Abstract
Tracheostomy is one of the more commonly performed procedures in critically ill patients under mechanical ventilation. Postoperative scarring is one of the bothersome sequelae of tracheostomies. Scars distort physical appearance, especially when found on the head and neck, which could have a negative impact on quality of life. The aim of this study was to evaluate and assess the impact of post‐tracheostomy scars on quality of life according to the tracheostomy method. A prospective, single‐center, observational, case‐control study was conducted. One hundred fifty‐six persons with a post‐tracheostomy surgical scar for more than four months were observed using the Patient and Observer Scar Assessment Scale and Dermatology Life Quality Index questionnaire. Persons were divided into two groups depending on the method of tracheostomy, and the duration of the cannulated period was considered in both groups. Statistical analyses were performed using SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA), and P values of <0.05 were considered significant. The patients who had a tracheostomic tube cannulation period of fewer than 15 days had better cosmetic results than those who had tracheostomic tubes for more than 15 days, regardless of the tracheostomy method: 6.64 ± 0.082 versus 16.15 ± 0.096 (P < 0.001) in the surgical tracheostomy group and 7.26 ± 0.211 versus 14.17 ± 0.379 (P < 0.05) in the percutaneous dilatational group. The Dermatology Life Quality Index scores had a mean value of 0.6 ± 0.01, which means that post‐tracheostomy scarring in the present study had no effect on the person's quality of life. The aesthetic outcomes of post‐tracheostomy scars after the open surgical tracheostomy technique did not significantly differ from those of the percutaneous dilatational technique in the present study. Persons with a long duration of tracheostomic tube ventilation showed worse aesthetic outcomes than those with short‐term tracheostomic cannulation, which was not dependent on the tracheostomy technique. The Dermatology Life Quality Index showed that post‐ttracheostomy scarring in the present study had no effect on the person's quality of life.
Keywords: POSAS, quality of life, scar assessment, scarring, tracheostomy
1. INTRODUCTION
Tracheostomy is a surgical procedure routinely performed in critically ill patients requiring prolonged mechanical ventilatory support. 1 , 2 , 3 Both open surgical tracheostomy (OST) and percutaneous dilatational tracheostomy (PDT) are methods used to perform tracheostomies in select individuals. The best technique for performing tracheostomy remains a matter of debate. 1 , 2 , 3 , 4 However, tracheotomies could have adverse effects, such as procedure‐related complications and future cosmetic concerns. 5 , 6 , 7 Post‐operational scarring is one of the bothersome sequelae of tracheostomies. Since the defect resulting from a tracheostomy is often allowed to heal spontaneously by secondary intention, hypertrophic scar formation is a frequent consequence. 8 A depressed tracheostomy scar can be aesthetically unacceptable and affects a person's quality of life. 9 Thus, postsurgical scar assessment and analysis of its impact on a person's quality of life is fundamental for a complete functional evaluation and as an outcome measure. 10 Long‐term complications of PDT and OST, including postoperative scarring, have been explored by several authors. 6 , 11 , 12 , 13 , 14 However, available studies have only determined the percentage of tracheostomy‐related cosmetic deformities without individual scar assessments and their impact on quality of life.
The aim of this study was to evaluate and assess the impact of post‐tracheostomy scars on quality of life according to the tracheostomy method.
2. METHODS
This prospective, single‐center, observational, case‐control study was conducted from May 2019 to March 2020. Persons were recruited from the ENT and Maxillofacial Surgery Department of “Heratsi” №1 University Hospital in Yerevan, Republic of Armenia.
Written informed consent was obtained from all participants after providing written and oral information about the study. The study protocol was approved by the Ethics Committee of Yerevan State Medical University, and the trial “Quality of life in patients undergoing tracheostomy” was registered in ISRCTN24668317 (IRB no. YSMU №7/18–19). Date of registration April 23, 2019.
The Patient and Observer Scar Assessment Scale (POSAS), which was developed by Draaijers et al., 15 was designed for a subjective evaluation of various types of scar formation and is an appropriate subjective tool for the evaluation of linear scars. 16 , 17
The Dermatology Life Quality Index (DLQI) was developed in 1994 by Finlay and Khan 18 and was designed to measure the impact of skin conditions on quality of life. It has been a widely used questionnaire for life quality assessment and translated into 85 languages.
One hundred fifty‐six persons with post‐tracheostomy surgical scars were observed using the POSAS scale. The time period of these persons' treatment was 2013–2018 years. The follow‐up range was from 12 to 84 months, with an average time of 39.6 months. All participants provided written informed consent for trial participation. The inclusion criteria were (1) persons undergoing tracheostomy in the ICU of Heratsi University Hospital from January 2013 to December 2018. Long‐term mechanical ventilation in intensive care units was the main indication for tracheostomy in participants. The exclusion criteria were (1) persons undergoing emergency tracheostomy, (2) persons with a previous history of neck trauma and scarring, (3) persons with previous radiotherapy, (4) persons with keloid or hypertrophic scar history, and (5) persons with autoimmune diseases.
Of the 156 recruited persons, 76 underwent OST (Group 1), and 80 underwent PDT (Group 2). All persons were Armenians with a mean age of 54.7 years (range, 16–87 years).
As demonstrated in Figure 1, the majority of patients undergoing tracheostomy were in the age range of 51–80 years. The male to female ratio was 2:1. All OSTs were performed by the same surgeon with a 2–3 cm length linear horizontal skin incision made more than 1.5 cm inferiorly to cricoid cartilage. After skin division, the underlying platysma and strap muscle were bluntly dissected vertically by Mosquito hemostats and laterally retracted. The overlying thyroid isthmus was mobilised and retracted inferiorly by a narrow Henahan retractor. A cricoid hook was used to provide upward tracheal traction to improve exposure. The second‐third tracheal rings were incised vertically with a scalpel, and tracheal entrance was dilated by Trousseau tracheal dilator. A tracheostomic tube was inserted after the intubation tube was removed. No skin sutures were needed for wound closure. Betadine dressing was put under tube flange. The PDT was performed by a 2–2.5 sm transverse skin incision. Subcutaneous fat and pretracheal tissues were bluntly dissected with Mosquito hemostats. A 14‐gauge sheathed introducer needle was inserted into the trachea under bronchoscope visualisation. Tracheal placement of the needle was confirmed by aspirating air bubbles into the saline‐filled syringe attached to the needle and by direct visualisation through the bronchoscope. When the needle was withdrawn, a guidewire was inserted through the plastic sheath. The insertion site was dilated with small tracheal dilators. After adequate dilatation, the dilator was removed, and a tracheostomy tube with an appropriate adapter was inserted into the trachea over the guided catheter. The placement of the tracheostomy tube was confirmed by direct bronchoscope visualisation. Betadine dressing was put under tube flange. The decannulation technique was the same in both groups.
FIGURE 1.
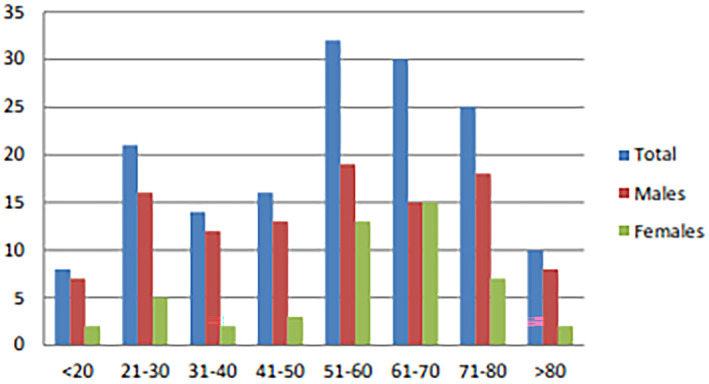
Age and sex distribution of recruited persons undergoing tracheostomy
After deflation of the tracheotomy cuff, the tube was removed, and the opening was covered with sterile dressings. The wound healed spontaneously under sterile dressing, which was changed once or several times per day, depending on tracheal secretion.
The ICU/Ward nursing wound care was uniform across all patients: they were treated in the same ICU clinic with the same nursing personnel. The skin around the stoma was cleaned once per day with antiseptics. Sterile gauze with a ‘T’ shape cut into them was used around the tracheostomic tube in all persons and changed once per day after antiseptic cleaning of the wound and flanges.
The tracheostomy cannulation period varied from 3 days to 2.5 months, with an average timing of one month. Observations showed that with the same operation technique, the aesthetic appearance of the scar differed in the studied groups and did not depend on aging, anatomy, or illness severity. This fact prompted the idea of the influence of the duration of the cannulated period on further post‐decannulation secondary wound healing and the aesthetic and functional outcome of the formed scar. Considering that the average cannulated period was one month, we conditionally separated the groups into less than fifteen days of cannulation and more than fifteen days of cannulation. Fifty‐nine persons (37.8%) noted that they were decannulated in less than fifteen days, and 97 persons (62.2%) noted more than fifteen days of tracheostomic cannulation.
Three observers (two residents and one medical doctor in the ENT and the maxillofacial surgery department) independently assessed post‐tracheostomy scars using the POSAS scar scale on the same day.
The POSAS consists of two scales: the patient and observer scales. Both scales contain six items that are scored numerically. Each of the six items on both scales has a ten‐step score, with 10 indicating the worst imaginable scar or sensation. The total score of both scales consists of adding the scores for each of the six items (range: 6 to 60). The lowest score, 6, reflects normal skin, whereas the highest score of 60 reflects the worst imaginable scar.
The observed component was composed of six parameters of scars: vascularity, pigmentation, thickness, relief, pliability, and surface area. Each parameter consisted of several categories. The degree of vascularity might be difficult to measure visually when there is pigmentation of the wound. A 7 × 3 cm piece of 3‐mm‐thick Plexiglas was used to compress the blood vessels and assess the amount of blood return after blanching and to assess the degree of pigmentation since it eliminates the effect of vascularity.
The patients assessed their own scars using the patient component of the POSAS on the same day. The patient component consisted of six parameters: scar‐related pain, itchiness, colour, stiffness, thickness, and irregularity.
Internal consistency was assessed using Cronbach's alpha statistics, which considered values greater than or equal to 0.70 to be acceptable. Interobserver reliability was defined as “the extent of agreement between three observers” and was assessed by computing the intraclass correlation coefficient (ICC) using a two‐way mixed model with measures of consistency. An ICC within the range of 0 to 0.20 was considered “slight”, 0.21 to 0.40 as “fair”, 0.41 to 0.60 as “moderate”, 0.61 to 0.80 as “substantial”, and 0.81 to 1.0 as “almost perfect”. 17
The DLQI questionnaire was used to evaluate post‐tracheostomy scars and their impact on quality of life. The DLQI is designed for use in adults. It is self‐explanatory and can be simply handed to the patient who is asked to complete it. The questionnaire consists of ten questions related to symptoms and feelings, daily activities, leisure, work and study, personal relationships, and treatment. The scores ranged from 0 to 30. Scores of 0 to 1 indicated no effect on the patient's life, 2 to 5 indicated a mild effect, 6 to 10 indicated a moderate effect, 11 to 20 indicated a high effect, and 21 to 30 indicated an extremely high effect. 18
All statistical analyses were performed using SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA), and P values of <0.05 were considered significant.
3. RESULTS
The internal consistency was acceptable for the observer components of the POSAS, with Cronbach's alpha values of 0.889, and 0.770 respectively.
The interobserver reliability (Table 1) was “almost perfect” for the OST group and “substantial” for the PDT group for the observer component of the POSAS in terms of the total score (the average ICCs were 0.876 and 0.728, respectively). For the individual observer component of the POSAS in the OST group, interobserver reliability was “almost perfect” for vascularity, relief, and surface area (0.913, 0.913, and 0.947, respectively) and “substantial” for pigmentation, thickness, and pliability (0.768, 0.802, and 0.627, respectively). In the PDT group, interobserver reliability was “substantial” for pigmentation, thickness, relief, pliability,y, and surface area (0.647, 0.672, 0.779 0.693, and 0.713, respectively), and was “moderate” for vascularity (0.440).
TABLE 1.
Interobserver reliability of the observer component of the patient and observer scar assessment scale
| Observer component of the POSAS | Single measure ICC (95% CI), OST group | Average measure ICC (95% CI), OST group | Single measure ICC (95% CI), PDT group | Average measure ICC (95% CI), PDT group |
|---|---|---|---|---|
| Vascularity | 0.777 (0.689 ~ 0.845) | 0.913 (0.849 ~ 0.943) | 0.207 (0.078 ~ 0.350) | 0.440 (0.201 ~ 0.618) |
| Pigmentation | 0.525 (0.395 ~ 0.646) | 0.76 (0.662 ~ 0.846) | 0.380 (0.230 ~ 0.525) | 0.647 (0.473 ~ 0.768) |
| Thickness | 0.575 (0.431 ~ 0.696) | 0.80 (0.694 ~ 0.873) | 0.405 (0.253 ~ 0.550) | 0.672 (0.503 ~ 0.786) |
| Relief | 0.777 (0.681 ~ 0.849) | 0.91 (0.865 ~ 0.944) | 0.541 0.394 ~ 0.668) | 0.779 (0.661 ~ 0.858) |
| Pliability | 0.359 (0.219 ~ 0.501) | 0.62 (0.456 ~ 0.751) | 0.429 (0.275 ~ 0.573) | 0.693 (0.532 ~ 0.801) |
| Surface area | 0.856 (0.781 ~ 0.906) | 0.947 (0.914 ~ 0.967) | 0.453 (0.311 ~ 0.588) | 0.713 (0.575 ~ 0.811) |
| Total | 0.702 (0.585 ~ 0.793) | 0.876 (0.809 ~ 0.920) | 0.472 (0.303 ~ 0.618) | 0.728 (0.566 ~ 0.829) |
Abbreviations: average measure ICC, intraclass correlation coefficient for the group of three observers; CI, confidence interval; single measure ICC, intraclass correlation coeficient for a single observer.
The total observer scale parameter values varied from 6 (min) to 35 (max) in the OST group and from 6 (min) to 22 (max) in the PDT group. As shown in Table 2, the mean total values of the POSAS observer scale and the separate parameters (except vascularity) were not significantly different in either group. The mean total score using the observer component of POSAS for OST post‐tracheostomic scars was 12.00 ± 0.370 and that for the PDT group was 12.08 ± 0.280 (P = 0.86) (Table 2).
TABLE 2.
POSAS observer scale parameters mean values
| Observer scale parameters | POSAS observer scale value, OST group | POSAS observer scale values, PDT group | P value |
|---|---|---|---|
| Vascularity | 1.79 ± 0.053 | 1.67 ± 0.037 | 0.18 |
| Pigmentation | 1.79 ± 0.007 | 1.87 ± 0.051 | 0.40 |
| Thickness | 1.94 ± 0.075 | 1.97 ± 0.050 | 0.76 |
| Relief | 2.38 ± 0.093 | 2.45 ± 0.073 | 0.56 |
| Pliability | 1.79 ± 0.068 | 1.90 ± 0.055 | 0.20 |
| Surface | 2.30 ± 0.096 | 2.48 ± 0.081 | 0.16 |
| Total score | 12.00 ± 0.370 | 12.08 ± 0.280 | 0.86 |
There were no differences between groups by sex, body habitus, or age in the present study. Differing cosmetic results were found on the POSAS Observer Scale data for persons with different durations of tracheostomic tube cannulation time. The results showed that the patients who had a tracheostomic tube cannulation period of less than 15 days had better cosmetic results than those who had tracheostomic tubes for more than 15 days, regardless of the tracheostomy method. Thus, 36 patients from the OST group were cannulated for less than 15 days, and the average Observer Scale total score was 6.64 ± 0.082 compared with 40 patients with a cannulation period of more than 15 days and an Observer Scale total score of 16.15 ± 0.096 (P < 0.001). Twenty‐three patients from the PDT group had a tracheostomic tube cannulation duration of less than 15 days with an average Observer Scale total score of 7.26 ± 0.211 compared with patients who underwent a cannulation period of more than 15 days (14.17 ± 0.379) (P < 0.05). Persons that were cannulated for more than 15 days had the worst results in both groups and showed values of 16.15 ± 1.130 and 14.17 ± 0.379, respectively (Table 3).
TABLE 3.
POSAS observer and patient scale values depending on duration of tracheostomic tube cannulation
| Parameters | Less than 15 days cannulation | More than 15 days cannulation |
|---|---|---|
| POSAS observer scale mean total value, OST group | 6.64 ± 0.13 | 16.15 ± 1.13* |
| POSAS observer scale mean total value, PDT group | 7.26 ± 0.21 | 14.17 ± 0.38 |
| POSAS patient scale mean total value, OST group | 6.76 ± 0.23 | 13.12 ± 0.44 |
| POSAS patient scale mean total value, PDT group | 7.08 ± 0.19 | 12.50 ± 0.69 |
Note: P < 0.05.
P < 0.001.
This finding could be the consequence of tracheostomic tube flanges extending pressure on tissues around the incision zone, which results in tissue atrophy and the worst cosmetic results, especially in the relief and surface parameters of the POSAS Observer Scale (Figures 2A,B and 3A,B).
FIGURE 2.
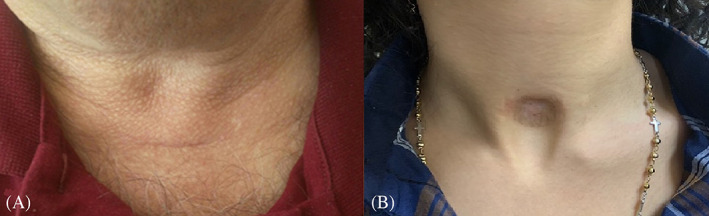
Post‐tracheostomy scar of person who underwent OST A, with less than15 days cannulation period, B, with more than15 days cannulation period
FIGURE 3.
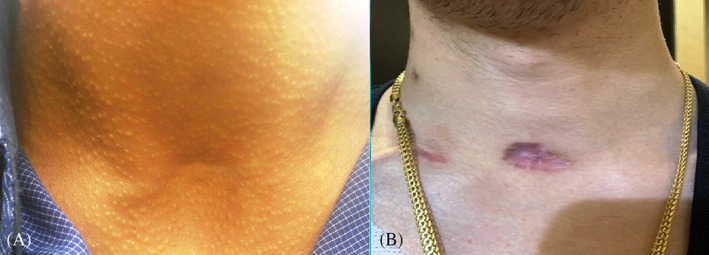
Post‐tracheostomy scar of person who underwent PDT A, with less than15 days cannulation period, B, with more than15 days cannulation period
3.1. Patient POSAS component self‐assessment tool
On simple linear regression analysis, the patients' overall opinion regarding their own scars was significantly influenced by scar‐related itchiness, colour, stiffness, thickness, and irregularity (P < 0.005; Table 4).
TABLE 4.
Simple linear regression analysis of variables associated with patient scar assessment scale scores
| Items | Slope coefficient, OST | P‐value, OST | Slope coefficient, PDT | P‐value, PDT |
|---|---|---|---|---|
| Pain | 0.65 | 0.000 | 0.65 | 0.000 |
| Itchiness | 0.66 | 0.000 | 0.75 | 0.000 |
| Colour | 0.81 | 0.000 | 0.80 | 0.000 |
| Stiffness | 0.83 | 0.000 | 0.89 | 0.000 |
| Thickness | 0.88 | 0.000 | 0.91 | 0.000 |
| Irregularity | 0.81 | 0.000 | 0.89 | 0.000 |
As demonstrated in Table 5, the total scores for patients' self‐assessment parameters in both groups were also not significantly different. They were 9.51 ± 0.441 in the OST group and 9.49 ± 0.392 in the PDT group. The minimal value of the total scores for each group was 6, and the maximum was 60. Therefore, generally, the total score for both groups could be interpreted as close to normal skin.
TABLE 5.
POSAS patient scale parameters values
| Patient scale parameters | OST group | PDT group | P‐value |
|---|---|---|---|
| Pain | 1.22 ± 0.069 | 1.18 ± 0.049 | 0.64 |
| Itchiness | 1.24 ± 0.065 | 1.14 ± 0.041 | 0.23 |
| Colour | 1.58 ± 0.088 | 1.54 ± 0.091 | 0.74 |
| Stiffness | 1.74 ± 0.098 | 1.75 ± 0.082 | 0.99 |
| Thickness | 1.71 ± 0.104 | 1.83 ± 0.085 | 0.38 |
| Irregularity | 1.80 ± 0.118 | 2.05 ± 0.117 | 0.13 |
| Total score | 9.51 ± 0.441 | 9.49 ± 0.392 | 0.96 |
However, when comparing the total scores of the patient assessment scale between persons who underwent tracheostomy intubation for less than 15 days with those who were cannulated for more than 15 days, the results were different, similar to the observer assessment scale: 6.76 ± 0.232 for the OST group and 7.08 ± 0.396 in persons with less than 15 days of cannulation period and 13.12 ± 0.442 and 12.5 ± 0.696, respectively, for persons cannulated for longer than 15 days.
The DLQI scores showed a mean value of 0.6 ± 0.013, which means that post‐tracheostomic scarring in the present study had no effect on the patient's life.
Questions 3, 5, 6, 7, 8, and 9 of the questionnaire had a value of 0 for all 156 persons. Point 10 (over the last week, how much of a problem has the treatment for your skin been, for example, by making your home messy or by taking up time?) was not relevant for all participants involved. Question 1 (over the last week, how itchy, sore, painful, or stinging has your skin?) had a maximum value of 1, which is scored as little impact for 39 persons. Questions 2 and 4 of the questionnaire had a maximum value of 1 for 11 persons.
4. DISCUSSION
Every surgical incision renders a scar, which may lead to an array of functional, cosmetic, and psychological consequences. 16 Scar tissue usually differs from healthy skin by an aberrant colour, thickening, surface irregularity, loss of elasticity, and contraction or expansion of the surface area. The patient frequently suffers from itching and pain, especially if the scar has become hypertrophic. The scar properties depend on aetiology, size, location, suturing technique, wound treatment, individual age, race, and genetic predisposition. 19 Postsurgical scar assessment and analysis of its impact on a person's quality of life is fundamental for a complete functional evaluation and as an outcome measure and may lead to an improvement in scar treatment and prevention. 10 , 16
Tracheostomy is one of the more commonly performed procedures in critically ill patients, but the optimal method of performing tracheostomies in this population remains to be established. 4 The length of the skin incision for OST varies from 2 to 5 cm in the horizontal or vertical direction between the cricoid cartilage and sternal notch, depending on the surgeon's technique. 2 , 6 , 20 While a horizontal incision allows for improved healing and better cosmetic appearance, a vertical incision allows for extension of the incision and avoidance of anterior jugular vein damage but has the worst cosmetic appearance. 6 , 21
Postoperative scarring is one of the bothersome sequelae of tracheostomies. Scars distort physical appearance, especially when found on the head and neck, which could cause a negative impact on quality of life. 22 Since the defect resulting from tracheostomy is allowed to repair spontaneously by secondary intention, hypertrophic scar formation is a frequent consequence. 8 Depression also occurs due to the loss of subcutaneous tissue. If the contracted scar tissue comes into contact with the trachea, it adheres to the trachea. A depressed and fixed tracheal scar produces a “tracheal tug”, which occurs when the skin and trachea move concurrently. The tracheal tug can cause dysphagia and pain with either lateral or vertical head movement in addition to an aesthetically unpleasing scar. 8 , 9 , 23 , 24 In the present study, all persons in the OST group underwent a small 2.5–3 cm horizontal neck incision and vertical tracheal ring incision without Bjork flap formation. This procedure prevents contact between the skin and the trachea and thus “tracheal tug” formation. However, extended tracheostomic tube cannulation showed the worst aesthetic results in both groups compared with short‐term cannulation: 6.64 ± 0.082 compared with 16.15 ± 0.096 (P < 0.001) in the OST group and 7.26 ± 0.092 compared with 13.94 ± 0.096 (P < 0.05) in the PDT group using the observers' total score. The worst aesthetic values were on the parameters of relief and surface area. We can suppose that long‐term pressure on tissues around the incision zone could result in tissue atrophy and the worst cosmetic results.
A depressed tracheostomy scar can be aesthetically unacceptable, very distressing to the patient, and affect the quality of life. 6 , 9 Minimally invasive tracheostomy techniques with short horizontal skin incisions usually heal with a small horizontal linear scar without significant puckering or irregularity. 25 Some studies have noted that OST has higher postoperative scarring incidence than PDT, but none of them describes the technique for the tracheostomies in their works (the length of incision, horizontal or vertical direction). 6 , 12 , 14 , 21 In the present study, no statistically significant difference was observed in the OST and PDT groups by the observer or patient components of the POSAS.
De Kleijn et al. 14 conducted a retrospective study in which 305 consecutive patients undergoing tracheotomy between 2003 and 2013 were included. Long‐term complications of PDT and OST, including postoperative scarring, were explored by the authors. A study of 171 patients in the total population showed 1.1% scarring in the PDT group and 10.7% scarring in the OST group. However, in the “high‐risk” patients who underwent pre‐or postoperative radiation therapy, previous neck surgery, thoracic surgery, or a previous tracheotomy, the scarring rates were 14.3% for PDT and 6.5% for OST. In this retrospective study, OSTs were performed using a Björk flap to prevent false routes when changing the tracheotomy tube. There was no information regarding the length of the incision. In the present study of 76 patients who underwent OST, all tracheotomies were performed by tracheal ring vertical incision in length appropriate to tube diameter. Thus, the values for scarring were almost identical in both groups. Therefore, conclusions about the worst scarring from surgical tracheostomies are not reliable in general.
Another meta‐analysis comparing PDT and OST included pooled data on 973 patients from 15 randomised trials and found that PDT reduced long‐term scarring compared with open surgical techniques. 12 Hazard et al. 11 reported 25% cosmetic deformity in patients with OPS and 9% in patients who underwent PDT. Gysin et al. 13 reported an unaesthetic scar in 40% of patients who underwent OST and 20% of patients who underwent PDT. In the noted article, the skin incision for OST was performed horizontally. However, the incision length was not marked. An inferior tracheal flap was made according to Björk, and the flap was then sutured to the skin, which could have contributed to “tracheal tug” formation and the worst cosmetic appearance. The abovementioned studies determined only the percentage of tracheostomy‐related cosmetic deformities without individual scar assessment.
In Table 2 of the present study, vascularity was statistically significantly different between the OST and PDT groups. Changes in vascularity are indicators of scar maturation. Different types of assessment tools are used to measure the vascularity in the scar. However, it is difficult for scar assessment scales to detect subtle changes in scar colour and monitor scar progress resulting from the limitations of the naked eye. 26 Both pigmentation and vascularity contribute to skin colour and interfere with each other. The accuracy of vascularity measurement using these assessment tools is not clear. As a commonly reported limitation, it is difficult for raters to distinguish scar vascularity from pigmentation, especially for scars with hyperpigmentation and increased vascularity at the same time. Mosterd et al. found that vascularity and pigmentation in the POSAS were the most predictive parameters of overall scar quality. 27 Since the PDT technique implies inserting a tube through a dilated small opening and such tissues are under tension more than separated ones, we believe that after decannulation, angiogenesis in the crushed tissues will begin later and will take longer. This situation is just our assumption, and more objective tools, such as DermaSpectrometer or Chromameter assessment or further histological verification, are necessary for the scientific justification of this assumption.
Despite the fact that the Patient and Observer Scar Assessment Scale is a subjective tool for the evaluation of linear scars, it is appropriate for detailed tracheostomy scar comparative analysis. 16 The POSAS is a standardised, validated, and comprehensive scar assessment tool in clinical care. 17 However, it does not assess the impact of scars on people's quality of life. Thus, the DLQI was used to evaluate the impact of post‐tracheostomy scars on quality of life. The results of a present study of 156 persons showed that post‐tracheostomy scarring had “no effect at all” on the patient's quality of life (0.6 ± 0.013).
We do not find any literature about studies that performed comparative OST and PDT scar assessments by any scar assessment or quality of life evaluation scales. To our knowledge, this study is the first attempt to evaluate post‐tracheostomy scars using POSAS and understand how it impacts quality of life.
The POSAS had more advantages, as its observer component showed better correlation with the patient's rating. The POSAS reflected the patient's perspective about scar‐related symptoms, such as pain and itchiness, and its own position regarding the scar's aesthetic view. Thus, most of the persons who underwent tracheostomy did not complain of pain and itching and were more concerned about scar thickness and irregularity, but these concerns generally did not affect their quality of life. The results of the present, observational study have shown that aesthetic outcomes of post‐tracheostomy scars after open surgical tracheostomy could be close to the PDT technique if it was performed using a minimally invasive approach. Depressed post‐tracheostomy scars appeared in both groups. In our opinion, this condition is the result of long‐term tracheostomic tube cannulation. In the present study, persons undergoing tracheostomy cannulation for more than 15 days had the worst aesthetic results and depressed scars in both groups.
Unfortunately, there is no way to reduce scarring in cases with prolonged tracheostomies. Like most wounds, if the wound is open longer than 2–3 weeks, then scarring is much worse. However, there are methods for aesthetic and functional correction of tracheostomy scars. Tracheostomy scar management is aimed at filling lost deep tissue bulk, the correction of tracheal skin tug, and a tension‐free closure that falls more naturally into the neck folds. 10 , 24 , 28
Many methods have been developed for the correction of tracheal tug and scar depression. Early authors did well in correcting scar depression, but the correction of tracheal tug was more difficult. Fat grafting proved to be a safe, minimally invasive, and effective procedure for the treatment of the tracheostomy scars both for functional and aesthetic purposes. It can be considered as a valid alternative to major open surgery. 9
The major reconstructive principles of tracheostomy scar correction are re‐approximation of individual layers of the neck for improved contour and release of tracheal skin tug, filling of tissue deficit, using scar de‐epithelialization, muscle flaps, or acellular dermal grafts, excision of hypertrophic scarring or keloids, and horizontal wound closure using simple closure or local skin flaps such as z‐plasty. 10 , 25 , 28 It is important to treat each case individually and to provide treatment that is best suited to the patient's needs. 24
5. STUDY LIMITATIONS
The limitations of this study include a small sample size. The lack of haematological parameters could directly or indirectly affect the wound healing process. Fixing a 15‐day period for scar evaluation is arbitrary and not scientifically based.
6. CONCLUSIONS
The aesthetic outcomes of post‐tracheostomy scars after the open surgical tracheostomy technique did not significantly differ from those of the percutaneous dilatational technique in the present study. Persons with a long duration of tracheostomic tube ventilation showed worse aesthetic outcomes than those with short‐term tracheostomic cannulation, which was not dependent on the tracheostomy technique.
The Dermatology Life Quality Index showed that post‐ttracheostomy scarring in the present study had no effect on the person's quality of life.
FUNDING INFORMATION
Investigator initiated and funded.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
ETHICS STATEMENT
IRB no.YSMU №7/18–19. Include appropriate statements in Supplementary files.
Shahparonyan RG, Poghosyan AY, Minasyan AM, et al. Evaluation of post‐tracheostomy scars and their impact on persons' quality of life: A case‐control study. Int Wound J. 2023;20(2):372‐380. doi: 10.1111/iwj.13885
DATA AVAILABILITY STATEMENT
All data generated or analysed during this study are included in this published article and its supplementary information files. https://doi.org/10.1186/ISRCTN24668317
REFERENCES
- 1. De Leyn P, Bedert L, Delcroix M, et al. Tracheotomy: clinical review and guidelines. Eur J Cardiothorac Surg. 2007;32(3):412‐421. [DOI] [PubMed] [Google Scholar]
- 2. Cheung NH, Napolitano LM. Tracheostomy: epidemiology, indications, timing, technique, and outcomes. Respir Care. 2014;59(6):895‐915. discussion 6–9. [DOI] [PubMed] [Google Scholar]
- 3. Hosokawa K, Nishimura M, Egi M, Vincent JL. Timing of tracheotomy in ICU patients: a systematic review of randomized controlled trials. Crit Care. 2015;19:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Delaney A, Bagshaw SM, Nalos M. Percutaneous dilatational tracheostomy versus surgical tracheostomy in critically ill patients: a systematic review and meta‐analysis. Crit Care. 2006;10(2):R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sofi K, Wani T. Effect of tracheostomy on pulmonary mechanics: an observational study. Saudi J Anaesth. 2010;4(1):2‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Engels PT, Bagshaw SM, Meier M, Brindley PG. Tracheostomy: from insertion to decannulation. Can J Surg. 2009;52(5):427‐433. [PMC free article] [PubMed] [Google Scholar]
- 7. Cipriano A, Mao ML, Hon HH, et al. An overview of complications associated with open and percutaneous tracheostomy procedures. Int J Crit Illn Inj Sci. 2015;5(3):179‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Halum SL, Ting JY, Plowman EK, et al. A multi‐institutional analysis of tracheotomy complications. Laryngoscope. 2012;122(1):38‐45. [DOI] [PubMed] [Google Scholar]
- 9. Mazzola IC, Cantarella G, Mazzola RF. Management of tracheostomy scar by autologous fat transplantation: a minimally invasive new approach. J Craniofac Surg. 2013;24(4):1361‐1364. [DOI] [PubMed] [Google Scholar]
- 10. Ha JH, Kim SW. Tracheostomy scar management by repositioning platysma muscle and applying an acellular dermal substitute. Head Neck. 2019;41(8):2671‐2675. [DOI] [PubMed] [Google Scholar]
- 11. Vercelli S, Ferriero G, Sartorio F, Stissi V, Franchignoni F. How to assess postsurgical scars: a review of outcome measures. Disabil Rehabil. 2009;31(25):2055‐2063. [DOI] [PubMed] [Google Scholar]
- 12. Hazard P, Jones C, Benitone J. Comparative clinical trial of standard operative tracheostomy with percutaneous tracheostomy. Crit Care Med. 1991;19(8):1018‐1024. [DOI] [PubMed] [Google Scholar]
- 13. Higgins KM, Punthakee X. Meta‐analysis comparison of open versus percutaneous tracheostomy. Laryngoscope. 2007;117(3):447‐454. [DOI] [PubMed] [Google Scholar]
- 14. Gysin C, Dulguerov P, Guyot JP, Perneger TV, Abajo B, Chevrolet JC. Percutaneous versus surgical tracheostomy: a double‐blind randomized trial. Ann Surg. 1999;230(5):708‐714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Kleijn BJ, Wedman J, Zijlstra JG, Dikkers FG, van der Laan B. Short‐ and long‐term complications of surgical and percutaneous dilatation tracheotomies: a large single‐Centre retrospective cohort study. Eur Arch Otorhinolaryngol. 2019;276(6):1823‐1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Draaijers LJ, Tempelman FR, Botman YA, et al. The patient and observer scar assessment scale: a reliable and feasible tool for scar evaluation. Plast Reconstr Surg. 2004;113(7):1960‐1965. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 17. van de Kar AL, Corion LU, Smeulders MJ, Draaijers LJ, van der Horst CM, van Zuijlen PP. Reliable and feasible evaluation of linear scars by the patient and observer scar assessment scale. Plast Reconstr Surg. 2005;116(2):514‐522. [DOI] [PubMed] [Google Scholar]
- 18. Chae JK, Kim JH, Kim EJ, Park K. Values of a patient and observer scar assessment scale to evaluate the facial skin graft scar. Ann Dermatol. 2016;28(5):615‐623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Finlay AY, Khan GK. Dermatology life quality index (DLQI)‐‐a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210‐216. [DOI] [PubMed] [Google Scholar]
- 20. Niessen FB, Spauwen PH, Schalkwijk J, Kon M. On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg. 1999;104(5):1435‐1458. [DOI] [PubMed] [Google Scholar]
- 21. Durbin CG Jr. Techniques for performing tracheostomy. Respir Care. 2005;50(4):488‐496. [PubMed] [Google Scholar]
- 22. Sanji RR, Channegowda C, Patil SB. Comparison of elective minimally invasive with conventional surgical tracheostomy in adults. Indian J Otolaryngol Head Neck Surg. 2017;69(1):11‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Choi Y, Lee JH, Kim YH, et al. Impact of postthyroidectomy scar on the quality of life of thyroid cancer patients. Ann Dermatol. 2014;26(6):693‐699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Skigen AL, Bedrock RD, Stopperich PS. Correction of the depressed, retracted, post‐tracheostomy scar. Plast Reconstr Surg. 1999;103(6):1703‐1705. [DOI] [PubMed] [Google Scholar]
- 25. Kurt Yazar S, Yüce E, Serin M, et al. A new technique in management of depressed posttracheostomy scars. Ann Plast Surg. 2018;81(3):311‐315. [DOI] [PubMed] [Google Scholar]
- 26. Lee KC, Dretzke J, Grover L, Logan A, Moiemen N. A systematic review of objective burn scar measurements. Burns Trauma. 2016. Apr;27(4):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mosterd K, Arits AH, Nelemans PJ, Kelleners‐Smeets NW. Aesthetic evaluation after non‐invasive treatment for superficial basal cell carcinoma. J Eur Acad Dermatol Venereol. 2013;27(5):647‐650. [DOI] [PubMed] [Google Scholar]
- 28. Grant N, Davison SP. Management of the post‐tracheostomy scar. Laryngoscope. 2007;117(12):2107‐2109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files. https://doi.org/10.1186/ISRCTN24668317


