Abstract
Background
Although hemoglobin A1c (HbA1c) is closely related to diabetes, its relationship with the incidence of hypertension is still unknown, so we aimed to evaluate the relationship between HbA1c and the incidence of hypertension in the general population.
Method
In this large prospective cohort study with a median follow-up of 2 years, we included 4,074 participants from the China Health and Nutrition Survey (CHNS). Multivariate COX regression, subgroup analysis, receiver operator characteristic (ROC) curve and restricted cubic spline (RCS) were used to evaluate the relationship between HbA1c and incidental hypertension.
Results
Compared with participants without incident hypertension, participants with incident hypertension had higher levels of HbA1c (P < 0.05). In univariate COX regression analysis, HbA1c was associated with the risk of hypertension (HR: 1.161, 95% CI: 1.105-1.221, P < 0.001). In multivariate COX regression analysis adjusted for confounding variables, HbA1c was still closely related to the risk of hypertension (HR: 1.102, 95% CI: 1.006-1.206, P = 0.037). And subgroup analysis showed that the relationship between HbA1c and hypertension remained significant in female, lower than high school and non-obese subgroups (P < 0.05). ROC curve also showed that HbA1c could predict the risk of hypertension (AUC = 0.583, 95% CI: 0.568-0.598, P < 0.001). Further RCS analysis showed that HbA1c was positively correlated with the risk of hypertension (P for nonlinearity = 0.642).
Conclusion
HbA1c was linearly and positively associated with the incidence of hypertension.
Keywords: glycated hemoglobin, hemoglobin A1c, hypertension, prevalence, incidence
1. Introduction
Currently, arterial hypertension (hereinafter referred to as hypertension) is a very common disease and one of the most important risk factors for cardiovascular disease and premature death (1). In 2 years, it is expected that more than 1.5 billion people will suffer from hypertension, which undoubtedly indicates that hypertension has gradually become a major global public health problem (2). The reasons for the dramatic increase in hypertension stem not only from an aging population, unhealthy lifestyles and unhealthy diets, but also from other metabolic problems, such as fluctuating blood glucose or diabetes (3).
Similar to hypertension, at present, diabetes is also a major global public health problem. In 2019, nearly 463 million people developed diabetes (9.3%), and this percentage is expected to rise by 0.9% and 1.6% by 2030 and 2045, respectively (4). As two major risk factors for cardiovascular disease and mortality, hypertension and diabetes tend to coexist in the same metabolically dysregulated individual and they share some common abnormal metabolic pathways, such as obesity, insulin resistance, inflammation and oxidative stress (5–7). Current evidence has shown that diabetes is closely related to hypertension (8, 9), while it is uncertain whether blood glucose fluctuations are associated with hypertension. Hemoglobin A1c (HbA1c) is not only one of the most important tools for diagnosing diabetes superior to fasting blood glucose, but also an indicator of blood glucose fluctuations and the efficacy of glycemic control over the last 3 months (10, 11). Although the relationship between HbA1c and cardiovascular disease and mortality has been reported in many studies (12–14), the research on its relationship with the prevalence and incidence of hypertension is still few and not unified. As mentioned earlier, the harm of hypertension and diabetes is great, so it is necessary to control the incidence of hypertension and diabetes to reduce the socio-economic burden and public health. HbA1c is not only a diagnostic factor of diabetes, but also has been proved to be closely related to cardiovascular disease and mortality. Assuming that there is a causal relationship between HbA1c and the incidence of hypertension, then controlling the level of HbA1c not only reduces the incidence of hypertension but also reduces the burden of diabetes, which is not only a treatment of killing two birds with one stone, but also has far-reaching significance in reducing the economic burden, the incidence of metabolic-related diseases and the reduction of premature death.
Therefore, in order to enrich this research area and provide more evidence for evidence-based medicine, this study aimed to explore the relationship between HbA1c and the incidence of hypertension in a general population in the Chinese community.
2. Subjects, materials and methods
2.1. Study population
This was a large prospective cohort study based on community populations, with all participants from the 2009 China Health and Nutrition Survey (CHNS 2009). After excluding individuals with baseline hypertension and those without HbA1c and follow-up data, a total of 4,074 individuals were enrolled in the study ( Figure 1 ). The CHNS was approved by the institutional review committees at the University of North Carolina at Chapel Hill and the National Institute of Nutrition and Food Safety, Chinese Center for Disease Control and Prevention. Every participant signed a written informed consent form when participating in the CHNS, and the study protocol was carried out in accordance with the Declaration of Helsinki.
Figure 1.
Flow chart of the study population. CHNS, China Health and Nutrition Survey; HbA1c, hemoglobin A1c.
2.2. Data collection and definitions
All the data included in this study were from CHNS 2009, including demographic data, complications data, drug treatment data, biomarker data and follow-up data, in which the educational level was divided into three groups: lower than high school, high school and higher than high school. Marital status was divided into two groups: married and non-married. Smoking status was divided into three groups: now, ever and never. Drinking status was divided into five groups: every day, 3-4 times/week, 1-2 times/week, ≤ 2 times/month and no drinking (15). Diabetes was defined as fasting blood glucose ≥ 7.0 mmol/L, HbA1c ≥ 6.5%, or using hypoglycemic drugs, or having a history of diabetes diagnosis (16). Incidental hypertension was defined as newly diagnosed hypertension when non-hypertensive individuals participating in CHNS 2009 re-participated in CHNS 2011 and 2015 by asking for medical history and blood pressure measurements, such as systolic blood pressure (SBP) and/or diastolic blood pressure (DBP) ≥ 140/90 mmHg. Anthropometric data, including body mass index (BMI), SBP, DBP, were measured by trained staff from CHNS in accordance with standard measurement procedures. Blood markers, including triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), apolipoprotein A1 (ApoA1), apolipoprotein B (ApoB), creatinine (CR), fasting plasma glucose (FPG), HbA1c and high-sensitivity C-reactive protein (Hs-CRP), were collected by trained CHNS staff and sent to a standard laboratory for determination according to standard operating procedures, of which HbA1c was determined by high performance liquid chromatography (model HLC-723 G7; Tosoh Corporation, Tokyo, Japan), and the levels of FPG, blood lipids and Hs-CRP were measured by GODPAP method (Randox Laboratories Ltd., UK), glycerol-phosphate oxidase method and the PEG-modified enzyme method (Kyowa Medex Co., Ltd, Tokyo, Japan), and immunoturbidimetric method (Hitachi 7600 automated analyzer, Hitachi Inc., Tokyo, Japan) respectively (17).
2.3. Statistical analysis
The continuous variables with normal or skewed distribution were expressed by mean ± standard deviation or median (first quartile, third quartile), respectively, and the differences between groups were tested by independent sample T test or Mann-Whitney U test. The classification variables were presented by frequency (percentage), and the differences between groups were compared by chi-square test and Fisher’s exact test. Univariate COX regression analysis was used to evaluate the relationship between each variable and the incidence of hypertension, and then the covariates with P < 0.05 and significant variables were selected to construct a multivariate COX proportional hazard regression model to evaluate the relationship between HbA1c and the incidence of hypertension. Subgroup analysis based on age, sex, educational level, diabetes, and obesity was used to evaluate the relationship between HbA1c and the incidence of hypertension in these subgroups and the potential interaction between HbA1c and these stratified variables. Receiver operator characteristic (ROC) curve was used to evaluate the ability of HbA1c to distinguish hypertension. Restricted cubic spline (RCS) was used to explore the potential nonlinear association between HbA1c and the risk of hypertension. Using SPSS 26.0, MedCalc 19.6.1 and R 3.6.3 for statistical analysis. A two-tailed P value < 0.05 was determined to be statistically significant.
3. Results
3.1. Baseline characteristics of study population
As shown in Table 1 , participants with incident hypertension had higher age, higher rates of education below high school, current smoking, daily alcohol consumption, diabetes, and hypoglycemic drugs use, and higher levels of BMI, SBP, DBP, TG, TC, LDL-C, ApoB, uric acid, FPG, Hs-CRP, and HbA1c compared with participants without incident hypertension (P < 0.05). However, there was no significant difference in marital status, HDL-C, ApoA1 and CR between the two groups (P > 0.05).
Table 1.
Baseline characteristics of participants stratified by the hypertension.
| Total population | Non-hypertension | Hypertension | P value | |
|---|---|---|---|---|
| Age, years | 48.25 ± 13.14 | 45.80 ± 12.82 | 53.13 ± 12.39 | < 0.001 |
| Sex, male, n (%) | 1868 (45.90%) | 1195 (44.10%) | 673 (49.40%) | 0.001 |
| Educational level, n (%) | < 0.001 | |||
| Lower than high school | 3139 (77.00%) | 2037 (75.10%) | 1102 (80.90%) | |
| High School | 499 (12.20%) | 350 (12.90%) | 149 (10.90%) | |
| Higher than high school | 431 (10.60%) | 321 (11.80%) | 110 (8.10%) | |
| Marital status, n (%) | 0.769 | |||
| Married | 3630 (89.10%) | 2411 (88.90%) | 1219 (89.50%) | |
| Non-married | 436 (10.70%) | 295 (10.90%) | 141 (10.40%) | |
| Smoking status, n (%) | 0.035 | |||
| Now | 1170 (28.70%) | 750 (27.70%) | 420 (30.80%) | |
| Ever | 98 (2.40%) | 58 (2.10%) | 40 (2.90%) | |
| Never | 2804 (68.80%) | 1902 (70.10%) | 902 (66.20%) | |
| Drinking status, n (%) | < 0.001 | |||
| Every day | 369 (9.10%) | 205 (7.60%) | 164 (12.00%) | |
| 3-4 times/week | 181 (4.40%) | 110 (4.10%) | 71 (5.20%) | |
| 1-2 times/week | 325 (8.00%) | 203 (7.50%) | 122 (9.00%) | |
| ≤ 2 times/month | 475 (11.70%) | 345 (12.70%) | 130 (9.50%) | |
| No drinking | 2724 (66.90%) | 1849 (68.20%) | 875 (64.20%) | |
| Diabetes, n (%) | 309 (7.60%) | 168 (6.20%) | 141 (10.40%) | < 0.001 |
| Hypoglycemic drugs, n (%) | 47 (1.20%) | 21 (0.80%) | 26 (1.90%) | 0.004 |
| BMI, kg/m2 | 22.98 ± 3.23 | 22.58 ± 3.09 | 23.78 ± 3.35 | < 0.001 |
| SBP, mmHg | 116.60 ± 11.23 | 114.45 ± 11.03 | 121.05 ± 10.30 | < 0.001 |
| DBP, mmHg | 76.01 ± 7.46 | 74.89 ± 7.51 | 78.35 ± 6.78 | < 0.001 |
| TG, mmol/L | 1.19 (0.81, 1.81) | 1.13 (0.78, 1.72) | 1.33 (0.91, 2.02) | < 0.001 |
| TC, mmol/L | 4.80 ± 0.96 | 4.73 ± 0.93 | 4.95 ± 1.01 | < 0.001 |
| LDL−C, mmol/L | 2.93 ± 0.91 | 2.87 ± 0.86 | 3.05 ± 1.01 | < 0.001 |
| HDL−C, mmol/L | 1.45 ± 0.46 | 1.45 ± 0.47 | 1.44 ± 0.44 | 0.442 |
| ApoA1, g/L | 1.15 ± 0.37 | 1.15 ± 0.35 | 1.17 ± 0.41 | 0.074 |
| ApoB, g/L | 0.89 ± 0.25 | 0.87 ± 0.24 | 0.93 ± 0.26 | < 0.001 |
| CR, umol/L | 86.20 ± 21.32 | 85.83 ± 23.52 | 86.94 ± 16.05 | 0.118 |
| Uric acid, umol/L | 299.12 ± 100.95 | 294.89 ± 103.64 | 307.54 ± 94.82 | < 0.001 |
| FPG, mmol/L | 5.28 ± 1.29 | 5.18 ± 1.15 | 5.46 ± 1.51 | < 0.001 |
| HbA1c, % | 5.54 ± 0.78 | 5.47 ± 0.68 | 5.68 ± 0.94 | < 0.001 |
| Hs-CRP, mg/L | 1.00 (0, 2.00) | 1.00 (0, 2.00) | 1.00 (1.00, 2.75) | < 0.001 |
| Follow-up time, years | 2.00 (2.00, 6.00) | 2.00 (2.00, 2.00) | 6.00 (2.00, 6.00) | < 0.001 |
Data were expressed as mean ± SD, median (interquartile range), or n (%). BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglycerides; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; CR, creatinine; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; Hs-CRP, high-sensitivity C-reactive protein.
3.2. Association of HbA1c with the incidence of hypertension
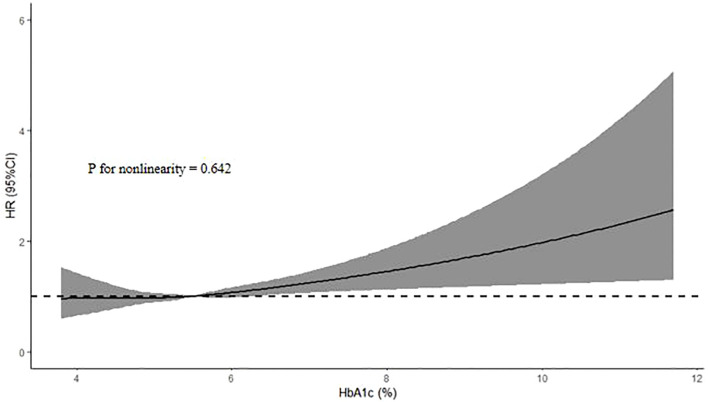
As shown in Table 2 , HbA1c was associated with the risk of hypertension in univariate COX regression analysis (HR: 1.161, 95% CI: 1.105-1.221, P < 0.001), and age, sex, educational level, smoking status, drinking status, diabetes, hypoglycemic drugs, BMI, SBP, DBP, TG, TC, LDL-C, ApoB, CR, uric acid, FPG and Hs-CRP were also associated with the risk of hypertension (P < 0.05). In multivariate COX regression analysis, higher HbA1c was still associated with higher risk of hypertension after adjusting for age, sex, educational level, marital status, smoking status, drinking status, diabetes, hypoglycemic drugs, BMI, SBP, DBP, TG, TC, LDL-C, ApoB, CR, uric acid, FPG and Hs-CRP (HR: 1.102, 95% CI: 1.006-1.206, P = 0.037). And in the subgroup analysis of Table 3 , the association was still significant in women, lower than high school and non-obese subgroups (HR: 1.158, 95% CI: 1.007-1.331, P = 0.039; HR: 1.127, 95% CI: 1.022-1.243, P = 0.017; HR: 1.073, 95% CI: 1.007-1.142, P = 0.029; respectively), whlie the relationship between HbA1c and new-onset hypertension no longer existed in the subgroups of < 60 years, ≥ 60 years, male, high school, higher than high school, diabetes, non-diabetes and obesity (P > 0.05). In addition, ROC analysis showed that HbA1c could predict the occurrence of hypertension (AUC = 0.583, 95% CI: 0.568-0.598, P < 0.001) ( Figure 2 ). Further RCS analysis showed that there was a positive linear correlation between HbA1c and the risk of hypertension (P for nonlinearity = 0.642) ( Figure 3 ). In addition, as shown in Table 4 , we conducted a sensitivity analysis showing that higher HbA1c was still associated with a higher risk of hypertension (P < 0.05).
Table 2.
Univariate and multivariate COX regression analysis of incident hypertension.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.025 (1.020, 1.029) | < 0.001 | 1.022 (1.017, 1.027) | < 0.001 |
| Male | 1.135 (1.021, 1.263) | 0.019 | 1.081 (0.914, 1.279) | 0.361 |
| Higher than high school | 0.737 (0.606, 0.897) | 0.002 | 0.809 (0.662, 0.989) | 0.038 |
| Married | 0.964 (0.810, 1.148) | 0.684 | 0.925 (0.774, 1.105) | 0.389 |
| Smoking status: Never | 0.888 (0.791, 0.997) | 0.045 | 0.941 (0.811, 1.091) | 0.420 |
| Drinking status: No drinking | 0.776 (0.657, 0.917) | 0.003 | 0.899 (0.743, 1.087) | 0.270 |
| Diabetes | 1.390 (1.168, 1.655) | < 0.001 | 0.889 (0.696, 1.135) | 0.346 |
| Hypoglycemic drugs | 1.712 (1.161, 2.523) | 0.007 | 1.151 (0.738, 1.796) | 0.534 |
| BMI | 1.048 (1.031, 1.066) | < 0.001 | 1.039 (1.020, 1.058) | < 0.001 |
| SBP | 1.032 (1.026, 1.038) | < 0.001 | 1.010 (1.004, 1.016) | 0.001 |
| DBP | 1.035 (1.026, 1.045) | < 0.001 | 1.009 (0.999, 1.018) | 0.064 |
| TG | 1.056 (1.022, 1.091) | 0.001 | 1.027 (0.972, 1.084) | 0.349 |
| TC | 1.115 (1.058, 1.176) | < 0.001 | 0.982 (0.874, 1.103) | 0.754 |
| LDL−C | 1.085 (1.030, 1.143) | 0.002 | 0.992 (0.877, 1.123) | 0.902 |
| HDL−C | 0.971 (0.864, 1.092) | 0.626 | ||
| ApoA1 | 1.077 (0.961, 1.208) | 0.203 | ||
| ApoB | 1.610 (1.312, 1.976) | < 0.001 | 1.133 (0.720, 1.783) | 0.588 |
| CR | 1.002 (1.000, 1.004) | 0.033 | 1.000 (0.996, 1.004) | 0.987 |
| Uric acid | 1.001 (1.000, 1.001) | 0.007 | 1.000 (0.999, 1.001) | 0.840 |
| FPG | 1.075 (1.041, 1.111) | < 0.001 | 0.984 (0.929, 1.043) | 0.590 |
| Hs-CRP | 1.010 (1.004, 1.016) | 0.002 | 1.005 (0.997, 1.012) | 0.208 |
| HbA1c | 1.161 (1.105, 1.221) | < 0.001 | 1.102 (1.006, 1.206) | 0.037 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglycerides; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; CR, creatinine; FPG, fasting plasma glucose; Hs-CRP, high-sensitivity C-reactive protein; HbA1c, hemoglobin A1c; HR, hazard ratio; CI, confidence interval.
Table 3.
Subgroups analyses for the association between HbA1c and the incidence of hypertension.
| HR (95% CI) | P value | P for interaction | |
|---|---|---|---|
| Age | 0.270 | ||
| < 60 years | 1.082 (0.961, 1.217) | 0.191 | |
| ≥ 60 years | 1.139 (0.979, 1.324) | 0.092 | |
| Sex | 0.672 | ||
| Male | 1.062 (0.938, 1.201) | 0.342 | |
| Female | 1.158 (1.007, 1.331) | 0.039 | |
| Educational level | 0.901 | ||
| Lower than high school | 1.127 (1.022, 1.243) | 0.017 | |
| High School | 0.981 (0.671, 1.434) | 0.921 | |
| Higher than high school | 0.922 (0.627, 1.355) | 0.678 | |
| Diabetes | 0.925 | ||
| Yes | 1.119 (0.972, 1.290) | 0.118 | |
| No | 1.096 (0.954, 1.259) | 0.197 | |
| Obesity | 0.423 | ||
| Yes | 1.307 (0.881, 1.938) | 0.183 | |
| No | 1.073 (1.007, 1.142) | 0.029 |
The multivariate adjusted model used in the subgroups analysis consisted of all covariates used in the multivariate adjusted models in Table 2 except for the variable (as a categorical variable) that was used for stratification. The HR was examined by per 1-unit increase of HbA1c. The interaction of HbA1c and variables used for stratification was examined by likelihood ratio tests. HbA1c, hemoglobin A1c; HR, hazard ratio; CI, confidence interval.
Figure 2.
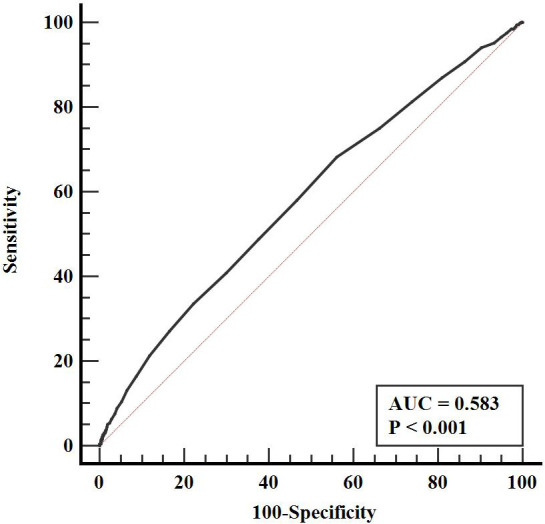
ROC curve evaluating diagnostic performance of HbA1c for incident hypertension. HbA1c, hemoglobin A1c; ROC, receiver operator characteristic; AUC, area under the curve.
Figure 3.
HR (95% CI) for the incidence of hypertension according to HbA1c. The association was adjusted for variables included in the multivariate adjusted models in Table 2 . HbA1c, hemoglobin A1c; HR, hazard ratio; CI, confidence interval.
Table 4.
Sensitivity analysis for the association between HbA1c and the incidence of hypertension.
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.022 (1.018, 1.027) | < 0.001 | 1.023 (1.019, 1.028) | < 0.001 |
| Male | 1.081 (0.929, 1.258) | 0.315 | 1.153 (1.036, 1.285) | 0.009 |
| Higher than high school | 0.804 (0.658, 0.983) | 0.033 | ||
| Married | 0.927 (0.776, 1.107) | 0.401 | ||
| Smoking status: Never | 0.944 (0.814, 1.095) | 0.446 | ||
| Drinking status: No drinking | 0.893 (0.738, 1.080) | 0.242 | ||
| Diabetes | 0.889 (0.696, 1.135) | 0.345 | 0.899 (0.708, 1.142) | 0.382 |
| Hypoglycemic drugs | 1.136 (0.729, 1.771) | 0.574 | ||
| BMI | 1.040 (1.022, 1.059) | < 0.001 | 1.040 (1.022, 1.058) | < 0.001 |
| SBP | 1.010 (1.004, 1.016) | 0.001 | 1.010 (1.004, 1.016) | 0.001 |
| DBP | 1.009 (0.999, 1.018) | 0.064 | 1.009 (1.000, 1.018) | 0.059 |
| TG | 1.022 (0.973, 1.073) | 0.389 | 1.023 (0.973, 1.074) | 0.376 |
| TC | 0.981 (0.873, 1.101) | 0.743 | 0.990 (0.881, 1.112) | 0.862 |
| LDL−C | 0.988 (0.872, 1.119) | 0.848 | 0.986 (0.867, 1.121) | 0.832 |
| HDL−C | ||||
| ApoA1 | ||||
| ApoB | 1.144 (0.729, 1.795) | 0.559 | 1.102 (0.702, 1.731) | 0.674 |
| CR | ||||
| Uric acid | ||||
| FPG | 0.987 (0.932, 1.045) | 0.652 | 0.988 (0.933, 1.046) | 0.669 |
| Hs-CRP | ||||
| HbA1c | 1.100 (1.006, 1.204) | 0.037 | 1.101 (1.007, 1.205) | 0.035 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglycerides; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; CR, creatinine; FPG, fasting plasma glucose; Hs-CRP, high-sensitivity C-reactive protein; HbA1c, hemoglobin A1c; HR, hazard ratio; CI, confidence interval.
4. Discussion
Although there is evidence that HbA1c is associated with hypertension, the relationship between them in people from CHNS is unknown. In this large prospective cohort study, we not only confirmed that HbA1c was closely related to the incidence of hypertension during follow-up, but also confirmed that this significant correlation still existed in women, lower than high school and non-obese subgroups, and further confirmed that there was a linear positive correlation between HbA1c and the risk of hypertension, which not only filled the knowledge gap of CHNS, but also stabilized the stability of the relationship between HbA1c and hypertension in Chinese population.
Although our study had made meaningful findings, there are still few studies on the relationship between HbA1c and hypertension and no unified conclusion has been reached. For example, Britton et al. found in a large prospective cohort study of 19,858 women in 2011 that higher HbA1c was closely associated with the risk of developing hypertension during an average follow-up period of 11.6 years, while this correlation could not be independent of BMI (18). A large longitudinal study from Japan also found no independent association between HbA1c and future new-onset hypertension (19). The evidence from a large medical center also only revealed the relationship between fasting blood glucose and the incidence of hypertension in prediabetes, and did not confirm the independent predictive effect of HbA1c on the incidence of hypertension (20). Similar to the above studies, Tatsumi et al. also only found an independent predictive effect of fasting blood glucose on new-onset hypertension in the cohort from Japan, and failed to confirm the independent correlation between HbA1c and new-onset hypertension (21). A Mendelian randomized study showed that in a univariate linear Mendelian random analysis, each 1 mmol/mol increase in HbA1c predicted by the gene increased the risk of hypertension by 2%, but this correlation no longer existed after adjusting for hemoglobin (22). Besides, another multicenter clinical study from China showed that the higher baseline HbA1c was not an independent risk factor for the incidence of hypertension in the multivariate adjusted model, while the absolute rate of change in HbA1c levels was independently associated with the risk of hypertension (23). However, Omar et al. confirmed a positive correlation between HbA1c levels and the risk of newly diagnosed hypertension in a small cross-sectional study (24). And in a study involving 9,603 middle-aged people, Julie et al. showed that higher HbA1c was not only independently associated with the prevalence of hypertension, but also with the incidence of hypertension (25). And a Mendelian randomized study using the UK Biobank data showed that higher HbA1c was not only closely associated with the risk of hypertension, but also positively correlated with SBP (26). Furthermore, Song et al. not only confirmed a strong correlation between HbA1c and the risk of hypertension in a Chinese population, but also unexpectedly found that it could also increase the risk of isolated systolic hypertension (27). Thus it can be seen that the relationship between HbA1c and the prevalence and incidence of hypertension has not reached a unified conclusion, and the causal relationship between HbA1c and hypertension has not been determined. What is encouraging is that our study found meaningful results that higher HbA1c was closely associated with a higher risk of hypertension, independent of traditional cardiovascular risk factors, including age, SBP, and BMI. In addition, The annual incidence of hypertension in this study was 10%, while the annual incidence of hypertension in the cohort study conducted by Lou et al. was 2.64% (28), and the probability in the study conducted by Heianza et al. was 2.29% (19). It can be seen that the incidence of hypertension in our study participants was higher than that in other studies, which was mainly related to the heterogeneity of the study population, and their study participants are mainly people without diabetes, which means that the cardiovascular metabolic risk of these people is relatively low, so the incidence of hypertension is relatively low.
In addition to hypertension, some studies have also shown that HbA1c is not only closely related to cardiovascular disease and poor cardiovascular outcomes (12–14, 22), but also inextricably related to all-cause mortality (14). These findings suggest that controlling HbA1c in the best range can not only reduce the incidence of diabetes, but also reduce the incidence of diabetic complications, cardiovascular disease morbidity, cardiovascular mortality and all-cause mortality, and further reduce the socio-economic burden and the health burden of the people, which is undoubtedly a great blessing for public health problems.
Additionally, not only the association between HbA1c and hypertension has not been agreed, but also the pathological mechanism of the harmful effects of higher HbA1c on hypertension is still unknown. There may be the following mechanisms involved in the pathogenic effect of HbA1c on hypertension. For example, higher HbA1c often reflects insulin resistance, and there is evidence that insulin resistance can promote the release of inflammatory factors, which in turn leads to endothelial dysfunction and the increase of sympathetic nerve tension, and may accelerate the reabsorption of sodium and water by renal tubules at the same time, eventually leading to the occurrence and development of hypertension (29–31). In addition, high HbA1c can reflect the state of continuously rising blood glucose, while persistently high glucose may induce the formation of advanced glycation end products, promote oxidative stress and activate protein kinase, thus damaging the stability and balance of endothelial cells and smooth muscle cells, leading to hypertension (32–35).
Despite our valuable results, there were several limitations that warrant discussion. First, although this was a prospective cohort study, it was still an observational study, so the causal link between HbA1c and hypertension was unknown in this population. Second, because the variable of physical activity was missing more in our study, we did not include this variable in our analysis and could not determine the extent of its effect on the association between HbA1c and hypertension. Third, due to the limitations of the data, we were unable to evaluate the effects of chronic kidney disease and hypothyroidism on the association between HbA1c with hypertension. Additionally, since HbA1c was measured only once at baseline, it was not possible to evaluate the impact of the trajectory of HbA1c change on hypertension. Besides, because our study lacked the variable of family history of hypertension, we were unable to evaluate the effect of family history of hypertension on the association between HbA1c and hypertension risk. And in this study, the annual incidence of hypertension was higher than that of other studies, so according to the epidemiological diagnostic criteria, the incidence of hypertension might not be so accurate. Finally, since all participants were from the CHNS, there was no systematic assessment of the causes of hypertension, so secondary hypertension could not be ruled out.
5. Conclusion
In this prospective cohort study from Chinese population, we found that there was a close linear positive correlation between HbA1c and the risk of hypertension, which not only further strengthened the close relationship between blood glucose fluctuation and the risk of hypertension, but also reminded us to pay attention not only to the traditional risk factors of hypertension, but also to the effect of blood glucose fluctuation on blood pressure level or hypertension.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by institutional review committees at the University of North Carolina at Chapel Hill and the National Institute of Nutrition and Food Safety, Chinese Center for Disease Control and Prevention. The patients/participants provided their written informed consent to participate in this study.
Author contributions
XH conceived, designed the study. CQ contributed to initial data analysis and interpretation. XH and XG drafted the initial manuscript. CQ, FC, and CT revised the manuscript. FC and CT were the guarantor of this work and had full access to all the data in the study and take responsibility for its integrity and the accuracy of the data analysis. All authors read and approved the final manuscript.
Acknowledgments
This research uses data from China Health and Nutrition Survey (CHNS). We are grateful to research grant funding from the National Institute for Health (NIH), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) for R01 HD30880, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) for R01DK104371 and R01HL108427, the NIH Fogarty grant D43 TW009077 for financial support for the CHNS data collection and analysis files since 1989, and the China-Japan Friendship Hospital, Ministry of Health for support for CHNS 2009, Chinese National Human Genome Center at Shanghai since 2009, and Beijing Municipal Center for Disease Prevention and Control since 2011. We thank the National Institute for Nutrition and Health, China Center for Disease Control and Prevention, Beijing Municipal Center for Disease Control and Prevention, and the Chinese National Human Genome Center at Shanghai.
Funding Statement
This work was supported by the National Natural Science Foundation of China (81820108019, and 91939303), Innovative Project of Chinese PLA General Hospital (CX19028), Key Health Care Projects of National Health Commission (2020ZD05), Basic Research Reinforcement Project (2021-JCJQ-JJ-1079). National Key Research and Development Projects (2022YFC3602400); the Key Health Care Projects of National Health Commission (2020ZD05); Basic Research Reinforcement Project (2022-JCJQ-ZD-079-00), and Project of National Clinical Research Center for Geriatric Diseases (NCRCG-PLAGH-2022007).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Lewington S, Lacey B, Clarke R, Guo Y, Kong XL, Yang L, et al. The burden of hypertension and associated risk for cardiovascular mortality in China. JAMA Intern Med (2016) 176(4):524–32. doi: 10.1001/jamainternmed.2016.0190 [DOI] [PubMed] [Google Scholar]
- 2. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: Analysis of worldwide data. Lancet (2005) 365(9455):217–23. doi: 10.1016/S0140-6736(05)17741-1 [DOI] [PubMed] [Google Scholar]
- 3. Zhou B, Perel P, Mensah GA, Ezzati M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat Rev Cardiol (2021) 18(11):785–802. doi: 10.1038/s41569-021-00559-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Res Clin Pract (2019) 157:107843. doi: 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- 5. Ferrannini E, Cushman WC. Diabetes and hypertension: The bad companions. Lancet (2012) 380(9841):601–10. doi: 10.1016/S0140-6736(12)60987-8 [DOI] [PubMed] [Google Scholar]
- 6. Yildiz M, Esenboğa K, Oktay AA. Hypertension and diabetes mellitus: Highlights of a complex relationship. Curr Opin Cardiol (2020) 35(4):397–404. doi: 10.1097/HCO.0000000000000748 [DOI] [PubMed] [Google Scholar]
- 7. Cheung BM, Li C. Diabetes and hypertension: is there a common metabolic pathway? Curr Atheroscler Rep (2012) 14(2):160–6. doi: 10.1007/s11883-012-0227-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun D, Zhou T, Heianza Y, Li X, Fan M, Fonseca VA, et al. Type 2 diabetes and hypertension. Circ Res (2019) 124(6):930–7. doi: 10.1161/CIRCRESAHA.118.314487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheung BM, Wat NM, Tso AW, Tam S, Thomas GN, Leung GM, et al. Association between raised blood pressure and dysglycemia in Hong Kong Chinese. Diabetes Care (2008) 31(9):1889–91. doi: 10.2337/dc08-0405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yazdanpanah S, Rabiee M, Tahriri M, Abdolrahim M, Rajab A, Jazayeri HE, et al. Evaluation of glycated albumin (GA) and GA/HbA1c ratio for diagnosis of diabetes and glycemic control: A comprehensive review. Crit Rev Clin Lab Sci (2017) 54(4):219–32. doi: 10.1080/10408363.2017.1299684 [DOI] [PubMed] [Google Scholar]
- 11. Ding L, Xu Y, Liu S, Bi Y, Xu Y. Hemoglobin A1c and diagnosis of diabetes. J Diabetes (2018) 10(5):365–72. doi: 10.1111/1753-0407.12640 [DOI] [PubMed] [Google Scholar]
- 12. Ikeda F, Doi Y, Ninomiya T, Hirakawa Y, Mukai N, Hata J, et al. Haemoglobin A1c even within non-diabetic level is a predictor of cardiovascular disease in a general Japanese population: The hisayama study. Cardiovasc Diabetol (2013) 12:164. doi: 10.1186/1475-2840-12-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sinning C, Makarova N, Völzke H, Schnabel RB, Ojeda F, Dörr M, et al. Association of glycated hemoglobin A1c levels with cardiovascular outcomes in the general population: Results from the BiomarCaRE (Biomarker for cardiovascular risk assessment in Europe) consortium. Cardiovasc Diabetol (2021) 20(1):223. doi: 10.1186/s12933-021-01413-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cavero-Redondo I, Peleteiro B, Álvarez-Bueno C, Rodriguez-Artalejo F, Martínez-Vizcaíno V. Glycated haemoglobin A1c as a risk factor of cardiovascular outcomes and all-cause mortality in diabetic and non-diabetic populations: A systematic review and meta-analysis. BMJ Open (2017) 7(7):e015949. doi: 10.1136/bmjopen-2017-015949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suebsamran P, Choenchoopon H, Rojanasaksothorn S, Loiha S, Chamnan P. Association between alcohol consumption and pre-diabetes among 383,442 Thai population aged 15 years and older in ubon ratchathani: Analytical cross-sectional study. J Med Assoc Thai (2016) 99(Suppl 1):S35–42. [PubMed] [Google Scholar]
- 16. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care (2013) 36(Suppl 1):S67–74. doi: 10.2337/dc13-S067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yan S, Li J, Li S, Zhang B, Du S, Gordon-Larsen P, et al. The expanding burden of cardiometabolic risk in China: The China health and nutrition survey. Obes Rev (2012) 13(9):810–21. doi: 10.1111/j.1467-789X.2012.01016.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Britton KA, Pradhan AD, Gaziano JM, Manson JE, Ridker PM, Buring JE, et al. Hemoglobin A1c, body mass index, and the risk of hypertension in women. Am J Hypertens (2011) 24(3):328–34. doi: 10.1038/ajh.2010.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heianza Y, Arase Y, Kodama S, Hsieh SD, Tsuji H, Saito K, et al. Fasting glucose and HbA1c levels as risk factors for the development of hypertension in Japanese individuals: Toranomon hospital health management center study 16 (TOPICS 16). J Hum Hypertens (2015) 29(4):254–9. doi: 10.1038/jhh.2014.77 [DOI] [PubMed] [Google Scholar]
- 20. Geva M, Shlomai G, Berkovich A, Maor E, Leibowitz A, Tenenbaum A, et al. The association between fasting plasma glucose and glycated hemoglobin in the prediabetes range and future development of hypertension. Cardiovasc Diabetol (2019) 18(1):53. doi: 10.1186/s12933-019-0859-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tatsumi Y, Morimoto A, Asayama K, Sonoda N, Miyamatsu N, Ohno Y, et al. Fasting blood glucose predicts incidence of hypertension independent of HbA1c levels and insulin resistance in middle-aged Japanese: The saku study. Am J Hypertens (2019) 32(12):1178–85. doi: 10.1093/ajh/hpz123 [DOI] [PubMed] [Google Scholar]
- 22. Luo S, Au Yeung SL, Schooling CM. Assessing the linear and non-linear association of HbA1c with cardiovascular disease: A mendelian randomisation study. Diabetologia (2021) 64(11):2502–10. doi: 10.1007/s00125-021-05537-w [DOI] [PubMed] [Google Scholar]
- 23. Liu L, Zhen D, Fu S, Sun W, Li H, Zhao N, et al. Associations of the baseline level and change in glycosylated hemoglobin A1c with incident hypertension in non-diabetic individuals: A 3-year cohort study. Diabetol Metab Syndr (2022) 14(1):54. doi: 10.1186/s13098-022-00827-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Omar SM, Musa IR, Abdelbagi O, Sharif ME, Adam I. The association between glycosylated haemoglobin and newly diagnosed hypertension in a non-diabetic Sudanese population: A cross-sectional study. BMC Cardiovasc Disord (2022) 22(1):208. doi: 10.1186/s12872-022-02649-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bower JK, Appel LJ, Matsushita K, Young JH, Alonso A, Brancati FL, et al. Glycated hemoglobin and risk of hypertension in the atherosclerosis risk in communities study. Diabetes Care (2012) 35(5):1031–7. doi: 10.2337/dc11-2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Au Yeung SL, Luo S, Schooling CM. The impact of glycated hemoglobin on risk of hypertension: A mendelian randomization study using UK biobank. J Hypertens (2020) 38(1):38–44. doi: 10.1097/HJH.0000000000002210 [DOI] [PubMed] [Google Scholar]
- 27. Song J, Wei N, Zhao Y, Jiang Y, Wu X, Gao H. Elevated glycosylated hemoglobin levels and their interactive effects on hypertension risk in nondiabetic Chinese population: A cross-sectional survey. BMC Cardiovasc Disord (2020) 20(1):218. doi: 10.1186/s12872-020-01501-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lou Y, Zhang Y, Zhao P, Qin P, Wang C, Ma J, et al. Association of fasting plasma glucose change trajectory and risk of hypertension: A cohort study in China. Endocr Connect (2022) 11(1):e210464. doi: 10.1530/EC-21-0464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang C. The role of inflammatory cytokines in endothelial dysfunction. Basic Res Cardiol (2008) 103(5):398–406. doi: 10.1007/s00395-008-0733-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vedovato M, Lepore G, Coracina A, Dodesini AR, Jori E, Tiengo A, et al. Effect of sodium intake on blood pressure and albuminuria in type 2 diabetic patients: The role of insulin resistance. Diabetologia (2004) 47(2):300–3. doi: 10.1007/s00125-003-1303-5 [DOI] [PubMed] [Google Scholar]
- 31. Griffin TP, Wall D, Browne GA, Dennedy MC, O’Shea PM. Associations between glycaemic control and activation of the renin-angiotensin-aldosterone system in participants with type 2 diabetes mellitus and hypertension. Ann Clin Biochem (2018) 55(3):373–84. doi: 10.1177/0004563217728964 [DOI] [PubMed] [Google Scholar]
- 32. Nowotny K, Jung T, Höhn A, Weber D, Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules (2015) 5(1):194–222. doi: 10.3390/biom5010194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saisho Y. Glycemic variability and oxidative stress: a link between diabetes and cardiovascular disease? Int J Mol Sci (2014) 15(10):18381–406. doi: 10.3390/ijms151018381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen Y, Huang Y, Li X, Xu M, Bi Y, Zhang Y, et al. Association of arterial stiffness with HbA1c in 1,000 type 2 diabetic patients with or without hypertension. Endocrine (2009) 36(2):262–7. doi: 10.1007/s12020-009-9221-z [DOI] [PubMed] [Google Scholar]
- 35. Zeng Q, Dong SY, Wang ML, Wang WM, Li JM, Dai ZX, et al. Serum glycated albumin, glycated hemoglobin, and arterial stiffness in a general Chinese population. Clin Chim Acta (2017) 468:33–8. doi: 10.1016/j.cca.2017.02.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.