Abstract
We used a panel of nine fusion proteins that contain different Duffy binding-like α (DBL-α) domains of Plasmodium falciparum-infected erythrocyte membrane protein 1 to assess the levels of antibody activity in serum samples obtained from semi-immune or nonimmune individuals from Lambaréné, Gabon. Recognition was measured in terms of either the prevalence or the magnitude of the response. A strong correlation between the immune status of the patients and reactivity with recombinant proteins was observed, which was interpreted as a reflection of the number of infections acquired over time. The antibody responses were predominantly directed toward variable epitopes of the DBL-α domain. Antibody responses could be reduced by preincubation of the sera with various fusion proteins. A portion of individuals who exhibited high-level responses to all fusion proteins also had antibodies which recognized conserved epitopes. The possibility that a synergizing effect of anti-DBL-α domain antibodies could support chemotherapy is discussed.
The human malaria parasite Plasmodium falciparum is responsible for severe forms of the disease and nearly all of the malaria-related mortality. Upon invasion of human erythrocytes, P. falciparum parasites cause significant structural changes to the surfaces of erythrocytes; the major change is the insertion of parasite-derived proteins in knob-like protrusions on the surfaces (1, 25). A variant antigen, designated P. falciparum erythrocyte membrane protein 1 (PfEMP-1), is anchored in these knobs and mediates adhesion to various host endothelial receptors (11; reviewed in references 6 and 14). PfEMP-1 is encoded by a family of 40 to 50 var genes, only one of which is expressed at any one time (5, 20), and variant forms of the protein differ from each other in their adhesive properties.
In addition to mediating cytoadherence, PfEMP-1 is thought to undergo clonal antigenic variation as a means of immune evasion. Although polymorphic in terms of sequence, all PfEMP-1 proteins have a common structure; they contain up to seven Duffy binding-like (DBL) domains and at least one cysteine-rich interdomain region (22). It has been shown that the DBL-α domain is involved in the formation of rosettes, in which infected erythrocytes are surrounded by uninfected red blood cells (10, 27). The rosette formation caused by parasite isolates correlates with the most severe forms of malaria (3, 12, 18). Recombinant DBL-α domains can block rosette formation (4), as can antibodies in the sera of malaria patients (26).
Epidemiological data have demonstrated that anti-PfEMP-1 antibodies provide protection against disease (2, 8, 9, 16); however, despite this apparent role in the development of antimalarial immunity, the use of PfEMP-1 in vaccine development is hampered by the extensive polymorphism in the var gene family. This polymorphism is generated by mitotic crossover events, which can lead to totally new variant forms (7), and by an unknown mechanism in which expression of one PfEMP-1 molecule can switch to expression of another.
In this study, we carried out an extensive analysis of immune responses to DBL-α domains of P. falciparum isolates obtained from malaria patients in Gabon. The cloned sequences encode not only conserved amino acids characteristic of PfEMP-1 DBL-α domains but also highly variable regions. Recombinant proteins were expressed in Escherichia coli, and human antibody responses to these purified proteins were assessed by using serum samples collected from semi-immune and nonimmune individuals from this region.
MATERIALS AND METHODS
Parasites and human sera.
Parasites were collected from samples taken from P. falciparum-infected individuals at the Albert Schweitzer Hospital in Lambaréné, Gabon, an area where P. falciparum is hyperendemic and transmission is intense (24, 29). Human sera were obtained from 100 semi-immune individuals who were between 15 and 64 years old (median age, 32 ± 16.5 years) and from 100 children who were between 1.3 and 6.5 years old living in the study area (median age, 3.9 ± 1.7 years). The definition of a semi-immune person is a person living in an area of malaria endemicity who is at least 15 years old and does not have any symptoms even if he or she is infected by P. falciparum. The children used in this study did not have this status; they were in fact nonimmune. The semi-immune individuals were from a cross-sectional study and apparently were asymptomatic. The nonimmune group was part of a chemotherapy trial in which the children received a 3-day course of either amodiaquine or artesunate plus amodiaquine for the treatment of uncomplicated falciparum malaria. This was defined as a falciparum parasitemia with a concentration between 1,000 and 200,000 parasites/μl, a body temperature of more than 37.5°C, or a history of fever in the previous 24 h. Individuals showing signs of severe malaria were excluded. Blood samples were taken before the initiation of treatment. Ethical clearance was obtained from the Ethics Committees of the International Foundation of the Albert Schweitzer Hospital. All study participants or their parents or guardians gave informed consent.
Cloning of DBL-α domains.
Genomic DNA of P. falciparum parasites originating from a number of P. falciparum-infected individuals were extracted with a Qiagen QIAmp blood kit (Qiagen, Hilden, Germany). The DNAs were used as templates to amplify DBL-α domain-specific fragments by PCR using standard procedures (19). Oligonucleotide primers were constructed around consensus sequences, and amplifications were carried out with a forward primer (5′-TTG GAT CCT AGA CGA TTA CAT YAT TGT GAT -3′; carrying a BamHI restriction site) and a reverse primer (5′-TTT GAG CTC TTA TTC GKC CCA TTC STC GAA CCA -3′; with a SacI restriction site) (where K is G or T, Y is C or T, and S is C or G).
Gel-purified DBL-α domain fragments were subsequently digested with appropriate restriction enzymes and ligated to pGexZ′ DNA that had been pretreated accordingly. Expression vector pGexZ′ was derived from the previously described vector pGexA and forms a fusion polypeptide with the glutathione S-transferase (GST) (28). The DNA sequence corresponding to the α-peptide of the β-galactosidase was inserted into the vector in order to allow blue-white selection in the presence of isopropyl-β-d-thiogalactoside (IPTG) and 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal). Positive recombinant clones were identified by a loss of α-complementation.
Transformants were checked by PCR amplification performed with vector-specific primers for the presence of DBL-α domain-specific DNA. Nucleotide sequences of the inserts were determined by DNA sequencing by using an ABI 373 Prism automated sequencing system with a Big Dye terminator sequencing kit (Applied Biosystems, Foster City, Calif.). Sequence identities were confirmed by BLAST analysis. Deduced amino acid alignments based on these sequences were constructed by using GCG pileup (EST Cluster Programme, Heidelberg, Germany)
Recombinant DBL-α domains in E. coli and synthetic peptides.
E. coli DH5α cells were grown to an optical density at 600 nm (OD600) of 0.7 to 0.9 at 30°C and then induced to express GST–DBL-α domain fusion proteins in the presence of 0.1 mM IPTG for an additional 4 h. The fusion proteins were solubilized in 7 M urea, refolded by dropwise dilution with 100 mM Tris (pH 8.0), and affinity purified on glutathione Sepharose 4B columns (Pharmacia, Upsala, Sweden) as described previously (21). Protein purity was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis with 10% polyacrylamide. Quantities were determined by performing a protein assay (Bio-Rad, Munich, Germany) as recommended by the supplier. Purified fusion proteins were stored in aliquots in elution buffer (50 mM Tris-HCl [pH 8.0], 10 mM reduced glutathione) at −80°C until they were needed. The molecular masses of the resulting GST–DBL-α domain fusion proteins were approximately 48 to 51 kDa, 26 kDa of which was the molecular mass of the carrier GST protein. The calculated protein yields varied from 0.1 to 1.0 mg per 50-ml induced culture.
Three synthetic peptides, corresponding to conserved regions of DBL-α domains, were obtained commercially coupled to bovine serum albumin (BSA) (ThermoHybaid GmbH, Ulm, Germany). The peptides were activated by N-3′-dimethylaminopropyl-N-ethylcarbodiimid, and 0.2 μmol of peptide was coupled to 0.1 μmol of BSA. The amino acid sequences of the peptides used were as follows: P1, GACAPYRRLHLCD; P2, LARSFADIGDIVRGKDLY; and P3, VPQYLRWFEEWAEDFCRK.
Analysis of variant-specific antibodies in humans.
An enzyme-linked immunosorbent assay (ELISA) was used to assess antibody reactivity with the purified recombinant proteins and with the synthetic peptides in human sera. The wells of 96-well microtiter plates (Costar, Corning, N.Y.) were each coated with 50 μl of recombinant GST–DBL-α domain fusion protein at a concentration of 5 μg/ml of coating buffer (15 mM Na2CO3, 35 mM NaHCO3; pH 9.3 to 9.6) and left overnight at 4°C. Control wells were coated with GST alone. The plates were washed extensively with washing buffer (0.5% Tween 20, phosphate-buffered saline [PBS]) and blocked with blocking buffer (4% BSA, PBS) for 2 h at room temperature. Then 50 μl of human serum (diluted 1:200 in 1% BSA in washing buffer) per well was added to duplicate wells, and the preparations were incubated for 2 h at room temperature. After three washes, 50 μl of horseradish peroxidase-conjugated rabbit anti-human immunoglobulin G (diluted 1:12,000; Sigma Chemical Co., St. Louis, Mo.) was added to each well, the preparations were incubated for 1.5 h and washed again, and the reaction mixtures were developed in the presence of 3,3′5,5′-tetramethylbenzidine substrate (Sigma) mixed with hydrogen peroxide (1:1) for 10 to 15 min at room temperature. Each reaction was stopped with 1 M H3PO4, and A450 was measured with a 550-nm reference filter by using a Hightech Digiscan (Asys, Eugendorf, Austria).
OD450 values specific for antibody reactivity with the recombinant DBL-α domain proteins were obtained by subtracting average OD450 values for GST from average OD450 values for the GST–DBL-α domain hybrid protein. High levels of GST reactivity in a small number of the serum samples resulted in negative OD450 values, and for statistical analyses these values were set at 0. The negative-to-positive cutoff value was calculated by determining the mean OD450 value ± 2 standard deviations based on the reactivities of sera of 20 German blood donors who had not been exposed to malaria. The same procedure was used to analyze the reactivities of the synthetic peptides; the coupled peptides were used at a concentration of 5 μg/ml, and the control wells were coated with 4% BSA.
A pool of sera from adult Gabonese individuals who were living in a hyperendemic area of malaria and were semi-immune to malaria served as a positive standard in the ELISA, and this pool was tested in parallel in all plates to account for test-to-test and day-to-day variations. Additionally, a pool of sera from German blood donors served as a negative control.
Competition ELISA.
To confirm the presence of variant-specific antibodies in adult sera, competition ELISAs were performed with different DBL-α domain recombinant proteins. For technical reasons the ELISAs were performed with a limited number of sera. Two different serum samples that were highly reactive with all fusion proteins were chosen and tested against three different fusion proteins (G15c, G17d, and G21d). Blocking of the sera was done with 0.1, 1, 5, and 10 μg of fusion protein. The sera used in the assays performed with plates coated with G15c and G17d were blocked with fusion proteins G15c, G17d, G21d, and G41a, while G21d was tested against itself.
Ninety-six-well plates were coated with recombinant fusion proteins and incubated overnight at 4°C. The plates were washed and blocked in blocking buffer (PBS, 4% BSA). The test sera were diluted 1:200 in dilution buffer (1% BSA in PBS–0.5% Tween 20) to which recombinant proteins at a range of concentrations were added, and the preparations were incubated for 2 h at room temperature. The blocked sera were added to plates coated with heterologous recombinant proteins, and the rest of the assay was performed as described above. Similar assys were performed to determine whether conserved peptides in solution could inhibit binding of immune sera to the DBL-α domain proteins. As described above, different amounts (0.1, 1, 5, and 10 μg) of the conserved peptides were added to diluted immune sera, and the preparations were incubated for 2 h at room temperature. The peptide-blocked sera were then added to the wells coated with recombinant proteins. The ELISA was performed as described above. As a control the sera were preincubated with the same recombinant protein, which was used to coat a 96-well plate.
Statistical analysis.
A statistical analysis was done by using nonparametric tests. The reactivities of individual serum samples with pairs of antigens were compared by using the Spearman nonparametric rank correlation test. Differences between groups were analyzed by using the Mann-Whitney test.
Nucleotide sequence accession numbers.
The nucleotide sequences encoding the deduced amino acid sequences of the DBL-α domains expressed as proteins fused to GST have been deposited in the GenBank database under the following accession numbers: DBL-G15, AY028941; DBL-G8d, AF366354; DBL-G41a, AF366355; DBL-G31a, AF366356; DBL-G23a, AF366357; DBL-G22, AF366358; DBL-G21d, AF366359; DBL-G17d, AF366360; and DBL-G15c, AF366361.
RESULTS
Analysis of DBL-α domain sequences from field isolates.
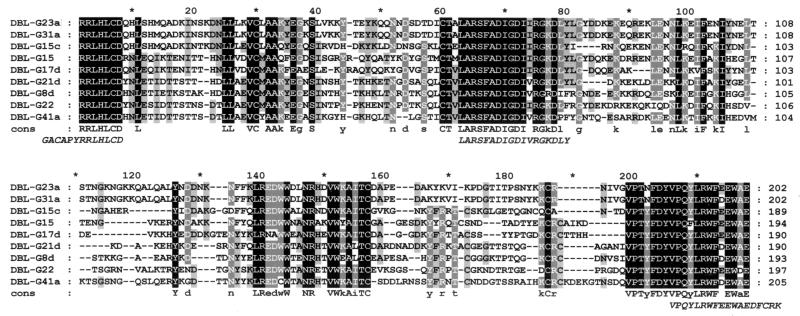
Cloned DBL-α domain fragments of different lengths between 660 and 690 bp encoded open reading frames estimated to have molecular masses of approximately 22 to 25 kDa. An alignment of the deduced amino acid sequences of the nine clones used in this study is shown in Fig. 1. Clones were found to vary in their degrees of identity, which ranged between 47.7 and 75.0% except for two sequences that were 99.5% identical to each other.
FIG. 1.
Alignment of the deduced amino acid sequences of the DBL-α domains expressed as proteins fused to GST. The shading indicates the levels of identity between polypeptides. White letters on a black background represent amino acid residues that are 100% identical in the sequences; white letters on a grey background represent amino acid residues that are 80% identical; and black letters on a grey background represent amino acid residues that are 60% identical. A consensus sequence (cons) is shown below the alignment; residues that are 100% identical are in uppercase letters, and residues that are 80% identical are in lowercase letters. The peptides used in the ELISA are shown at the bottom in italics. Oligonucleotide primers were constructed for amino acids located at the extreme 5′ and 3′ ends of the sequences.
Immunoreactivity to recombinant DBL-α domain polypeptides.
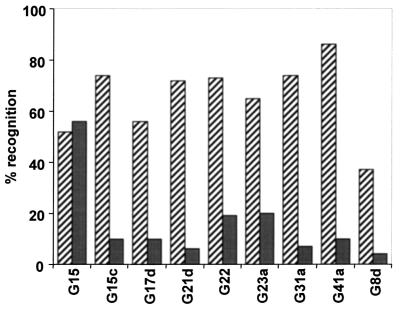
Nine recombinant DBL-α domain antigens were chosen for testing with ELISAs. The reactivities of the fusion proteins with serum immunoglobulin G antibodies were analyzed, and the frequencies of recognition of the recombinant proteins for the two groups of human sera are shown in Fig. 2. Most of the DBL-α domain variants tested were recognized by 50 to 80% of the sera from semi-immune individuals; the only exception was G8d, which was recognized by only 38% of the sera. In comparison, the fusion proteins were only poorly recognized by sera from the nonimmune children (between 5 and 20% of the sera except for G15) (Fig. 2). For each of the fusion proteins except G15 the semi-immune group exhibited significantly higher antibody reactivity than the nonimmune group (P < 0.01) (Table 1); for fusion protein G15 no significant difference was observed.
FIG. 2.
Frequencies of recognition of recombinant proteins. The reactivities of the proteins were examined by using serum samples obtained from 100 individuals classified as being semi-immune to malaria (cross-hatched bars) and from 100 individuals belonging to the nonimmune group (grey bars). The percentages of serum positivity were calculated by using OD450 values obtained for each fusion protein above the cutoff values.
TABLE 1.
Comparison of the median OD450 values and semi-interquartile ranges of the OD450 values for nonimmune and semi-immune sera with the different fusion proteins obtained by ELISA
| Fusion protein | Sera | OD450a
|
Pb | |
|---|---|---|---|---|
| Median | Semi-interquartile range | |||
| G41a | Total | 0.53 | 0.62 | |
| Nonimmune | 0.08 | 0.15 | ||
| Semi-immune | 0.59 | 0.53 | <0.01 | |
| G17 | Total | 0.28 | 0.41 | |
| Nonimmune | 0.08 | 0.16 | ||
| Semi-immune | 0.33 | 0.43 | <0.01 | |
| G22 | Total | 0.27 | 0.45 | |
| Nonimmune | 0.11 | 0.14 | ||
| Semi-immune | 0.38 | 0.48 | <0.01 | |
| G15c | Total | 0.28 | 0.44 | |
| Nonimmune | 0.05 | 0.10 | ||
| Semi-immune | 0.33 | 0.49 | <0.01 | |
| G21d | Total | 0.28 | 0.60 | |
| Nonimmune | 0.05 | 0.06 | ||
| Semi-immune | 0.30 | 0.59 | <0.01 | |
| G31a | Total | 0.33 | 0.60 | |
| Nonimmune | 0.04 | 0.10 | ||
| Semi-immune | 0.38 | 0.58 | <0.01 | |
| G8d | Total | 0.13 | 0.23 | |
| Nonimmune | 0.01 | 0.02 | ||
| Semi-immune | 0.17 | 0.23 | <0.01 | |
| G15 | Total | 0.20 | 0.29 | |
| Nonimmune | 0.18 | 0.22 | ||
| Semi-immune | 0.24 | 0.34 | NS | |
| G23a | Total | 0.24 | 0.47 | |
| Nonimmune | 0.05 | 0.16 | ||
| Semi-immune | 0.35 | 0.42 | <0.01 | |
The OD450 values were calculated by using OD450 values obtained for each fusion protein above the cutoff values.
The P values for comparisons of the two patient groups are given; only positive sera were included. NS, not significant.
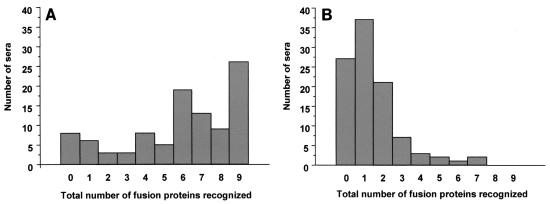
Further analysis of the human responses for the semi-immune group showed that while 26 of the 100 serum samples recognized all nine recombinant proteins, 8 of the 100 individuals examined did not respond to any of the fusion proteins (Fig. 3A). In contrast, none of the nonimmune sera recognized all or almost all of the fusion proteins; most of the group reacted with none (27 sera), one (37 sera), or two (21 sera) of the fusion proteins (Fig. 3B). Children tended to respond to smaller numbers of DBL-α domain variants than adults.
FIG. 3.
Differences in the patterns of recognition of fusion proteins by human sera. The total numbers of human sera from members of the semi-immune group (A) and the nonimmune group (B) capable of reacting with 0 to 9 fusion proteins at any one time were determined based on the calculated cutoff values.
We examined more closely each of the nine fusion proteins which showed the highest levels of reactivity in terms of OD450 values with individual serum samples. Six fusion proteins, G8d, G15, G15c, G21d, G22, and G41a, were recognized at high titers by individual sera. These same sera also recognized each of the other eight recombinant proteins. The serum that strongly reacted with fusion protein G17d also cross-reacted strongly with seven other fusion proteins. Finally, the serum that reacted most strongly with fusion proteins G23a and G31a also cross-reacted with three other proteins. Given the degree of DBL-α domain variation observed in the sequences, this pattern of recognition suggests that at least parts of the acquired antibodies may be directed towards the more conserved regions of the variant molecules.
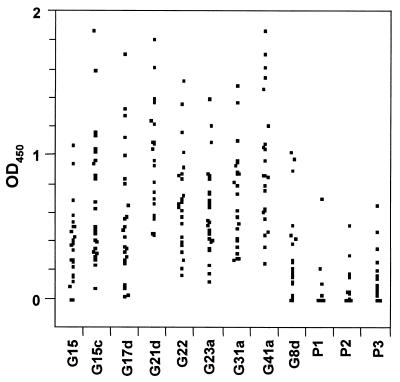
The next question addressed was whether the semi-immune responders recognized conserved regions in the expressed DBL-α domain proteins. Thus, three synthetic peptides corresponding to conserved amino acid sequences were tested for reactivity by performing an ELISA. For the semi-immune group, 25 individual serum samples which recognized all of the fusion proteins were chosen for these experiments (Fig. 4). Overall, the synthetic peptides were found to be less reactive than the entire fusion proteins. P3 was the most reactive peptide and was recognized by 14 sera, P2 was recognized by 8 sera, and P1 was recognized by only 5 sera. Interestingly, a serum that was positive with P1 was also positive with P2 and P3, and a serum that reacted with P2 also detected peptide P3. The most likely explanation for this observation involves the immunogenicity of P3 compared to the immunogenicities of the other two peptides. There was not a significant correlation between antirecombinant antibodies and antipeptide antibodies, and the finding that the highly reactive sera from adults were less reactive with the conserved peptides suggested that the DBL-α domain-specific antibodies were predominantly directed against variant regions of the protein rather than the conserved epitopes.
FIG. 4.
Scattergram showing ELISA seroreactivities of serum samples. Twenty-five highly reactive sera from semi-immune individuals were tested in an ELISA against all nine GST fusion proteins (indicated at the bottom), as well as against peptides P1, P2, and P3, which cover conserved amino acids from the DBL-α domain. The OD450 values were calculated by using OD450 values obtained for each fusion protein above the cutoff values.
Inhibition of immune sera by recombinant DBL-α domain proteins and conserved peptides.
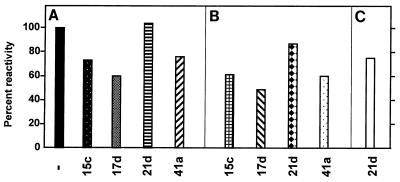
To determine whether sera that contained high levels of antibodies recognized variant regions of the recombinant proteins or conserved peptide motifs present in all fusion proteins, we performed competition ELISA. In every case but one preincubation of the sera with recombinant proteins resulted in a reduction in the OD450 similar to the reduction observed with the homologous protein (Fig. 5). Notably, none of the homologous proteins was capable of completely eliminating the reactivity, indicating that cross-reactive epitopes were present. One of the heterologous proteins (G21d) did not have any effect on the reactivity, except when it was tested against itself (Fig. 5C). Obviously, there are no commonly recognized epitopes that are shared by G21d and the other fusion proteins tested.
FIG. 5.
Competition ELISA with various fusion proteins. ELISA plates were coated with fusion proteins G15c (A), G17d (B), and G21d (C). A serum sample positive with all of the fusion proteins was added to the plates after preincubation with 10-μg portions of the fusion proteins, as indicated at the bottom. Levels of inhibition were expressed as percentages of the OD450 without inhibitor (−).
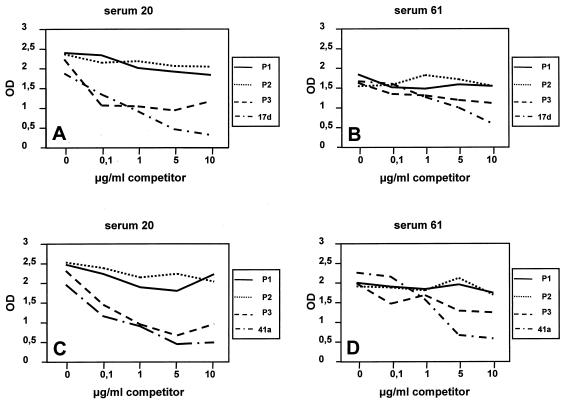
To determine whether the conserved peptides in solution could inhibit binding of immune sera to DBL-α domain proteins, the three conserved peptides were used as inhibitors in a competition ELISA. Preincubation of sera with either the P1 or P2 conserved peptide had no effect on the level of binding of any of the recombinant proteins tested, showing that the sera recognized epitopes in the recombinant proteins which were distinct from the epitopes in P1 or P2 (Fig. 6). Preincubation of the sera with the P3 conserved peptide clearly reduced the level of binding (Fig. 6). The competition ELISA results suggested that antibodies against variant-specific and conserved epitopes can be found in sera of immune persons.
FIG. 6.
Competition ELISA with three conserved peptides derived from the DBL-α domain. ELISA plates were coated either with fusion protein G17d (A and B) or with fusion protein G41a (C and D). Sera that were positive with all of the fusion proteins were added to the plates after they were incubated with 0, 0.1, 1.0, 5, and 10 μg of peptide. The competitors used are indicated on the right of each graph. OD, optical density at 450 nm.
Antigenicity of DBL-α domain fusion proteins.
In order to determine whether recognition of DBL-α domain variants was due to a single population of antibodies which reacted with epitopes common to both proteins or to two separate non-cross-reacting populations of antibodies, we compared the reactivities of individual serum samples with pairs of fusion proteins. In this analysis we used only sera from members of the semi-immune group, since the number of positively reacting sera from members of the nonimmune group was too small to analyze.
The levels of identity for pairs of fusion proteins and their calculated ρ values with the semi-immune group are shown in Table 2. When fusion proteins G23a and G31a, in which 99.5% of the amino acids are identical, were compared, the ρ value determined by the Spearman rank test was 0.88. For the G21d-G8d fusion protein pair, which exhibited 75.0% identity, the ρ value was only 0.59. In addition, the G41a and G8d proteins, with a level of identity of 63.5%, gave a calculated ρ value of 0.36. Thus, there was not a strong correlation between the levels of identity of the fusion proteins and the ρ values calculated from the reactivities with semi-immune sera (P = 0.107). From this analysis it appeared that the serum samples reacted predominantly with different epitopes on the proteins.
TABLE 2.
Comparison of pairs of fusion proteins for amino acid identity
| Fusion protein | % Identity with:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| G23a | G31a | G8d | G21d | G22 | G15 | G15c | G17d | G41a | |
| G23a | 0.00 | ||||||||
| G31a | 99.51 (0.88) | 0.00 | |||||||
| G8d | 53.89 (0.54) | 54.40 (0.52) | 0.00 | ||||||
| G21d | 54.79 (0.57) | 55.32 (0.73) | 75.00 (0.59) | 0.00 | |||||
| G22 | 49.75 (0.55) | 49.24 (0.55) | 64.10 (0.53) | 66.67 (0.62) | 0.00 | ||||
| G15 | 55.15 (0.61) | 54.64 (0.53) | 57.81 (0.62) | 60.64 (0.52) | 54.08 (0.57) | 0.00 | |||
| G15c | 62.03 (0.54) | 61.50 (0.70) | 54.35 (0.51) | 58.92 (0.77) | 54.01 (0.58) | 58.82 (0.55) | 0.00 | ||
| G17d | 56.15 (0.49) | 55.61 (0.48) | 54.89 (0.56) | 61.08 (0.49) | 56.68 (0.77) | 65.24 (0.50) | 55.26 (0.50) | 0.00 | |
| G41a | 47.72 (0.56) | 48.22 (0.59) | 63.54 (0.36) | 61.70 (0.49) | 62.76 (0.67) | 56.48 (0.44) | 52.97 (0.50) | 56.22 (0.63) | 0.00 |
The values in parentheses are ρ values as calculated by Spearman rank analysis for each pair of fusion proteins reacting with the group of semi-immune human sera.
DISCUSSION
Understanding the naturally occurring immune reponses to various parasite antigens, including the immunological significance of amino acid polymorphism, is an important aspect of evaluating PfEMP-1 as a potential component of a subunit malaria vaccine. Because of the availability of degenerate primers capable of amplifying DBL-α domain sequences, the DBL-α domain is becoming the target of immunoepidemiological studies to analyze diversity in parasite populations worldwide. Here we describe the use of bacterially synthesized PfEMP-1 domains for detection of antibodies in sera of malaria patients by an ELISA technique. DBL-α domain sequences were amplified from parasite DNA prepared from randomly selected P. falciparum-infected individuals from Lambaréné, Gabon. Importantly, DNA was prepared from ex vivo parasites that were directly frozen in the peripheral blood of the patients and had not gone through in vitro culture and selection. By comparing the antibody response to cloned DBL-α domain antigens with the degree of agglutination of infected erythrocytes, we were able to conclude that the antibodies that recognize the recombinant DBL-α domain antigens in the ELISA were of the same nature as agglutinating antibodies which recognize domains expressed on the surfaces of infected erythrocytes.
Two previously described techniques readily detect the presence of antibodies to surface-associated plasmodial antigens; these two techniques are agglutination of parasite-infected erythrocytes and flow cytometry (15, 17, 23). The current view is that the major immunogen detected on the surface is the PfEMP-1 molecule. Our results confirm and extend this view, even though none of the studies have ruled out the possibility that antibodies to other surface-exposed antigens, such as rifins, are also present in human sera (13).
A significant difference in the antibody responses to the DBL-α domain of the two groups of sera (semi-immune and nonimmune human sera) was observed. The semi-immune sera were highly reactive, while the nonimmune sera were much less reactive. Relatively low ELISA reactivities to all three synthetic peptides from conserved regions of the DBL-α domain were also observed when we used sera from 25 highly responsive semi-immune adults, indicating that antibodies to conserved regions can be acquired upon infection with P. falciparum. Moreover, we observed a hierarchy of recognition with our peptides (P3 > P2 > P1). This hierarchy coincided with HLA epitope predictions (when the epitope prediction program syfpeithi [http://syfpeithi.de/] was used), which demonstrated that an HLA class II epitope in P3 had a higher predictive value than an HLA class II epitope in P2 had. P3, therefore, seemed to be generally more immunogenic. P1 was too short to be analyzed with the same prediction program. The higher immunogenicity was also reflected by the results of the competition ELISA, which showed that P3 had an an inhibitory effect but P2 or P1 did not.
The role of antibodies to conserved regions in protective immunity is not yet clear. Individuals appear to acquire antibodies to conserved regions on PfEMP-1, as well as to variant-specific domains, as an individual living in an area of endemicity becomes more PfEMP-1 responsive, as reported by other workers (23). In view of the lack of correlation between the degree of identity between fusion proteins and antibody reactivity, we concluded that most of the antibodies are directed against the variant parts of the DBL-α domains. This conclusion is supported by the fact that in the competition experiments the reactivity of sera could not be eliminated completely by homologous or heterologous fusion proteins and was completely absent with one fusion protein. Similar observations were made when conserved peptides were used. Thus, our study based on the use of recombinant antigens provided additional evidence which supports the view that the semi-immune status of an individual is the result of an accumulation of variant-specific antibodies against PfEMP-1 molecules.
Although our data is not extensive, we provide the first evidence that points to a positive influence of anti-DBL-α domain antibodies in nonimmune malaria patients. Among the group of nonimmune individuals were 10 children in whom recrudescent parasites appeared 3 to 4 weeks after chemotherapy. It is interesting that in the sera of these children lower antibody titers to the fusion proteins were generally observed (the mean OD450 for cured individuals was 0.160 [semi-interquartile OD450 range, 0.039], compared to a mean OD450 for treatment failures of 0.001 [semi-interquartile OD450 range, 0.006] [P = 0.02]). It is thought that a patient with a higher level of antibody against DBL-α domains is more likely to be cured by chemotherapy than an individual who has a lower level of antibody and that the presence of PfEMP-1 antibodies could aid in parasite clearance. A more detailed analysis of the ability of antibodies to synergize with chemotherapy may shed light on this possibility.
It is not known whether the DBL-α domains investigated in this study were expressed at any one time or how frequently they were expressed, but the finding that some sera have very strong responses to some of the fusion proteins indeed suggests that the individuals were exposed frequently and possibly at an early stage to these domains. An alternative explanation for the strong responses is that they are the result of cross-reacting antibodies. In our study area, every child experiences between two and four malaria attacks per year, and based on the known large repertoire of PfEMP-1 variants, it is likely that each infection is acompanied by a different PfEMP-1. It follows that a high number of infections (between 30 and 60) is necessary for an individual to acquire the relevant repertoire of anti-PfEMP-1 antibodies. This calculation is consistent with the time that it usually takes (10 to 15 years) to gain semi-immunity to malarial infections in an area where malaria is hyperendemic and transmission of malaria is stable. If the geographic distribution and differences reported for malaria parasite isolates are taken into consideration, it is reasonable to expect that the PfEMP-1 variation from area to area is very extensive. In terms of developing vaccination strategies and understanding how protective immunity is acquired in children, it is therefore important that sera from different areas of endemicity be screened for the presence of cross-reacting anti-PfEMP-1 antibodies. The use of specific recombinant PfEMP-1 domains to perform this work has clear advantages over agglutination assays or fluorescence-activated cell sorter analysis, since recombinant proteins are easier to handle and can be prepared in unlimited and reproducible amounts. Therefore, such proteins are more amenable substrates for immunoepidemiological analyses.
ACKNOWLEDGMENTS
We thank Adrian Luty and Francine Ntoumi for critical reading of the manuscript and Silvelia Grummes and Andrea Weierich for technical assistance.
This work received financial support from the fortüne-Progamme of the Medical Faculty of the University of Tübingen, from the European Commission (QLK2-CT-1999-01293 and ERBIC-18-CT-980359), and from a fellowship to R.M.O. from the Deutsche Akademische Austauschdienst.
REFERENCES
- 1.Aikawa M, Rabbege J R, Udeinya I, Miller L H. Electron microscopy of knobs in Plasmodium falciparum-infected erythrocytes. J Parasitol. 1983;69:435–437. [PubMed] [Google Scholar]
- 2.Bull P C, Lowe B S, Kortok M, Molyneux C S, Newbold C I, Marsh K. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat Med. 1998;4:358–360. doi: 10.1038/nm0398-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlson J, Helmby H, Hill A V, Brewster D, Greenwood B M, Wahlgren M. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet. 1990;336:1457–1460. doi: 10.1016/0140-6736(90)93174-n. [DOI] [PubMed] [Google Scholar]
- 4.Chen Q, Barragan A, Fernandez V, Sundstrom A, Schlichtherle M, Sahlen A, Carlson J, Datta S, Wahlgren M. Identification of Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) as the rosetting ligand of the malaria parasite P. falciparum. J Exp Med. 1998;187:15–23. doi: 10.1084/jem.187.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Q, Fernandez V, Sundstrom A, Schlichtherle M, Datta S, Hagblom P, Wahlgren M. Developmental selection of var gene expression in Plasmodium falciparum. Nature. 1998;394:392–395. doi: 10.1038/28660. [DOI] [PubMed] [Google Scholar]
- 6.Deitsch K W, Wellems T E. Membrane modifications in erythrocytes parasitized by Plasmodium falciparum. Mol Biochem Parasitol. 1996;76:1–10. doi: 10.1016/0166-6851(95)02575-8. [DOI] [PubMed] [Google Scholar]
- 7.Freitas-Junior L H, Bottius E, Pirrit L A, Deitsch K W, Scheidig C, Guinet F, Nehrbass U, Wellems T E, Scherf A. Frequent ectopic recombination of virulence factor genes in telomeric chromosome clusters of P. falciparum. Nature. 2000;407:1018–1022. doi: 10.1038/35039531. [DOI] [PubMed] [Google Scholar]
- 8.Giha H A, Staalsoe T, Dodoo D, Elhassan I M, Roper C, Satti G M, Arnot D E, Theander T G, Hviid L. Nine-year longitudinal study of antibodies to variant antigens on the surface of Plasmodium falciparum-infected erythrocytes. Infect Immun. 1999;67:4092–4098. doi: 10.1128/iai.67.8.4092-4098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta S, Snow R W, Donnelly C A, Marsh K, Newbold C. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat Med. 1999;5:340–343. doi: 10.1038/6560. [DOI] [PubMed] [Google Scholar]
- 10.Handunnetti S M, David P H, Perera K L, Mendis K N. Uninfected erythrocytes form “rosettes” around Plasmodium falciparum infected erythrocytes. Am J Trop Med Hyg. 1989;40:115–118. doi: 10.4269/ajtmh.1989.40.115. [DOI] [PubMed] [Google Scholar]
- 11.Howard R J, Barnwell J W, Rock E P, Neequaye J, Ofori Adjel D, Maloy W L, Lyon J A, Saul A. Two approximately 300 kilodalton Plasmodium falciparum proteins at the surface membrane of infected erythrocytes. Mol Biochem Parasitol. 1988;27:207–223. doi: 10.1016/0166-6851(88)90040-0. [DOI] [PubMed] [Google Scholar]
- 12.Kun J F J, Schmidt-Ott R, Lehman L G, Lell B, Luckner D, Greve B, Matousek P, Kremsner P G. Merozoite surface antigen 1 and 2 genotyping and rosetting of Plasmodium falciparum in severe versus mild malaria in Lambaréné, Gabon. Trans R Soc Trop Med Hyg. 1998;92:110–114. doi: 10.1016/s0035-9203(98)90979-8. [DOI] [PubMed] [Google Scholar]
- 13.Kyes S A, Rowe J A, Kriek N, Newbold C I. Rifins: a second family of clonally variant proteins expressed on the surface of red cells infected with Plasmodium falciparum. Proc Natl Acad Sci USA. 1999;96:9333–9338. doi: 10.1073/pnas.96.16.9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newbold C I, Craig A G, Kyes S, Berendt A R, Snow R W, Peshu N, Marsh K. PfEMP1, polymorphism and pathogenesis. Ann Trop Med Parasitol. 1997;91:551–557. doi: 10.1080/00034989760923. [DOI] [PubMed] [Google Scholar]
- 15.Newbold C I, Pinches R, Roberts D J, Marsh K. Plasmodium falciparum: the human agglutinating antibody response to the infected red cell surface is predominantly variant specific. Exp Parasitol. 1992;75:281–292. doi: 10.1016/0014-4894(92)90213-t. [DOI] [PubMed] [Google Scholar]
- 16.Piper K P, Hayward R E, Cox M J, Day K P. Malaria transmission and naturally acquired immunity to PfEMP-1. Infect Immun. 1999;67:6369–6374. doi: 10.1128/iai.67.12.6369-6374.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piper K P, Roberts D J, Day K P. Plasmodium falciparum: analysis of the antibody specificity to the surface of the trophozoite-infected erythrocyte. Exp Parasitol. 1999;91:161–169. doi: 10.1006/expr.1998.4368. [DOI] [PubMed] [Google Scholar]
- 18.Rowe A, Obeiro J, Newbold C I, Marsh K. Plasmodium falciparum rosetting is associated with malaria severity in Kenya. Infect Immun. 1995;63:2323–2326. doi: 10.1128/iai.63.6.2323-2326.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saiki R K, Gelfand D H, Stoffel S, Scharf S J, Higuchi R, Horn G T, Mullis K B, Erlich H A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988;239:487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- 20.Scherf A, Hernandez-Rivas R, Buffet P, Bottius E, Benatar C, Pouvelle B, Gysin J, Lanzer M. Antigenic variation in malaria: in situ switching, relaxed and mutually exclusive transcription of var genes during intra-erythrocytic development in Plasmodium falciparum. EMBO J. 1998;17:5418–5426. doi: 10.1093/emboj/17.18.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith D B, Johnson K S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 22.Smith J D, Craig A G, Kriek N, Hudson T D, Kyes S, Fagen T, Pinches R, Baruch D I, Newbold C I, Miller L H. Identification of a Plasmodium falciparum intercellular adhesion molecule-1 binding domain: a parasite adhesion trait implicated in cerebral malaria. Proc Natl Acad Sci USA. 2000;97:1766–1771. doi: 10.1073/pnas.040545897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staalso T, Khalil E A, Elhassan I M, Zijlstra E E, Elhassan A M, Giha H A, Theander T G, Jakobsen P H. Antibody reactivity to conserved linear epitopes of Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) Immunol Lett. 1998;60:121–126. doi: 10.1016/s0165-2478(97)00143-0. [DOI] [PubMed] [Google Scholar]
- 24.Sylla E H K, Kun J F J, Kremsner P G. Mosquito distribution and entomological inoculation rates in three malaria-endemic areas in Gabon. Trans R Soc Trop Med Hyg. 2000;94:652–656. doi: 10.1016/s0035-9203(00)90219-0. [DOI] [PubMed] [Google Scholar]
- 25.Trager W, Rudzinska M A, Bradbury P C. The fine structure of Plasmodium falciparum and its host erythrocytes in natural malarial infections in man. Bull W H O. 1966;35:883–885. [PMC free article] [PubMed] [Google Scholar]
- 26.Treutiger C J, Hedlund I, Helmby H, Carlson J, Jepson A, Twumasi P, Kwiatkowski D, Greenwood B M, Wahlgren M. Rosette formation in Plasmodium falciparum isolates and anti-rosette activity of sera from Gambians with cerebral or uncomplicated malaria. Am J Trop Med Hyg. 1992;46:503–510. doi: 10.4269/ajtmh.1992.46.503. [DOI] [PubMed] [Google Scholar]
- 27.Udomsangpetch R, Wahlin B, Carlson J, Berzins K, Torii M, Aikawa M, Perlmann P, Wahlgren M. Plasmodium falciparum-infected erythrocytes form spontaneous erythrocyte rosettes. J Exp Med. 1989;169:1835–1840. doi: 10.1084/jem.169.5.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valle D, Kun J, Linss J, Garcia E d S, Goldenberg S. cDNA cloning and expression of Rhodnius prolixus vitellogenin. Insect Biochem Mol Biol. 1993;23:457–465. doi: 10.1016/0965-1748(93)90053-u. [DOI] [PubMed] [Google Scholar]
- 29.Wildling E, Winkler S, Kremsner P G, Brandts C, Jenne L, Wernsdorfer W H. Malaria epidemiology in the province of Moyen Ogooué, Gabon. Trop Med Parasitol. 1995;46:77–82. [PubMed] [Google Scholar]