Abstract
Background:
Bacteria pathogens constitute a significant proportion of diarrhoea-causing food contaminants. Transmission of antibiotic resistant foodborne pathogens to humans is a major threat to food safety, especially in developing countries where quality hygiene and sanitation facilities are lacking. Factors related to antibiotic use, sanitation and hand hygiene have been associated with the spread of infectious diseases as well as antibiotic resistant bacteria. Proper food handling ensures that food is not contaminated with potential pathogenic bacteria. This study assessed the carriage of antibiotic resistant bacteria and associated factors.
Methods:
A cross-sectional study was conducted among food handlers who sell ready to eat food in the Tamale metropolis of the Northern Region of Ghana. Food vending stations with huge customer base were randomly selected and the food handlers recruited using written informed consent. Structured questionnaires were used to collect participants sociodemographic details and information on sanitation, hand hygiene practice and antibiotic use. Sterile cotton swabs soaked in phosphate buffered saline was used to swab the palms of participating food handlers for bacteria isolation. All identified bacteria were tested for susceptibility to 12 antibiotics.
Results:
In all, 406 food handlers participated in this study, the mean (SD) age was 26.5 (2.64) years. Bacteria isolated were predominantly Staphylococci 60 (14.8%) and Escherichia coli 54 (13.3%). All the isolates were resistant to at least one antibiotic tested. The isolates showed high resistance to broad-spectrum antibiotics such as ampicillin (40.0%-75.0%), tetracycline (40.0%-80.0%), amoxiclav (20.0%-80.0%) and chloramphenicol (7.7%-50.0%). Logistic regression model revealed that the carriage of antibiotic resistant bacteria by food handlers was significantly associated with age, educational level, years on the job, training in food preparation, hygiene practice, water source, type of toilet facility used and antibiotic use.
Conclusion:
Street food handlers could be potential sources of food-borne transmission of antibiotic resistant bacteria.
Keywords: Carriage, antibiotic resistant bacteria, associated factors, food safety, food handlers, Tamale Metropolis, Ghana
Introduction
Safe and nutritious food and food products are key to the sustenance and promotion of good health among human beings. However, when food is not handled hygienically, it becomes contaminated and unsafe for consumption.1 Food can become contaminated at any point during the process of production and distribution, and therefore the primary responsibility to ensure that food sold is not contaminated lies with food handlers.1-3
In Ghana, street food is very popular among school children, workers and many households.4 The typical street food handler is very likely not to have received formal training in food preparation but would have acquired their culinary skills informally from home5 without any formal training in food hygiene practices. As a result, many street food outlets are characterized by unsanitary cooking areas, improper washing of utensils, exposure of food to flies and dust, lack of toilet and water facilities and improper hand washing techniques.5,6 Unhygienic and unsafe food handling can be a potential conduit for the transmission of enteropathogenic bacteria to consumers. Food and food receptacles become contaminated with microorganisms through direct contact with contaminated hands of food handlers. Contaminated hands play an enormous role in transporting pathogens to consumers through food, especially if served with bare hands. Pathogenic bacteria commonly implicated in foodborne infections include Salmonella, Shigella, Escherichia coli (E. coli), Listeria, Campylobacter species, Enterobacter spp. and enterotoxigenic Staphylococcus aureus (S. aureus).1,3,7,8
Globally, unsafe food poses health threats, endangering the life of consumers. Infants, young children, pregnant women, the elderly and those with underlying illness are particularly vulnerable.1,9-11 Unsafe food containing harmful pathogens has been the cause of over 200 diseases including diarrhoea.12 Globally 600 million people become sick each year after consuming contaminated food with 420 000 people dying as a result.12
It has been reported that a large proportion of foodborne disease incidents emanate from improperly prepared or mishandling of food at home, in food service establishments, or at markets. Also, about 97% of reported food poisoning cases are due to the improper handling of food by persons involved in catering services.13,14 Infections associated with pathogens such as Salmonella, Shigella and Shiga-toxin producing E.coli (STEC) are among the major public health problems in tropical and subtropical regions of the world.8,15 The World Health Organization (WHO) estimates that foodborne and water-borne diarrheal diseases together kill around a 2.2 million people annually. The Center for Disease Control and Prevention (CDC), USA reports that the etiology of millions of illnesses throughout the world can be traced to foodborne pathogens. One of the major consequences of consuming contaminated food is diarrhoea, which is the leading cause of death, especially among children in developing and resource-poor countries. In Ghana, diarrhoeal diseases are common (despite it being under-reported) and often associated with the consumption of contaminated food.16 Therefore, the importance of food safety cannot be overemphasized in developing countries such as Ghana where weak health systems exist17 and institutions mandated to enforce regulations and by-laws on food safety and hygiene are poorly resourced.
Coupled with this, the threats of infections caused by antibiotic resistant bacteria is increasing in several communities and health care settings in many developing nations.18-20 Antibiotic resistance is a major public health challenge facing health care systems in low-and-middle-income countries like Ghana.18 Infections that result from these resistant bacteria are difficult to treat or sometimes impossible to cure.
This study assessed the carriage of antibiotic resistant bacteria and associated factors among food handlers in Tamale Metropolis, Ghana.
Method
Study design and study area
This cross-sectional study was conducted among food handlers who sell ready to eat food in the Tamale metropolis of the Northern region of Ghana from November 2020 to March 2021. Tamale is the capital of the Northern Region of Ghana, and it is located geographically between latitudes 09°24′27″ and 9.40750° North and longitudes 00°51′12″ and 0.85333° West. Tamale metropolis covers a total area of about 750 km2 21and has a population of 127 978.22 The metropolis is regarded as the fastest-growing city in the West Africa23 and by its strategic location, Tamale has a market potential for local goods from the agricultural and commerce sectors from other districts in the Region.21
Vending site selection
Food vending stations with huge customer base were randomly selected. A total of 406 food handlers were recruited from the vending sites using written informed consent. Two palm swabs were taken from each enrolled respondent.
Data and sample collection
Each food vending station was visited twice. Day 1 was used for sample collection (palm swabs) and questionnaire administration. Day 2 was used to verify information recorded through observation of their practices.
On the first day of the visit, the study was explained to the food handlers and those who agreed to participate, were recruited and given a unique identification number. Questionnaires were used to record participants’ demographic details, sanitary practices and antibiotic use. Palms were swabbed by a trained laboratory scientist. A sterile polyester-tipped applicator was moistened in sterile phosphate-buffered saline (PBS) and rolled over the palm of each participant’s right hand, in between the fingers and under the nails. The swab was then immersed into a vial containing 1ml of sterile PBS, capped and labelled appropriately. The same was done for the left hand. All the samples collected were placed on ice packs and transported to the laboratory for bacteriological analysis within 6 hours of collection.
On the second day, we confirmed the type of antibiotics consumed by study participants by comparing medication packages presented to us with the choice made in the questionnaire.
Sample processing, culture and bacteria identification
The palm swabs in PBS were homogenized, and 500 µl of the homogenate aspirated was transferred into sterile microtubes and centrifuged at 2700×g for 15 minutes. The sediment (50 µl) was spread onto 90-mm MacConkey, blood and chocolate agar plates using sterile beads as described by Omulo et al.24
Colony characteristics, Gram reaction and biochemical test reactions were used for presumptive identification of bacterial isolates.25,26 All identified isolates were later confirmed using API 20E and API 20NE (BioMérieux® sa, Marcy-l’Etoile, France) following the instructions of the manufacturer.
Antimicrobial susceptibility test
Antimicrobial susceptibility test was performed on all isolates using the Kirby-Bauer disk diffusion method according to the Clinical & Laboratory Standards Institute (CLSI) protocol.27 The following antibiotics were used; Amikacin (30 µg), Amoxicillin-clavulanic acid (30 µg), Ampicillin (30 µg), Chloram-phenicol(30 µg), Ceftriaxone (30 µg), Ciprofloxacin (10 µg), Erythromycin (15 µg), Gentamicin (10 µg), Trimethoprim-sulfamethoxazole (25 µg), Cefoxitin(20 µg), Tetracycline (30 µg) and Vancomycin(30 µg). E. coli ATCC 25922, Klebsiella pneumoniae ATCC 13883 and S. aureus ATCC 25923 were used as controls.
Data analysis
All data was double-checked for accuracy and completeness within a day of collection. SPSS Version 26 was used for all statistical analyses. The main outcome of interest was antibiotic resistance. Demographic details, hygiene practices, antibiotic use, water source and sanitation related variables were tested as the main predictors for the carriage of antibiotic-resistant bacteria.
For hygiene practices, we measured the hygiene score (H-score) as the number of correct answers out of 8 assessment questions. We therefore calculated the mean H-score and defined good hygiene practice as H-score equal to or above the mean H-score and a poor H-Score as below the mean H-score.
Data analysis of the study included descriptive and analytic components. For descriptive analysis we calculated frequencies, proportions, mean and SD for the H-score after checking for its normal distribution. We reported proportions of answers for hygiene practices.
The analytic component compared proportions of baseline characteristics, H-score and carriage of antibiotic resistant bacteria by using the chi-squared test. In addition, we conducted a logistic regression model to identify predictors of carriage of antibiotic-resistant bacteria. A P-value <.05 was considered statistically significant.
Results
Demographic characteristics, hand hygiene practice and carriage of antibiotic-resistant bacteria
In all, 406 food handlers participated in this study, of which 268 (66.0%) were female and 138 (34.0%) were male. The mean (SD) age of the food handlers was 26.5 (2.6) years with a majority 103 (25.4) within the age group of 21 to 25 years. Majority (62.8%) had up to primary school as their highest level of education and 161 (39.7%) had spent less than 2 years at their current workplace (Table 1).
Table 1.
Participants characteristics and practices associated with carriage of antibiotic resistant bacteria.
| Characteristics | N (%) | Antibiotic-resistant bacteria | χ2, df | P-value | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| n (%) | n (%) | ||||
| Age (y) | |||||
| <20 | 94 (23.2) | 24 (25.5) | 70 (74.5) | 20.79, 6 | .002 |
| 21-25 | 103 (25.4) | 34 (33.0) | 69 (67.0) | ||
| 26-30 | 72 (17.7) | 11 (15.3) | 61 (84.7) | ||
| 31-35 | 57 (14.0) | 8 (14.0) | 49 (86.0) | ||
| 36-40 | 39 (9.6) | 6 (15.4) | 33 (84.6) | ||
| 41-45 | 25 (6.2) | 2 (8.0) | 23 (92.0) | ||
| >45 | 16 (3.9) | 0 (0.0) | 16 (100.0) | ||
| Sex | |||||
| Male | 138 (34.0) | 28 (20.3) | 110 (79.7) | .8184 | |
| Female | 268 (66.0) | 57 (21.3) | 211 (78.7) | ||
| Education | |||||
| None | 132 (32.5) | 41 (31.1) | 91 (68.9) | 13.94, 4 | .0075 |
| Primary | 123 (30.3) | 21 (17.1) | 102 (82.9) | ||
| JHS | 103 (25.4) | 18 (17.5) | 85 (82.5) | ||
| SHS | 38 (9.4) | 3 (7.9) | 35 (92.1) | ||
| Tertiary | 10 (2.5) | 2 (20.0) | 8 (80.0) | ||
| Role | |||||
| Server | 124 (30.5) | 34 (27.4) | 90 (72.6) | 4.55, 2 | .1029 |
| Cook | 223 (54.9) | 40 (17.9) | 183 (82.1) | ||
| Supervisor | 59 (14.5) | 11 (18.6) | 48 (81.4) | ||
| Duration of work (y) | |||||
| <2 | 161 (39.7) | 41 (25.5) | 120 (74.5) | 11.92, 4 | .0179 |
| 2-4 | 121 (29.8) | 31 (25.6) | 90 (74.4) | ||
| 5-7 | 79 (19.5) | 8 (10.1) | 71 (89.9) | ||
| 8-10 | 31 (7.6) | 3 (9.7) | 28 (90.3) | ||
| >10 | 14 (3.4) | 2 (14.3) | 12 (85.7) | ||
| Food handling traininga | |||||
| Yes | 82 (20.2) | 10 (12.2) | 72 (87.8) | .0329 | |
| No | 324 (79.8) | 75 (23.1) | 249 (76.9) | ||
| Medical checkup (last 6 mo) | |||||
| Yes | 94 (23.2) | 21 (22.3) | 73 (77.7) | .7026 | |
| No | 312 (76.8) | 64 (20.5) | 248 (79.5) | ||
| Hygiene practices | |||||
| Good | 98 (24.1) | 12 (12.2) | 86 (87.8) | .0153 | |
| Poor | 308 (75.9) | 73 (23.7) | 235 (76.3) | ||
| Water source | |||||
| Pipe borne water | 120 (29.6) | 13 (10.8) | 107 (89.2) | 11.99, 4 | .0174 |
| Water tanker supplied | 99 (24.4) | 28 (28.3) | 71 (71.7) | ||
| Borehole | 67 (16.5) | 15 (22.4) | 52 (77.6) | ||
| No idea | 48 (11.8) | 11 (22.9) | 37 (77.1) | ||
| Dam | 68 (16.7) | 18 (26.5) | 50 (73.5) | ||
| Defecation facility | |||||
| Private | 69 (17.0) | 6 (8.7) | 63 (91.3) | 23.44, 2 | <.0001 |
| Public | 248 (61.1) | 45 (18.1) | 203 (81.9) | ||
| Open | 89 (21.9) | 34 (38.2) | 55 (61.8) | ||
| Antibiotic use | |||||
| Yes | 285 70.2 | 71 (24.9) | 214 (75.1) | .0021 | |
| No | 121 29.8 | 14 (11.6) | 107 (88.4) | ||
Training organized by accredited institutions or in-service training on good food hygiene practices.
Only a fifth, 82 (20.2%) had undergone formal training in food production while 94 (23.2%) had undergone medical check-up within the last 6 months. Out of the total, 120 (29.6%) indicated that their main water source was tap water, while 68 (16.7%) relied on dam water. More than half of the food handlers (61.1%) used public toilet facilities while 89 (21.9%) and 69 (17.0%) used open defecation and private toilet facility respectively while at work (Table 1).
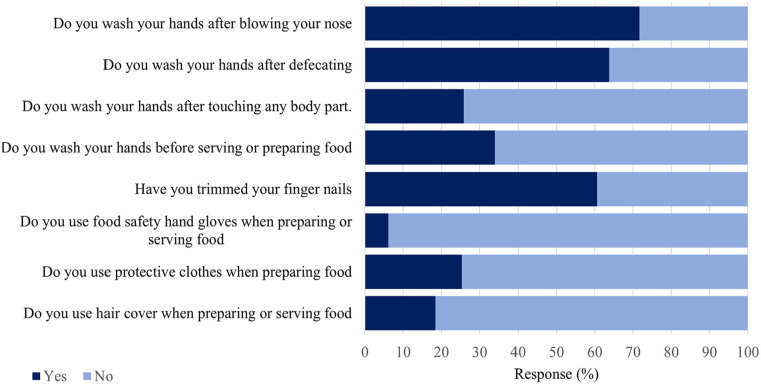
In terms of food hygiene practice, the food handlers had an overall mean score of 5.5 (SD 1.2) for questions that assessed their compliance with food handling hygiene practices. One hundred and thirty-eight (34.0%) wash their hands before serving or preparing food, with 105 (25.9%) washing their hands after touching any body parts. Also, 259 (63.8%) indicated that they wash their hands after defecation while 291 (71.7%) wash their hands after blowing their nose (Figure 1). Some, 25 (6.3%) used food safety hand gloves, 75 (18.5%) hair cover, 103 (25.4%) protective clothes and 246 (60.6%) had trimmed fingernails (Figure 1).
Figure 1.
Food handling and hand hygiene practices among food handlers.
Antibiotic usage
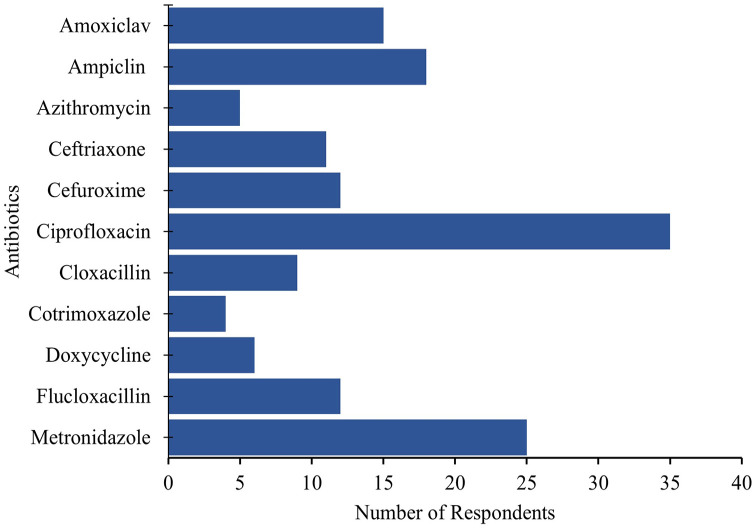
In all, 152 (37.8%) participants indicated that they had used an antibiotic within the past one month. Ten different antibiotics were used with ciprofloxacin (23.03%; 35/152) being most used, followed by metronidazole (16.45%; 25/152), ampicillin (11.84%; 18/152), amoxiclav (9.87%; 15/152), cefuroxime (7.89%; 12/152), flucloxacillin (7.89%; 12/152) and ceftriaxone (7.24%; 11/152). The study confirmed the identities of 85% antibiotics used by respondents by examining the package presented to the study team for verification (Figure 2).
Figure 2.
List of antibiotics used by food handlers.
Prevalence of antibiotic-resistant bacteria among food handlers
The overall prevalence of carriage of antibiotic resistant bacteria among food handlers was 20.9%. Majority (56.3%) of the bacteria isolated were Gram negative predominantly E. coli (n = 54, 13.3%). Other isolates were Klebsiella species 13 (3.2%), Citrobacter species 5 (1.2%), Proteus species 5 (1.2%), Enterobacter species 4 (1.0%) and Pseudomonas aeruginosa 4 (1.0%). Gram positive bacteria isolated were coagulase negative Staphylococci (CoNS) 36 (8.9%), Staphylococcus aureus 24 (5.9%) and Enterococcus species 6 (1.5%).
Antibiotic resistance pattern of isolates
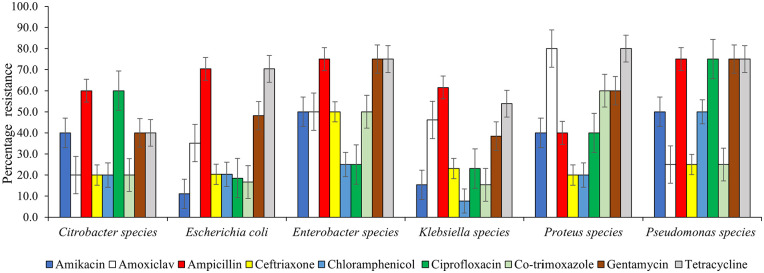
The antibiotic resistance pattern is presented in Figure 3. Generally, drug resistance was commonly detected among Gram negative bacteria. All the isolates were resistant to at least one antibiotic used. Gram negative isolates showed high resistance to the broad-spectrum antibiotics such as ampicillin (40.0%-75.0%), tetracycline (40.0%-80.0%), amoxiclav (20.0%-80.0%) and chloramphenicol (7.7%-50.0%). Resistance to Co-trimoxazole and gentamicin ranged from 15.4% to 60.0% and 38.5% to 75.0% respectively. Furthermore, the resistance to ceftriaxone and ciprofloxacin detected was 50.0% % and 75.0% respectively.
Figure 3.
Antimicrobial resistance pattern of Gram negative bacteria isolates.
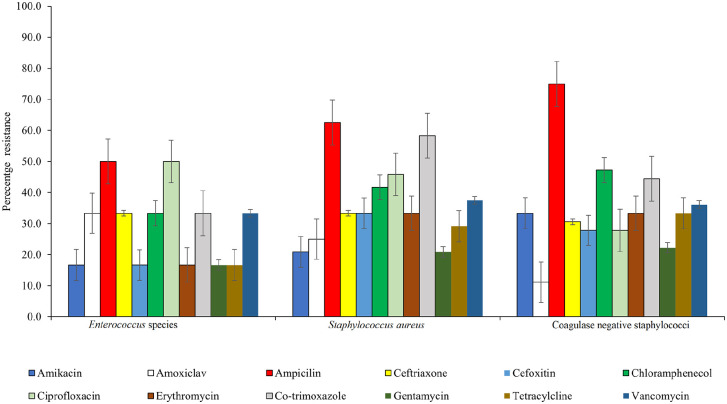
About 16.7% to 33.3% and 33.3% to 66.7% of the Gram positive bacteria, showed resistance to cefoxitin and vancomycin respectively Figure 4.
Figure 4.
Antimicrobial resistance pattern of Gram positive bacteria isolates.
Factors associated with carriage of antibiotic resistant bacteria
The study noted that the carriage of antibiotic resistant bacteria was significantly associated with age, educational level, duration of work, food production training, hygiene practice, water source, toilet facility used and antibiotic use (Table 1).
In a logistic regression analysis, higher education level and antibiotic usage were significantly associated with reduced likelihood of carriage of antibiotic resistant bacteria. Primary level of education (OR = 0.457, CI = 0.251-0.8140, P = .0127), JHS (OR = 0.47, CI = 0.2539-0.8814, p = .0226), SHS (OR = 0.1902,0.05897-0.6355, P = .0031) and Tertiary (OR = 0.5540, CI = 0.1149-2.457, P = .0723) were associated with 54%, 53%, 81% and 45% decrease in the odds of carriage of antibiotic resistant bacteria respectively. Those who had not used antibiotics had a 60% reduction in odds of carrying antibiotic resistant bacteria. Those with no training in food production and had poor hygiene practice were 2 times more likely to carry antibiotic resistant bacteria on their hands. Using water sources like tanker supplied water, borehole and dam was associated with 3-fold higher odds of carriage of antibiotic resistant bacteria. Food handlers who used public toilet and open defecation while at work had a 2-fold and 6-fold higher odds of carriage of antibiotic resistant bacteria respectively (Table 2).
Table 2.
Logistic regression analysis for carriage of antibiotic resistant bacteria.
| Characteristics | OR | 95% CI | P-value |
|---|---|---|---|
| Variable | |||
| Age (y) | |||
| <20 | 1 | ||
| 21-25 | 1.437 | 0.7589-2.677 | .2759 |
| 26-30 | 0.526 | 0.2452-1.126 | .1261 |
| 31-35 | 0.476 | 0.2060-1.162 | .1045 |
| 36-40 | 0.530 | 0.2019-1.398 | .2574 |
| 41-45 | 0.254 | 0.05604-1.003 | .0991 |
| >45 | 0.182 | 0.01667-1.060 | .1128 |
| Education | |||
| None | 1 | ||
| Primary | 0.457 | 0.2511-0.8140 | .0127 |
| JHS | 0.470 | 0.2539-0.8814 | .0226 |
| SHS | 0.190 | 0.05897-0.6355 | .0031 |
| Tertiary | 0.554 | 0.1149-2.457 | .0723 |
| Duration of work (y) | |||
| <2 | 1 | ||
| 2-4 | 1.008 | 0.5821-1.707 | >.9999 |
| 5-7 | 0.330 | 0.1546-0.7262 | .0061 |
| 8-10 | 0.314 | 0.09641-0.9716 | .0631 |
| >10 | 0.488 | 0.1054-2.101 | .522 |
| Food handling traininga | |||
| Yes | 1 | ||
| No | 2.169 | 1.070-4.407 | .0329 |
| Hygiene practices | |||
| Good | 1 | ||
| Poor | 2.226 | 1.186-4.279 | .0153 |
| Water source | |||
| Pipe borne water | 1 | ||
| Water tanker supplied | 3.246 | 1.612-6.398 | .0015 |
| Borehole | 2.374 | 1.085-5.382 | .0527 |
| No idea | 2.829 | 1.167-6.529 | .0252 |
| Dam | 2.963 | 1.334-6.473 | .0076 |
| Defecation facility | |||
| Private | 1 | ||
| Public | 2.328 | 0.9557-5.368 | .0648 |
| Open | 6.491 | 2.551-15.85 | <.0001 |
| Antibiotic use | |||
| Yes | 1 | ||
| No | 0.394 | 0.2182-0.7233 | .0021 |
Training organized by accredited institutions or in-service training on good food hygiene practices.
Discussion
We set out to assess the carriage of antibiotic resistant bacteria among food handlers and the associated factors.
In this study, E. coli was detected among 13.3% (54/406) of food handlers, which is higher than the 10.9% detected among food handlers in Ethiopia by Assefa et al28 and lower than 41.7% detected by Allam et al.10 Lower detection rates ranging from 1.8% to 7.8% have been reported in several studies.29-31 E. coli is an important cause of food poisoning and an indicator of faecal contamination. The isolation of E. coli from palm swabs may indicate that food handlers are not practicing hand hygiene and proper glove use. The presence of other potential pathogens like Klebsiella species, Citrobacter species, Proteus species, Enterobacter species and Pseudomonas aeruginosa on the hands of food handlers may be due to contact with contaminated raw animal products, fruits and vegetables.32
S. aureus forms part of the skin microflora and up to 50% of healthy humans carry them in the anterior nostrils32-37 as such, the 21.7 % carriage detected in the study is not surprising. It is similar to the 20% detected by Nasrolahei et al32 among food handlers in Iran and the 20.5% detected by Dagnew et al38 among food handlers in Ethiopia. However, it is lower than 27% detected by Beyene et al9 and the 30.1% detected by Alhashimi et al39 S. aureus, a common cause of food poisoning can easily contaminate food if food handlers accidentally sneeze or cough during food preparation or serving. More importantly, when they do not wash their hands properly after touching their nose or after using the toilet.28,32,35,36,40 S. aureus may produce heat-stable enterotoxins when in or on food products.35,41 Improving hand hygiene and the use of gloves has the potential of reducing skin contamination and subsequent shedding of S. aureus into food.42,43
Bacteria contamination of the hands of food handlers is a food safety issue of public health concern especially when these bacteria are resistant to some antibiotics. The high resistance to broad-spectrum antibiotics such as ampicillin, tetracycline, amoxiclav and chloramphenicol may be due to their availability over the counter and high prescription rate.44 Also, the low purchasing power of these food handlers may lure them to purchase presumably cheaper drugs assuming they are bioequivalent.45 Furthermore, some food handlers may truncate the course of therapy because of their inability to pay for the full course of medication.45,46 The use of antibiotics for less period of time than is required, can exert selective pressure on bacterial populations which contributes to their resistance.44,45 Resistance to beta-lactams and fluoroquinolones must be of great concern as they are preferred to aminoglycosides for the treatment of serious infections because of their high efficacy and low toxicity.47,48
In Ghana, multidrug resistant (MDR) bacteria have been reported among food handlers.49,50 The proportion of MDR bacteria detected in this study, is lower than that reported in studies in Ethiopia where MDR bacteria isolated from food handlers ranged from 42.2% to 85.7%.8,51 MDR bacteria detected among the food handlers is of great public health concern because of possible transmission to patrons of the food. MDR bacteria have been associated with high treatment failure, adverse effects or increased mortality among hospitalized patients.48,52
Factors related to sanitation and hand hygiene have been associated with the spread of infectious diseases and the spread of antibiotic resistant bacteria.24 In our study, while most of the food handlers used public toilet facilities, 36.2% did not practice handwashing with soap after defecation. Public toilet facilities have been reported be to epicentres for the transmission of bacteria.53 Only 23.2% of food handlers had done their mandatory medical examination within the last 6 months. This is lower than the 57%28 and 63.2%54 in studies conducted in Ethiopia. Food handlers’ medical examination is mandatory in many countries to ensure the protection of consumers55 and there is the need to ensure compliance in Ghana.
In this study 324 (79.8%) of the food handlers had no formal training in food safety and hygiene which is higher than the 30.4% reported by Assefa et al.28 This may be because some food handlers regard food safety as a common sense approach, which is reflected in their interest in food safety training or lack of it.56 In a food preparation environment, there are many potential risks for contamination, therefore good hygiene practices within such an environment helps to reduce or eliminate them. Food hygiene training equips food handlers with enough knowledge and skills to prevent cross-contamination and food poisoning. Human hands play a vital role in transmission of pathogens in the food industry and domestic environment.57 The importance of hand hygiene in infection prevention and control cannot be underestimated.
Conclusion
The relatively high prevalence of antibiotic resistant bacteria detected in this study indicates that food handlers could be a potential source of food contamination and eventual transmission to consumers. The high number of MDR enteric pathogens could increase the transmission of these difficult to treat enteric bacterial pathogens among persons who patronize food from these food vendors.
The study recommends strict monitoring and enforcement of proper hand hygiene and food safety by food handlers. Regular training and medical examination for food handlers should be encouraged. Also, the public must be informed about proper hygiene and food safety practice, and this will drive the demand for quality and safe food.
Acknowledgments
The authors thank participants for agreeing to participate in this research.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author Contributions: EKV and GIM designed the study and wrote the protocol. EKV, DBA, VCK and MOB did the sample and data collection. EKV, DBA, VCK and KOA performed the bacteria isolation, identification and antimicrobial susceptibility tests. GIM, EKV, MOB and KOA performed the data analysis. GIM, EKV, KOA and MOB wrote the first draft. GIM, EKV, KOA and MOB reviewed the manuscript. All authors read and approved the final manuscript.
Ethical Approval: The study was approved by The Tamale Teaching Hospital ethical review committee. Consent to participate in the study was obtained from all participants. Information obtained in the study was kept confidential.
Significance Statement: • Ciprofloxacin was the most used antibiotic (23.03%; 35/152) among food handlers interviewed.
• The overall prevalence of carriage of antibiotic resistant bacteria among food handlers was 20.9%.
• The odds of carrying antibiotic resistant bacteria reduced by 60% in the absence of antibiotic usage
References
- 1. World Health Organization. WHO estimates of the global burden of foodborne diseases: foodborne disease burden epidemiology reference group 2007-2015. World Health Organization; 2015. [Google Scholar]
- 2. Yambi O, Rocha C, Jacobs N. Unravelling the food-health nexus to build healthier food systems. World Rev Nutr Diet. 2020;121:1-8. [DOI] [PubMed] [Google Scholar]
- 3. Mama M, Alemu G. Prevalence, antimicrobial susceptibility patterns and associated risk factors of Shigella and Salmonella among food handlers in Arba Minch University, South Ethiopia. BMC Infect Dis. 2016;16:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mensah JO, Aidoo R, Teye AN. Analysis of street food consumption across various income groups in the Kumasi Metropolis of Ghana. Int Rev Manag Bus Res. 2013;2:951. [Google Scholar]
- 5. Monney I, Agyei D, Owusu W. Hygienic practices among food vendors in educational institutions in Ghana: the case of Konongo. Foods. 2013;2:282-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. El-Nemr I, Mushtaha M, Irungu P, et al. Assessment of food safety knowledge, self-reported practices, and microbiological hand hygiene levels of produce handlers in Qatar. J Food Protect. 2019;82:561-569. [DOI] [PubMed] [Google Scholar]
- 7. Mengist A, Mengistu G, Reta A. Prevalence and antimicrobial susceptibility pattern of Salmonella and Shigella among food handlers in catering establishments at Debre Markos University, Northwest Ethiopia. Int J Infect Dis. 2018;75:74-79. [DOI] [PubMed] [Google Scholar]
- 8. Getie M, Abebe W, Tessema B. Prevalence of enteric bacteria and their antimicrobial susceptibility patterns among food handlers in Gondar town, Northwest Ethiopia. Antimicrob Resist Infect Control. 2019;8:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beyene G, Mamo G, Kassa T, Tasew G, Mereta ST. Nasal and hand carriage rate of Staphylococcus aureus among food handlers working in Jimma Town, Southwest Ethiopia. Ethiop J Health Sci. 2019;29:605-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Allam H, Al-Batanony M, Seif A, Awad E. Hand contamination among food handlers. J Pure Appl Microbiol. 2021;15:1536-1546. [Google Scholar]
- 11. Gyansah MA. Salmonella Carriage Among Food Handlers and Patients Attending St. Joseph’s Hospital, Jirapa, Upper West Region. 2017. http://ir.knust.edu.gh/handle/12345678910123
- 12. World Health Organisation. WHO: Food safety- key facts. 2019. Accessed May 7, 2019. https://www.who.int/news-room/fact-sheets/detail/food-safety
- 13. Greig JD, Todd EC, Bartleson CA, Michaels BS. Outbreaks where food workers have been implicated in the spread of foodborne disease. Part 1. Description of the problem, methods, and agents involved. J Food Protect. 2007;70:1752-1761. [DOI] [PubMed] [Google Scholar]
- 14. Akabanda F, Hlortsi EH, Owusu-Kwarteng J. Food safety knowledge, attitudes and practices of institutional food-handlers in Ghana. BMC Public Health. 2017;17:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Girma G. Prevalence, antibiogram and growth potential of Salmonella and Shigella in Ethiopia: implications for public health. A review. Prevalence. 2015;33:288-307. [Google Scholar]
- 16. Songsore J. The complex interplay between everyday risks and disaster risks: the case of the 2014 cholera pandemic and 2015 flood disaster in Accra, Ghana. Int J Disaster Risk Reduct. 2017;26:43-50. [Google Scholar]
- 17. Odeyemi OA, Sani NA. Antibiotic resistance and burden of foodborne diseases in developing countries. Future Sci. 2016;2;FSO139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hassan MM. Scenario of antibiotic resistance in developing countries. In: Mareș M, Lim SHE, Lai K-S, Cristina R-T, eds. Antimicrobial Resistance-A One Health Perspective. IntechOpen; 2020. doi:10.5777/intechopen.94957 [Google Scholar]
- 19. Ayukekbong JA, Ntemgwa M, Atabe AN. The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrob Resist Infect Control. 2017;6:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Worku T, Jejaw A, Kannan S, Wondafrash B. Isolation and antimicrobial sensitivity patterns of enteric bacterial pathogens from asymptomatic food handlers, Jimma, Ethiopia. Am J Heal Res. 2016;3:399-406. [Google Scholar]
- 21. Ghana Statistics Service. 2010 Population & Housing Census: National Analytical Report. Ghana Statistics Service; 2013. [Google Scholar]
- 22. Service GS. 2020. Population and Housing Census: General Report volume 3A. Population of Regions and Districts; 2021. [Google Scholar]
- 23. Fuseini I, Yaro JA, Yiran GAJC. City profile: Tamale, Ghana. Cities. 2017;60:64-74. [Google Scholar]
- 24. Omulo S, Lofgren ET, Lockwood S, et al. Carriage of antimicrobial-resistant bacteria in a high-density informal settlement in Kenya is associated with environmental risk-factors. Antimicrob Resist Infect Control. 2021;10:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Delost MD. Introduction to Diagnostic Microbiology for the Laboratory Sciences. Jones & Bartlett Learning; 2020. [Google Scholar]
- 26. Hall GS. Bailey & Scott’s Diagnostic Microbiology. 13th ed. American Society for Clinical Pathology; 2013. [Google Scholar]
- 27. CLSI. Performance Standards for Antimicrobial Susceptibility Testing: 25th Informational Supplement. CLSI document M100-S25 Clinical and Laboratory Standards Institute; 2015. [Google Scholar]
- 28. Assefa T, Tasew H, Wondafrash B, Beker J. Contamination of bacteria and associated factors among food handlers working in the student cafeterias of Jimma University Main Campus, Jimma, South West Ethiopia. Altern Integr Med. 2015;2015:1-8. [Google Scholar]
- 29. Ifeadike C, Ironkwe O, Adogu P, et al. Prevalence and pattern of bacteria and intestinal parasites among food handlers in the Federal Capital Territory of Nigeria. Niger Med J. 2012;53:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fawzi M, Gomaa NF, Bakr WM. Assessment of hand washing facilities, personal hygiene and the bacteriological quality of hand washes in some grocery and dairy shops in Alexandria, Egypt. Egypt Public Health Assoc. 2009;84:2. [PubMed] [Google Scholar]
- 31. Souza CVSd, Azevedo PRMd, Seabra LMAJ. Food safety in Brazilian popular public restaurants: food handlers’ knowledge and practices. J Food Safety. 2018;38:e12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nasrolahei M, Mirshafiee S, Kholdi S, Salehian M, Nasrolahei M. Bacterial assessment of food handlers in Sari City, Mazandaran Province, north of Iran. J Infect Public Health. 2017;10:171-176. [DOI] [PubMed] [Google Scholar]
- 33. Brown AF, Leech JM, Rogers TR, McLoughlin RM. Staphylococcus aureus colonization: modulation of host immune response and impact on human vaccine design. Front Immunol. 2014;4:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Belkum A, Verkaik NJ, De Vogel CP, et al. Reclassification of Staphylococcus aureus nasal carriage types. J Infect Dis. 2009;199:1820-1826. [DOI] [PubMed] [Google Scholar]
- 35. Schlossberg D. Clinical Infectious Disease. Cambridge University Press; 2015. [Google Scholar]
- 36. Wei H-L, Chiou C-S. Molecular subtyping of Staphylococcus aureus from an outbreak associated with a food handler. Epidemiol Infect. 2002;128:15-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sakr A, Brégeon F, Mège J-L, Rolain J-M, Blin O. Staphylococcus aureus nasal colonization: an update on mechanisms, epidemiology, risk factors, and subsequent infections. Front Microbiol. 2018;9:2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dagnew M, Tiruneh M, Moges F, Tekeste Z. Survey of nasal carriage of Staphylococcus aureus and intestinal parasites among food handlers working at Gondar University, Northwest Ethiopia. BMC Public Health. 2012;12:837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alhashimi HMM, Ahmed MM, Mustafa JM. Nasal carriage of enterotoxigenic Staphylococcus aureus among food handlers in Kerbala city. Karbala Int J Mod Sci. 2017;3:69-74. [Google Scholar]
- 40. Isara A, Isah E, Lofor P, Ojide C. Food contamination in fast food restaurants in Benin City, Edo State, Nigeria: implications for food hygiene and safety. Public Health. 2010;124:467-471. [DOI] [PubMed] [Google Scholar]
- 41. Bennett SD, Walsh KA, Gould LH. Foodborne disease outbreaks caused by Bacillus cereus, Clostridium perfringens, and Staphylococcus aureus—United States, 1998–2008. Clin Infect Dis. 2013;57:425-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wertheim HF, Melles DC, Vos MC, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5:751-762. [DOI] [PubMed] [Google Scholar]
- 43. Ho J, Boost M, O’Donoghue M. Sustainable reduction of nasal colonization and hand contamination with Staphylococcus aureus in food handlers, 2002–2011. Epidemiol Infect. 2015;143:1751-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chokshi A, Sifri Z, Cennimo D, Horng H. Global contributors to antibiotic resistance. J Glob Infect Dis. 2019;11:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Planta MB. The role of poverty in antimicrobial resistance. J Am Board Fam Med. 2007;20:533-539. [DOI] [PubMed] [Google Scholar]
- 46. King T, Schindler R, Chavda S, Conly JJAR, Control I. Dimensions of poverty as risk factors for antimicrobial resistant organisms in Canada: a structured narrative review. Antimicrob Resist Infect Control. 2022;11:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Morata L, Cobos-Trigueros N, Martínez JA, et al. Influence of multidrug resistance and appropriate empirical therapy on the 30-day mortality rate of Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother. 2012;56:4833-4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huh K, Chung DR, Ha YE, et al. Impact of difficult-to-treat resistance in gram-negative bacteremia on mortality: retrospective analysis of nationwide surveillance data. Clin Infect Dis. 2020;71:e487-e496. [DOI] [PubMed] [Google Scholar]
- 49. Dsani E, Afari EA, Danso-Appiah A, Kenu E, Kaburi BB, Egyir B. Antimicrobial resistance and molecular detection of extended spectrum β-lactamase producing Escherichia coli isolates from raw meat in Greater Accra region, Ghana. BMC Microbiol. 2020;20:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Courage S, Akosua Bonsu K, Enoch Y, Patrick T, Stephen K. Multidrug resistant Salmonella spp. and Escherichia coli from a popular Ready-to-Eat local food (Fufu) from commercial food vendors in Ghana. J Food Saf Hyg. 2020;5:214-219. [Google Scholar]
- 51. Marami D, Hailu K, Tolera M. Prevalence and antimicrobial susceptibility pattern of Salmonella and Shigella species among asymptomatic food handlers working in Haramaya University cafeterias, Eastern Ethiopia. BMC Res Notes. 2018;11:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Giamarellou H. Multidrug-resistant Gram-negative bacteria: how to treat and for how long. Int J Antimicrob Agents. 2010;36:S50-S54. [DOI] [PubMed] [Google Scholar]
- 53. Greed C. The role of the public toilet: pathogen transmitter or health facilitator? Build Serv Eng Res Technol. 2006;27:127-139. [Google Scholar]
- 54. Abera B, Biadegelgen F, Bezabih B. Prevalence of Salmonella typhi and intestinal parasites among food handlers in Bahir Dar Town, Northwest Ethiopia. Ethiop J Health Dev. 2010;24:45-48. [Google Scholar]
- 55. Moyo D, Moyo F. Outcomes of food handlers’ medical examinations conducted at an occupational health clinic in Zimbabwe. Preprint. Posted online August 19, 2020. doi: 10.20944/preprints202008.0416.v1 [DOI] [Google Scholar]
- 56. Jevšnik M, Raspor P. Food safety knowledge and behaviour among food handlers in catering establishments: a case study. Br Food J. 2021;124:3293-3307. [Google Scholar]
- 57. Jumaa P. Hand hygiene: simple and complex. Int J Infect Dis. 2005;9:3-14. [DOI] [PubMed] [Google Scholar]