Abstract
Background:
Preterm birth is associated with pulmonary complications early in life; however, long-term risks of asthma into adulthood are unclear.
Objective:
To determine asthma risks from childhood into adulthood associated with gestational age at birth in a large population-based cohort.
Methods:
A national cohort study was conducted of all 4,079,878 singletons born in Sweden during 1973–2013, followed up for asthma identified from primary care, specialty outpatient, and inpatient diagnoses in nationwide registries through 2018 (up to 46 years). Cox regression was used to adjust for potential confounders, and co-sibling analyses assessed the influence of unmeasured shared familial (genetic and/or environmental) factors.
Results:
In 91.9 million person-years of follow-up, 607,760 (14.9%) persons were diagnosed with asthma. Preterm birth was associated with increased risk of asthma at ages <10 years (adjusted hazard ratio [HR], 1.73; 95% CI, 1.70–1.75), 10–17 years (1.29; 1.27–1.32), and 18–46 years (1.19; 1.17–1.22). Across all ages, adjusted HRs further stratified were 3.01 (95% CI, 2.88–3.15) for extremely preterm (22–27 weeks), 1.76 (1.72–1.79) for very or moderately preterm (28–33 weeks), 1.31 (1.29–1.32) for late preterm (34–36 weeks), and 1.13 (1.12–1.14) for early term (37–38 weeks), compared with full-term (39–41 weeks) birth. These findings were not explained by shared familial factors. Asthma risks were elevated after spontaneous or medically indicated preterm birth, and with or without perinatal respiratory complications.
Conclusions:
In this large national cohort, preterm and early term birth were associated with increased risks of asthma from childhood into mid-adulthood. Persons born prematurely need long-term follow-up into adulthood for timely detection and treatment of asthma.
INTRODUCTION
Asthma has increased in prevalence over the past few decades and now affects 300 million children and adults worldwide.1–3 In addition to environmental airborne exposures and genetic heritability,1 developmental factors such as preterm birth have been identified as potential risk factors. Preterm birth (gestational age <37 weeks) may potentially lead to higher long-term risks of asthma because of impaired lung function from immature development or complications of early treatment. Approximately 11% of all births worldwide occur preterm,4 and >95% of preterm infants who receive modern neonatal care now survive into adulthood.5 6 As a result, clinicians will increasingly encounter preterm birth survivors of all ages and will need to understand their long-term risks of asthma to guide risk stratification and clinical care across the life course.
Most prior studies of long-term respiratory sequelae have focused on childhood and reported increased frequency of wheezing and bronchodilator use in preterm children.7 Fewer studies have explored whether outcomes persist into adulthood, and their findings are inconsistent. Some have reported that preterm birth, with or without bronchopulmonary dysplasia (BPD), is associated with reduced expiratory airflow that persists into early adulthood.8–12 However, most epidemiologic studies have not found significantly increased risks of asthma in adulthood,13–18 but were limited in follow-up, asthma ascertainment, and/or statistical power. Sex-specific differences1 and potential confounding by shared familial factors are also important to assess but remain unknown. Large cohort studies with more complete ascertainment of asthma and several decades of follow-up are needed to inform long-term risk stratification and clinical care in preterm birth survivors.
To address these knowledge gaps, we conducted a national cohort study of over 4 million persons in Sweden. Our goals were to examine: (1) associations between gestational age at birth and risk of asthma up to age 46 years, the maximum follow-up currently possible in this large cohort; (2) potential sex-specific differences; and (3) potential confounding by shared familial (genetic and/or early life environmental) factors using co-sibling analyses. The results will help guide long-term risk stratification, secondary prevention, and clinical monitoring for asthma in the growing populations worldwide who were born prematurely.
METHODS
Study Population
The Swedish Birth Registry contains prenatal and birth information for nearly all births nationwide since 1973. Using this registry, we identified all 4,088,503 singleton live births in Sweden during 1973–2013. Singleton births were chosen to improve internal comparability, given the higher prevalence of preterm birth and its different underlying causes among multiple births. We excluded 8,625 (0.2%) persons who had missing information for gestational age, leaving 4,079,878 persons (99.8% of the original cohort) for inclusion in the study. This study was approved by the Regional Ethical Review Board in Lund, Sweden. Participant consent was not required because this study used only pseudonymized registry data.
Gestational Age at Birth Ascertainment
Gestational age at birth was identified from the Swedish Birth Registry based on maternal report of last menstrual period in the 1970s and ultrasonography estimation starting in the 1980s and onward. This was analyzed alternatively as a continuous variable or categorical variable with 6 groups: extremely preterm (22–27 weeks), very or moderately preterm (28–33 weeks), late preterm (34–36 weeks), early term (37–38 weeks), full-term (39–41 weeks, used as the reference group), and post-term (≥42 weeks). Early term birth (37–38 weeks) was examined as a separate category because it has previously been associated with increased respiratory mortality from childhood into adulthood compared with full-term birth.5 19 In addition, the first 3 groups were combined to provide summary estimates for preterm birth.
Asthma Ascertainment
The study cohort was followed up for diagnosis of asthma from birth through the end of follow-up in 2018 (maximum age 46 years; median 24.5 years). Asthma was defined based on International Classification of Diseases (ICD), Versions 8, 9, and 10 (ICD-8/9 code 493; ICD-10 codes J45-J46). All ICD codes were identified from primary or secondary diagnoses in the Swedish Hospital and Outpatient Registries or from primary care records. The Swedish Hospital Registry was started in 1964 and covered nearly 80% of the national population in 1973 (beginning of the current follow-up period) and was subsequently expanded to include >99% by 1987.20 Asthma diagnoses in this registry have been reported to have a positive predictive value of 93%.20 The Swedish Outpatient Registry contains all outpatient diagnoses from specialty clinics nationwide starting in 2001. Primary care records previously collected by our group21 initially included all primary care diagnoses from two populous counties covering 20% of the national population starting in 1998, then was gradually expanded to cover 90% of the national population from 2008 and onward.
Covariates
Other perinatal and parental characteristics that may be associated with gestational age at birth and asthma were identified using the Swedish Birth Registry and national census data, which were linked using a pseudonymous personal serial number.5 6 22–25 The following were included as adjustment variables: birth year (continuous and categorical by decade), sex, birth order (1, 2, ≥3), maternal and paternal age (continuous), maternal and paternal education level (≤9, 10–12, >12 years), maternal body mass index (BMI; continuous), maternal smoking (0, 1–9, ≥10 cigarettes/day), and maternal and paternal asthma (yes/no, ascertained from nationwide diagnoses as described above).
Maternal BMI and smoking were assessed at the beginning of prenatal care starting in 1982, and were available for 60.1% and 73.6% of the cohort, respectively. Data were >99% complete for all other variables. Gestational age at birth was similar in persons with or without any missing covariate data (median, 40.1 and 40.0 weeks, respectively; Supplemental Table 1). Missing data for each covariate were treated as a separate category in the main analyses, and sensitivity analyses were performed that (1) restricted to persons with complete data (N=2,390,663), or (2) multiply imputed missing data using 20 imputations with all other covariates and asthma as predictors.
Statistical Analysis
Cox proportional hazards regression was used to compute hazard ratios (HRs) and 95% confidence intervals (CIs) for associations between gestational age at birth and risk of asthma. These associations were examined from birth to age 46 years and within narrower age intervals (<10, 10–17, 18–46 years) among persons still living in Sweden and without a prior diagnosis of asthma at the beginning of the respective interval. The data were analyzed as time-to-event with attained age as the Cox model time axis. Individuals were censored at death as identified in the Swedish Death Registry (n=41,605; 1.0%) or emigration as determined by absence of a Swedish residential address in census data (n=238,800; 5.9%). Analyses were conducted first adjusting only for attained age (as the model time axis) and then additionally for all covariates (as defined above). The proportional hazards assumption was assessed by examining log-log plots, and was met in each model.
Potential sex-specific differences were assessed by performing sex-stratified analyses and formally testing interactions between preterm or early term birth and sex on the additive and multiplicative scale. Additive interactions were assessed by testing whether the relative excess risk due to interaction (RERI) was significantly different from zero.26 Absolute risk differences and 95% CIs, attributable fraction in the exposed (AFe), and population attributable fraction (PAF) were computed for each gestational age group compared with full-term.27 In a sensitivity analysis, competing risks models were performed to account for death as a competing event, as an alternative to censoring at death.
Co-sibling analyses were performed to assess for potential confounding effects of unmeasured shared familial (genetic and/or environmental) factors among all participants with at least one full sibling (N=3,465,649; 84.9% of the cohort).5 6 22–25 These analyses can help further elucidate whether associations observed in the main analyses are due to direct effects of preterm birth as opposed to genetic or environmental factors within families that are shared determinants of preterm birth and asthma. Relevant environmental factors within families may include ambient airborne exposures such as passive smoking or air pollution. These analyses used stratified Cox regression with a separate stratum for each family as identified by the mother’s and father’s pseudonymous serial numbers. In the stratified Cox model, each set of siblings had its own baseline hazard function that reflects the family’s shared genetic and environmental factors, and thus associations between gestational age at birth and asthma were examined within families, controlling for their shared exposures. In addition, these analyses were further adjusted for the same covariates as in the main analyses. The co-sibling design includes only persons with siblings. In a sensitivity analysis, generalizability to persons without siblings was explored by repeating the primary analyses while restricting alternatively to persons with siblings (N=3,465,649) or those without (N=614,229).
Secondary analyses were performed to examine: (1) asthma risks associated with spontaneous (71.4%) vs. medically indicated (28.6%) preterm birth, which was systematically recorded starting in 1990 (N=2,422,584; maximum age 29 years at end of follow-up); (2) potential modification of risks by perinatal respiratory complications (respiratory distress, BPD, or other chronic respiratory conditions originating in the perinatal period); (3) associations between fetal growth and asthma risk (fetal growth was defined as small for gestational age [SGA; <10th percentile], appropriate for gestational age [AGA; 10th-90th percentile], or large for gestational age [LGA; >90th percentile]); and (4) stratified analyses by birth decade to assess for potential time trends, with follow-up to age 15 years to allow a similar follow-up time for each group. All statistical tests were two-sided and used a significance level of 0.05. All analyses were conducted using Stata version 15.1.
RESULTS
Table 1 shows perinatal and parental characteristics by gestational age at birth. Preterm infants were more likely than full-term infants to be male or first-born; and their parents were more likely to be at the extremes of age or have low education level. In addition, their mothers were more likely to smoke or have high BMI or asthma at the beginning of prenatal care.
Table 1.
Characteristics of study participants by gestational age at birth, Sweden, 1973–2013.
| Extremely preterm | Very or moderately preterm | Late preterm | Early term | Full-term | Post-term | |
|---|---|---|---|---|---|---|
| (22–27 weeks) | (28–33 weeks) | (34–36 weeks) | (37–38 weeks) | (39–41 weeks) | (≥42 weeks) | |
| N=7,966 (0.2%) | N=42,911 (1.1%) | N=152,824 (3.7%) | N=719,495 (17.6%) | N=2,818,414 (69.1%) | N=338,268 (8.3%) | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Child characteristics | ||||||
| Sex | ||||||
| Male | 4,327 (54.3) | 23,914 (55.7) | 83,186 (54.4) | 370,720 (51.5) | 1,431,661 (50.8) | 183,665 (54.3) |
| Female | 3,639 (45.7) | 18,997 (44.3) | 69,638 (45.6) | 348,775 (48.5) | 1,386,753 (49.2) | 154,603 (45.7) |
| Birth order | ||||||
| 1 | 4,033 (50.6) | 22,247 (51.8) | 76,395 (50.0) | 289,701 (40.3) | 1,185,001 (42.0) | 169,260 (50.0) |
| 2 | 2,238 (28.1) | 12,002 (28.0) | 45,344 (29.7) | 263,235 (36.6) | 1,059,293 (37.6) | 108,051 (31.9) |
| ≥3 | 1,695 (21.3) | 8,662 (20.2) | 31,085 (20.3) | 166,559 (23.1) | 574,120 (20.4) | 60,957 (18.0) |
| Maternal characteristics | ||||||
| Age (years) | ||||||
| <20 | 351 (4.4) | 2,039 (4.7) | 6,416 (4.2) | 21,822 (3.0) | 83,174 (2.9) | 12,884 (3.8) |
| 20–24 | 1,522 (19.1) | 8,762 (20.4) | 32,639 (21.4) | 136,535 (19.0) | 570,888 (20.3) | 75,361 (22.3) |
| 25–29 | 2,335 (29.3) | 13,296 (31.0) | 49,865 (32.6) | 237,088 (33.0) | 994,449 (35.3) | 118,844 (35.1) |
| 30–34 | 2,162 (27.1) | 11,394 (26.6) | 40,134 (26.3) | 204,989 (28.5) | 795,092 (28.2) | 90,043 (25.6) |
| 35–39 | 1,256 (15.8) | 5,929 (13.8) | 19,331 (12.6) | 97,092 (13.5) | 317,606 (11.3) | 35,437 (10.5) |
| ≥40 | 340 (4.3) | 1,491 (3.5) | 4,439 (2.9) | 21,969 (3.0) | 57,205 (2.0) | 5,699 (1.7) |
| Education (years) | ||||||
| ≤9 | 1,281 (16.1) | 7,000 (16.3) | 23,537 (15.4) | 100,488 (14.0) | 356,257 (12.6) | 47,313 (14.0) |
| 10–12 | 3,755 (47.1) | 20,361 (47.5) | 72,084 (47.2) | 328,911 (45.7) | 1,269,526 (45.0) | 153,329 (45.3) |
| >12 | 2,809 (35.3) | 15,035 (35.0) | 55,869 (36.6) | 285,065 (39.6) | 1,175,366 (41.7) | 135,261 (40.0) |
| Unknown | 121 (1.5) | 515 (1.2) | 1,334 (0.9) | 5,031 (0.7) | 17,265 (0.6) | 2,365 (0.7) |
| Body mass index (kg/m 2 ) | ||||||
| <18.5 | 137 (1.7) | 1,088 (2.5) | 4,653 (3.0) | 21,206 (2.9) | 63,625 (2.3) | 4,517 (1.3) |
| 18.5–24.9 | 2,160 (27.1) | 13,960 (32.5) | 56,201 (36.8) | 296,688 (41.2) | 1,146,382 (40.7) | 108,829 (32.2) |
| 25.0–29.9 | 968 (12.2) | 5,064 (11.8) | 18,757 (12.3) | 94,759 (13.2) | 362,997 (12.9) | 42,499 (12.6) |
| ≥30.0 | 582 (7.3) | 2,639 (6.2) | 8,863 (5.8) | 40,853 (5.7) | 137,795 (4.9) | 18,580 (5.5) |
| Unknown | 4,119 (51.7) | 20,160 (47.0) | 64,350 (42.1) | 265,989 (37.0) | 1,107,615 (39.3) | 163,843 (48.4) |
| Smoking (cigarettes/day) | ||||||
| 0 | 4,094 (51.4) | 23,260 (54.2) | 88,867 (58.2) | 456,860 (63.5) | 1,758,709 (62.4) | 180,716 (53.4) |
| 1–9 | 717 (9.0) | 4,267 (9.9) | 14,084 (9.2) | 63,674 (8.8) | 214,638 (7.6) | 21,860 (6.5) |
| ≥10 | 493 (6.2) | 2,744 (6.4) | 8,921 (5.8) | 36,642 (5.1) | 111,944 (4.0) | 10,890 (3.2) |
| Unknown | 2,662 (33.4) | 12,640 (29.5) | 40,952 (26.8) | 162,319 (22.6) | 733,123 (26.0) | 124,802 (36.9) |
| Asthma | 1,103 (13.9) | 5,948 (13.9) | 21,363 (14.0) | 94,429 (13.1) | 331,120 (11.8) | 40,189 (11.9) |
| Paternal characteristics | ||||||
| Age (years) | ||||||
| <20 | 572 (7.2) | 5,156 (12.0) | 19,474 (12.7) | 76,449 (10.6) | 306,989 (10.9) | 42,689 (12.6) |
| 20–24 | 1,227 (15.4) | 10,617 (24.7) | 42,670 (27.9) | 194,631 (27.0) | 818,000 (29.0) | 102,961 (30.4) |
| 25–29 | 1,414 (17.7) | 11,200 (26.1) | 43,771 (28.6) | 220,588 (30.7) | 891,131 (31.6) | 102,324 (30.3) |
| 30–34 | 986 (12.4) | 7,094 (16.5) | 25,831 (16.9) | 134,272 (18.7) | 495,406 (17.6) | 54,273 (16.0) |
| 35–39 | 475 (6.0) | 3,123 (7.3) | 10,720 (7.0) | 54,814 (7.6) | 184,293 (6.5) | 20,582 (6.1) |
| ≥40 | 257 (3.2) | 1,573 (3.7) | 5,214 (3.4) | 25,151 (3.5) | 79,642 (2.8) | 9,168 (2.7) |
| Unknown | 3,035 (38.1) | 4,148 (9.7) | 5,144 (3.4) | 13,590 (1.9) | 42,953 (1.5) | 6,271 (1.9) |
| Education (years) | ||||||
| ≤9 | 916 (11.5) | 8,248 (19.2) | 30,891 (20.2) | 137,435 (19.1) | 524,381 (18.6) | 68,985 (20.4) |
| 10–12 | 2,471 (31.0) | 19,256 (44.9) | 73,088 (47.8) | 343,457 (47.7) | 1,331,389 (47.2) | 156,871 (46.4) |
| >12 | 1,540 (19.3) | 11,208 (26.1) | 43,574 (28.5) | 224,465 (31.2) | 917,185 (32.5) | 105,692 (31.2) |
| Unknown | 3,039 (38.2) | 4,199 (9.8) | 5,271 (3.5) | 14,138 (2.0) | 45,459 (1.6) | 6,720 (2.0) |
| Asthma | 425 (5.3) | 3,288 (7.7) | 12,811 (8.4) | 60,126 (8.4) | 229,089 (8.1) | 26.620 (7.9) |
In 91.9 million person-years of follow-up, 607,760 (14.9%) persons were diagnosed with asthma. The median age at the first registered asthma diagnosis was 9.1 years, and median age at the end of follow-up was 24.5 years. The cumulative incidence of asthma was 19.3% in persons born preterm, 16.3% in those born early term, and 14.4% in those born full-term. Because the earliest available primary care data started in 1998 and specialty outpatient data in 2001, the age at earliest registration of asthma does not necessarily correspond to age at first onset (see Supplemental Figure 1 for cumulative hazard functions by birth decade).
Associations Between Gestational Age at Birth and Asthma
Gestational age at birth was inversely associated with asthma risk (Table 2). Across all ages (up to 46 years), preterm birth was associated with a 1.4-fold risk of asthma (adjusted HR, 1.44; 95% CI, 1.42–1.45). Each additional week of gestational age was associated with a 5% lower risk on average (adjusted HR per additional week, 0.95; 95% CI, 0.95–0.95).
Table 2.
Associations between gestational age at birth and risk of asthma, Sweden, 1973–2018.
| Cases | Ratea | Age-adjusted | Full modelb | Risk differencec | AFe | PAF | |
|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | (95% CI) | % | % | |||
| Attained ages up to 46 years | |||||||
| Preterm (<37 wks) | 39,284 | 933.4 | 1.48 (1.46, 1.49) | 1.44 (1.42, 1.45) | 298.5 (289.1, 307.9) | 32.0 | 2.8 |
| Extremely preterm (22–27 wks) | 1,889 | 2,533.9 | 3.07 (2.94, 3.22) | 3.01 (2.88, 3.15) | 1,889.0 (1,785.8, 2,013.3) | 74.9 | 0.3 |
| Very or moderately preterm (28–33 wks) | 9,457 | 1,165.4 | 1.81 (1.77, 1.85) | 1.76 (1.72, 1.79) | 530.5 (506.9, 554.1) | 45.5 | 1.0 |
| Late preterm (34–36 wks) | 27,938 | 840.8 | 1.35 (1.33, 1.36) | 1.31 (1.29, 1.32) | 205.9 (195.9, 216.0) | 24.5 | 1.6 |
| Early term (37–38 wks) | 117,455 | 757.3 | 1.14 (1.13, 1.15) | 1.13 (1.12, 1.14) | 122.4 (117.6, 127.1) | 16.2 | 3.6 |
| Full-term (39–41 wks) | 404,844 | 634.9 | Reference | Reference | Reference | ||
| Post-term (≥42 wks) | 46,187 | 546.6 | 0.99 (0.98, 1.00) | 0.99 (0.98, 1.00) | −88.3 (−93.7, −83.0) | (13.9)d | (1.6)e |
| Per additional week (trend) | 0.94 (0.94, 0.94) | 0.95 (0.95, 0.95) | |||||
| Attained ages <10 years | |||||||
| Preterm (<37 wks) | 24,485 | 1,452.5 | 1.80 (1.77, 1.82) | 1.73 (1.70, 1.75) | 632.8 (614.3, 65132) | 43.6 | 4.6 |
| Extremely preterm (22–27 wks) | 1,595 | 4,555.3 | 4.14 (3.94, 4.35) | 4.05 (3.85, 4.25) | 3,735.6 (3,512.0, 3,959.2) | 82.0 | 0.6 |
| Very or moderately preterm (28–33 wks) | 6,432 | 1,953.7 | 2.35 (2.30, 2.41) | 2.26 (2.20, 2.31) | 1,134.0 (1,086.1, 1,181.9) | 58.0 | 1.7 |
| Late preterm (34–36 wks) | 16,458 | 1,245.4 | 1.57 (1.54, 1.59) | 1.51 (1.48, 1.53) | 425.7 (406.4, 445.1) | 34.2 | 2.5 |
| Early term (37–38 wks) | 66,376 | 1,040.1 | 1.22 (1.21, 1.23) | 1.20 (1.19, 1.21) | 220.5 (211.8, 229.1) | 21.2 | 5.1 |
| Full-term (39–41 wks) | 208,644 | 819.7 | Reference | Reference | Reference | ||
| Post-term (≥42 wks) | 21,150 | 682.6 | 0.98 (0.96, 0.99) | 0.95 (0.94, 0.97) | −137.1 (−146.9, −127.3) | (16.7)d | (1.8)e |
| Per additional week (trend) | 0.92 (0.91, 0.92) | 0.92 (0.92, 0.92) | |||||
| Attained ages 10–17 years | |||||||
| Preterm (<37 wks) | 6,169 | 600.5 | 1.31 (1.28, 1.33) | 1.29 (1.27, 1.32) | 65.9 (50.5, 81.3) | 11.0 | 0.8 |
| Extremely preterm (22–27 wks) | 143 | 788.5 | 1.82 (1.65, 2.00) | 1.86 (1.69, 2.04) | 253.9 (124.6, 383.2) | 32.2 | 0.1 |
| Very or moderately preterm (28–33 wks) | 1,273 | 642.6 | 1.43 (1.37, 1.48) | 1.40 (1.34, 1.46) | 108.0 (72.5, 143.5) | 16.8 | 0.3 |
| Late preterm (34–36 wks) | 4,753 | 586.0 | 1.26 (1.23, 1.29) | 1.23 (1.20, 1.26) | 51.4 (34.3, 68.4) | 8.8 | 0.5 |
| Early term (37–38 wks) | 22,788 | 595.4 | 1.10 (1.09, 1.11) | 1.09 (1.08, 1.10) | 60.8 (52.2, 69.3) | 10.2 | 2.2 |
| Full-term (39–41 wks) | 83,132 | 534.6 | Reference | Reference | Reference | ||
| Post-term (≥42 wks) | 9,019 | 453.8 | 0.99 (0.97, 1.01) | 0.98 (0.96, 0.99) | −80.8 (−90.9,−70.8) | (15.1)d | (1.7)e |
| Per additional week (trend) | 0.96 (0.96, 0.96) | 0.97 (0.97, 0.97) | |||||
| Attained ages 18–46 years | |||||||
| Preterm (<37 wks) | 8,630 | 577.0 | 1.21 (1.19, 1.23) | 1.19 (1.17, 1.22) | 80.3 (67.8, 92.8) | 15.7 | 1.2 |
| Extremely preterm (22–27 wks) | 151 | 705.7 | 1.53 (1.37, 1.71) | 1.46 (1.30, 1.63) | 209.0 (96.4, 321.6) | 29.6 | <0.1 |
| Very or moderately preterm (28–33 wks) | 1,752 | 616.6 | 1.34 (1.29, 1.39) | 1.31 (1.26, 1.36) | 119.9 (90.8, 148.9) | 19.4 | 0.3 |
| Late preterm (34–36 wks) | 6,727 | 565.3 | 1.17 (1.15, 1.20) | 1.16 (1.14, 1.18) | 68.6 (54.7, 82.4) | 12.1 | 0.7 |
| Early term (37–38 wks) | 28,291 | 533.7 | 1.07 (1.06, 1.08) | 1.07 (1.05, 1.08) | 37.0 (30.1, 43.8) | 6.9 | 1.4 |
| Full-term (39–41 wks) | 113,058 | 496.7 | Reference | Reference | Reference | ||
| Post-term (≥42 wks) | 16,018 | 476.1 | 1.00 (0.99, 1.02) | 1.00 (0.98, 1.01) | −20.6 (−28.5, −12.7) | (4.1)d | (0.5)e |
| Per additional week (trend) | 0.97 (0.97, 0.98) | 0.98 (0.98, 0.98) |
Incidence rate per 100,000 person-years.
Adjusted for child characteristics (birth year, sex, birth order), maternal characteristics (age, education, smoking, BMI, asthma), and paternal characteristics (age, education, asthma).
Incidence rate difference per 100,000 person-years.
Preventable fraction in the exposed.
Population preventable fraction.
These risks were strongest in childhood, yet modest associations persisted into adolescence and adulthood. At ages <10 years, the adjusted HR for asthma associated with preterm birth was 1.73 (95% CI, 1.70–1.75). Further stratified by gestational age, the adjusted HRs were 4.05 (95% CI, 3.85–4.25) for extremely preterm, 2.26 (2.20–2.31) for very or moderately preterm, 1.51 (1.48–1.53) for late preterm, and 1.20 (1.19–1.21) for early term, compared with full-term birth. At ages 10–17 years, the corresponding HRs for the same gestational age groups were reduced to 1.86 (95% CI, 1.69–2.04), 1.40 (1.34–1.46), 1.23 (1.20–1.26), and 1.09 (1.08–1.10), respectively. At ages 18–46 years they were further reduced but still significantly elevated: 1.46 (95% CI, 1.30–1.63), 1.31 (1.26–1.36), 1.16 (1.14–1.18), and 1.07 (1.05–1.08), respectively. In all models, the HRs from the full model were similar to those adjusted only for attained age (i.e., the covariates were not major confounders). Figure 1 shows adjusted HRs for asthma from birth to age 46 years by gestational age at birth.
Figure 1.
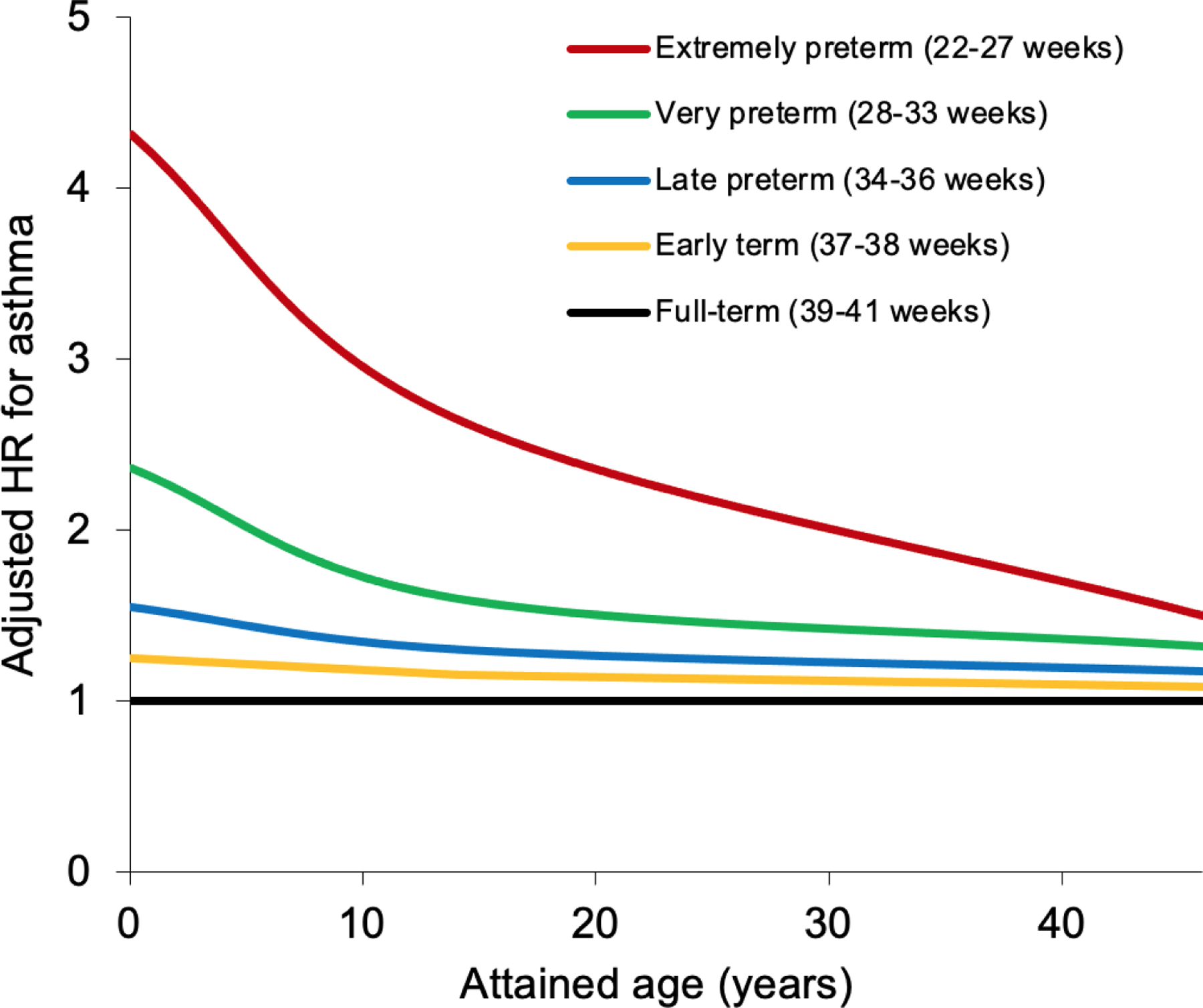
Adjusted hazard ratios for asthma by gestational age at birth compared with full-term birth, Sweden, 1973–2018.
Across the entire follow-up, preterm birth accounted for an estimated 32.0% of all asthma cases among persons born preterm (i.e., AFe) and 2.8% of asthma cases in the entire population (i.e., PAF). Early term birth accounted for 16.2% of all asthma cases among persons born at early term and 3.6% of asthma cases in the population (Table 2, AFe and PAF). The PAF for preterm or early term birth together was 4.9%. By comparison, maternal smoking had an AFe of 16.4% and PAF of 3.3%.
Sex-Specific Analyses
Gestational age at birth was inversely associated with asthma risk among males or females, with only a slightly stronger association in males (Supplemental Table 2). Across all ages (up to 46 years), the adjusted HR for asthma associated with preterm birth was 1.47 (95% CI, 1.45–1.49) in males and 1.40 (1.38–1.42) in females. Positive additive and multiplicative interactions were found between male sex and either preterm or early term birth (i.e., the combined effect of these factors on asthma risk exceeded the sum or product of their separate effects, P<0.001 for each) (Supplemental Table 3). The positive additive interactions indicate that preterm and early term birth accounted for significantly more asthma cases in males than females.
Co-Sibling Analyses
Co-sibling analyses that control for unmeasured shared familial factors resulted in only modest attenuation of risk estimates (Table 3). For example, at ages up to 46 years, the adjusted HR for asthma associated with preterm birth was 1.44 (95% CI, 1.42–1.45) in the primary analysis and 1.40 (1.38–1.43) in the co-sibling analysis. The corresponding HRs were 1.73 (95% CI, 1.70–1.75) and 1.72 (1.67–1.76) at ages <10 years, 1.29 (1.27–1.32) and 1.21 (1.17–1.26) at ages 10–17 years, and 1.19 (1.17–1.22) and 1.13 (1.09–1.17) at ages 18–46 years.
Table 3.
Co-sibling analyses for gestational age at birth in relation to asthma risk, Sweden, 1973–2018.
| Gestational age at birth | Cases | HR (95% CI)a |
|---|---|---|
| Attained ages up to 46 years | ||
| Preterm (<37 wks) | 31,945 | 1.40 (1.38, 1.43) |
| Extremely preterm (22–27 wks) | 1,417 | 3.28 (2.95, 3.64) |
| Very or moderately preterm (28–33 wks) | 7,416 | 1.79 (1.72, 1.86) |
| Late preterm (34–36 wks) | 23,112 | 1.28 (1.25, 1.31) |
| Early term (37–38 wks) | 101,100 | 1.09 (1.08, 1.11) |
| Full-term (39–41 wks) | 349,147 | Reference |
| Post-term (≥42 wks) | 38,358 | 0.99 (0.97, 1.00) |
| Per additional week (trend) | 0.95 (0.95, 0.95) | |
| Attained ages <10 years | ||
| Preterm (<37 wks) | 19,931 | 1.72 (1.67, 1.76) |
| Extremely preterm (22–27 wks) | 1,192 | 4.50 (3.97, 5.10) |
| Very or moderately preterm (28–33 wks) | 5,069 | 2.38 (2.26, 2.51) |
| Late preterm (34–36 wks) | 13,670 | 1.50 (1.46, 1.55) |
| Early term (37–38 wks) | 57,290 | 1.15 (1.13, 1.17) |
| Full-term (39–41 wks) | 180,163 | Reference |
| Post-term (≥42 wks) | 17,611 | 0.97 (0.94, 0.99) |
| Per additional week (trend) | 0.92 (0.92, 0.92) | |
| Attained ages 10–17 years | ||
| Preterm (<37 wks) | 5,266 | 1.21 (1.17, 1.26) |
| Extremely preterm (22–27 wks) | 110 | 1.74 (1.42, 2.11) |
| Very or moderately preterm (28–33 wks) | 1,052 | 1.40 (1.30, 1.51) |
| Late preterm (34–36 wks) | 4,104 | 1.16 (1.11, 1.21) |
| Early term (37–38 wks) | 20,476 | 1.05 (1.03, 1.07) |
| Full-term (39–41 wks) | 75,513 | Reference |
| Post-term (≥42 wks) | 7,991 | 1.01 (0.98, 1.04) |
| Per additional week (trend) | 0.97 (0.97, 0.98) | |
| Attained ages 18–46 years | ||
| Preterm (<37 wks) | 10,478 | 1.13 (1.09, 1.17) |
| Extremely preterm (22–27 wks) | 233 | 1.37 (1.09, 1.71) |
| Very or moderately preterm (28–33 wks) | 2,170 | 1.24 (1.16, 1.34) |
| Late preterm (34–36 wks) | 8,075 | 1.10 (1.06, 1.15) |
| Early term (37–38 wks) | 34,902 | 1.04 (1.02, 1.07) |
| Full-term (39–41 wks) | 133,729 | Reference |
| Post-term (≥42 wks) | 17,075 | 0.99 (0.96, 1.01) |
| Per additional week (trend) | 0.98 (0.98, 0.98) |
Adjusted for shared familial (genetic and/or environmental) factors, in addition to child characteristics (birth year, sex, birth order), maternal characteristics (age, education, smoking, BMI, asthma), and paternal characteristics (age, education, asthma).
Secondary Analyses
Both spontaneous and medically indicated preterm birth were associated with substantially increased risk of asthma compared with full-term birth (adjusted HRs, 1.46; 95% CI, 1.44–1.48; and 1.51; 1.45–1.57, respectively), with similar risk magnitudes (P=0.11 for difference in HRs) (Supplemental Table 4).
Preterm birth was associated with significantly elevated risk of asthma in persons with known perinatal respiratory complications (adjusted HR, 2.05; 95% CI, 1.69–2.49; n=1,391) and also in those without (1.43; 1.41–1.44), compared with full-term birth (P<0.001 for difference in these HRs). There were too few reported BPD diagnoses (n=458) to enable separate analyses in this subgroup.
In analyses of fetal growth, SGA was associated with only a slightly increased risk for asthma (e.g., adjusted HR at ages up to 46 years: 1.06; 95% CI, 1.05–1.07) (Supplemental Table 5), and was a much weaker risk factor than preterm birth (P<0.001 for difference in HRs).
Across all decades from the 1970s through 2000s, preterm birth was associated with substantially increased risks of asthma up to age 15 years. The adjusted HR for asthma associated with preterm birth was 1.37 (95% CI, 1.24–1.52) for those born in 1973–1979, compared with 1.56 (1.49–1.63) for those born in 1980–1989, 1.54 (1.50–1.58) for those born in 1990–1999, and 1.59 (1.56–1.62) for those born in 2000–2009 (Supplemental Table 6).
Sensitivity Analyses
Competing risks models that accounted for death as a competing event yielded nearly identical risk estimates as the main analyses that used censoring at death (e.g., preterm vs. full-term, adjusted HR at ages up to 46 years, 1.43; 95% CI, 1.41–1.44). As alternative approaches for missing data, a complete case analysis and multiple imputation also resulted in little change in risk estimates (e.g., preterm vs. full-term, adjusted HR at ages up to 46 years, 1.49; 95% CI, 1.47–1.51; and 1.44; 1.42–1.45, respectively). In the complete case analysis, maternal smoking and BMI were not important confounders (i.e., adjustment made negligible difference in risk estimates). Appropriateness of the co-sibling design was further assessed by exploring generalizability of the main analysis results to persons without siblings. The risk estimates were nearly identical in persons with siblings (e.g., preterm vs. full-term, adjusted HR at ages up to 46 years: 1.44; 95% CI, 1.42–1.46) and those without siblings (1.43; 1.40–1.47; P for interaction = 0.87).
DISCUSSION
In this large national cohort study, preterm and early term birth were associated with increased risks of asthma from childhood into mid-adulthood. Preterm and early term birth, respectively, were associated with 73% and 20% higher risks of asthma at ages <10 years, 29% and 9% higher risks at 10–17 years, and 19% and 7% higher risks at 18–46 years, compared with full-term birth. Persons born extremely preterm had substantially higher risks (4-, 1.9-, and 1.5-fold at ages <10, 10–17, and 18–46 years, respectively). These risks were only slightly higher in males than females and were largely not explained by shared genetic or environmental determinants in families.
Prior studies have suggested that preterm birth, with or without BPD, is associated with increased frequency of respiratory symptoms and bronchodilator use early in life.7 However, longer-term studies into adulthood have yielded inconsistent findings. A Swedish national cohort study of 622,616 persons born in 1973–1979 found that extremely (but not later) preterm birth was associated with a >2-fold risk of asthma medication prescription at ages 25–35 years.28 A Danish study of 1.7 million persons born in 1980–2009 reported strong inverse associations between gestational age at birth and purchase of asthma medications in childhood or adolescence, which were non-significant in young adulthood (ages 25–31 years).13 Other smaller studies of young adults in Norway (ages 20–24 years)14 and the UK (ages 18–25 years)15 reported non-significant associations between preterm birth and asthma symptoms or diagnosis. A survey of 5,192 Finnish adults aged 31 years reported no association between preterm birth and asthma.16 A survey of 1,534 Australians reported significant associations between preterm birth and asthma in childhood but not adulthood (age 30–43 years).17 A retrospective cohort study of 149,398 Swedish males aged 17–20 years also found no association between preterm birth and physician-diagnosed asthma.18
Most smaller clinical studies of lung function have reported that preterm birth survivors have reduced expiratory outflow that persists into early adulthood. A Finnish study with 719 participants reported that early preterm (<34 weeks) but not late preterm (34–36 weeks) birth in the presurfactant era was associated with substantially reduced expiratory airflow in young adulthood (mean age 23 years), even after excluding those with BPD.8 An Australian study with 294 participants found that extremely preterm birth in the postsurfactant era also was linked with substantially reduced expiratory airflow at age 25 years, which was more severe in those with BPD.11 A meta-analysis of 11 studies reported that persons born very preterm or with very low birthweight (mostly in the presurfactant era) had substantially reduced expiratory airflow at a mean age of 23 years.12
To our knowledge, the present study is the largest to date of preterm or early term birth and asthma risks with follow-up into adulthood, and the first to assess for potential familial confounding. We found an inverse dose-response relationship between gestational age at birth and asthma risk that was not explained by measured covariates nor unmeasured shared familial (genetic and/or early-life environmental) factors. Preterm and early term birth together were estimated to account for more asthma cases in this population than maternal smoking. Asthma risks were elevated after either spontaneous or medically indicated preterm birth, and with or without perinatal respiratory complications. Preterm birth was more strongly associated with asthma risk among persons born in the 1980s through 2000s, including the post-surfactant era, likely related to improved survival of extremely and very preterm infants.6 In contrast with preterm birth, low fetal growth had a much weaker association with asthma risk, consistent with prior evidence.29 The present study adds to prior evidence for long-term sequelae of preterm birth, including other respiratory outcomes such as sleep-disordered breathing22 and respiratory mortality5 in this cohort.
Early complications of preterm birth include respiratory distress syndrome, reported in >85% of extremely preterm neonates,30 and BPD, reported in 88% and 61% of infants born at 23 and 26 weeks, respectively, in Sweden during 2004–200731 32 and similar proportions in the US.33 Consistent with prior studies,10 13 our findings suggest that these complications do not explain most subsequent risks of asthma. All preterm infants, even those without early respiratory complications, may have impaired lung development and alveolarization that increases long-term risks of asthma. Contributing factors may include perinatal infections, aspiration, inadequate nutrition,34 or tobacco smoke exposure, which have been associated with preterm birth and poor lung development or subsequent asthma.35 Hormone-related differences in airway development also lead to relatively narrower airways and higher risk of asthma in boys than girls, followed by reversal of this sex difference and a female predominance after further airway maturation in adolescence.36 In preterm birth survivors, the resulting lung disease is highly variable in phenotype but includes obstructive disease with both structural and reactive inflammatory components.37 Preterm birth combined with harmful environmental exposures may also potentially lead to chronic obstructive pulmonary disease (COPD) later in adulthood.38 A recent Australian study with 1,445 participants reported a 2.9-fold odds (95% CI, 1.1–1.7) of COPD at age 53 years among those born at 28 to <34 weeks compared with ≥37 weeks, after adjusting for sociodemographic factors and parental smoking.39
Most preterm infants now survive into adulthood,5 and thus clinicians will need to understand their long-term asthma risks to guide risk stratification and clinical monitoring across the life course. Clinicians and affected families should now recognize preterm birth as a chronic condition that predisposes to the development of asthma from childhood into mid-adulthood, especially among those born at the earliest gestational ages. Medical records and history-taking in patients of all ages should routinely encompass birth history, including gestational age and perinatal complications.40 Such information can help identify those born prematurely and facilitate clinical monitoring for timely detection and treatment of asthma in those at highest risk. Secondary preventive efforts should include avoidance of smoking, air pollution, or occupations that involve airborne exposures, and optimal management of asthma across the life course.41 Access to high-quality preconception and prenatal care is also needed to help reduce the occurrence of preterm birth.42
A key strength of this study was the ability to examine gestational age at birth in relation to asthma in a large national cohort with follow-up into mid-adulthood. This study design minimizes potential selection or ascertainment biases and enables more robust risk estimates based on a national population.5 6 22–25 The availability of primary care diagnoses enabled more complete ascertainment of asthma than has been possible in most prior studies. The large sample size enabled well-powered assessment of narrowly defined gestational age groups and sex-specific differences. The analyses also included thorough assessment and control for confounding by measured covariates, including maternal smoking intensity, as well as unmeasured familial factors. The overall asthma prevalence in this cohort (14.9%) was similar to previously published prevalences based on clinical assessments in Sweden and in most other European countries and the US.2
This study also had several limitations. First, asthma lacks a gold standard for diagnosis,1 and pulmonary function data were unavailable. However, high positive predictive values (>90%) have been reported for asthma and most chronic disorders in the Swedish registries,20 and our observed prevalences were similar to those reported in other high-income countries.2 3 Some asthma diagnoses could be misdiagnoses of reduced expiratory airflow that is unresponsive to bronchodilators in preterm birth survivors with neonatal lung pathology. Outpatient data were unavailable until 1998 from primary care settings and 2001 from specialty outpatient settings. It is possible that persons born prematurely are more likely to be diagnosed with asthma because of greater contact with the health care system (i.e., detection bias). However, this is less likely for highly symptomatic disorders such as asthma, or for persons born early term in whom increased risks also were found. Death was a competing event that precluded diagnosis of asthma, but was uncommon (1.0%) in this cohort, and competing risks models suggested that it had a negligible effect on risk estimates. Other analytic models to examine discordant and concordant sibling pairs could also be considered to further address potential familial confounding.43 Lastly, this study was limited to Sweden and will need replication in other diverse populations when feasible.
In summary, preterm and early term birth were associated with increased risks of asthma from childhood into mid-adulthood in a large population-based cohort. Persons born prematurely need long-term clinical follow-up into adulthood for timely detection and treatment of asthma.
Supplementary Material
What is already known on this topic
Preterm birth (gestational age <37 weeks), with or without perinatal respiratory complications, has been associated with reduced lung function early in life. However, long-term risks of asthma into adulthood after preterm or early term (37–38 weeks) birth, and potential confounding by familial factors, are unknown.
What this study adds
In this national cohort study of >4 million persons, preterm and early term birth, respectively, were associated with 73% and 20% higher risks of asthma at ages <10 years, 29% and 9% higher risks at 10–17 years, and 19% and 7% higher risks at 18–46 years, compared with full-term birth, after adjusting for covariates. These findings were largely not explained by shared familial (genetic and/or environmental) factors.
How this study might affect research, practice or policy
Persons born prematurely need long-term clinical follow-up into adulthood for timely detection and treatment of asthma.
Funding:
This work was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health (R01 HL139536); the Swedish Research Council; the Swedish Heart-Lung Foundation; and ALF project grant, Region Skåne/Lund University, Sweden. The funding agencies had no role in the design and conduct of the study; collection, analysis, and interpretation of data; or the writing of the manuscript or the decision to submit it for publication. There were no conflicts of interest.
Footnotes
Competing Interests: All authors declare no support from other organisations for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Patient Consent: Patient consent was not required as this study used only de-identified registry-based secondary data.
Ethical Approval: This study was approved by the ethics committee of Lund University in Sweden (No. 2008/471 with amendment 2010/512 for inclusion of more recent data).
Data Sharing: Due to legal concerns, supporting data cannot be made openly available. Further information about the health data registries is available from the Swedish National Board of Health and Welfare: https://www.socialstyrelsen.se/en/statistics-and-data/registers/.
Transparency: The corresponding author (CC) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported, no important aspects of the study have been omitted, and any discrepancies from the study as planned have been explained.
Patient and Public Involvement Statement: Patients and the public were not involved in the planning or conduct of this study, because of data protection restrictions and the highly technical methods required to do a data linkage analysis.
Dissemination: The results will be disseminated to patients and relevant communities through a website and press releases suitable for a non-specialized audience.
Contributor Information
Casey Crump, Icahn School of Medicine at Mount Sinai, Departments of Family Medicine and Community Health and of Population Health Science and Policy, One Gustave L. Levy Place, New York, New York 10029, USA..
Jan Sundquist, Lund University, Department of Clinical Sciences, Clinical Research Centre (CRC), building 28, floor 11, Jan Waldenströms gata 35, Skåne University Hospital, SE-205 02 Malmö, Sweden.
Kristina Sundquist, Lund University, Department of Clinical Sciences, Clinical Research Centre (CRC), building 28, floor 11, Jan Waldenströms gata 35, Skåne University Hospital, SE-205 02 Malmö, Sweden.
REFERENCES
- 1.Stern J, Pier J, Litonjua AA. Asthma epidemiology and risk factors. Semin Immunopathol 2020;42(1):5–15. doi: 10.1007/s00281-020-00785-1 [published Online First: 2020/02/06] [DOI] [PubMed] [Google Scholar]
- 2.Asher MI, Garcia-Marcos L, Pearce NE, et al. Trends in worldwide asthma prevalence. Eur Respir J 2020;56(6):2002094. doi: 10.1183/13993003.02094-2020 [published Online First: 2020/09/26] [DOI] [PubMed] [Google Scholar]
- 3.Kuruvilla ME, Vanijcharoenkarn K, Shih JA, et al. Epidemiology and risk factors for asthma. Respir Med 2019;149:16–22. doi: 10.1016/j.rmed.2019.01.014 [published Online First: 2019/03/20] [DOI] [PubMed] [Google Scholar]
- 4.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012;379(9832):2162–72. doi: 10.1016/S0140-6736(12)60820-4 [published Online First: 2012/06/12] [DOI] [PubMed] [Google Scholar]
- 5.Crump C, Sundquist J, Winkleby MA, et al. Gestational age at birth and mortality from infancy into mid-adulthood: a national cohort study. Lancet Child Adolesc Health 2019;3(6):408–17. doi: 10.1016/S2352-4642(19)30108-7 [published Online First: 2019/04/09] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crump C, Winkleby MA, Sundquist J, et al. Prevalence of Survival Without Major Comorbidities Among Adults Born Prematurely. JAMA 2019;322(16):1580–88. doi: 10.1001/jama.2019.15040 [published Online First: 2019/10/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Been JV, Lugtenberg MJ, Smets E, et al. Preterm birth and childhood wheezing disorders: a systematic review and meta-analysis. PLoS Med 2014;11(1):e1001596. doi: 10.1371/journal.pmed.1001596 [published Online First: 2014/02/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nasanen-Gilmore P, Sipola-Leppanen M, Tikanmaki M, et al. Lung function in adults born preterm. PLoS One 2018;13(10):e0205979. doi: 10.1371/journal.pone.0205979 [published Online First: 2018/10/20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vollsaeter M, Roksund OD, Eide GE, et al. Lung function after preterm birth: development from mid-childhood to adulthood. Thorax 2013;68(8):767–76. doi: 10.1136/thoraxjnl-2012-202980 [published Online First: 2013/06/12] [DOI] [PubMed] [Google Scholar]
- 10.Kotecha SJ, Edwards MO, Watkins WJ, et al. Effect of preterm birth on later FEV1: a systematic review and meta-analysis. Thorax 2013;68(8):760–6. doi: 10.1136/thoraxjnl-2012-203079 [published Online First: 2013/04/23] [DOI] [PubMed] [Google Scholar]
- 11.Doyle LW, Irving L, Haikerwal A, et al. Airway obstruction in young adults born extremely preterm or extremely low birth weight in the postsurfactant era. Thorax 2019;74(12):1147–53. doi: 10.1136/thoraxjnl-2019-213757 [published Online First: 2019/09/29] [DOI] [PubMed] [Google Scholar]
- 12.Doyle LW, Andersson S, Bush A, et al. Expiratory airflow in late adolescence and early adulthood in individuals born very preterm or with very low birthweight compared with controls born at term or with normal birthweight: a meta-analysis of individual participant data. Lancet Respir Med 2019;7(8):677–86. doi: 10.1016/S2213-2600(18)30530-7 [published Online First: 2019/05/13] [DOI] [PubMed] [Google Scholar]
- 13.Damgaard AL, Hansen BM, Mathiasen R, et al. Prematurity and prescription asthma medication from childhood to young adulthood: a Danish national cohort study. PLoS One 2015;10(2):e0117253. doi: 10.1371/journal.pone.0117253 [published Online First: 2015/02/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Svanes C, Omenaas E, Heuch JM, et al. Birth characteristics and asthma symptoms in young adults: results from a population-based cohort study in Norway. Eur Respir J 1998;12(6):1366–70. doi: 10.1183/09031936.98.12061366 [published Online First: 1999/01/07] [DOI] [PubMed] [Google Scholar]
- 15.Mallen CD, Mottram S, Wynne-Jones G, et al. Birth-related exposures and asthma and allergy in adulthood: a population-based cross-sectional study of young adults in North Staffordshire. J Asthma 2008;45(4):309–12. doi: 10.1080/02770900801911194 [published Online First: 2008/05/01] [DOI] [PubMed] [Google Scholar]
- 16.Pekkanen J, Xu B, Jarvelin MR. Gestational age and occurrence of atopy at age 31--a prospective birth cohort study in Finland. Clin Exp Allergy 2001;31(1):95–102. [published Online First: 2001/02/13] [PubMed] [Google Scholar]
- 17.Matheson MC, AL DO, Burgess JA, et al. Preterm birth and low birth weight continue to increase the risk of asthma from age 7 to 43. J Asthma 2017;54(6):616–23. doi: 10.1080/02770903.2016.1249284 [published Online First: 2016/10/30] [DOI] [PubMed] [Google Scholar]
- 18.Braback L, Hedberg A. Perinatal risk factors for atopic disease in conscripts. Clin Exp Allergy 1998;28(8):936–42. doi: 10.1046/j.1365-2222.1998.00282.x [published Online First: 1998/10/02] [DOI] [PubMed] [Google Scholar]
- 19.Crump C, Sundquist K, Winkleby MA, et al. Early-term birth (37–38 weeks) and mortality in young adulthood. Epidemiology 2013;24(2):270–6. doi: 10.1097/EDE.0b013e318280da0f [DOI] [PubMed] [Google Scholar]
- 20.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. doi: 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sundquist J, Ohlsson H, Sundquist K, et al. Common adult psychiatric disorders in Swedish primary care where most mental health patients are treated. BMC Psychiatry 2017;17(1):235. doi: 10.1186/s12888-017-1381-4 [published Online First: 2017/07/02] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crump C, Friberg D, Li X, et al. Preterm birth and risk of sleep-disordered breathing from childhood into mid-adulthood. Int J Epidemiol 2019;48(6):2039–49. doi: 10.1093/ije/dyz075 [published Online First: 2019/04/22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crump C, Howell EA, Stroustrup A, et al. Association of Preterm Birth With Risk of Ischemic Heart Disease in Adulthood. JAMA Pediatr 2019;173(8):736–43. doi: 10.1001/jamapediatrics.2019.1327 [published Online First: 2019/06/04] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crump C, Groves A, Sundquist J, et al. Association of Preterm Birth With Long-term Risk of Heart Failure Into Adulthood. JAMA Pediatr 2021;175(7):689–97. doi: 10.1001/jamapediatrics.2021.0131 [published Online First: 2021/04/06] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crump C, Sundquist J, Sundquist K. Preterm birth and risk of type 1 and type 2 diabetes: a national cohort study. Diabetologia 2020;63(3):508–18. doi: 10.1007/s00125-019-05044-z [published Online First: 2019/12/06] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanderweele TJ, Knol MJ. A tutorial on interaction. Epidemiol Methods 2014;3:33–72. [Google Scholar]
- 27.Kleinbaum DG, Kupper LL, Morgenstern H. Epidemiologic Research: Principles and Quantitative Methods. New York: Wiley; 1982. [Google Scholar]
- 28.Crump C, Winkleby MA, Sundquist J, et al. Risk of asthma in young adults who were born preterm: a Swedish national cohort study. Pediatrics 2011;127(4):e913–20. doi: 10.1542/peds.2010-2603 [published Online First: 2011/03/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Zhang Z, Chen O. What is the impact of birth weight corrected for gestational age on later onset asthma: a meta-analysis. Allergy Asthma Clin Immunol 2022;18(1):1. doi: 10.1186/s13223-021-00633-3 [published Online First: 2022/01/06] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010;126(3):443–56. doi: 10.1542/peds.2009-2959 [published Online First: 2010/08/25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Express Group, Fellman V, Hellstrom-Westas L, et al. One-year survival of extremely preterm infants after active perinatal care in Sweden. JAMA 2009;301(21):2225–33. doi: 10.1001/jama.2009.771 [published Online First: 2009/06/06] [DOI] [PubMed] [Google Scholar]
- 32.Siffel C, Kistler KD, Lewis JFM, et al. Global incidence of bronchopulmonary dysplasia among extremely preterm infants: a systematic literature review. J Matern Fetal Neonatal Med 2019:1–11. doi: 10.1080/14767058.2019.1646240 [published Online First: 2019/08/10] [DOI] [PubMed] [Google Scholar]
- 33.Stoll BJ, Hansen NI, Bell EF, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA 2015;314(10):1039–51. doi: 10.1001/jama.2015.10244 [published Online First: 2015/09/09] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhatia J, Parish A. Nutrition and the lung. Neonatology 2009;95(4):362–7. doi: 10.1159/000209302 [published Online First: 2009/06/06] [DOI] [PubMed] [Google Scholar]
- 35.Jaakkola JJ, Gissler M. Maternal smoking in pregnancy, fetal development, and childhood asthma. Am J Public Health 2004;94(1):136–40. doi: 10.2105/ajph.94.1.136 [published Online First: 2004/01/10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carey MA, Card JW, Voltz JW, et al. It’s all about sex: gender, lung development and lung disease. Trends Endocrinol Metab 2007;18(8):308–13. doi: 10.1016/j.tem.2007.08.003 [published Online First: 2007/09/04] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collaco JM, McGrath-Morrow SA. Respiratory Phenotypes for Preterm Infants, Children, and Adults: Bronchopulmonary Dysplasia and More. Ann Am Thorac Soc 2018;15(5):530–38. doi: 10.1513/AnnalsATS.201709-756FR [published Online First: 2018/01/13] [DOI] [PubMed] [Google Scholar]
- 38.Martinez FD. Early-Life Origins of Chronic Obstructive Pulmonary Disease. N Engl J Med 2016;375(9):871–8. doi: 10.1056/NEJMra1603287 [published Online First: 2016/09/01] [DOI] [PubMed] [Google Scholar]
- 39.Bui DS, Perret JL, Walters EH, et al. Association between very to moderate preterm births, lung function deficits, and COPD at age 53 years: analysis of a prospective cohort study. Lancet Respir Med 2022;10(5):478–84. doi: 10.1016/S2213-2600(21)00508-7 [published Online First: 2022/02/22] [DOI] [PubMed] [Google Scholar]
- 40.Crump C Medical history taking in adults should include questions about preterm birth. BMJ 2014;349:g4860. doi: 10.1136/bmj.g4860 [DOI] [PubMed] [Google Scholar]
- 41.Dharmage SC, Bui DS, Perret JL, et al. Lung function deficits of adults born very preterm and with very low birthweight. Lancet Respir Med 2019;7(8):643–45. doi: 10.1016/S2213-2600(19)30042-6 [published Online First: 2019/05/13] [DOI] [PubMed] [Google Scholar]
- 42.Shapiro-Mendoza CK, Barfield WD, Henderson Z, et al. CDC Grand Rounds: Public Health Strategies to Prevent Preterm Birth. MMWR Morb Mortal Wkly Rep 2016;65(32):826–30. doi: 10.15585/mmwr.mm6532a4 [published Online First: 2016/08/19] [DOI] [PubMed] [Google Scholar]
- 43.Begg MD, Parides MK. Separation of individual-level and cluster-level covariate effects in regression analysis of correlated data. Stat Med 2003;22(16):2591–602. doi: 10.1002/sim.1524 [published Online First: 2003/08/05] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


