Abstract
In most areas of the world women comprise the majority of older persons (especially at the most advanced ages), but the additional longevity (globally it is 4.8 years) women have often comes with poorer health status compared to age-matched men. This article draws attention to four distinct ways an applied gerontological intervention designed to increase the human healthspan via “rate (of ageing) control” could positively impact the health and wellbeing of women in today’s ageing world. The four benefits examined are: (1) improving women’s health in late life; (2) increasing reproductive longevity and improving maternal health, (3) reducing the financial vulnerability many women experience at advanced ages (especially in the developing world); and (4) reducing the caring burdens which typically fall, at least disproportionately, on daughters to care for their ageing parents. Highlighting these factors is important as is helps focus geroscience advocacy not only on the potential health dividend age retardation could confer on those in late life, but also the distributional effects on health throughout the lifespan (e.g. improving maternal health) and on helping to ameliorate other important inequalities (e.g. reducing the financial vulnerabilities of late life and easing the burdens on the care givers for ageing parents). By making vivid the benefits “rate (of ageing) control” could confer on women, especially in the developing world, the goal of retarding biological ageing can be rightly construed as a pressing public health priority for the 21st century.
Keywords : Ageing, Geroscience, Healthspan, Longevity, Women
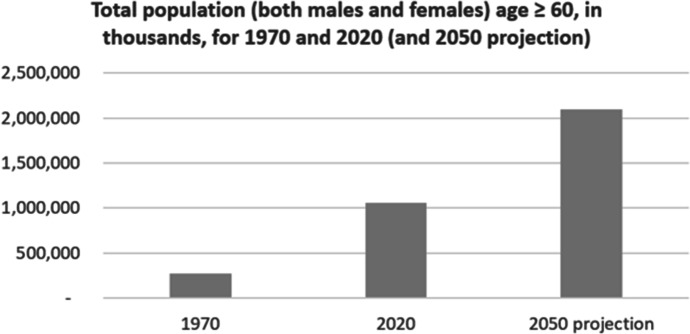
Population ageing is both a momentous achievement and it presents one of the most significant challenges to science and medicine in the 21st century. The ageing of the world’s populations is a significant success story because it reflects the enormous achievements that public health has made in reducing early and mid-life mortality. Humans “had a life expectancy at birth of 30 years of less for more than 99.9% of the time that we have inhabited this planet” (Hayflick, 2000). And now life expectancy at birth for a baby born in the world is currently age 73 (WHO, 2021a), and is expected to rise to age 81 by the end of the century (UN, 2011). In 1970 the number of persons age ≥ 60 was approximately 278 million (Fig. 1) (UN, 2022a, b). By the year 2020 that number had risen to over a billion people. The World Health Organization predicts that by 2050 the number of persons age ≥ 60 will surpass 2 billion, and 80% of them will be living in low- and middle-income countries (WHO, 2021b). People are surviving into late life, and having less children. The phenomenon of population ageing presents a unique challenge to the medical sciences as saving people from the extrinsic (environmental) risks which cause premature death (e.g. infectious diseases, poverty, smoking, obesity, violence, climate change, etc.) is quite distinct from redressing the intrinsic risks that arise because of the inborn ageing process itself.
Fig. 1.
World Population Ageing
October 1st is the United Nation’s International Day of Older Persons, and the 2022 theme was “The Resilience and Contributions of Older Women”. Medical science must be innovative in its approach to basic scientific health research if it aspires to improve the health prospects of today’s ageing populations vs simply increasing life expectancy by preventing death in late life. Longevity scientists (Comfort, 1969; Neugarten & Havighurst, 1977; Holliday, 1999; Butler et al., 2008, Rae et al., 2010; Kaeberlein et al., 2015; Olshansky, 2018; Barzilai et al., 2020, Santesmasses et al., 2020) have long argued that the development of an applied gerontological intervention which slowed the molecular and cellular processes of biological ageing would constitute a novel and significant approach to human longevity. Deploying biomedical science to target the ageing process itself is a novel approach to public health as the dominant strategy employed by epidemiology over the past two centuries has been a strategy of disease control.
A specific focus on the potential impact an applied gerontological intervention would have on women is timely given the 2022 theme of the UN’s International Day of Older Persons. This article considers the potential impact an ageing intervention could have on (1) women’s health in late life, (2) reproductive longevity and maternal health, (3) the financial vulnerability many women experience in later life (especially in developing countries) and (4) the familial caring duties which typically fall, at least disproportionately, upon daughters to care for their ageing parents. These considerations reveal the significance of geroscience for the health and wellbeing of the women in today’s ageing world.
Before addressing the specifics of how advances in longevity science could positively impact the health and wellbeing of women, it is important to first take seriously the ethical and distributive justice concerns that typically arise with respect to debates in bioethics and public health ethics, such as concerns for intergenerational equality and global justice. Globally, the women of the world live longer than men. So focusing on the importance of longevity science, especially for women, will naturally raise the question: “Can we justify trying to extend the lives of those who have more life already?”
Fair Innings, Empirical Ethics and Longevity Science
A concern some commentators have expressed about aspiring to make further advances in increasing the human lifespan concerns the potential distributive impacts, from the perspective of both intergenerational equality and global justice, on the distribution of community health. These concerns arise when the following question is posed- can we justify trying to extend the lives of those who have more life already? The theoretical discussions and debates addressing this general question tend to function at an abstract level of analysis, pitting “the haves” against “the have nots”, such as “saving the young” vs “saving older persons”, and/or “saving lives in developing countries” vs “saving lives in developed countries”.
To date, these bioethical frameworks and arguments addressing longevity science have presumed that the strategy deployed to increase the lifespan is one of disease control. And this raises many intransient predicaments given that realities of both limited healthcare resources as well as the empirical fact that any reduction, or even the elimination of, one disease risk for older persons does not reduce or eliminate the risks of other age-related health vulnerabilities. Eliminating prostate cancer, for example, does not reduce the risks of lung cancer, or breast cancer, or heart disease or Alzheimer’s disease. Aspiring to increase further the lifespan of older populations by prioritizing the strategy of tackling each single disease of late life, one at a time, exacerbates the problems of distributive equality given the reality of co-morbidity in late life.
Faced with these predicaments, many different conclusions have been proposed with respect to addressing the concerns longevity science poses to distributive equality. Some have argued that biomedical research should not pursue any further advances in human longevity. The most prominent bioethicist to take this line of reasoning was Callahan (1998). In False Hopes: Why America’s Quest for Perfect Health Is a Recipe for Failure he argued:
The average person in good health in the developed countries of the world (and living in a reasonably safe environment), already lives long enough to accomplish most reasonable ends. . . . Neither the human species as a whole, nor most individuals, need more than the present average life expectancy in the developed countries (the mid-seventies to low-eighties) for a perfectly satisfactory life. This idea of a steady-state life expectancy at its present level would establish, happily, a finite and attainable goal: ‘‘Enough, already.’’ (Callahan, 1998)
And Williams (1997, 1999) invokes a “fair innings” principle to help adjudicate priority setting among health research which would benefit younger vs older persons. This principle focuses on the sum of a person’s past accumulation of health (their quality adjusted life years (QALYs)) and their expected health related quality of-life at each year of age in the future. Williams notes that the folklore reference to “three-score-years-and-ten” (70 years) has been the generally held notion in distributive justice. And the “fair innings” principle maintains that “on grounds of equity we want to give some extra weight to the claims of those who are unlikely to achieve it, and lower weight to the claims of those who have already achieved it or who are likely to do so” (Williams, 1999).
Bognar (2015) further elucidates the fair innings principle, distinguishing between consequentialist and fairness-based versions of the principle. What he refers to as the “utilitarian ageist” version of the fair innings principle relies on moral sentiments to prioritize the young over older patients in cases like the following scenario:
Suppose you have to decide between giving a life saving drug to just one of the following two patients:
(A) a 20-year old patient who will live for many years if she gets the drug; or
(B) a 70-year old patient who will live for only a few more years if she gets the drug. (Bognar, 2015)
The moral sentiments activated by such scenarios are amplified by the salient empirical constraints designed into the thought experiment. For example, in the scenario described above, to elicit “utilitarian ageist” sentiments health is construed of as a zero-sum situation. In a zero-sum situation giving an advantage to one side is equivalent to a loss for the other side. The scenario Bognar provides primes “utilitarian ageist” intuitions because we can only save either the young patient or the older patient, but we cannot save both. Furthermore, the numbers of vulnerable patients in these two categories are comparable. That is, we are asked to consider the moral imperative to prioritize a medical treatment that would save either one young patient or save one older patient versus a scenario (more closely representing today’s ageing world) where there are significantly more patients at risks of disease in late life than there are at risk of early-life morbidity and mortality.
When placed in the context of global justice, many these same egalitarian sentiments have been expressed as reasons for weighing more heavily the potential adverse distributional effects additional longevity may have on community health. Mauron (2005) and Pijnenburg and Leget (2007), for example, argue that the unequal death that exists between the developed and developing world presents an ethical obstacle to pursuing further life extension.
In contrast to the prevalence of normative principles and theories addressing the distributive effects of increasing longevity via the strategy of disease control, there is something of a theoretical lacuna when it comes to addressing the potential distributive effects of an ageing intervention. The existence of this theoretical lacuna is understandable given that, at least historically, the prospect of successfully intervening in the ageing process was more science fiction than a likely future medical reality. But today an applied gerontological intervention is considered not only a feasible medical intervention, but the ultimate form of preventative medicine by many experts in geroscience (Kaeberlein et al., 2015). This is the case because slowing biological ageing would have a greater effect on the quality of life of older persons compared to what could be expected with the benefits yielded by the disease-specific approach to human longevity.
The likely distributional effects of slowly ageing on community health and wellbeing has not been extensively studied, and thus bioethical analyses of ageing research typically presume an ageing intervention is synonymous with an intervention that extends the lifespan by postponing death via disease control. To help redress the lacuna in normative theorizing about rate of ageing control, this article advances an empirical ethics analysis of longevity science by focusing on the potential impact on women’s health and wellbeing. Empirical ethics has two central commitments. Firstly, its primary aim is to “improve the context sensitivity of ethics” (Musschenga, 2005). Secondly, it is an approach to ethics that is interdisciplinary in its methodology, for it maintains that “intellectually responsible philosophical ethics is one that continuously engages the relevant empirical literature” (Doris & Stich, 2005).
A focus on the impact an applied gerontological intervention could have on women’s health and wellbeing facilitates both a contextually-sensitive normative analysis of longevity science, as well as an interdisciplinary approach. These two aspects of empirical ethics help ensure that an ethical analysis of longevity science attends to contextual realities like the female-male survivor paradox, as well as the impact ageing has on reproductive longevity and maternal health, financial wellbeing in late life and the distribution of the caring burdens for ageing parents. Additionally, empirical ethics will be better attuned to both the limits of the “disease-control” paradigm of the medical sciences (given the impact ageing has on the risks of multi-morbidity in late life), as well as the potential to widely diffuse the benefits of an ageing intervention.
Rather than making the question “Can we justify trying to extend the lives of those who have more life already?” the central focus of an abstract justice-based analysis, empirical ethics brings precision to more concrete, relevant and pressing questions regarding the aspiration to modify the rate of biological ageing. So the question “Can we justify trying to extend the lives of those who have more life already?” can be re-framed, once filtered through an empirical ethical analysis of the impact of longevity science on women’s health and wellbeing, into the more specific questions:
Can we justify trying to improve the quality of life of older persons by increasing the humanspan (thus reducing the risks of disease, frailty and disability in late life)?
Can we justify trying to increase reproductive longevity, and improving maternal health?
Can we justify trying the reduce the financial hardships persons typically face in late life?
Can we justify trying to reduce the caring burdens that typically fall on daughters to care for their older parents?
The arguments presented in this article suggest that there is a strong presumption in favour of answering “yes!” to all four of these questions.
There is one further point, a critical point proponents of the “fair innings” position might raise against the empirical ethical analysis I deploy in this paper, that is worth addressing. And that critical interjection is to note that, globally, women live longer than men. Not only do women live longer than men, women also enjoy more years of healthy life expectancy. According to the World Health Organization’s “healthy life expectancy (HALE) at birth” estimates (WHO, 2022b), females born in the world have a healthy life expectancy at birth of 64.9 years, compared to only 62.5 for males. Thus the critic might, reasonably, ask why the empirical analysis of this article focuses so much on women’s health and wellbeing, when men do not, on average, live as long as women?
To be clear, the argument advanced in this paper does not deny that there is also a pressing moral imperative to slow the ageing process in men, just as there is for women. One could certainly deploy an ethical argument that focused on attending to the 2.4 year differential in healthy life expectancy for males and females. Tsuchiya and Williams (2005), for example, do this. But deploying such an argument would be of more limited use, in terms of illustrating the different societal benefits of slowing biological ageing (e.g. for reproductive health, reducing the burdens of caregiving for older parents, etc.). So there are compelling reasons, from a geroscience advocacy perspective, for drawing specific attention to the diverse benefits an applied gerontological intervention would offer women.
Furthermore, because men already enjoy more socio-economic benefits and opportunities than women, making the lower health prospects of men the primary focus of a normative analysis of longevity science risks conflating an egalitarian concern for health with a concern for equality of overall wellbeing. The latter is a much more significant, multifaceted and complex issue than health alone.
Egalitarian advocacy has been shown, at least in some contexts, to actually increase ageism (Martin & North, 2022). And advocates of geroscience should consider this when framing the benefits of an ageing intervention and advocating for the field. In their study of “egalitarian advocacy” Martin and North (2022) define the term as compromising a motivation to take action and enact equality-based change. And their study found that, while egalitarian advocacy helps reduce sexism and racism, it predicts greater likelihood to support “Succession”-based ageism. That is, the belief that older adults should step aside to free up coveted opportunities. Robert Butler, the first director of the NIH’s National Institutes of Aging, coined the term ‘‘ageism’’ in 1969, which means a systematic stereotyping of, and discrimination against, people because they are old.
Martin and North (2022) found that egalitarian advocates endorse less prejudice toward, and show more support for, women and racial minorities, but harbor more prejudice, and show less advocacy for, older individuals. If the benefits of longevity science are construed as something that only likely benefits rich old men, for example, then the moral justification for increasing lifespan will not be persuasive in a world with stark health inequalities. By contrast, by highlighting the diverse ways geroscience could improve the health and wellbeing of women throughout the lifespan, rate (of ageing) control is more likely to be construed as an important and morally laudable public health strategy for today’s ageing populations.
Two Approaches to Human Longevity: Disease Control vs Rate (of ageing) Control
Longevity scientists have identified two broad strategies for increasing the lifespan. The gerontologist Comfort (1969) described these two different approaches to human longevity as follows:
Science can be expected to affect human longevity favourably in two quite distinct ways. It already does so by suppressing causes of premature death, through the repertoire of applications which now render our lives less nasty, brutish and short than they would otherwise be. It could also affect longevity by postponing the process which causes our liability to disease and death to increase logarithmically with time. The first of these two influences already means that in privileged countries more and more people reach the so-called “specific age” (75~80 years), but it has not altered that age appreciably. The second, which is now in the stage of active research, would aim at postponement or slowing of ageing itself.
This same distinction was also noted by Neugarten and Havighurst (1977) when they argued, in Extending the Human Life Span: Social Policy and Social Ethics, that two strategies for lengthening the lifespan were being pursued by scientists. One strategy they described as the continuing goal of conquering disease. By contrast the second strategy attempted “to identify the intrinsic biological processes that are thought to underlie aging… then to alter the biological clock that is presumably programmed into the human species” (1977). This second approach they referred to as “rate control”, in contrast to the first strategy which they described as “disease control”.
More recently Carnes et al. (2003) made a similar distinction when describing two different two ways of manufacturing survival time: (1) by reclaiming survival time by reducing avoidable mortality (e.g., death from starvation, malaria, accident, cancer, heart disease, etc.); and (2) reclaiming survival time by extending the biological warranty period- that is, the period of time when intrinsic failures of our biology are not expected. In their extensive cross-cultural examination of longevity in hunter-gatherers, Gurven and Kaplan (2007) conclude that “human bodies are designed to function well for about seven decades in the environment in which our species evolved.” Within this seven decade period of time humans could, if they escaped the extrinsic threats to their lives, reach sexual maturity, produce and nurture their offspring. And Gurven and Kaplan estimate that, for at least one-fourth of the adult population living in such conditions (e.g., with no sanitation, immunizations, or medicine), they likely lived 15–20 years as grandparents. The biological warranty period of approximately seven decades is not selected for; rather, it is an “inadvertent by product of evolutionary neglect, and genetic programs for growth, development and reproduction” (Carnes et al., 2003).
The disease control strategy (see Box 1), first developed in the 19th century, prioritizes mitigating the proximate risk factors of infectious diseases like cholera, small pox, polio, diphtheria, dysentery, HIV/AIDS, malaria, COVID-19 etc. The prudence of such a strategy is evident by considering, for example, the leading causes of death for females in the year 1900. In the year 1900 life expectancy at birth in the United States (for all races, both sexes) was age 47.3 (CDC, 2010) and the top three leading causes of death for females were pneumonia and influenza (198.5 per 100,000), tuberculosis (187.8 per 100,000), and enteritis and diarrhea (134.9 per 100,000) (Hahn et al., 2018). A century later none of those infectious diseases remained leading causes of death for females in the United States. But what replaced the top three leading causes of female mortality in the United States? By the year 2010 the following three chronic diseases were the leading causes of female mortality- heart disease (184.9 per 100,000), all cancers (168.2 per 100,000), and stroke (49.1 per 100,000) (Hahn et al., 2018). The shift in the leading mortality causes for females in the United Statues over the course of the twentieth century is indicative of the shift in global mortality for the twenty-first century. Namely, a shift from infectious diseases as the leading cause of death early in life to chronic disease death in later life, and this is one of the significant health challenges facing developing countries in the foreseeable future. This is so because ageing is the major risk factor for all the chronic diseases that cause most death among women today in the developed world.
The public health strategy of disease control that was successful in mitigating the early-life mortality of infectious disease was also applied to the most prevalent chronic diseases, like cancer, heart disease and stroke, as well as other preventable forms of injury, disability and death (e.g. motor vehicle accidents, gun violence, etc.). While genuine progress has been made, and continues to be made, with reducing mortality by targeting the proximate causes of chronic diseases like cancer (e.g. tobacco, unhealthy diet, etc.), the disease control strategy faces significant challenges as the world’s populations age. As Olshansky notes (2018), “finding a cure for one of the late onset diseases like cancer can mean that more debilitating diseases can become more prevalent because the hazard in old age is not so much that one disease displaces another but that the new diseases are often much more debilitating”.
Unlike a cure for one specific disease of late life, an ageing intervention would be a unique and significant biomedical advancement if it can safely, cheaply and effectively increase the human healthspan, that is, “the period of life spent in good health, free from the chronic diseases and disabilities of ageing” (Kaeberlein, 2018). Olshansky (2016) describes the concerted and coordinated effort to accelerate the pace of translation from the basic biology of ageing into clinic interventions that improve quality of life at all ages, but especially at older ages, as the Longevity Dividend Initiative Consortium.
The goal of slowing biological ageing is the aspiration of the field of science known as “biogerontology” or, more recently, “geroscience”. Geroscience is “an interdisciplinary scientific field of inquiry which strives to understand how ageing enables chronic disease and seeks to develop novel multi-disease preventative and therapeutic approaches” (Kennedy et al., 2014). This article details the reasons why geroscience ought to be considered an integral scientific priority for promoting women’s health and wellbeing. By emphasizing the unique health, economic and societal benefits age retardation offers the women of an ageing world the case for targeting biological ageing can be more persuasively made as a significant scientific priority for the twenty-first century.
Women’s Health and Advanced Age
With global declines in early and mid-life mortality, the world’s populations are now surviving into advanced age. Significant improvements in public health- ranging from sanitation, vaccinations, the provision of maternal healthcare and advances in neonatal medicine, to increases in material prosperity and changes in behaviour- have helped to reduce many of the most common causes of early and mid-life mortality. From 1990 to 2020, for example, the global under-5 mortality rate dropped by 60%, from 1 in 11 children dying before reaching age 5 in 1990, to 1 in 27 by the year 2020 (WHO, 2022a). A consequence of the success in preventing early and mid-life mortality is that the inborn ageing process emerged as the major risk factor for disease and death in developed countries (Harman, 1991) and this is expected to apply globally as the populations of low- and middle-income countries survive into later life. Most of the 2 billion persons over age 60 by the year 2050 will be living in the developing world.
A focus on women’s ageing processes is particularly important as women live longer than men in most areas of the world, and comprise the majority of older persons, especially at more advanced ages (Kerr, 2021). The longevity advantage women have over men is evident by many measures, and is present in many distinct cultural settings. The United Nation’s World Population Ageing 2019 Report notes that, at the global level, in 2015–2020, women’s life expectancy at birth exceeded that of men by 4.8 years. And the female advantage in average longevity is largest in Latin America and the Caribbean (6.5 years), Europe and Northern America (6.1 years), and Eastern and South-Eastern Asia (5.3 years) (UN, 2019). Alberts et al. (2014) contend that there is strong evidence that “female survival advantage is a typical feature of nearly all human populations for which such vital-statistics data are available”.
Furthermore, at the most advanced ages (age ≥ 110) women far outnumber men in terms of survival. Of the 53 supercentarians that were alive in the year 2015, 51 were female (Dulken & Brunet, 2015). The oldest person ever recorded with a valid birth record was Jeanne Calment, who lived to be 122 years old (Robine et al., 2019). By contrast the longest-lived man was Jiroemon Kimura, who lived to age 116 (Gondo et al., 2017). And while male centenarians (age ≥ 100) are far fewer in number than female centenarians, male centenarians tend to have significantly better cognition and physical function that their female counterparts (Terry et al., 2008).
Unfortunately the longer life expectancy enjoyed by women are not additional years composed of only healthy years of life. On the contrary, compared with age-matched men, women tend to have poorer health status (i.e., they are more frail) (Collard et al., 2012; Gordon & Hubbard, 2020; Hägg & Jylhävä, 2021). Frailty can be defined as “a state of vulnerability to poor resolution of homoeostasis after a stressor event and is a consequence of cumulative decline in many physiological systems during a lifetime” (Clegg et al., 2013). Alberts et al. (2014) conclude that “research on contemporary populations generally suggests that men are physically stronger, report fewer diseases, and have fewer limitations in the activities of daily living at older ages than women”.
This phenomenon is known as the “male–female health-survival” paradox. There are many different potential explanations for the male–female health-survival paradox, which include biological, social and psychological factors. Oksuzyan et al. (2008), for example, contend that this paradox is likely due to multiple causes, which include fundamental differences between the sexes (such as genetic factors, immune system responses, hormones, and disease patterns), behavioral differences such as risk-taking and reluctance to seek and comply with medical treatment, and methodological challenges, such as selective non-participation and under-reporting of health problems, and delayed seeking of treatment by men. And in their study of disability among persons age 85 + , Kingston et al. (2014) conclude that the disability-survival paradox is still evident in the very old and appears due to sex differences in the types and impacts of disease.
Because the longevity survival that women enjoy over men is associated with poorer health status for women, the imperative to increase the “healthspan” via an ageing intervention is an even more urgent one for the women of today’s ageing world given that “women in modern societies can expect to live nearly one-third of their adult lives in a postreproductive state” (Austad, 1997). The empirical basis for believing that age retardation could delay and potentially compress diminished health status in advanced age comes from ageing studies in animal models as well as studies on the health status of exceptionally long-lived humans, such as centenarians ((age ≥ 100),) and supercentenarians (age ≥ 110). In their study of survival, disability and morbidity among centenarians (age 100–104 years), semisupercentenarians (age 105–109 years), and supercentenarians (age 110–119 years) Andersen et al. (2012) observed that the older the age group, generally, the later the onset of diseases, such as cancer, cardiovascular disease, dementia, and stroke, as well as of cognitive and functional decline. As the limit of human life span was effectively approached with supercentenarians, compression of morbidity was generally observed. An ageing intervention that enabled most people, and especially women, to age more like supercentenarians age would mean more years of healthy life could become the norm, as well as a compression of morbidity in late life. This is a morally laudable aspiration for the medical sciences of an ageing world.
Reproductive Longevity and Maternal Health
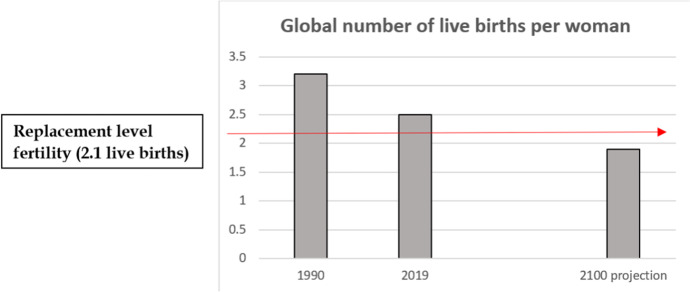
Population ageing is driven by the dual empirical realities that persons are living longer and the global birth rate is declining. Global fertility declined from 3.2 live births per woman in 1990 to 2.5 in 2019 (Fig. 2). The United Nation’s (UN, 2020) World Fertility and Family Planning 2020: Highlights predicts that global fertility will continue to decline this century, reaching 1.9 by the year 2100. This would be below the “replacement level fertility” of an average of 2.1 children per woman. In the United States, the average age of first birth increased from age 21 in 1970 to 26.9 in 2018, and the proportion of women having their first child after age 35 increased nearly 10-fold since 1970 (Llarena & Hine, 2021).
Fig. 2.
Declining Global Fertility
Other specific aspects of the biology of ageing that are of particular importance to women’s health, that go beyond the general age-related health risks of disease, frailty and disability in late life, concern both reproductive longevity and maternal health. In the twentieth century the female life expectancy in developed countries increased by 30 years but this did not prolong the fertile years of women (Brody et al., 2000). Within an evolutionary framework, ageing and reproduction are intrinsically linked (Levine et al., 2016). Biological ageing eventually reduces both fertility and maternal health, and these empirical realities amplify why geroscience is so important to the women of an ageing world.
Most (though not all) women desire to have children they are biologically related to, and, like in the US, the average age of mothers having their first child is getting older in many countries. This trend is also occurring in developing countries, as the level of girls’ education is positively associated with the time of reproductive events like the mean ages at first birth and first marriage (Bongaarts et al., 2017). Biological ageing influences fertility, maternal health and the health of potential offspring.
With respect to fertility, for example, a woman that is younger than age 30 has an 85% chance of conceiving within a year of trying, and this is reduced to 75% at the age of 30, to 66% at the age of 35 and 44% at the age of 40 due to the effect of ageing on the ovary and eggs (Delbaere et al., 2020). Furthermore, as Peterson et al. (2012) note, there is a growing body of research indicating that many people, especially women pursuing higher education (Lampic et al., 2006; Virtala et al., 2011), underestimate the impact aging has on fertility. And many women erroneously believe that IVF can overcome the effects of ageing (Maheshwari et al., 2008).
In the year 1900 the maternal mortality rate in the US was approximately 850 per 100,000 births, and over a century later in most high income countries approximately 10–20 per 100,000 women died in conjunction with childbirth, almost a 99% reduction due to modern medicine (Goldenberg & McClure, 2011). However, pregnancy among older mothers is accompanied by a higher incidence of pregnancy complications (e.g. failure to progress in labour, postpartum haemorrhage) that can endanger the health of the prospective mother. When compared to women under age 40 there is an overall increased risk of maternal mortality (Garcia et al., 2022).
Furthermore, in women 35 years and older the incidences of aneuploidy and birth defects dramatically increase (Llarena & Hine, 2021). Maternal age increases the risk of chromosomal disorders in offspring. For example, the risk of Down syndrome is estimated to be 0.87 per 1000 live births for the maternal age group 25–29, and this risk rises to 4.58 for the maternal age group 35–39, and 15.71 and 33.50, respectively, for the maternal age groups 40–44 and 45–49 (Moorthie et al., 2018).
Llarena and Hine (2021) explore geroscience approaches to maintain long-term ovarian fitness. Caloric restriction (CR), for example, has been studied for decades in a variety of species (like mice) and extends lifespan by altering the rate of biological ageing. CR induces stress response pathways in organisms, which results in longer life by slowing the rate of molecular and cellular decline. Llarena and Hine (2021) cite studies (Li et al., 2015; Selesniemi et al., 2008) of CR in mice when noting that CR increases ovarian reserve, improves oocyte quality, and prolongs the reproductive life span in mammals. CR is unlikely to be a feasible and safe public health intervention to retard primary ageing in humans. But geroscience is exploring a variety of molecules that may mimic the health benefits of CR. Two promising candidates are rapamycin and metformin, and both may improve reproductive longevity.
Rapamycin is a macrolide compound that has been used as a drug to help prevent the rejection of transplanted organs for patients undergoing organ transplant. But recent experiments have found that consuming rapamycin can extend lifespan, including in mammals. Many studies have shown that rapamycin increased the lifespans of male and female mice and these studies on mouse data “demonstrate that this molecule is effective in preventing, even reversing, a broad rage of age-related conditions and thus warrants being described as an “anti-ageing” intervention” (Selvarani et al., 2021). Short-term rapamycin treatment in young and middle-aged female mice was shown to increase ovarian lifespan (Dou et al., 2017).
Metformin has been safely utilized as a pharmacological intervention to help control type 2 diabetes for decades. In experiments on animals, metformin has been shown to slow ageing and exerts its therapeutic effects “through a number of mechanisms and physiological pathways that resemble those generated by caloric restriction” (Novelle et al., 2016). Because of its low cost and proven safety over many decades, metformin is among one of the top candidates for a likely first-generation of applied gerontological interventions. TAME (Targeting Aging with Meformin) is a clinical trial to test the drug metformin as a safe and effective intervention against several age-related diseases (Barzilai et al., 2016; Kulkarni et al., 2022).
With respect to metformin’s potential to positively impact ovarian ageing, Llarena and Hine (2021) conclude that further research is needed, though they do note that metformin has been shown to safely and effectively improve ovulation rates in the polycystic ovarian syndrome population, and increasing evidence demonstrates safety during the first trimester of pregnancy (Penzias et al., 2017). However studies on metformin’s impact on the reproductive longevity of mice have been conflicting.
Economic Vulnerability and Familial Caring Duties
Improving the health of women in later life is also an economic, as well as public health, imperative. In most societies, but especially developing countries, a person’s financial security is linked to their ability to work in paid employment. “In many countries, the absence of social protection systems with high coverage and adequate benefits means that a person’s assets and savings, when savings exist, are usually not sufficient to guarantee adequate income security until the end of their lives” (UN, 2022a, b). Suffering the disease, frailty and disability of late life often imposes economic hardship and vulnerability on older populations. And because women have a longevity advantage over men, and this additional survival is associated with poorer health status for women in terms of frailty and disability, an ageing intervention that helped expand the healthspan of women could improve the economic situation of women by delaying and compressing the amount of time they are most economically vulnerable in the post-reproductive stage of the lifespan.
Another societal benefit of retarding ageing, which would confer significant benefits on women in particular, concerns the expected decline in the familial caring burdens that would be associated with delaying and compressing the disease, frailty and disability of late life. The combination of increasing life expectancy and the legacy of patriarchal practices concerning the unpaid care labour daughters are still expected to provide to their ageing parents means that millions of women in the world today provide a disproportionate amount of the (unpaid) care for their ageing parents.
Worldwide, nearly 70% to 80% of the impaired older persons are cared for at home by family members, and varying estimates across different countries indicate that the majority of all caregivers of older persons are women (Sharma et al., 2016). A recent US study of caregiving to older parents found that sons provide relatively less care to their own parents if they have sisters, whereas daughters provide relatively more care if they have brothers (Grigoryeva, 2017). Grigoryeva (2017) estimated that, on average, daughters provide 13.6 h of parent care per month, compared to sons’ 5.9 h, which means daughters spend more than twice as much time providing care to parents than do sons. Furthermore, the study also found that daughters will likely provide more care to mothers than sons will to fathers. Given that women survive longer than men this means that a disproportionate share of parental caring duties for older persons will typically fall upon women, especially in developing countries where less people can afford to pay for long-term care. Increasing the human healthspan would reduce the burdens of care for ageing parents, and this would help abate the gender inequality of these caring burdens as well as permit women to spend that time instead on other activities which could be beneficial to them, such as paid employment or leisure activities (which women have less time for than men (Codina & Pestana, 2019)).
Conclusion
Science advocacy is an integral part of the scientific enterprise, public health and democratic politics. Such advocacy influences the share of public funding distinct areas of scientific research are allocated (e.g. research on ageing vs Alzheimer’s disease) and the public health measures mandated (e.g. quarantine, wearing of face masks when indoors, immunizations) or encouraged (e.g. smoking cessation, condom use, diet and lifestyle) by the government. When scientific advocacy is credible and transparent, it can be both an expression of, and instrument for, civic stewardship by enabling democratic societies to better harness the benefits of science to realize a better future for everyone.
The philosopher of science Kitcher (2004) endorses this inclusive vision of science when he argues that “responsible biology” entails that scientists have an obligation to reflect on the ends, and not just the means, of scientific research and to conceive of themselves as artisans working for the public good. Advocating for the disease control approach to human longevity easily coheres with Kitcher’s ideal of “responsible biology”. The aspiration to cure cancer, for example, epitomizes the ideal of scientists as artisans working for the public good (Farrelly, 2021a, b). In 1971 US President Richard Nixon asked for an appropriation of an extra $100 million to launch the so-called “war on cancer”. At the time cancer was the second leading cause of death in America. Half a century later and the 2021 annual budget for the National Cancer Institute had grown to $6.35 billion dollars (National Cancer Institute, 2022) and cancer still remains the second leading cause of death.
There is a legitimate egalitarian concern to address in terms of the opportunity costs of prioritizing the goal of rate (of ageing) control over the continued focus on disease control. Medical resources, including funding for basic scientific research, are limited. If the goal of accelerating the pace of translation from basic biology of ageing into clinic interventions that slowed ageing diverted the limited funding committed to tackling early onset health disadvantages this would exacerbate the existing inequalities between the vulnerable young and older persons. I believe this would constitute an injustice. But that is not what the strategy of the Longevity Dividend Initiative (Olshansky, 2016) proposes, as its focus is on diverting some of the attention and resources currently invested into research on the specific diseases of late life (like cancer and Alzheimer’s) into the major risk factor for all of those conditions. This would mean that, at least in the short and medium term, some of the funding committed to pursuing novel therapeutics for specific diseases and conditions that occur in late life ought to be diverted into research that targets ageing itself as a form of preventative medicine against the current health vulnerabilities of late life.
Pursuing further lifespan extension for older populations via disease control will yield diminishing health dividends because a new treatment for one specific disease of ageing does not reduce the other risks of multi-morbidity in late life. The longer we live, the greater the influence on disease expression (Olshansky, 2016). If an applied gerontological intervention can be developed that delayed and compressed the morbidity, frailty and disability of late life, then the current strains placed on health care resources and basic scientific research could be dramatically reduced. And this would enable more resources to be invested in mitigating early life mortality and morbidity risks. It is important to acknowledge that slowing the rate of biological ageing would not of course be a panacea for all diseases or a solution to health inequalities. The social determinates of health like education, employment, social inclusion, and housing, for example, would remain critical for achieving more equitable health outcomes for the global population. And egalitarian advocates are justified in raising the concern over how accessible an ageing intervention is likely to be, both to the poor in rich countries and to the global poor. The cost and accessibility of such an intervention will depend largely on the kind of technology it is. If such an intervention required genome editing in a world-class medical facility with specialized surgeons, for example, then the obstacles to ensuring the fair diffusion of the technology would be very significant. The encouraging news is that a fertile source for therapies slowing ageing is FDA approved drugs whose safety has been investigated (Snell et al., 2016). Repurposed drugs that have been in existence for many decades will be off-patent (which means they can be developed at a fraction of the original costs) and have an extensive track-record for safety.
Like the COVID-19 vaccines, an “anti-ageing drug” would be a critical public health intervention that all persons in the world should have access to. Rather than object to an ageing intervention on the grounds that it will exacerbate inequality, the more appropriate response would be to recognize how important an ageing intervention is to the public health of the world’s ageing populations and champion both its development and its fair diffusion. The four ways ageing impacts women identified in this article- the existence and persistence of the “male–female health-survival” paradox, fertility and maternal health, the economic vulnerability of women in late life (especially in developing countries), and the caring burdens which typically fall (at least disproportionately) on daughters to care for their ageing parents- ought to buttress geroscience’s prominence as an important instrument in promoting equality. This article has detailed the empirical bases for supporting this conclusion by emphasising the impact an applied gerontological impact could have on the nearly 4 billion girls and women currently alive.
Acknowledgements
I am very grateful to two anonymous referees for their helpful feedback and useful suggestions on earlier versions of this paper.
Declarations
Conflict of Interest
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alberts SC, Archie EA, Gesquiere LR, et al. The male-female health-survival paradox: A comparative perspective on sex differences in aging and mortality. In: Weinstein M, Lane M, et al., editors. Sociality, hierarchy, health: Comparative biodemography: A collection of papers. National Academies Press; 2014. pp. 339–364. [PubMed] [Google Scholar]
- Andersen SL, Sebastiani P, Dworkis DA, Feldman L, Perls TT. Health span approximates life span among many supercentenarians: compression of morbidity at the approximate limit of life span. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2012;67(4):395–405. doi: 10.1093/gerona/glr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austad SN. Postreproductive survival and the role of the elderly in nature. In: Wachter K, Finch C, editors. Between Zeus and the salmon: The biodemography of longevity. National Academy Press; 1997. pp. 161–174. [PubMed] [Google Scholar]
- Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a tool to target aging. Cell Metabolism. 2016;23(6):1060–1065. doi: 10.1016/j.cmet.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, Appleby JC, Austad SN, Cuervo AM, Kaeberlein M, Gonzalez-Billault C, Lederman S, Stambler I, Sierra F. Geroscience in the Age of COVID-19. Aging and Disease. 2020;11(4):725–729. doi: 10.14336/AD.2020.0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongaarts J, Mensch BS, Blanc AK. Trends in the age at reproductive transitions in the developing world: The role of education. Population Studies. 2017;71(2):139–154. doi: 10.1080/00324728.2017.1291986. [DOI] [PubMed] [Google Scholar]
- Butler RN. Age-ism: Another form of bigotry. The Gerontologist. 1969;9:243–246. doi: 10.1093/geront/9.4_Part_1.243. [DOI] [PubMed] [Google Scholar]
- Butler RN, Miller RA, Perry D, Carnes BA, Williams TF, Cassel C, Brody J, Bernard MA, Partridge L, Kirkwood T, Martin GM, Olshansky SJ. New model of health promotion and disease prevention for the 21st century. BMJ (Clinical research ed.) 2008;337(7662):a399. doi: 10.1136/bmj.a399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bognar G. Fair innings. Bioethics. 2015;29(4):251–261. doi: 10.1111/bioe.12101. [DOI] [PubMed] [Google Scholar]
- Brody JA, Grant MD, Frateschi LJ, Miller SC, Zhang H. Reproductive longevity and increased life expectancy. Age and Ageing. 2000;29(1):75–78. doi: 10.1093/ageing/29.1.75. [DOI] [PubMed] [Google Scholar]
- Callahan D. False hopes: Why America’s quest for perfect health is a recipe for failure. Simon and Schuster; 1998. [Google Scholar]
- Carnes BA, Olshansky SJ, Grahn D. Biological evidence for limits to the duration of life. Biogerontology. 2003;4(1):31–45. doi: 10.1023/a:1022425317536. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2010). Table 22: Life expectancy at birth, at 65 years of age, and at 75 years of age, by race and sex: United States, selected years 1900–2007. https://www.cdc.gov/nchs/data/hus/2010/022.pdf. Accessed 24 Feb 2021.
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet (London, England) 2013;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codina N, Pestana JV. Time matters differently in leisure experience for men and women: Leisure dedication and time perspective. International Journal of Environmental Research and Public Health. 2019;16(14):2513. doi: 10.3390/ijerph16142513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: A systematic review. Journal of the American Geriatrics Society. 2012;60(8):1487–1492. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- Comfort A. Longer life by 1990? New Scientist. 1969;11:549–551. [Google Scholar]
- Delbaere I, Verbiest S, Tydén T. Knowledge about the impact of age on fertility: A brief review. Upsala Journal of Medical Sciences. 2020;125(2):167–174. doi: 10.1080/03009734.2019.1707913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doris J, Stich S. As a matter of fact: Empirical perspectives on ethics. In: Jackson F, Smith M, editors. Oxford handbook of contemporary analytic philosophy. Oxford University Press; 2005. pp. 114–152. [Google Scholar]
- Dou X, Sun Y, Li J, Zhang J, Hao D, Liu W, Wu R, Kong F, Peng X, Li J. Short-term rapamycin treatment increases ovarian lifespan in young and middle-aged female mice. Aging Cell. 2017;16(4):825–836. doi: 10.1111/acel.12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulken B, Brunet A. Stem cell aging and sex: Are we missing something? Cell Stem Cell. 2015;16(6):588–590. doi: 10.1016/j.stem.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrelly C. 50 years of the "war on cancer": Lessons for public health and geroscience. GeroScience. 2021;43(3):1229–1235. doi: 10.1007/s11357-021-00366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrelly C. Responsible biology, aging populations and the 50th anniversary of the "War on Cancer". Biogerontology. 2021;22(4):429–440. doi: 10.1007/s10522-021-09925-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M, Walker K, Thornton J. Management of pregnancy complications in women of advanced maternal age. Obstetrics, Gynaecology & Reproductive Medicine. 2022;32(6):101–104. doi: 10.1016/j.ogrm.2022.04.001. [DOI] [Google Scholar]
- Goldenberg RL, McClure EM. Maternal mortality. American Journal of Obstetrics and Gynecology. 2011;205(4):293–295. doi: 10.1016/j.ajog.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondo Y, Hirose N, Yasumoto S, Arai Y, Saito Y. Age verification of the longest lived man in the world. Experimental Gerontology. 2017;99:7–17. doi: 10.1016/j.exger.2017.08.030. [DOI] [PubMed] [Google Scholar]
- Gordon EH, Hubbard RE. Differences in frailty in older men and women. The Medical Journal of Australia. 2020;212(4):183–188. doi: 10.5694/mja2.50466. [DOI] [PubMed] [Google Scholar]
- Gurven M, Kaplan H. Longevity among hunter-gatherers: A cross-cultural examination. Population and Development Review. 2007;33(2):321–365. doi: 10.1111/j.1728-4457.2007.00171.x. [DOI] [Google Scholar]
- Grigoryeva A. Own gender, sibling’s gender, parent’s gender: The division of elderly parent care among adult children. American Sociological Review. 2017;82(1):116–146. doi: 10.1177/0003122416686521. [DOI] [Google Scholar]
- Hägg S, Jylhävä J. Sex differences in biological aging with a focus on human studies. eLife. 2021;10:e63425. doi: 10.7554/eLife.63425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn RA, Chang MH, Parrish RG, Teutsch SM, Jones WK. Trends in mortality among females in the United States, 1900–2010: Progress and challenges. Preventing Chronic Disease. 2018;15:E30. doi: 10.5888/pcd15.170284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. The aging process: Major risk factor for disease and death. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(12):5360–5363. doi: 10.1073/pnas.88.12.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L. The future of ageing. Nature. 2000;408:267–269. doi: 10.1038/35041709. [DOI] [PubMed] [Google Scholar]
- Holliday R. Ageing in the 21st century. Lancet. 1999;354 Suppl:SIV4. doi: 10.1016/s0140-6736(99)90347-1. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M. How healthy is the healthspan concept? GeroScience. 2018;40(4):361–364. doi: 10.1007/s11357-018-0036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Rabinovitch PS, Martin GM. Healthy aging: The ultimate preventative medicine. Science (New York, N.Y.) 2015;350(6265):1191–1193. doi: 10.1126/science.aad3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss-Coray T, Sierra F. Geroscience: Linking aging to chronic disease. Cell. 2014;159(4):709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr CL. Geroscience approaches to women's health in an aging world. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2021;76(9):1531–1532. doi: 10.1093/gerona/glab173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston A, Davies K, Collerton J, Robinson L, Duncan R, Bond J, Kirkwood TB, Jagger C. The contribution of diseases to the male-female disability-survival paradox in the very old: results from the Newcastle 85+ study. PloS one. 2014;9(2):e88016. doi: 10.1371/journal.pone.0088016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitcher P. Responsible biology. BioScience. 2004;54(4):331–336. doi: 10.1641/0006-3568(2004)054[0331:RB]2.0.CO;2. [DOI] [Google Scholar]
- Kulkarni AS, Aleksic S, Berger DM, Sierra F, Kuchel GA, Barzilai N. Geroscience-guided repurposing of FDA-approved drugs to target aging: A proposed process and prioritization. Aging Cell. 2022;21(4):e13596. doi: 10.1111/acel.13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, Lu AT, Chen BH, Hernandez DG, Singleton AB, Ferrucci L, Bandinelli S, Salfati E, Manson JE, Quach A, Kusters CD, Kuh D, Wong A, Teschendorff AE, Widschwendter M, Ritz BR, Absher D, Assimes TL, Horvath S. Menopause accelerates biological aging. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(33):9327–9332. doi: 10.1073/pnas.1604558113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampic C, Svanberg AS, Karlström P, Tydén T. Fertility awareness, intentions concerning childbearing, and attitudes towards parenthood among female and male academics. Human Reproduction (Oxford, England) 2006;21(2):558–564. doi: 10.1093/humrep/dei367. [DOI] [PubMed] [Google Scholar]
- Li L, Fu YC, Xu JJ, Lin XH, Chen XC, Zhang XM, Luo LL. Caloric restriction promotes the reserve of follicle pool in adult female rats by inhibiting the activation of mammalian target of rapamycin signaling. Reproductive Sciences (Thousand Oaks, Calif.) 2015;22(1):60–67. doi: 10.1177/1933719114542016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llarena N, Hine C. Reproductive longevity and aging: geroscience approaches to maintain long-term ovarian fitness. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2021;76(9):1551–1560. doi: 10.1093/gerona/glaa204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari A, Porter M, Shetty A, Bhattacharya S. Women's awareness and perceptions of delay in childbearing. Fertility and Sterility. 2008;90(4):1036–1042. doi: 10.1016/j.fertnstert.2007.07.1338. [DOI] [PubMed] [Google Scholar]
- Martin AE, North MS. Equality for (almost) all: Egalitarian advocacy predicts lower endorsement of sexism and racism, but not ageism. Journal of Personality and Social Psychology. 2022;123(2):373–399. doi: 10.1037/pspi0000262. [DOI] [PubMed] [Google Scholar]
- Mauron A. The choosy reaper. From the myth of eternal youth to the reality of unequal death. EMBO Reports. 2005;6 Spec No(Suppl 1):S67–S71. doi: 10.1038/sj.embor.7400432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorthie S, Blencowe H, Darlison MW, Gibbons S, Lawn JE, Mastroiacovo P, Morris JK, Modell B, Congenital Disorders Expert Group Chromosomal disorders: estimating baseline birth prevalence and pregnancy outcomes worldwide. Journal of Community Genetics. 2018;9(4):377–386. doi: 10.1007/s12687-017-0336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musschenga AW. Empirical ethics, context-sensitivity, and contextualism. The Journal of Medicine and Philosophy. 2005;30(5):467–490. doi: 10.1080/03605310500253030. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. (2022). NCI Budget Fact Book. Available from: https://www.cancer.gov/about-nci/budget/fact-book. Accessed 7 July 2022.
- Neugarten B, Havighurst R. Extending the human life span: social policy and social ethics. National Science Foundation; 1977. [Google Scholar]
- Novelle MG, Ali A, Diéguez C, Bernier M, de Cabo R. Metformin: A hopeful promise in aging research. Cold Spring Harbor Perspectives in Medicine. 2016;6(3):a025932. doi: 10.1101/cshperspect.a025932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksuzyan A, Juel K, Vaupel JW, Christensen K. Men: good health and high mortality. Sex differences in health and aging. Aging Clinical and Experimental Research. 2008;20(2):91–102. doi: 10.1007/BF03324754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshansky SJ. Articulating the case for the longevity dividend. Cold Spring Harbor Perspectives in Medicine. 2016;6(2):a025940. doi: 10.1101/cshperspect.a025940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshansky SJ. From Lifespan to Healthspan. Journal of the American Medical Association. 2018;320:1323–1324. doi: 10.1001/jama.2018.12621. [DOI] [PubMed] [Google Scholar]
- Peterson BD, Pirritano M, Tucker L, Lampic C. Fertility awareness and parenting attitudes among American male and female undergraduate university students. Human Reproduction (Oxford, England) 2012;27(5):1375–1382. doi: 10.1093/humrep/des011. [DOI] [PubMed] [Google Scholar]
- Penzias A, Bendikson K, Butts S, et al. Role of metformin for ovulation induction in infertile patients with polycystic ovary syndrome (PCOS): a guideline. Fertility and Sterility. 2017;108(3):426–441. doi: 10.1016/j.fertnstert.2017.06.026. [DOI] [PubMed] [Google Scholar]
- Pijnenburg MA, Leget C. Who wants to live forever? Three arguments against extending the human lifespan. Journal of Medical Ethics. 2007;33(10):585–587. doi: 10.1136/jme.2006.017822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae MJ, Butler RN, Campisi J, de Grey AD, Finch CE, Gough M, Martin GM, Vijg J, Perrott KM, Logan BJ. The demographic and biomedical case for late-life interventions in aging. Science Translational Medicine. 2010;2(40):40cm21. doi: 10.1126/scitranslmed.3000822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robine JM, Allard M, Herrmann FR, Jeune B. The real facts supporting Jeanne Calment as the oldest ever human. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2019;74(Suppl_1):S13–S20. doi: 10.1093/gerona/glz198. [DOI] [PubMed] [Google Scholar]
- Santesmasses D, Castro JP, Zenin AA, Shindyapina AV, Gerashchenko MV, Zhang B, Kerepesi C, Yim SH, Fedichev PO, Gladyshev VN. COVID-19 is an emergent disease of aging. Aging Cell. 2020;19(10):e13230. doi: 10.1111/acel.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selesniemi K, Lee HJ, Tilly JL. Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age. Aging Cell. 2008;7(5):622–629. doi: 10.1111/j.1474-9726.2008.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvarani R, Mohammed S, Richardson A. Effect of rapamycin on aging and age-related diseases-past and future. GeroScience. 2021;43(3):1135–1158. doi: 10.1007/s11357-020-00274-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Chakrabarti S, Grover S. Gender differences in caregiving among family - caregivers of people with mental illnesses. World Journal of Psychiatry. 2016;6(1):7–17. doi: 10.5498/wjp.v6.i1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell TW, Johnston RK, Srinivasan B, Zhou H, Gao M, Skolnick J. Repurposing FDA-approved drugs for anti-aging therapies. Biogerontology. 2016;17(5–6):907–920. doi: 10.1007/s10522-016-9660-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry DF, Sebastiani P, Andersen SL, Perls TT. Disentangling the roles of disability and morbidity in survival to exceptional old age. Archives of Internal Medicine. 2008;168(3):277–283. doi: 10.1001/archinternmed.2007.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya A, Williams A. A "fair innings" between the sexes: are men being treated inequitably? Social Science & Medicine. 2005;60(2):277–286. doi: 10.1016/j.socscimed.2004.04.035. [DOI] [PubMed] [Google Scholar]
- United Nations, Department of Economic and Social Affairs, Population Division. (2011). World population prospects: the 2010 revision, volume I: comprehensive tables. ST/ESA/SER.A/313. https://www.un.org/en/development/desa/population/publications/pdf/trends/WPP2010/WPP2010_Volume-I_Comprehensive-Tables.pdf. Accessed 7 July 2022.
- United Nations, Department of Economic and Social Affairs, Population Division. (2019). World Population Ageing 2019: Highlights (ST/ESA/SER.A/430). https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf. Accessed 7 July 2022.
- United Nations, Department of Economic and Social Affairs, Population Division. (2020). World fertility and family planning 2020: Highlights (ST/ESA/SER.A/440). https://www.un.org/en/development/desa/population/publications/pdf/family/World_Fertility_and_Family_Planning_2020_Highlights.pdf. Accessed 7 July 2022.
- United Nations, Department of Economic and Social Affairs, Population Division. (2022a). World Population Prospects 2022, Online edition. Available from: https://population.un.org/wpp/Download/Standard/Population/. Accessed 7 July 2022.
- United Nations, Department of Economic and Social Affairs programme on ageing. (2022b). The focal point on ageing in the United Nations system. Income Poverty in Old Age: An Emerging Development Priority. Available from: https://www.un.org/esa/socdev/ageing/documents/PovertyIssuePaperAgeing.pdf. Accessed 7 July 2022.
- Virtala A, Vilska S, Huttunen T, Kunttu K. Childbearing, the desire to have children, and awareness about the impact of age on female fertility among Finnish university students. The European Journal of Contraception & Reproductive Health Care : The Official Journal of the European Society of Contraception. 2011;16(2):108–115. doi: 10.3109/13625187.2011.553295. [DOI] [PubMed] [Google Scholar]
- Williams A. Intergenerational equity: An exploration of the 'fair innings' argument. Health Economics. 1997;6(2):117–132. doi: 10.1002/(sici)1099-1050(199703)6:2<117::aid-hec256>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Williams A. Inequalities in health and intergenerational equity. Ethical Theory and Moral Practice : An International Forum. 1999;2(1):47–55. doi: 10.1023/a:1009922327634. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2021a). Global health exploratory [internet]. Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/life-expectancy-at-birth-(years). [cited 2022 July 7].
- World Health Organization. (2021b). Ageing and health. Available from: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health. [cited 2022 July 7].
- World Health Organization. (2022a). Child mortality and causes of death. Available from: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/child-mortality-and-causes-of-death. [cited 2022 July 7].
- World Health Organization. (2022b). World health observatory: Healthy life expectancy (HALE) at birth (years). Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/gho-ghe-hale-healthy-life-expectancy-at-birth. [cited 2022 November 22].