Abstract
Study objectives
Obstructive sleep apnea (OSA) severity has been suggested in aldosterone elevation in resistant hypertension, whereas it is undetermined in the rest population. We explored the association of OSA parameters with plasma aldosterone concentration (PAC) in participants with and without hypertension.
Methods
We enrolled clinically hypertensive patients with polysomnography and PAC data under no interfering agents, compared (log) PAC, and assessed the linearity of log PAC by tertiles (T1/2/3) of sleep parameters and their association using linear regression by gender and age. We enrolled participants with and without hypertension who had No-SAS scale and PAC data from the community and duplicated the observations from clinical setting considering age, gender, and presence of hypertension.
Results
Of the 2,066 clinical patients with hypertension (1,546 with OSA), men participants (n=1,412), log apnea–hypopnea index (p=0.043), apnea index (AI, p=0.010), and lowest oxygen saturation (LSaO2, p=0.013) showed significant linearity with log PAC. Log AI (B=0.04, 95%CI: 0.01,0.07, p=0.022) and log LSaO2 (B=−0.39, 95%CI: −0.78,−0.01, p=0.044) showed significant positive and negative linear associations with log PAC in regression. In community dwellers, 6,417 participants with untreated hypertension (2,642 with OSA) and 18,951 normotensive participants (3,000 with OSA) were included. Of the men participants with and without hypertension, the OSA group showed significantly higher (log) PAC than did their counterparts, and log No-SAS score showed positive association with log PAC (hypertension: B=0.072, 95%CI: 0.002,0.142, p=0.043; normotension: B=0.103, 95%CI: 0.067,0.139, p<0.001) in linear regression analysis, which were consistent in all age groups.
Conclusions
OSA parameters were positively associated with PAC in normotensive and hypertensive participants, indicating that OSA may increase circulating aldosterone, especially in men.
Keywords: plasma aldosterone concentration, lowest saturation of oxygen, apnea index, no-SAS scale, obstructive sleep apnea, normotension
Introduction
Obstructive sleep apnea (OSA) is a chronic sleep disorder characterized by repetitive episodes of upper airway collapse during sleep, resulting in intermittent hypoxia, hypercapnia, sleep arousal, sleep fragmentation, and daytime sleepiness (1, 2). OSA is independently associated with increased risk for hypertension and cardiovascular morbidity and mortality (3, 4). Approximately 46%–53% of patients with moderate to severe OSA have a diagnosis of hypertension (5). Therefore, OSA represents a tremendous threat to the global health.
OSA is considered as a multi-factorial disease, closely related to genetic, epigenetic environmental, and developmental factors including obesity (6, 7). However, even if the above factors are controlled, OSA still exists (8), suggesting the existence of residual factors affecting the occurrence or severity of OSA. Aldosterone, a steroid hormone produced by adrenal glands and playing important roles in maintaining body fluid and homeostasis, has obtained increasing attention on this aspect recently (9–11).
It has been suggested that aldosterone is involved in OSA and/or its severity through the following suggested mechanisms. Sodium water reabsorption followed by excess aldosterone elevates overnight fluid shifting to the neck in supine position, which results in pharyngeal edema and upper airway obstruction and further contributes to OSA pathogenesis or severity (12). The presence of aldosterone receptors on upper airway smooth muscle cells membranes also supports a direct local role played by aldosterone in increasing para-pharyngeal edema and favoring OSA or aggravating its severity (13). Aldosterone-excess-related potassium and glucose metabolism dysregulation leads to neuropathy development, which affect the central control of respiration and consequently upper airway neural reflexes, further favoring OSA (14). Furthermore, adipose tissue per se and adipocyte-derived factors, such as leptin and complement-C1q TNF-related protein-1, obesity, a well-known risk factor for OSA, induce aldosterone overproduction, independent of renin angiotensin aldosterone system (RAAS) and sympathetic nervous systems (15). Emerging evidence shows that mineralocorticoid receptor antagonists appear to reduce the disease severity in some OSA patients (16, 17). Therefore, it is reasonable to speculate that circulating aldosterone might be involved in OSA severity.
The relationship between OSA and aldosterone in patients with hypertension has been investigated in some studies, reporting a positive correlation between OSA severity and plasma or 24-h urinary aldosterone in resistant hypertension (18–21). However, most studies focused on clinical-resistant hypertension with small sample and failed to exclude effects of interfering agents on aldosterone measurements (9). These limitations might hinder the extrapolation of the results. In addition, the association of OSA severity and aldosterone is less studied in the general population with hypertension (22). Furthermore, studies that assess the association between OSA and plasma aldosterone concentration (PAC) in individuals without hypertension are lacking. In a meta-analysis, researchers performed subgroup analyses separately for participants with and without hypertension (n = 120), and results suggest that OSA is associated with higher PAC in the presence of hypertension (23).
Therefore, to test the hypothesis that there would be linear association between OSA severity parameters and PAC from non-OSA to OSA status in population with and without hypertension, we conducted a gender-stratified cross-sectional study in clinical patients with hypertension who had polysomnography (PSG) data and PAC measurements under no interfering agents and in community dwellers with and without hypertension who had data on No-SAS scale and PAC measured under no interfering agents by considering age, gender, and presence of hypertension. It would be beneficial to identify the association, as the prevalence of OSA remains high and contributes to an increasing risk of adverse outcomes and aldosterone antagonist treatment is effective (16, 17, 24).
Materials and methods
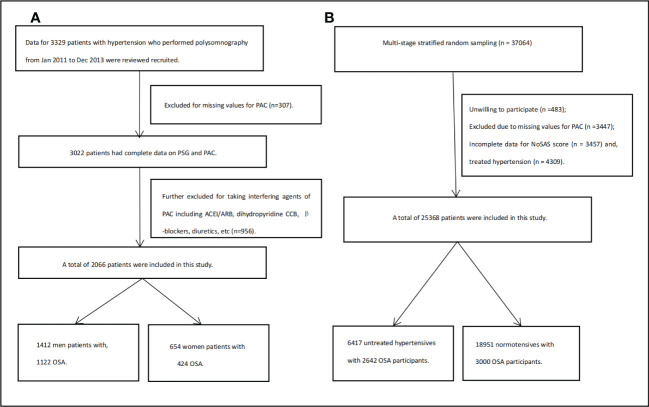
As in the flow chart ( Figure 1A ), in clinical data, participants were the patients in the Urumqi Research on Sleep Apnea and Hypertension (UROSAH) cohort, as described recently (25). Briefly, UROSAH is a single-center, retrospective, observational study to assess the association of OSA with long-term cardiovascular outcomes in patients with hypertension. Data for hypertensive patients aged ≥18 years who visited the Hypertension Center between January 2011 and December 2013 were reviewed. For the current study, we further excluded those with missing PSG and or PAC data and those who underwent PAC measurement when taking antihypertensive agents that would affect RAAS.
Figure 1.
Flow chart for clinical participants (A) and for general population (B).
As in flow chart ( Figure 1B ), in general population, we used a multi-stage stratified sampling method to enroll study participants aged ≥15 years from Emin county Xinjiang, as described in our previous studies (26–28). For the current study, we excluded participants with hypertension under any antihypertensive agent treatment at the time of survey.
The present study was approved by the Ethics Committee at People’s Hospital of Xinjiang Uygur Autonomous Region. All participants or their legal representatives signed written consent forms.
Data collection
In clinical data, all patients underwent in-laboratory overnight PSG (Compumedics E series, Australia) examination consistent with our previous studies (29). PSG evaluation included monitoring airflow with nasal pressure, respiratory effort with piezoelectric bands at abdominal and chest locations, oxygen saturation measurement with pulse oximetry, surface electrodes connected with standard techniques to obtain chin electromyography, and electrooculography. Data were scored by sleep technologists licensed by the American Academy of Sleep Medicine. Total sleep time, sleep efficiency, apnea hypopnea index (AHI), apnea index (AI), hypopnea index, mean and lowest oxygen saturation (LSaO2), time spent with oxygen saturation <90% (T90), maximum duration of hypoventilation, average duration of hypoventilation, oxygen desaturation index 3 and 4, and wake after sleep onset and arousal index were recorded or calculated. AHI is defined as the total number of hypopneas and apneas that occur per hour of sleep. OSA was defined if AHI was ≥5 events per hour, and mild, moderate, and severe OSA were defined as AHI=5–15, 15–30, and ≥30 events per hour, respectively.
In general population, No-SAS scale was completed in participants by trained investigators. No-SAS score is a recent developed screening tool (30, 31). It ranges from 0 to 17 and allocates 4 points for having a neck circumference of >40 cm, 3 points for having a body mass index (BMI) of 25–30 kg/m2, or 5 points for having a BMI of ≥30 kg/m² or higher, 2 points for snoring, 4 points for being >55 years, and 2 points for being male. OSA was defined if the No-SAS score was ≥8 points (30).
Measurements of PAC in clinical and general population
In clinical data, fasting blood samples were drawn at 9:00 a.m. after patients had been ambulant for at least 2 h and seated for 30 min. Patients were requested to stop taking antihypertensive agents for 4–6 weeks before hormone testing. For patients with BP ≥160/100 mmHg, we switched their antihypertensive agents to alpha-blockers and/or calcium channel blockers (verapamil) for 4–6 weeks before hormone testing. In the presence of severe or symptomatic hypertension, the workup was made under antihypertensive medications known as poorly affecting measurements of PAC and plasma renin activity (PRA). In addition, if patients suffered from hypokalemia, serum potassium (K) was corrected with oral K supplements, as close as possible to 3.9–4.0 mmol/L. All patients were recommended to maintain free diet as usual with no restriction on salt intake.
In the general population, fasting venous blood samples were collected, centrifugated onsite, and transported to Xinjiang Hypertension Institute (located in Urumqi, 500–600 km in distance) in portable refrigerators and were stored at −70°C until measurement in 2021.
PAC was measured using radioimmunoassay (DSL-8600 ACTIVE® Aldosterone Coated Tube Radioimmunoassay Kit; Diagnostic Systems Laboratories, Webster, TX, USA) with the intra- and inter-assay coefficients of variation of 5.6% and 8.5% in both data. In clinical data, PRA was also measured by radioimmunoassay (RIA) using commercial kits (Center of Beifang Biology Technique, Beijing, China), and the intra- and inter-assay coefficients of variation were less than 10% and 15%, respectively. The details of the measurements are described in previous studies from our center (32, 33).
Other variables
In clinical population, in addition to PSG parameters, PAC, PRA, and aldosterone to renin activity ratio (ARR), data on age, gender, BMI, abdominal circumference, cigarette consumption and alcohol intake (yes/no), diabetes mellitus (DM, yes/no), chronic kidney disease (CKD, yes/no), systolic and diastolic blood pressure (SBP, DBP), fasting plasma glucose (FPG), alanine aminotransferase (ALT), serum creatinine, total cholesterol (TC), triglyceride (TG), serum and 24-h urinary K and sodium, and menopausal status (yes/no) for women participants were included. Seated office BP was measured on the upper arm after patients rested quietly for 10 min at least with a mercury sphygmomanometer using international recommendations at hospitalization. The mean value of two measurements was recorded and used for analysis.
In the general population, data on age, gender, cigarette and alcohol consumption, hypertension, coronary heart disease and stroke, and relevant treatments were also collected. Height, weight, neck and abdominal circumference, and BP were measured. BP was measured three times with a Professional Portable BP Monitor (OMRON HBP-1300, Kyoto, Japan) on the right arm positioned at heart level after the participant was sitting at rest for 5 min, with 30 s between each measurement, and the average of three values was used. Fasting venous blood samples were tested for serum creatinine, FPG, TC, TG, ALT, and aspartate aminotransferase (AST). BMI was calculated as weight (kg)/height2 (m).
Definitions
In both population, hypertension was defined as SBP ≥140 mmHg or DBP ≥90 mmHg, or under antihypertensive therapy. Estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI equation, and CKD was defined if eGFR<60ml/min/1.73m2. DM was defined if fasting glucose ≥7.0 mmol/L, if using any glucose-lowering medication, or if there was self-reported history of diabetes.
In general population data, for men participants, age was categorized into <45, 45–60, and ≥60 years according to the criteria from the World Health Organization for developing countries (25, 27); for women participants, age was categorized into <51 and ≥51 years as a cutoff age for menopausal status based on previous studies (34). Educational attainment status was categorized into <9 and ≥9 years. Alcohol consumption is defined as consuming an alcoholic beverage at least once per week in the past month. Cigarette consumption is defined as smoking at least 20 packets of cigarettes in their lifetime and currently smoking cigarettes. Abdominal obesity is defined as having an abdominal circumference ≥90 cm for men and ≥85 cm for women. Cardiovascular disease is defined as self-reported medical history of coronary heart disease and stroke.
Statistical analyses
In clinical data, characteristics of participants were provided by non-, mild, moderate, and severe OSA groups. PAC and log PAC were compared by tertiles of PSG-based sleep parameters as T1, T2, and T3 groups by gender (men and women), and linearity of log PAC was explored among the tertile groups. In the general population, participants were divided into two groups by presence of OSA; PAC and log PAC was also compared between non-OSA and OSA groups in age-specific men (<45, 45–60, and ≥60 years) and women participants (<51 and ≥51 years) by untreated hypertension and normotension. Continuous variables with a normal distribution were presented as mean ± standard and compared between groups using analysis of univariable ANOVA test or Student’s t-test; otherwise, they were presented as median (interquartile range, IQR) and compared between groups using nonparametric test. Kolmogorov–Smirnov test was used to check the data normality. We provided both PAC and log PAC for among- or between-group comparison. Categorical variables were expressed as frequency and percentage, and chi-square test was used for among- or between-group comparison. Multivariate linear regression was used to evaluate the relationship of log-transformed PSG sleep parameters and log No-SAS score with log PAC by calculating B and 95% confidence intervals (95%CI) due to their skewed distribution. Variables to be adjusted were selected using univariate linear regression (p<0.10). Tolerance and variance inflation factor of multiple linear regression were used to evaluate collinearity among variables. Collinearity was considered when tolerance <0.1 or variance inflation factor >10. Data were analyzed using SPSS statistical software (version 25.0, SPSS Inc., Chicago, IL). All analyses were two-tailed, and p-value<0.05 was statistically significant.
Results
Clinical population
As given in the flow chart ( Figure 1A ), of the 3,329 participants in UROSAH, 2,066 participants with hypertension, who underwent PAC measurement under no interfering agents, comprising the current analytical sample aged 46 years and 68.3% (n=1,412) were men. There were 520 non-OSA, 627 mild, 465 moderate, and 454 severe OSA participants ( Table 1 ).
Table 1.
Characteristics of hypertensive participants by total and by severity of obstructive sleep apnea (OSA) with measured plasma aldosterone under no interfering agents in UROSAH cohort.
| Total | Non-OSA | Mild OSA | Moderate OSA | Severe OSA | p | |
|---|---|---|---|---|---|---|
| N | 2,066 | 520 (25.2) | 627 (30.3) | 465 (22.5) | 454 (22.0) | |
| Age (years) | 46.0 (40.0,52.0) | 43.0 (37.0,48.0) | 47.0 (41.0,52.0) | 48.0 (41.0,54.0) | 47.0 (40.0,53.0) | <0.001 |
| Men (n,%) | 1412.0 (68.3) | 290.0 (55.8) | 394.0 (62.8) | 346.0 (74.4) | 382.0 (84.1) | <0.001 |
| Women (n,%) | 654 (31.7) | 230 (44.2) | 233 (37.2) | 119 (25.6) | 72 (15.9) | <0.001 |
| Cigarette consumption (n,%) | 735.0 (35.6) | 133.0 (25.6) | 213.0 (34.0) | 181.0 (38.9) | 208.0 (45.8) | <0.001 |
| Alcohol intake (n,%) | 730.0 (35.3) | 146.0 (28.1) | 208.0 (33.2) | 171.0 (36.8) | 205.0 (45.2) | <0.001 |
| Diabetes (n,%) | 271.0 (13.1) | 40.0 (7.7) | 82.0 (13.1) | 67.0 (14.4) | 82.0 (18.1) | <0.001 |
| Chronic kidney disease (n,%) | 70.0 (3.4) | 13.0 (2.5) | 22.0 (3.5) | 23.0 (4.9) | 12.0 (2.6) | 0.139 |
| Body mass index (kg/m2) | 27.6 (25.4,30.1) | 26.2 (24.1,28.6) | 27.3 (25.4,29.8) | 28.1 (25.9,30.7) | 29.0 (26.7,31.5) | <0.001 |
| Abdominal circumference (cm) | 99.0 (92.0,105.0) | 94.0 (87.0,100.0) | 98.0 (92.0,104.0) | 101.0 (95.0,107.0) | 103.0 (97.0,110.0) | <0.001 |
| ALT (U/L) | 24.0 (17.0,35.0) | 23.0 (16.0,33.0) | 24.0 (17.0,35.0) | 22.0 (16.0,33.0) | 26.0 (18.0,36.0) | <0.001 |
| eGFR (ml/min/1.73 m2) | 100.4 (86.2,109.9) | 103.5 (87.6,113.0) | 99.3 (85.4,108.8) | 98.7 (87.6,107.9) | 101.2 (86.5,110.3) | 0.002 |
| Fasting plasma glucose (mmol/L) | 4.9 (4.5,5.3) | 4.8 (4.4,5.3) | 4.9 (4.5,5.3) | 4.9 (4.5,5.3) | 5.0 (4.5,5.5) | 0.018 |
| Serum potassium (mmol/L) | 3.9 (3.7,4.1) | 4.0 (3.7,4.1) | 3.9 (3.7,4.1) | 3.9 (3.7,4.1) | 3.9 (3.7,4.1) | 0.006 |
| Serum sodium (mmol/L) | 141.0 (139.0,143.0) | 141.0 (139.0,143.0) | 141.0 (139.0,143.0) | 141.0 (139.0,143.0) | 141.0 (139.0,143.0) | 0.417 |
| Plasma renin activity (ng/ml per h) | 1.4 (0.6,2.7) | 1.5 (0.7,2.8) | 1.3 (0.6,2.6) | 1.3 (0.6,2.7) | 1.5 (0.6,2.7) | 0.388 |
| Plasma aldosterone concentration (ng/dl) | 13.5 (9.2,19.6) | 13.1 (9.0,18.8) | 13.2 (9.0,19.7) | 13.8 (9.1,20.3) | 14.2 (9.7,19.5) | 0.206 |
| Aldosterone renin ratio | 9.8 (5.0,21.4) | 9.1 (4.6,19.1) | 9.9 (5.1,21.4) | 10.7 (52,23.4) | 9.5 (5.3,22.9) | 0.223 |
| Total cholesterol (mmol/L) | 4.5 (3.9,5.0) | 4.4 (3.8,5.0) | 4.5 (3.8,5.1) | 4.8 (3.9,5.0) | 4.5 (4.0,5.1) | 0.515 |
| Triglyceride (mmol/L) | 1.7 (1.2,2.4) | 1.6 (1.1,2.2) | 1.7 (1.2,2.4) | 1.8 (1.2,2.4) | 1.9 (1.3,2.6) | <0.001 |
| Systolic blood pressure (mmHg) | 140.0 (130.0,150.0) | 140.0 (126.0,150.0) | 140.0 (130.0,150.0) | 140.0 (130.0,150.0) | 140.0 (130.0,150.0) | 0.046 |
| Diastolic blood pressure (mmHg) | 90.0 (83.4,100.0) | 90.0 (82.0,100.0) | 90.0 (80.0,100.0) | 90.0 (82.0,100.0) | 94.0 (88.0,100.0) | 0.025 |
| Total sleep time (min) | 379.0 (334.0,419.0) | 379.8 (333.6,422.0) | 375.0 (331.0,413.0) | 371.5 (329.5,408.3) | 389.5 (341.4,429.5) | 0.001 |
| Sleep efficiency (%) | 72.0 (63.2,79.9) | 71.5 (63.0,80.0) | 71.6 (63.1,78.9) | 70.1 (61.8,78.8) | 74.8 (65.6,81.9) | <0.001 |
| 24 h urinary potassium (mmol/l) | 34.0 (23.7,44.2) | 32.9 (23.7,42.8) | 34.0 (24.4,44.1) | 35.5 (22.3,45.3) | 34.5 (23.7,45.4) | 0.362 |
| 24 h urinary sodium (mmol/l) | 164.8 (106.1,230.9) | 159.0 (106.2,220.6) | 166.1 (106.4,232.2) | 165.3 (103.8,239.2) | 171.2 (105.1,235.8) | 0.537 |
| Apnea hypopnea index (event/h) | 12.3 (4.8,27.0) | 1.6 (0.7,3.0) | 8.9 (6.6,11.6) | 20.4 (17.6,25.0) | 46.5 (6.0,60.8) | <0.001 |
| Apnea index (event/h) | 1.1 (0.1,7.1) | 0.0 (0.0,0.2) | 0.6 (0.1,2.0) | 3.6 (0.9,8.2) | 21.2 (8.5,36.4) | <0.001 |
| Hypopnea index (event/h) | 8.5 (3.4,17.0) | 1.4 (0.5,2.7) | 7.3 (5.5,9.9) | 16.2 (12.2,19.6) | 24.8 (14.0,34.4) | <0.001 |
| Lowest desaturation of oxygen (%) | 82.0 (77.0,87.0) | 88.0 (87.0,90.0) | 83.0 (80.0,86.0) | 80.0 (75.0,83.0) | 74.0 (67.0,78.0) | <0.001 |
| Mean desaturation of oxygenSaO2 (%) | 93.0 (91.0,94.0) | 94.0 (93.0,95.0) | 93.0 (92.0,94.0) | 92.0 (91.0,94.0) | 91.0 (90.0,93.0) | <0.001 |
| T90 (min) | 9.4 (1.0,50.3) | 0.4 (0.0,1.6) | 6.7 (2.0,21.1) | 22.0 (8.4,52.9) | 73.9 (30.9,129.2) | <0.001 |
| Oxygen desaturation index ≥3% (event/h) | 28.7 (15.4,49.2) | 11.9 (7.3,18.4) | 24.0 (18.2,30.9) | 38.9 (31.9,48.5) | 67.2 (53.4,84.5) | <0.001 |
| Oxygen desaturation index ≥4% (event/h) | 15.8 (6.7,32.1) | 4.6 (2.5,7.4) | 12.7 (9.0,16.8) | 25.0 (19.9,30.9) | 52.2 (40.0,70.4) | <0.001 |
| Maximum hypoventilation duration (min) | 33.9 (25.4,44.2) | 22.1 (17.6,27.9) | 31.6 (26.4,38.9) | 39.0 (32.6,47.3) | 45.8 (37.4,56.4) | <0.001 |
| Average hypoventilation duration (min) | 19.6 (17.5,22.1) | 17.4 (15.3,19.7) | 19.5 (17.9,21.8) | 20.5 (18.5,22.9) | 21.2 (18.6,24.1) | <0.001 |
| Wake after sleep onset (min) | 62.5 (33.5,101.5) | 61.0 (34.0,98.4) | 63.8 (33.9,104.1) | 67.0 (37.3,105.8) | 55.3 (,30.5,94.8) | 0.015 |
| Arousal index (min) | 14.0 (9.0,20.0) | 13.0 (9.0,18.0) | 13.5 (9.0,9.0,19.0) | 14.0 (9.0,20.0) | 14.0 (8.3,21.0) | 0.594 |
OSA, obstructive sleep apnea; UROSAH, Urumqi Research on Sleep Apnea and Hypertension; ALT, alanine aminotransferase; eGFR, estimated glomerular filtration rate; T90, time spent with oxygen saturation <90%.
As in Table 2 , in men participants, the T3 group of AI (the highest tertile T3 vs. the lowest T1: 14.5 vs. 13.0 ng/dl, p=0.027) and of LSaO2 (the lowest T3 vs. the highest T1: 14.1 vs. 12.9 ng/dl, p=0.016) showed significantly higher PAC, compared with the T1 group, and log PAC showed significant linearity among tertiles of AHI (p=0.043), AI (p=0.010), and LSaO2 (p=0.013), whereas these were not in women participants ( Supplementary Table S1 ). Sample size and range of PAC by tertiles of PSG parameters are provided in Supplementary Table S2 .
Table 2.
Comparison of plasma aldosterone concentration by tertile of polysomnography sleep parameters in men participants with hypertension under no interfering agents with Bonferroni correction for between group comparison.
| Parameters | T1 | T2 | T3 | p | p1 | p2 | p3 | p for trend |
|---|---|---|---|---|---|---|---|---|
| Log PAC (mean ± SD) | ||||||||
| AHI tertile | 1.11 ± 0.22 | 1.13 ± 0.23 | 1.14 ± 0.23 | 0.125 | 0.682 | 0.128 | 1.00 | 0043 |
| AI tertile | 1.11 ± 0.22 | 1.12 ± 0.23 | 1.15 ± 0.22 | 0.035 | 0.875 | 0.030 | 0.383 | 0.010 |
| HI tertile | 1.12 ± 0.22 | 1.13 ± 0.23 | 1.12 ± 0.23 | 0.425 | 0.581 | 1.00 | 1.00 | 0.601 |
| LSaO2 tertile | 1.10 ± 0.23 | 1.13 ± 0.23 | 1.14 ± 0.22 | 0.044 | 0.519 | 0.039 | 0.849 | 0.013 |
| MSaO2 tertile | 1.12 ± 0.22 | 1.13 ± 0.22 | 1.13 ± 0.23 | 0.670 | 1.000 | 1.000 | 1.000 | 0.384 |
| T90% tertile | 1.13 ± 0.22 | 1.12 ± 0.23 | 1.13 ± 0.23 | 0.509 | 1.000 | 1.000 | 1.000 | 0.831 |
| ODI3 tertile | 1.13 ± 0.22 | 1.12 ± 0.23 | 1.13 ± 0.23 | 0.658 | 0.754 | 0.622 | 0.970 | 0.819 |
| ODI4 tertile | 1.13 ± 0.22 | 1.11 ± 0.24 | 1.13 ± 0.22 | 0.401 | 0.947 | 0.642 | 0.481 | 0.825 |
| Max hypo duration tertile | 1.12 ± 0.23 | 1.12 ± 0.23 | 1.14 ± 0.22 | 0.162 | 1.000 | 0.361 | 0.251 | 0.119 |
| Aver hypo duration tertile | 1.12 ± 0.23 | 1.13 ± 0.23 | 1.13 ± 0.22 | 0.501 | 0.818 | 1.000 | 1.000 | 0.359 |
| WASO tertile | 1.12 ± 0.21 | 1.13 ± 0.22 | 1.12 ± 0.24 | 0.447 | 0.662 | 1.000 | 1.000 | 0.746 |
| Arousal index | 1.12 ± 0.22 | 1.13 ± 0.23 | 1.13 ± 0.22 | 0.630 | 1.000 | 1.000 | 1.000 | 0.640 |
| PAC (median, IQR) | ||||||||
| AHI tertile | 13.1 (9.0,18.9) | 13.3 (9.2,19.8) | 14.2 (9.6,19.6) | 0.435 | 0.512 | 0.555 | 0.198 | / |
| AI tertile | 13.0 (8.9,18.9) | 13.0 (8.8,19.1) | 14.5 (10.1,19.7) | 0.032 | 0.270 | 0.027 | 0.053 | / |
| HI tertile | 13.1 (9.1,18.5) | 13.9 (9.4,20.1) | 13.6 (9.1,19.6) | 0.250 | 0.114 | 0.972 | 0.126 | / |
| LSaO2 tertile | 12.9 (8.7,19.0) | 13.7 (9.5,19.7) | 14.1 (10.1,19.6) | 0.046 | 0.806 | 0.016 | 0.068 | / |
| MSaO2 tertile | 13.2 (9.3,18.8) | 13.4 (9.1,19.6) | 14.3 (9.1,20.0) | 0.550 | 0.851 | 0.465 | 0.252 | / |
| T90% tertile | 13.3 (9.1,19.1) | 13.2 (9.2,19.7) | 13.9 (9.1,19.5) | 0.731 | 0.628 | 0.548 | 0.628 | / |
| ODI ≥3% tertile | 13.3 (9.1,19.8) | 13.3 (9.1,19.0) | 13.8 (9.3,19.6) | 0.764 | 0.832 | 0.795 | 0.952 | / |
| ODI ≥4% tertile | 13.5 (9.1,19.7) | 13.1 (9.0,19.2) | 13.9 (9.5,19.5) | 0.548 | 0.786 | 0.445 | 0.589 | / |
| Max hypo duration tertile | 13.5 (8.9,19.6) | 13.3 (9.0,19.7) | 13.7 (9.9,19.5) | 0.198 | 0.856 | 0.140 | 0.098 | / |
| Aver hypo duration tertile | 13.5 (8.7,19.5) | 13.4 (9.1,19.6) | 13.6 (9.9,19.5) | 0.054 | 0.315 | 0.246 | 0.011 | / |
| WASO | 13.4 (9.2,19.0) | 13.9 (9.5,20.1) | 13.2 (8.9,19.2) | 0.726 | 0.662 | 0.428 | 0.758 | / |
| Arousal index | 13.5 (9.2,19.0) | 13.6 (9.5,19.8) | 13.3 (8.9,19.5) | 0.528 | 0.625 | 0.465 | 0.338 | / |
PAC, plasma aldosterone concentration; AHI, apnea hypopnea index; AI, apnea index; HI, hypopnea index; LSaO2, lowest saturation of oxygen; MSaO2, mean saturation of oxygen; T90, time spent with oxygen saturation <90%; ODI, Oxygen Desaturation Index; hypo, hypoventilation; WASO, wake after sleep onset p, for among group comparison; p1, for T1 vs. T2 comparison; P2, for T1 vs. T3 comparison; P3, for T2 vs. T3 comparison.
As in Table 3 , in the linear regression analysis for men participants, log AI showed significant positive association with log PAC in the crude model (B=0.02, 95%CI: 0.01,0.04, p=0.049), and the association became stronger in the model (B=0.04, 95%CI: 0.01,0.07, p=0.022) adjusted for log age, BMI, SBP, DBP, ALT, PRA, and serum and 24-h urinary K, which were selected using univariate linear regression and tested for collinearity ( Supplementary Table S3 ). In addition, log LSaO2 showed negative association with log PAC marginally in crude model (B=−0.17, 95%CI: −0.35,0.01, p=0.067) and significantly in the adjusted model for the above variables (B=−0.39, 95%CI: −0.78,−0.01, p=0.044). Moreover, log T90 showed marginally positive association with log PAC in adjusted model (B=0.02, 95%CI: −0.01,0.05, p=0.083).
Table 3.
Liner regression analysis for the relationship of log-transformed PSG-based sleep parameters with log-transformed plasma aldosterone concentration in men participants with hypertension without interfering agents (B, 95%CI, p).
| Log plasma aldosterone concentration | Crude model | Adjusted model |
|---|---|---|
| Log apnea hypopnea index | 0.01 (−0.01,0.04), 0.146 | 0.01 (−0.03,0.05), 0.738 |
| Log apnea index | 0.02 (0.01,0.04), 0.049 | 0.04 (0.01,0.07), 0.022 |
| Log hypopnea index | 0.01 (−0.02,0.03), 0.757 | −0.04 (−0.08,0.01), 0.129 |
| Log lowest desaturation of oxygen | −0.17 (−0.35,0.01), 0.067 | −0.39 (−0.78,−0.01), 0.044 |
| Log mean oxygen saturation | −0.12 (−0.60,0.36), 0.638 | 0.12 (−0.48,0.86), 0.582 |
| Log time spent with oxygen saturation <90% | 0.01 (−0.01,0.02), 0.540 | 0.02 (−0.01,0.05), 0.083 |
| Log oxygen desaturation index ≥3% | 0.00 (−0.03,0.03), 0.994 | −0.01 (−0.05,0.02), 0.454 |
| Log oxygen desaturation index ≥4% | 0.01 (−0.02,0.03), 0.739 | −0.01 (−0.03,0.02), 0.642 |
| Log maximum duration of hypoventilation | 0.03 (−0.02,0.09), 0.245 | 0.04 (−0.07,0.15), 0.509 |
| Log average duration of hypoventilation | 0.03 (−0.05,0.12), 0.472 | 0.04 (−0.13,0.21), 0.653 |
| Log wake after sleep onset | −0.01 (−0.04,0.02), 0.642 | 0.02 (−0.03,0.08), 0.410 |
| Log arousal time | 0.01 (−0.03,0.056), 0.707 | 0.02 (−0.05,0.10), 0.575 |
Adjusted for log-transformed age, body mass index, systolic and diastolic blood pressure, alanine aminotransferase, plasma renin activity, serum potassium, and 24-h urinary potassium
Community-based population
As given in the flow chart ( Figure 1B ), 6,417 participants with untreated hypertension (2,642 with OSA) and 18,951 participants without hypertension (3,000 with OSA) with complete No-SAS scale and PAC data were included ( Table 4 ).
Table 4.
Characteristics of non-OSA and OSA participants by presence of hypertension in community dwellers.
| Untreated hypertension | Normotension | |||||
|---|---|---|---|---|---|---|
| Non-OSA | OSA | p | Non-OSA | OSA | p | |
| N | 3,775 | 2,642 | 15,951 | 3,000 | ||
| Gender, men, n,% | 1,771 (46.9) | 2,059 (77.9) | <0.001 | 5,379 (33.7) | 2,449 (81.6) | <0.001 |
| Women,n,% | 2,004 (53.1) | 583 (22.1) | 10,572 (66.3) | 551 (18.4) | ||
| Age (years) | 49.0 (25.0,75.0) | 58.0 (47.0,66.0) | <0.001 | 41.0 (32.0,49.0) | 56.0 (40.0,56.0) | <0.001 |
| <45 years (n,%) | 1,177 (31.2) | 538 (20.4) | <0.001 | 9,469 (59.4) | 953 (31.8) | <0.001 |
| 45–60 years (n,%) | 1,910 (50.6) | 935 (35.4) | 5,576 (35.0) | 1,068 (35.6) | ||
| ≥60 years (n,%) | 688 (18.2) | 1,169 (44.2) | 906 (5.7) | 979 (32.6) | ||
| Education <9 years (n,%) | 2,926 (78.2) | 1,906 (74.8) | 0.003 | 10,400 (66.2) | 1,965 (67.3) | 0.039 |
| ≥9 years | 814 (21.8) | 640 (25.1) | 5,320 (33.8) | 954 (32.7) | ||
| Cigarette use (yes, n,%) | 999 (26.6) | 925 (36.4) | <0.001 | 3,263 (20.5) | 1,361 (46.8) | <0.001 |
| Alcohol intake (yes, n,%) | 1,267 (33.8) | 1,269 (50.1) | <0.001 | 4,002 (25.2) | 1,517 (52.2) | <0.001 |
| CVD (n,%) | 90 (2.4) | 107 (4.0) | <0.001 | 194 (1.2) | 97 (3.2) | <0.001 |
| Neck circumference (cm) | 35.4 (33.4,37.5) | 40.1 (37.4,42.0) | <0.001 | 34.2 (32.2,36.5) | 40.0 (37.6,42.1) | <0.001 |
| Body mass index (kg/m2) | 24.9 (23.0,27.5) | 29.1 (26.7,31.8) | <0.001 | 23.8 (21.6,26.2) | 28.2 (26.1,30.9) | <0.001 |
| AC (cm) | 88.3 (82.0,94.6) | 100.0 (93.6,106.5) | <0.001 | 83.4 (76.8,90.4) | 97.8 (92.0,104.0) | <0.001 |
| Abdominal obesity (n,%) | 2,050 (54.4) | 2,266 (87.7) | <0.001 | 5,869 (37.1) | 2,485 (83.8) | <0.001 |
| Systolic BP (mmHg) | 143.0 (134.0,152.0) | 146.0 (138.0,158.0) | <0.001 | 115.0 (107.0,123.0) | 122.0 (115.0,130.0) | <0.001 |
| Diastolic BP (mmHg) | 91.0 (85.0,96.0) | 92.0 (86.0,98.0) | <0.001 | 74.0 (69.0,80.0) | 78.0 (70.0,82.0) | <0.001 |
| FPG (mmol/L) | 5.31 (4.84,5.89) | 5.53 (5.00,6.30) | <0.001 | 5.16 (4.71,5.64) | 5.41 (4.90,6.06) | <0.001 |
| ALT (U/L) | 22.0 (16.0,29.5) | 23.0 (17.0,31.0) | <0.001 | 19.0 (15.0,26.0) | 22.0 (17.0,30.0) | <0.001 |
| AST (U/L) | 22.0 (19.0,27.0) | 22.0 (18.0,28.0) | <0.001 | 21.0 (17.0,25.0) | 22.0 (18.0,26.0) | 0.896 |
| TC (mmol/L) | 4.90 (4.20,5.70) | 5.00 (4.30,5.70) | <0.001 | 4.50 (3.88,5.30) | 4.80 (4.10,5.60) | <0.001 |
| TG (mmol/L) | 1.24 (0.90,1.80) | 1.48 (1.00,2.17) | <0.001 | 1.10 (0.80,1.60) | 1.40 (1.00,2.00) | <0.001 |
| Serum Cr (mmol/l) | 69.0 (58.7,79.9) | 72.7 (61.2,84.9) | <0.001 | 67.6 (56.8,79.0) | 72.0 (61.9,84.0) | <0.001 |
| No-SAS score | 4.0 (2.0,6.0) | 11.0 (9.0,11.0) | <0.001 | 2.0 (0.0,5.0) | 9.0 (9.0,11.0) | <0.001 |
OSA, obstructive sleep apnea; CVD, cardiovascular disease; AC, abdominal circumference; BP, blood pressure; FPG, fasting plasma glucose; TC, total cholesterol; TG, triglyceride; Cr, creatinine; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
As in Table 5 , in the OSA group, young and middle-aged men participants showed significantly higher PAC and log PAC in untreated hypertension and in nornotension. However, women participants (1,603 post-menopausal status) with OSA and untreated hypertension showed significantly lower PAC and log PAC, compared with their counterparts.
Table 5.
Comparison of PAC and log PAC between total, men, and women participants with and without OSA by presence of hypertension and by age in community-based data.
| Untreated hypertension | Normotension | |||||
|---|---|---|---|---|---|---|
| Non-OSA | OSA | P | Non-OSA | OSA | P | |
| N | 3,775 | 2,642 | 15,951 | 3,000 | ||
| PAC (median, interquartile range) | ||||||
| Men | 12.2 (8.7,17.5) | 13.4 (9.3,19.2) | <0.001 | 13.0 (9.2,18.4) | 14.4 (10.1,20.4) | <0.001 |
| <45 years | 12.8 (9.1,18.4) | 14.0 (9.5,20.0) | 0.025 | 12.9 (9.1,18.4) | 15.6 (10.6,21.0) | <0.001 |
| 45–60 years | 12.2 (8.6,17.4) | 14.0 (9.6,20.0) | <0.001 | 13.2 (9.3,18.5) | 14.9 (10.3,21.1) | <0.001 |
| ≥60 years | 10.3 (7.9,15.8) | 12.5 (8.9,17.3) | <0.001 | 12.7 (9.1,17.5) | 13.0 (9.4,18.2) | 0.403 |
| Women | 15.0 (10.8,20.9) | 14.2 (10.5,19.7) | 0.010 | 16.0 (11.2,23.1) | 14.0 (10.6,18.9) | <0.001 |
| <51 years | 17.3 (12.2,22.8) | 16.3 (11.4,23.6) | 0.007 | 16.6 (11.5,24.1) | 17.8 (11.7,23.4) | 0.841 |
| ≥51 years | 14.6 (10.7,20.3) | 14.10 (10.5,19.3) | <0.001 | 14.3 (10.7,19.9) | 14.0 (10.4,18.5) | 0.061 |
| Log plasma aldosterone concentration (mean ± standard deviation) | ||||||
| Men | 2.09 ± 0.20 | 2.13 ± 0.20 | <0.001 | 2.12 ± 0.20 | 2.16 ± 0.20 | <0.001 |
| <45 years | 2.11 ± 0.20 | 2.14 ± 0.20 | 0.043 | 2.12 ± 0.20 | 2.18 ± 0.20 | <0.001 |
| 45-60 years | 2.09 ± 0.20 | 2.15 ± 0.20 | <0.001 | 2.12 ± 0.20 | 2.17 ± 0.20 | <0.001 |
| ≥60 years | 2.05 ± 0.20 | 2.10 ± 0.19 | 0.001 | 2.11 ± 0.20 | 2.13 ± 0.20 | 0.362 |
| Women | 2.18 ± 0.20 | 2.17 ± 0.20 | 0.015 | 2.22 ± 0.23 | 2.16 ± 0.19 | <0.001 |
| <51 years | 2.23 ± 0.23 | 2.20 ± 0.20 | 0.001 | 2.23 ± 0.23 | 2.23 ± 0.18 | 0.915 |
| ≥51 years | 2.12 ± 0.18 | 2.17 ± 0.19 | <0.001 | 2.16 ± 0.19 | 2.15 ± 0.19 | 0.043 |
PAC, plasma aldosterone concentration.
In the linear regression analysis shown in Table 6 , log No-SAS score showed significant positive association with log PAC in men with hypertension (B=0.068, 95%CI: 0.044,0.092, p<0.001) and without hypertension (B=0.060, 95%CI: 0.045,0.076, p<0.001) in the crude model and in the adjusted model (hypertension: B=0.072, 95%CI: 0.002,0.142, p=0.043; normotension: B=0.103, 95%CI: 0.067,0.139, p<0.001) for variables age, education attainment status, cigarette consumption, alcohol intake, cardiovascular disease, SBP, DBP, BMI, abdominal circumference, FPG, serum creatinine, TC, TG, ALT, and AST selected using univariate regression analysis ( Supplementary Table S4 ).
Table 6.
Liner regression for the relationship of log No-SAS score with and log plasma aldosterone concentration in total, men, and women participants by presence of hypertension and by age in community-based participants (B, 95%CI, p).
| Crude model | Adjusted model | |
|---|---|---|
| Untreated hypertension | ||
| Men | 0.068 (0.044,0.092), <0.001 | 0.072 (0.002,0.142), 0.043 |
| <45 years | 0.058 (0.020,0.095), 0.003 | 0.093 (0.023,0.162), 0.009 |
| 45–60 years | 0.105 (0.069,0.141), <0.001 | 0.155 (0.096,0.214), <0.001 |
| ≥60 years | 0.212 (0.129,0.294), <0.001 | 0.295 (0.162,0.429), <0.001 |
| Women | −0.035 (−0.063,−0.006), 0.017 | 0.030 (−0.029,0.090), 0.316 |
| <51 years | 0.039 (−0.026,0.104), 0.242 | 0.145 (0.015,0.275), 0.028 |
| ≥51 years | −0.052 (−0.101,−0.003), 0.038 | 0.011 (−0.062,0.083), 0.773 |
| Normotension | ||
| Men | 0.060 (0.045,0.076), <0.001 | 0.103 (0.067,0.139), <0.001 |
| <45 years | 0.066 (0.044,0.087), <0.001 | 0.100 (0.063,0.137), <0.001 |
| 45–60 years | 0.086 (0.059,0.112), <0.001 | 0.087 (0.045,0.129), <0.001 |
| ≥60 years | 0.111 (0.010,0.211), 0.031 | 0.267 (0.117,0.416), 0.020 |
| Women | −0.106 (−0.134,−0.078), <0.001 | 0.009 (−0.029,0.047), 0.654 |
| <51 years | −0.071 (−0.116,−0.027), 0.002 | 0.048 (−0.020,0.116), 0.165 |
| ≥51 years | −0.053 (−0.090,−0.016), 0.005 | 0.033 (−0.054,0.054), 0.998 |
Adjusted for log-transformed age, systolic and diastolic blood pressure, body mass index, abdominal circumference, fasting blood glucose, serum creatinine, total cholesterol, triglyceride, alanine aminotransferase, aspartate aminotransferase, and education, cigarette consumption, alcohol intake, and cardiovascular disease
This association between log No-SAS score and log PAC in men participants with and without hypertension also stayed consistent in young, middle-aged, and old age groups.
In women participants, log No-SAS score showed significant association with log PAC only in the aged <51 years with hypertension after adjustment for above variables (B=0.145, 95%CI: 0.015,0.275, p=0.028).
Discussion
Elevated circulating aldosterone has consistently been shown to be a risk factor for vascular disease (10, 33, 35–37) and renal dysfunction (32) in various population. It is undetermined whether circulating aldosterone is elevated in OSA, a well-known risk factor for CVD (3, 4). Therefore, we assessed the association of objective and subjective OSA parameters with PAC in hypertensive and normotensive general population in this cross-sectional study.
We observed the following. First, men with hypertension and with the highest and the lowest tertile of AI and LSaO2 contain higher PAC, and log PAC showed significant linearity with AHI, AI, and LSaO2. Second, log AI and log LSaO2 showed significant positive and negative association with log PAC in crude and adjusted linear regression analysis; log T90 showed marginally positive association with log PAC in adjusted model. Third, in community dwellers, the OSA group in young and middle-aged men participants showed significantly higher PAC and log PAC in stratification by untreated hypertension and by nornotension. Fourth, in linear regression analysis, log No-SAS score showed significant positive association with log PAC in men participants with and without hypertension, which is consistent in young, middle-aged, and old age groups. Fifth, in women participants from the community, log No-SAS score showed significant association with log PAC only in those aged <51 years with hypertension after adjustment for confounding variables.
Current observations may add some evidence on uncertainty on the association of OSA with circulating aldosterone and extend previous observations in resistant hypertension OSA to general hypertensive and normotensive population, in which PAC was measured under no interfering antihypertensive agents. That is, consistent with previous studies mainly conducted in resistant hypertension (18–20), PSG-based objective (AI, LSaO2) and questionnaire-based subjective (No-SAS score) OSA parameters showed significant linear association with PAC in participants with and without hypertension from the clinical setting and from the community.
In clinical data, one of the differences in the present study is that PAC was measured under no interfering agents in patients included in the study, which previous studies failed to consider (18–20). Based on current evidence, most antihypertensive agents affect PAC measurement (29) or even increase the PAC (38). The other difference is that we performed this study in general hypertension clinical and community setting, since those from the clinical setting were given antihypertensive agent, which would not affect RAAS at the time of PAC measurement, indicating that most of them were not resistant hypertension. In addition, all the participants with hypertension included from the community were untreated at the time of blood sample collection. Total and all age group men with hypertension and OSA showed significantly higher PAC and log PAC than did those without OSA. In addition, a positive independent linear association between log No-SAS score and log PAC was observed, consistent in age-stratified analysis. Previous relevant studies were conducted mainly in resistant hypertension, in which PA accounts for as high as 36% (25) and as high as 22.0% of general hypertension (26, 27). These results are consistent with those of our clinical data and with those of previous studies (18–20, 24). Therefore, these factors may make the current results more convincing.
In normotensive population from the community, more importantly, total and all age group men with OSA showed significantly higher PAC than did those without OSA, and regression analysis showed a positive independent linear association of log No-SAS score with log PAC in total men participants. The results are consistent with those of a study conducted in resistant hypertensive patients, which showed that those at high risk for OSA by Berlin Questionnaire are characterized by increased 24-h urinary aldosterone excretion (21). This observation might be of significance when considering the harmful effects of both aldosterone and OSA. Previous studies have reported that higher circulating aldosterone leads to elevation in BP and to the development of hypertension (39); current results may implicate that elevated aldosterone in normotensive OSA population might exert some roles in BP elevation and hypertension development in this specific population group. In addition, OSA population without hypertension also showed increased risk for CVD, and thus, it might be reasonable to speculate that elevated circulating aldosterone in this specific population may also play some role in this process, based on previous studies that elevation in circulating aldosterone is a risk factor for CVD (10, 35–37). However, longitudinal studies are needed, especially in those without hypertension or other CV risk factors at baseline, since most relevant studies are performed in hypertensive or high-risk population (10, 35–37). Several studies reported that mineralocorticoid antagonists could improve BP control and OSA severity (16, 28–30). Therefore, aldosterone blockade may be a useful strategy as a supplementary treatment for hypertensive patients with OSA, but further research is still needed to prove it.
We considered the effects of gender on the results. In women participants in total, pre- and post-menopausal status from clinical data, we failed to generate an association between sleep parameters and PAC. However, in our clinical sample, men participants account for 66.1%, possibly making the women sample under-powered to observe a thin kind subtle association. In population-based data, a positive linear association was observed between log No-SAS score with log PAC in the participants aged <51 years with hypertension after adjustment for confounders. We selected 51 years as the cutoff for age stratification analysis in women participants, since we considered that this age might be a marker for pre- and post-menopausal status, based on previous studies (34). Therefore, this association may indicate that female patients with hypertension and OSA may be able to affect aldosterone secretion. In addition, previous studies show that aldosterone secretion in older population is prone to be independent of regulators such as adrenocorticotropic hormone, renin, and K due to aldosterone-producing cluster cells in the adrenal gland (40). Therefore, in older women participants with hypertension and OSA, the effects of aldosterone-producing cell clusters on PAC may outweigh that of OSA. However, further studies are needed.
In the current study, we observed an independent association between LSaO2 (significantly) and T90 (marginally after adjustment for confounding variables) with circulating aldosterone, consistent with previous studies. Previous human, animal, and in vitro studies show that chronic intermittent hypoxia, a pathophysiological manifestation in OSA, induces sympathetic outflow to the kidney, stimulates renin release, and leads to elevated circulating levels of angiotensin II (Ang II) (41, 42), both of which are important regulators for aldosterone secretion from the adrenal gland. In addition, it has been shown that Ang II is higher in OSA patients than in healthy controls (23). In addition, we observed that AI is in significant positive linear association with PAC, partially consistent with previous studies (18–20). Indeed, AI is a part of AHI, a mechanistic complete obstruction of upper airway during sleep in OSA, followed by hypoxic events and lowering of circulating oxygen saturation, which might be the trigger for increase in circulating aldosterone. In addition, there might be a bidirectional relationship between OSA and circulating aldosterone. OSA stimulates aldosterone secretion, which in turn leads to excess fluid retention and displacement of fluid from the lower extremities to the neck during sleep, resulting in upper airway narrowing and or obstruction (43). In another study conducted in patients with diastolic heart failure, hypertension, and OSA, diuretic treatment improves upper airway caliber and OSA severity, further implicating pharyngeal edema as a cause of upper airway collapse during sleep (44). Furthermore, aldosterone, related to sarcopenia (45), might affect the mass and function of upper dilator muscles, and lower skeletal muscle mass was found to be higher in patients with primary aldosteronism (46). Therefore, elevated aldosterone may also increase the apnea events through above mechanisms. Luckily, in a recent study of mice model, the non-steroidal mineralocorticoid receptor antagonist (finerenone) brought significant improvements in clinically relevant functional parameters in skeletal muscle (47).
The following aspects may merit the current study. First, we enrolled patients with hypertension with a larger sample from clinical and community settings, in which PAC was measured under no interfering agents, and we considered the effects of gender and age, which may reflect objectiveness of current results. Second, we replicated the clinical observation in large community-based population and extended previous observations to normotensive population. Third, we considered important regulators of PAC including PRA and 24-h UK, reflecting K intake, and confounders like 24-h urinary sodium. However, the current study contains some limitations, which should be considered when interpreting the observations. First, the cross-sectional nature of the study would not enable us to draw casual association between parameters of OSA and PAC, whereas current observations are consistent with previous ones in resistant hypertension. Second, the number of women participants in clinical data was relatively smaller, which may explain, at least in part, the null association between PSG parameters and PAC. Third, in community-based data, OSA was diagnosed by questionnaires, which may have brought some bias to the results when considering the subjectivity of questionnaire-based diagnosis of a disease. Although these questionnaires can diagnose sleep disordered breathing, an umbrella term with a prevalence of 24.0%–83.8% (48–51), OSA is the most frequent type (52). In addition, No-SAS score has shown high sensitivity and specificity for OSA screening, compared with polysomnography, and has been validated in a Chinese population (31, 53). Moreover, it is not feasible to perform PSG in population-based studies due to its cost and technique dependence.
In conclusion, objective and subjective parameters of OSA showed significant independent linear association with PAC in clinical and general hypertensive and normotensive population, indicating that OSA is involved in aldosterone over-secretion and that aldosterone blockade might be a complementary therapy in patients with OSA and hypertension.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: The data that support the findings of this study are available upon request from the corresponding author. Requests to access these datasets should be directed to lnanfang2016@sina.com; morale118@126.com/1017663289@qq.com.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee at People’s Hospital of Xinjiang Uygur Autonomous Region. The patients/participants provided their written informed consent to participate in this study.
Author contributions
NL and MH conceived the idea, designed the study, performed data collection and analysis and performed supervision of the study, participated in the paper writing, and gave crucial suggestions. HW and MH performed data collection and analysis and wrote the first draft. LG, MYL, WY, ML, LY, MML, AM, SL, ZW, ZX, LT, YL, QL and JH performed data collection and analysis and participated in sample measurement and in paper writing. All authors read and approved the final article. All authors contributed to the article and approved the submitted version.
Funding Statement
This study was funded by Key R&D projects in Xinjiang Uygur Autonomous Region (2022B03009-1).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.1016804/full#supplementary-material
References
- 1. West SD, Turnbull C. Obstructive sleep apnoea. Eye (Lond) (2018) 32(5):889–903. doi: 10.1038/s41433-017-0006-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, et al. Sleep apnea and cardiovascular disease: An American heart association/american college of cardiology foundation scientific statement from the American heart association council for high blood pressure research professional education committee, council on clinical cardiology, stroke council, and council on cardiovascular nursing. Circulation (2008) 118(10):1080–111. doi: 10.1161/CIRCULATIONAHA.107.189375 [DOI] [PubMed] [Google Scholar]
- 3. McNicholas WT, Bonsigore MR. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J (2007) 29(1):156–78. doi: 10.1183/09031936.00027406 [DOI] [PubMed] [Google Scholar]
- 4. Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American college of Cardiology/American heart association task force on clinical practice guidelines. Hypertension (2018) 71(6):e13–e115. doi: 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 5. Lavie P, Herer P, Hoffstein V. Obstructive sleep apnea syndrome as a risk factor for hypertension: population study. BMJ. (2000) 320(7233):479–82. doi: 10.1136/bmj.320.7233.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Redline S, Tishler PV. The genetics of sleep apnea. Sleep Med Rev (2000) 4(6):583–602. doi: 10.1053/smrv.2000.0120 [DOI] [PubMed] [Google Scholar]
- 7. Panahi L, Udeani G, Ho S, Knox B, Maille J. Review of the management of obstructive sleep apnea and pharmacological symptom management. Medicina (Kaunas) (2021) 57(11):1173. doi: 10.3390/medicina57111173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sim JJ, Yan EH, Liu IL, Rasgon SA, Kalantar-Zadeh K, Calhoun DA, et al. Positive relationship of sleep apnea to hyperaldosteronism in an ethnically diverse population. J Hypertens (2011) 29(8):1553–9. doi: 10.1097/HJH.0b013e3283492219 [DOI] [PubMed] [Google Scholar]
- 9. Wang Y, Li CX, Lin YN, Zhang LY, Li SQ, Zhang L, et al. The role of aldosterone in OSA and OSA-related hypertension. Front Endocrinol (Lausanne) (2022) 12:801689. doi: 10.3389/fendo.2021.801689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Inoue K, Goldwater D, Allison M, Seeman T, Kestenbaum BR, Watson KE. Serum aldosterone concentration, blood pressure, and coronary artery calcium : The multi-ethnic study of atherosclerosis. Hypertension. (2020) 76(1):113–20. doi: 10.1161/HYPERTENSIONAHA.120.15006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Struthers AD, MacDonald TM. Review of aldosterone- and angiotensin II-induced target organ damage and prevention. Cardiovasc Res (2004) 61(4):663–70. doi: 10.1016/j.cardiores.2003.11.037 [DOI] [PubMed] [Google Scholar]
- 12. Friedman O, Bradley TD, Chan CT, Parkes R, Logan AG. Relationship between overnight rostral fluid shift and obstructive sleep apnea in drug-resistant hypertension. Hypertension. (2010) 56(6):1077–82. doi: 10.1161/HYPERTENSIONAHA.110.154427 [DOI] [PubMed] [Google Scholar]
- 13. Di Murro A, Petramala L, Cotesta D, Zinnamosca L, Crescenzi E, Marinelli C, et al. Renin-angiotensin-aldosterone system in patients with sleep apnoea: prevalence of primary aldosteronism. J Renin Angiotensin Aldosterone Syst (2010) 11(3):165–72. doi: 10.1177/1470320310366581 [DOI] [PubMed] [Google Scholar]
- 14. Ehrhart-Bornstein M, Lamounier-Zepter V, Schraven A, Langenbach J, Willenberg HS, Barthel A, et al. Human adipocytes secrete mineralocorticoid-releasing factors. Proc Natl Acad Sci U S A (2003) 100(24):14211–6. doi: 10.1073/pnas.2336140100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huby AC, Antonova G, Groenendyk J, Gomez-Sanchez CE, Bollag WB, Filosa JA, et al. Adipocyte-derived hormone leptin is a direct regulator of aldosterone secretion, which promotes endothelial dysfunction and cardiac fibrosis. Circulation. (2015) 132(22):2134–45. doi: 10.1161/CIRCULATIONAHA.115.018226 [DOI] [PubMed] [Google Scholar]
- 16. Yang L, Zhang H, Cai M, Zou Y, Jiang X, Song L, et al. Effect of spironolactone on patients with resistant hypertension and obstructive sleep apnea. Clin Exp Hypertens (2016) 38(5):464–8. doi: 10.3109/10641963.2015.1131290 [DOI] [PubMed] [Google Scholar]
- 17. Gaddam K, Pimenta E, Thomas SJ, Cofield SS, Oparil S, Harding SM. Spironolactone reduces severity of obstructive sleep apnoea in patients with resistant hypertension: a preliminary report. J Hum Hypertens (2010) 24(8):532–7. doi: 10.1038/jhh.2009.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pratt-Ubunama MN, Nishizaka MK, Boedefeld RL, Cofield SS, Harding SM, Calhoun DA. Plasma aldosterone is related to severity of obstructive sleep apnea in subjects with resistant hypertension. Chest. (2007) 131(2):453–9. doi: 10.1378/chest.06-1442 [DOI] [PubMed] [Google Scholar]
- 19. Gonzaga CC, Gaddam KK, Ahmed MI, Pimenta E, Thomas SJ, Harding SM, et al. Severity of obstructive sleep apnea is related to aldosterone status in subjects with resistant hypertension. J Clin Sleep Med (2010) 6(4):363–8. [PMC free article] [PubMed] [Google Scholar]
- 20. Ke X, Guo W, Peng H, Hu C, Zhang H, Peng C, et al. Association of aldosterone excess and apnea-hypopnea index in patients with resistant hypertension. Sci Rep (2017) 7:45241. doi: 10.1038/srep45241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Calhoun DA, Nishizaka MK, Zaman MA, Harding SM. Aldosterone excretion among subjects with resistant hypertension and symptoms of sleep apnea. Chest. (2004) 125(1):112–7. doi: 10.1378/chest.125.1.112 [DOI] [PubMed] [Google Scholar]
- 22. Pecori A, Buffolo F, Pieroni J, Forestiero V, Sconfienza E, Veglio F, et al. Primary aldosteronism and obstructive sleep apnea: Casual association or pathophysiological link? Horm Metab Res (2020) 52(6):366–72. doi: 10.1055/a-1133-7255 [DOI] [PubMed] [Google Scholar]
- 23. Jin ZN, Wei YX. Meta-analysis of effects of obstructive sleep apnea on the renin-angiotensin-aldosterone system. J Geriatr Cardiol (2016) 13(4):333–43. doi: 10.11909/j.issn.1671-5411.2016.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med (2019) 7(8):687–98. doi: 10.1016/S2213-2600(19)30198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cai X, Li N, Hu J, Wen W, Yao X, Zhu Q, et al. Nonlinear relationship between Chinese visceral adiposity index and new-onset myocardial infarction in patients with hypertension and obstructive sleep apnoea: Insights from a cohort study. J Inflammation Res (2022) 15:687–700. doi: 10.2147/JIR.S351238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang Z, Heizhati M, Wang L, Li M, Yang Z, Lin M, et al. Poor sleep quality is negatively associated with low cognitive performance in general population independent of self-reported sleep disordered breathing. BMC Public Health (2022) 22(1):3. doi: 10.1186/s12889-021-12417-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heizhati M, Li N, Wang L, Hong J, Li M, Yang W, et al. Association of hypertension with mild cognitive impairment in population from less-developed areas of multiethnic Northwest China. Neuroepidemiology. (2021) 55(5):407–15. doi: 10.1159/000517956 [DOI] [PubMed] [Google Scholar]
- 28. Yang Z, Heizhati M, Wang L, Li M, Pan F, Wang Z, et al. Subjective poor sleep quality is associated with higher blood pressure and prevalent hypertension in general population independent of sleep disordered breathing. Nat Sci Sleep (2021) 13:1759–70. doi: 10.2147/NSS.S329024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang W, Shao L, Heizhati M, Wu T, Yao X, Wang Y, et al. Oropharyngeal microbiome in obstructive sleep apnea: Decreased diversity and abundance. J ClinSleep Med (2019) 15(12):1777–88. doi: 10.5664/jcsm.8084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marti-Soler H, Hirotsu C, Marques-Vidal P, Vollenweider P, Waeber G, Preisig M, et al. TThe NoSAS score for screening of sleep-disordered breathing: a derivation and validation study. Lancet Respir Med (2016) 4(9):742–8. doi: 10.1016/S2213-2600(16)30075-3 [DOI] [PubMed] [Google Scholar]
- 31. Hong C, Chen R, Qing S, Kuang A, Yang H, Su X, et al. Validation of the NoSAS score for the screening of sleep-disordered breathing: A hospital-based retrospective study in China. J Clin Sleep Med (2018) 14(2):191–7. doi: 10.5664/jcsm.6930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin M, Heizhati M, Gan L, Yao X, Luo Q, Zhang D, et al. Higher aldosterone is associated with increased renal impairment risk in patients with hypertension and abnormal glucose metabolism: a longitudinal study. J Hypertens (2022) 40(3):561–9. doi: 10.1097/HJH.0000000000003049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhu Q, Heizhati M, Lin M, Wang M, Yao X, Gan L, et al. Higher plasma aldosterone concentrations are associated with elevated risk of aortic dissection and aneurysm: a case-control study. Hypertension. (2022) 79(4):736–46. doi: 10.1161/HYPERTENSIONAHA.121.18342 [DOI] [PubMed] [Google Scholar]
- 34. Shukri MZ, Tan JW, Manosroi W, Pojoga LH, Rivera A, Williams JS, et al. Biological sex modulates the adrenal and blood pressure responses to angiotensin II. Hypertension. (2018) 71(6):1083–90. doi: 10.1161/HYPERTENSIONAHA.117.11087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tomaschitz A, Pilz S, Ritz E, Meinitzer A, Boehm BO, März W. Plasma aldosterone levels are associated with increased cardiovascular mortality: the ludwigshafen risk and cardiovascular health (LURIC) study. Eur Heart J (2010) 31(10):1237–47. doi: 10.1093/eurheartj/ehq019 [DOI] [PubMed] [Google Scholar]
- 36. Joseph JJ, Echouffo-Tcheugui JB, Kalyani RR, Yeh HC, Bertoni AG, Effoe VS, et al. Aldosterone, renin, cardiovascular events, and all-cause mortality among African americans: The Jackson heart study. JACC Heart Fail (2017) 5(9):642–51. doi: 10.1016/j.jchf.2017.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hu J, Shen H, Huo P, Yang J, Fuller PJ, Wang K, et al. Heightened cardiovascular risk in hypertension associated with renin-independent aldosteronism versus renin-dependent aldosteronism: A collaborative study. J Am Heart Assoc (2021) 10(24):e023082. doi: 10.1161/JAHA.121.023082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cannone V, Buglioni A, Sangaralingham SJ, Scott C, Bailey KR, Rodeheffer R, et al. Aldosterone, hypertension, and antihypertensive therapy: Insights from a general population. Mayo Clin Proc (2018) 93(8):980–90. doi: 10.1016/j.mayocp.2018.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vasan RS, Evans JC, Larson MG, Wilson PW, Meigs JB, Rifai N. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med (2004) 351(1):33–41. doi: 10.1056/NEJMoa033263 [DOI] [PubMed] [Google Scholar]
- 40. Pauzi FA, Azizan EA. Functional characteristic and significance of aldosterone-producing cell clusters in primary aldosteronism and age-related hypertension. Front Endocrinol (Lausanne) (2021) 12:631848. doi: 10.3389/fendo.2021.631848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev (2010) 90(1):47–112. doi: 10.1152/physrev.00043.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Takeda Y, Itaya-Hironaka A, Yamauchi A, Makino M, Sakuramoto-Tsuchida S, Ota H, et al. Intermittent hypoxia upregulates the renin and Cd38 mRNAs in renin-producing cells via the downregulation of miR-203. Int J Mol Sci (2021) 22(18):10127. doi: 10.3390/ijms221810127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shiota S, Ryan CM, Chiu KL, Ruttanaumpawan P, Haight J, Arzt M, et al. Alterations in upper airway cross-sectional area in response to lower body positive pressure in healthy subjects. Thorax. (2007) 62(10):868–72. doi: 10.1136/thx.2006.071183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bucca CB, Brussino L, Battisti A, Mutani R, Rolla G, Mangiardi L, et al. Diuretics in obstructive sleep apnea with diastolic heart failure. Chest. (2007) 132(2):440–6. doi: 10.1378/chest.07-0311 [DOI] [PubMed] [Google Scholar]
- 45. Burton LA, McMurdo ME, Struthers AD. Mineralocorticoid antagonism: a novel way to treat sarcopenia and physical impairment in older people? Clin Endocrinol (Oxf) (2011) 75(6):725–9. doi: 10.1111/j.1365-2265.2011.04148.x [DOI] [PubMed] [Google Scholar]
- 46. Kwak MK, Lee SE, Cho YY, Suh S, Kim BJ, Song KH, et al. The differential effect of excess aldosterone on skeletal muscle mass by sex. Front Endocrinol (Lausanne) (2019) 10:195. doi: 10.3389/fendo.2019.00195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lowe J, Kolkhof P, Haupt MJ, Peczkowski KK, Rastogi N, Hauck JS, et al. Mineralocorticoid receptor antagonism by finerenone is sufficient to improve function in preclinical muscular dystrophy. ESC Heart Fail (2020) 7(6):3983–95. doi: 10.1002/ehf2.12996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med (2015) 3(4):310–8. doi: 10.1016/S2213-2600(15)00043-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Redline S, Sotres-Alvarez D, Loredo J, Hall M, Patel SR, Ramos A, et al. Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. the Hispanic community health Study/Study of latinos. Am J Respir Crit Care Med (2014) 189(3):335–44. doi: 10.1164/rccm.201309-1735OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pływaczewski R, Bednarek M, Jonczak L, Zieliński J. Sleep-disordered breathing in a middle-aged and older polish urban population. J Sleep Res (2008) 17(1):73–81. doi: 10.1111/j.1365-2869.2008.00632.x [DOI] [PubMed] [Google Scholar]
- 51. Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. (2014) 146(5):1387–94. doi: 10.1378/chest.14-0970 [DOI] [PubMed] [Google Scholar]
- 52. Gessner V, Bitter T, Horstkotte D, Oldenburg O, Fox H. Impact of sleep- disordered breathing in patients with acute myocardial infarction: a retrospective analysis. J Sleep Res (2017) 26(5):657–64. doi: 10.1111/jsr.12540 [DOI] [PubMed] [Google Scholar]
- 53. Rong Y, Wang S, Wang H, Wang F, Tang J, Kang X, et al. Validation of the NoSAS score for the screening of sleep-disordered breathing in a sleep clinic. Can Respir J (2020), 4936423. doi: 10.1155/2020/4936423 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: The data that support the findings of this study are available upon request from the corresponding author. Requests to access these datasets should be directed to lnanfang2016@sina.com; morale118@126.com/1017663289@qq.com.