Abstract
Protection against infections with Streptococcus pneumoniae depends on the presence of antibodies against capsular polysaccharides that facilitate phagocytosis. Asplenic patients are at increased risk for pneumococcal infections, since both phagocytosis and the initiation of the antibody response to polysaccharides take place in the spleen. Therefore, vaccination with pneumococcal polysaccharide vaccines is recommended prior to splenectomy, which, as in the case of trauma, is not always feasible. We show that in rats, vaccination with a pneumococcal conjugate vaccine can induce good antibody responses even after splenectomy, particularly after a second dose. The spleen remains necessary for a fast, primary response to (blood-borne) polysaccharides, even when they are presented in a conjugated form. Coadministration of a conjugate vaccine with additional nonconjugated polysaccharides of other serotypes did not improve the response to the nonconjugated polysaccharides. We conclude that pneumococcal conjugate vaccines can be of value in protecting asplenic or hyposplenic patients against pneumococcal infections.
Protection against infections with encapsulated bacteria such as Streptococcus pneumoniae depends on the presence of antibodies against capsular polysaccharides. These serotype-specific antibodies facilitate phagocytosis, the main mode of host defense against S. pneumoniae. The spleen is an important organ in the immune response to S. pneumoniae, since it contains both antibody-producing B cells and phagocytes (4, 26). Asplenic patients are therefore at increased risk for invasive infections with S. pneumoniae (13). Although most infections occur within the first few years after splenectomy, the risk of overwhelming postsplenectomy infections is lifelong (9, 36). Therefore, vaccination against S. pneumoniae is indicated for this group. At present, the vaccine available for this aim is the 23-valent pneumococcal polysaccharide (PPS) vaccine (1). The immunogenicity of PPS vaccine in splenectomized patients has been assessed in a number of trials. Some studies have shown the effectiveness of vaccination in inducing protective concentrations of specific antibodies (5, 10, 24, 33), while others have shown limited serological responses (15). As the initiation of the antibody response to polysaccharides seems to depend on the presence of splenic tissue, and in particular on a functional marginal-zone B-cell compartment (3, 14, 18, 34), it may be expected that polysaccharide vaccines are of limited use in asplenic patients.
The physical coupling of a polysaccharide to a carrier protein greatly improves the immunogenicity of the polysaccharide, a principle first described in 1929 (11). Conjugated polysaccharides overcome the antipolysaccharide unresponsiveness in early life (2, 8), which is thought to be due to a still immature splenic marginal-zone B-cell compartment (2). The induction of antipolysaccharide antibodies by conjugate vaccines at a young age suggests that conjugates might initiate the antipolysaccharide antibody response in the absence of a functional splenic marginal-zone B-cell compartment. The recently developed pneumococcal conjugate vaccines (PCV) may thus be of value in the management of hypo- and asplenic patients.
To test the hypothesis of the relative splenic independence of the antipolysaccharide antibody response after conjugate vaccination, we studied the immunogenicity of a tetravalent PCV after splenectomy. We used a rat model for which it was previously shown that splenectomy had a clearly negative effect on the antipolysaccharide antibody response (21). Because PCV contain only a limited number (4 to 11) of pneumococcal serotypes compared with the number contained by 23-valent PPS vaccines, we studied the potential benefit of admixture of additional polysaccharides with the PCV. We also studied the effect of booster immunization. Since the spleen is the only site of specific antibody production very early after exposure to blood-borne antigens, we compared antibody responses after immunization via two different routes, the subcutaneous (s.c.) and intravenous (i.v.) routes, or by the s.c. route alone.
MATERIALS AND METHODS
Animals, splenectomy, and immunization.
This study was approved by the University of Groningen Institutional Review Board. Young adult male Wistar rats (n = 60; Harlan, Horst, The Netherlands) of approximately 200 g each were housed under standard laboratory conditions on a 12-h light–12-h dark cycle. They were fed standard laboratory rat food (Hope Farms, Inc., Woerden, The Netherlands) and tap water ad libitum. Splenectomy was performed 6 weeks before vaccination on 30 rats via an upper midline incision under clean, but not sterile, conditions (27). At time zero and 28 days later, both the splenectomized rats and control rats were immunized with one of the vaccines described below. In the first series, animals were simultaneously immunized with 0.5 ml of vaccine s.c. as well as 0.5 ml i.v. in the tail vein. In the second series of experiments, the vaccine (0.5 ml) was administered s.c. only. Each experimental group consisted of five rats. All rats were marked individually to allow antibody titers to be analyzed longitudinally for single rats.
At time zero and at days 5, 28, 33 (5 days after the second vaccination), and 56 (28 days after the second vaccination), blood was obtained from the tail vein (time points were based on previous experience [Breukels et al., unpublished data]). Blood was allowed to clot on ice for 2 h. Serum samples were stored at −20°C until time of analysis.
Vaccines. (i) PPS.
PPS serotypes 4, 6B, 9V, 14, 19F, and 23F (American Type Culture Collection [ATCC], Manassas, Va.) were dissolved in 0.9% NaCl to reach a concentration of 5 μg/ml for each serotype. Each 0.5-ml dose of this PPS vaccine compares to 1/10 of the human dose.
(ii) PCV.
We used a tetravalent PCV (National Institute of Public Health and the Environment, Bilthoven, The Netherlands) consisting of 50-kDa polysaccharides of pneumococcal serotypes 6B, 14, 19F, and 23F, conjugated to tetanus toxoid carrier protein via a cysteamine linker (28), with aluminium phosphate as an adjuvant. This conjugate was then mixed with unconjugated polysaccharide serotypes 4 and 9V (ATCC). Each 0.5-ml dose of this vaccine (PCV) contained 0.36 μg of polysaccharides of serotypes 14, 19F, and 23F; 1.08 μg of polysaccharides of serotype 6B; and 2.5 μg of the unconjugated PPSs of serotypes 4 and 9V.
Type-specific anti-pneumococcal antibody determinations.
Levels of serum immunoglobulin G (IgG) against all six polysaccharides included in the two vaccines (serotypes 4, 6B, 9V, 14, 19F, and 23) were measured by enzyme-linked immunosorbent assay (ELISA). All serum samples were preincubated overnight with excess free common cell wall polysaccharide to neutralize anti-common cell wall polysaccharide antibodies (12, 25).
Microtiter plates (Greiner Labortechnik, Langerthal, Germany) were coated with pneumococcal capsular polysaccharides (ATCC; 10 μg/ml in 0.9% NaCl) and stored overnight at 37°C. Subsequently, plates were washed with phosphate-buffered saline (PBS)–0.05% (vol/vol) Tween 20 and incubated with serial dilutions of serum samples in PBS–0.05% Tween 20–1% (vol/vol) bovine serum albumin. After being washed (PBS–0.05% Tween 20), the plates were incubated for 2 h (37°C) with peroxidase-labeled goat anti-rat IgG (Southern Biotechnology Associates, Birmingham, Ala.). After the plates were washed and incubated with an enzyme substrate for 20 min at room temperature, absorbance was read at 450 nm on a Millennia ELISA reader (Flow Laboratories, Inc., Irvine, Calif.).
Serum samples from individual rats obtained prevaccination and at the different time points after vaccination were analyzed simultaneously. The antibody concentrations in the serum samples were calculated by comparison with the antibody concentrations of a rat hyperimmune serum pool generated from the sera of rats vaccinated with a heptavalent PCV (Prevnar; Wyeth Lederle Vaccines and Pediatrics, Rochester, N.Y.; containing serotypes 4, 6B, 9F, 14, 18C, 19F, and 23F) or a 23-valent PPS vaccine (Pneumovax; Merck, West Point, Pa.). This pool was included in every ELISA run as a standard. The antibody concentrations in this hyperimmune pool were considered to be 100 U/ml (100%) for each serotype; antibody titers were expressed in units per milliliter. Based on comparison of optical density readings, type-specific antibody concentrations in this hyperimmune standard were highest for serotypes 4, 9F, and 19F and lower for serotypes 6B and 23F. Both the hyperimmune serum pool and the individual postimmunization samples showed low optical density readings for serotype 14. No reliable anti-polysaccharide 14 antibody titers could therefore be assigned to sera after vaccination; only trends in antibody titers could be documented.
Statistical analysis.
Serological data from rats of the different vaccine groups (five rats per group) were analyzed and compared for statistically significant differences by using the Mann-Whitney U test. P values of <0.05 were considered significant.
RESULTS
Splenectomy impairs the antibody response to PPS.
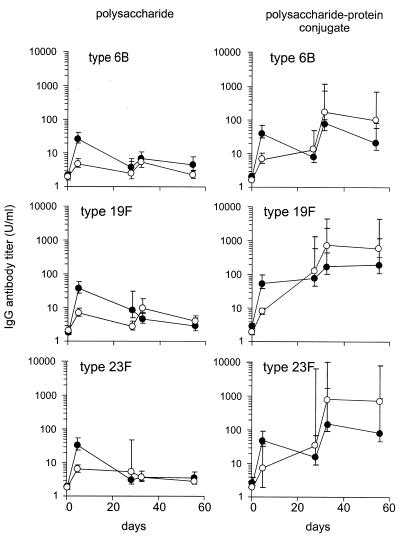
In control rats, vaccination with the 6-valent PPS mixture induced a clear IgG response to all serotypes except serotype 14 (Fig. 1, left panels; data for serotype 14 are not shown). Maximal geometric mean antibody titers, obtained on day 5, ranged from 21 to 57 U/ml. In splenectomized rats, maximum geometric mean antibody titers were significantly (P < 0.01) lower and remained <10 U/ml for the five serotypes (range, 2 to 10 U/ml). A second vaccination with the PPS mixture did not improve the antibody response.
FIG. 1.
IgG responses to vaccination with PPSs (left panels) and PPS-protein conjugates (right panels) in control and splenectomized rats. Control rats (filled symbols) and splenectomized rats (open symbols) were vaccinated i.v. and s.c. at days 0 and 28 either with 2.5 μg of each of PPS serotypes 4, 6B, 9V, 14, 19F, and 23F or with a tetravalent, PCV consisting of polysaccharides of pneumococcal serotypes 6B, 14, 19F, and 23F conjugated to tetanus toxoid. Left panels show IgG responses at days 0, 5, 28, 33, and 56 after vaccination with PPS. Antibody responses to serotypes 4 and 9V are not shown but displayed a course similar to that for serotype 6B. Right panels show IgG responses at days 0, 5, 28, 33, and 56 after vaccination with PCV. Antibody titers are expressed in units per milliliter. Shown are geometric mean antibody titers and standard errors of the means.
Antibody responses to PCV.
In control rats, the primary response after vaccination with PCV resulted in serotype-specific antibody titers at day 5 that were comparable with those acquired after one dose of PPS vaccine (geometric mean antibody titers, 27 to 83 U/ml) (Fig. 1, right panels). After splenectomy, IgG titers at day 5 were significantly (P < 0.01) lower than those in controls 5 days after vaccination with PCV. However, in the period between days 5 and 28 after PCV injection, IgG titers increased steadily and reached levels comparable with those in control rats at day 28.
Revaccination with PCV 4 weeks after the first PCV dose resulted in a further increase in antibody concentration in control as well as in splenectomized rats. In splenectomized rats, antibody titers after the second dose of PCV were significantly (P < 0.05) higher than the maximal antibody titers reached after PPS vaccination. Thus, it can be concluded that PCV can overcome polysaccharide unresponsiveness in splenectomized rats.
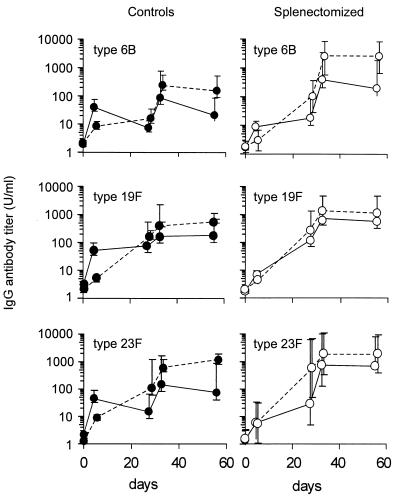
Results of s.c. compared with results of combined i.v. and s.c. injection of PCV.
After s.c. injection of PCV in control rats, the titers of specific antibodies to serotypes 6B, 19F, and 23F at day 5 were significantly (P < 0.05) lower than after combined i.v. and s.c. vaccination. However, antibody titers rose gradually over the first 4 weeks to reach titers equal to the maximal titers already achieved at day 5 after combined i.v. and s.c. injection (Fig. 2). After splenectomy, this response pattern (a gradual rise in antibody titer over 4 weeks) was seen after s.c. as well as after combined i.v. and s.c. vaccination. No significant differences were seen in antibody responses between both vaccination routes, nor did antibody patterns after s.c. vaccination differ significantly between control and splenectomized rats. These data indicate that the spleen is important in the fast, primary response to blood-borne polysaccharide antigens, even if presented in conjugate form. The response patterns after a second vaccination with PCV did not differ among the four groups: a rise in antibody titer was already observed after 5 days.
FIG. 2.
Antipneumococcal IgG response after s.c. vaccination (left panels) compared with that of combined s.c. and i.v. vaccination with PCV (right panels). Control rats (filled symbols) and splenectomized rats (open symbols) were vaccinated s.c. (broken lines) or i.v. as well as s.c. (solid lines) at days 0 and 28 with PCV. See Fig. 1 legend for further details.
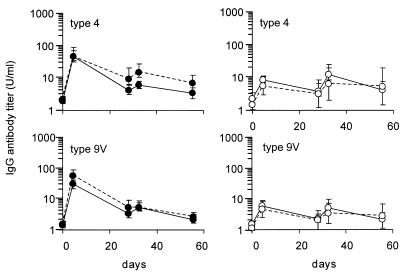
Coadministration of nonconjugated polysaccharide with PCV.
The above data indicate that in splenectomized rats, PCV induce higher antibody responses than PPS vaccines do. We administered PPS vaccine together with PCV to see whether admixture of PPS vaccine with PCV would improve the antibody response to PPS vaccine and thus increase the limited serotype coverage of PCV. No differences in antibody responses were detected after admixture of PPS serotypes 4 and 9V with the 4-valent PCV and after admixture with unconjugated forms of the PPS serotypes 6B, 14, 19F, and 23F (Fig. 3). The poor immunogenicity of nonconjugated polysaccharides in splenectomized rats therefore cannot be improved by concomitant immunization with a conjugate vaccine.
FIG. 3.
Antibody response to serotype 4 and 9V polysaccharides mixed with native or conjugated polysaccharides. Control rats (filled symbols) and splenectomized rats (open symbols) were i.v. and s.c. vaccinated on days 0 and 28 with 2.5 μg of the PPS serotypes 4 and 9V mixed with conjugated (broken lines) or native (solid lines) polysaccharides of serotypes 6B, 14, 19F, and 23F. See Fig. 1 legend for further details.
DISCUSSION
Despite the wide range of antibodies available, invasive pneumococcal infections remain a substantial cause of morbidity and mortality (17, 23). Patients with anatomical or functional asplenia are particularly at risk (13, 32) because the spleen is the most important site of phagocytosis of poorly opsonized antigens in the early phase of bacterial invasion, before sufficient amounts of specific antibodies have been produced (26). After a splenectomy, the liver will partially take over the phagocytic function of the spleen. However, the liver needs a higher level of opsonization because of the relatively high velocity of blood flow (22). Because of the splenic dependency of the antipolysaccharide antibody response, the opsonization of encapsulated bacteria may be suboptimal after splenectomy (4). Therefore, immunization with PPS vaccine is recommended prior to elective splenectomy (1), although efficacy data are scarce (7). However, vaccination prior to splenectomy is not always feasible, for instance, after trauma.
PCV are developed to enhance the immune response to PPSs. Asplenic or hyposplenic patients might benefit from vaccination with PCV because polysaccharide-protein conjugates can initiate an antibody response to the polysaccharide without the need for a functional spleen (19, 35, 37). We therefore tested the immunogenicity of PCV in splenectomized rats and showed that a series of two doses of PCV given 6 weeks after splenectomy resulted in antibody titers that were at least as high as in control rats but that the antibody response to PPS was impaired by splenectomy (Fig. 1). This indicates that the immunogenicity of PCV, in contrast with that of PPS vaccine, is not impaired by splenectomy.
The spleen is of particular importance not only for its antibody response to polysaccharide antigens, as is indicated above, but also as the site of antibody generation very early after exposure to all blood-borne antigens. We have shown that the spleen produces a fast primary antibody response to PCV injected into the bloodstream. After i.v. vaccination of control animals with PCV, maximum antibody titers were reached by day 5 (Fig. 1 and 2). However, in the absence of splenic tissue, the antibody response to i.v. injected PCV was delayed and no difference was observed between the s.c. and i.v. routes of vaccination: antibody titers rose gradually to reach a maximum after 28 days. These data indicate that extrasplenic lymphoid tissues (lymph nodes) are able to generate a primary antibody response to PCV, but this response is slower than the splenic response. The antibody response patterns to a second dose of PCV, either s.c. or i.v. injected, are similar in the presence and in the absence of splenic tissue, implying a less prominent role for the spleen in the secondary response.
PCVs, as we have shown, have as an advantage that they can induce antibody responses in the absence of a spleen but have as a major drawback that the number of serotypes that can be included is limited. The licensed heptavalent PCV (Prevnar; Wyeth Lederle Vaccines and Pediatrics) includes seven serotypes. Although 11-valent PCVs are under evaluation, they do not have the same coverage of serotypes as that of the 23-valent PPS vaccine. Theoretically, admixture of the PPS vaccine with the PCV could be an approach to broaden the coverage. Conjugation of a polysaccharide to a protein changes the nature of the antipolysaccharide antibody response, from a T-cell-independent to a T-cell-dependent response (16, 29). Antigen-presenting B cells probably take up the conjugated polysaccharide-protein molecule and internalize it via membrane Igs and present the peptides of the protein to T-helper cells in association with major histocompatibility complex class II molecules on their surfaces. This induces T-helper cells to stimulate polysaccharide-specific B cells to mature into antibody-producing plasma cells or into memory cells (20, 30, 31). According to this model, activation of polysaccharide-specific B cells could take place either via direct physical interaction with peptide-specific T-helper cells or via soluble factors secreted by peptide-specific T cells (6). Our experimental data indicate that responses to PPS serotypes 4 and 9V do not improve by admixture with 4-valent PCV containing serotypes 6B, 14, 19F, and 23F (Fig. 3). Distinct differences in localization may explain this finding: in the splenic marginal zone, PPS specifically localizes on marginal-zone B cells, whereas for PCV, this localization on marginal-zone B cells is far less pronounced (Breukels et al., unpublished data). Physical interaction of serotype 4- and 9V-specific B cells with peptide-specific T cells may not take place, and soluble factors secreted by peptide-specific T cells may be short-range-acting cytokines unable to stimulate more distantly located B cells.
It should be noted that both the capacity to respond to bacterial polysaccharide antigens and the unique capacity of the spleen to phagocytose poorly opsonized bacteria are lost by splenectomy. Spleen-saving procedures or splenic autotransplantation at the time of splenectomy may potentially preserve or restore at least part of the splenic immune function and should be pursued. Protection provided by conjugate vaccines is limited to the antigens present in the vaccine. The clinical efficacy of PCVs for asplenic and splenectomized patients in preventing overwhelming postsplenectomy infections therefore remains to be established, but this type of vaccine, extended with more vaccine antigens, seems at present to be the best option for patients with (functional) asplenia.
Our data indicate that the spleen is of prime importance in the antibody response to PPS vaccine. In the absence of splenic tissue, the ability to mount a primary antibody response to PPS vaccine is lost. This may explain the greatly increased risk of invasive infections with encapsulated bacteria like S. pneumoniae after splenectomy. We have shown that the antibody response to PCV is independent of the spleen: in control as well as in splenectomized rats and after i.v. as well as s.c. pneumococcal conjugate vaccination, antibody responses are generated. We therefore conclude that PCV may be of value in the management of anatomic as well as functional asplenic patients.
ACKNOWLEDGMENTS
M.A.B. is financially supported by The Netherlands Medical Research Foundation, grant 920-02-091. Financial support for this study was given by the Groningen Foundation for Pediatric Oncology Research and the J. K. de Cock Foundation.
Wyeth Lederle Vaccines and Pediatrics, Rochester, N.Y., provided the heptavalent PCV for these studies.
REFERENCES
- 1.Advisory Committee on Immunization Practices. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP) Morb Mortal Wkly Rep. 1997;46:1–24. [PubMed] [Google Scholar]
- 2.Ahman H, Kayhty H, Tamminen P, Vuorela A, Malinoski F, Eskola J. Pentavalent pneumococcal oligosaccharide conjugate vaccine PncCRM is well-tolerated and able to induce an antibody response in infants. Pediatr Infect Dis J. 1996;15:134–139. doi: 10.1097/00006454-199602000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Amlot P L, Grennan D, Humphrey J H. Splenic dependence of the antibody response to thymus-independent (TI-2) antigens. Eur J Immunol. 1985;15:508–512. doi: 10.1002/eji.1830150516. [DOI] [PubMed] [Google Scholar]
- 4.Amlot P L, Hayes A E. Impaired human antibody response to the thymus-independent antigen, DNP-Ficoll, after splenectomy. Implications for post-splenectomy infections. Lancet. 1985;i:1008–1011. doi: 10.1016/s0140-6736(85)91613-7. [DOI] [PubMed] [Google Scholar]
- 5.Ammann A J, Addiego J, Wara D W, Lubin B, Smith W B, Mentzer W C. Polyvalent pneumococcal-polysaccharide immunization of patients with sickle-cell anemia and patients with splenectomy. N Engl J Med. 1977;297:897–900. doi: 10.1056/NEJM197710272971701. [DOI] [PubMed] [Google Scholar]
- 6.Breukels M A, Rijkers G T, Voorhorst-Ogink M M, Zegers B J M. Regulatory T cells in the antibody response to Haemophilus influenzae type b polysaccharide. Infect Immun. 1999;67:789–793. doi: 10.1128/iai.67.2.789-793.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler J C, Breiman R F, Campbell J F, Lipman H B, Broome C V, Facklam R R. Pneumococcal polysaccharide vaccine efficacy. An evaluation of current recommendations. JAMA. 1993;270:1826–1831. [PubMed] [Google Scholar]
- 8.Eskola J, Kayhty H, Takala A K, Peltola H, Ronnberg P R, Kela E, Pekkanen E, McVerry P H, Makela P H. A randomized, prospective field trial of a conjugate vaccine in the protection of infants and young children against invasive Haemophilus influenzae type b disease. N Engl J Med. 1990;323:1381–1387. doi: 10.1056/NEJM199011153232004. [DOI] [PubMed] [Google Scholar]
- 9.Evans D I. Postsplenectomy sepsis 10 years or more after operation. J Clin Pathol. 1985;38:309–311. doi: 10.1136/jcp.38.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giebink G S, Foker J E, Kim Y, Schiffman G. Serum antibody and opsonic responses to vaccination with pneumococcal capsular polysaccharide in normal and splenectomized children. J Infect Dis. 1980;141:404–412. doi: 10.1093/infdis/141.3.404. [DOI] [PubMed] [Google Scholar]
- 11.Goebel W F, Avery O T. Chemo-immunological studies on conjugated carbohydrate proteins. II. Immunological specificity of synthetic sugar proteins. J Exp Med. 1929;50:533–550. doi: 10.1084/jem.50.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldblatt D, Levinsky R J, Turner M W. Role of cell wall polysaccharide in the assessment of IgG antibodies to the capsular polysaccharides of Streptococcus pneumoniae in childhood. J Infect Dis. 1992;166:632–634. doi: 10.1093/infdis/166.3.632. [DOI] [PubMed] [Google Scholar]
- 13.Gopal V, Bisno A L. Fulminant pneumococcal infections in ‘normal’ asplenic hosts. Arch Intern Med. 1977;137:1526–1530. [PubMed] [Google Scholar]
- 14.Guinamard R, Okigaki M, Schlessinger J, Ravetch J V. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat Immunol. 2000;1:31–36. doi: 10.1038/76882. [DOI] [PubMed] [Google Scholar]
- 15.Hosea S W, Burch C G, Brown E J, Berg R A, Frank M M. Impaired immune response of splenectomised patients to polyvalent pneumococcal vaccine. Lancet. 1981;i:804–807. doi: 10.1016/s0140-6736(81)92681-7. [DOI] [PubMed] [Google Scholar]
- 16.Insel R A, Anderson P W. Response to oligosaccharide-protein conjugate vaccine against Haemophilus influenzae b in two patients with IgG2 deficiency unresponsive to capsular polysaccharide vaccine. N Engl J Med. 1986;315:499–503. doi: 10.1056/NEJM198608213150807. [DOI] [PubMed] [Google Scholar]
- 17.Klein J O. The epidemiology of pneumococcal disease in infants and children. Rev Infect Dis. 1981;3:246–253. doi: 10.1093/clinids/3.2.246. [DOI] [PubMed] [Google Scholar]
- 18.Kraal G, Ter Hart H, Meelhuizen C, Venneker G, Claassen E. Marginal zone macrophages and their role in the immune response against T-independent type 2 antigens: modulation of the cells with specific antibody. Eur J Immunol. 1989;19:675–680. doi: 10.1002/eji.1830190416. [DOI] [PubMed] [Google Scholar]
- 19.Kristensen K. Antibody response to a Haemophilus influenzae type b polysaccharide tetanus toxoid conjugate vaccine in splenectomized children and adolescents. Scand J Infect Dis. 1992;24:629–632. doi: 10.3109/00365549209054649. [DOI] [PubMed] [Google Scholar]
- 20.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 21.Leemans R, Harms G, Rijkers G T, Timens W. Spleen autotransplantation provides restoration of functional splenic lymphoid compartments and improves the humoral immune response to pneumococcal polysaccharide vaccine. Clin Exp Immunol. 1999;117:596–604. doi: 10.1046/j.1365-2249.1999.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lockwood C M. Immunological functions of the spleen. Clin Haematol. 1983;12:449–465. [PubMed] [Google Scholar]
- 23.Macfarlane J T, Finch R G, Ward M J, Macrae A D. Hospital study of adult community-acquired pneumonia. Lancet. 1982;ii:255–258. doi: 10.1016/s0140-6736(82)90334-8. [DOI] [PubMed] [Google Scholar]
- 24.Molrine D C, Siber G R, Samra Y, Shevy D S, MacDonald K, Cieri R, Ambrosino D M. Normal IgG and impaired IgM responses to polysaccharide vaccines in asplenic patients. J Infect Dis. 1999;179:513–517. doi: 10.1086/314582. [DOI] [PubMed] [Google Scholar]
- 25.Musher D M, Luchi M J, Watson D A, Hamilton R, Baughn R E. Pneumococcal polysaccharide vaccine in young adults and older bronchitics: determination of IgG responses by ELISA and the effect of adsorption of serum with non-type-specific cell wall polysaccharide. J Infect Dis. 1990;161:728–735. doi: 10.1093/infdis/161.4.728. [DOI] [PubMed] [Google Scholar]
- 26.Pabst R. The spleen in lymphocyte migration. Immunol Today. 1988;9:43–45. doi: 10.1016/0167-5699(88)91258-3. [DOI] [PubMed] [Google Scholar]
- 27.Pabst R, Hafke R, Hillebrand J. Enhanced regeneration of transplanted splenic tissue by increased work load to the splenic compartments. J Trauma. 1985;25:326–328. doi: 10.1097/00005373-198504000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Pawlowski A, Kallenius G, Svenson S B. A new method of non-cross-linking conjugation of polysaccharides to proteins via thioether bonds for the preparation of saccharide-protein conjugate vaccines. Vaccine. 1999;17:1474–1483. doi: 10.1016/s0264-410x(98)00385-5. [DOI] [PubMed] [Google Scholar]
- 29.Robbins J B, Schneerson R. Polysaccharide-protein conjugates: a new generation of vaccines. J Infect Dis. 1990;161:821–832. doi: 10.1093/infdis/161.5.821. [DOI] [PubMed] [Google Scholar]
- 30.Schneerson R, Barrera O, Sutton A, Robbins J B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980;152:361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siber G R. Pneumococcal disease: prospects for a new generation of vaccines. Science. 1994;265:1385–1387. doi: 10.1126/science.8073278. [DOI] [PubMed] [Google Scholar]
- 32.Spickett G P, Bullimore J, Wallis J, Smith S, Saunders P. Northern region asplenia register: analysis of first two years. J Clin Pathol. 1999;52:424–429. doi: 10.1136/jcp.52.6.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan J L, Ochs H D, Schiffman G, Hammerschlag M R, Miser J, Vichinsky E, Wedgwood R J. Immune response after splenectomy. Lancet. 1978;i:178–181. doi: 10.1016/s0140-6736(78)90612-8. [DOI] [PubMed] [Google Scholar]
- 34.Timens W, Boes A, Poppema S. Human marginal zone B cells are not an activated B cell subset: strong expression of CD21 as a putative mediator for rapid B cell activation. Eur J Immunol. 1989;19:2163–2166. doi: 10.1002/eji.1830191129. [DOI] [PubMed] [Google Scholar]
- 35.van den Dobbelsteen G P, Kroes H, van Rees E P. Characteristics of immune responses to native and protein conjugated pneumococcal polysaccharide type 14. Scand J Immunol. 1995;41:273–280. doi: 10.1111/j.1365-3083.1995.tb03564.x. [DOI] [PubMed] [Google Scholar]
- 36.Waghorn D J, Mayon-White R T. A study of 42 episodes of overwhelming post-splenectomy infection: is current guidance for asplenic individuals being followed? J Infect. 1997;35:289–294. doi: 10.1016/s0163-4453(97)93232-1. [DOI] [PubMed] [Google Scholar]
- 37.Webber S A, Sandor G G, Patterson M W, Mitchell L A, Scheifele D, Ochnio J J, McVerry P H. Immunogenicity of Haemophilus influenzae type b conjugate vaccine in children with congenital asplenia. J Infect Dis. 1993;167:1210–1212. doi: 10.1093/infdis/167.5.1210. [DOI] [PubMed] [Google Scholar]