Abstract
Background
Immunocompromised patients are at increased risk of SARS-CoV-2 infections. Patients undergoing chimeric antigen receptor (CAR) T-cell therapy for relapsed/refractory B-cell malignancies are uniquely immunosuppressed due to CAR T-mediated B-cell aplasia (BCA). While SARS-CoV-2 mortality rates of 33%–40% are reported in adult CAR T-cell recipients, outcomes in pediatric and young adult CAR T-cell recipients are limited.
Methods
We created an international retrospective registry of CAR T recipients aged 0–30 years infected with SARS-CoV-2 within 2 months prior to or any time after CAR T infusion. SARS-CoV-2-associated illness was graded as asymptomatic, mild, moderate, or severe COVID-19, or multisystem inflammatory syndrome in children (MIS-C). To assess for risk factors associated with significant SARS-CoV-2 infections (infections requiring hospital admission for respiratory distress or supplemental oxygen), univariate and multivariable regression analyses were performed.
Results
Nine centers contributed 78 infections in 75 patients. Of 70 SARS-CoV-2 infections occurring after CAR T infusion, 13 (18.6%) were classified as asymptomatic, 37 (52.9%) mild, 11 (15.7%) moderate, and 6 (8.6%) severe COVID-19. Three (4.3%) were classified as MIS-C. BCA was not significantly associated with infection severity. Prior to the emergence of the Omicron variant, of 47 infections, 19 (40.4%) resulted in hospital admission and 7 (14.9%) required intensive care, while after the emergence of the Omicron variant, of 23 infections, only 1 (4.3%) required admission and the remaining 22 (95.7%) had asymptomatic or mild COVID-19. Death occurred in 3 of 70 (4.3%); each death involved coinfection or life-threatening condition. In a multivariable model, factors associated with significant SARS-CoV-2 infection included having two or more comorbidities (OR 7.73, CI 1.05 to 74.8, p=0.048) and age ≥18 years (OR 9.51, CI 1.90 to 82.2, p=0.014). In the eight patients infected with SARS-CoV-2 before CAR T, half of these patients had their CAR T infusion delayed by 15–30 days.
Conclusions
In a large international cohort of pediatric and young adult CAR-T recipients, SARS-CoV-2 infections resulted in frequent hospital and intensive care unit admissions and were associated with mortality in 4.3%. Patients with two or more comorbidities or aged ≥18 years were more likely to experience significant illness. Suspected Omicron infections were associated with milder disease.
Keywords: Immunotherapy; Pediatrics; COVID-19; Hematologic Neoplasms; Receptors, Chimeric Antigen
WHAT IS ALREADY KNOWN ON THIS TOPIC
SARS-CoV-2 infections pose an increased risk in immunocompromised populations. Chimeric antigen receptor (CAR) T-cell recipients are a uniquely immunocompromised group due to frequent B-cell aplasia, history of hematological malignancy, and receipt of multiple lines of chemotherapy and/or hematopoietic stem cell transplant. However, information on the outcomes of CAR T-cell recipients who have been infected with SARS-CoV-2 is primarily limited to adults prior to the emergence of the Omicron (B1.1.529) variant.
WHAT THIS STUDY ADDS
This study describes outcomes and clinical features of children and young adults who developed SARS-CoV-2 infections after undergoing CAR T-cell therapy and identifies risk factors for significant disease within this population.
HOW THIS STUDY MAY AFFECT RESEARCH, PRACTICE OR POLICY
This study may influence practice by identifying patients following CAR T-cell therapy at higher risk of hospitalization secondary to SARS-CoV-2 infections who would benefit from preventative measures, such as vaccination or prophylaxis strategies, as well as early treatment with antiviral therapy.
Introduction
Patients undergoing chimeric antigen receptor (CAR) T-cell therapy for relapsed/refractory (r/r) B-acute lymphoblastic leukemia (B-ALL) or B-non-Hodgkin's lymphoma (B-NHL) comprise a cohort of pediatric oncology patients with distinct immunological deficiencies. Currently, CAR T-cell therapies for pediatric B-ALL include one commercially available product, tisagenlecleucel, which targets CD19, and investigational CAR T-cell products targeting CD19 and/or CD22.1–4 Tisagenlecleucel is also available for treatment of mature B-cell lymphomas in adult patients,5 in addition to other Food and Drug Administration (FDA)-approved CD19-directed CAR T-cell therapies for this indication, including axicabtagene ciloleucel6 and lisocabtagene maraleucel.7 CAR T-cell recipients are immunocompromised due to underlying hematological malignancies, receipt of multiple lines of chemotherapy and immunotherapies, history of hematopoietic stem cell transplant (SCT), and/or comorbidities that might have precluded SCT. Furthermore, prior to CAR T-cell therapy, patients typically undergo lymphodepleting chemotherapy, leading to further immunosuppression, and following CAR T-cell infusion, CD19-directed and/or CD22-directed CAR T cells target and eliminate both malignant and healthy B cells, resulting in B-cell aplasia (BCA).8 9 CAR-associated inflammatory toxicities such as cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome frequently necessitate anti-inflammatory therapies and are associated with protracted cytopenias that can predispose to infections.3 10 Finally, those with inadequate responses to CAR T-cell therapies require further cancer-directed therapies, increasing their degree of immunosuppression.
Since its discovery in December 2019, SARS-CoV-2 has resulted in more than 1 000 000 deaths in the USA.11 Just over 0.01% of recorded pediatric cases (ages 0–17) have resulted in death, but children remain at risk for severe sequelae including multisystem inflammatory syndrome in children (MIS-C).11–14 While risks to healthy children are relatively low, a study of 917 pediatric oncology patients confirmed increased susceptibility to severe outcomes after SARS-CoV-2 infection, including a 31% hospitalization rate and 1.6% death rate.15 Risks for severe disease were significantly higher for patients with comorbidities, hematological malignancies, and age ≥11 years. Conversely, while the Omicron (B.1.1.529) SARS-CoV-2 variant has been associated with increased transmissibility, several studies have suggested decreased pathogenicity in the general population.11 16–21 Whether pathogenicity is also decreased in immunocompromised patients is not known.
The immune deficits and limited serological responses to SARS-CoV-2 vaccination in patients after CD19-directed CAR T raise concerns for increased risk of infection, ineffective immune responses to SARS-CoV-2 infection, and heightened infection severity in this population.22 23 In adults with hematological malignancies who have undergone CAR T-cell therapy, mortality from SARS-CoV-2 infections has been reported in as many as 33%–40% of patients.24 25 However, outcomes in children and young adults undergoing CAR T-cell therapies are not well described. Furthermore, given the relapsed and refractory nature of the hematological malignancies for which CAR T-cells are indicated, an important consideration is the impact on SARS-CoV-2 infection on delaying this potentially life-saving therapy. Primary objectives of this study were (1) to describe the outcomes, laboratory and imaging findings of SARS CoV-2 infections in children and young adult recipients of CAR T-cell therapies; (2) to evaluate patient factors associated with significant infections in this population, including the relative severity of infections before and after the emergence of the Omicron variant; and (3) to survey the delays in delivery of CAR T therapy for those infected before anticipated infusions.
Methods
Study design and participating sites
This is an international multicenter retrospective study that included patients aged 0–30 years with a diagnosis of r/r B-ALL or B-NHL who underwent or were planning to undergo CAR T-cell therapy within 2 months of a documented SARS-CoV-2 infection. Infections between the dates of 1 March 2020 and 1 March 2022 were included. Nine institutions contributed retrospective deidentified patient data: Children’s Hospital of Philadelphia (n=48), Lucile Packard Children’s Hospital Stanford (n=3), Children’s Hospital Los Angeles (n=8), Princess Maxima Center for Pediatric Oncology (n=5), Johns Hopkins All Children’s Hospital (n=4), Medical College of Wisconsin (n=3), Clinica Pediatrica Università degli Studi di Milano Bicocca (n=3), Children’s National Hospital (n=2), and Hackensack University Medical Center (n=2) (online supplemental figure S1). Data were collected from the medical record, deidentified, and entered retrospectively via a REDCap data collection tool in a registry housed at the Children’s Hospital of Philadelphia.
jitc-2022-005957supp002.pdf (1.3MB, pdf)
Data collected
Baseline information on CAR T-cell recipients included age, sex, leukemia/lymphoma history (diagnosis, remission status at time of infection, and receipt of chemotherapy when diagnosed with SARS-CoV-2 infection), presence of comorbidities compiled from the Centers for Disease Control and Prevention (CDC) list of underlying medical conditions associated with higher risk of severe COVID-19 (type 1 or 2 diabetes, asthma, chronic lung disease, pulmonary hypertension, on immunosuppressive medicines, chronic kidney disease, liver disease, congenital heart disease, pre-existing hypertension, trisomy 21, overweight or obese, cancer not in remission), history of SCT, and CAR T-cell infusion details (CAR T-cell product and history of CAR T-cell-related toxicities).16
Data collected about the clinical course of SARS-CoV-2 infections included symptoms experienced, hospitalization to the acute care or intensive care unit (ICU), respiratory support, presence of hypotension, laboratory findings (maximum and minimum values during illness), chest imaging findings (X-ray and CT), echocardiogram findings, and SARS-CoV-2-directed treatments. SARS-CoV-2 positivity was defined as a positive PCR or antigen test. In patients who had multiple SARS-CoV-2 PCR or antigen tests, duration of positivity and time to clearance were measured. Duration of SARS-CoV-2 positivity was defined as the number of days from first to last positive result on serial tests. Time to SARS-CoV-2 clearance, or the number of days from the first positive to the first negative SARS-CoV-2 test, was not reported because of significant variability in the practice of testing until SARS-CoV-2 negativity.
Infection severity classification
Based on symptoms and degree of support required, patients were categorized into five clinical groups: (1) asymptomatic, no symptoms of COVID-19; (2) mild COVID-19, symptomatic but no requirement for hospital admission; (3) moderate COVID-19, symptomatic and required admission but not meeting criteria for severe disease; (4) severe COVID-19, symptomatic and required intensive care admission and/or high-flow nasal cannula, non-invasive positive-pressure ventilation, advanced airway and mechanical ventilation, vasopressor support; and (5) MIS-C for those who met the CDC definition of MIS-C.26 As MIS-C is a severe complication of SARS-CoV-2 infection, these patients were grouped with the severe COVID-19 category for further analysis.
To evaluate for factors associated with more versus less significant SARS-CoV-2 infection, moderate COVID-19 requiring oxygen supplementation or admission for respiratory distress, severe COVID-19, and MIS-C were classified as significant infections. Factors previously found to be associated with more severe SARS-CoV-2 infections (age, cytopenias and comorbidities) and variables suspected to contribute to more severe infections in CAR T-cell recipients (BCA, receipt of chemotherapy, history of SCT, presence of coinfection) or less severe SARS-CoV-2 infections (infection following emergence of the Omicron B.1.1.529 variant) were included in the analysis.15 25 Omicron became the dominant SARS-CoV-2 strain in the USA following 11 December 2021.27 Patients diagnosed with SARS-CoV-2 infection following this date in the USA were classified as having suspected Omicron variant infections in this study. In Europe, Omicron became the dominant form of SARS-CoV-2 in mid-January 2022, but no infections classified as Omicron in this study were identified outside of the USA.
Delays in cancer-directed therapy
To evaluate for delays in CAR T-cell therapy due to SARS-CoV-2 infection, patients diagnosed within 2 months prior to CAR T-cell infusion were evaluated for length of delay to CAR T-cell infusion, duration of SARS-CoV-2 test positivity, and severity of illness.
Statistical analysis
Statistical analyses were performed using GraphPad Prism V.9.3.1 (San Diego, California, USA) and SPSS V.28.0 and R V.4.2. Groups were compared via Kruskal-Wallis test for continuous variables and Fisher’s exact or χ2 tests for categorical variables. Univariable and multivariable logistic regressions were used to determine factors associated with the development of significant SARS-CoV-2 infections. The multivariable model included variables that were significant in the univariable analysis without many missing values. Odds ratios (ORs) and their 95% CIs were reported. A two-sided p value of <0.05 was considered statistically significant.
Results
Timing and classification of SARS-CoV-2 infections
Seventy-five pediatric/young adult patients with r/r B-ALL or B-NHL treated with CAR T-cell therapy experienced 78 unique SARS-CoV-2 infections (3 patients had two distinct infectious episodes separated by 3–12 months). Eight infections occurred within 2 months prior to CAR T-cell infusion, and 70 infections occurred following infusion. Of the 70 postinfusion infections, 13 (18.6%) resulted in asymptomatic disease; 37 (52.9%) resulted in mild COVID-19; 11 (15.7%) resulted in moderate COVID-19; 6(8.6%) resulted in severe COVID-19; and 3 (4.3%) resulted in MIS-C (table 1).
Table 1.
Characteristics of children and young adults infected with SARS-CoV-2 following CAR T
| Asymptomatic infection (N=13) |
Mild COVID-19 (N=37) |
Moderate COVID-19 (N=11) |
Severe COVID-19 (n=6)+MIS-C (N=3) | Total (N=70) | P value | |
| Age (years), median (range) | 9.0 (4.0–18.0) | 16 (1.6–25.0) | 21 (11.0–27.0) | 17 (1.0–20.0) | 16.0 (1.0–27.0) | 0.0008 |
| Sex (% female) | 6/31 (19.4) | 17/31 (54.8) | 4/31 (12.9) | 4/31 (12.9) | 31/70 (44.3) | p=0.9524 |
| BMI percentile, n (%) | p=0.3133 | |||||
| <85% | 6/47 (12.8) | 26/47 (55.3) | 9/47 (19.1) | 6/47 (12.8) | 47/70 (67.1) | |
| 85%–94% (overweight) | 3/13 (23.1) | 6/13 (46.2%) | 1/13 (7.7%) | 3/13 (23.1) | 13/70 (18.6) | |
| 95%–100% (obese) | 4/10 (40.0) | 5/10 (50.0) | 1/10 (10.0) | 0/10 (0) | 10/70 (14.3) | |
| Cancer in remission at time of SARS-CoV-2 infection, n (%) | 13/67 (19.4) | 36/67 (53.7) | 11/67 (16.4%) | 7/67 (10.4) | 67/70 (95.7) | p=0.0838 |
| Undergoing cancer chemotherapy at the time of SARS-CoV-2 infection, n (%) | 0/8 (0) | 5/8 (62.5) | 1/8 (12.5) | 2/8 (25.0) | 8/70 (11.4) | p=0.4856 |
| In B-cell aplasia at the time of infection, n (%) | 10/57 (17.5) | 28/57 (49.1) | 10/57 (17.5) | 9/57 (15.8) | 57/70 (81.4) | 0.2969 |
| On immunoglobulin replacement at time of infection, n (%) | 13/60 (21.7) | 29/60 (48.3) | 11/60 (18.3) | 7/60 (11.7) | 60/70 (85.7) | 0.1047 |
| Infection during Omicron dominant strain (12/11/21+), n (%) | 5/23 (21.7) | 17/23 (73.9) | 1/23 (4.3) | 0/23 (0) | 23/70 (32.9) | 0.0163 |
| 2+ comorbidities present, n (%) | 1/14 (7.1) | 3/14 (21.4) | 3/14 (21.4) | 7/14 (50.0) | 14/70 (20.0) | <0.0001 |
| Comorbidities (n), median (range) | 1.0 (0.0–3.0) | 0.0 (0.0–4.0) | 0.0 [0.0–3.0) | 2.0 (0.0–3.0) | 1.0 (0.0–1.0) | 0.0526 |
| History of SCT prior to infection (% yes) | 3/24 (12.5) | 11/24 (45.8) | 7/24 (29.2) | 3/24 (12.5) | 24/70 (34.3) | 0.1527 |
| Timing of SCT relative to CAR T-cell infusion, n (%) | 0.2503 | |||||
| No SCT | 10/46 (21.7) | 26/46 (56.5) | 4/46 (8.7) | 6/46 (13.0) | 46/70 (65.7) | |
| SCT pre-CAR T-cell infusion | 2/17 (11.8) | 9/17 (52.9) | 5/17 (29.4) | 1/17 (5.9) | 17/70 (24.2) | |
| SCT post-CAR T-cell infusion | 1/7 (14.3) | 2/7 (28.6) | 2/7 (28.6) | 2/7 (28.6) | 7/70 (10) | |
| CAR T product received most recently, n (%) | 0.0809 | |||||
| Tisagenlecleucel (CTL019, Kymriah) | 7/47 (14.9) | 23/47 (48.9) | 10/47 (21.3) | 6/47 (12.8) | 47/70 (67.1) | |
| Humanized CART19 (CTL119 Penn) | 5/17 (29.4) | 11/17 (64.7) | 0/17 (0.0) | 1/17 (5.9) | 17/70 (24.3) | |
| CART 22 (Penn) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 0/1 (0) | 1/70 (1.4) | |
| Seattle Children’s Research Institute CAR19, JCAR017 | 1/4 (25.0) | 2/4 (50.0 | 0/4 (0) | 1/4 (25.0) | 4/70 (5.7) | |
| Other | 0/1 (0) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 1/70 (1.4) |
Patients are grouped into asymptomatic infection (no symptoms), mild COVID-19 (experienced symptoms but did not require admission), moderate COVID-19 (required admission for fever or oxygen therapy, including nasal cannula or blow-by oxygen), severe COVID-19 (symptomatic and required intensive care admission and/or high flow nasal cannula, non-invasive positive-pressure ventilation, advanced airway and mechanical ventilation, and vasopressor support), and MIS-C (those who met the CDC definition of MIS-C). The severe COVID-19 and MIS-C groups were combined as they both represent severe manifestations of SARS-CoV-2 infections. Groups are compared with Kruskal-Wallis or χ2 or Fisher’s exact tests with significance set as p value of p<0.05.
BMI, body mass index; CAR, chimeric antigen receptor; SCT, stem cell transplant.
The median age of those who developed SARS-CoV-2 infections post-CAR T-cell infusions was 16 years (range 1.0–27.0). All patients with infection following CAR T-cell infusion had r/r B-ALL as an indication for CAR T-cell therapy, and 67 of 70 (95.7%) were in remission at the time of SARS-CoV-2 infection. Of the 70 patients, 57 (81.4%) had BCA at the time of SARS-CoV-2 infection and 60 (85.7%) were receiving support with immunoglobulin replacement. BCA status, immunoglobulin replacement, and type of CAR T-cell product were not associated with infection severity (table 1). Overall, of the 70 patients, 24 (34.3%) had undergone SCT prior to SARS-CoV-2 infection without significant differences among groups (table 1). Overweight or obese status was present in 32.9% of our cohort (table 1).
Infection characteristics
SARS-CoV-2 infections occurred a median of 435 days (range 20–3180 days) following CAR T-cell infusion (table 2). As the early post-CAR T-cell period is often associated with BCA, neutropenia, and lymphopenia, early infections (occurring ≤90 days after CAR T-cell infusion) (n=14) were evaluated for associated severity. Infections early in the post-CAR T course were most frequently asymptomatic (50%) or mild (35.7%), while moderate (0%) and severe COVID-19/MIS-C (14.3%) were found less frequently. For infections occurring >90 days after CAR T, infections were mild in 57%, asymptomatic in 10.7%, moderate in 0%, and severe COVID-19/MIS-C in 11.5%. There was a known exposure to someone with SARS-CoV-2 in 37 (54.4%) of 68 patients, a rate similar to that reported in adult cell therapy recipients (45%).25 Eighteen of 70 (25.7%) patients had serial positive SARS-CoV-2 tests recorded; for these patients, duration of documented SARS-CoV-2 test positivity ranged from 8 to 215 days, with a median of 30 days, and duration of SARS-CoV-2 positivity did not differ significantly between severity groups (table 2 and online supplemental figure S2A). However, there were several outliers with protracted SARS-CoV-2 positivity in the severe COVID-19 group; one patient with more than nine consecutive positive tests and 215 days of positivity who never had documented clearance, and one patient with 94 days of positivity on four consecutive tests who had documented clearance at day 123. Median duration of SARS-CoV-2 test positivity for patients with BCA was 30 days (range 10–215, n=14), while it was 29.5 days in those with B-cell recovery (BCR) (range 8–39 days, n=4) (online supplemental figure S2B). The median duration of SARS-CoV-2 positivity was 38.5 days (range 15–215, n=4) in patients with an early (≤90 days from CAR T) infection and 30 days (range 8–94, n=14) in those with a late (>90 days from CAR T) infection (online supplemental figure S2C). Formal comparisons were not performed for SARS-CoV-2 positivity due to missing values.
Table 2.
SARS-CoV-2 infection characteristics and supportive care requirements
| Asymptomatic infection (n=13) |
Mild COVID-19 (n=37) |
Moderate COVID-19 (n=11) |
Severe COVID-19 (n=6)+MIS-C (n=3) | Total | P value | |
| Days following most recent CAR T-cell infusion, median (range) | 90 (21–780) | 732 (21–3180) | 630 (450–2130) | 210.0 (20.0–1230) | 435 (20–3180) | 0.0010 |
| ≤90 days post-CAR T | 7/14 (50.0%) | 5/14 (35.7%) | 0/14 (0.0%) | 2/14 (14.3%) | 14/70 (20.0%) | 0.0040 |
| >90 days post-CAR T | 6/56 (10.7%) | 32/56 (57.1%) | 11/56 (19.6%) | 7/56 (12.5%) | 56/70 (80.0%) | 0.0045 |
| Duration of SARS-CoV-2 Positivity, median (range) (n) | 18.0 (15–21) (2) | 29.5 (8–56) (6) | 30.0 (10–44) (5) | 56.0 (14–215) (5) | 30.0 (8–215) (18) | 0.3786 |
| Time to first negative test, median (range) (n) | 28.0 (0–35) (5) | 75.0 (15–147) (8) | 41.0 (1–52) (5) | 34.0 (20–123) (6) | 32.5 (0–147.0) (24) | 0.3568 |
| SARS-CoV-2 antibody test positivity (%) | 1/5 (20.0) | 3/5 (60.0) | 0/5 (0) | 1/5 (20) | 5/17 (29.4) | 0.2451 |
| Coinfection present (%) | 0/9 (0) | 0/9 (0) | 4/9 (44.4) | 5/9 (55.6) | 9/69 (13.0) | <0.0001 |
| Coinfection bacterial | 3/6 (50.0) | 3/6 (50.0) | 6/9 (66.7) | |||
| Coinfection viral | 0 | 0 | 0 | |||
| Coinfection fungal | 1/1 (100.0) | 0/1 (0.0) | 1/9 (11.1) | |||
| Multiple types of coinfection | 0/2 (0.0) | 2/2 (100.0) | 2/9 (22.2) | |||
| Required hospital admission (%) | 0/20 (0) | 0/20 (0) | 11/20 (55) | 9/20 (45) | 20/70 (28.6) | <0.0001 |
| Reason for admission | 0.0922 | |||||
| Fever | 5/10 (50%) | 5/10 (50%) | 10/20 (20%) | |||
| Respiratory distress | 6/7 (85.7%) | 1/7 (14.3%) | 7/20 (35%) | |||
| Shock | 0/2 (0%) | 2/2 (100%) | 2/20 (10%) | |||
| Other | 0/1 (0%) | 1/1 (100%) | 1/20 (5%) | |||
| Days from SARS-CoV-2+ test to admission, median (range) (n) | 1.0 (0–59) (10) | 11.0 (0–29) (8) | 4.0 (0–59) (18) | 0.9811 | ||
| Duration of hospitalization, median (range) (n) | 5.0 (1–22) (11) | 32 (11–95) (9) | 13.0 (1–95) (20) | 0.0002 | ||
| Required ICU admission | 1/8 (12.5%) | 7/8 (87.5%) | 8/70 (11.4%) | 0.0045 | ||
| Duration of ICU admission, median days (range) (n) | 1 (1–1) (1) | 9.0 (4–85) (7) | 7.0 (1–85) (8) | NA | ||
| Respiratory support required | ||||||
| None | 13/58 (22.4%) | 37/58 (63.8%) | 7/58 (12.1%) | 1/58 (1.7%) | 58/70 (82.9%) | |
| Nasal canula | 4/4 (100%) | 0/4 (0%) | 4/70 (5.7%) | |||
| High-flow nasal canula | 0/2 (0%) | 2/2 (100%) | 2/70 (2.9%) | |||
| Intubation and mechanical ventilation (conventional vent) | 0/2 (0%) | 2/2 (100%) | 2/70 (2.9%) | |||
| Intubation and mechanical ventilation (oscillator) | 0/1 (0%) | 1/1 (100%) | 1/70 (1.4%) | |||
| ECMO | 0/1 (0%) | 1/1 (100%) | 1/70 (1.4%) | |||
| Other | 0/2 (0%) | 2/2 (100%) | 2/70 (2.9%) | |||
| Hypotension/shock | 0/5 (0%) | 0/5 (0%) | 0/5 (0%) | 5/5 (100%) | 5/70 (7.1%) | <0.0001 |
| Required intravenous fluid boluses for hypotension | 0/4 (0%) | 0/4 (0%) | 0/4 (0%) | 4/4 (100%) | 4/70 (5.7%) | <0.0001 |
| Vasopressors required | 0/4 (0%) | 0/4 (0%) | 0/4 (0%) | 4/4 (100%) | 4/70 (5.7%) | <0.0001 |
| Experienced death with SAR-CoV-2 infection | 0/3 (0%) | 0/3 (0%) | 1/3 (33.3%) | 2/3 (66.7%) | 3/70 (4.3%) |
Characteristics of SARS-CoV-2 infections in children and young adult patients who have experienced infection with SARS-CoV-2 following CAR T-cell therapy. Groups are compared with Kruskal-Wallis, χ2 or Fisher’s exact tests with significance of p<0.05.
CAR, chimeric antigen receptor; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; MIS-C, multisystem inflammatory syndrome in children; NA, not available.
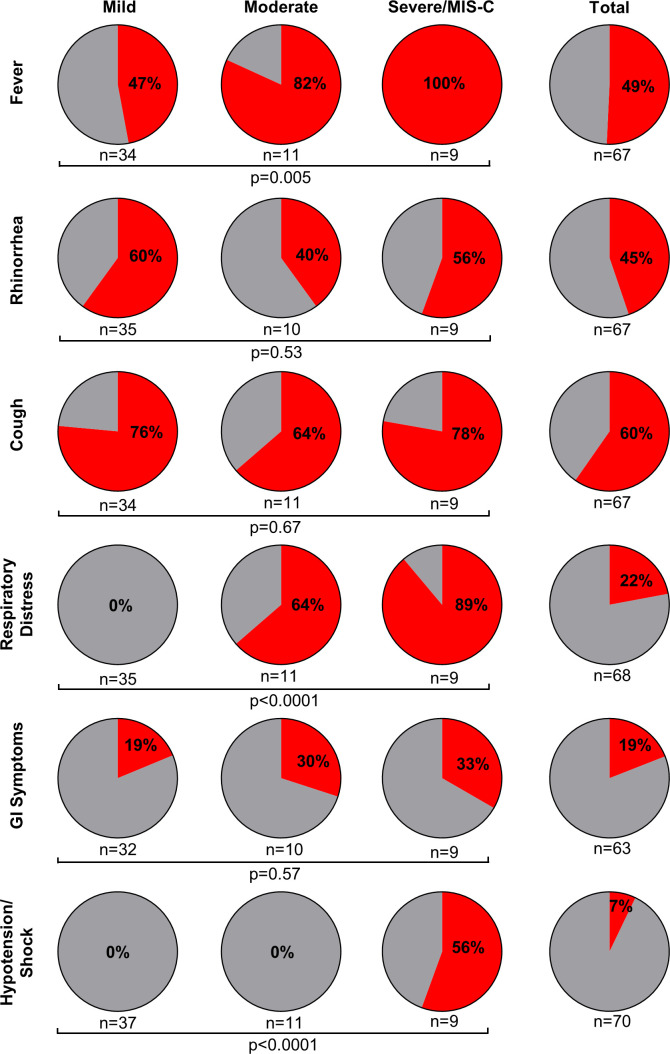
The most common symptoms of SARS-CoV-2 infection in our cohort were cough, fever, and rhinorrhea (figure 1). Gastrointestinal symptoms occurred at similar rates among the mild COVID-19, moderate COVID-19, and severe COVID-19/MIS-C groups, while fever and respiratory distress occurred more commonly in the moderate COVID-19 and severe COVID-19/MIS-C groups, as expected based on defining criteria.
Figure 1.
Symptoms of SARS-CoV-2 infections in CAR T-cell recipients. The proportion of children and young adults experiencing listed symptoms from SARS-CoV-2 infections following CAR T-cell infusions is shown in groups with mild COVID-19 (mild), moderate COVID-19 (moderate), severe COVID-19, and MIS-C (severe/MIS-C). The group with asymptomatic infections is not shown but is included in the total column. Total patients reported is 70 for symptoms of fever, rhinorrhea, cough, respiratory distress, and GI symptoms due to missing data. Comparisons between mild, moderate, and severe/MIS-C groups are via χ2 test with significance of p<0.05. CAR, chimeric antigen receptor; GI, gastrointestinal; MIS-C, multisystem inflammatory syndrome in children.
Laboratory and imaging findings
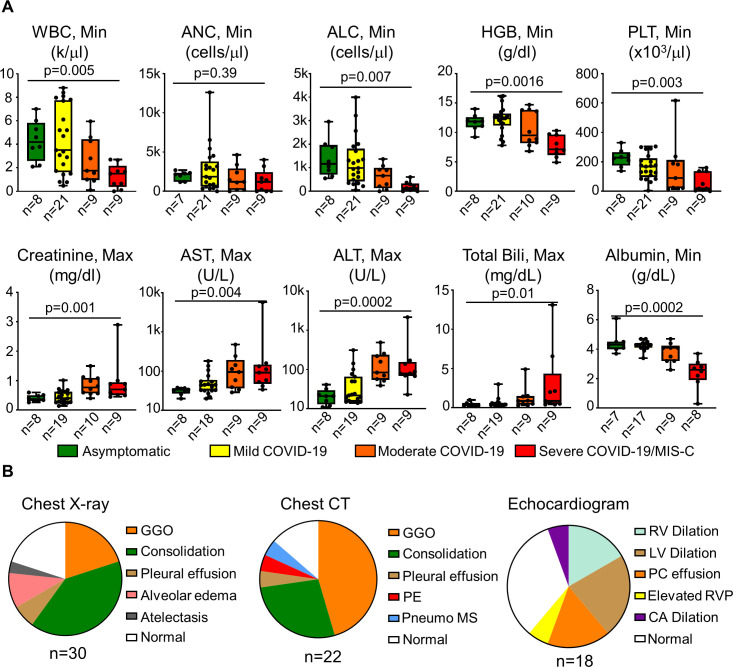
There were significant differences in minimum white blood cell counts, absolute lymphocyte counts (ALCs), hemoglobin, and platelet counts between asymptomatic, mild, moderate, and severe/MIS-C groups, while there were no significant differences in absolute neutrophil counts (figure 2). Maximum creatinine, aspartate aminotransferase (AST), alanine transaminase (ALT), total bilirubin, and minimum albumin values were also significantly different (figure 2A). Coagulation parameters and inflammatory markers were assessed in a smaller subset of patients: prothombin time, partial thromboplastin time, D-dimer, C reactive protein, ferritin, and lactate dehydrogenase were elevated in the severe COVID-19/MIS-C group (online supplemental figure S3); however, statistical comparisons between groups were not possible for coagulation parameters and inflammatory markers due to missing values in asymptomatic and mild COVID-19 groups.
Figure 2.
Laboratory and imaging findings in CAR T-cell recipients with COVID-19. (A) Laboratory findings in asymptomatic SARS-CoV-2 infections, mild COVID-19, moderate COVID-19, and severe COVID-19/MIS-C groups. Min or max laboratory values over the course of illness are reported. Bars show the median and 25th–75th IQR. Comparisons between groups are with Kruskal-Wallis test with significance of p<0.05. (B) Distribution of abnormal imaging findings on chest X-ray, chest CT, and echocardiogram is demonstrated. ALT, alanine transaminase; ANC, absolute neutrophil count; AST, aspartate aminotransferase; bili, bilirubin; CA, coronary artery; GGO, ground-glass opacity; Hgb, hemoglobin; LV, left ventricular; max, maximum; min, minimum; MIS-C, multisystem inflammatory syndrome in children; PE, pulmonary embolism; PLT, platelet count; pneumo MS, pneumomediastinum; n, number of values; RV, right ventricular; RVP, right ventricular pressure; WBC, white blood cell.
We assessed imaging findings obtained throughout infectious course. When obtained, chest X-ray or chest CT did not reveal abnormal findings in patients with mild COVID-19. However, in patients with moderate and severe COVID-19 or MIS-C, imaging findings included ground-glass opacities, consolidations, pleural effusions, alveolar edema, and atelectasis, pulmonary embolism, and pneumomediastinum (figure 2B). Echocardiograms were more commonly obtained in patients with moderate COVID-19 (with two of five abnormal) and severe COVID-19/MIS-C (with seven of nine abnormal), while only one patient with mild COVID-19 had an echocardiogram performed and it was normal. Abnormal echocardiography findings included right ventricular dysfunction, left ventricular dysfunction, pericardial effusion, elevated right ventricular pressure, and coronary artery dilation (figure 2B).
Severity and outcomes
Hospital admissions occurred in 20 of 70 (28.6%) and ICU admissions occurred in 8 of 70 (11.4%) patients infected following CAR T-cell infusion. Admissions occurred a median of 4 days (range 0–59) following detection of SARS-CoV-2 infection. Of the 20 admissions, reasons for admission included fever in 10 (50%), respiratory distress in 7 (35%), shock in 2 (10%), or other in 1 (5%). Median duration of hospital admission was 13 days for the admitted cohort (range 1–95 days), and median length of ICU stay was 7 days in the ICU-admitted cohort (n=8) (table 2).
Most patients did not require supplemental oxygen; however, 2.9% required high-flow nasal cannula; 4.3% required intubation and mechanical ventilation; and one patient (1.4%) required extracorporeal membrane oxygenation (table 2). Five of seventy (7.1%) patients developed hypotension or shock. Four of these five patients required intravenous fluids and vasopressors (table 2).
In our cohort, 3 of the 70 (4.3%) patients died with SARS-CoV-2 infection. Importantly, all three patients had a co-occurring infection or life-threatening condition (one with multispecies bacteremia and Aspergillus pneumonia, one with progressive leukemia, bacteremia, and osteomyelitis, and one with sinusoidal obstruction syndrome occurring after SCT). Coinfections occurred in 9 of 69 (13.0%) of patients overall: of the nine coinfections, zero (0%) ccurred in those with asymptomatic or mild disease; four (44.4%) occurred in those with moderate COVID-19; and five (55.6%) occurred in those with severe COVID-19 or MIS-C (p<0.0001) (table 2). Of the nine coinfections, six were bacterial (66.7%); one was fungal (11.1%); and two were multiple types (22.2%) (table 2).
Differences in infection severity pre-Omicron and post-Omicron variant
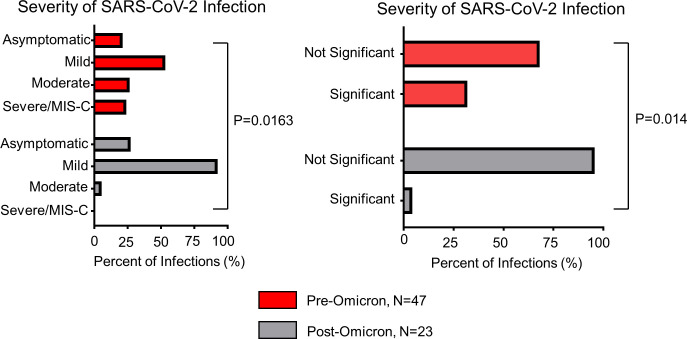
For infections occurring prior to the emergence of Omicron as the dominant SARS-CoV-2 variant, admission occurred in 19 of 47 (40.4%) patients, and ICU admissions occurred in 6 of 47 (12.7%). For infections following Omicron, hospital and ICU admission occurred in 1 of the 23 (4.3%) patients infected, for the development of a pulmonary embolism. The remaining 22 of 23 (95.7%) had asymptomatic (n=5) or mild-COVID-19 (n=17). The OR for admission following the emergence of the Omicron variant (with reference pre-Omicron) was 0.067 (95% CI 0.0062 to 0.4086, p=0.0016) (table 2). Infection severity categorization pre-Omicron and post-Omicron is depicted in figure 3.
Figure 3.
Severity of SARS-CoV-2 infection before and after the emergence of Omicron variant. The severity of SARS-CoV-2 infections by category (asymptomatic, mild COVID-19, moderate COVID-19, and severe COVID-19/MIS-C) and by significant (those with moderate COVID-19 requiring oxygen supplementation or admitted for respiratory distress, severe COVID-19, and MIS-C) versus not significant infections in the pre-Omicron (n=47) and post-Omicron (n=23) time periods. Groups are compared with χ2 and Fisher’s exact test with significance set as p=0.05. MIS-C, multisystem inflammatory syndrome in children.
Treatments
As the SARS-CoV-2 pandemic has evolved, significant research efforts have been made to identify effective COVID-19-directed treatments. Interventions can be grouped broadly into two categories: (1) medications that target the ‘detectable viral replication phase’ including direct antivirals (remdesivir or nirmatrelvir/ritonavir) and those that provide passive immunity (convalescent plasma and monoclonal antibodies (mAbs)) and (2) those that target the ‘inflammatory phase’, including steroids or other more targeted immune-modulating agents (tocilizumab and anakinra).28
Patients who had asymptomatic or mild disease were more likely to be observed clinically than to undergo any therapy. One of thirteen (7.7%) patients with asymptomatic disease and 8 of 37 (21.6%) patients with mild disease received one or more SARS-CoV-2-specific mAbs including indevimab+casirivimab, bamlanivimab, and sotrovimab (online supplemental table S1). mAb treatment for these patients likely reflects adoption of an early intervention strategy by treating physicians and the changing landscape of available treatments throughout the study. None of the patients with asymptomatic or mild COVID-19 received anti-inflammatory medications. Patients with moderate disease were more often treated with at least one medication directed at viral replication (6 of 11, 55%) and inflammatory (5 of 11, 45%) phases of infection. Eight out of nine patients with severe COVID-19 or MIS-C were treated with both categories of medications. Of the four patients who experienced SARS-CoV-2 positivity for greater than 50 days on sequential testing (range 56–215 days), all received interventions to provide passive immunity (convalescent plasma and/or mAb), and those who had severe COVID-19 also received remdesivir and/or steroids.
Factors associated with severity of disease
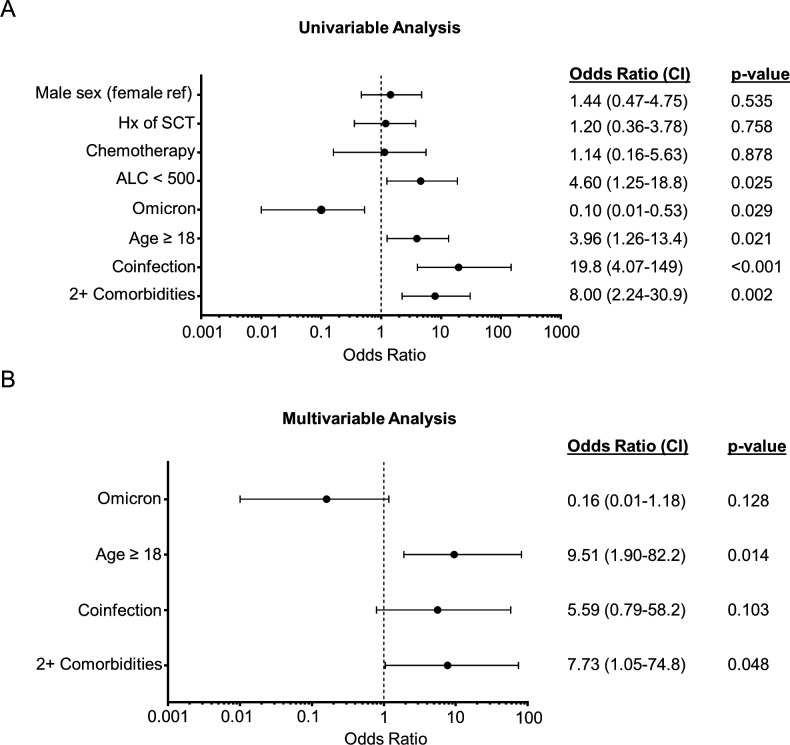
To identify patient and infection features associated with more severe disease, patients were classified as having either significant (n=16) SARS-CoV-2 infection (including groups with severe COVID-19, MIS-C, and moderate COVID-19 with oxygen supplementation or respiratory distress) or not (n=54) (figure 4).
Figure 4.
Univariable and multivariable regression analyses for significant SARS-CoV-2 infections. Significant SARS-CoV-2 infections were defined as infections resulting in admission for respiratory distress or requiring oxygen supplementation, those admitted to the ICU, or meeting criteria for MIS-C, while not significant infections were defined as infections not meeting these criteria. (A) Forest plot demonstrating variables evaluated for association with significant infection in univariable regression analysis. Factors evaluated included ALC under 500 cells/µL (ALC <500 cells/µL), receipt of chemotherapy at time of infection (chemotherapy), presence of coinfection, age ≥18, suspected infection with Omicron variant (Omicron), Hx of hematopoietic SCT, and sex. BCA was not included because all patients with significant infection had BCA so OR could not be reported. (B) Variables significant in the univariable analysis were included in a multivariable regression model. ORs with 95% CIs are shown. P values: two-tailed significance was set as p=0.05. ALC, absolute lymphocyte count; BCA, B-cell aplasia; Hx, history; MIS-C, multisystem inflammatory syndrome in children; SCT, stem cell transplant.
In a univariable logistic model, factors most strongly associated with significant SARS-CoV-2 infection included possessing at least two comorbidities (OR 8.00, 95% CI 2.24 to 30.9, p=0.002), presence of a coinfection (OR 19.8, 95% CI 4.07 to 149, p<0.001), age ≥18 years (OR 3.96, 95% CI 1.26 to 13.4, p=0.021), and minimum ALC of <500 cells/µL during infection (OR 4.60, 95% CI 1.25 to 18.8, p=0.025) (figure 4A). Of note, ALC values were available for 14 of 16 significant infections, but only 33 of 54 non-significant infections. Infection in the period when Omicron was the dominant SARS-CoV-2 variant was protective against significant infection (OR 0.10, 95% CI 0.01 to 0.53, p=0.029). Factors not associated with significant SARS-CoV-2 infection included receipt of chemotherapy at the time of infection, history of SCT, and sex (figure 4A). BCA was not evaluable in regression analysis because every patient in the significant infection group had BCA at the time of infection. Comorbidities were evaluated individually to identify high-risk features (online supplemental tables S2, S3). The two comorbidities that were more highly represented in the severe-COVID-19/MIS-C group were trisomy 21 and having cancer not in remission at the time of SARS-CoV-2 infection (online supplemental table S2), but no single comorbidity was significantly associated with significant disease, although there was a trend toward significance with trisomy 21 (online supplemental table S3).
Variables significant in the univariable analysis mentioned earlier were included in a multivariable regression model. ALC of <500 cells/µL was omitted due to missing data in the group without significant infections. In this multivariable model, factors significantly associated with severe infection included ≥2 comorbidities (OR 7.73, 95% CI 1.05 to 74.8, p=0.048) and age ≥18 years (OR 9.51, 95% CI 1.90 to 82.2, p=0.014). Point estimates of OR for infection during the Omicron-dominant period suggested a protective effect, while the presence of coinfection suggested an association with significant infections, but these variables were no longer statistically significant in the multivariable model (figure 4B).
Changes to cancer-directed therapy
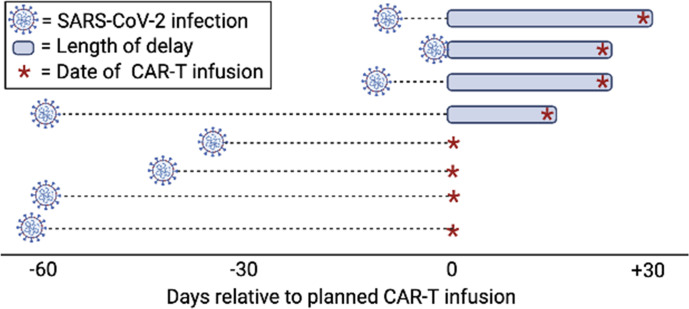
Multiple studies have demonstrated delays or modifications in cancer-directed care for pediatric oncology patients infected with SARS-CoV-2.11 29 30 In patients with r/r B-ALL where disease burden prior to CAR T-cell therapy has been shown to impact outcomes, it is ideal to proceed to CAR T-cell therapy without substantial delays to avoid disease progression.31–33 In this registry, eight patients were infected with SARS-CoV-2 within 2 months prior to a planned CAR-T infusion, six with r/r B-ALL, and two with r/r B-NHL. SARS-CoV-2 infections occurred a median of 39 days (range 1–60) prior to infection. Infections delayed CAR T-cell infusions in four patients (two patients with asymptomatic infections and two patients with moderate COVID-19) by 15–30 days (figure 5). Of note, all patients who had a positive test for SARS-CoV-2 within 10 days of planned infusion (three of three) had delays in CAR-T infusions between 24 days and 30 days, whereas four of five patients who had a positive test more than 30 days prior to planned CAR-T cell infusion did not have any delays. Three of eight (37.5%) patients were in remission at the time of infection, while the remaining five (62.5%) had active B-ALL or B-NHL. Of the eight patients, five (62.5%) were classified as having asymptomatic SARS-CoV-2 infections, and three (37.5%) experienced moderate COVID-19 requiring hospitalization. In this group, the number of days of documented SARS-CoV-2 positivity was 21.5 days (range 3–60 days, n=8). None of the patients with asymptomatic infections received SARS-CoV-2-directed therapies, including one patient who had a positive test 8 days prior to planned CAR T-cell infusion. All of the patients with moderate disease received remdesivir and/or steroids.
Figure 5.
SARS-CoV-2 infections prior to anticipated CAR T-cell therapy. The timing of SARS-CoV-2 infection prior to planned CAR T-cell infusion and the delay in administration of planned CAR T-cell product is shown for eight patients, with each row representing one patient. CAR, chimeric antigen receptor.
Discussion
We present an analysis of SARS-CoV-2 infections in a large cohort of children and young adult CAR T-cell recipients using a retrospective international registry. Our data provide critical information on the severity of SARS-CoV-2 infections in children and young adults undergoing CAR T-cell therapy before and after the emergence of the Omicron variant, summarize common laboratory and imaging findings in this uniquely immunocompromised population, and identify factors associated with infection severity.
Hospital admission was common in our cohort, with approximately 30% requiring admission and 10% requiring intensive care. However, children and young adult CAR T-cell recipients experienced decreased morbidity and mortality compared with an analogous adult cohort. In contrast to the 33%–40% COVID-19-related mortality rate reported in adults by the European Society for Blood and Marrow Transplantation Infectious Diseases Working Party and the European Hematology Association Lymphoma groups, the mortality rate reported in our cohort (median age 16.0 years) was 4.3% (3 of 70).24 25 34 Additionally, deaths in the present study occurred exclusively in patients with significant coinfections or concomitant medical conditions, highlighting the influence of age on SARS-CoV-2 risk. Outcomes in our patient population mirrored outcomes for pediatric oncology patients in general, suggesting that patients who underwent CAR T-cell therapy are at higher risk of severe disease than the general pediatric population but not clearly higher than the oncology population at large.15 BCA was expectedly common in our cohort, making contribution to severity of infection difficult to determine. Interestingly, BCA was not associated with longer carriage of the SARS-CoV-2 virus compared with patients who had experienced BCR, although it is important to note a small number of patients had BCR at the time of infection and SARS-CoV-2 testing was not obtained at standardized intervals in this study. Interestingly, SARS-CoV-2 infections occurring within 90 days of CAR T-cell infusion, a period when CAR T-cell recipients are frequently recovering from cytopenias and T-cell lymphopenia in combination with BCA, were most commonly asymptomatic.10 Likely contributing to this finding was the greater frequency of bone marrow aspiration/biopsy and lumbar puncture procedures in the first 3 months following CAR T-cell infusions, and the associated preprocedural SARS-CoV-2 testing required at many institutions. Those with severe COVID-19 or MIS-C frequently had leukopenia, lymphopenia, anemia, and thrombocytopenia, along with elevations in serum AST, ALT, and bilirubin, demonstrating the occurrence of bone marrow suppression and hepatic toxicity in these groups. Chest imaging findings were expectedly common in those with moderate to severe COVID-19 or MIS-C, and echocardiogram abnormalities were present in seven of nine patients with severe COVID-19 or MIS-C, demonstrating the common occurrence of cardiac changes in severely-affected patients.
In a univariable analysis for factors associated with significant SARS-CoV-2 infections, the most critical factors included having two or more comorbidities, a coinfection, age ≥18 years, and minimum ALC of <500 cells/µL, while infection occurring after Omicron became the dominant SARS-CoV-2 variant was protective against significant disease. Importantly, vaccination status was available in only 8 of 23 patients infected with suspected Omicron variant, with five having undergone SARS-CoV-2 vaccination and three not having undergone SARS-CoV-2 vaccination. A potentially greater proportion of CAR T-cell recipients being vaccinated against SARS-CoV-2 following the emergence of the Omicron variant is therefore an important unmeasured confounding variable. Additionally, limited availability of SARS-CoV-2 PCR testing earlier in the pandemic could have contributed to a higher proportion of moderate and severe infections identified prior to emergence of the Omicron variant. In the multivariate model, having two or more comorbidities and age ≥18 years were the primary determinants of disease severity, similar to risk factors identified in a pediatric oncology cohort and in pediatric patients in general.15 35 36 Although the numbers were small, it appeared that trisomy 21 and cancer not in remission were comorbidities more highly represented in the severe COVID-19/MIS-C group. While this finding certainly warrants validation, given the limited sample size, it is in line with previous data and suggests these may be higher-risk comorbidities for severe infections.
BCA was common in this cohort, both in mild and significant forms of infection, making its contribution to the more severe infections difficult to determine. However, for the patients who had serial positive SARS-CoV-2 tests with BCA, we demonstrate a median duration of SARS-CoV-2 positivity of 30 days, reinforcing prior findings that SARS-CoV-2 carriage is prolonged in SCT and CAR T-cell recipients.25 Prior reports demonstrate that those with BCA are less likely to develop seroconversion and are therefore more likely to rely on cellular responses to clear SARS-CoV-2 infections.22 23 In adult CAR T-cell recipients, only 11% of those with BCA at the time of BNT162b2 mRNA COVID-19 vaccination had serological conversion after two doses of vaccine, compared with 66% who had BCR following CAR T.22 However, cellular responses to SARS-CoV-2 vaccination with the BNT162b2 or mRNA-1273 vaccines have been demonstrated in 50%–67% of CD19 or CD19/22-directed CAR T-cell recipients, illustrating the utility of vaccination in this population, even in patients with BCA.22 23 Additionally, passive immunity measures such as mAbs for prophylaxis, such as tixagevimab and cilgavimab, likely carry more importance in those without expected humoral responses to vaccination, although recommendations regarding their use evolve based on their neutralizing activity against emerging variants of concern. Finally, intravenous immune globulin (IVIG) products likely provide a degree of passive immunity as neutralizing antibodies against SARS-CoV-2 viruses have been documented in pooled IVIG products up to September 2021.37 The prevalence of BCA and high rate of hospital admission in this cohort highlights the increased risk of SARS-CoV-2 infection and the importance of vaccination, passive immunity measures, and early antiviral treatments such as ritonavir-boosted nirmatrelvir to prevent severe infections in this group, when available.
SARS-CoV-2 infections occurring after Omicron became the dominant variant were most often categorized as asymptomatic and mild COVID-19 in our cohort. Additionally, the admission rate for SARS-CoV-2 infections was nearly 10 times higher prior to Omicron compared with after (40.4% vs 4.3%), suggesting Omicron is associated with less severe disease, consistent with observations in immunocompetent populations.17–20 While genetic characterization of patient samples by sequencing was not done in this study, it is highly likely that cases in the USA after 11 December 2021 were due to Omicron, given dominance of the Omicron variant after that date.27 As SARS-CoV-2 continues to evolve and new variants emerge, it is critical to understand their pathogenicity not only in the general population but also in immunocompromised patients.
Limitations of this study include the potential for recall bias with retrospective data collection. Many of the patients in the asymptomatic and mild COVID-19 groups had missing laboratory data as laboratory studies were less often obtained for clinical decision-making in these groups. The duration of SARS-CoV-2 positivity was determined based on tests performed as part of routine care and therefore was not obtained at regular intervals. There were several potential confounders for our assessment of Omicron severity. The proportion of patients who had undergone vaccination against SARS-CoV-2 was not tracked as part of this study and is certain to have increased as vaccines became available. Additionally, treatment options evolved over the course of the SARS-CoV-2 pandemic. Although remdesivir was available early in the pandemic (FDA approved on 10 October 2020), dexamethasone was shown to be effective in patients hospitalized with COVID-19 (RECOVERY trial38 published online on 17 July 2020), and bamlanivimab and casirivimab+imdevimab received emergency use authorization (EUA) for mild to moderate COVID-19 (November 2020), the increased availability of mAbs and increased provider familiarity of SARS-CoV-2 management following the emergence of the Omicron variant are other potential confounders. FDA EUA of nirmatrelvir and ritonavir (22 December 2021) is another potential confounder, although no patients included in the registry were reported to have received nirmatrelvir+ritonavir at the time of study entry. Strengths of this study included the large number of patients who have undergone CAR T-cell therapy in BCA at the time of infection, allowing for an evaluation of factors associated with significant infection in this uniquely immunocompromised pediatric and young adult population.
Conclusions
As SARS-CoV-2 persists in the population, the data presented here provide a framework for understanding the risks of SARS-CoV-2 infections for young patients undergoing CAR T-cell therapies for hematological malignancies. Children and young adults undergoing CAR T-cell therapy who are 18 years or older or who have two or more comorbidities are at higher risk of significant SARS-CoV-2 infections and represent a population for which preventative measures such as vaccination and/or passive immunity, as well as early treatment with antiviral therapy, should be considered.
jitc-2022-005957supp001.pdf (458.5KB, pdf)
Footnotes
Twitter: @KevinMcNerneyMD
Contributors: KOM, RMR, KLD and SLM conceived of and created the registry for data collection, performed the data analysis, and wrote and edited the manuscript. KOM, RMR, PA-H, FGC, J-AT, AM, AB, JK, HD, AV and CC performed the chart review, data extraction and manuscript editing. YL and HL performed the statistical analysis and manuscript review and editing. KOM will serve as the guarantor of this work. KLD and SLM conceived of the project, guided the project, performed the data analysis and edited the manuscript.
Funding: KOM is supported by contributions from the V Foundation.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein) or of any geographical or locational reference does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: AB: Novartis, Amgen and Medac, speaker’s bureau; Medac, Neovii and Novartis meeting support. SLM: Novartis, advisory boards, study steering committees, clinical trial support; Wugen: advisory board.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Requests for data from the present study should be addressed to the corresponding author at kmcnern1@jhmi.edu. All proposals requesting data access will need to specify how the data will be used, and all proposals will need the approval of the study investigator team before data release.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was reviewed by the Children’s Hospital of Philadelphia's Committee for the Protection of Human Subjects' institutional review board (IRB) which determined the registry protocol meets the exemption criteria per 45 CFR 46.104(d) 4(ii). Nine participating institutions obtained IRB approval or waiver.
References
- 1.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018;378:439–48. 10.1056/NEJMoa1709866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med 2018;24:20–8. 10.1038/nm.4441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 2017;129:3322–31. 10.1182/blood-2017-02-769208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah NN, Highfill SL, Shalabi H, et al. CD4/CD8 T-cell selection affects chimeric antigen receptor (CAR) T-cell potency and toxicity: updated results from a phase I anti-CD22 CAR T-cell trial. J Clin Oncol 2020;38:1938–50. 10.1200/JCO.19.03279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 2019;380:45–56. 10.1056/NEJMoa1804980 [DOI] [PubMed] [Google Scholar]
- 6.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017;377:2531–44. 10.1056/NEJMoa1707447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. The Lancet 2020;396:839–52. 10.1016/S0140-6736(20)31366-0 [DOI] [PubMed] [Google Scholar]
- 8.Vora SB, Waghmare A, Englund JA, et al. Infectious complications following CD19 chimeric antigen receptor T-cell therapy for children, adolescents, and young adults. Open Forum Infect Dis 2020;7:ofaa121. 10.1093/ofid/ofaa121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507–17. 10.1056/NEJMoa1407222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strati P, Varma A, Adkins S, et al. Hematopoietic recovery and immune reconstitution after axicabtagene ciloleucel in patients with large B-cell lymphoma. Haematologica 2021;106:2667–72. 10.3324/haematol.2020.254045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CDC . COVID data Tracker, 2020. Available: https://covid.cdc.gov/covid-data-tracker [Accessed 05 Jun 2022].
- 12.Castagnoli R, Votto M, Licari A, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr 2020;174:882. 10.1001/jamapediatrics.2020.1467 [DOI] [PubMed] [Google Scholar]
- 13.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 2020;395:1771–8. 10.1016/S0140-6736(20)31103-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 2020;383:334–46. 10.1056/NEJMoa2021680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston EE, Martinez I, Davis ES, et al. SARS-CoV-2 in childhood cancer in 2020: a disease of disparities. J Clin Oncol 2021;39:3778–88. 10.1200/JCO.21.00702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CDC . Healthcare workers, 2020. Available: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html [Accessed 26 May 2022].
- 17.Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet 2022;399:437–46. 10.1016/S0140-6736(22)00017-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies M-A, Kassanjee R, Rousseau P, et al. Outcomes of laboratory-confirmed SARS-CoV-2 infection in the Omicron-driven fourth wave compared with previous waves in the Western Cape Province, South Africa. Trop Med Int Health 2022;27:564–73. 10.1111/tmi.13752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ulloa AC, Buchan SA, Daneman N, et al. Estimates of SARS-CoV-2 omicron variant severity in Ontario, Canada. JAMA 2022;327:1286. 10.1001/jama.2022.2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veneti L, Bøås H, Bråthen Kristoffersen A, et al. Reduced risk of hospitalisation among reported COVID-19 cases infected with the SARS-CoV-2 omicron BA.1 variant compared with the delta variant, Norway, December 2021 to January 2022. Eurosurveillance 2022;27:27. 10.2807/1560-7917.ES.2022.27.4.2200077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iuliano AD, Brunkard JM, Boehmer TK, et al. Trends in disease severity and health care utilization during the early omicron variant period compared with previous SARS-CoV-2 high transmission periods - United States, December 2020-January 2022. MMWR Morb Mortal Wkly Rep 2022;71:146–52. 10.15585/mmwr.mm7104e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ram R, Hagin D, Kikozashvilli N, et al. Safety and immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in patients after allogeneic HCT or CD19-based CART therapy-A single-center prospective cohort study. Transplant Cell Ther 2021;27:788–94. 10.1016/j.jtct.2021.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parvathaneni K, Toress-Rodriguez K, Meng W, et al. Adoptive immune responses to Sars-Cov2 vaccination in CART19 treated patients. Blood 2021;138:1757. 10.1182/blood-2021-15373034041523 [DOI] [Google Scholar]
- 24.Busca A, Salmanton-García J, Corradini P, et al. COVID-19 and CAR T cells: a report on current challenges and future directions from the EPICOVIDEHA survey by EHA-IDWP. Blood Adv 2022;6:2427–33. 10.1182/bloodadvances.2021005616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah GL, DeWolf S, Lee YJ, et al. Favorable outcomes of COVID-19 in recipients of hematopoietic cell transplantation. J Clin Invest 2020;130:6656–67. 10.1172/JCI141777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CDC . Multisystem inflammatory syndrome (MIS), 2020. Available: https://www.cdc.gov/mis/mis-c/hcp/index.html [Accessed 05 Jun 2022].
- 27.CDC . Omicron variant: what you need to know, 2022. Available: https://www.cdc.gov/coronavirus/2019-ncov/variants/omicron-variant.html [Accessed 05 Jun 2022].
- 28.Griffin DO, Brennan-Rieder D, Ngo B, et al. The importance of understanding the stages of COVID-19 in treatment and trials. AIDS Rev 2021;23:5803. 10.24875/AIDSRev.200001261 [DOI] [PubMed] [Google Scholar]
- 29.Madhusoodhan PP, Pierro J, Musante J, et al. Characterization of COVID‐19 disease in pediatric oncology patients: the new York‐New Jersey regional experience. Pediatr Blood Cancer 2021;68. 10.1002/pbc.28843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howden K, Glidden C, Romanescu RG, et al. A cross-sectional survey exploring the impact of the COVID-19 pandemic on the cancer care of adolescents and young adults. Curr Oncol 2021;28:3201–13. 10.3390/curroncol28040278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ravich JW, Huang S, Zhou Y, et al. Impact of high disease burden on survival in pediatric patients with B-ALL treated with Tisagenlecleucel. Transplant Cell Ther 2022;28:73.e1–73.e9. 10.1016/j.jtct.2021.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schultz LM, Baggott C, Prabhu S, et al. Disease burden affects outcomes in pediatric and young adult B-cell lymphoblastic leukemia after commercial Tisagenlecleucel: a pediatric real-world chimeric antigen receptor Consortium report. J Clin Oncol 2022;40:945–55. 10.1200/JCO.20.03585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myers RM, Taraseviciute A, Steinberg SM, et al. Blinatumomab nonresponse and High-Disease burden are associated with inferior outcomes after CD19-CAR for B-ALL. J Clin Oncol 2022;40:932–44. 10.1200/JCO.21.01405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spanjaart AM, Ljungman P, de La Camara R, et al. Poor outcome of patients with COVID-19 after car T-cell therapy for B-cell malignancies: results of a multicenter study on behalf of the European Society for blood and marrow transplantation (EBMT) infectious diseases Working Party and the European hematology association (EHA) lymphoma group. Leukemia 2021;35:3585–8. 10.1038/s41375-021-01466-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhalala US, Gist KM, Tripathi S, et al. Characterization and outcomes of hospitalized children with coronavirus disease 2019: a report from a multicenter, viral infection and respiratory illness universal study (coronavirus disease 2019) registry. Crit Care Med 2022;50:e40–51. 10.1097/CCM.0000000000005232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tripathi S, Sayed IA, Dapul H, et al. Risk factors for critical coronavirus disease 2019 and mortality in hospitalized young adults: an analysis of the Society of critical care medicine discovery viral infection and respiratory illness universal study (virus) coronavirus disease 2019 registry. Crit Care Explor 2021;3:e0514. 10.1097/CCE.0000000000000514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romero C, Díez J-M, Gajardo R. Anti-SARS-CoV-2 antibodies in healthy donor plasma pools and IVIG products-an update. Lancet Infect Dis 2022;22:19. 10.1016/S1473-3099(21)00755-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021;384:693–704. 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2022-005957supp002.pdf (1.3MB, pdf)
jitc-2022-005957supp001.pdf (458.5KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Requests for data from the present study should be addressed to the corresponding author at kmcnern1@jhmi.edu. All proposals requesting data access will need to specify how the data will be used, and all proposals will need the approval of the study investigator team before data release.