Abstract
Salmonella strains that lack or overproduce DNA adenine methylase (Dam) elicit a protective immune response to different Salmonella species. To generate vaccines against other bacterial pathogens, the dam genes of Yersinia pseudotuberculosis and Vibrio cholerae were disrupted but found to be essential for viability. Overproduction of Dam significantly attenuated the virulence of these two pathogens, leading to, in Yersinia, the ectopic secretion of virulence proteins (Yersinia outer proteins) and a fully protective immune response in vaccinated hosts. Dysregulation of Dam activity may provide a means for the development of vaccines against varied bacterial pathogens.
Salmonella DNA adenine methylase (Dam) mutants ectopically express multiple genes that are normally induced during infection (18, 20, 27). These Dam mutants are markedly attenuated but highly effective as live vaccines against murine typhoid fever (12, 20). DNA adenine methylases are highly conserved in many pathogens such as Vibrio cholerae (http://www.tigr.org), Salmonella enterica serovar Typhi (http://www.sanger.ac.uk), pathogenic Escherichia coli (2), Yersinia pestis (http: //www.tigr.org), and Haemophilus influenzae (10). The goal of this study was to determine whether the findings regarding Dam's role in Salmonella pathogenesis could be extended to V. cholerae and Yersinia pseudotuberculosis, the causative agents of human cholera and gastroenteritis, respectively; additionally, Y. pseudotuberculosis causes a fatal bacteremia in mice. In contrast to Salmonella, which is a facultative intracellular parasite, both Y. pseudotuberculosis and V. cholerae are principally extracellular pathogens. Yersinia sp. pathogenesis is dependent upon virulence proteins called Yops (for Yersinia outer proteins) (6, 15, 37), which, upon host contact, are injected directly into the host cell cytoplasm, where they act as effectors to inhibit phagocytosis and proinflammatory cytokine release (31, 32, 36, 38). In contrast, V. cholerae is a mucosal pathogen that expresses virulence factors, including cholera toxin and toxin coregulated pilus, in the small intestine (9).
In this report, we show that Dam is essential for the viability of Y. pseudotuberculosis and V. cholerae. Overproduction of Dam was not lethal and attenuated the virulence of both pathogens. Additionally, Dam-overproducing strains of Yersinia ectopically secreted Yop virulence proteins in vitro and conferred full protection against Yersinia bacteremia in vaccinated hosts.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Derivatives of Y. pseudotuberculosis strain YPIIIpYV (obtained from Stanley Falkow) and V. cholerae strain 0395 (classical, Ogawa serotype; obtained from John Mekalanos) (Table 1) were grown overnight with shaking at 28 and 37°C, respectively. The following antibiotics were used at the indicated concentrations: for V. cholerae, kanamycin (50 μg/ml), ampicillin (50 μg/ml), tetracycline (1.2 μg/ml), and streptomycin (100 μg/ml); and for Y. pseudotuberculosis, kanamycin (50 μg/ml), ampicillin (50 μg/ml), tetracycline (5 μg/ml), and chloramphenicol (20 μg/ml).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype or description | Source or reference |
|---|---|---|
| Strains | ||
| Y. pseudotuberculosis | ||
| YPIIIpYV | Wild type | Stanley Falkow |
| MT2294 | dam::Kn + pTP166-Cm | This work |
| MT2357 | DUP[φ(dam::Kn)∗pCVD442∗(dam+)] | This work |
| MT2358 | DUP[φ(dam::Kn)∗pCVD442∗(dam+)] pWKS30-Tc + Y. pseudotuberculosis dam+ | This work |
| MT2359 | DUP[φ(dam::Kn)∗pCVD442∗(dam+)] pWKS30-Tc + E. coli dam+ | This work |
| MT2360 | DUP[φ(dam::Kn)∗pCVD442∗(dam+)] pWKS30-Tc | This work |
| V. cholerae | ||
| O395 | Wild type; classical | John Mekalanos |
| MT2284 | dam::Kn pWKS30-Tc + E. coli dam+ | This work |
| MT2361 | DUP[φ(dam::Kn)∗pCVD442∗(dam+)] | This work |
| MT2362 | DUP[φ(dam::Kn)∗pCVD442∗(dam+)] pWKS30-Tc + V. cholerae dam+ | This work |
| MT2363 | DUP[φ(dam::Kn)∗pCVD442∗(dam+)] pWKS30-Tc + E. coli dam+ | This work |
| MT2364 | DUP[φ(dam::Kn)∗pCVD442∗(dam+)] pWKS30-Tc | This work |
| S. enterica serovar Typhimurium | ||
| MT2188 | damΔ232 | 20 |
| Plasmids | ||
| pTP166-Cm | E. coli dam under tac promoter control; chloramphenicol-resistant (Aps) derivative of pTP166 | 29; this work |
| pWKS30-Tc | Tetracycline-resistant (Aps) derivative of pWKS30 | 43; this work |
Construction of genomic DNA libraries.
Genomic DNA libraries for Y. pseudotuberculosis and V. cholerae were constructed using approximately 2 to 5 μg of genomic DNA that was partially digested with Sau3AI and size fractionated to 2.5 to 6 kb on a 0.8% agarose gel. The size-fractionated DNA was ligated into the vector pWKS30 (ampicillin resistant [Apr] [43]) that was previously cleaved with BamHI. The recombinant plasmids were introduced into E. coli DH5α λpir by electroporation; we then pooled the Apr recombinant clones, from which plasmid DNA was isolated.
Construction of plasmids containing dam derived from V. cholerae, Y. pseudotuberculosis, and E. coli.
Plasmid pWKS30Tc is a tetracycline-resistant derivative of pWKS30 (43). The tet gene and promoter from pBR322 were excised as an AvaI/EcoRI fragment, treated with the Klenow fragment to produce blunt ends, and cloned into plasmid pWKS30, which had been partially digested with BglII and SspI (removing the bla gene) and treated with the Klenow fragment to produce pWKS30Tc. The V. cholerae dam gene was cloned into the blunt-ended SmaI site of pWKS30Tc by ligating a 1.2-kb DraIII/EcoRI chromosomal DNA fragment containing V. cholerae dam that had been treated with the Klenow fragment, generating a pWKS30Tc plasmid harboring V. cholerae dam. The Y. pseudotuberculosis dam gene was cloned into the blunt-ended SmaI site of pWKS30Tc by ligating a 1.3-kb NcoI/PvuII chromosomal DNA fragment containing Y. pseudotuberculosis dam that had been treated with the Klenow fragment, generating a pWKS30Tc plasmid harboring Y. pseudotuberculosis dam. The E. coli dam gene was cloned into the blunt-ended SmaI site of pWKS30Tc by ligating a 1.3-kb XbaI/PvuII chromosomal DNA fragment containing E. coli dam that had been treated with the Klenow fragment, generating a pWKS30Tc plasmid harboring E. coli dam. Plasmid clones containing the putative V. cholerae, Y. pseudotuberculosis, and E. coli dam genes were introduced into an E. coli dam mutant strain. Recovered plasmids were found to be resistant to the methylation-sensitive restriction enzyme MboI, indicating that the recombinant clones encode Dam.
Plasmid pTP166Cm is a chloramphenicol-resistant derivative of pTP166 (Apr), which overproduces E. coli dam from a Ptac promoter (29). A blunt-ended 1.4-kb BsaAI fragment containing the chloramphenicol resistance gene and its promoter from pACYC184 was ligated to a pTP166 plasmid derivative that had been digested with DraI and AatII (removing the bla gene) and treated with the Klenow fragment. The resulting chloramphenicol-resistant clone, pTP166Cm, overproduced E. coli dam, as evidenced by the DNA methylase assay (see below).
DNA sequencing and protein sequence analysis.
The nucleotide sequences of the dam genes were determined using a Big Dye Terminator reaction kit, and samples were analyzed on a model 310 genetic analyzer (PE Biosystems). Protein sequence alignment was performed using the CLUSTAL W method available at the European Bioinformatics website (http://www2.ebi.ac.uk/clustalw/).
DNA adenine methylase assays.
Dam activity was measured by a modified tritiated S-adenosylmethionine ([3H]SAM) incorporation assay (13) wherein SAM serves as a methyl donor for DNA methylases. Briefly, cells from an overnight grown culture (10 ml) were collected by centrifugation, quick frozen under liquid nitrogen, washed once in 1× Tris-EDTA buffer (10 mM Tris HCl [pH 8.0]–1 mM EDTA [pH 8.0]), and resuspended in 10× Tris-EDTA. Lysozyme (0.05 mg) was added, and the cells were disrupted by sonication. Cell debris was removed by centrifugation, and the cell extracts were recovered. The total amount of protein in each cell extract was determined by a Bradford protein assay. GATC-specific methylase activity was quantified by adding 0.1 ml of cell extract to 7 μl of the following methylase reaction mixture: 0.015 mM SAM, 34 mM dithiothreitol, 0.5 μg of RNase A, 4 μg of bovine serum albumin, 5 μg of double-stranded GATC containing the DNA substrate (5′-CAGGATCCATGCGATCAACCGATCAAGGATCCAC-3′), and 0.55 μCi of [methyl-3H]SAM. The reaction mixture was incubated for 1 h at 37°C, after which an excess of unlabeled SAM was added to reach a final concentration of 3.0 mM to stop the reaction. A 0.08-ml sample of each reaction mixture was transferred to Whatman DE81 filter paper and washed several times with 0.4 M NH4HCO3, followed by one wash with 95% ethyl alcohol. The filters were allowed to dry, and the amount of methyl-3H incorporated onto the DNA substrate was determined by scintillation counting. Methylase activity in each cell extract was calculated as counts per minute of methyl-3H per microgram of total protein in the cell extract.
Virulence, colonization, and protection assays. (i) LD50 assay.
For Yersinia, an assay was used to determine the lethal dose required to kill 50% of the animals (LD50); this virulence assay was performed as described in reference 20. Briefly, mutant and wild-type Yersinia species grown overnight in Luria broth at 28°C with shaking were washed in 0.15 M NaCl, diluted in 0.2 ml of 0.2 M phosphate buffer (pH 8.0), and used to perorally infect BALB/c mice by gastrointubation. The protective capacity of Dam derivatives was determined by challenging immunized mice with the virulent parental strain. Mice were examined daily following challenge for morbidity and mortality. To determine the number of bacteria in host tissues, moribund mice were sacrificed and bacteria were recovered from host tissues and plated for colony counts. Host tissues assayed included Peyer's patches (the four Peyer's patches proximal to the ileal-cecal junction), mesenteric lymph nodes, and spleens.
(ii) Competitive index assay.
The competitive index is the ratio of mutant to wild-type organisms recovered from host tissue after infection. For Y. pseudotuberculosis infection, 6- to 8-week-old BALB/c mice were gastrointubated with 8.0 × 108 cells of mutant organisms and 8.0 × 108 cells of wild-type organisms. After 7 days, mice were sacrificed, spleens were recovered and homogenized, and bacteria were enumerated by direct colony count as described previously (5). For V. cholerae infection, 5-day-old CD-1 suckling mice were coinoculated perorally with approximately 105 mutant organisms and 105 wild-type organisms; 24 h postinfection, mice were sacrificed and bacterial numbers were isolated from the intestine as described previously (7).
Nucleotide sequence accession numbers.
The sequence data for V. cholerae dam and Y. pseudotuberculosis dam have been submitted to the DDBJ, EMBL, and GenBank databases under accession numbers AF274317 and AF274318, respectively.
RESULTS
Characterization of dam from Y. pseudotuberculosis and V. cholerae.
To clone the dam gene from Y. pseudotuberculosis and V. cholerae, recombinant plasmids derived from a genomic DNA library constructed from both pathogens were screened for the ability to complement the 2 aminopurine (2-AP) sensitivity phenotype of an S. enterica serovar Typhimurium dam mutant strain (2-AP is a purine analog which is toxic to dam mutants [14]). A 1.3-kb NcoI/PvuII DNA fragment from Y. pseudotuberculosis conferred 2-AP resistance to a dam mutant serovar Typhimurium strain and encoded DNA adenine methylase activity as evidenced by resistance of the recombinant plasmid (recovered from dam mutant E. coli) to digestion with the restriction enzyme MboI, which cleaves only nonmethylated GATC sequences. Sequence analysis revealed an open reading frame (ORF) encoding a putative 271-amino-acid protein exhibiting 71% identity to the entire E. coli Dam protein. Taken together, these data indicate that this ORF encoded Y. pseudotuberculosis Dam activity.
A 1.2-kb EcoRI/DraIII V. cholerae DNA fragment conferred 2-AP resistance to dam mutant serovar Typhimurium and encoded DNA adenine methylase activity as judged by resistance of the plasmid clone to MboI cleavage. Sequence analysis of the insert DNA revealed an ORF encoding a 277-amino-acid protein which displays 63.5% identity over the entire E. coli Dam protein. The V. cholerae dam gene described in this study differs from a previously published V. cholerae dam sequence, which partially overlaps and is in the opposite orientation to that of the dam gene identified here and has only 30 to 35% identity over the entire E. coli Dam sequence (1). Moreover, a recombinant plasmid containing the previously identified dam gene was unable to confer 2-AP resistance to a dam mutant serovar Typhimurium strain, and when this plasmid was recovered from dam mutant E. coli, it was completely digested by MboI, indicating the lack of DNA methylase activity (data not shown). These data suggest that the dam gene identified in this study encodes V. cholerae DNA adenine methylase activity that is specific for GATC sequences.
dam is essential for viability in Y. pseudotuberculosis and V. cholerae.
Standard genetic procedures to remove the dam gene from Y. pseudotuberculosis and V. cholerae were unsuccessful, suggesting that, in contrast to Salmonella and E. coli, dam is essential for viability in Yersinia and Vibrio. To confirm the requirement of dam for growth, suicide plasmids containing dam deletion mutations were integrated into the native chromosomal dam locus of Y. pseudotuberculosis and V. cholerae. The chromosome integration event generates a duplication of the dam locus, in which one copy is dam+ and one copy has a mutation (22). Essentiality (on rich medium) was demonstrated by showing that the generation of the dam mutation haploid state from the parental dam duplication is dependent upon the presence of a dam+ gene provided in trans. The dam deletion structure in the chromosomes of Y. pseudotuberculosis and V. cholerae was confirmed by both PCR and Southern analysis according to the method of Julio et al. (22). Table 2 shows that dam mutant segregants of Y. pseudotuberculosis and V. cholerae were obtained only in the presence of a wild-type copy of dam provided in trans. These data indicate that dam is essential for viability in Y. pseudotuberculosis and in V. cholerae, similar to the essential role of the cell cycle-regulated methyltransferase CcrM in Caulobacter crescentus, Rhizobium meliloti, Brucella abortus, and Agrobacterium tumefaciens (23, 33, 35, 44).
TABLE 2.
Dam is essential for viability in Y. pseudotuberculosis and V. choleraea
| Strain | Plasmid | No. of colonies with the indicated genotype at the dam locus after excision of pCVD442
|
|
|---|---|---|---|
| dam+ | Δdam | ||
| Y. pseudotuberculosis | |||
| MT2357 | None | 200 | 0 |
| MT2358 | dam+ (Y. pseudotuberculosis) | 135 | 44 |
| MT2359 | dam+ (E. coli) | 151 | 21 |
| MT2360 | Vector alone | 200 | 0 |
| V. cholerae | |||
| MT2361 | None | 196 | 0 |
| MT2362 | dam+ (V. cholerae) | 153 | 47 |
| MT2363 | dam+ (E. coli) | 118 | 76 |
| MT2364 | Vector alone | 194 | 0 |
Integration of the suicide plasmid pCVD442 (8) into the Y. pseudotuberculosis or V. cholerae chromosome results in a duplication of the dam locus wherein one copy contains the wild-type dam gene and the other copy contains dam with a deletion associated with a kanamycin resistance marker (22). Selection for the excision of pCVD442 on plates containing 10% sucrose result in either dam+ or dam mutant (Δdam::Kn) segregants (8). The dam mutant segregants of Y. pseudotuberculosis or V. cholerae are associated with chromosomal internal deletions of 314 or 324 bp of the dam sequence, respectively. For plasmid complementation experiments, pWKS30-Tc (43) was used to provide the dam gene from Y. pseudotuberculosis, V. cholerae, or E. coli as indicated.
Dam overproduction attenuates the virulence of Y. pseudotuberculosis and V. cholerae.
To examine whether altered levels of Dam activity affected the virulence of Y. pseudotuberculosis and V. cholerae, recombinant plasmids that overproduced E. coli Dam were introduced into both pathogens, and the resulting strains were assayed for virulence. Note that loss of the Dam-overproducing plasmid in a dam+ parental background would result in a virulent (wild-type) strain. Thus, these virulence studies were performed with dam mutant parental backgrounds since dam is essential for viability in Y. pseudotuberculosis and V. cholerae and loss of the Dam-overproducing plasmids in dam mutant backgrounds is lethal for both pathogens.
Overproduction of E. coli Dam from a recombinant plasmid in Y. pseudotuberculosis (MT2294) and V. cholerae (MT2284), resulting in 74- and 53-fold increases in Dam activity, respectively, was not lethal but significantly attenuated the virulence of these two pathogens. That is, Dam overproduction results in a >6,000-fold attenuation in a Y. pseudotuberculosis murine bacteremia infection model and a 5-fold defect (P < 0.05) in V. cholerae colonization in a suckling mouse model (Table 3). The attenuation in both organisms was not due to a general growth defect since the Dam-overproducing strains showed growth rates in vitro similar to that of the wild type (data not shown). Relevant to these findings, CcrM overproduction was recently shown to attenuate the intracellular replication of B. abortus in murine macrophages (35).
TABLE 3.
Dam overproduction confers a virulence defect in Y. pseudotuberculosis and V. cholerae
| Strain | Relevant genotypea | Oral LD50 ratiob (mutant/wild type) | Competitive indexc |
|---|---|---|---|
| MT2294 | DamOPY. pseudotuberculosis | >6,000 | <10−4 |
| MT2284 | DamOPV. cholerae | ND | 0.218 |
Bacterial strains are derivatives of Y. pseudotuberculosis strain YPIIIpYV and V. cholerae strain O395. Dam-overproducing (DamOP) strains of Y. pseudotuberculosis (MT2294) and V. cholerae (MT2284) contain E. coli dam on chloramphenicol- and tetracycline-resistant derivatives of the high-copy-number recombinant plasmid pTP166 (29) and the medium-copy-number plasmid pWKS30 (43), respectively, in Dam mutant (Δdam::Kn) genetic backgrounds. (Dam overproduction from the high-copy-number plasmid pTP166-Cm in V. cholerae was deleterious to V. cholerae cell growth as evidenced by a 50% decrease in the rate of growth on rich medium.) Since dam is essential for viability in Y. pseudotuberculosis and V. cholerae, the loss of the Dam-overproducing plasmids in dam mutant backgrounds is lethal for both pathogens.
The oral LD50 ratio (the LD50 of the Dam-overproducing strain divided by the LD50 of wild-type bacteria) was determined by infecting 18 BALB/c mice with 1.56 × 1011 cells of the Y. pseudotuberculosis Dam-overproducing strain MT2294 as described previously (20); 18 of 18 mice survived this challenge dose, and no visible signs of infection were observed. The peroral LD50 of wild-type Y. pseudotuberculosis (2.5 × 107) was determined by Monack et al. (30). ND, not determined.
For Y. pseudotuberculosis infection, six BALB/c mice were gastrointubated with 8.0 × 108 Yersinia dam mutant cells containing the Dam-overproducing plasmid from strain MT2294 and 8.0 × 108 cells of the wild type. After 7 days, mice were sacrificed, spleens were recovered and homogenized, and bacteria were enumerated by direct colony counting as described previously (5). Of the 106 to 107 Yersinia organisms recovered from each of six spleens, none contained the Dam-overproducing plasmid. For V. cholerae infection, six CD-1 mice were gastrointubated with a 1:1 ratio of Dam-overproducing cells (MT2284) to wild-type cells; 24 h postinfection, mice were sacrificed and bacterial numbers were determined from the intestine as described previously (7). The attenuation conferred was significant according to the two-tailed Fisher exact test (P < 0.05).
Dam-overproducing Y. pseudotuberculosis ectopically secretes Yops.
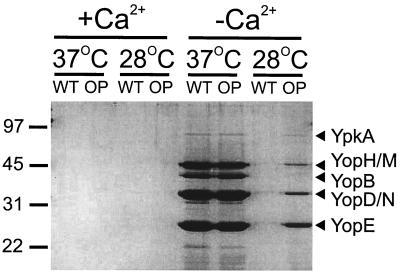
To understand the mechanism of Yersinia virulence attenuation, we questioned whether Dam-overproducing Y. pseudotuberculosis showed altered secretion of Yops. Yops, which play essential roles in Yersinia virulence, are normally under strict regulatory control by the low calcium response, whereby Yop secretion occurs in vitro only at 37°C under conditions of low calcium (40). However, overproduction of Dam in Y. pseudotuberculosis resulted in the relaxation of the temperature, but not the low-calcium, dependence of Yop secretion (Fig. 1). These data indicate that Dam participates in the environmental regulation of the secretion of Yersinia virulence proteins.
FIG. 1.
Dam overproducer Y. pseudotuberculosis strains ectopically secrete Yops. Proteins were isolated from culture supernatants from wild-type (WT) and Dam overproducer (OP) Yersinia strains grown at the indicated temperatures in the presence (+Ca2+) or absence (−Ca2+) of calcium according to the method of Forsberg et al. (11). Cells were separated from the culture supernatant by centrifugation, supernatant proteins were precipitated with ammonium sulfate, and proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and visualized by Coomassie blue staining. Yops were identified according to the method of Hakansson et al. (16). Numbers refer to molecular weight standards (in thousands).
Dam-overproducing Y. pseudotuberculosis strains confer protective immune responses in mice.
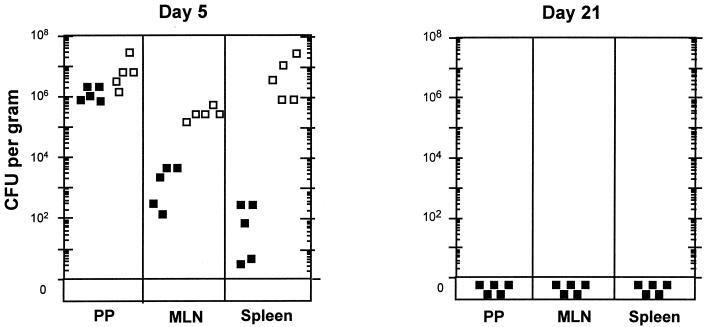
Because Dam-overproducing Y. pseudotuberculosis strains were attenuated for virulence, we determined whether they could serve as live attenuated vaccines against murine bacteremia. BALB/c mice were perorally immunized via gastrointubation with a dose of 9.3 × 109 cells of the Y. pseudotuberculosis Dam-overproducing strain MT2294, as described in footnote a of Table 2. Eight weeks later, the immunized mice were challenged perorally with 2.6 × 1010 wild-type Y. pseudotuberculosis cells. All (13 of 13) mice immunized with Dam-overproducing Y. pseudotuberculosis survived a wild-type Yersinia challenge of >1,000-fold above the LD50. None of 14 unimmunized mice survived the challenge dose. The protection conferred was significant according to the two-tailed Fisher exact test (P < 0.05). Mice immunized with the Yersinia Dam-overproducing strain were cleared of the vaccine between day 5 and day 21 and thus Dam-overproducing Y. pseudotuberculosis cells were not present at the time of challenge (S. M. Julio et al., submitted for publication). Moreover, vaccinated animals blocked the proliferation of virulent (Dam+) Yersinia in Peyer's patches, mesenteric lymph nodes, and spleens and cleared these virulent bacteria by 21 days postinfection (Fig. 2). These data suggest that mice vaccinated with Yersinia Dam-overproducing strains hinder the proliferation of virulent Yersinia in mucosal and systemic tissues, similar to what has been observed in mice vaccinated with Salmonella Dam mutants (19).
FIG. 2.
Immunization with Dam overproducer Yersinia strains blocks the proliferation of virulent Y. pseudotuberculosis in mucosal and systemic tissues in mice. Virulent Y. pseudotuberculosis cells (3.2 × 109) were orally administered to nonvaccinated BALB/c mice (open boxes) or to mice vaccinated with 7.6 × 109 Dam overproducer Y. pseudotuberculosis cells (closed boxes). Vaccinated mice were orally challenged 8 weeks postimmunization. The fate of the virulent organisms was determined in host tissues at the times indicated after challenge. All nonvaccinated mice were dead by day 10. Mice immunized with the Yersinia Dam-overproducing strain were cleared of the vaccine between day 5 and day 21 and thus Dam-overproducing Y. pseudotuberculosis cells were not present at the time of challenge (Julio et al., submitted). PP, Peyer's patches; MLN, mesenteric lymph nodes.
DISCUSSION
DNA adenine methylase plays a pivotal role in many bacterial functions, including the replication, repair, transposition, and segregation of chromosomal DNA (26, 28). Additionally, in Salmonella, Dam is a global regulator of bacterial gene expression and plays a critical role in virulence, and mutants with altered levels of Dam activity elicit protective immune responses to murine typhoid fever (12, 20). Here we explored the role of Dam in the pathogenesis of two other enteric bacteria, Y. pseudotuberculosis and V. cholerae. In contrast to results of studies performed with E. coli and Salmonella (4, 28), Dam was found to be essential for viability in Yersinia and Vibrio. Dam overproduction attenuated the virulence of Y. pseudotuberculosis and V. cholerae, leading to, in Yersinia, a fully protective immune response in vaccinated hosts. Since mutations in Dam attenuate the virulence of several diverse pathogens, the role of DNA methylation in virulence may emerge as a common theme in bacterial pathogenesis.
Dam's essential role in the viability of Y. pseudotuberculosis and V. cholerae, which are members of the gamma subdivision of proteobacteria, parallels the essential role of CcrM (cell cycle-regulated methyltransferase) for the viability of several proteobacteria of the alpha subdivision, including C. crescentus, R. meliloti, B. abortus, and A. tumefaciens (23, 35, 39, 44). Both Dam and CcrM catalyze the transfer of a methyl group from SAM to the N-6 position of adenine at specific target sequences within DNA. However, the target sequences of these two enzymes are different: Dam methylates GATC sequences, and CcrM methylates GANTC sequences. Moreover, both the catalytic and SAM binding domains of Dam and CcrM are arranged in a different linear order. For these reasons, Dam and CcrM belong to different methyltransferase groups. Despite these differences, DNA adenine methylation may exert its effects on diverse bacteria via its role as a global regulator of gene expression. That is, Dam regulates many (>20) Salmonella genes that are specifically induced during infection (20), and CcrM autoregulates ccrM transcription and has been implicated in the regulation of a number of genes involved in normal cell cycle progression (33, 34). Thus, the role of DNA methylation in regulating gene expression may explain, in part, Dam's function in many cellular processes of diverse bacteria.
DNA methylation plays a role in the virulence of a wide range of pathogens, including Salmonella spp. and B. abortus (which causes fetal-calf abortion) via Dam and CcrM activity, respectively (20, 26, 35). Dam also plays a role in Salmonella invasion, M-cell cytotoxicity, and the secretion of Salmonella virulence-associated proteins (12). Here we show that overproduction of Dam completely attenuated Y. pseudotuberculosis virulence and resulted in the export of Yops under conditions not normally permissive for secretion. Specifically, Dam overproduction relaxed the temperature dependence but not the low calcium dependence of Yop secretion, suggesting that Dam contributes to the strict environmental regulation governing the synthesis and/or secretion of Yersinia virulence proteins. Such altered protein secretion may attenuate the virulence of Y. pseudotuberculosis, as has been suggested for Salmonella (12). Moreover, the ectopic secretion of immunogens may contribute to the heightened immunity in hosts vaccinated with Dam mutants of Yersinia or Salmonella (19, 20).
The role of Dam in virulence and in the elicitation of protective immune responses may rely on its capacity as a global regulator of gene expression (19, 20, 24–26). Insights into the regulatory role of Dam have resulted from studies involving the E. coli pyelonephritis-associated pilus (pap) operon, which encodes pili that are required for infection of the urinary tract. The expression of pap genes is reversibly switched between the unexpressed state and the expressed state by a methylation-sensitive process termed phase variation (42). The reversible transition from non-pilus expressing to pilus expressing may allow the bacteria to attach and detach from urogenital tissues, enabling initial colonization and infection of the bladder and subsequent colonization and infection of the kidney, causing cystitis and pyelonephritis, respectively. Dam target sites (GATC sequences) in the pap promoter are protected from methylation by the binding of regulatory proteins at or near these sites, forming specific DNA methylation patterns analogous to what has been observed in eukaryotes (3, 17, 21, 41).
DNA methylation can modulate gene expression by altering the affinity of regulatory proteins for DNA, and, conversely, regulatory proteins can bind to nonmethylated Dam target sites, protecting these sites from methylation. Dysregulation of Dam activity can disable the ability of a pathogen to cause disease via aberrant virulence gene expression and contribute to the heightened immunity in vaccinated hosts through the ectopic production of an expanded repertoire of potential antigens. While a concern of this approach is that Dam overproducer strains can revert to wild-type virulence by mutation, insertion of multiple nontandem copies of Dam-overproducing cassettes in the chromosome should reduce the likelihood of this undesired scenario. Because the Dam methylase is essential for bacterial virulence or viability in multiple gram-negative pathogens (24, 26), Dam inhibitors are a promising target for antimicrobial drug development.
ACKNOWLEDGMENTS
This work was supported by private donations from Jim and Deanna Dehlsen, University of California Biotech Program, the Santa Barbara Cottage Hospital Research Program, USDA grant 2000-02539 (to M.J.M.), National Institutes of Health (NIH) grant AI23348 (to D.A.L.), NIH grant AI43486 (to K.E.K.), NIH training grant AI07271-15 (to D.P.), and a postdoctoral grant from the Cancer Center of Santa Barbara (to D.M.H.).
REFERENCES
- 1.Bandyopadhyay R, Das J. The DNA adenine methyltransferase-encoding gene (dam) of Vibrio cholerae. Gene. 1994;140:67–71. doi: 10.1016/0378-1119(94)90732-3. [DOI] [PubMed] [Google Scholar]
- 2.Blattner F R, Plunkett III G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 3.Braaten B A, Nou X, Kaltenbach L S, Low D A. Methylation patterns in pap regulatory DNA control pyelonephritis-associated pili phase variation in E. coli. Cell. 1994;76:577–588. doi: 10.1016/0092-8674(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 4.Casadesus J, Torreblanca J. Methylation-related epigenetic signals in bacterial DNA. In: Russo V E A, Martienssen R A, Riggs A D, editors. Epigenetic mechanisms of gene regulation. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 141–153. [Google Scholar]
- 5.Conner C P, Heithoff D M, Julio S M, Sinsheimer R L, Mahan M J. Differential patterns of acquired virulence genes distinguish Salmonella strains. Proc Natl Acad Sci USA. 1998;95:4641–4645. doi: 10.1073/pnas.95.8.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornelis G R, Boland A, Boyd A P, Geuijen C, Iriarte M, Neyt C, Sory M P, Stainier I. The virulence plasmid of Yersinia, an antihost genome. Microbiol Mol Biol Rev. 1998;62:1315–1352. doi: 10.1128/mmbr.62.4.1315-1352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Correa N E, Lauriano C M, McGee R, Klose K E. Phosphorylation of the flagellar regulatory protein FlrC is necessary for Vibrio cholerae motility and enhanced colonization. Mol Microbiol. 2000;35:743–755. doi: 10.1046/j.1365-2958.2000.01745.x. [DOI] [PubMed] [Google Scholar]
- 8.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faruque S M, Albert M J, Mekalanos J J. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol Mol Biol Rev. 1998;62:1301–1314. doi: 10.1128/mmbr.62.4.1301-1314.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J F, Dougherty B A, Merrick J M, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 11.Forsberg A, Bolin I, Norlander L, Wolf-Watz H. Molecular cloning and expression of calcium-regulated, plasmid-coded proteins of Y. pseudotuberculosis. Microb Pathog. 1987;2:123–137. doi: 10.1016/0882-4010(87)90104-5. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Del Portillo F, Pucciarelli M G, Casadesus J. DNA adenine methylase mutants of Salmonella typhimurium show defects in protein secretion, cell invasion, and M cell cytotoxicity. Proc Natl Acad Sci USA. 1999;96:11578–11583. doi: 10.1073/pnas.96.20.11578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geier G E, Modrich P. Recognition sequence of the dam methylase of Escherichia coli K12 and mode of cleavage of Dpn I endonuclease. J Biol Chem. 1979;254:1408–1413. [PubMed] [Google Scholar]
- 14.Glickman B, van den Elsen P, Radman M. Induced mutagenesis in dam− mutants of Escherichia coli: a role for 6-methyladenine residues in mutation avoidance. Mol Gen Genet. 1978;163:307–312. doi: 10.1007/BF00271960. [DOI] [PubMed] [Google Scholar]
- 15.Guan K L, Dixon J E. Protein tyrosine phosphatase activity of an essential virulence determinant in Yersinia. Science. 1990;249:553–556. doi: 10.1126/science.2166336. [DOI] [PubMed] [Google Scholar]
- 16.Hakansson S, Schesser K, Persson C, Galyov E E, Rosqvist R, Homble F, Wolf-Watz H. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact-dependent membrane disrupting activity. EMBO J. 1996;15:5812–5823. [PMC free article] [PubMed] [Google Scholar]
- 17.Hale W B, van der Woude M W, Low D A. Analysis of nonmethylated GATC sites in the Escherichia coli chromosome and identification of sites that are differentially methylated in response to environmental stimuli. J Bacteriol. 1994;176:3438–3441. doi: 10.1128/jb.176.11.3438-3441.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heithoff D M, Conner C P, Hanna P C, Julio S M, Hentschel U, Mahan M J. Bacterial infection as assessed by in vivo gene expression. Proc Natl Acad Sci USA. 1997;94:934–939. doi: 10.1073/pnas.94.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heithoff D M, Enioutina E Y, Daynes R A, Sinsheimer R L, Low D A, Mahan M J. Salmonella DNA adenine methylase mutants confer cross-protective immunity. Infect Immun. 2001;69:6725–6730. doi: 10.1128/IAI.69.11.6725-6730.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heithoff D M, Sinsheimer R L, Low D A, Mahan M J. An essential role for DNA adenine methylation in bacterial virulence. Science. 1999;284:967–970. doi: 10.1126/science.284.5416.967. [DOI] [PubMed] [Google Scholar]
- 21.Hendrich B, Bird A. Mammalian methyltransferases and methyl-CpG-binding domains: proteins involved in DNA methylation. Curr Top Microbiol Immunol. 2000;249:55–74. doi: 10.1007/978-3-642-59696-4_4. [DOI] [PubMed] [Google Scholar]
- 22.Julio S M, Conner C P, Heithoff D M, Mahan M J. Directed formation of chromosomal deletions in Salmonella typhimurium: targeting of specific genes induced during infection. Mol Gen Genet. 1998;258:178–181. doi: 10.1007/s004380050721. [DOI] [PubMed] [Google Scholar]
- 23.Kahng L S, Shapiro L. The CcrM DNA methyltransferase of Agrobacterium tumefaciens is essential, and its activity is cell cycle regulated. J Bacteriol. 2001;183:3065–3075. doi: 10.1128/JB.183.10.3065-3075.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Low D A, Weyland N J, Mahan M J. Roles of DNA methylation in regulating bacterial gene expression and virulence. Infect Immun. 2001;69:7197–7204. doi: 10.1128/IAI.69.12.7197-7204.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahan M J, Heithoff D M, Sinsheimer R L, Low D A. Assessment of bacterial pathogenesis by analysis of gene expression in the host. Annu Rev Genet. 2000;34:139–164. doi: 10.1146/annurev.genet.34.1.139. [DOI] [PubMed] [Google Scholar]
- 26.Mahan M J, Low D A. DNA methylation regulates bacterial gene expression and virulence. ASM News. 2001;67:356–361. [Google Scholar]
- 27.Mahan M J, Slauch J M, Mekalanos J J. Selection of bacterial virulence genes that are specifically induced in host tissues. Science. 1993;259:686–688. doi: 10.1126/science.8430319. [DOI] [PubMed] [Google Scholar]
- 28.Marinus M G. Methylation of DNA, edition VIII. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 782–791. [Google Scholar]
- 29.Marinus M G, Poteete A, Arraj J A. Correlation of DNA adenine methylase activity with spontaneous mutability in Escherichia coli K-12. Gene. 1984;28:123–125. doi: 10.1016/0378-1119(84)90095-7. [DOI] [PubMed] [Google Scholar]
- 30.Monack D M, Mecsas J, Bouley D, Falkow S. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J Exp Med. 1998;188:2127–2137. doi: 10.1084/jem.188.11.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmer L E, Hobbie S, Galan J E, Bliska J B. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNF-alpha production and downregulation of the MAP kinases p38 and JNK. Mol Microbiol. 1998;27:953–965. doi: 10.1046/j.1365-2958.1998.00740.x. [DOI] [PubMed] [Google Scholar]
- 32.Persson C, Nordfelth R, Holmstrom A, Hakansson S, Rosqvist R, Wolf-Watz H. Cell-surface-bound Yersinia translocate the protein tyrosine phosphatase YopH by a polarized mechanism into the target cell. Mol Microbiol. 1995;18:135–150. doi: 10.1111/j.1365-2958.1995.mmi_18010135.x. [DOI] [PubMed] [Google Scholar]
- 33.Reisenauer A, Kahng L S, McCollum S, Shapiro L. Bacterial DNA methylation: a cell cycle regulator? J Bacteriol. 1999;181:5135–5139. doi: 10.1128/jb.181.17.5135-5139.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reisenauer A, Quon K, Shapiro L. The CtrA response regulator mediates temporal control of gene expression during the Caulobacter cell cycle. J Bacteriol. 1999;181:2430–2439. doi: 10.1128/jb.181.8.2430-2439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robertson G T, Reisenauer A, Wright R, Jensen R B, Jensen A, Shapiro L, Roop R M., II The Brucella abortus CcrM DNA methyltransferase is essential for viability, and its overexpression attenuates intracellular replication in murine macrophages. J Bacteriol. 2000;182:3482–3489. doi: 10.1128/jb.182.12.3482-3489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosqvist R, Bölin I, Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect Immun. 1988;56:2139–2143. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosqvist R, Forsberg A, Rimpilainen M, Bergman T, Wolf-Watz H. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol Microbiol. 1990;4:657–667. doi: 10.1111/j.1365-2958.1990.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 38.Rosqvist R, Magnusson K E, Wolf-Watz H. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 1994;13:964–972. doi: 10.1002/j.1460-2075.1994.tb06341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stephens C, Reisenauer A, Wright R, Shapiro L. A cell cycle-regulated bacterial DNA methyltransferase is essential for viability. Proc Natl Acad Sci USA. 1996;93:1210–1214. doi: 10.1073/pnas.93.3.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Straley S C, Plano G V, Skrzypek E, Haddix P L, Fields K A. Regulation by Ca2+ in the Yersinia low-Ca2+ response. Mol Microbiol. 1993;8:1005–1010. doi: 10.1111/j.1365-2958.1993.tb01644.x. [DOI] [PubMed] [Google Scholar]
- 41.Tavazoie S, Church G M. Quantitative whole-genome analysis of DNA-protein interactions by in vivo methylase protection in E. coli. Nat Biotechnol. 1998;16:566–571. doi: 10.1038/nbt0698-566. [DOI] [PubMed] [Google Scholar]
- 42.van der Woude M, Braaten B, Low D. Epigenetic phase variation of the pap operon in Escherichia coli. Trends Microbiol. 1996;4:5–9. doi: 10.1016/0966-842x(96)81498-3. [DOI] [PubMed] [Google Scholar]
- 43.Wang R F, Kushner S R. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene. 1991;100:195–199. [PubMed] [Google Scholar]
- 44.Wright R, Stephens C, Shapiro L. The CcrM DNA methyltransferase is widespread in the alpha subdivision of proteobacteria, and its essential functions are conserved in Rhizobium meliloti and Caulobacter crescentus. J Bacteriol. 1997;179:5869–5877. doi: 10.1128/jb.179.18.5869-5877.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]