]
- 36.Hashmonai M, Cameron AEP, Connery CP, Perin N, Licht PB. The etiology of primary hyperhidrosis: a systematic review. Clin Auton Res. 2017;27(6):379–383. doi: 10.1007/s10286-017-0456-0. [DOI] [PubMed] [Google Scholar]
- 37.Schestatsky P, Callejas MA, Valls-Sole J. Abnormal modulation of electrodermal activity by thermoalgesic stimuli in patients with primary palmar hyperhidrosis. J Neurol Neurosurg Psychiatry. 2011;82(1):92–96. doi: 10.1136/jnnp.2009.203687. [DOI] [PubMed] [Google Scholar]
- 38.Solish N, Bertucci V, Dansereau A, et al. A comprehensive approach to the recognition, diagnosis, and severity-based treatment of focal hyperhidrosis: recommendations of the Canadian Hyperhidrosis Advisory Committee. Dermatol Surg. 2007;33(8):908–923. doi: 10.1111/j.1524-4725.2007.33192.x. [DOI] [PubMed] [Google Scholar]
- 39.Lee JB, Kim TW, Shin YO, Min YK, Yang HM. Effect of the heat-exposure on peripheral sudomotor activity including the density of active sweat glands and single sweat gland output. Korean J Physiol Pharmacol. 2010;14(5):273–278. doi: 10.4196/kjpp.2010.14.5.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirakawa N, Higashimoto I, Takamori A, Tsukamoto E, Uemura Y. The impact of endoscopic thoracic sympathectomy on sudomotor function in patients with palmar hyperhidrosis. Clin Auton Res. 2021;31(2):225–230. doi: 10.1007/s10286-020-00685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathias CJ. Sympathetic nervous system disorders in man. Baillire's Clin Endocrinol Metab. 1993;7(2):465–490. doi: 10.1016/S0950-351X(05)80184-3. [DOI] [PubMed] [Google Scholar]
- 42.Moura NB, Jr, das-Neves-Pereira JC, De Oliveira FRG, et al. Expression of acetylcholine and its receptor in human sympathetic ganglia in primary hyperhidrosis. Ann Thorac Surg. 2013;95(2):465–470. doi: 10.1016/j.athoracsur.2012.10.068. [DOI] [PubMed] [Google Scholar]
- 43.De Oliveira FRG, Moura NB, Jr, de Campos JRM, et al. Morphometric analysis of thoracic ganglion neurons in subjects with and without primary palmar hyperhidrosis. Ann Vasc Surg. 2014;28(4):1023–1029. doi: 10.1016/j.avsg.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Du Q, Lin M, Yang JH, Chen JF, Tu YR. Overexpression of AQP5 was detected in axillary sweat glands of primary focal hyperhidrosis patients. Dermatology. 2016;232(2):150–155. doi: 10.1159/000444081. [DOI] [PubMed] [Google Scholar]
- 45.Lin JB, Chen JF, Lai FC, et al. Involvement of activin a receptor type 1 (ACVR1) in the pathogenesis of primary focal hyperhidrosis. Biochem Biophys Res Commun. 2020;528(2):299–304. doi: 10.1016/j.bbrc.2020.05.052. [DOI] [PubMed] [Google Scholar]
- 46.Lin JB, Kang MQ, Huang LP, Zhuo Y, Li X, Lai FC. CHRNA1 promotes the pathogenesis of primary focal hyperhidrosis. Mol Cell Neurosci. 2021;111:103598. doi: 10.1016/j.mcn.2021.103598. [DOI] [PubMed] [Google Scholar]
- 47.Henning MA, Pedersen OB, Jemec GB. Genetic disposition to primary hyperhidrosis: a review of literature. Arch Dermatol Res. 2019;311(10):735–740. doi: 10.1007/s00403-019-01966-1. [DOI] [PubMed] [Google Scholar]
- 48.Kaufmann H, Saadia D, Polin C, Hague S, Singleton A, Singleton A. Primary hyperhidrosis—evidence for autosomal dominant inheritance. Clin Auton Res. 2003;13(2):96–98. doi: 10.1007/s10286-003-0082-x. [DOI] [PubMed] [Google Scholar]
- 49.Higashimoto I, Yoshiura K, Hirakawa N, et al. Primary palmar hyperhidrosis locus maps to 14q11.2–q13. Am J Med Genet A. 2006;140(6):567–572. doi: 10.1002/ajmg.a.31127. [DOI] [PubMed] [Google Scholar]
- 50.Del Sorbo F, Brancati F, De Joanna G, Valente EM, Lauria G, Albanese A. Primary focal hyperhidrosis in a new family not linked to known loci. Dermatology. 2011;223(4):335–342. doi: 10.1159/000334936. [DOI] [PubMed] [Google Scholar]
- 51.Chen J, Lin M, Chen X, et al. A novel locus for primary focal hyperhidrosis mapped on chromosome 2q31.1. Br J Dermatol. 2015;172(4):1150–1153. doi: 10.1111/bjd.13383. [DOI] [PubMed] [Google Scholar]
- 52.Schote AB, Schiel F, Schmitt B, et al. Genome-wide linkage analysis of families with primary hyperhidrosis. PLoS One. 2020;15(12):e0244565. doi: 10.1371/journal.pone.0244565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simes BC, Moore JP, Brown TC, Rushforth TJ, Bookout AL, Richardson CL. Genetic polymorphism analysis of patients with primary hyperhidrosis. Clin Cosmet Investig Dermatol. 2018;11:477–483. doi: 10.2147/CCID.S176842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: a comprehensive review. Part II. Therapeutic options. J Am Acad Dermatol. 2019;81(3):669–680. doi: 10.1016/j.jaad.2018.11.066. [DOI] [PubMed] [Google Scholar]
- 55.Bechara FG, Sand M, Altmeyer P. Characteristics of refractory sweating areas following minimally invasive surgery for axillary hyperhidrosis. Aesthetic Plast Surg. 2009;33(3):308–311. doi: 10.1007/s00266-008-9261-4. [DOI] [PubMed] [Google Scholar]
- 56.Bechara FG, Sand M, Hoffmann K, Boorboor P, Altmeyer P, Stuecker M. Histological and clinical findings in different surgical strategies for focal axillary hyperhidrosis. Dermatol Surg. 2008;34(8):1001–1009. doi: 10.1111/j.1524-4725.2008.34198.x. [DOI] [PubMed] [Google Scholar]
- 57.Cervantes J, Perper M, Eber AE, Fertig RM, Tsatalis JP, Nouri K. Laser treatment of primary axillary hyperhidrosis: a review of the literature. Lasers Med Sci. 2018;33(3):675–681. doi: 10.1007/s10103-017-2434-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article/as supplementary information files.