Abstract
To investigate the relationship between small non‐coding RNA‐204‐3p (miR‐204‐3p) and the onset and wound healing of diabetic foot ulcers (DFU) and the underlying molecular mechanism, sixty four newly diagnosed patients with T2DM without DFU (T2DM group), 82 T2DM patients with DFU (DFU group), and 60 controls with normal glucose tolerance (NC group) were included. Quantitative real‐time PCR (qRT‐PCR) method was used to determine miR‐204‐3p expression levels in peripheral blood and wound margin tissue of subjects, and to analyse the relationship between the expression of miR‐204‐3p and wound healing. In vitro experiments were also performed to understand the effect of miR‐204‐3p on high glucose induced injury of HaCaT cells (human keratinocytes). The results showed that miR‐204‐3p expression level of peripheral blood in the T2DM group was marked lower than that in the NC group [2.38 (1.31‐5.04) vs 3.27 (1.51‐6.98)] (P < .05). Similarly, the miR‐204‐3p expression level of peripheral blood in the DFU group was significantly lower than the T2DM group [1.15 (0.78‐2.89) vs 2.38 (1.31‐5.04)] (P < .01). The expression level of miR‐204‐3p in peripheral blood and wound margin tissues of DFU patients was positively correlated with the healing rate of foot ulcers after 8 weeks (P < .05). Multifactorial logistic regression analysis showed that decreased expression of miR‐204‐3p in peripheral blood was an independent risk factor for DFU (OR = 2.95, P < .05). The results of in vitro experiments showed that miR‐204‐3p could improve the proliferation and migration of HKC cells and reduce the proportion of apoptosis of HKC cells by targeted regulation of zinc finger protein Kruppel like factor 6 (KLF6) in high glucose environment. Therefore, the decreased expression of miR‐204‐3p in peripheral blood and wound tissue of T2DM patients is closely related to the occurrence and poor wound healing of DFU. The down‐regulated expression of miR‐204‐3p can reduce its ability to antagonise the functional damage of keratinocytes induced by high‐glucose conditions. These results will provide potential targets for the treatment of DFU.
Keywords: foot ulcer, human keratinocytes, KLF6, miR‐204‐3p, type 2 diabetes
Abbreviations
- ABI
ankle brachial index
- AGEs
advanced glycation end products
- CRP
C‐reactive protein
- DFU
diabetic foot ulcer; ESR, erythrocyte sedimentation rate
- FPG
fasting plasma glucose
- HbA1c
glycosylated haemoglobin A1c
- HKCs
human keratinocytes
- IL‐1
interleukin‐1
- KLF6
zinc‐finger transcription factor Krüppel‐like factor 6
- MMP‐9
matrix metalloproteinase‐9
- qRT‐PCRq
quantitative real‐time PCR assays
- SUC
skin ulcer control with normal glucose tolerance
- TGF‐β
transforming growth factor β
- WBC
white blood cell count
1. INTRODUCTION
Diabetes mellitus is a metabolic disease characterised by elevated blood glucose levels. Long‐term high blood glucose levels can cause a variety of chronic complications in patients with diabetes mellitus, and diabetic foot ulcers (DFUs) are one such complication. A one‐year observational cohort study of 1333 diabetic patients older than 50 years in eight hospitals in China showed that the annual incidence of DFU was 8.1%. 1 DFU is one of the leading causes of non‐traumatic amputation. Due to the adverse effects of high‐glucose conditions in diabetic patients and obvious changes in the skin microenvironment, the healing of DFUs becomes delayed or difficult, rendering healing chronically challenging. 2
Cutaneous wound healing is a dynamic and orderly physiological process including several stages of the inflammatory response, such as cell proliferation or connective tissue formation, wound contraction and wound covering, with re‐epithelialisation as an important part of wound repair. 3 , 4 Human keratinocytes (HKCs) are the main constituent cells of the epidermis, accounting for more than 90% of epidermal cells. Recent studies have shown that the interactions between the epidermis and mesenchyme, especially keratinocyte‐fibroblast interactions, play an extremely important role in wound healing and scar formation. 5 Keratinocytes affect the wound healing process by secreting many cytokines, including transforming growth factor β (TGF‐β), interleukin‐1 (IL‐1), and matrix metalloproteinases. 6 Among these cytokines, TGF‐β can promote fibroblast proliferation and collagen synthesis and secretion, and matrix metalloproteinase‐9 (MMP‐9) is an enzyme with the largest relative molecular weight among the matrix metalloproteinase superfamily and can degrade extracellular matrix components. 7
In vitro studies have shown that hyperglycaemia can inhibit the migration and proliferation of HKCs and promote their apoptosis. 8 , 9 Lafosse et al also found that the ability of HKCs to secrete factors that promote wound healing was reduced under high‐glucose conditions. 10 Advanced glycation end products (AGEs) are stable covalent adducts produced by a series of reactions between the amino groups of macromolecular substances (such as proteins, lipids, and nucleic acids) and aldehyde groups of glucose or other reducing sugars under non‐enzymatic conditions. In a high‐glucose environment, AGEs can accumulate and act on the AGE receptor (RAGE), affecting cell structure and function. Studies have shown that AGEs can inhibit the migration and proliferation of HKCs 11 and upregulate the expression of MMP‐9 in HKCs. 12 GADD45a is a gene whose expression is induced by DNA damage and can regulate many signalling pathways, such as those of apoptosis and survival. Zhou et al 13 found that the expression of GADD45a is increased in the foot ulcer wounds of diabetic patients and the skin wounds of diabetic model rats; furthermore, in vitro studies indicated that AGEs can dramatically upregulate the expression of GADD45a in HKCs, promote demethylation of the MMP‐9 promoter and its corresponding expression, and thus affect the healing of diabetic foot wounds. These studies indicate that hyperglycaemia can give rise to HKC impairment and then affect wound repair.
MicroRNAs (miRNAs) are a class of endogenous non‐coding small RNAs that regulate the expression of genes and/or proteins through posttranscriptional levels and participate in various biological processes, such as cell proliferation, apoptosis, cell cycle progression, and differentiation. 14 Studies 15 , 16 have confirmed that loss of the Dicer enzyme or the DGCR8 gene required for miRNA biosynthesis in the skin of newborn mice can lead to defects in skin barrier function, poor hair follicle development, and the excessive proliferation of basal cells of the epidermis, suggesting that miRNAs play critical roles in epidermal development and functional maintenance.
Etich et al 17 found that the expression of miR‐204 changed significantly during wound healing, but it is unclear whether it is involved in the regulation of wound healing. In an animal experiment, An et al 18 observed that miR‐204 expression is downregulated during the healing process of rat corneal ulcers; subsequently, in vitro studies showed that inhibiting miR‐204 expression can suppress the proliferation of rat corneal epithelial cells and promote apoptosis. Previous studies suggest that miR‐204 has beneficial effects on various physiological and pathological conditions, including cancer and cardiovascular disease. 19 , 20 Recently, miR‐204‐3p was reported to potentially play a protective role in high glucose‐induced podocyte apoptosis and dysfunction by downregulating the expression of the bradykinin B2 receptor (Bdkrb2). 21 However, studies assessing whether miR‐204‐3p can ameliorate or inhibit high glucose‐induced HKC dysfunction have not been reported.
Krüppel‐like factors (KLFs) are highly conserved zinc‐finger proteins that bind specific DNA sequences and regulate cell proliferation, apoptosis, differentiation, and metabolism. 22 Zinc‐finger transcription factor Krüppel‐like factor 6 (KLF6) is a member of the KLF family, is expressed abnormally in cutaneous melanoma 23 and psoriasis, 24 and is associated with wound healing. 25 At present, the research on whether KLF6 is involved in the functional changes of HKC in high glucose environment has also not been published.
Nowadays, there are few reports about the clinical research on the relationship between miR‐204‐3p and the incidence of DFU. Therefore, we are designed to explain the changes in the miR‐204‐3p expression level in the peripheral blood and wound margin tissue of DFU patients and their relationship with the onset and treatment outcome of DFU. Additionally, the possible mechanism of miR‐204‐3p involved in wound healing of DFU was explored in vitro experiments.
2. MATERIALS AND METHODS
2.1. Study subjects
A total of 82 patients with type 2 diabetes (T2DM) and DFU who were hospitalised in the Department of Endocrinology, the First Affiliated Hospital of Anhui Medical University, between April 2019 and December 2020, had a course of foot ulcer ≥4 weeks, an ankle‐brachial index (ABI) of 0.7 to 1.3, a ulcer area within 2 to 20 cm2 and a Wagner classification at II‐III grade (DFU group). Additionally, 64 newly diagnosed T2DM patients admitted to the Department of Endocrinology at our hospital at the corresponding period were selected as T2DM group. These patients had a course of T2MD ranging from 1 week to 5 months with no DFU, no lower extremity atherosclerosis disease, and no diabetic peripheral neuropathy. Moreover, 60 healthy people undergoing physical examination were selected from the Health Management Centre of our hospital in the same period as the normal control group (NC group). These participants established normal glucose tolerance by undergoing 75 g oral glucose tolerance test (OGTT), with a fasting plasma glucose (FPG) < 6.1 mmol/L and blood glucose <7.8 mmol/L 2 hours after glucose load. All subjects had no severe heart, liver, or kidney dysfunction or cancerous ulcer wounds. The study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Anhui Medical University, and all subjects provided informed consent.
3. STUDY METHODS
3.1. Treatment of diabetic foot ulcer and collection of ulcer margin tissue samples
As described by previous study, 26 all subjects underwent wound debridement after admission. During the debridement process, the full‐sickness skin tissue within 0.5 cm of the wound margin was cut by skilled surgeons using tissue scissors according to the sampling protocol. Meanwhile, all patients were given routine systemic treatment, including anti‐infection treatment, antihypertensive treatment, hypoglycemic treatment, correction of hypoproteinemia, neuroprotection treatment, etc. Offloading and negative‐pressure wound therapy could be administered in accordance with the specific conditions of patients. The complete wound healing of DFU patients was noted after 8 weeks of treatment.
3.2. Detection of observational indexes
Venous blood was drawn from the elbow of each subject at 8:00 to 8:30 am on the morning after a 10‐hours fast, and was collected into anticoagulant tubes or non‐anticoagulant tubes for determination of fasting plasma glucose (FPG), glycosylated haemoglobin A1c (HbA1c), white blood cell count (WBC), C‐reactive protein (CRP), erythrocyte sedimentation rate (ESR), plasma miR‐204‐3p (P‐miR‐204‐3p), lipid indicators, etc. In detail, the blood glucose and the blood lipid were measured using automatic biochemistry analyser (MODULE P800, Roche, Switzerland), FPG was detected by the glucose oxidase method, and the total cholesterol (TCH), triglyceride (TG), high‐density lipoprotein cholesterol (HDL‐C), and low‐density lipoprotein cholesterol (LDL‐C) were distinguished by oxidised enzyme‐linked colorimetry. Additionally, HbA1c by high‐pressure liquid chromatography, CRP by latex‐enhanced scattering immunoturbidimetric assay, and ESR by Westergren method. Ulcer area was measured by digital photography combined with Image J medical image analysis software (Image J‐ij133‐jdk15; National Institutes of Health, Bethesda, Maryland), and ABI was measured by Doppler blood flow detector (DPL‐03; Hangzhou Yuanxiang Medical Equipment Co., Ltd, China).
3.3. Cell culture
HKCs were obtained from the Cell Resource Centre of the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences. RPMI‐1640 medium (Gibco, USA) was supplemented with 10% foetal bovine serum (FBS, Sigma‐Aldrich, USA). HKCs were inoculated in a culture flask containing 10 mL of medium and cultured in a humidified incubator (Thermo, USA) at 37°C in a 5% CO2 and 95% air atmosphere, and the medium was changed every 2 to 3 days. Serum starvation was performed for 24 hours to synchronise the cells before experiments were performed.
3.4. Cell grouping and transfection
Synchronised HKCs were collected and treated with D‐glucose (Sigma‐Aldrich). Then, the cells were randomly divided into the normal glucose group (NG group, D‐glucose 5 mmol/L), the high‐glucose treatment group (HG group, D‐glucose 30 mmol/L), the miR‐204‐3p overexpression group and its relative control groups (the HG + miR‐204‐3p group, HG + miR‐NC group, and miR‐204‐3p‐ and miR‐NC‐transfected high glucose‐treated group), the KLF6 expression‐inhibition group and its control groups (the HG + si‐KLF6 group, HG + si‐NC6 group, and si‐KLF6‐ and si‐NC‐transfected high glucose‐treated group), and the miR‐204‐3p and KLF6 overexpression group and its relative control groups (the HG + miR‐204‐3p + pcDNA‐KLF6 group, HG + miR‐204‐3p + pcDNA group, and miR‐204‐3p and pcDNA‐KLF6 or miR‐204‐3p and pcDNA cotransfected high glucose‐treated group). Cell transfection was performed with Lipofectamine 2000 reagent (Invitrogen, USA) according to the manufacturer's instructions, and HKCs were cultured for 48 hours after transfection. Hsa‐miR‐204‐3p lentivirus (miR‐204‐3p) and negative control lentivirus (miR‐NC), as well as si‐KLF6, si‐NC, pcDNA, and pcDNA‐KLF6, were synthesised by GenePharma (Shanghai, China).
3.5. Apoptosis detection by flow cytometry
Cells from each group were digested with 0.25% trypsin (Sigma‐Aldrich), collected (1 × 106 cell/mL), and centrifuged at 1000 rpm for 5 minutes at room temperature. Then, the cells were resuspended in 500 μL of Annexin V binding buffer and 5 μL of Annexin V‐fluorescein isothiocyanate (FITC) stock solution (Beyotime, China), and the cells were incubated in the dark for 30 minutes at 4°C. Then, 5 μL of propidium iodide (PI; Beyotime) was added, and the cells were incubated for another 10 minutes. HKC apoptosis was quantified by flow cytometry (BD Biosciences).
3.6. Cell proliferation analysis by CCK‐8
Each group of cells in the logarithmic growth phase was digested with 0.25% trypsin and seeded in a 96‐well plate at 2000 cells/well. Then, the cells were incubated for 24 hours. The medium in each well was replaced with fresh medium, and 10 μL of CCK‐8 solution (Beyotime) was added. Subsequently, the plate was incubated in an incubator at 37°C in a 5% CO2 and 95% air atmosphere for 2 hours. The absorbance (OD) was recorded at 450 nm using a microplate reader (Eppendorf, Germany).
3.7. Cell migration assessment by transwell assay
Cells from each group were digested with 0.25% trypsin and collected (3 × 105 cell/mL). A 100‐μL cell suspension was seeded in the upper chamber of the Transwell chamber (8 μm, Corning, USA), 600 μL of medium was added to the lower chamber, and then the plate was incubated at 37°C for 12 hours. The Transwell chamber was removed from the incubator, the medium was discarded, the chamber was washed twice with PBS, fixed in 4% paraformaldehyde for 15 minutes, air‐dried at room temperature and stained with 0.1% crystal violet for 30 minutes. Cells on the upper surface of the Transwell filter were swabbed off and washed three times with PBS. Cells in five randomly selected fields were counted, and the cell migration rate was calculated by dividing the number of cells remaining on the filter by the initial number of cells seeded.
3.8. Detection of miR‐204‐3p and KLF6 expression by real‐time quantitative PCR assays
Real‐time quantitative PCR assays (qRT‐PCR) were performed to detect the expression of plasma miR‐204‐3p, miR‐204‐3p, and KLF6 in wound margin tissue and HKCs. RNA was extracted from peripheral blood, 50 mg of wound margin tissue or cultured HKCs according to the instructions of the miRcute miRNA extraction and separation kit (TIANGEN, Beijing, China), respectively. cDNA was then synthesised according to the instructions of miRcute miRNA cDNA synthesis kit (TIANGEN, Beijing, China). The primer sequences of miR‐204‐3p and KLF6 were as follows: miR‐204‐3p: forward primer 5′‐AGC TGT ACA AGT AAG CCT GAT CAT GTA CCC ATA GG‐3′ and reverse primer 5′‐GGG AGA GGG GCT TAG CTT ATG GGA CAG TTA TGG GC‐3′; U6: forward primer 5′‐GCT TCG GCA CAT ATA CTA AAA‐3′ and reverse primer 5′‐CGC TTC ACG AAT TTG CCT GTC AT‐3′; KLF6: forward primer 5′‐TGC TAC TTG AAA GCA CGC CA‐3′ and reverse primer 5′‐CAG CCT TGC CTT GAC CAT CT‐3′; GAPDH: forward primer 5′‐GGA GCG AGA TCC CTC CAA AAT‐3′ and reverse primer 5′‐GGC TGT CAT ACT TCT CAT GG‐3′.
qRT‐PCR was carried out according to the instructions of the miRcute miRNA fluorescence quantitative detection kit (TIANGEN, Beijing, China). The cycling conditions for miR‐204‐3p were: initial denaturation at 95°C for 5 minutes, denaturation at 95°C for 10 seconds, annealing at 65°C for 15 seconds, and extension at 72°C for 30 seconds, for a total of 40 cycles. With U6 as the internal reference, the relative expression of miR‐204‐3p was calculated based on the 2−ΔΔCt method. In addition, the cycling conditions for KLF6 were: initial denaturation at 95°C for 10 minutes, denaturation at 95°C for 15 seconds, annealing at 60°C for 50 seconds, and extension at 74°C for 30 seconds, for a total of 45 cycles. With GAPDH as the internal reference, the relative expression of KLF6 was calculated based on the 2−ΔΔCt method.
3.9. Protein expression detected by western blot
Proteins were extracted with radioimmunoprecipitation assay (RIPA) buffer (Beyotime Institute of Biotechnology) in cultured HKCs. Protein from each sample was separated on a 10% SDS‐PAGE gel at 55 V for approximately 30 minutes, followed by 110 V for 1 hours when the bromophenol blue indicator reached the separation gel, and then transferred to nitrocellulose membranes at 200 mA for 2 hours. The membranes were blocked with blocking solution containing 5% non‐fat dry milk (NFDM) prepared with TBST for 1 hour at room temperature and washed three times with TBST for 10 minutes per wash. The membranes were incubated with primary antibodies against KLF6 (dilution: 1:1000), MMP‐9 (dilution: 1:400), TGF‐β (dilution: 1:400), Bax (dilution: 1:1000), Bcl‐2 (dilution: 1:1000), and cleaved caspase‐3 (dilution: 1:1000) at 4°C overnight. Subsequently, the membranes were incubated with the appropriate horseradish peroxidase‐conjugated secondary antibody (dilution: 1:8000; Beyotime) at room temperature for 1 hours. Then, the developer (Thermo Fisher Scientific, Inc.) was added dropwise for development, and pictures were taken. GAPDH was used as the internal reference protein to calculate the relative expression of the proteins. The above primary antibodies were purchased from Cell Signalling Technology, Inc. USA.
3.10. Dual‐luciferase reporter gene assay
The nucleotide sequence complementary to miR‐204‐3p in the 3′‐untranslated region (3′‐UTR) of KLF6 was obtained using the online prediction software TargetScan (www.targetscan.org). The 3′‐UTR luciferase reporter gene vectors of wild‐type KLF6 (WT‐KLF6) and mutant KLF6 (MUT‐KLF6) were constructed and cotransfected with miR‐204‐3p or anti‐miR‐204‐3p, respectively. Then, luciferase activity was assessed with a luciferase reporter assay kit (GenePharma) according to the manufacturer's protocol.
3.11. Statistical analysis
SPSS version 22.0 (IBM SPSS Company, USA) was used for the statistical analysis. Normal measurement data is expressed by mean ± standard deviation, and non‐normal measurement data is expressed by median (interquartile range) [M (P 25, P75)]. The t‐test was used to analyse differences between two groups, and the comparison between multiple groups used the analysis of variance test, followed by the SNK‐q test. The count data was expressed as a percentage, and the χ2 test was performed. Spearman correlation analysis was used to evaluate the correlation between the expression of miR‐204‐3p and other clinical variables. A multivariate unconditional logistic regression method was used to assess whether miR‐204‐3p is an independent risk factor for DFU. All statistical tests were two‐sided, and P < .05 represents a significant difference. Each in vitro independent experiment was repeated at least three times.
4. RESULTS
4.1. Comparisons of clinical data among NC group, T2DM group, and DFU group
There were no significant differences found in the gender composition, age, SBP, DBP, TCH, and LDL‐C among the three groups (P > .05). Compared with the NC group, there was a significant increase in FPG, HbA1c, TG levels in the T2DM group and DFU group (P < .05), while HDL‐C level and P‐miR‐204‐3p decreased significantly (P < .05). Besides, compared with the NC group, inflammatory indices (CRP, WBC, ESR) were significantly increased, while ABI was significantly decreased in the DFU group (P < .05). No significant difference was observed in ABI and inflammatory indices (CRP, WBC, ESR) between the NC group and T2DM group. Additionally, compared with the T2DM group, the duration of diabetes, FPG, HbA1c, and inflammatory indices (CRP, WBC, ESR) in the DFU group were significantly increased (P < .05), while ABI, HDL‐C level and P‐miR‐204‐3p expression were significantly decreased (P < .05), and there were no significant differences observed in TG between the T2DM group and DFU group (P > .05). The detailed results are shown in Table 1.
TABLE 1.
Comparison of clinical data among NC group, T2DM group, and DFU group [n (%), ( ± s), M (P25, P75)]
| Variable | NC group (n = 60) | T2DM group (n = 64) | DFU group (n = 82) | P value |
|---|---|---|---|---|
| Male [n (%)] | 33 (55.0) | 34 (53.1) | 44 (53.7) | .795 |
| Age (year) | 52.1 ± 8.9 | 53.3 ± 8.7 | 52.6 ± 8.5 | .308 |
| Duration of diabetes (year) | ‐ | 0.4 ± 0.2 | 11.7 ± 5.8 | <.001 |
| Ulcer duration (week) | ‐ | ‐ | 7.8 ± 2.4 | ‐ |
| Ulcer area (cm2) | ‐ | ‐ | 10.7 ± 3.6 | ‐ |
| SBP (mm Hg) | 119 ± 9 | 123 ± 12 | 125 ± 13 | .103 |
| DBP (mm Hg) | 73 ± 12 | 76 ± 13 | 77 ± 11 | .361 |
| FPG (mmol/L) | 4.9 ± 0.5 | 9.7 ± 2.3b | 11.5 ± 3.3bd | <.001 |
| HbA1c (%) | 5.2 ± 0.3 | 8.2 ± 1.4b | 9.4 ± 1.4bd | <.001 |
| TG (mmol/L) | 1.5 ± 0.7 | 1.7 ± 0.5a | 1.8 ± 0.7b | .031 |
| TCH (mmol/L) | 4.3 ± 0.7 | 4.9 ± 0.6 | 4.7 ± 0.5 | .187 |
| LDL‐C (mmol/L) | 2.5 ± 0.2 | 3.2 ± 0.4 | 2.6 ± 0.3 | .096 |
| HDL‐C (mmol/L) | 1.4 ± 0.2 | 1.2 ± 0.3a | 0.9 ± 0.4bd | <.001 |
| ABI | 1.1 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.2bd | <.001 |
| CRP (mg/L) | 6.3 ± 0.9 | 8.7 ± 1.1 | 30.5 ± 2.4bd | <.001 |
| WBC (×109) | 4.3 ± 0.7 | 4.6 ± 0.8 | 10.2 ± 2.2bd | <.001 |
| ESR (mm/h) | 10.3 ± 2.2 | 12.6 ± 2.8 | 45.7 ± 15.9bd | <.001 |
| P‐miR‐204‐3p | 3.27 (1.51‐6.98) | 2.38 (1.31‐5.04)a | 1.15 (0.78‐2.89)bd | <.001 |
| T‐miR‐204‐3p | ‐ | ‐ | 2.29 (1.11‐4.57) | ‐ |
| T‐KLF6 | ‐ | ‐ | 2.924 (1.47‐4.02) | ‐ |
Note: Data are presented mean ± standard deviations or numbers (%) or median with IQR; Differences among four groups analysed using one‐way analysis of variance or x 2 test, and the SNK‐q test analysis was used for comparison between the two groups. Versus NC group, a P < .05, b P < .01; vs T2DM group, c P < .05, d P < .01. T‐miR‐204‐3p, miR‐204‐3p expression in the wound margin tissue; T‐KLF6, KLF6 mRNA expression in the wound margin tissue.
Abbreviations: ABI, ankle brachial index; CRP, C‐reactive protein; DBP, diastolic blood pressure; DFU, diabetic foot ulcer; ESR, erythrocyte sedimentation rate; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; MiR, MicroRNA; NC, normal control; P‐miR‐204‐3p, plasma miR‐204‐3p expression; SBP, systolic blood pressure; T2DM, type 2 diabetes; TCH, total cholesterol; TG, triacylglycerol; WBC, white blood cell.
4.2. Correlations between miR‐204‐3p expression level in plasma and wound margin tissue and other clinical data
As shown in Tables 2 and 3, both in the T2DM group and DFU group, P‐miR‐204‐3p and T‐miR‐204‐3p levels were negatively correlated with FPG, HbA1c (P < .05). In addition, in the DFU group, P‐miR‐204‐3p level was negatively correlated with T‐KLF6 level, and positively correlated with T‐miR‐204‐3p level, while P‐miR‐204‐3p and T‐miR‐204‐3p levels had no significant correlation with other indicators in the two groups (P > .05). Moreover, in the NC group, P‐miR‐204‐3p had no significant correlation with other indicators (P > .05).
TABLE 2.
Correlations between the expression levels of miR‐204‐3p in peripheral blood and other clinical data among NC group, T2DM group, and DFU group (r)
| Variables | NC (n = 60) | T2DM (n = 64) | DFU (n = 82) | |||
|---|---|---|---|---|---|---|
| r | P value | r | P value | r | P value | |
| Age | 0.039 | .621 | 0.018 | .726 | 0.057 | .501 |
| Sex | 0.084 | .458 | 0.027 | .603 | 0.096 | .351 |
| Duration of diabetes | ‐ | ‐ | −0.045 | .279 | −0.105 | .268 |
| Ulcer duration | ‐ | ‐ | ‐ | ‐ | −0.125 | .103 |
| Ulcer area | ‐ | ‐ | ‐ | ‐ | −0.104 | .278 |
| FPG | −0.179 | .086 | −0.363 | <.001 | −0.314 | .001 |
| HbA1c | −0.152 | .112 | −0.302 | .003 | −0.298 | .009 |
| TG | 0.037 | .655 | −0.168 | .105 | −0.098 | .232 |
| TCH | 0.048 | .583 | 0.088 | .237 | 0.071 | .311 |
| LDL‐C | 0.094 | .226 | 0.016 | .752 | 0.078 | .485 |
| HDL‐C | 0.028 | .702 | 0.148 | .115 | 0.114 | .124 |
| ABI | 0.081 | .473 | 0.084 | .326 | 0.198 | .068 |
| CRP | 0.084 | .448 | −0.079 | .396 | −0.119 | .115 |
| WBC | 0.049 | .383 | 0.018 | .673 | −0.136 | .104 |
| ESR | 0.031 | .742 | 0.026 | .582 | −0.128 | .112 |
| T‐miR‐204‐3p | ‐ | ‐ | ‐ | ‐ | 0.483 | <.001 |
| T‐KLF6 | ‐ | ‐ | ‐ | ‐ | −0.373 | <.001 |
Note: T‐miR‐204‐3p, miR‐204‐3p expression in the wound margin tissue; T‐KLF6, KLF6 mRNA expression in the wound margin tissue.
Abbreviations: ABI, ankle brachial index; CRP, C‐reactive protein; DBP, diastolic blood pressure; DFU, diabetic foot ulcer; ESR, erythrocyte sedimentation rate; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; MiR, MicroRNA. P‐miR‐204‐3p, plasma miR‐204‐3p expression; NC, normal control; SBP, systolic blood pressure; T2DM, type 2 diabetes; TCH, total cholesterol; TG, triacylglycerol; WBC, white blood cell.
TABLE 3.
Correlations between the expression levels of miR‐204‐3p in wound margin tissue and other clinical data in the DFU group (r)
| Variables | DFU (n = 82) | |
|---|---|---|
| r | P value | |
| Age | 0.023 | .625 |
| Sex | 0.058 | .414 |
| Duration of diabetes | −0.097 | .231 |
| Ulcer duration | −0.101 | .167 |
| Ulcer area | −0.087 | .301 |
| FPG | −0.352 | .001 |
| HbA1c | −0.327 | .007 |
| TG | −0.106 | .121 |
| TCH | 0.089 | .296 |
| LDL‐C | 0.045 | .503 |
| HDL‐C | 0.119 | .102 |
| ABI | −0.118 | .109 |
| CRP | −0.109 | .147 |
| WBC | −0.127 | .101 |
| ESR | −0.101 | .152 |
| P‐miR‐204‐3p | 0.483 | <.001 |
| T‐KLF6 | −0.373 | <.001 |
Note: T‐KLF6, KLF6 mRNA expression in the wound margin tissue.
Abbreviations: ABI, ankle brachial index; CRP, C‐reactive protein; DBP, diastolic blood pressure; DFU, diabetic foot ulcer group; ESR, erythrocyte sedimentation rate; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; MiR, MicroRNA; NC, control group; P‐miR‐204‐3p, plasma miR‐204‐3p expression; SBP, systolic blood pressure; T2DM, type 2 diabetes group; TCH, total cholesterol; TG, triacylglycerol; WBC, white blood cell.
4.3. Relationship between miR‐204‐3p levels in plasma and wound margin tissue and the clinical features of diabetic foot ulcers
To further investigate the clinical significance of altered miR‐204‐3p expression levels in plasma and wound margin tissue, the median values of P‐miR‐204‐3p, and T‐miR‐204‐3p in the DFU group were used as the cut‐off point for grouping, namely, the low expression group (lower than the cut‐off point) and the high expression group (higher than or equal to the cut‐off point). It was found that P‐miR‐204‐3p and T‐miR‐204‐3p were positively correlated with the healing rate of foot ulcer after 8 weeks (for P‐miR‐204‐3p, P = .022; for T‐miR‐204‐3p, P = .010). There was no correlation between P‐miR‐204‐3p and T‐miR‐204‐3p and other clinical features of foot ulcers (Tables 4 and 5).
TABLE 4.
Relationship between the expression levels of miR‐204‐3p in peripheral blood and clinical features of DFU [( ± s), n (%)]
| DFU | |||
|---|---|---|---|
| High expression group (n = 43) | Low expression group (n = 39) | P value | |
| Age (year) | 52.3 ± 8.7 | 53.1 ± 9.5 | .236 |
| Sex | .681 | ||
| Male | 24 (55.8) | 20 (51.3) | |
| Female | 19 (44.2) | 19 (48.7) | |
| Ulcer area (cm2) | .967 | ||
| ≤5 | 8 (18.6) | 7 (17.9) | |
| 5‐10 | 22 (51.2) | 20 (51.3) | |
| >10 | 13 (30.2) | 12 (30.8) | |
| Ulcer duration (week) | .273 | ||
| ≤6 | 13 (30.2) | 6 (15.4) | |
| 6‐10 | 20 (46.5) | 23 (59.0) | |
| >10 | 10 (23.3) | 10 (25.6) | |
| Wagner grade | .985 | ||
| II | 10 (23.3) | 9 (23.1) | |
| III | 33 (66.7) | 30 (66.9) | |
| Outcome of ulcer after 8 weeks | .022 | ||
| Healing | 24 (55.8) | 12 (30.8) | |
| Non‐healing | 19 (44.2) | 27 (69.2) | |
Note: Data are presented mean ± standard deviations or numbers (%); Differences between two groups analysed using t test or χ2 test.
Abbreviations: DFU, diabetic foot ulcer; MiR, MicroRNA.
TABLE 5.
Relationship between the expression levels of miR‐204‐3p in wound margin tissue and clinical features of DFU [( ± s), n (%)]
| DFU | |||
|---|---|---|---|
| High expression group (n = 37) | Low expression group (n = 45) | P value | |
| Age (year) | 52.7 ± 9.2 | 53.5 ± 8.4 | .194 |
| Sex | .948 | ||
| Male | 20 (54.1) | 24 (53.3) | |
| Female | 17 (45.9) | 21 (46.7) | |
| Ulcer area (cm2) | .734 | ||
| ≤5 | 5 (13.5) | 9 (20.0) | |
| 5‐10 | 20 (54.1) | 23 (51.1) | |
| >10 | 12 (32.4) | 13 (28.9) | |
| Ulcer duration (week) | .542 | ||
| ≤6 | 10 (30.2) | 8 (15.4) | |
| 6‐10 | 19 (46.5) | 24 (59.0) | |
| >10 | 8 (23.3) | 13 (25.6) | |
| Wagner grade | .295 | ||
| II | 7 (23.3) | 13 (23.1) | |
| III | 30 (66.7) | 32 (66.9) | |
| Outcome of ulcer after 8 weeks | .010 | ||
| Healing | 22 (59.5) | 14 (31.1) | |
| Non‐healing | 15 (40.5) | 31 (68.9) | |
Note: Data are presented mean ± standard deviations or numbers (%); Differences between two groups analysed using t test or χ2 test.
Abbreviations: DFU, diabetic foot ulcer; MiR, MicroRNA.
4.4. Analysis of risk factors for a diatic foot ulcer
In diabetic patients, multifactorial unconditional logistic regression analysis was performed with DFU as the dependent variable, and gender, age, diabetic duration, SBP, DBP, FPG, HbA1c, TG, TCH, LDL‐C, HDL‐C, ABI, CRP, WBC, ESR, and P‐miR‐204‐3p as independent variables, respectively. The results showed that the duration of diabetes, HbA1c, ABI, CRP, and low expression of P‐miR‐204‐3p were all independent risk factors for DFU (Table 6).
TABLE 6.
Multiple logistic regression analysis of risk factors for diabetic foot ulcers
| Variable | β | SE | Wald | OR | 95% CI | P value |
|---|---|---|---|---|---|---|
| Diabetic duration (year) | 0.56 | 0.19 | 8.75 | 4.21 | 1.24‐9.25 | <.001 |
| HbA1c (%) | 0.39 | 0.22 | 3.26 | 1.83 | 1.13‐7.81 | .037 |
| CRP (mg/L) | 0.26 | 0.11 | 2.92 | 1.65 | 1.06‐10.42 | .041 |
| ABI | 0.32 | 0.18 | 3.86 | 1.77 | 1.12‐9.89 | .036 |
| P‐miR‐204‐3p | 0.44 | 0.26 | 5.72 | 2.95 | 1.18‐10.97 | .008 |
Note: Multivariate unconditional logistic regression analysis adjusted for sex, age, diabetic duration, ulcer duration, SBP, DBP, FPG, HbA1c, TG, TCH, LDL‐C, HDL‐C, ABI, CRP, WBC count, ESR, ulcer area, P‐miR‐204‐3p.
Abbreviations: ABI, ankle brachial index; CRP, C‐reactive protein; DBP, diastolic blood pressure; ESR, erythrocyte sedimentation rate; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; MiR, MicroRNA; P‐miR‐204‐3p, plasma miR‐204‐3p expression; SBP, systolic blood pressure; TCH, total cholesterol; TG, triacylglycerol; WBC, white blood cell.
4.5. High‐glucose conditions severely disturb expression patterns in HKCs
To explore the detrimental effects of high‐glucose levels on HKCs, we exposed HKCs to high‐glucose medium (30 mM) for 48 hours. The apoptosis rate was significantly increased in high glucose‐treated HKCs compared with the NG group (Figure 1A). High‐glucose conditions inhibited cell proliferation and migration (Figure 1B,C). To further elucidate the regulatory mechanisms involved, we measured the expression of TGF‐β, MMP‐9, Bcl‐2, Bax, and cleaved caspase‐3. Among these proteins, TGF‐β and MMP‐9 play crucial roles in wound healing, and Bcl‐2, Bax, and cleaved caspase‐3 are factors strongly associated with apoptosis. 27 , 28 The mRNA and protein expression of TGF‐β and Bcl‐2 were markedly reduced in high glucose‐treated HKCs, whereas the expression of MMP‐9, Bax, and cleaved caspase‐3 was increased (Figure 1H,I). Meanwhile, the expression levels of miR‐204‐3p and KLF6 were evaluated, showing a significant reduction in miR‐204‐3p (Figure 1D), whereas KLF6 (protein and mRNA) was correspondingly elevated (Figure 1E‐G).
FIGURE 1.
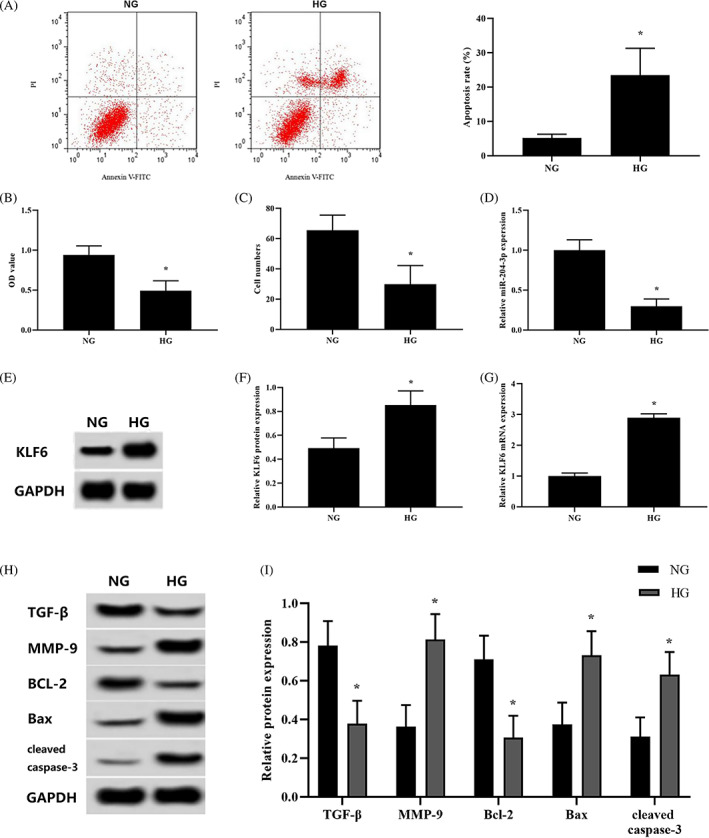
Response of HKCs to high‐glucose conditions. (A) Apoptosis was analysed using flow cytometry. (B) Cell viability was monitored by CCK‐8 assay. (C) Cell migration was tested using Transwell inserts. (D) The expression of miR‐204‐3p was detected by qRT‐PCR. (E‐G) The mRNA and protein expression of KLF6 were detected by qRT‐PCR and western blotting, respectively. (H and I) The protein expression of TGF‐β, MMP‐9, Bcl‐2, Bax, and cleaved caspase‐3 was detected by western blotting. *P < .05 vs the NG group; n = 3 in each group. Normal glucose, NG; high glucose, HG
4.6. Overexpression of miR‐204‐3p attenuates high glucose effects
To further investigate the role of miR‐204‐3p in high glucose‐treated HKCs, miR‐204‐3p lentivirus was transfected into high glucose‐treated HKCs. High glucose‐induced apoptosis was significantly reduced by overexpression of miR‐204‐3p (Figure 2A,B). The proliferation and migration of HKCs were observably improved (Figure 2C,D). In addition, overexpression of miR‐204‐3p prevented the high glucose‐induced downregulation of TGF‐β and Bcl‐2 protein expression in HKCs but markedly suppressed MMP‐9, Bax, and cleaved caspase‐3 protein expression (Figure 2F‐K).
FIGURE 2.
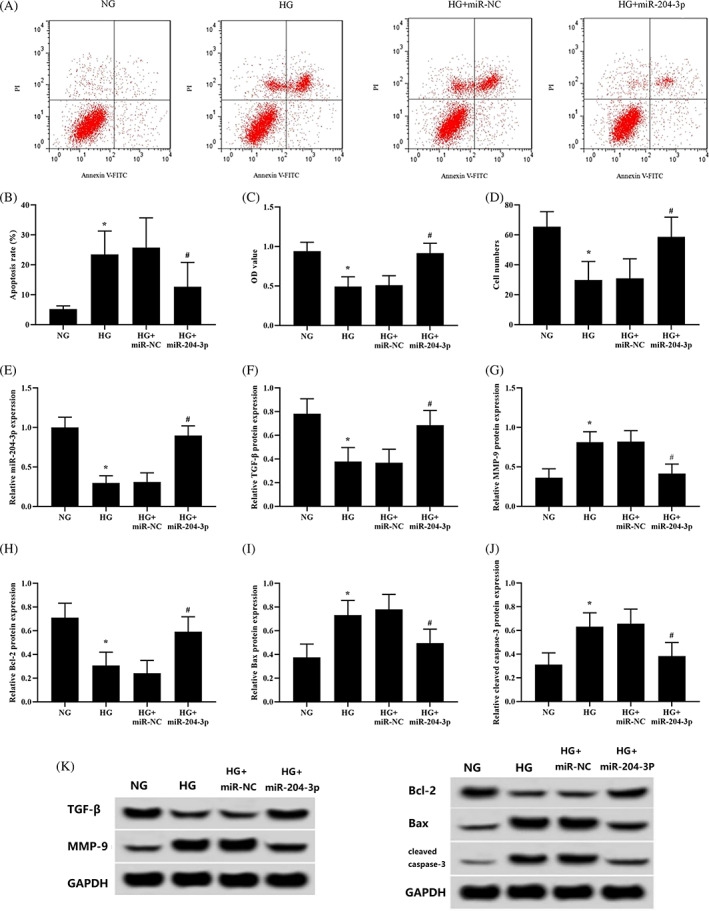
Overexpression of miR‐204‐3p attenuates HKCs' response to high‐glucose conditions. High glucose‐treated keratinocytes transfected with miR‐NC or miR‐204‐3p for 48 hours. (A and B) Apoptosis was analysed using flow cytometry (the NG group and HG group were the negative controls in the transfection experiment). (C) Cell proliferation was monitored by CCK‐8 assay. (D) Cell migration was tested using Transwell inserts. (E) The expression of miR‐204‐3p was detected by qRT‐PCR. (F‐K) The protein expression of TGF‐β, MMP‐9, Bcl‐2, Bax, and cleaved caspase‐3 was detected by western blotting; *P < .05 vs the NG group; # P < .05 vs the HG + miR‐NC group; n = 3 in each group
4.7. KLF6 inhibition alleviates high glucose effects
To evaluate the role KLF6 in high glucose‐induced HKC dysfunction, si‐NC‐ or si‐KLF6‐transfected HKCs were exposed to high‐glucose conditions (30 mM). High glucose‐induced apoptosis was significantly reduced (Figure 3A), and cell proliferation and migration were clearly enhanced (Figure 3B,C) when KLF6 expression was inhibited. Then, we detected the expression of TGF‐β, MMP‐9, and apoptosis‐related proteins by western blotting and discovered that the reduced expression of KLF6 (Figure 3D,I) significantly reduced the protein expression of MMP‐9 and Bax (Figure 3F,H,I). Correspondingly, the protein expression of TGF‐β, which promotes wound healing, and Bcl‐2, which inhibits apoptosis, was markedly increased (Figure 3E,G,I).
FIGURE 3.
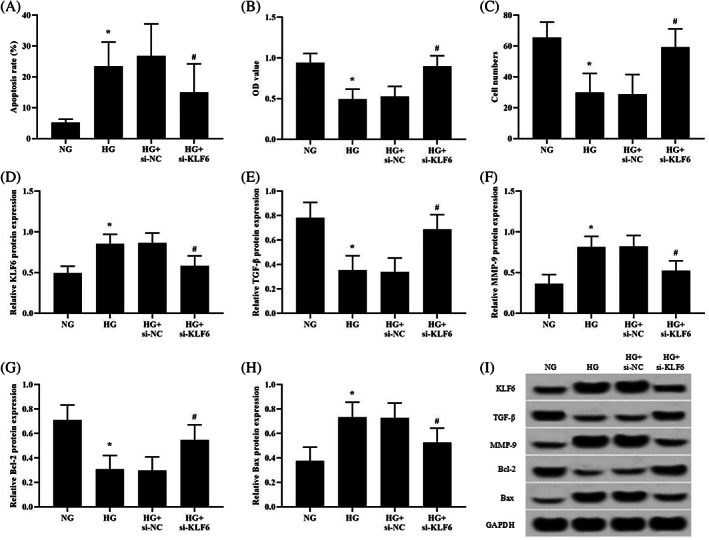
KLF6 inhibition attenuates the high glucose response of HKCs. (A) Apoptosis was analysed using flow cytometry (the NG group and HG group were the negative controls in the transfection experiment). (B) Cell viability was monitored by CCK‐8 assay. (C) Cell migration was tested using Transwell inserts. (D‐I) The protein expression of KLF6, TGF‐β, MMP‐9, Bcl‐2, and Bax was detected by western blotting. *P < .05 vs the NG group; # P < .05 vs the HG + si‐NC group; n = 3 in each group
4.8. The KLF6 gene has a direct miR‐204‐3p binding site
The DNA bases in the 3′‐UTR of KLF6 can form complementary pairs with those of miR‐204‐3p and were predicted using the online prediction software TargetScan (www.targetscan.org). We hypothesized that miR‐204‐3p has a regulatory effect on KLF6 expression. The putative miR‐204‐3p binding site in the 3′‐UTR of KLF6 is shown in Figure 4A. The wild‐type (WT) sequence of KLF6 or its mutant‐type (MUT) sequence was cotransfected with miR‐NC, miR‐204‐3p, anti‐miR‐NC, or anti‐miR‐204‐3p into luciferase reporter plasmids to verify that miR‐204‐3p targets the 3′‐UTR of KLF6. The luciferase reporter assay showed that transfection of miR‐204‐3p significantly decreased luciferase enzymatic activity using the WT‐ but not the MUT‐UTR (Figure 4B). The level of KLF6 was reduced accordingly, while after transfection with anti‐miR‐204‐3p, the level was upregulated (Figure 4C,D). Taken together, these results confirm that KLF6 is a direct target gene of miR‐204‐3p.
FIGURE 4.
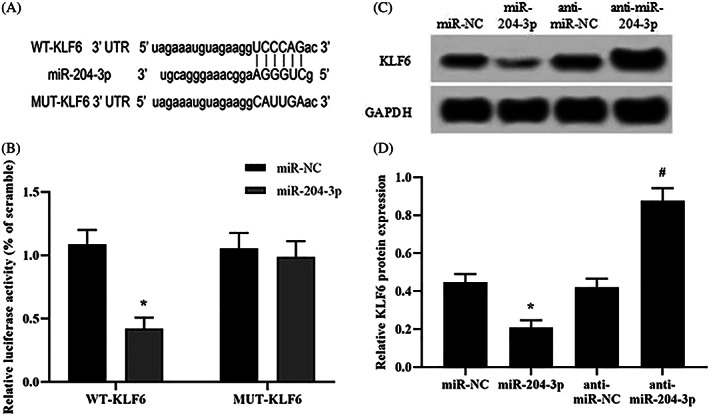
Functional proof for miR‐204 binding to the KLF6 UTR. (A) The putative binding sites in the 3′‐UTR of KLF6 and miR‐204‐3p predicted by online software. (B) The luciferase reporter assay was performed in HKCs with the wild‐type (WT) sequence of KLF6 or its mutant‐type (MUT) sequence and cotransfected with miR‐NC, miR‐204‐3p, anti‐miR‐NC or anti‐miR‐204‐3p in the luciferase reporter plasmids. (C and D) The protein expression of KLF6 was detected in HKCs transfected with miR‐204‐3p or anti‐miR‐204‐3p. *P < .05 vs the miR‐NC group; # P < .05 vs the anti‐miR‐NC group; n = 3 in each group
4.9. KLF6 overexpression neutralises the protective effect of miR‐204‐3p
We designed a study to further elucidate the role of KLF6 in the protective effect of miR‐204‐3p on high glucose‐induced HKCs. KLF6 overexpression abolished the protective effect of miR‐204‐3p overexpression on HKC apoptosis (Figure 5A). Moreover, the upregulation of cell proliferation and migration by miR‐204‐3p transfection was also reversed by KLF6 overexpression (Figure 5B,C). Additionally, KLF6 overexpression inhibited the upregulation of TGF‐β and Bcl‐2 protein expression and the downregulation of MMP‐9 and Bax protein expression in miR‐204‐3p‐transfected cells (Figure 5D‐I).
FIGURE 5.
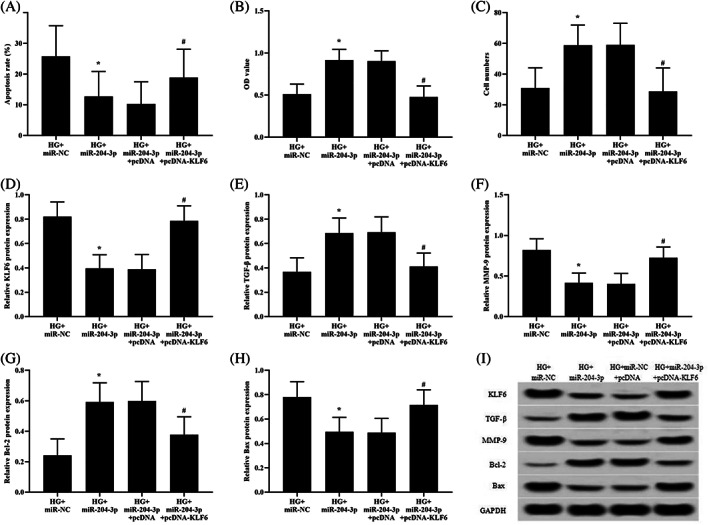
KLF6 overexpression neutralises the protective effect of miR‐204‐3p in high glucose‐treated HKCs. High glucose‐treated HKCs were cotransfected with miR‐204‐3p and pcDNA or miR‐204‐3p and pcDNA‐KLF6 for 48 hours. (A) Apoptosis was analysed using flow cytometry. (B) Cell viability was monitored by CCK‐8 assay. (C) Cell migration was tested using Transwell inserts. (D‐I) The protein levels of KLF6, TGF‐β, MMP‐9, Bcl‐2, and Bax were determined by western blotting. *P < .05 vs the NG + miR‐NC group; # P < .05 vs the HG + miR‐204‐3p group; n = 3 in each group
5. DISCUSSION
In this study, we confirmed that the expression level of miR‐204‐3p in peripheral blood of newly diagnosed T2DM patients was significantly lower than that of people with normal glucose tolerance. Moreover, the expression level of miR‐204‐3p in peripheral blood of T2DM patients associated with DFU was significantly lower than that in T2DM patients without DFU. Multivariate logistic regression analysis illustrated that the lower expression of miR‐204‐3p was an independent risk factor for DFU. Further analysis showed that the expression level of miR‐204‐3p in peripheral blood and wound margin tissue of patients with DFU was closely related to the healing rate of foot ulcer. Specifically, low expression of miR‐204‐3p was associated with lower healing rate of foot ulcer in T2DM patients associated with DFU. These results suggested that the decreased expression of miR‐204‐3p was not only a strong risk factor for the onset of DFU but also as a potential biomarker for predicting the ending of foot ulcer healing. To the best of our knowledge, this is the first study that looked into the relationship between changes of miR‐204‐3p expression and the pathogenesis and treatment outcomes of DFU in patients with T2DM.
Surprisingly, in the present study, in comparison with the controls with normal glucose tolerance, the miR‐204‐3p level in peripheral blood of T2DM patients was significantly decreased. Furthermore, correlation analysis demonstrated that the expression level of miR‐204‐3p was negatively correlated with FPG, HbA1c levels in both T2DM patients and T2DM patients associated with DFU. These results suggest that the low expression of miR‐204‐3p in the peripheral blood and wound margin tissues of T2DM patients may be related to hyperglycemia. We can not explain the reason for the down‐regulation of miR‐204‐3p expression in T2DM patients. However, a previous study demonstrated that high blood sugar potentially downregulates the expression of miR‐24 by inducing the activation of c‐Myc, 29 Therefore, more studies are needed to clarify the mechanism of miR‐204‐3p expression change in high glucose environment.
In fact, in the present study, the course of DFU was at least 4 weeks, which was chronic refractory wound, including the following clinical features: long history of DM and poor long‐term blood glucose control, combined with varying degrees of abnormal lipid metabolism, peripheral vascular lesion, and infectious inflammation status. Multivariate regression analysis showed that the courses of DM, HbA1c, ABI, and CRP were independent influencing factors for the occurrence of foot ulcer, which was consistent with findings of the previous studies. 30 , 31 , 32 Notably, further analysis showed that the peripheral blood miR‐204‐3p level in DFU group was significantly lower than that in T2DM group. Multivariate regression analysis provided evidence that the low expression of miR‐204‐3p was an independent influencing factor in the occurrence of DFU, correlations analysis showed that low expression of miR‐204‐3p was associated with lower wound healing rate in T2DM patients associated with DFU. These results suggest that decreased expression of miR‐204‐3p was associated with poor wound healing of DFU, miR‐204‐3p is involved in the pathogenesis of DFU. However, the specific mechanism is not clear.
It is generally believed that DFU is harder to heal than non‐diabetic chronic skin wounds, and hyperglycemia is an important adverse factor. In view of the decreased expression of miR‐204‐3p in T2DM patients, which is related to hyperglycemia, and the change of miR‐204‐3p expression may affect the occurrence and healing of DFU, we believe that hyperglycemia may have a detrimental effect on DFU by regulating the role of miR‐204‐3p. In order to further verify the above hypothesis, we carried out in vitro experiments.
Known TGF‐β is an important cytokine secreted by HKCs and can act on fibroblasts to promote wound healing. In diabetic chronic wounds, increased expression of MMP‐9 is considered a critical factor leading to delayed wound healing, and MMP‐9 has, therefore, been addressed as a vital therapeutic target using a specific inhibitor. 27 In addition, Bcl‐2, Bax, and cleaved caspase‐3 are all important factors closely linked to apoptosis, with Bcl‐2 inhibiting apoptosis, and Bax and caspase‐3 promoting apoptosis. 28 In the present study, in vitro experiments showed that the proliferation and migration of high glucose‐treated HKCs cells were visibly decreased, while the proportion of apoptotic cells was significantly increased, which is consistent with previous reports. 8 , 9 Moreover, the expression of miR‐204‐3p, TGF‐β, and Bcl‐2 was markedly reduced. Further studies showed that miR‐204‐3p overexpression can significantly upregulate the expression of TGF‐β and Bcl‐2 proteins in HKCs treated with high‐glucose medium, increase the proliferation and migration of HKCs, and reduce the protein levels of MMP‐9, Bax, and cleaved caspase‐3 and the apoptosis rate. These results indicate that miR‐204‐3p can combat the functional impairment of HKCs under high‐glucose conditions and may participate in the process of diabetic chronic wound repair.
It is found that high‐glucose conditions are an important influencing factor in regulating KLF6 expression. KLF6 is overexpressed in proximal tubular epithelial cells in a diabetic rat model 33 and is obviously increased in a diabetic pulmonary fibrosis rat model. Furthermore, in vitro studies have shown that the expression of KLF6 can be induced by high‐glucose conditions. 34 Consistent with these results, we found that the protein and mRNA levels of KLF6 were markedly increased in high glucose‐treated HKCs in the present study. Inhibiting KLF6 expression significantly increased the protein levels of TGF‐β and Bcl‐2 in high‐glucose culture of HKCs, enhancing their proliferation and migration, and reduced Bax expression and the HKC apoptosis rate. These findings indicate that KLF6 may play a pivotal role in HKC injury under high‐glucose conditions.
In this study, we found that there was a negative correlation between the expression of KLF6 and the expression of miR‐204‐3p in the wound margin tissues of DFU group. In addition, in vitro studies showed that the increased protein and mRNA levels of KLF6 in HKCs were attenuated by overexpression of miR‐204‐3p. To further explore whether overexpression of miR‐204‐3p can prevent HKC impairment induced by high‐glucose treatment by targeting KLF6, we used bioinformatics prediction and a dual‐luciferase reporter gene to verify that the UTR of the KLF6 gene is a target of miR‐204‐3p. Upregulating or downregulating the expression of miR‐204‐3p significantly altered KLF6 levels, indicating that miR‐204‐3p has a negative regulatory effect on KLF6 expression. Conversely, KLF6 overexpression reversed the effect of miR‐204‐3p overexpression on the expression of TGF‐β and Bcl‐2 and the proliferation and migration of HKC and reduced the inhibitory effects of miR‐204‐3p overexpression on the expression of MMP‐9 and Bax and apoptosis. These results confirm that miR‐204‐3p can directly target KLF6 to protect HKCs against functional damage in a high‐glucose environment.
In summary, we found that the decreased expression of miR‐204‐3p in peripheral blood and wound tissue may be associated with the pathogenesis and poor healing of DFU, and demonstrate that miR‐204‐3p improves high glucose‐induced apoptosis and dysfunction in HKCs in vitro by targeting the reduction of KLF6 expression, so as to participate in wound healing. However, there are some limitations in our study: (a) the single‐centre study had relatively small sample size, and there was an inevitable selection bias in this study, thus more research is needed to confirm the findings in our study; (b) The mechanism of miR‐204‐3p involved in wound healing process is relatively preliminary; (c) The results of this study are derived only from biochemical and functional studies based on a two‐dimensional monoculture system in vitro. If these results are extended to the therapeutic concepts, considerable limitations remain. Therefore, in future, more studies are needed to explore target cell and tissue interactions in appropriate models in vitro and in vivo.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data. Xiaotong Zhao and Murong Xu collected data and prepared materials. Dandan Xie and Lili Deng analysis data. The first draft of the manuscript was written by Xiaotong Zhao and Ying Tang. Revised the manuscript and determined the final published version to be complete by Mingwei Chen and Youmin Wang. All authors agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
ETHICS STATEMENT
All procedures performed in this study involving human participants have been approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University and performed in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
INFORMED CONSENT
Written informed consent was obtained from all the respondents.
ACKNOWLEDGEMENTS
We are grateful to the all patients for participating in the study. We thank the participants of this study including the doctors, nurses, and researchers from the Department of Endocrinology and the Health Management Center of the First Affiliated Hospital of Anhui Medical University. This study was supported by the Natural Science Foundation of Anhui Province in China (2108085MH269) and the Natural Science Research Project of Colleges and Universities in Anhui Province (KJ2021A0274).
Zhao X, Xu M, Tang Y, et al. Decreased expression of miR‐204‐3p in peripheral blood and wound margin tissue associated with the onset and poor wound healing of diabetic foot ulcers. Int Wound J. 2023;20(2):413‐429. doi: 10.1111/iwj.13890
The funding body had no role in the design of the study, or the collection, analysis, and interpretation of data, or in writing the manuscript.
Funding information Natural Science Research Project of Colleges and Universities in Anhui Province, Grant/Award Number: KJ2021A0274; Natural Science Foundation of Anhui Province in China, Grant/Award Number: 2108085MH269
Contributor Information
Mingwei Chen, Email: chmw1@163.com.
Youmin Wang, Email: 971359183@qq.com.
DATA AVAILABILITY STATEMENT
The data sets generated during the study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Jiang Y, Wang X, Xia L, et al. A cohort study of diabetic patients and diabetic foot ulceration patients in China. Wound Repair Regen. 2015;23(2):222‐230. [DOI] [PubMed] [Google Scholar]
- 2. Salazar JJ, Ennis WJ, Koh TJ. Diabetes medications:impact on inflammation and wound healing. J Diabetes Complicat. 2016;30(4):746‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martin P. Wound healing–aiming for perfect skin regeneration. Science. 1997;276(5309):75‐81. [DOI] [PubMed] [Google Scholar]
- 4. Sorg H, Tilkorn DJ, Hager S, Hauser J, Mirastschijski U. Skin wound healing: an update on the current knowledge and concepts. Eur Surg Res. 2017;58(1–2):81‐94. [DOI] [PubMed] [Google Scholar]
- 5. Werner S, Krieg T, Smola H. Keratinocyte‐fibroblast interactions in wound healing. J Invest Dermatol. 2007;127(5):998‐1008. [DOI] [PubMed] [Google Scholar]
- 6. Wang PH, Huang BS, Horng HC, Yeh CC, Chen YJ. Wound healing. J Chin Med Assoc. 2018;81(2):94‐101. [DOI] [PubMed] [Google Scholar]
- 7. Yen YH, Pu CM, Liu CW, et al. Curcumin accelerates cutaneous wound healing via multiple biological actions: the involvement of TNF‐α, MMP‐9, α‐SMA, and collagen. Int Wound J. 2018;15(4):605‐617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Terashi H, Izumi K, Deveci M, Rhodes LM, Marcelo CL. High glucose inhibits human epidermal keratinocyte proliferation for cellular studies on diabetes mellitus. Int Wound J. 2005;2(4):298‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deveci M, Gilmont RR, Dunham WR, Mudge BP, Smith DJ, Marcelo CL. Glutathione enhances fibroblast collagen contraction and protects keratinocytes from apoptosis in hyperglycaemic culture. Br J Dermatol. 2005;152(2):217‐224. [DOI] [PubMed] [Google Scholar]
- 10. Lafosse A, Dufeys C, Beauloye C, Horman S, Dufrane D. Impact of hyperglycemia and low oxygen tension on adipose‐derived stem cells compared with dermal fibroblasts and keratinocytes: importance for wound healing in type 2 diabetes. PLoS One. 2016;11(12):e0168058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhu P, Yang C, Chen LH, Ren M, Lao GJ, Yan L. Impairment of human keratinocyte mobility and proliferation by advanced glycation end products‐modified BSA. Arch Dermatol Res. 2011;303(5):339‐350. [DOI] [PubMed] [Google Scholar]
- 12. Zhu P, Ren M, Yang C, Hu YX, Ran JM, Yan L. Involvement of RAGE, MAPK and NF‐κB pathways in AGEs‐induced MMP‐9 activation in HaCaT keratinocytes. Exp Dermatol. 2012;21(2):123‐129. [DOI] [PubMed] [Google Scholar]
- 13. Zhou L, Wang W, Yang C, et al. GADD45a promotes active DNA demethylation of the MMP‐9 promoter via base excision repair pathway in AGEs‐treated keratinocytes and in diabetic male rat skin. Endocrinology. 2018;159(2):1172‐1186. [DOI] [PubMed] [Google Scholar]
- 14. Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11(9):597‐610. [DOI] [PubMed] [Google Scholar]
- 15. Andl T, Murchison EP, Liu F, et al. The miRNA‐processing enzyme dicer is essential for the morphogenesis and maintenance of hair follicles. Curr Biol. 2006;16(10):1041‐1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yi R, Pasolli HA, Landthaler M, et al. Fuchs E.DGCR8‐dependent microRNA biogenesis is essential for skin development. Proc Natl Acad Sci U S A. 2009;106(2):498‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Etich J, Bergmeier V, Pitzler L, Brachvogel B. Identification of a reference gene for the quantification of mRNA and miRNA expression during skin wound healing. Connect Tissue Res. 2017;58(2):196‐207. [DOI] [PubMed] [Google Scholar]
- 18. An J, Chen X, Chen W, et al. MicroRNA expression profile and the role of miR‐204 in corneal wound healing. Invest Ophthalmol Vis Sci. 2015;56(6):3673‐3683. [DOI] [PubMed] [Google Scholar]
- 19. Yu SY, Dong B, Fang ZF, Hu XQ, Tang L, Zhou SH. Knockdown of lncRNA AK139328 alleviates myocardial ischaemia/reperfusion injury in diabetic mice via modulating miR‐204‐3p and inhibiting autophagy. J Cell Mol Med. 2018;22(10):4886‐4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xi X, Teng M, Zhang L, Xia L, Chen J, Cui Z. MicroRNA‐204‐3p represses colon cancer cells proliferation, migration, and invasion by targeting HMGA2. J Cell Physiol. 2020;235(2):1330‐1338. [DOI] [PubMed] [Google Scholar]
- 21. Han X, Li Q, Wang C, Li Y. MicroRNA‐204‐3p attenuates high glucose‐induced MPC5 podocytes apoptosis by targeting braykinin B2 receptor. Exp Clin Endocrinol Diabetes. 2019;127(6):387‐395. [DOI] [PubMed] [Google Scholar]
- 22. McConnell BB, Yang VW. Mammalian Krüppel‐like factors in health and diseases. Physiol Rev. 2010;90(4):1337‐1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huh SJ, Chen YL, Friedman SL, et al. KLF6 gene and early melanoma development in a collagen I‐rich extracellular environment. J Natl Cancer Inst. 2010;102(15):1131‐1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palau N, Julià A, Ferrándiz C, et al. Genome‐wide transcriptional analysis of T cell activation reveals differential gene expression associated with psoriasis. MC Genomics. 2013;14:825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jennings J, Chen D, Feldman D. Transcriptional response of dermal fibroblasts in direct current electric fields. Bioelectromagnetics. 2008;29(5):394‐405. [DOI] [PubMed] [Google Scholar]
- 26. Li X, Tang Y, Jia Z, Zhao X, Chen M. Decreased expression of miR‐24 in peripheral plasma of type 2 diabetes mellitus patients associated with diabetic foot ulcer. Wound Repair Regen. 2020;28(6):728‐738. [DOI] [PubMed] [Google Scholar]
- 27. Nguyen TT, Ding D, Wolter WR, et al. Validation of matrix metalloproteinase‐9 (MMP‐9) as a novel target for treatment of diabetic foot ulcers in humans and discovery of a potent and selective small‐molecule MMP‐9 inhibitor that accelerates healing. J Med Chem. 2018;61(19):8825‐8837. [DOI] [PubMed] [Google Scholar]
- 28. Dolka I, Król M, Sapierzyński R. Evaluation of apoptosis‐associated protein (Bcl‐2, Bax, cleaved caspase‐3 and p53) expression in canine mammary tumors: an immunohistochemical and prognostic study. Res Vet Sci. 2016;105:124‐133. [DOI] [PubMed] [Google Scholar]
- 29. Xiang Y, Cheng J, Wang D, et al. Hyperglycemia repression of miR‐24 coordinately upregulates endothelial cell expression and secretion of von Willebrand factor. Blood. 2015;125(22):3377‐3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Noor S, Zubair M, Ahmad J. Diabetic foot ulcer–a review on pathophysiology, classification and microbial etiology. Diabetes Metab Syndr. 2015;9(3):192‐199. [DOI] [PubMed] [Google Scholar]
- 31. Ladurner R, Küper M, Königsrainer I, et al. Predictive value of routine transcutaneous tissue oxygen tension (tcpO2) measurement for the riskof non‐healing and amputation in diabetic foot ulcer patients with non‐palpable pedal pulses. Med Sci Monit. 2010;16(6):CR273‐CR277. [PubMed] [Google Scholar]
- 32. Tecilazich F, Dinh T, Pradhan‐Nabzdyk L, et al. Role of endothelial progenitor cells and inflammatory cytokines in healing of diabetic foot ulcers. PLoS One. 2013;8(12):e83314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Holian J, Qi W, Kelly DJ, et al. Role of Kruppel‐like factor 6 in transforming growth factor‐beta1‐ induced epithelial‐mesenchymal transition of proximal tubule cells. Am J Physiol Renal Physiol. 2008;295(5):F1388‐F1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zou XZ, Gong ZC, Liu T, et al. Involvement of epithelial‐mesenchymal transition afforded by activation of LOX‐1/TGF‐β1/KLF6 signaling pathway in diabetic pulmonary fibrosis. Pulm Pharmacol Ther. 2017;44:70‐77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during the study are available from the corresponding author on reasonable request.


