Abstract
Erdheim-Chester disease (ECD) is a non-Langerhans cell histiocytosis characterised by clonal expansion of histiocytes in various organs. These induce an inflammatory environment, which leads to damage of the affected areas. Recently, a new disease entity was proposed encompassing key features of ECD but also of Rosai-Dorfman-Destombes disease, another histiocytosis. Mitogen-activated protein kinase kinase 1 (MAP2K1) mutations seem to present a specific genetic lesion for this subtype.
Here, we describe a case of this new disease entity with clinical, radiological and genetic findings compatible with ECD but histological findings compatible with Rosai-Dorfman-Destombes disease. In particular, there were intraabdominal and retroperitoneal lesions, which tested positive for a (c.167A>C; p.Q56P) mutation of the MAP2K1 gene. On histological examination, S100-positive, giant histiocytes with focal emperipolesis of haematological cells in addition to infiltration by lymphocytes and granulocytes were seen.
As described for this rare variant of ECD, there was also bilateral testicular infiltration. We also describe a manifestation of oligoarthritis in this patient with ECD.
The patient was treated with methotrexate and prednisolone. While radiological response to this regime was excellent, arthritis persisted. We added anakinra, which induced a response of the arthritis for more than a year. Due to treatment failure therapy was switched to upadacitinib, which induced a remission of the arthritis as well.
This case adds a rare phenotype to an already rare presentation of ECD. The patient responded to immunosuppressive therapy.
Keywords: Arthritis, Autoimmune Diseases, Biological Therapy, Glucocorticoids, Magnetic Resonance Imaging
WHAT IS ALREADY KNOWN ON THIS TOPIC
Histiocytoses are potential differential diagnoses in patients presenting with persistent arthralgias.
Erdheim-Chester disease (ECD) can mimic both other histiocytoses (such as Rosai-Dorfmann-Destombes disease) as well as more common inflammatory diseases.
WHAT THIS STUDY ADDS
Immunosuppressive treatment can be attempted in ECD with targeted therapy as an alternative strategy in case of failure.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
ECD might cause oligoarthritis in certain patients. However, this needs to be confirmed in more patients.
Introduction
Erdheim-Chester disease (ECD) is a non-Langerhans cell histiocytosis (LCH) defined as ‘a clonal disorder marked by frequent hyperactivation of mitogen-activated protein kinase signalling in which an inflammatory milieu is important in the pathogenesis and clinical manifestations of the disease’.1 Common manifestations include xanthelasma, perirenal infiltration (with ‘hairy kidney’ sign on CT scan)2 and infiltration of the femora and tibiae by sclerotic lesions.1 3 However, a plethora of clinical manifestations has been reported over the past years usually caused by infiltration of a corresponding variety of organs by histiocytes. Importantly, these histiocytes induce an inflammatory environment, which causes ECD-associated organ damage.4 Mutations causing hyperactivation of histiocytes have been detected in BRAFV600E, MAP2K1 and a variety of other genes.5 It belongs to the L-Group of histiocytoses according to the Histiocyte Society classification criteria, characterised by MAPK mutations (>80%/cases).5
Rosai-Dorfmann-Destombes disease (RDD) is a non-LCH usually found in children and usually presenting with nodal infiltration by histiocytes.6 Extranodal involvement is present in half of cases and comprises a wide variety of organs including the testes.7 Genetic abnormalities can be present in MAP2K1, KRAS proto-oncogene, GTPase (KRAS).8 It belongs to the R-Group of histiocytoses, characterised by histological findings such as abundant emperipolesis with specific immunohistological staining.5
Both diseases are diagnosed by histological criteria and genetic testing of lesions for defining mutations (via next-generation sequencing or testing for specific mutations) after specific radiological findings have been documented on Positron emission tomography–computed tomography (PET-CT).9
Razanamahery et al10 reported a novel entity consisting of histologic findings consistent with RDD, clinical manifestations of ECD and specific MAP2K1 mutations (ECD/RDD).
Case report
Our patient initially presented with acute pain of the entire back as well as the right groin starting in 2010. Pain episodes lasted for 1–7 days and the patient described them as muscle soreness. The pain was not relieved by over-the-counter analgesics and was present several times a year. Fatigue was also present.
In 2012, the pain started to affect additional joints (both shoulders, multiple metacarpophalangeal (MCP) joints of both hands and several proximal interphalangeal joints and both ankles). Treatment with non-steroidal anti-inflammatory drugs and physiotherapy did not improve the arthralgia.
Several visits to local hospitals and practices led to a number of diagnostic tests without conclusive results. These included an arthroscopy of the right hip, MRI of both hip joints and a CT scan (CT) of the abdomen.
Starting in August 2015, the patient noticed induration of both testes and consulted an urologist. An open biopsy of the left testis yielded a histological finding of chronic fibrosing granulomatous orchitis. An MRI of both testes and pelvis showed oedema and contrast enhancement of the left spermatic funiculus.
Follow-up CTs and MRI performed in August 2016 (CT) and January 2017 (MRI) showed no changes of the testicular lesions but did show several new peritoneal infiltrations in the upper abdomen partially surrounding the spleen. These had not been present in a CT scan performed in 2010. No lymphadenopathy was noted.
A laparoscopic biopsy of the abdominal lesions was performed in January 2017. The intraoperative inspection of the peritoneum demonstrated diffuse white infiltration with several nodular lesions of the major omentum, multiple sites on the parietal peritoneum and the visceral peritoneum of several organs (ie, small intestine, colon, liver) of which samples were collected. A small amount of ascites was present as well. The lesions had a soft consistency. On visual inspection, no enlarged lymph nodes were present.
Initial and subsequent histological and immunohistochemical examinations showed fibrosis, ectatic blood vessels and infiltration of the peritoneum by S100-positive giant histiocytes with focal emperipolesis (ie, intact intracellular presence) of haematological cells. Inflammatory infiltrates by both lymphocytes and granulocytes were associated with these histiocytes.
S100 staining of the testicular biopsies taken in 2015 was performed, which showed the same infiltrates. Additional staining demonstrated positivity for CD 45 of the entire infiltrate, the histiocytes were positive for CD163 and CD68. However, subsequent NRAS, HRAS, BRAF and KIT sequencing of the peritoneal lesions did not show any abnormalities.
Additional sequencing of BRAF, EGFR, KRAS, NRAS, PIK3CA, ALK, AKT1, ERBB2, ERBB4, CTNNB1, FBXW7, FGFR1, FGFR2, FGFR3, PTEN, SMAD4, STK11, TP53, MET, NOTCH1, MAP2K1 and DDR2 revealed a gain-of- function mutation (c.167A>C; p.Q56P) of the MAP2K1 gene.
A repeat MRI of the abdomen in late 2019 (see figure 1) demonstrated expansion of the testicular and abdominal lesions, which now consisted of several large lesions along the lateral abdominal wall and the inguinal canal.
Figure 1.
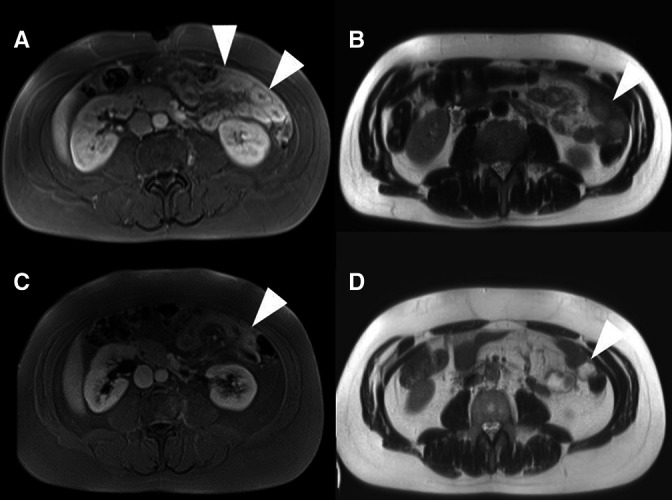
Radiological presentation: (A) and (B) representative MRI images (A=T1 VIBE fat-saturated images postcontrast B=T2 haste) of the intraabdominal manifestations present in our patient before initiation of therapy with prednisone and MTX (C) and (D) follow-up MRI images after approx. 1 month of therapy with prednisone and MTX. (C=T1 VIBE fat-saturated images postcontrast D=T2 HASTE) The lesions (marked with Δ) show strong contrast uptake and are T2 hypointense. Additional diffusion weighted sequences (not shown) show no significant restriction of diffusion. MTX, methotrexate.
Consequently, a diagnosis of ECD with Rosai-Dorfman like lesions was made.
We initiated a therapy with 1 mg/kg of prednisolone orally, which was tapered to 5 mg once daily. Additionally, methotrexate (MTX) sc was started at a dose of 15 mg once weekly. Due to persistent arthritis, the MTX dosage was increased to 20 mg once weekly after 1 month and prednisolone was increased to 1 mg/kg with tapering to 5 mg over 6 weeks.
A repeat MRI was performed before the prednisolone dose was increased again (1 month after initiation of therapy). This showed almost total regression of the abdominal lesions (see figure 1). However, there was also synovitis of the right hip joint documented with MRI, as well as persistent articular pain. Notably, periarticular intraosseus sclerosis was absent. Sonographic signs of tendovaginitis of the long biceps tendon and both knees were present as well. There was no periarticular fibrosis. In addition, systemic inflammation was present and documented for the first time (C-reactive protein 77.9 mg/L (ref. <5 mg/L), Erythrocyte sedimentation rate 42 mm/1 hour).
We added anakinra 100 mg once daily to the treatment regimen, which led to a significant reduction in arthralgias, fatigue and inflammation parameters for more than 1 year.
In February 2021, there was a recurrence of arthritis of several MCP joints as well as both knees. Immunosuppressive therapy was switched from MTX and anakinra to MTX and upadacitinib 15 mg once daily which was effective. Arthritis did not reoccur under upadacitinib during 8 months of follow-up.
The most recent follow-up MRI in February 2022 demonstrated complete resolution of the abdominal findings, demonstrating robust response to therapy.
Discussion
Here, we report a case of ECD/RDD. This new entity has been described for the first timeby Razanamahery et al.10 Consistent with their report, our patient presented with lesions consistent with ECD but histological features suggestive of RDD, testicular involvement and a MAP2K1 mutation.
The detected mutation has also been described in the context of LCH.11 Thus, a differential diagnosis of ECD, LCH, RDD or indeed ECD/RDD can be considered. Our patient presented with histology consistent with RDD (S100-positive giant histiocytes with focal emperipolesis), multilocular involvement consistent with both ECD (abdominal; close to the spleen) and RDD (testes) but not with LCH.5 This specific presentation taken together with the mutation previously described by Razanamahery et al.10 supports ECD/RDD to represent a distinct disease entity driven by specific MAP2K1 mutations. An atypical presentation of RDD can not be discounted, although the detected mutation has, to our knowledge, not been described in this context.
Uniquely, however, our patient presented with arthritis of several joints without intraosseous sclerosis. There have been reports of arthropathy in patients with ECD,12 as well as arthritis and arthropathy in patients with RDD.13–15 Monarthritis has been described before, with synovial infiltration of the affected joint by histiocytes.16 Oligoarthritis has been described in the context of RDD.17 To our knowledge, this is the first report of a patient with ECD presenting with arthritis of more than one joint.
While case reports can not be generalised some facets of this case might still be of interest, especially in such a rare entity.
Oligoarthritis in addition to arthralgia due to bone infiltration may be a manifestation associated with this novel subtype of ECD. This possible association should be investigated further for confirmation.
Treatment recommendations for ECD1 18 can be classified into four approaches:
Targeted therapies (eg, vemurafenib, cobimetinib, imatinib) targeting common molecular alterations in ECD and great efficacy in patients with these mutations and even some where the precise mutation is not known.
Interferon-α with the largest available evidence prior to targeted therapies but limited by toxicities.
Anticytokine therapies (eg, infliximab, anakinra and tocilizumab) with fewer reported toxicities than interferon-α but less evidence.
Corticosteroids and MTX19 with little evidence but with a broad, non-targeted mechanism of action and surgery with a direct and immediate effect on critical lesions
In this patient, we eventually chose treatment with upadacitinib, a Janus-Kinase (JAK) inhibitor targeting JAK1 for its efficacy in treating arthritis, present in our patient, for example, rheumatoid arthritis,20 as well as its mechanism of action, JAK-STAT inhibition. This pathway can be activated by IL-1, TNF-α and IL-6,20 targets of established anticytokine therapies for ECD.
Good response of our patient to immunosuppressiv therapy suggests the need for structured investigations regarding immunosuppressive management of this novel entity.
Thus, our case suggests that oligoarthritis might be a manifestation of ECD/RDD which in this case was managed with immunosuppressive therapy, that is, prednisolone, MTX, upadacitinib and anakinra.
Footnotes
Contributors: All authors were involved in drafting the article or revising it critically for important intellectual content. All authors approved the final manuscript to be. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. LR and MS contributed equally to this paper.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: JP received travel grants from AbbVie, EuroImmun, CSL-Behring, Galapagos and Janssen-Cilag. MG received travel grants, speaker’s fees or compensation for advisory boards from AbbVie, Eli Lilly, Hexal, Janssen-Cilag, Novartis, Pfizer and Takeda. AH has no competing interests to declare. AR has no competing interests to declare. SH received travel grants from Jannsen-Cilag. LR has no competing interests to declare. MS received compensation for his work as an advisor or consultant by Chugai/ Roche, Hexal/Sandoz, Gilead, AbbVie, Janssen-Cilag and Boehringer/Ingelheim, for his work as a lecturer by Novartis, AbbVie, AstraZeneca, Chugai/Roche and Janssen-Cilag and for his work relating to medical conventions by Chugai/Roche, Boehringer/Ingelheim, Celgene, Medac and UCB.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Diamond EL, Dagna L, Hyman DM, et al. Consensus guidelines for the diagnosis and clinical management of Erdheim-Chester disease. Blood 2014;124:483–92. 10.1182/blood-2014-03-561381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haroche J, Amoura Z, Wechsler B, et al. [Erdheim-Chester disease]. Presse Med 2007;36:1663–8. 10.1016/j.lpm.2007.04.032 [DOI] [PubMed] [Google Scholar]
- 3.Liew JW, Starkebaum G. Bone lesions in Erdheim-Chester disease. Arthritis Rheumatol 2019;71:1206. 10.1002/art.40845 [DOI] [PubMed] [Google Scholar]
- 4.Papo M, Emile J-F, Maciel TT, et al. Erdheim-Chester disease: a Concise review. Curr Rheumatol Rep 2019;21:66. 10.1007/s11926-019-0865-2 [DOI] [PubMed] [Google Scholar]
- 5.Emile J-F, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood 2016;127:2672–81. 10.1182/blood-2016-01-690636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abla O, Jacobsen E, Picarsic J, et al. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood 2018;131:2877–90. 10.1182/blood-2018-03-839753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Gobbo A, Moltrasio F, Young RH, et al. Involvement of the testis and related structures by Rosai-Dorfman disease: report of 2 new cases and review of the literature. Am J Surg Pathol 2013;37:1871–5. 10.1097/PAS.0b013e31829e2509 [DOI] [PubMed] [Google Scholar]
- 8.Garces S, Medeiros LJ, Patel KP, et al. Mutually exclusive recurrent KRAS and MAP2K1 mutations in Rosai-Dorfman disease. Mod Pathol 2017;30:1367–77. 10.1038/modpathol.2017.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goyal G, Young JR, Koster MJ, et al. The Mayo clinic histiocytosis Working group consensus statement for the diagnosis and evaluation of adult patients with histiocytic neoplasms: Erdheim-Chester disease, Langerhans cell histiocytosis, and Rosai-Dorfman disease. Mayo Clin Proc 2019;94:2054–71. 10.1016/j.mayocp.2019.02.023 [DOI] [PubMed] [Google Scholar]
- 10.Razanamahery J, Diamond EL, Cohen-Aubart F, et al. Erdheim-Chester disease with concomitant Rosai-Dorfman like lesions: a distinct entity mainly driven by MAP2K1. Haematologica 2020;105:e5–8. 10.3324/haematol.2019.216937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakraborty R, Hampton OA, Shen X, et al. Mutually exclusive recurrent somatic mutations in MAP2K1 and BRAF support a central role for ERK activation in LCH pathogenesis. Blood 2014;124:3007–15. 10.1182/blood-2014-05-577825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khokhar K, Fascia D, Scullion D, et al. Erdheim-Chester disease. Arthritis Rheumatol 2016;68:163. 10.1002/art.39426 [DOI] [PubMed] [Google Scholar]
- 13.Pessler F, Paessler ME, Lambert M, et al. Polyarthritis in a child with Rosai-Dorfman disease. Clin Exp Rheumatol 2007;25:645–8. [PubMed] [Google Scholar]
- 14.Gupta S, Finzel KC, Grubber BL. Rosai-Dorfman disease masquerading as chronic ankle arthritis: a case report and review of the literature. Rheumatology 2004;43:811–2. 10.1093/rheumatology/keh179 [DOI] [PubMed] [Google Scholar]
- 15.Razanamahery J, Dresco F, Emile JF, et al. Sacroiliitis in a patient with Rosai-Dorfman disease: new bone location or overlap with axial spondylarthritis? Rheumatology 2020;59:2168–70. 10.1093/rheumatology/kez675 [DOI] [PubMed] [Google Scholar]
- 16.Kroot EJA, Weel AEAM, Hazes JMW, et al. Diagnostic value of blind synovial biopsy in clinical practice. Rheumatology 2006;45:192–5. 10.1093/rheumatology/kei117 [DOI] [PubMed] [Google Scholar]
- 17.Haghighat Jahromi A, Goodman AM, Rosai-Dorfman-Destombes HCK. RDD) disease presenting as palindromic rheumatism. BMC Med Imaging 2021;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goyal G, Heaney ML, Collin M, et al. Erdheim-Chester disease: consensus recommendations for evaluation, diagnosis, and treatment in the molecular era. Blood 2020;135:1929–45. 10.1182/blood.2019003507 [DOI] [PubMed] [Google Scholar]
- 19.Goyal G, Shah MV, Call TG, et al. Clinical and radiological responses to oral methotrexate alone or in combination with other agents in Erdheim-Chester disease. Blood Cancer J 2017;7. 10.1038/s41408-017-0034-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serhal L, Edwards CJ. Upadacitinib for the treatment of rheumatoid arthritis. Expert Rev Clin Immunol 2019;15:13–25. 10.1080/1744666X.2019.1544892 [DOI] [PubMed] [Google Scholar]


