Abstract
Transthyretin cardiac amyloidosis (ATTR-CA) is an overlooked cause of heart failure, with substantial morbidity and mortality. The emergence of several novel therapies has fueled the interest in early and accurate diagnosis of ATTR-CA so that potentially life-saving pharmacologic therapy can be administered in a timely manner. The most promising imaging modality and biomarker is SPECT imaging with technetium 99m (99mTc)–radiolabeled bone-seeking tracers, which have high specificity in the diagnosis of ATTR-CA, potentially obviating biopsy. In this article, the authors provide a focused review on the use of 99mTc pyrophosphate (PYP), 3,3-diphosphono-1,2-propanodicarboxylic acid (DPD), and hydroxymethylene diphosphonate (HMDP) for diagnosis of ATTR-CA, present a systematic approach to interpretation of the scans, and highlight several common pitfalls to illustrate important diagnostic principles for accurate interpretation of these images. The authors indicate when to use endomyocardial biopsy for the diagnosis of cardiac amyloidosis and conclude with a section on quantitation of 99mTc-PYP/DPD/HMDP imaging.
© RSNA, 2022
Summary
This article provides a focused review of transthyretin cardiac amyloidosis and a stepwise approach to accurately and definitively diagnosing it using technetium 99m pyrophosphate, 3,3-diphosphono-1,2-propanodicarboxylic acid, or hydroxymethylene diphosphonate cardiac scintigraphy.
Essentials
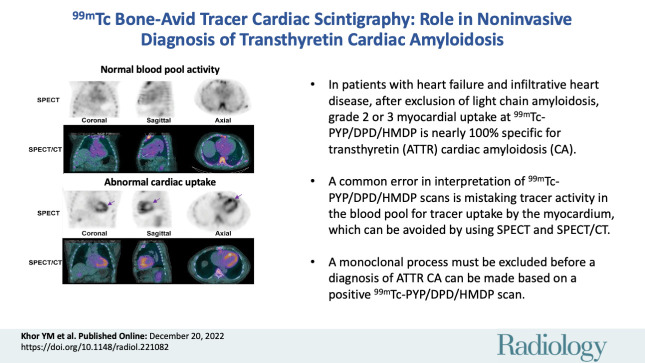
■ Over the last 5 years, nuclear cardiology has witnessed tremendous growth in the use of technetium 99m (99mTc) bone-avid tracer cardiac scintigraphy for clinical and research applications.
■ A seminal publication confirmed the high diagnostic accuracy of 99mTc pyrophosphate (PYP), 3,3-diphosphono-1,2-propanodicarboxylic acid (DPD), and hydroxymethylene diphosphonate (HMDP) imaging: In patients with heart failure and typical imaging features of infiltrative heart disease, after exclusion of light chain (AL) amyloidosis, grade 2 or grade 3 myocardial uptake at 99mTc-PYP/DPD/HMDP imaging is nearly 100% specific for transthyretin cardiac amyloidosis (ATTR-CA).
■ A common error in the interpretation of 99mTc-PYP/DPD/HMDP scans is mistaking tracer activity in the blood pool for tracer uptake by the myocardium, which can be avoided by using SPECT and SPECT/CT.
■ A missed or delayed diagnosis of AL amyloidosis is likely fatal; hence, a monoclonal process must be excluded with serum/urine immunofixation electrophoresis and serum free AL assay before a diagnosis of ATTR-CA can be made based on a positive 99mTc-PYP/DPD/HMDP scan.
■ The planar heart-to–contralateral lung uptake (H/CL) ratio is prone to errors and should be used solely to diagnose ATTR-CA at bone-avid tracer cardiac scintigraphy. In our clinical practice, we omit the H/CL ratio and report the visual impression based on SPECT/CT images at 2–3 hours after tracer injection.
Introduction
Over the last 5 years, nuclear cardiology has witnessed tremendous growth in the use of technetium 99m (99mTc) bone-avid tracer cardiac scintigraphy for clinical and research applications. Its substantial clinical value for evaluation of patients with heart failure with preserved ejection fraction (HFpEF) has motivated many hospitals to start cardiac amyloidosis imaging programs. However, experience with performance, interpretation, and reporting of 99mTc bone-avid tracer cardiac scintigraphy remains limited. High-quality scan acquisition, accurate interpretation, and definitive reporting are critical for realization of the full potential of this modality. This review will provide an overview of cardiac amyloidosis, summarize the emerging literature on bone-avid tracer cardiac scintigraphy, and illustrate a step-by-step approach to accurate interpretation and reporting of 99mTc bone-avid tracer cardiac scintigraphy findings.
Cardiac Amyloidosis
Amyloidoses are protein misfolding disorders characterized by extracellular deposition of insoluble amyloid fibrils in various organs, including the heart (1). Amyloid deposits in the myocardium expand the extracellular space, increase myocardial mass, impair diastolic and systolic function, and result in restrictive heart failure (2). The most common forms of cardiac amyloidosis result from misfolded immunoglobulin light chain (AL) or transthyretin (ATTR) proteins and are thus referred to as AL cardiac amyloidosis and ATTR cardiac amyloidosis (ATTR-CA), respectively (1).
AL amyloidosis is caused by a plasma cell dyscrasia. Cardiac involvement, if untreated, is highly fatal, with a median survival of less than 12 months (3). With successful anti–plasma cell therapies, including daratumumab (a monoclonal CD38-targeting antibody), superior survival and organ response have been reported (4). Therefore, early and accurate diagnosis is crucial to improve outcomes in AL amyloidosis.
There are two forms of ATTR amyloidosis. One is a hereditary form caused by an autosomal dominant variant in the ATTR gene (hereditary ATTR [ATTRv] amyloidosis; v for variant ATTR). Patients with ATTRv amyloidosis typically present after 30 years of age (age of symptom onset is determined by the type of variant), with predominantly neuropathic, cardiomyopathic, or mixed manifestations (5). The other, more common form of ATTR amyloidosis, called wildtype ATTR cardiac amyloidosis (ATTRwt-CA), is related to aging and other yet unknown pathophysiologic insults (5). Patients with ATTRwt-CA are predominantly men over age 70 years and often present with heart failure. After the onset of heart failure, median survival of patients with ATTRwt-CA is 4–5 years (6). Until recently, a biopsy was required to diagnose ATTR-CA; because of the low yield of fat pad biopsy (15% for wildtype ATTR and 45% for ATTRv) (7), endomyocardial biopsy was often performed. Advances in imaging have transformed the evaluation of patients with cardiac amyloidosis, and endomyocardial biopsy is now reserved for challenging cases.
Imaging Evaluation of HFpEF in Older Adults
Suspicion of cardiac amyloidosis in a patient with heart failure is based on typical echocardiographic and/or cardiac MRI features (8): increased ventricular wall thickness, increased myocardial mass, abnormal global longitudinal strain with apical sparing, characteristic alterations in gadolinium kinetics, diffuse late gadolinium enhancement, and expansion of the extracellular volume (2). However, cardiac MRI structural and functional imaging features are not specific for amyloidosis and cannot be used to identify amyloidosis at early stages or distinguish the AL and ATTR forms of cardiac amyloidosis (2). Although cardiac uptake of bone-avid trascers has been known since the early 1980s to represent cardiac amyloidosis (9), multiple studies reported variable diagnostic sensitivity, probably because patients with both AL and ATTR forms of cardiac amyloidosis were included in these studies. More than a decade ago, researchers recognized that these tracers provide a highly sensitive signal for ATTR-CA but not for AL cardiac amyloidosis (10). With this new knowledge, subsequent studies focused on diagnosing ATTR-CA. Multiple studies using bone-avid tracer cardiac scintigraphy have confirmed its very high specificity (nearly 100%), allowing noninvasive diagnosis without endomyocardial biopsy (11,12).
Noninvasive Diagnosis of ATTR-CA without Biopsy
Currently, the most established molecular imaging modality for diagnosis of ATTR-CA is scintigraphy with 99mTc-radiolabeled bone-seeking tracers that are bisphosphonate derivatives, namely 99mTc pyrophosphate (PYP), 99mTc 3,3-diphosphono-1,2-propanodicarboxylic acid (DPD), and 99mTc hydroxymethylene diphosphonate (HMDP). Multiple single-center studies have shown high diagnostic accuracy of 99mTc-PYP/DPD/HMDP for ATTR-CA (11,13). A seminal publication by Gillmore et al (12), which included 1217 patients with suspected amyloidosis from five countries and seven major amyloidosis centers, confirmed the high diagnostic accuracy of 99mTc-PYP/DPD/HMDP imaging: In patients with heart failure and typical imaging features of infiltrative pathologic abnormalities, after exclusion of AL amyloidosis, grade 2 or grade 3 myocardial uptake (see following section) on 99mTc-PYP/DPD/HMDP images was nearly 100% specific for ATTR-CA (12).
Mechanism of Myocardial Uptake of Bone-Avid Tracers in Amyloidosis
The mechanism of myocardial uptake of 99mTc-radiolabeled bone-seeking tracers in ATTR-CA is not fully understood. A probable mechanism is that these tracers bind to the calcium content within the myocardium affected by amyloidosis, akin to their affinity to the calcium in the bones at sites of active bone formation. In the past, 99mTc-PYP was used as an imaging tool for diagnosis of myocardial infarct because it binds to the calcium deposits in infarcted myocardium, and the uptake correlates with calcium content in the injured and necrotic myocytes (14). Stats and Stone (15) reported greater microcalcification densities in endomyocardial biopsies from patients with ATTR-CA than those with AL cardiac amyloidosis, which might account for the observed preferential binding of these bone-seeking tracers to ATTR-CA compared with AL cardiac amyloidosis. However, fluorine 18 sodium fluoride, a tracer targeting microcalcification, had not shown consistent or significant myocardial uptake in AL amyloidosis or ATTR-CA (16–18). Interestingly, another commonly used 99mTc bisphosphonate derivative for bone scintigraphy, 99mTc methyl diphosphate, was not avid for cardiac amyloidosis (19–21). Although case reports had described varying degree of myocardial uptake in ATTR-CA in a small number of patients (22,23), current recommendations do not advocate the use of 99mTc methyl diphosphate for cardiac amyloidosis due to its very low sensitivity.
99mTc-PYP/DPD/HMDP Scintigraphy for Risk Assessment in ATTR-CA
Several studies have reported on the utility of 99mTc-PYP/DPD/HMDP scintigraphy for risk stratification in ATTR-CA. Although abnormal myocardial uptake of 99mTc-PYP, 99mTc-DPD, or 99mTc-HMDP, compared with no uptake, indicated worse prognosis (24,25), there was no difference in prognosis for visually assessed mildly, moderately, or severely abnormal scans (24). On planar images, semiquantitative metrics of heart-to–contralateral lung uptake ratio (H/CL ratio) greater than 1.6 (see section “How to Interpret 99mTc-PYP/DPD/HMDP Images: A Systematic Approach”) (26) and heart-to–whole body ratio greater than 7.5 (25) were associated with worse major adverse event-free survival. Heart-to–whole body ratio was obtained by dividing the counts in the heart by the counts in whole-body image at 3 hours after injection of 99mTc-DPD (25).
99mTc-PYP/DPD/HMDP Scintigraphy to Unravel the Prevalence of ATTR-CA in Select at-Risk Cohorts
Autopsy studies revealed that ATTR amyloid deposits are prevalent in older adults, with more than 20% of persons showing evidence of myocardial ATTR amyloid deposition after age 80 years. However, a clinical diagnosis is made in far fewer patients (27). Clinical diagnosis using bone-avid tracer cardiac scintigraphy to screen at-risk populations (withoutendomyocardial biopsy) has provided new knowledge on the clinical epidemiology of ATTR-CA. In older adults (age >60 years) hospitalized with HFpEF and increased left ventricular wall thickness (>12 mm), ATTR-CA was found in 13%–21% of patients (28–30). ATTR-CA was also identified among patients with severe aortic valve stenosis: 6%–9% of those undergoing surgical aortic valve replacement (31,32), as many as 9%–13% of patients undergoing transcatheter aortic valve replacement (33–37), and nearly 30% of patients with low-flow low-gradient aortic stenosis (35). Finally, in a large community-based screening study, 6.3% of patients over 60 years of age with HFpEF and increased wall thickness had ATTR-CA (38). 99mTc bone-avid tracer scintigraphy has revealed that ATTR-CA is likely underdiagnosed and affects about 10% of older adults with HFpEF and increased left ventricular wall thickness.
Novel Targeted Therapies for ATTR-CA
Transthyretin protein normally exists in a homotetrameric form. Aging or ATTR gene variants can destabilize the transthyretin tetramer, causing a dissociation into aggregation-prone monomers that form ATTR amyloid fibrils (5). Until 2018, ATTR amyloidosis was untreatable, but three highly effective therapies are now approved by the U.S. Food and Drug Administration. Inotersen (an antisense oligonucleotide inhibitor) (39) and patisiran (a double-stranded small interfering RNA gene-silencing therapy) (40) halt production of variant ATTR protein and are approved for ATTRv neuropathy. Tafamidis (41), an ATTR-stabilizing agent that prevents the breakdown of ATTR, is approved for ATTR cardiomyopathy (wildtype ATTR or ATTRv). Tafamidis was found to be less effective in patients with advanced ATTR-CA (41). Next-generation gene silencers, and even a CRISPR/Cas9 (42) in vivo gene-editing therapy for treatment of ATTRv amyloidosis, are currently under development. Most of the current ATTR-CA therapies focus on the precursor protein (43), and no approved therapies are directed against the amyloid fibril. One year after ATTR stabilization or silencing therapy in ATTRv amyloidosis, minimal changes in cardiac structure and function were described at echocardiography (44) or cardiac MRI (45), but a 20% reduction in cardiac uptake of bone-avid tracers was described (45). Whether improvement in cardiac uptake of bone-avid tracers represents improvement in myocardial amyloidosis is not known. This is because current treatments target the precursor protein and improvements in cardiac structure and function are small, as they represent natural regression of amyloid. As the mechanism of tracer binding to amyloid is not known, the significance of decrease in myocardial radiotracer uptake after treatment with ATTR silencing therapy is not known. Newer therapies directed at the amyloid fibril are currently under investigation (43). Molecular amyloid imaging is likely to play an important role in identifying candidates for antifibril-based therapies as well as in the assessment of response to therapy.
In summary, we now have the capability for noninvasive, definitive diagnosis of ATTR-CA using widely available 99mTc bone-avid tracer cardiac scintigraphy. In combination with new and effective disease-modifying therapies, the use of 99mTc-PYP/DPD/HMDP imaging has dramatically increased worldwide. In the following sections, we focus on interpretation of 99mTc-PYP/DPD/HMDP imaging, highlight important diagnostic principles, and discuss common pitfalls that confound image interpretation. The images in this article are primarily 99mTc-PYP scans, but the principles of evaluation and diagnostic performance are similar for all three tracers (99mTc-PYP, 99mTc-DPD, and 99mTc-HMDP) (46).
Clinical Vignette
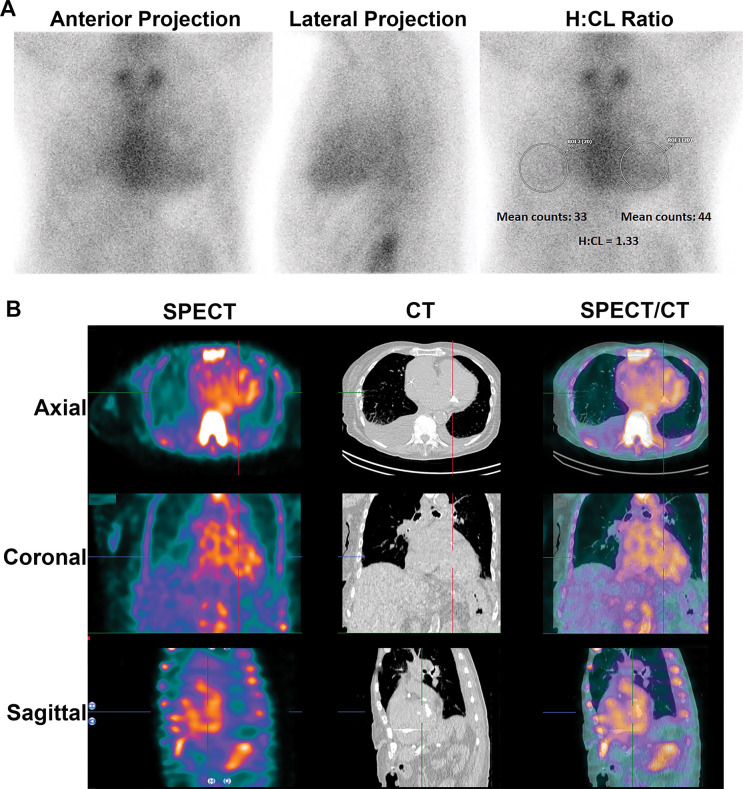
A 77-year-old man with HFpEF was referred to our hospital for management of newly diagnosed ATTR-CA. His echocardiogram showed normal left ventricular ejection fraction and increased left ventricular wall thickness with restrictive filling characteristics. Global longitudinal strain was reduced at −14.8%. His troponin-T level was 28 ng/L (reference range: 0–14 ng/L), and his N-terminal pro–B-type natriuretic peptide level was 602 pg/mL (reference level: less than 450 pg/mL). He underwent 99mTc-PYP scintigraphy at an outside hospital, and the scan was interpreted as showing grade 3 myocardial uptake (Fig 1A) that was suggestive of ATTR-CA. His serum free AL levels and serum and urine immunofixation electrophoresis findings were normal. He was referred to our hospital for further management with a view to initiate pharmacologic treatment with an ATTR stabilizer.
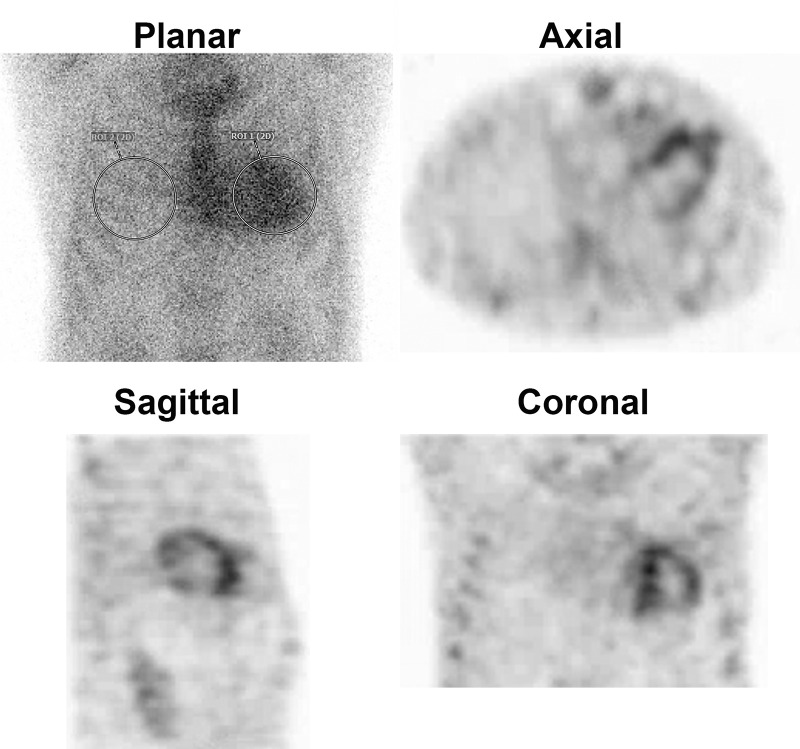
Figure 1:
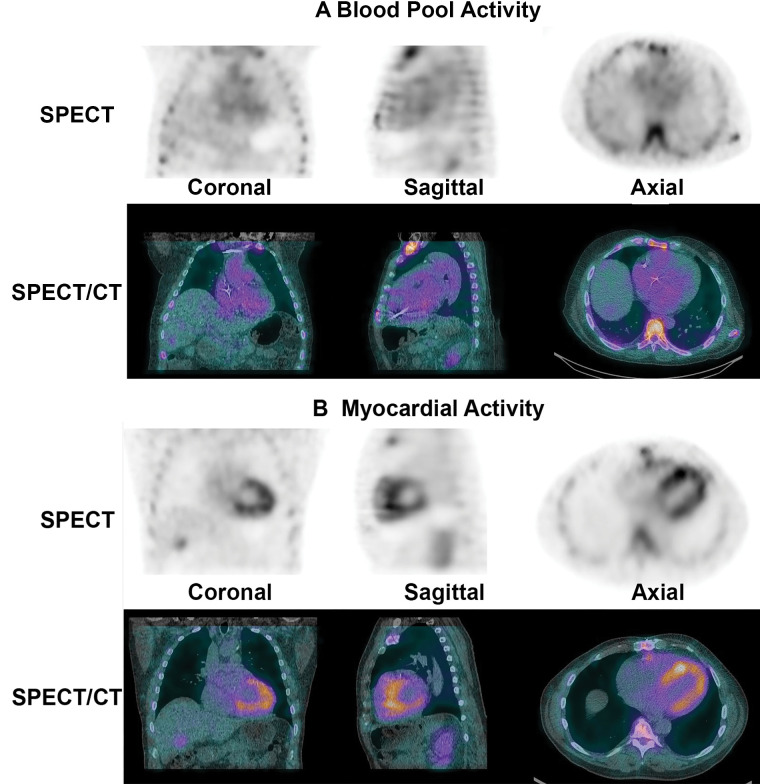
Planar and SPECT/CT technetium 99m (99mTc) pyrophosphate (PYP) images obtained for suspected transthyretin cardiac amyloidosis (ATTR-CA). (A) A 77-year-old White man with new onset of symptoms of heart failure underwent 99mTc-PYP planar scanning. The scan was reported as showing grade 3 myocardial uptake suggestive of ATTR-CA, and the patient was referred to our institution for initiation of tafamidis therapy. A careful review of the 99mTc-PYP planar images, which were reported to be acquired 1 hour after injection of radiotracer, suggested that tracer activity was concentrated in the blood pool and not in the myocardium because the typical appearance of a central clearing was not evident. Absence of rib uptake of tracer suggested that imaging was performed too early, making the images uninterpretable (see also Fig 2). H/CL = heart-to–contralateral lung uptake ratio, ROI = region of interest, 2D = two-dimensional. (B) SPECT/CT imaging at 3 hours is the preferred protocol at our institution, and it clearly demonstrates blood pool activity, with no myocardial 99mTc-PYP uptake in the rib. A definitive diagnosis of ATTR-CA based on interpretation of 99mTc-PYP planar images alone is not possible even for experienced readers.
A careful review of the 99mTc-PYP planar images, which were acquired 1 hour after radiotracer injection, suggested tracer activity was concentrated in the blood pool and not in the myocardium. To confirm, we repeated the 99mTc-PYP scanning using SPECT/CT at 3 hours after tracer injection (Fig 1B). The images confirmed blood pool activity with no myocardial 99mTc-PYP uptake. Diagnostically, the onset of his heart failure symptoms coincided with the new diagnosis of atrial fibrillation, suggesting the primary cause of his symptoms was most likely atrial fibrillation superimposed on a stiff ventricle from a combination of diabetes, prior coronary artery disease, and hypertension.
How to Acquire 99mTc-PYP/DPD/HMDP Images
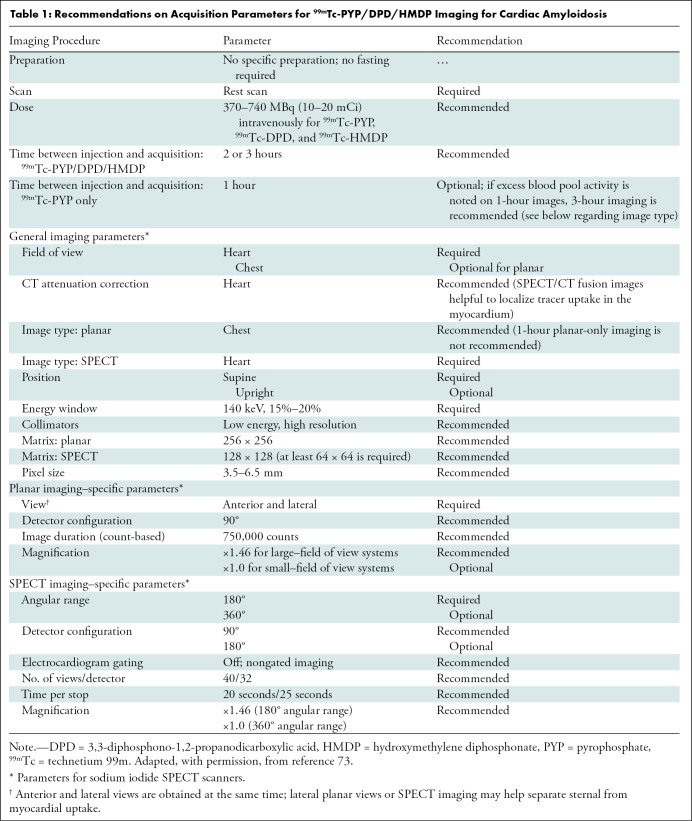
Guidelines on image acquisition and scan protocols for 99mTc-PYP/DPD/HMDP studies based on expert consensus are described in Table 1. We highlight that the recommended time of imaging is 2–3 hours after injection of 99mTc-PYP/DPD/HMDP; imaging at 1 hour is optional for laboratories with vast experience with both 1-hour and 3-hour 99mTc-PYP imaging. SPECT, or preferably SPECT/CT, is required, as it is the only definitive way to visualize 99mTc-PYP/DPD/HMDP uptake in the myocardium and differentiate it from blood pool activity (47).
Table 1:
Recommendations on Acquisition Parameters for 99mTc-PYP/DPD/HMDP Imaging for Cardiac Amyloidosis
How to Interpret 99mTc-PYP/DPD/HMDP Images: A Systematic Approach
Step 1: Perform Quality Control
As with any other radiopharmaceutical agent, strict compliance with quality control regulations for radiochemical purity is necessary before intravenous administration of 99mTc-PYP/DPD/HDP. Dissociation of 99mTc-PYP can occur after prolonged standing (more than 6 hours) or a change in pH, where free dissociated 99mTc pertechnetate is seen as unexpected activity in the thyroid and stomach (48). Interpretation of the planar images becomes challenging due to proximity of the stomach to the heart, but this may be overcome on SPECT images. Excessive free 99mTc pertechnetate may result in false-negative scans. Laboratories that are new to radiolabeling 99mTc-PYP should be aware that the stannous PYP kit is not only used for bone and myocardial imaging but can also be used for gated blood pool imaging if reconstituted and administered differently. Both preparation methods are detailed in the product package insert, and confusing one for the other will result in a radiolabeled product that remains in the blood pool with no localization to the myocardium or bone.
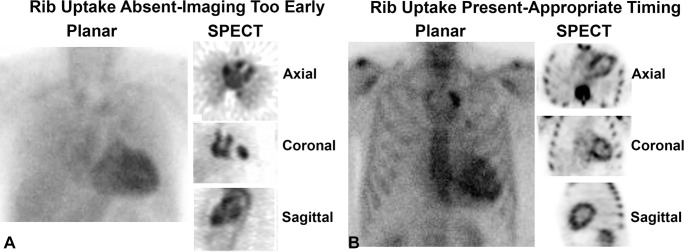
Step 2: Confirm the Presence of Rib Uptake as a Check for Appropriate Timing of Scan Acquisition
An important concern is mistaking tracer activity in the blood pool for tracer uptake by the myocardium on planar images. A common reason for this is performing the scan too early after injection of radiotracer. The absence of tracer uptake by the ribs, especially with 99mTc-PYP, is an indicator that images were obtained too early for an interpretable scan (Fig 2A). When scans from a later time point are obtained, they clearly demonstrate symmetric tracer uptake in the bones (Fig 2B). Any tracer uptake in the region of the heart on planar images should raise suspicion for myocardial uptake and must be confirmed with use of SPECT. Substantial soft-tissue uptake of 99mTc-DPD, predominantly in skeletal muscles, has been reported in patients with ATTR-CA; this can sometimes attenuate visualization of radiotracer uptake by the bone (49).
Figure 2:
Rib uptake as an indication of appropriate timing of scan acquisition in technetium 99m pyrophosphate cardiac scintigraphy. (A) In the early phase shortly after tracer injection, blood pool activity within the heart is expected, while the ribs and other skeletal structures are not clearly visible. The absence of tracer uptake by the ribs is a good indicator that it is too early to interpret the scan, as the tracer is still circulating in the blood pool and has not entered the myocardium or bound to the bones. (B) In contrast, late images acquired 3 hours after radiotracer injection clearly show tracer uptake in the ribs. Any tracer uptake in the heart on 3-hour images should raise suspicion for myocardial uptake.
Step 3: Distinguish Myocardial 99mTc-PYP Uptake from Blood Pool Activity
A common misinterpretation of 99mTc-PYP cardiac images is mistaking tracer activity in the blood pool for tracer uptake by the myocardium, as illustrated in the case vignette. SPECT, and SPECT/CT if available, can be used to confirm 99mTc-PYP uptake in the myocardium and differentiate it from blood pool radioactivity. It is important for imaging physicians to be familiar with the appearance of blood pool activity on SPECT images (Fig 3).
Figure 3:
SPECT and SPECT/CT to distinguish myocardial technetium 99m pyrophosphate uptake from blood pool activity. (A) On SPECT and SPECT/CT images, tracer activity in the blood pool has an amorphous appearance that is not clearly separable from the mediastinum. (B) True-positive tracer uptake by the myocardium is characterized by a U shape or horseshoe shape on the axial and coronal planes and a donut shape on the sagittal plane.
Step 4: Interpret Myocardial Uptake: Visual Assessment and Grading of 99mTc-PYP Images
99mTc-PYP/DPD/HMDP chest images are optimally reviewed in transaxial, sagittal, and coronal projections. Reorientation of the images into cardiac projections can be challenging in negative scans, and in positive scans, myocardial activity may be scaled higher when using the cardiac projections alone. When 99mTc-PYP accumulates in the myocardium, the scan appearance should be similar to a normal myocardial perfusion scan, with high signal intensity in the myocardium and minimal, if any, activity in the blood pool. An inverse gray scale or any linear color scale can be used for SPECT interpretation. For SPECT/CT fusion images, CT is displayed in gray scale and SPECT in color scale.
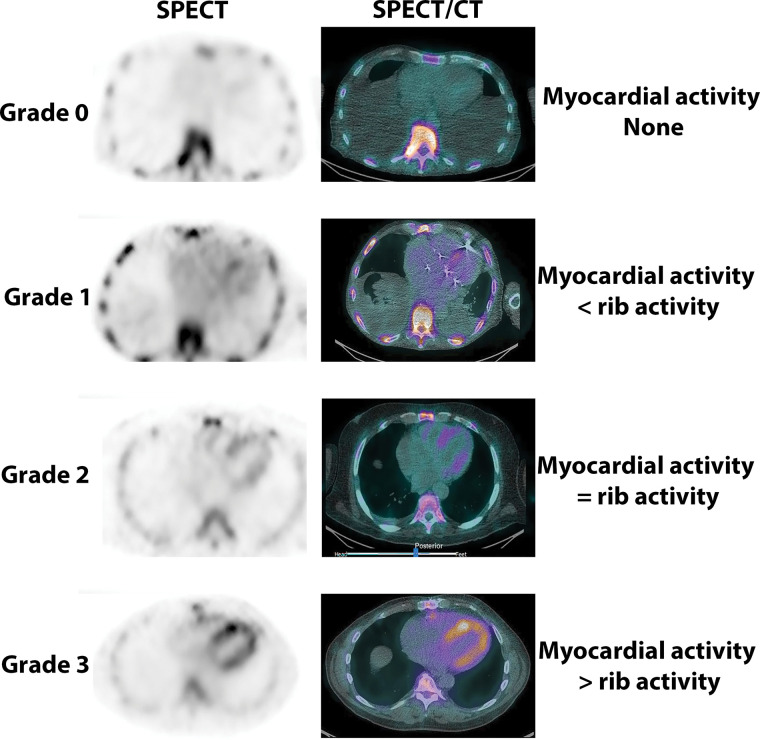
Once tracer uptake is ascertained to be in the myocardium, the degree of uptake can be categorized using a four-point visual scoring system, which is widely known as the Perugini score (Fig 4). Myocardial uptake of 99mTc-PYP at 3-hour imaging is graded as follows: grade 0, no myocardial uptake; grade 1, myocardial uptake less than bone uptake; grade 2, myocardial uptake comparable with bone uptake; and grade 3, myocardial uptake more than bone uptake, where bone uptake is defined by tracer uptake by the ribs (11).
Figure 4:
Visual grading of technetium 99m (99mTc) pyrophosphate (PYP) cardiac images. 99mTc-PYP axial SPECT images (left column) and SPECT/CT images (right column) demonstrate no myocardial uptake of PYP (grade 0) and myocardial tracer uptake less than rib uptake (grade 1), equal to rib uptake (grade 2), and greater than rib uptake (grade 3).
In patients with heart failure and typical imaging findings on echocardiographic or cardiac MRI scans, a finding of grade 2 or 3 myocardial uptake in the absence of a monoclonal protein in the serum or urine is nearly 100% specific for ATTR-CA, obviating endomyocardial biopsy (12). A visual score of 0 is not suggestive of ATTR-CA, while a visual score of 1 is equivocal for ATTR-CA (and may represent early ATTR-CA). A visual score of 0–3 can be present in patients with AL cardiac amyloidosis.
Optional Step for Interpretation: Semiquantitative Method of H/CL Ratio at Planar Imaging
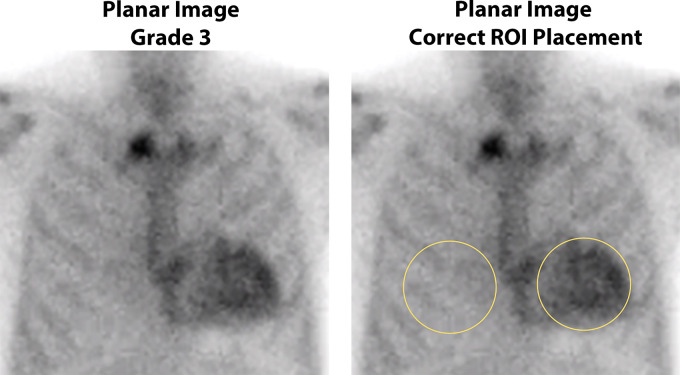
The H/CL ratio is defined as the ratio of mean counts obtained in the region of interest (ROI) drawn over the entire cardiac silhouette to the counts within a similar-sized ROI mirrored over the contralateral lung on planar images (Fig 5). The H/CL ratio is usually concordant with the visual grading at SPECT and is an optional adjunct to support decision-making. An H/CL ratio at 1-hour imaging greater than 1.5 was associated with worse survival (26) and was able to help distinguish ATTR-CA from AL amyloidosis (50). However, scanning 1 hour after injection of 99mTc-PYP/DPD/HMDP frequently produces images showing high blood pool activity and is not recommended (47). In the presence of high blood pool activity or low contralateral lung activity, as in pleural effusions, the H/CL ratio may be falsely elevated. Care should be taken during the placement of the ROI to avoid overlap with sternal and focal rib uptake as well as the adjacent lung. Common errors include drawing an ROI of a different size, wrong positioning of the ROI over the contralateral lung, or inclusion of focal rib uptake in the ROI. In some instances, it is acceptable to derive the H/CL ratio by placing an ROI of a different size or at a different site in the contralateral hemithorax to avoid the overlapping tracer uptake. Due to the many limitations, there is no clear added value of the planar H/CL ratio, and in our clinical practice, we omit the H/CL ratio and report solely the visual impression based on the SPECT/CT images at 2–3 hours after tracer injection.
Figure 5:
Semiquantitative method of heart-to–contralateral lung uptake ratio (H/CL ratio) uptake ratio on planar imaging at 1 hour after tracer injection. Anterior planar images acquired 1 hour after injection of technetium 99m pyrophosphate show grade 3 myocardial tracer uptake. Correct placement of regions of interest (ROIs) for the derivation of H/CL ratio is shown: The “heart” ROI should be drawn over the entire myocardium, and the “contralateral chest” ROI should be of the same size and mirrored over the contralateral chest on planar imaging at 1 hour. The ROI should be positioned to minimize overlap with sternal or focal rib uptake and maximize coverage of the heart without including adjacent lung. The H/CL ratio is optional and should only be reported if myocardial activity is noted on SPECT images.
Step 5: Exclude a Monoclonal Process with Serum/Urine Immunofixation Electrophoresis and a Serum Free AL Assay in All Patients with Suspected Cardiac Amyloidosis
Although 99mTc-PYP/DPD/HMDP scans are less sensitive for AL amyloidosis, with grade 0 uptake seen in nearly 60% of patients (Fig 6), more than 40% of patients with AL amyloidosis can demonstrate myocardial uptake of these tracers (Fig 7), with intense uptake (grade 2 or 3) in 20%–30% (12). If untreated, AL amyloidosis is a highly fatal disease with a median survival of less than 12 months, but outcomes are much better when it is diagnosed early and treated without delay (3). For these reasons, serum/urine immunofixation electrophoresis and serum free AL assay are recommended in all patients with suspected amyloidosis. Immunofixation is essential because the level of paraprotein in AL amyloidosis is often very low and is detectable with use of routine electrophoresis in only 50% of patients with AL amyloidosis (51). Therefore, a monoclonal process must be excluded with serum/urine immunofixation electrophoresis and serum free light chain assay before the confirmation or exclusion of a diagnosis of ATTR-CA based on a 99mTc-PYP/DPD/HMDP scan. In practice, patients referred for 99mTc-PYP/DPD/HMDP scans often have not had a complete evaluation for AL amyloidosis. If a patient has had any positive evaluation for AL amyloidosis (ie, serum/urine immunofixation electrophoresis or serum free AL assay), then involved organ biopsy, cardiac MRI, or an endomyocardial biopsy may be preferred over 99mTc-PYP/DPD/HMDP scintigraphy. Coexistent monoclonal gammopathy can be found in up to 40% of patients with ATTRwt-CA (52), and such patients require expert evaluation.
Figure 6:
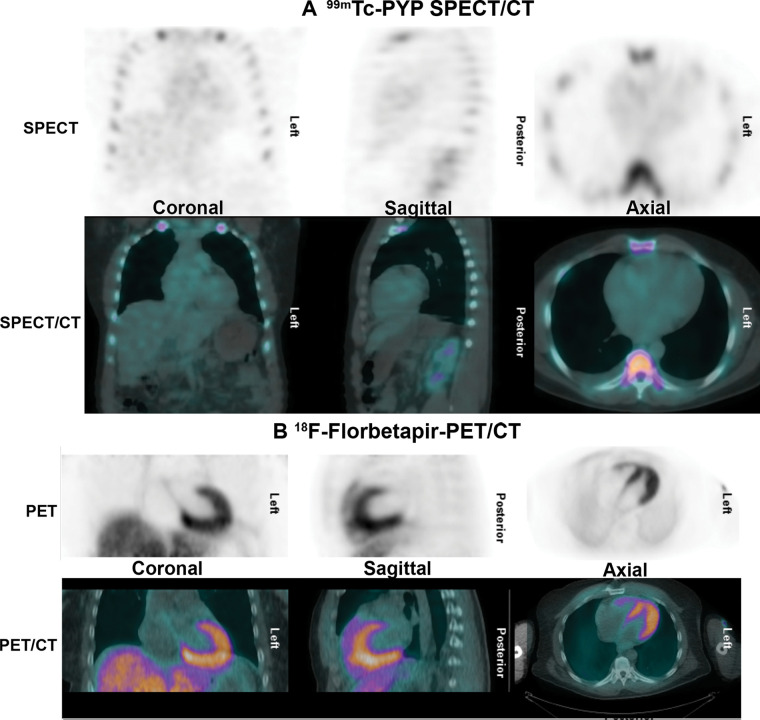
Negative technetium 99m (99mTc) pyrophosphate (PYP) scan in light chain (AL) amyloidosis. A 56-year-old African American man was evaluated with cardiac MRI for symptoms of heart failure. The cardiac MRI findings (not shown) were suggestive of amyloidosis, but (A) the 99mTc-PYP SPECT/CT images show grade 0 uptake. Further evaluation with serum and urine studies as well as bone marrow biopsy confirmed a diagnosis of systemic AL amyloidosis. (B) Fluorine 18 (18F) florbetapir PET/CT images acquired as a part of a research study demonstrate diffuse intense myocardial uptake consistent with AL amyloidosis. Amyloid PET tracers may be helpful to identify AL amyloidosis but are currently investigational.
Figure 7:
Technetium 99m (99mTc) pyrophosphate (PYP) scan positive for light chain (AL) amyloidosis. 99mTc-PYP planar images in a 67-year-old White man with suspected amyloidosis were strongly positive, with grade 3 uptake and an elevated heart-to–contralateral lung uptake ratio of 1.54. Myocardial uptake was confirmed on axial, sagittal, and coronal SPECT images. However, subsequent investigations confirmed the diagnosis of AL amyloidosis. Intense myocardial 99mTc-PYP uptake can be seen in more than 20%–30% of patients with AL amyloidosis; therefore, a monoclonal process must be excluded by means of serum/urine immunofixation electrophoresis and a serum free AL assay before a diagnosis of transthyretin cardiac amyloidosis is made based on a positive 99mTc-PYP scan. ROI = region of interest, 2D = two-dimensional.
Step 6: Review for Physiologic Tracer Distribution and Extracardiac Tracer Activity
As with all imaging modalities, physicians must exercise due diligence during interpretation and look beyond the organ of interest to examine the entire imaged volume for incidental, potentially actionable findings. Physiologic distribution of 99mTc-PYP/DPD/HMDP includes symmetric bone uptake, with activity in the bladder, kidney, and soft tissues (53). Asymmetric or focal increased bony tracer uptake is usually attributed to benign causes, such as rib fractures and degenerative changes, but may warrant further evaluation if a sinister pathologic origin is suspected, such as bone metastasis, metabolic bone disease, or Paget disease (Fig 8) (48). Extracardiac 99mTc-PYP activity has been reported, most commonly in the kidney (due to physiologic excretion), followed by the bone and breast (53).
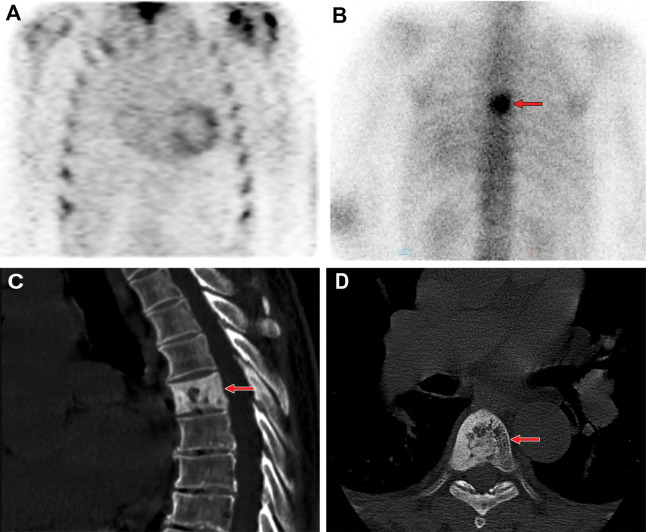
Figure 8:
Review of technetium 99m (99mTc) pyrophosphate (PYP) images for extracardiac tracer activity. (A) 99mTc-PYP SPECT chest images in a 76-year-old White man with suspected cardiac amyloidosis show grade 2 uptake (ie, a positive finding). (B) Review of the entire image volume revealed an incidental focus of extracardiac intense tracer uptake (arrow) in the thoracic spine. (C) Sagittal and (D) axial CT images, which were obtained from the recommended CT examination, show cortical thickening and sclerosis of the T6 vertebral body (arrow), with coarse trabecular thickening. A bone biopsy was performed, as there was concern for bone metastasis, and final histologic results coupled with serum biochemistry findings were compatible with Paget disease.
Challenges with 99mTc-PYP/DPD/HMDP Image Interpretation
False-Positive 99mTc-PYP/DPD/HMDP Scans
High blood pool activity.—By far the most common pitfall in the clinical interpretation of 99mTc-PYP/DPD/HMDP scintigraphy for suspected ATTR-CA is mistaking tracer activity in the blood pool for tracer uptake by the myocardium, resulting in a false-positive scan. 99mTc-PYP/DPD/HMDP activity in the blood pool is a frequent finding on whole-body or chest planar images, especially with 1-hour imaging. Immediately after intravenous injection, 99mTc-PYP circulates in the blood pool and is cleared from the blood by bone uptake and urinary excretion. In patients with intact renal clearance, 10% of tracer activity remains in the vascular system after 1 hour, and approximately 40%–50% of the injected dose will be taken up by the skeletal system after 1–2 hours (54). Therefore, images acquired shortly after tracer injection will show expected blood pool activity within the heart, with no visible uptake in skeletal structures (Fig 2A). The absence of tracer uptake by the ribs is an indicator that it is too early to interpret the scan, as the tracer is still predominantly in the vascular system and has not entered and bound to the bones. The most reliable method to distinguish between blood pool activity and myocardial uptake is to repeat imaging after a delay (3 hours after injection of radiotracer), which should show clearance of blood pool activity, with clear demonstration of tracer uptake in the bones (Fig 2B). Occasionally, 99mTc-PYP activity in the blood pool may persist on the 3-hour image. The predictors of high blood pool activity are not well understood, but we encounter this more commonly in older adults or patients with renal failure. The use of SPECT and, if available, SPECT/CT imaging helps distinguish myocardial from blood pool 99mTc-PYP activity.
On planar images, 99mTc-PYP activity in the blood pool typically manifests as diffuse and amorphous uptake in the region of the heart that can be mild, moderate, or intense. True myocardial 99mTc-PYP uptake demonstrates a central clearing, best visualized on the left anterior oblique projection. The latter may be challenging to ascertain in small hearts. At SPECT imaging, positive 99mTc-PYP uptake by the myocardial wall has an unmistakable U shape, or horseshoe shape, on axial and coronal images and a donut shape on sagittal images, with an appearance similar to myocardial perfusion images (Fig 3B), whereas 99mTc-PYP activity in the blood pool lacks a definite shape and is inseparable from the mediastinal activity (Fig 3A). In selected cases where blood pool activity persists on the 3-hour image and clinical suspicion remains high, further evaluation can be considered, including cardiac MRI or endomyocardial biopsy if clinically indicated.
Recent acute myocardial infarct and other myocardial injuries.—99mTc-PYP cardiac imaging was used in the 1970s for imaging acute myocardial injury from infarction (55) before it was replaced by serum cardiac biomarkers. Cell death during myocardial infarction is followed by an influx of calcium and leads to deposition of intramyocardial calcium complexes, which have high affinity for bone-avid tracers, such as 99mTc-PYP. Generally, tracer uptake in infarction is focal and localizes to an infarcted area following a typical coronary artery distribution. Lack of awareness of this obsolete use of 99mTc-PYP cardiac scintigraphy may lead to inappropriate timing in ordering and interpretation of the test in patients who experienced a recent acute myocardial infarction (Fig 9). Following a myocardial infarction, myocardial 99mTc-PYP uptake usually begins to diminish after 14 days as the infarcted area heals, but rarely, it may remain persistently positive beyond 6 months (56). Other instances of myocardial insult, such as myocarditis (57,58), postradiation injury (59), doxorubicin-induced cardiomyopathy (60), and hydroxychloroquine cardiotoxicity (61), have been shown in case reports to lead to diffusely increased myocardial uptake of 99mTc-PYP. Hence, it is important to note any relevant medical history that may otherwise give rise to a false-positive test result.
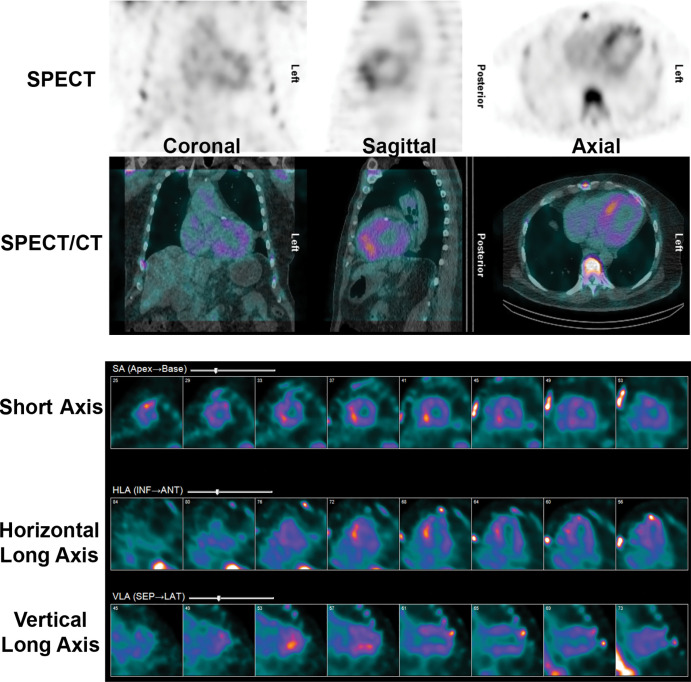
Figure 9:
False-positive 99m technetium pyrophosphate (99mTc-PYP) scan in a recent myocardial infarct. A 72-year-old White man underwent 99mTc-PYP SPECT/CT for evaluation of suspected transthyretin cardiac amyloidosis due to increased left ventricular wall thickening at echocardiography. His medical history was notable for an acute myocardial infarct from a thrombotic occlusion of the left anterior descending coronary artery 10 days before 99mTc-PYP SPECT/CT. The scan shows focal intense tracer uptake, most pronounced in the septum as a sequela of the recent myocardial infarction. 99mTc-PYP myocardial uptake is less specific for amyloidosis in the setting of an acute myocardial infarction and may remain positive for 6 months after infarct. ANT = anterior, HLA = horizontal long axis, INF = inferior, LAT = lateral, SA = short axis, SEP = septal, VLA = vertical long axis.
False-Negative 99mTc-PYP/DPD/HMDP Scans
The specificity of grade 2 or 3 uptake on 99mTc-PYP/DPD/HMDP scans combined with absence of clone is 100% for ATTR-CA, with 100% positive predictive value. This obviates endomyocardial biopsy in this group of patients. The sensitivity of a grade 2 or 3 scan for ATTR-CA is 71%, and this finding implies that almost 29% of patients with ATTR-CA have false-negative scans (12). Scans may be falsely negative in early-stage ATTR-CA disease, where amyloid infiltration is minimal (and perhaps clinically insignificant) or in patients with ATTRv amyloidosis with certain pathogenic ATTR variants, such as Phe64Leu (p.Phe84Leu) (62) and Glu61Ala (p.Glu81Ala) (63) variations. False-negative 99mTc-DPD scans are common in patients carrying Val30Met (p.Val50Met) variations with type B full-length amyloid fibrils (64), but rarely, in patients with Val122Ile (p.Val142Ile) variant, 99mTc-PYP scans can be negative (Fig 10). Therefore, in patients with high clinical suspicion of ATTR-CA but negative 99mTc-PYP/DPD/HMDP scans, further evaluation should be considered (see section “When to Refer for Further Evaluation Including Endomyocardial Biopsy”). Note that 99mTc methyl diphosphate, a commonly used 99mTc bisphosphonate derivative for bone scintigraphy, is not recommended for imaging of cardiac amyloidosis. 99mTc methyl diphosphate has poor sensitivity for detection of ATTR-CA (11) (Fig 11).
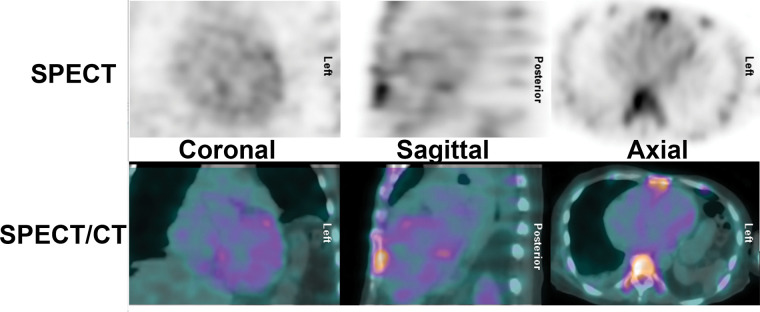
Figure 10:
False-negative technetium 99m (99mTc) pyrophosphate (PYP) scan in hereditary transthyretin (ATTRv) amyloidosis. An 82-year-old African American man presented with symptoms of heart failure. Investigations revealed typical echocardiographic features of an infiltrative pathologic abnormality, and he was found to carry the Val122Ile (p.Val142Ile) transthyretin (ATTR) gene variant. His 99mTc-PYP SPECT/CT scan was, however, surprisingly, negative. The diagnosis of ATTRv cardiomyopathy was confirmed approximately 6 months later postmortem when the autopsy revealed extensive ATTR amyloid fibril deposition in the myocardium. Rarely, in patients with the Val122Ile (p.Val142Ile) variant, 99mTc-PYP scans can be negative. False-negative scans are more commonly encountered in patients with ATTRv with certain pathogenic ATTR variants, such as Phe64Leu (p.Phe84Leu) and Val30Met (p.Val50Met).
Figure 11:
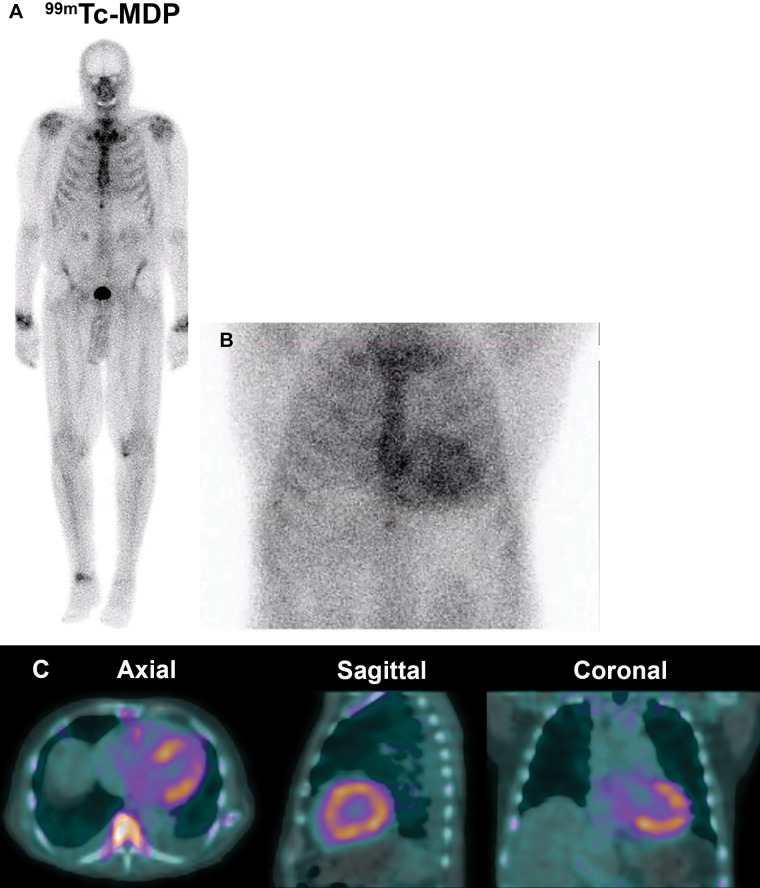
Negative technetium 99m (99mTc) methylene diphosphonate (MDP) bone scan in transthyretin cardiac amyloidosis (ATTR-CA). (A) 99mTc methylene diphosphonate planar whole-body bone scan for the evaluation of bone metastasis in a 79-year-old White man with newly diagnosed malignant neoplasm reveals no abnormal focal tracer uptake suspicious for bone metastasis and no appreciable tracer uptake in the heart. (B) 99mTc pyrophosphate planar scan and (C) SPECT/CT scan obtained 30 days later for the evaluation of ATTR-CA were strongly positive.
Clinical Reporting of 99mTc-PYP/DPD/HMDP Studies
Standardized reporting of 99mTc-PYP/DPD/HMDP scans for ATTR-CA should include patient demographic characteristics and image acquisition method, documenting the type of radiotracer used, dose activity, time interval between radiotracer injection and scan acquisition, and scan technique (planar, SPECT, or SPECT/CT). Reporting of scan findings should describe visual interpretation and semiquantitative interpretation in relation to rib uptake, while reporting of H/CL ratio is optional (8). Incidental positive ancillary findings should be included as well. The conclusion should provide an overall interpretation of the findings into categories of (a) not suggestive of ATTR-CA, (b) strongly suggestive of ATTR-CA, or (c) equivocal for ATTR-CA. The report should state that evaluation for AL amyloidosis with use of serum free AL assay and serum and urine immunofixation electrophoresis is recommended in all patients undergoing 99mTc-PYP/DPD/HMDP scintigraphy for cardiac amyloidosis.
When to Refer for Further Evaluation Including Endomyocardial Biopsy
There are several reasons for further evaluation after bone-avid tracer cardiac scintigraphy. In patients with monoclonal gammopathy, the specificity of 99mTc-PYP/DPD/HMDP is lower, and histologic typing with mass spectrometry–based proteomic analysis of the amyloid fibrils may be necessary (12). Per data from Gillmore et al (12), grade 2 or 3 99mTc-PYP/DPD/HMDP activity has modest sensitivity and negative predictive value for ATTR-CA. If clinical suspicio n is high (eg, a gene-positive patient with typical phenotype but a 99mTc-PYP scan showing grade 0 or 1 activity), there is discordance of the imaging result. In such cases, the imaging finding may represent early ATTR-CA (if AL amyloidosis is excluded). Management of these patients with possible early ATTR-CA is not well established and may include watchful waiting, interval 99mTc-PYP/DPD/HMDP imaging after several months, and, in select cases, endomyocardial biopsy to confirm the diagnosis (2) or initiation of targeted ATTR-CA therapy. It is advisable to refer patients with equivocal 99mTc-PYP/DPD/HMDP studies or difficult-to-interpret AL assay results to dedicated centers with high volume and multidisciplinary expertise in cardiac amyloidosis.
Future: Quantification of Cardiac Amyloidosis with Use of SPECT and SPECT/CT
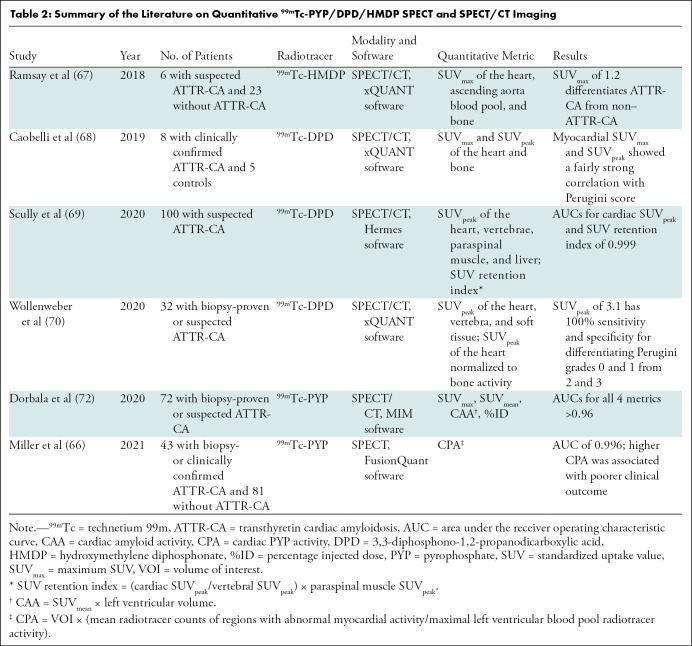
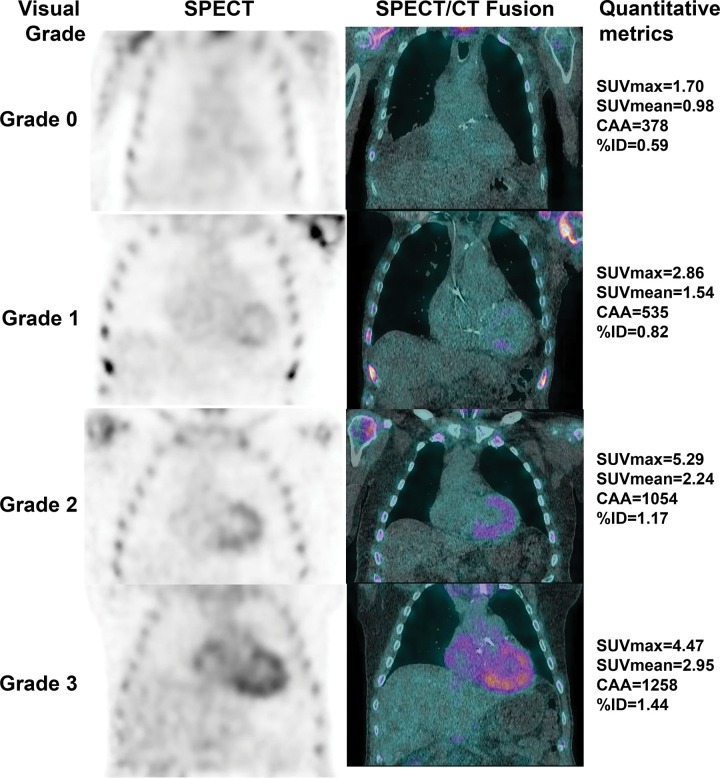
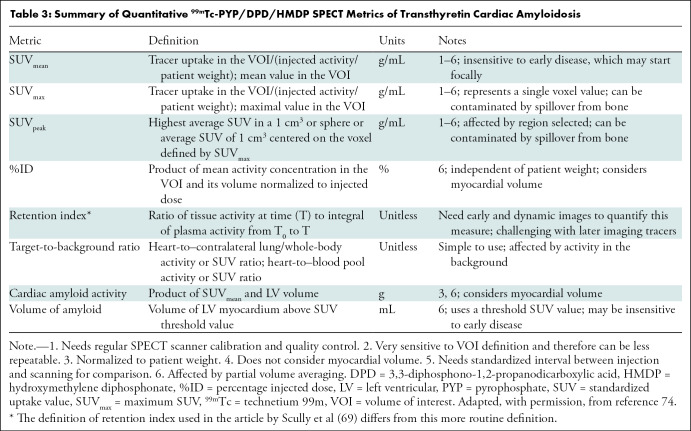
While the current methods of interpretation using visual grading are adequate for diagnosis, they are not able to fully address pertinent clinical matters, such as reliable detection of early disease, evaluation of treatment response, assessment of disease progression (65), and prognostication (24). Several groups have successfully explored the feasibility of deriving target-to-background ratios on SPECT images (66) as well as absolute quantification of cardiac technetium 99m pyrophosphate, 3,3-diphosphono-1,2-propanodicarboxylic acid, or hydroxymethylene diphosphonate uptake with use of SPECT/CT to reflect the amyloid burden in the myocardium (Table 2) (67–72). Advances in SPECT instrumentation, notably CT-based attenuation correction, scatter correction, improved reconstruction algorithms, advanced software, as well as cadmium zinc telluride crystals, make it possible to determine standardized uptake value–based and other advanced quantitative metrics (Fig 12, Table 3) (74). Quantitative bone-avid tracer cardiac scintigraphy may have the added advantages of excellent test-retest repeatability and less interobserver variability and paves the way for application of radiomics in the evaluation of cardiac amyloidosis. Further studies are underway to validate and harmonize the process of quantification using SPECT/CT to advance it from the realm of research to clinical application.
Table 2:
Summary of the Literature on Quantitative 99mTc-PYP/DPD/HMDP SPECT and SPECT/CT Imaging
Figure 12:
Visual grading and quantitative metrics from technetium 99m pyrophosphate SPECT/CT images. The left column shows attenuation-corrected SPECT images with visual grading labels; the right column shows SPECT/CT fusion images with corresponding maximum standardized uptake value (SUVmax), mean standardized uptake value (SUVmean), cardiac amyloid activity (CAA), and percentage injected dose (%ID) for each image.
Table 3:
Summary of Quantitative 99mTc-PYP/DPD/HMDP SPECT Metrics of Transthyretin Cardiac Amyloidosis
Supported by the National Institutes of Health (grants HL 130563 [R.H.F.]; and HL 130563, HL 150342, K24 HL157648, AHA19SRG34950011, and 16CSA28880004 [S.D]).
Disclosures of conflicts of interest: Y.M.K. No relevant relationships. S.A.M.C. Research funding from Pfizer; honoraria for lectures from Ionis, BridgeBio, and Pfizer; support for travel to meetings from Ionis. V.S. Research grant from the American Society of Nuclear Cardiology (ASNC)/Pfizer; member of the Pfizer speaker bureau; unpaid member of the Society of Cardiovascular Computed Tomography and ASNC committees. R.H.F. Research funding from GlaxoSmithKline and Akcea; consulting fees from Ionis, Alnylam Pharmaceuticals, and Caelum Biosciences. M.F.D.C. Grants to institution from Gilead Sciences and Spectrum Dynamics. S.D. Grants to institution from Pfizer, Attralus, GE Healthcare, Philips, the National Institutes of Health, and the American Heart Association; payment for lectures from Janssen and Ionetix.
Abbreviations:
- AL
- light chain
- ATTR
- transthyretin
- ATTR-CA
- ATTR cardiac amyloidosis
- ATTRv
- hereditary ATTR
- ATTRwt-CA
- wildtype ATTR cardiac amyloidosis
- DPD
- 3,3-diphosphono-1,2-propanodicarboxylic acid
- H/CL ratio
- heart-to–contralateral lung uptake ratio
- HFpEF
- heart failure with preserved ejection fraction
- HMDP
- hydroxymethylene diphosphonate
- PYP
- pyrophosphate
- ROI
- region of interest
- 99mTc
- technetium 99m
References
- 1. Merlini G , Bellotti V . Molecular mechanisms of amyloidosis . N Engl J Med 2003. ; 349 ( 6 ): 583 – 596 . [DOI] [PubMed] [Google Scholar]
- 2. Dorbala S , Cuddy S , Falk RH . How to image cardiac amyloidosis: a practical approach . JACC Cardiovasc Imaging 2020. ; 13 ( 6 ): 1368 – 1383 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Falk RH , Alexander KM , Liao R , Dorbala S . AL (light-chain) cardiac amyloidosis: a review of diagnosis and therapy . J Am Coll Cardiol 2016. ; 68 ( 12 ): 1323 – 1341 . [DOI] [PubMed] [Google Scholar]
- 4. Kastritis E , Palladini G , Minnema MC , et al . Daratumumab-based treatment for immunoglobulin light-chain amyloidosis . N Engl J Med 2021. ; 385 ( 1 ): 46 – 58 . [DOI] [PubMed] [Google Scholar]
- 5. Ruberg FL , Grogan M , Hanna M , Kelly JW , Maurer MS . Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review . J Am Coll Cardiol 2019. ; 73 ( 22 ): 2872 – 2891 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grogan M , Scott CG , Kyle RA , et al . Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system . J Am Coll Cardiol 2016. ; 68 ( 10 ): 1014 – 1020 . [Published correction appears in J Am Coll Cardiol 2017;69(23):2882.] [DOI] [PubMed] [Google Scholar]
- 7. Quarta CC , Gonzalez-Lopez E , Gilbertson JA , et al . Diagnostic sensitivity of abdominal fat aspiration in cardiac amyloidosis . Eur Heart J 2017. ; 38 ( 24 ): 1905 – 1908 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dorbala S , Ando Y , Bokhari S , et al . ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: part 1 of 2—evidence base and standardized methods of imaging . J Nucl Cardiol 2019. ; 26 ( 6 ): 2065 – 2123 . [Published correction appears in J Nucl Cardiol 2021;28(4):1761-1762.] [DOI] [PubMed] [Google Scholar]
- 9. Wizenberg TA , Muz J , Sohn YH , Samlowski W , Weissler AM . Value of positive myocardial technetium-99m-pyrophosphate scintigraphy in the noninvasive diagnosis of cardiac amyloidosis . Am Heart J 1982. ; 103 ( 4 Pt 1 ): 468 – 473 . [DOI] [PubMed] [Google Scholar]
- 10. Rapezzi C , Quarta CC , Guidalotti PL , et al . Usefulness and limitations of 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in the aetiological diagnosis of amyloidotic cardiomyopathy . Eur J Nucl Med Mol Imaging 2011. ; 38 ( 3 ): 470 – 478 . [DOI] [PubMed] [Google Scholar]
- 11. Perugini E , Guidalotti PL , Salvi F , et al . Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy . J Am Coll Cardiol 2005. ; 46 ( 6 ): 1076 – 1084 . [DOI] [PubMed] [Google Scholar]
- 12. Gillmore JD , Maurer MS , Falk RH , et al . Nonbiopsy diagnosis of cardiac transthyretin amyloidosis . Circulation 2016. ; 133 ( 24 ): 2404 – 2412 . [DOI] [PubMed] [Google Scholar]
- 13. Glaudemans AW , van Rheenen RW , van den Berg MP , et al . Bone scintigraphy with (99m)technetium-hydroxymethylene diphosphonate allows early diagnosis of cardiac involvement in patients with transthyretin-derived systemic amyloidosis . Amyloid 2014. ; 21 ( 1 ): 35 – 44 . [DOI] [PubMed] [Google Scholar]
- 14. Reimer KA , Martonffy K , Schumacher BL , Henkin RE , Quinn JL 3rd , Jennings RB . Localization of 99mTc-labeled pyrophosphate and calcium in mycoardial infarcts after temporary coronary occlusion in dogs . Proc Soc Exp Biol Med 1977. ; 156 ( 2 ): 272 – 276 . [DOI] [PubMed] [Google Scholar]
- 15. Stats MA , Stone JR . Varying levels of small microcalcifications and macrophages in ATTR and AL cardiac amyloidosis: implications for utilizing nuclear medicine studies to subtype amyloidosis . Cardiovasc Pathol 2016. ; 25 ( 5 ): 413 – 417 . [DOI] [PubMed] [Google Scholar]
- 16. Martineau P , Finnerty V , Giraldeau G , Authier S , Harel F , Pelletier-Galarneau M . Examining the sensitivity of 18F-NaF PET for the imaging of cardiac amyloidosis . J Nucl Cardiol 2021. ; 28 ( 1 ): 209 – 218 . [DOI] [PubMed] [Google Scholar]
- 17. Morgenstern R , Yeh R , Castano A , Maurer MS , Bokhari S . 18Fluorine sodium fluoride positron emission tomography, a potential biomarker of transthyretin cardiac amyloidosis . J Nucl Cardiol 2018. ; 25 ( 5 ): 1559 – 1567 . [DOI] [PubMed] [Google Scholar]
- 18. Trivieri MG , Dweck MR , Abgral R , et al . 18F-sodium fluoride PET/MR for the assessment of cardiac amyloidosis . J Am Coll Cardiol 2016. ; 68 ( 24 ): 2712 – 2714 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang JC , Fox J , Chen C , Yu AF . Cardiac ATTR amyloid nuclear imaging—not all bone scintigraphy radionuclide tracers are created equal . J Nucl Cardiol 2018. ; 25 ( 5 ): 1879 – 1884 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee VW , Caldarone AG , Falk RH , Rubinow A , Cohen AS . Amyloidosis of heart and liver: comparison of Tc-99m pyrophosphate and Tc-99m methylene diphosphonate for detection . Radiology 1983. ; 148 ( 1 ): 239 – 242 . [DOI] [PubMed] [Google Scholar]
- 21. Cuscaden C , Ramsay SC , Prasad S , Goodwin B , Smith J . Estimation of prevalence of transthyretin (ATTR) cardiac amyloidosis in an Australian subpopulation using bone scans with echocardiography and clinical correlation . J Nucl Cardiol 2021. ; 28 ( 6 ): 2845 – 2856 . [DOI] [PubMed] [Google Scholar]
- 22. Fathala A . Incidentally detected cardiac amyloidosis on 99mTc-MDP bone scintigraphy . Radiol Case Rep 2020. ; 15 ( 6 ): 705 – 708 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin H , Zhang X , Einstein AJ , Tang G . Serial Tc-99m MDP scintigraphy demonstrating increasing cardiac uptake over time in a patient with light-chain cardiac amyloidosis . J Nucl Cardiol 2022. ; 29 ( 4 ): 2024 – 2028 . [DOI] [PubMed] [Google Scholar]
- 24. Hutt DF , Fontana M , Burniston M , et al . Prognostic utility of the Perugini grading of 99mTc-DPD scintigraphy in transthyretin (ATTR) amyloidosis and its relationship with skeletal muscle and soft tissue amyloid . Eur Heart J Cardiovasc Imaging 2017. ; 18 ( 12 ): 1344 – 1350 . [DOI] [PubMed] [Google Scholar]
- 25. Rapezzi C , Quarta CC , Guidalotti PL , et al . Role of (99m)Tc-DPD scintigraphy in diagnosis and prognosis of hereditary transthyretin-related cardiac amyloidosis . JACC Cardiovasc Imaging 2011. ; 4 ( 6 ): 659 – 670 . [DOI] [PubMed] [Google Scholar]
- 26. Castano A , Haq M , Narotsky DL , et al . Multicenter study of planar technetium 99m pyrophosphate cardiac imaging: predicting survival for patients with ATTR cardiac amyloidosis . JAMA Cardiol 2016. ; 1 ( 8 ): 880 – 889 . [DOI] [PubMed] [Google Scholar]
- 27. Mohammed SF , Mirzoyev SA , Edwards WD , et al . Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction . JACC Heart Fail 2014. ; 2 ( 2 ): 113 – 122 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bennani Smires Y , Victor G , Ribes D , et al . Pilot study for left ventricular imaging phenotype of patients over 65 years old with heart failure and preserved ejection fraction: the high prevalence of amyloid cardiomyopathy . Int J Cardiovasc Imaging 2016. ; 32 ( 9 ): 1403 – 1413 . [DOI] [PubMed] [Google Scholar]
- 29. Devesa A , Camblor Blasco A , Pello Lázaro AM , et al . Prevalence of transthyretin amyloidosis in patients with heart failure and no left ventricular hypertrophy . ESC Heart Fail 2021. ; 8 ( 4 ): 2856 – 2865 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lindmark K , Pilebro B , Sundström T , Lindqvist P . Prevalence of wild type transtyrethin cardiac amyloidosis in a heart failure clinic . ESC Heart Fail 2021. ; 8 ( 1 ): 745 – 749 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Singal AK , Bansal R , Singh A , et al . Concomitant transthyretin amyloidosis and severe aortic stenosis in elderly Indian population: a pilot study . JACC CardioOncol 2021. ; 3 ( 4 ): 565 – 576 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Treibel TA , Fontana M , Gilbertson JA , et al . Occult transthyretin cardiac amyloid in severe calcific aortic stenosis: prevalence and prognosis in patients undergoing surgical aortic valve replacement . Circ Cardiovasc Imaging 2016. ; 9 ( 8 ): e005066 . [DOI] [PubMed] [Google Scholar]
- 33. Scully PR , Patel KP , Treibel TA , et al . Prevalence and outcome of dual aortic stenosis and cardiac amyloid pathology in patients referred for transcatheter aortic valve implantation . Eur Heart J 2020. ; 41 ( 29 ): 2759 – 2767 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rosenblum H , Masri A , Narotsky DL , et al . Unveiling outcomes in coexisting severe aortic stenosis and transthyretin cardiac amyloidosis . Eur J Heart Fail 2021. ; 23 ( 2 ): 250 – 258 . [DOI] [PubMed] [Google Scholar]
- 35. Castaño A , Narotsky DL , Hamid N , et al . Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement . Eur Heart J 2017. ; 38 ( 38 ): 2879 – 2887 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nitsche C , Aschauer S , Kammerlander AA , et al . Light-chain and transthyretin cardiac amyloidosis in severe aortic stenosis: prevalence, screening possibilities, and outcome . Eur J Heart Fail 2020. ; 22 ( 10 ): 1852 – 1862 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nitsche C , Scully PR , Patel KP , et al . Prevalence and outcomes of concomitant aortic stenosis and cardiac amyloidosis . J Am Coll Cardiol 2021. ; 77 ( 2 ): 128 – 139 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. AbouEzzeddine OF , Davies DR , Scott CG , et al . Prevalence of transthyretin amyloid cardiomyopathy in heart failure with preserved ejection fraction . JAMA Cardiol 2021. ; 6 ( 11 ): 1267 – 1274 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Benson MD , Waddington-Cruz M , Berk JL , et al . Inotersen treatment for patients with hereditary transthyretin amyloidosis . N Engl J Med 2018. ; 379 ( 1 ): 22 – 31 . [DOI] [PubMed] [Google Scholar]
- 40. Adams D , Gonzalez-Duarte A , O’Riordan WD , et al . Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis . N Engl J Med 2018. ; 379 ( 1 ): 11 – 21 . [DOI] [PubMed] [Google Scholar]
- 41. Maurer MS , Schwartz JH , Gundapaneni B , et al . Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy . N Engl J Med 2018. ; 379 ( 11 ): 1007 – 1016 . [DOI] [PubMed] [Google Scholar]
- 42. Gillmore JD , Gane E , Taubel J , et al . CRISPR-Cas9 in vivo gene editing for transthyretin amyloidosis . N Engl J Med 2021. ; 385 ( 6 ): 493 – 502 . [DOI] [PubMed] [Google Scholar]
- 43. Macedo AVS , Schwartzmann PV , de Gusmão BM , Melo MDT , Coelho-Filho OR . Advances in the treatment of cardiac amyloidosis . Curr Treat Options Oncol 2020. ; 21 ( 5 ): 36 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Solomon SD , Adams D , Kristen AV , et al . Effects of patisiran, an RNA interference therapeutic, on cardiac parameters in patients with hereditary transthyretin-mediated amyloidosis: an analysis of the APOLLO study . Circulation 2019. ; 139 ( 4 ): 431 – 443 . [DOI] [PubMed] [Google Scholar]
- 45. Fontana M , Martinez-Naharro A , Chacko L , et al . Reduction in CMR derived extracellular volume with patisiran indicates cardiac amyloid regression . JACC Cardiovasc Imaging 2021. ; 14 ( 1 ): 189 – 199 . [DOI] [PubMed] [Google Scholar]
- 46. Treglia G , Glaudemans AWJM , Bertagna F , et al . Diagnostic accuracy of bone scintigraphy in the assessment of cardiac transthyretin-related amyloidosis: a bivariate meta-analysis . Eur J Nucl Med Mol Imaging 2018. ; 45 ( 11 ): 1945 – 1955 . [DOI] [PubMed] [Google Scholar]
- 47. Dorbala S , Ando Y , Bokhari S , et al . Addendum to ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: Part 1 of 2—evidence base and standardized methods of imaging . J Nucl Cardiol 2021. ; 28 ( 4 ): 1769 – 1774 . [DOI] [PubMed] [Google Scholar]
- 48. Love C , Din AS , Tomas MB , Kalapparambath TP , Palestro CJ . Radionuclide bone imaging: an illustrative review . RadioGraphics 2003. ; 23 ( 2 ): 341 – 358 . [DOI] [PubMed] [Google Scholar]
- 49. Hutt DF , Quigley AM , Page J , et al . Utility and limitations of 3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in systemic amyloidosis . Eur Heart J Cardiovasc Imaging 2014. ; 15 ( 11 ): 1289 – 1298 . [DOI] [PubMed] [Google Scholar]
- 50. Bokhari S , Castaño A , Pozniakoff T , Deslisle S , Latif F , Maurer MS . (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses . Circ Cardiovasc Imaging 2013. ; 6 ( 2 ): 195 – 201 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gillmore JD , Wechalekar A , Bird J , et al . Guidelines on the diagnosis and investigation of AL amyloidosis . Br J Haematol 2015. ; 168 ( 2 ): 207 – 218 . [DOI] [PubMed] [Google Scholar]
- 52. Phull P , Sanchorawala V , Connors LH , et al . Monoclonal gammopathy of undetermined significance in systemic transthyretin amyloidosis (ATTR) . Amyloid 2018. ; 25 ( 1 ): 62 – 67 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Griffin JM , Rosenblum H , Teruya S , et al . Prevalence and significance of incidental findings on Pyp imaging in the Scan-mp study: the first 123 cases . J Card Fail 2020. ; 26 ( 10 Supplement ): S34 . [Google Scholar]
- 54. Mallinckrodt Pharmaceuticals . Technescan PYP Kit for the Preparation of Technetium Tc 99m Pyrophosphate Injection . https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018321s022lbl.pdf. Published 2017. Accessed November 26, 2022 .
- 55. Buja LM , Parkey RW , Stokely EM , Bonte FJ , Willerson JT . Pathophysiology of technetium-99m stannous pyrophosphate and thallium-201 scintigraphy of acute anterior myocardial infarcts in dogs . J Clin Invest 1976. ; 57 ( 6 ): 1508 – 1522 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Olson HG , Lyons KP , Aronow WS , Brown WT , Greenfield RS . Follow-up technetium-99m stannous pyrophosphate myocardial scintigrams after acute myocardial infarction . Circulation 1977. ; 56 ( 2 ): 181 – 187 . [DOI] [PubMed] [Google Scholar]
- 57. Mitsutake A , Nakamura M , Inou T , Kikuchi Y , Takeshita A , Fujimi S . Intense, persistent myocardial avid technetium-99m-pyrophosphate scintigraphy in acute myocarditis . Am Heart J 1981. ; 101 ( 5 ): 683 – 684 . [DOI] [PubMed] [Google Scholar]
- 58. Ahmad M , Dubiel JP . Tc-99m pyrophosphate myocardial imaging in perimyocarditis . J Nucl Med 1981. ; 22 ( 5 ): 452 – 454 . [PubMed] [Google Scholar]
- 59. Soin JS , Cox JD , Youker JE , Swartz HM . Cardiac localization of 99mTc-(Sn)-pyrophosphate following irradiation of the chest . Radiology 1977. ; 124 ( 1 ): 165 – 168 . [DOI] [PubMed] [Google Scholar]
- 60. Ziessman HA , O’Malley JP , Thrall JH . Cardiac System . In: Nuclear Medicine: The Requisites in Radiology . 4th ed . Philadelphia, Pa: : Saunders Elsevier; , 2014. . [Google Scholar]
- 61. Chang ICY , Bois JP , Bois MC , Maleszewski JJ , Johnson GB , Grogan M . Hydroxychloroquine-mediated cardiotoxicity with a false-positive 99mtechnetium-labeled pyrophosphate scan for transthyretin-related cardiac amyloidosis . Circ Cardiovasc Imaging 2018. ; 11 ( 1 ): e007059 . [DOI] [PubMed] [Google Scholar]
- 62. Musumeci MB , Cappelli F , Russo D , et al . Low sensitivity of bone scintigraphy in detecting Phe64Leu mutation-related transthyretin cardiac amyloidosis . JACC Cardiovasc Imaging 2020. ; 13 ( 6 ): 1314 – 1321 . [DOI] [PubMed] [Google Scholar]
- 63. Cuddy SAM , Dorbala S , Falk RH . Complexities and pitfalls in cardiac amyloidosis . Circulation 2020. ; 142 ( 4 ): 409 – 415 . [DOI] [PubMed] [Google Scholar]
- 64. Möckelind S , Axelsson J , Pilebro B , Lindqvist P , Suhr OB , Sundström T . Quantification of cardiac amyloid with [18F]flutemetamol in patients with V30M hereditary transthyretin amyloidosis . Amyloid 2020. ; 27 ( 3 ): 191 – 199 . [DOI] [PubMed] [Google Scholar]
- 65. Castaño A , DeLuca A , Weinberg R , et al . Serial scanning with technetium pyrophosphate (99mTc-PYP) in advanced ATTR cardiac amyloidosis . J Nucl Cardiol 2016. ; 23 ( 6 ): 1355 – 1363 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Miller RJH , Cadet S , Mah D , et al . Diagnostic and prognostic value of technetium-99m pyrophosphate uptake quantitation for transthyretin cardiac amyloidosis . J Nucl Cardiol 2021. ; 28 ( 5 ): 1835 – 1845 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ramsay SC , Lindsay K , Fong W , Patford S , Younger J , Atherton J . Tc-HDP quantitative SPECT/CT in transthyretin cardiac amyloid and the development of a reference interval for myocardial uptake in the non-affected population . Eur J Hybrid Imaging 2018. ; 2 ( 1 ): 17 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Caobelli F , Braun M , Haaf P , Wild D , Zellweger MJ . Quantitative 99mTc-DPD SPECT/CT in patients with suspected ATTR cardiac amyloidosis: feasibility and correlation with visual scores . J Nucl Cardiol 2020. ; 27 ( 5 ): 1456 – 1463 . [DOI] [PubMed] [Google Scholar]
- 69. Scully PR , Morris E , Patel KP , et al . DPD quantification in cardiac amyloidosis: a novel imaging biomarker . JACC Cardiovasc Imaging 2020. ; 13 ( 6 ): 1353 – 1363 . [Published correction appears in JACC Cardiovasc Imaging 2021;14(1):318-319.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wollenweber T , Rettl R , Kretschmer-Chott E , et al . In vivo quantification of myocardial amyloid deposits in patients with suspected transthyretin-related amyloidosis (ATTR) . J Clin Med 2020. ; 9 ( 11 ): 3446 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ross JC , Hutt DF , Burniston M , et al . Quantitation of 99mTc-DPD uptake in patients with transthyretin-related cardiac amyloidosis . Amyloid 2018. ; 25 ( 3 ): 203 – 210 . [DOI] [PubMed] [Google Scholar]
- 72. Dorbala S , Park MA , Cuddy S , et al . Absolute Quantitation of Cardiac 99mTc-Pyrophosphate Using Cadmium-Zinc-Telluride-Based SPECT/CT . J Nucl Med . 2021. ; 62 ( 5 ): 716 – 722 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dorbala S , Ando Y , Bokhari S , et al . Correction to: ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: Part 1 of 2-evidence base and standardized methods of imaging . J Nucl Cardiol 2021. ; 28 ( 4 ): 1761 – 1762 . [DOI] [PubMed] [Google Scholar]
- 74. Dorbala S , Kijewski MF , Park MA . Quantitative bone-avid tracer SPECT/CT for cardiac amyloidosis: a crucial step forward . JACC Cardiovasc Imaging 2020. ; 13 ( 6 ): 1364 – 1367 . [DOI] [PubMed] [Google Scholar]