Abstract
Background
This review’s goals were to investigate apremilast’s efficacy versus placebo in palmoplantar psoriasis (PP) and palmoplantar pustulosis (PPP), and apremilast’s efficacy versus methotrexate in PP.
Methods
A literature search was conducted in PubMed, clinicaltrials.gov, and Embase in July 2022. Publications investigating subjects with PP or PPP, treated with apremilast, which reported palmoplantar-specific outcomes were used. Exclusion criteria included cases of drug-induced PP/PPP, case studies, non-English texts, omission of palmoplantar-specific outcomes, and incomplete publications. Studies were assessed for risk of bias using Cochrane Review Manager application and CASP checklist. Primary endpoints were a 50% improvement of the Palmoplantar Psoriasis/Pustulosis Area and Severity Index (PPPASI 50) and improvement of the Palmoplantar Physician Global Assessment (PPPGA) to 0 or 1 in patients with baseline PPPGA ≥ 3.
Results
Seventeen original studies including five placebo-controlled randomized clinical trials (RCTs), one phase II clinical trial, two randomized methotrexate comparative trials, six cohort studies, and three case series were analyzed, totaling 1117 participants. Meta-analysis of four placebo-controlled RCTs investigating PP found apremilast treatment to be superior to placebo in achieving a PPPGA of 0/1 (baseline PPPGA of ≥ 3) after 16 weeks of treatment (n = 244; OR = 2.69 [1.39–5.22]). Apremilast was superior to placebo in achieving PPPASI 50 at week 16 in the only placebo-controlled RCT of PPP (78.3 vs. 40.9%) [P = 0.0003]. Apremilast was comparable to methotrexate in achieving PPPASI 50 at week 16 in PP (59.5 vs. 64.3%; n = 84; [P = 0.65]). Non-randomized studies generally showed marked improvement in PPPASI, PPPGA, and DLQI scores following apremilast treatment.
Discussion
Apremilast treatment in PP and PPP resulted in significant improvement in objective, palmoplantar-specific clinical parameters versus placebo, and comparable efficacy with methotrexate in PP. Limitations in interpreting these results include variations in palmoplantar-specific metrics used and risk of bias of included studies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-022-00877-w.
Keywords: Apremilast, Methotrexate, Otezla, Palmoplantar psoriasis, Palmoplantar pustulosis, Psoriasis
Key Summary Points
| Palmoplantar psoriasis and palmoplantar pustulosis are chronic, inflammatory skin diseases that are often resistant to topical corticosteroids or phototherapy. |
| This study investigated the efficacy of apremilast versus placebo and methotrexate in palmoplantar psoriasis and palmoplantar pustulosis. |
| Meta-analysis of placebo-controlled RCTs investigating apremilast in palmoplantar psoriasis found apremilast to be superior to placebo in achieving clinical clearance. |
| Apremilast was superior to placebo in achieving clinical improvements based on palmoplantar-specific metrics in palmoplantar pustulosis. |
| In patients with palmoplantar psoriasis, apremilast demonstrated comparable efficacy to methotrexate, and may be a preferred oral, non-biologic, systemic agent in patients with hepatic insufficiency. |
Introduction
Palmoplantar psoriasis (PP) is a chronic, debilitating, inflammatory skin disease of the palms and/or soles, with reported incidence of 2.8–40.9% in patients with psoriasis [1]. Despite the surface area of the palms and soles representing less than 5% of total body surface area (BSA), patients with PP experience significant impairments in quality of life. Compared to moderate-to– severe plaque psoriasis without palm and sole involvement, patients with PP have greater impairments in quality of life, evidenced by higher Dermatology Life Quality Index (DLQI) scores [2]. The presentation of PP varies widely, and lesions may present in isolation or with concomitant plaque psoriasis at other sites. The morphology of PP often resembles plaque psoriasis (i.e., well-defined, erythematous plaques with overlying thick, hyperkeratotic scales, often with accompanying fissures). PP may also present as cyclic, fluid-filled, sterile, pustular lesions in an erythematous background [3]. PP is typically classified into hyperkeratotic palmoplantar psoriasis and palmoplantar pustular psoriasis subgroups, respectively, based on these morphological distinctions [4]. Palmoplantar pustulosis (PPP) is often considered a distinct entity from PP and presents with concomitant psoriasis in other sites less frequently. Of note, PPP disproportionately affects smokers and women, with a female-to-male ratio of 8:2 [5]. The treatment of PP and PPP is challenging and may vary based on clinical presentation and morphological subtype. Traditional first-line therapies for psoriasis, including topical corticosteroids and phototherapy, are often ineffective due to the thickness of the stratum corneum in the palms and soles [6]. Some systemic medications and biologic therapies have shown promise, but many drugs in these classes are still under active investigation [7, 8].
Objective measures typically used to stratify disease severity in psoriasis, like the Psoriasis Area and Severity Index (PASI) and BSA, are unreliable measures of patient disability in PP and PPP, as they tend to underestimate disease severity [2]. Instead, modified assessments like the Palmoplantar Psoriasis Area and Severity Index (PPPASI) and the Palmoplantar Psoriasis Physician Global Assessment (PPPGA) are better suited for measuring disease severity in PP and PPP. Given the rapidly evolving landscape in the treatment of psoriasis, our aim was to assess the utility of apremilast in palmoplantar disease using these scoring tools.
Apremilast is an orally administered, selective inhibitor of phosphodiesterase-4 (PDE-4) used in the treatment of psoriasis and psoriatic arthritis. Recommended dosing of apremilast is 30 mg twice daily. PDE-4 inhibition prevents hydrolysis of cyclic adenosine monophosphate (cAMP), increasing intracellular cAMP levels, resulting in downstream effects on signaling pathways in various cell types including T cells, monocytes, keratinocytes, and synovial fibroblasts [9]. Increased intracellular cAMP due to PDE-4 inhibition leads to protein kinase A (PKA) activation and increased expression of IL-10, an anti-inflammatory cytokine [9]. Activation of cAMP responsive element (CRE) sites also causes inhibition of NF-kB and reduced production of downstream proinflammatory mediators implicated in the pathogenesis of psoriasis (e.g., TNF-α, IL-23) [10].
With traditional first-line therapies for psoriasis often proving ineffective in producing clinical improvement in patients with PP and PPP, non-biologic systemic agents and biologic treatments are often necessary. Regarding biologic treatments, the quality of evidence supporting their use is low to moderate, in part due to a lack of clinical trials investigating their use in PP and PPP [7, 8].
While the efficacy of apremilast in the treatment of plaque psoriasis is well established, randomized controlled trials (RCTs) evaluating the efficacy of apremilast in PP have demonstrated mixed results [11, 12]. Fewer studies have evaluated apremilast in PPP. The aim of this review is to investigate the efficacy of apremilast versus placebo in PP and PPP, and to compare the efficacy of apremilast versus another orally administered systemic agent, methotrexate, in PP.
Methods
Our protocol is reported in line with the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2020 guidelines. A literature search was conducted in PubMed (MEDLINE), Embase, and clinicaltrials.gov in July 2022 using a combination of the terms (‘palmoplantar’ OR ‘palms’ OR ‘soles’) AND (‘psoriasis’ OR ‘pustulosis’) AND (‘apremilast’ OR ‘otezla’). This search was replicated in August 2022 to include articles published in the interim. Our initial search was filtered using the “(clinical trial)” filter in the PubMed and Embase search options. This search was then modified to include all original, experimental prospective and retrospective studies, and nonexperimental descriptive studies for the purpose of this review. Comprehensive review articles were referenced to identify any additional studies that were missed during the initial searches.
Eligibility Criteria
Criteria for publications included in this review were those with subjects diagnosed with PP or PPP based on the opinion of the authors of each publication, and subjects who received treatment with apremilast. Publications were required to report results using palmoplantar-specific scoring systems, namely a 50% improvement in the Palmoplantar Psoriasis/Pustulosis Area and Severity Index (PPPASI 50) and an improvement to a score of 0 or 1 in the Palmoplantar Psoriasis/Pustulosis Physician Global Assessment (PPPGA). Studies investigating patients with plaque psoriasis with concomitant palmoplantar involvement were included if they reported specific outcomes in the palmoplantar-involved subpopulation using palmoplantar-specific scoring systems. Excluded studies were those not written in English, case studies, publications involving subjects with drug-induced PP/PPP, and incomplete or non-peer-reviewed publications (poster abstracts, preprint publications, etc.). Studies were grouped for pooled analyses by disease classification (i.e., PP, PPP). Study characteristics including author, year of publication, patient number, intervention, treatment duration, and results were obtained using a standardized table tailored to this review, with the tabulated information evaluated to determine eligibility.
Study Selection and Data Extraction
Initial screening of studies was performed manually by three independent reviewers (R.K.S., K.G.E., J.Q.J.). Discrepancies in eligibility criteria were resolved by an additional reviewer (W.L.). Data abstraction was performed by R.K.S. All randomized studies included for analysis were assessed for risk of bias by two independent authors (R.K.S., M.S.D.) through manual review of each paper.
Primary outcomes sought for the purpose of this review include PPPASI 50 and a PPPGA of 0 or 1 (i.e., clear or almost clear) in patients with a baseline score of ≥ 3. Secondary outcomes included a 75% improvement in the PPPASI score (PPPASI 75) and mean PPPASI improvement. Studies that used a Physician Global Assessment (PGA) in cases of palmoplantar pustulosis and palmoplantar pustular psoriasis, or a Hand and Foot Physician Global assessment (H&F PGA) were also considered. Other outcomes reported were mean improvement in the Dermatology Life Quality Index (DLQI) score, and drug survival time (months of drug until discontinuation). For studies where certain values (e.g., mean change, PPPASI 50) were unavailable but could be determined from baseline values, absolute change, or individual subject results, values were calculated manually. Primary and secondary endpoints measured at variable intervals amongst studies were defined and reported as they were in the original studies. Study characteristics were tabulated for each individual study.
Statistical Analysis
Using the Cochrane Review Manager 5.4 application, a meta-analysis was performed comparing the efficacy (PPPGA 0/1 rates) of apremilast versus placebo in PP, with an odds ratio being used as the effect measure in the synthesis of these results. Only randomized placebo-controlled trials were included for meta-analysis, with those deemed eligible for analysis having study intervention characteristics matching the planned inclusion criteria. To minimize the risk of reporting bias, only study results with double-blinded periods were included for meta-analysis. Forest plot analysis using the Mantel–Haenszel fixed-effect method was utilized in calculating the odds ratio. The Mantel–Haenszel statistical method was chosen over the Peto method as the latter performs best only when event occurrence is rare [13]. Significance of heterogeneity amongst studies was assessed using the χ2 test (P value < 0.1 as statistically significant) and presented as the I2 test (I2 > 50% indicates significant heterogeneity, I2 < 25% indicates non-significant heterogeneity). No additional methods to explore causes of heterogeneity were performed due to the calculated I2 value representing non-significant heterogeneity.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Results
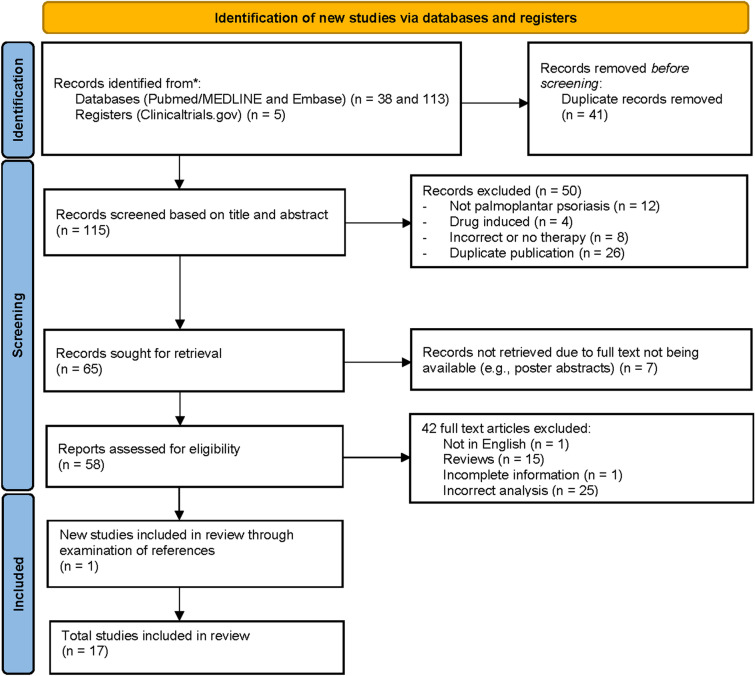
Initial search yielded 156 total articles; 41 duplicates were excluded, and 115 publications were screened based on title and abstract. Subsequently, 57 articles were excluded, leading to the identification of 58 articles for full-text review. Ultimately, 17 publications investigating treatment with apremilast in patients with PP or PPP were included after full text-review (Fig. 1). These 17 publications consisted of five placebo-controlled RCTs [11, 12, 14–17], one single-arm phase II clinical trial [18], two randomized methotrexate comparative trials [19, 20], six prospective/retrospective cohort studies [21–26], and three case series [27–29], totaling 1117 subjects with palmoplantar disease treated with apremilast or placebo. Of the 1117 subjects with palmoplantar disease, 948 cases of PP and 169 cases of PPP were represented.
Fig. 1.
PRISMA diagram showing study selection
All randomized studies included for analysis were manually assessed for risk of bias and compiled using the Cochrane Review Manager application. Six different domains of bias were assessed, including method of random sequence generation, allocation concealment, participant and personnel blinding, outcome assessment blinding, incomplete outcomes, and selective reporting (Fig. 2). Observational and retrospective cohort studies, and case series were assessed for risk of bias using the Critical Appraisal Skills Program (CASP) Cohort Study checklist (Supplemental Table 1).
Fig. 2.
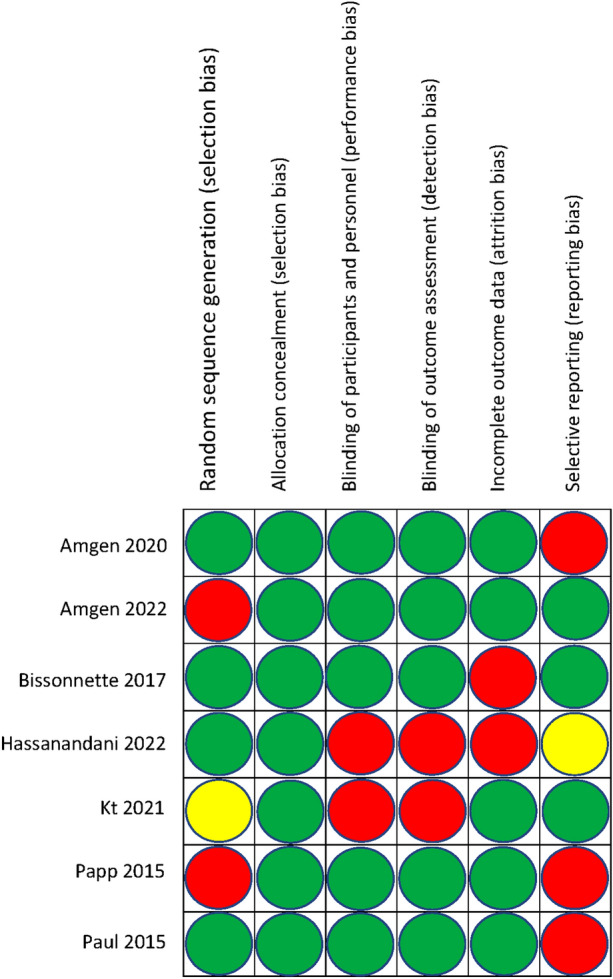
Risk of bias assessment for randomized clinical trials
Palmoplantar Psoriasis
A total of 12 publications investigated patients with PP treated with apremilast (APR) including four placebo (PBO)-controlled RCTs [11, 12, 14–16], two randomized methotrexate (MTX) comparative trials [19, 20], four prospective/retrospective cohort studies [22–24, 26], and two case series [27, 29] (Table 1).
Table 1.
Studies investigating apremilast in palmoplantar psoriasis (PP)
| Author, year, study name, study type | Comparison | Number of patients | PPPASI 50 (%) | PPPGA 0 or 1 (%) (baseline PPPGA ≥ 3) | Secondary outcomes |
|---|---|---|---|---|---|
|
PSOR-005 RCT |
Apremilast vs. placebo | 49 (19 baseline PPPGA ≥ 3) | 66.7 vs. 20.0 (APR vs. PBO) (week 16) | ||
|
ESTEEM 1 RCT |
Apremilast vs. placebo | 254 (83 baseline PPPGA ≥ 3) | 38.6 vs. 30.8 (APR vs. PBO) (week 16) | ||
|
ESTEEM 2 RCT |
Apremilast vs. placebo | 124 (42 baseline PPPGA ≥ 3) | 65.4 vs. 31.3 (APR vs. PBO) (week 16) | ||
|
Bissonnette et al. 2018 [12] RCT |
Apremilast vs. placebo | 100 (baseline PPPGA ≥ 3) | 36.0 vs 22.0 (APR vs. PBO) (week 16) [P = 0.119] | 14.0 vs. 4.0 (APR vs. PBO) (week 16) [P = 0.1595] |
Mean PPPASI improvement (%): 39.6 vs. 23.1 (APR vs. PBO) (week 16) [P = 0.0167] PPPASI 75 (%): 22.0 vs. 8.0 (APR vs. PBO) (week 16) [P = 0.0499] Mean DLQI improvement: 4.3 vs. 0.8 (APR vs. PBO) (week 16) [P = 0.0004] |
|
Kt et al. [19] 2021 MTX comparative trial |
Apremilast vs. methotrexate | 84 (76/84 PP, 8/84 PPP) | 59.5 vs. 64.3 (APR vs. MTX) (week 16) |
Median PPPASI improvement (%): 62.3 vs. 65.8 (APR vs. MTX) (week 16) [P = 0.39, (95% CI = – 4.2–2.1)] PPPASI 75 (%): 33.3 vs. 40.5 (APR vs. MTX) (week 16) [P = 0.49] Mean DLQI improvement (%): 50.0 vs. 47.6 (APR vs. MTX) (week 16) [P = 0.99 (95% CI = – 1.0–2.0)] |
|
|
Hassanandani et al. 2022 [20] MTX comparative trial |
Apremilast methotrexate combination vs. methotrexate | 60 (baseline PPPGA ≥ 3) | 80.0 vs. 60.0 (APR + MTX vs. MTX) (week 16) [P = < 0.05] |
Mean PPPASI improvement (%): 81.5 vs. 71.7 (APR + MTX vs. MTX) (week 16) [P = 0.002] PPPASI 75 (%): 43.3 vs. 30.0 (APR + MTX vs. MTX) (week 16) [P = 0.001] Mean DLQI improvement (%): 66.9 vs. 58.9 (APR + MTX vs. MTX) (week 16) [P = 0.001] |
|
|
Ioannides et al. 2021 [22] APRAISAL Prospective Cohort |
None | 111 (44 with baseline PPPGA ≥ 3) | 72.7 (week 52) [95% CI = 59.6–85.9] | Median DLQI score improvement (%): 75.0 (week 24) (includes non-palmoplantar psoriasis) | |
|
Del Alcázar et al. 2020 [23] Retrospective cohort |
None | 85 (mean baseline PPPGA 4.2) | 36.1 and 83.3 (weeks 12 and 52) (as observed) | Drug survival after 1 year of treatment (%): 54.9 (includes non-palmoplantar psoriasis) | |
|
Reich et al. 2019 [26] LAPIS-PSO Prospective cohort |
None | 67 (28 with baseline PPPGA ≥ 3) | PPPGA 0 or 1 (baseline PPPGA ≥ 1) (%): 62.7 and 71.7 (week 16 and 52) (full analysis set) | ||
|
Pavia et al. 2022 [24] Retrospective cohort |
None | 12 (11 with baseline PPPGA ≥ 3) | 90.9 (week 24) | ||
|
Ständer et al. 2020 [29] Case series |
None | 6 (palmoplantar pustular psoriasis) |
PGA of 0 or 1 (%): 83.3 (week 4) PGA 0 or 1 (%): 100.0 (week 12) |
||
|
Aceituno-Madera et al. 2018 [27] Case series |
None | 4 | PPPGA 0 or 1 (%): 50.0 (week 16) |
APR apremilast, DLQI Dermatology Quality of Life Index, MTX methotrexate, PBO placebo, PGA physician global assessment, PPPASI Palmoplantar Psoriasis Area and Severity Index, PPPGA palmoplantar physician global assessment, RCT randomized controlled trial
There were four placebo-controlled RCTs analyzing the use of apremilast in PP. A post hoc, pooled analysis of three randomized, placebo-controlled clinical trials (ESTEEM1, ESTEEM2, and PSOR-005) demonstrated the efficacy of apremilast monotherapy in patients with PP. In patients with moderate to severe PP (baseline PPPGA ≥ 3), apremilast resulted in significantly more clearance (PPPGA 0 or 1) versus placebo at week 16 (47.8 vs. 26.9%) (n = 144) [P = 0.021 (95% CI = 0.05–0.37)] [11, 14–16]. In all patients with PP regardless of severity (PPPGA ≥ 1), apremilast resulted in significantly more patients achieving PPPGA of 0 at week 16 (46.3 vs. 25.4%) (n = 427) (APR vs. PBO) [P = < 0.001 (95% CI = 0.12–0.30)] [11. 14–16]. In the placebo-controlled RCT performed by Bissonnette et al. there was no significant difference in the rates of PPPGA of 0 or 1 (14.0 vs. 4.0%) (n = 100) [P = 0.1595] or PPPASI 50 (36.0 vs. 22.0%) [P = 0.119] achievement between the apremilast and placebo groups at week 16 [12]. However, apremilast did result in significantly greater rates of PPPASI 75 (22.0 vs. 8.0%) [P = 0.0499] and mean PPPASI improvement (39.6 vs. 23.1%) [P = 0.0167] at week 16 versus placebo [12].
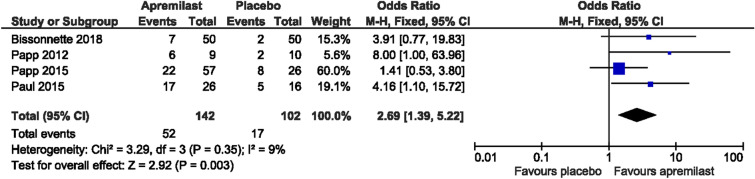
Meta-analysis of the four randomized placebo-controlled trials [12, 14–16] comparing the rates of clearance (PPPGA 0 or 1) in patients with moderate to severe PP (baseline PPPGA ≥ 3) (n = 244) demonstrated a statistically significant difference favoring apremilast versus placebo [odds ratio (OR) = 2.69 (95% confidence interval (CI) = 1.39–5.22)] (Fig. 3). The heterogenicity between the included randomized placebo-controlled clinical trials was found to be low (χ2 = 3.29; df = 3; P = 0.35; I2 = 9%).
Fig. 3.
Forest plot of apremilast vs. placebo in palmoplantar psoriasis (PP)
Two comparative trials investigated apremilast versus methotrexate in PP which demonstrated their similar efficacies and improved ability to produce clearance when given in combination. In a randomized comparative trial by Kt et al. [19], the use of apremilast and methotrexate in palmoplantar disease (PP, n = 76; PPP, n = 8) was investigated with the two drugs demonstrating comparable efficacy in achieving PPPASI 50 (59.5 vs. 64.3%) [P = 0.65] and PPPASI 75 (33.3 vs. 40.5%) [P = 0.49] at week 16. In a randomized comparative trial by Hassanandani et al. [20], apremilast in combination with methotrexate was shown to be more effective versus methotrexate monotherapy in achieving PPPGA of 0 or 1 at week 16 in patients with moderate to severe PP (baseline PPPGA ≥ 3) (80.0 vs. 60.0%) (n = 60) [P = < 0.05].
Four prospective/retrospective cohort studies evaluating apremilast in PP suggest that apremilast may be able to produce clearance over an extended period. APRAISAL, a 52-week prospective cohort study in Greece [22], demonstrated long– term clearance (PPPGA 0 or 1 at week 52) in 72.7% [95% CI = 59.6–85.9] of patients with moderate to severe PP treated with apremilast (n = 44). In a 52-week retrospective cohort study by the Spanish Psoriasis Group [23], the rates of patients treated with apremilast (n = 85, mean baseline PPPGA 4.2) achieving PPPGA 0 or 1 was 36.1 and 83.3% (as observed) at weeks 12 and 52, respectively. LAPIS-PSO, a 52-week, multicenter, observational cohort study in Germany investigating apremilast treatment for PP [26], resulted in 62.7 and 71.7% of patients (n = 67) achieving PPPGA 0 or 1 at weeks 16 and 52, respectively. A single-center retrospective cohort study by Pavia et al. [24] showed 90.9% (n = 12) of subjects with moderate to severe PP (baseline PPPGA ≥ 3) achieving clearance (PPPGA 0 or 1) after 24 weeks of apremilast treatment.
One publication proposed apremilast as viable treatment option in the pustular variant of PP, suggesting it may produce an immediate response in some patients. In a case series by Ständer et al. [29] a majority (5/6, i.e., 83.3%) of subjects with moderate-to-severe palmoplantar pustular psoriasis (baseline PGA ≥ 3) achieved a PGA of 0 or 1 at week 4 following apremilast initiation. Another case series by Aceituno-Madera et al. [27] showed half of patients with PP (n = 4) achieving clearance (PPPGA 0 or 1) at week 16.
Palmoplantar Pustulosis
Five publications investigated patients with PPP treated with apremilast (APR) including one placebo-controlled RCT [17], one single-arm phase II clinical trial [18], two retrospective cohort studies [21, 25], and one case series [28] (Table 2).
Table 2.
Studies investigating apremilast in palmoplantar pustulosis (PPP)
| Author, year, study name, study type | Comparison | Number of patients | PPPASI 50 (%) | PPPGA 0 or 1 (%) (baseline PPPGA ≥ 3) | Secondary outcomes |
|---|---|---|---|---|---|
|
Amgen 2022 [17] RCT |
Apremilast vs. placebo | 90 (86 with baseline PPPGA ≥ 3) | 78.3 vs. 40.9 (APR vs. PBO) (week 16) [P = 0.0003] |
Mean PPPASI improvement (%): 64.3 vs. 42.3 (APR vs. PBO) (week 16) PGA 0 or 1 (%): 19.6 vs. 4.5 (APR vs. PBO) (week 16) |
|
|
Reich et al. 2021 [18] APLANTUS Phase II clinical trial |
None | 21 (19 with baseline H&F PGA ≥ 3) | 33.3, 57.1, and 61.9 (weeks 4, 12 and 20) |
Median PPPASI improvement (%): 57.1 (week 20) [P = < 0.001] PPPASI 75 (%): 9.5, 28.6, and 14.3 (weeks 4, 12, and 20) Median DLQI improvement (%): 76.5 (week 20) [P = 0.03] |
|
|
Kato et al. 2021 [21] Retrospective cohort |
None | 10 | 80.0 (week 16) |
Mean PPPASI improvement (%): 61.9 (2 ± 1 weeks) [P = 0.013] PPPASI 75 (%): 50.0 (week 16) Mean DLQI improvement (%): 66.0 (2 ± 1 weeks) [P = 0.009] |
|
|
Kromer et al. 2019 [25] Retrospective cohort |
APR vs. cyclosporine vs. acitretin-PUVA vs. MTX vs. acitretin vs. alitretinoin vs. fumaric acid esters vs. certolizumab pegol vs. infliximab vs. golimumab vs. ustekinumab vs. adalimumab vs. secukinumab vs. etanercept) | 347 (35 treated with APR) |
Median drug survival (months): 15 vs. 12 vs. 9 vs. 8 vs. 6 vs. 5 vs. 3 vs. 47.4 vs. 26 vs. 22 vs. 21 vs. 18 vs. 9 vs. 8 (APR vs. cyclosporine vs. acitretin-PUVA vs. MTX vs. acitretin vs. alitretinoin vs. fumaric acid esters vs. certolizumab pegol vs. infliximab vs. golimumab vs. ustekinumab vs. adalimumab vs. secukinumab vs. etanercept) PPPASI 75 (%): 31.4 (APR) |
||
|
Kt et al. 2021 [28] Case Series |
None | 5 | 80.0 (average treatment duration 15.2 weeks) |
Mean PPPASI improvement (%): 72.9 (average treatment duration 15.2 weeks) PPPASI 75 (%): 40.0 (average treatment duration 15.2 weeks) |
APR apremilast, DLQI Dermatology Quality of Life Index, MTX methotrexate, PBO placebo, PGA physician global assessment, PPPASI Palmoplantar Pustulosis Area and Severity Index, PPPGA palmoplantar physician global assessment, PUVA psoralen plus ultraviolet A, RCT randomized controlled trial
In the only randomized, placebo-controlled clinical trial investigating apremilast in PPP (n = 90), apremilast showed significantly greater rates of PPPASI 50 achievement compared to placebo at week 16 (78.3 vs. 40.9%) [P = 0.0003] [17]. Similarly, apremilast also resulted in greater rates of clearance or near clearance (PGA of 0 or 1) at week 16 compared to placebo in patients with PPP (19.6 vs. 4.5%) (mean baseline PGA 3.7) [17]. APLANTUS, a single-arm phase II clinical trial investigating apremilast in patients with PPP (n = 21), resulted in 61.9% of patients achieving PPPASI 50 at week 20, including a median PPPASI improvement of 57.1% at week 20 [P < 0.001] [18].
Two retrospective cohort studies have examined the use of apremilast in PPP. In a retrospective cohort study by Kato et al. [21], apremilast induction resulted in an immediate response in patients with PPP, with a mean PPPASI reduction of 61.9% after 2 ± 1 weeks of treatment (n = 10) [P = 0.013] and 80.0% of patients achieving PPPASI 50 by week 16. A multicenter retrospective cohort study based in Germany [25] examined drug survival rates (i.e., time to drug discontinuation due to loss of efficacy or adverse effects) in 347 patients with PPP treated with various non-biologic systemic agents and biologic medications. Amongst the non-biologic systemic agents used in the treatment of PPP, patients treated with apremilast (n = 35) had the highest median drug survival rate in months at 15 months, followed by cyclosporine, acitretin-PUVA, methotrexate, acitretin monotherapy, alitretinoin, and fumaric acid esters [25]. Apremilast also had a greater median drug survival rate in months compared to two different biologic medications, secukinumab and etanercept [25]. In this study, apremilast produced a PPPASI 75 response in 31.4% of patients [25]. A lone case series by Kt et al. [28] demonstrated 80.0% of patients with PPP achieving PPPASI 50 after an average duration of treatment with apremilast for 15.2 weeks (n = 5).
Discussion
To our knowledge, this study is the first meta-analysis investigating the use of apremilast in PP and demonstrates a statistically significant difference in the rates of disease clearance/near clearance in patients treated with apremilast compared to placebo. Our findings are meaningful, as the results of these individual trials demonstrated mixed results with regard to statistical significance. According to our search, only one randomized placebo-controlled clinical trial has been performed investigating the use of apremilast in PPP, which showed significantly greater rates of PPPASI 50 achievement compared to placebo [17]. While cohort studies and case series have highlighted the promise of administering apremilast [21–29], additional RCTs investigating apremilast in palmoplantar disease, especially PPP, should be conducted for further confirmation.
Studies comparing apremilast with methotrexate demonstrated similar rates of improvement in PPPASI scores for PP between the two drugs [19] and higher median drug survival rates in PPP for apremilast versus methotrexate [25]. Higher drug survival rates may suggest apremilast as being better tolerated or more effective than alternative non-biologic, systemic drugs. Unlike other non-biologic systemic agents including methotrexate and cyclosporine, apremilast does not require dose adjustments in patients with hepatic insufficiency [30]. These studies support apremilast as a preferred oral, non-biologic, systemic agent for the treatment of palmoplantar disease, especially in patients with concomitant liver disease.
Our study has several limitations. Numerous studies that included patients with palmoplantar disease were excluded from this review due to the lack of use of palmoplantar-specific scoring systems. Furthermore, patients with PP and PPP often are excluded from participating in clinical trials due to limited BSA involvement or pustular morphologies, contributing to the lack of RCTs investigating patients with palmoplantar disease—especially PPP. Next, variations in treatment duration to primary endpoint measurement create difficulties in generalizing the results of studies with larger patient populations. The use of different palmoplantar-specific metrics amongst studies also provides an obstacle in comparing results between separate investigations. Some of the included randomized studies omitted pertinent information in their methods, which potentially limit the interpretation of our results and introduce biases, namely performance and detection bias. Reporting bias also warrants consideration, especially in case series. Differences in reporting methodology (i.e., intention to treat vs. per-protocol analysis) and omission of prespecified outcomes, also make reporting bias worth considering for included clinical trials and cohort studies.
The limitations of our study highlight several changes which may be made to future trials to gain a better understanding of palmoplantar disease. First, additional investigations of apremilast in PPP are necessary to evaluate its efficacy, as only one placebo-controlled RCT evaluated apremilast in PPP. Next, future studies’ inclusion criteria must be accommodating of patients with palmoplantar involvement, especially those pustular variants. Further, RCTs should incorporate standardized reporting for palmoplantar-specific metrics in order to better compare efficacy across studies (e.g., PPPASI 50 and PPPGA 0/1).
Conclusions
Apremilast is an effective, oral, non-biologic, systemic agent in the treatment of palmoplantar disease including PP and PPP. Patients with PP and PPP treated with apremilast showed significant improvement in objective, palmoplantar-specific clinical parameters versus placebo, with meta-analysis demonstrating superiority of apremilast in producing disease clearance in PP compared to placebo. Apremilast demonstrates comparable efficacy to methotrexate and may be used in combination with methotrexate to produce further clinical improvements, and represents an alternative option for an oral, non-biologic, systemic agent for the treatment of palmoplantar disease, which may be preferred in patients with hepatic insufficiency. Fewer studies investigating treatment with apremilast in PPP exist, but their results support apremilast as a viable option for these patients. Future research should be inclusive of patients with palmoplantar disease, especially with pustular involvement. Standardized metrics for the evaluation of palmoplantar disease severity amongst studies are necessary to aid in the generalizability of results.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. All named authors contributed to the final paper as follows: RKS contributed to the concept and design, statistical analysis, and drafting of the manuscript. KGE, JQJ, and MSD contributed to the statistical analysis and drafting of the manuscript. MH, TB, and WL contributed to the concept and design, with WL also contributing to the resolution of any discrepancies in study eligibility criteria.
Disclosures
JQJ is supported in part by research grant funding from the National Psoriasis Foundation. TB is a principal investigator for trials sponsored by AbbVie, Castle, CorEvitas, Dermavant, Galderma, Mindera, and Pfizer. She has received research grant funding from Novartis and Regeneron. She has been an advisor for AbbVie, Arcutis, Boehringer– Ingelheim, Bristol Myers Squibb, Janssen, Leo, Lilly, Novartis, Pfizer, Sun, and UCB. WL has received research grant funding from AbbVie, Amgen, Janssen, Leo, Novartis, Pfizer, Regeneron, and TRex Bio. No conflicts of interest are applicable to this project. Riley Kyle Spencer has nothing to disclose. Kareem Giovanni Elhage has nothing to disclose. Mitchell Sparling Davis has nothing to disclose. Marwa Hakimi has nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
IRB Approval Status
Not applicable.
References
- 1.Timotijević ZS, Trajković G, Jankovic J, et al. How frequently does palmoplantar psoriasis affect the palms and/or soles? A systematic review and meta-analysis. Postepy Dermatol Alergol. 2019;36(5):595–603. doi: 10.5114/ada.2019.89508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung J, Callis Duffin K, Takeshita J, et al. Palmoplantar psoriasis is associated with greater impairment of health-related quality of life compared with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2014;71(4):623–632. doi: 10.1016/j.jaad.2014.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffiths CE, Christophers E, Barker JN, et al. A classification of psoriasis vulgaris according to phenotype. Br J Dermatol. 2007;156(2):258–262. doi: 10.1111/j.1365-2133.2006.07675.x. [DOI] [PubMed] [Google Scholar]
- 4.Raposo I, Torres T. Palmoplantar psoriasis and palmoplantar pustulosis: current treatment and future prospects. Am J Clin Dermatol. 2016;17(4):349–358. doi: 10.1007/s40257-016-0191-7. [DOI] [PubMed] [Google Scholar]
- 5.Khandpur S, Singhal V, Sharma VK. Palmoplantar involvement in psoriasis: a clinical study. Indian J Dermatol Venereol Leprol. 2011;77(5):625. doi: 10.4103/0378-6323.84071. [DOI] [PubMed] [Google Scholar]
- 6.Spuls PI, Hadi S, Rivera L, Lebwohl M. Retrospective analysis of the treatment of psoriasis of the palms and soles. J Dermatolog Treat. 2003;14(Suppl 2):21–25. doi: 10.1080/jdt.14.s2.21.25. [DOI] [PubMed] [Google Scholar]
- 7.Obeid G, Do G, Kirby L, Hughes C, Sbidian E, Le Cleach L. Interventions for chronic palmoplantar pustulosis. Cochrane Database Syst Rev. 2020;1(1):CD011628. doi: 10.1002/14651858.CD011628.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanchez IM, Sorenson E, Levin E, Liao W. The efficacy of biologic therapy for the management of palmoplantar psoriasis and palmoplantar pustulosis: a systematic review. Dermatol Ther (Heidelb) 2017;7(4):425–446. doi: 10.1007/s13555-017-0207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schafer P, Parton A, Capone L, et al. Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell Signal. 2014;26(9):2016–2029. doi: 10.1016/j.cellsig.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83(12):1583–1590. doi: 10.1016/j.bcp.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Bissonnette R, Pariser DM, Wasel NR, et al. Apremilast, an oral phosphodiesterase-4 inhibitor, in the treatment of palmoplantar psoriasis: results of a pooled analysis from phase II PSOR-005 and phase III Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis (ESTEEM) clinical trials in patients with moderate to severe psoriasis. J Am Acad Dermatol. 2016;75(1):99–105. doi: 10.1016/j.jaad.2016.02.1164. [DOI] [PubMed] [Google Scholar]
- 12.Bissonnette R, Haydey R, Rosoph LA, et al. Apremilast for the treatment of moderate-to-severe palmoplantar psoriasis: results from a double-blind, placebo-controlled, randomized study. J Eur Acad Dermatol Venereol. 2018;32(3):403–410. doi: 10.1111/jdv.14647. [DOI] [PubMed] [Google Scholar]
- 13.Deeks JJ, Higgins JPT, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins J, Thomas J, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.1. The Cochrane Collaboration; 2020.
- 14.Papp KA, Kaufmann R, Thaçi D, Hu C, Sutherland D, Rohane P. Efficacy and safety of apremilast in subjects with moderate to severe plaque psoriasis: results from a phase II, multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison study. J Eur Acad Dermatol Venereol. 2013;27(3):e376–e383. doi: 10.1111/j.1468-3083.2012.04716.x. [DOI] [PubMed] [Google Scholar]
- 15.Papp K, Reich K, Craig L, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1) J Am Acad Dermatol. 2015;73(1):37–49. doi: 10.1016/j.jaad.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 16.Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2) Br J Dermatol. 2015;173(6):1387–1399. doi: 10.1111/bjd.14164. [DOI] [PubMed] [Google Scholar]
- 17.“A Study to Evaluate the Efficacy and Safety of Apremilast (CC-10004) in Japanese Subjects with Palmoplantar Pustulosis.” ClinicalTrials.gov, Amgen, 6 Jan. 2022, https://clinicaltrials.gov/ct2/show/NCT04057937.
- 18.“Apremilast in Patients with Moderate to Severe Palmoplantar Pustulosis (PPP) (APLANTUS) - Study Results.” ClinicalTrials.gov, Kristian Reich, 24 Sept. 2021, https://clinicaltrials.gov/ct2/show/results/NCT04572997.
- 19.Kt S, Thakur V, Narang T, Dogra S, Handa S. Comparison of the efficacy and safety of apremilast and methotrexate in patients with palmoplantar psoriasis: a randomized controlled trial. Am J Clin Dermatol. 2021;22(3):415–423. doi: 10.1007/s40257-021-00596-6. [DOI] [PubMed] [Google Scholar]
- 20.Hassanandani T, Panda M, Jena AK, Raj C. Methotrexate monotherapy versus methotrexate and apremilast combination therapy in the treatment of palmoplantar psoriasis: a prospective, randomised, assessor-blinded, comparative study. Indian J Dermatol Venereol Leprol. 2022;9:1–8. doi: 10.25259/ijdvl_843_2021. [DOI] [PubMed] [Google Scholar]
- 21.Kato N, Takama H, Ando Y, et al. Immediate response to apremilast in patients with palmoplantar pustulosis: a retrospective pilot study. Int J Dermatol. 2021;60(5):570–578. doi: 10.1111/ijd.15382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ioannides D, Antonakopoulos N, Georgiou S, et al. A real-world, non-interventional, prospective study of the effectiveness and safety of apremilast in bio-naïve adults with moderate plaque psoriasis treated in the routine care in Greece—the ‘ APRAISAL. J Eur Acad Dermatol Venereol. 2022;36(11):2055–2063. doi: 10.1111/jdv.18509. [DOI] [PubMed] [Google Scholar]
- 23.Del Alcázar E, Suárez-Pérez JA, Armesto S, et al. Real-world effectiveness and safety of apremilast in psoriasis at 52 weeks: a retrospective, observational, multicentre study by the Spanish Psoriasis Group. J Eur Acad Dermatol Venereol. 2020;34(12):2821–2829. doi: 10.1111/jdv.16439. [DOI] [PubMed] [Google Scholar]
- 24.Pavia G, Gargiulo L, Cortese A, et al. Apremilast for the treatment of palmo-plantar non-pustular psoriasis: a real-life single-center retrospective study. Dermatol Ther. 2022;35(2):e15253. doi: 10.1111/dth.15253. [DOI] [PubMed] [Google Scholar]
- 25.Kromer C, Wilsmann-Theis D, Gerdes S, et al. Drug survival and reasons for drug discontinuation in palmoplantar pustulosis: a retrospective multicenter study. J Dtsch Dermatol Ges. 2019;17(5):503–516. doi: 10.1111/ddg.13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reich K, Korge B, Magnolo N, et al. Quality-of-life outcomes, effectiveness and tolerability of apremilast in patients with plaque psoriasis and routine German dermatology care: results from LAPIS-PSO. Dermatol Ther (Heidelb) 2022;12(1):203–221. doi: 10.1007/s13555-021-00658-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aceituno-Madera P, Salazar-Nievas M, Moreno-Suarez F. Apremilast, an oral phosphodiesterase 4 inhibitor, in the treatment of palmoplantar psoriasis. Our experience in real clinical practice. J Am Acad Dermatol. 2019 doi: 10.1016/j.jaad.2018.05.225. [DOI] [Google Scholar]
- 28.Kt S, Thakur V, Narang T, Dogra S, Handa S. Apremilast in treatment of palmoplantar pustulosis—a case series. Int J Dermatol. 2021;60(6):e247–e248. doi: 10.1111/ijd.15398. [DOI] [PubMed] [Google Scholar]
- 29.Ständer S, Syring F, Ludwig RJ, Thaçi D. Successful treatment of refractory palmoplantar pustular psoriasis with apremilast: a case series. Front Med (Lausanne) 2020;15(7):543944. doi: 10.3389/fmed.2020.543944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dattola A, Del Duca E, Saraceno R, Gramiccia T, Bianchi L. Safety evaluation of apremilast for the treatment of psoriasis. Expert Opin Drug Safe. 2017;16(3):381–385. doi: 10.1080/14740338.2017.1288714. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.