Abstract
Cigarette smoking is associated with surgical complications, including wound healing and surgical site infection. However, the association between smoking status and postoperative wound complications is not completely understood. Our objective was to investigate the effect of smoking on postoperative wound complications for major surgeries. Data were collected from the 2013 to 2018 participant use files of the American College of Surgeons National Surgical Quality Improvement Program database. A propensity score matching procedure was used to create the balanced smoker and nonsmoker groups. Multivariable logistic regression was used to calculate the odds ratios (ORs) with 95% confidence intervals (CIs) for postoperative wound complications, pulmonary complications, and in‐hospital mortality associated with smokers. A total of 1 156 002 patients (578 001 smokers and 578 001 nonsmokers) were included in the propensity score matching analysis. Smoking was associated with a significantly increased risk of postoperative wound disruption (OR 1.65, 95% CI 1.56‐1.75), surgical site infection (OR 1.31, 95% CI 1.28‐1.34), reintubation (OR 1.47, 95% CI 1.40‐1.54), and in‐hospital mortality (OR 1.13, 95% CI 1.07‐1.19) compared with nonsmoking. The length of hospital stay was significantly increased in smokers compared with nonsmokers. Our analysis indicates that smoking is associated with an increased risk of surgical site infection, wound disruption, and postoperative pulmonary complications. The results may drive the clinicians to encourage patients to quit smoking before surgery.
Keywords: postoperative complications, propensity score, smoking, surgical site infection, wound dehiscence
1. INTRODUCTION
Smoking is recognised as a risk factor for perioperative cardiovascular, respiratory, and wound‐related complications. 1 , 2 Wound‐related complications can lengthen hospital stays, expand hospital resource utilisation, and affect patient recovery. 3 , 4 A brief statement about smoking impacting on wound healing was published by the American Society of Anesthesiologists' Task Force on Smoking Cessation. 5 The statement mentioned that smoking has a direct impact on postoperative outcomes, and patients receiving elective surgery should abstain from smoking for as long as possible before and after surgery. Recently, a consensus statement on perioperative smoking cessation by the Society for Perioperative Assessment and Quality Improvement (SPAQI) mentioned that smoking cessation should be done as soon as practicable with surgical scheduling. 6 Extended abstinence is associated with lower rates of wound healing complications. 7
Normal wound healing is the foundation of successful surgery. A comprehensive review article mentioned that smoking is one of the risk factors associated with wound complications. Other risk factors include infection, malnutrition, immobilisation, diabetes, drugs (such as steroids), and radiation. 8 The interaction between smoking and postoperative wound complications (wound infection, wound disruption) has been analysed in studies related to many different surgical procedures, including breast cancer surgery, 9 ambulatory surgery, 10 gastrointestinal cancer, and thoracic surgery, 11 and colorectal surgery. 12 They found surgical site infection (SSI) and delayed wound healing more frequently in smokers. However, not all the studies have consistent results. For example, there were two cohort studies about plastic surgery, and no difference was observed in wound complications between smokers and nonsmokers. 13 , 14 Recently, various tobacco and nicotine products have been accessible on the market, and one in vitro study disclosed the divergent effects on wound healing. 15 Accordingly, in this study, we attempted to understand the current impact of smoking on wound complications in surgical cases.
We conducted this propensity score‐matched study using the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database to investigate whether patients who were active smokers were more likely to have wound‐related complications postoperatively. We hypothesized that the current smokers have an increased risk of wound complications compared with nonsmokers.
2. METHOD
2.1. Data collection
This retrospective cohort study used the ACS NSQIP database to collect raw data from major surgical procedures. This database contains demographic data, comorbidities, perioperative surgical data, surgical outcomes, and complications. 16 Data were prospectively collected in a standardised way, and the accuracy and reproducibility were established. 17 This study was evaluated and approved by the Institutional Review Board of Taipei Medical University as exempt (TMU‐JIRB202110058). The study was performed in line with the STROCSS guidelines for cohort studies. 18 The protocol was also submitted to the international clinical trial registry (clinicaltrial.gov – registration number NCT05142956).
2.2. Study population
We used the 2013 to 2018 NSQIP participant user file to identify all codes and output variables required for surgical cases. Only patients with complete information for baseline parameters and without preoperative open wound infections were included. A total of 4 176 663 patients were initially chosen. (Figure 1).
FIGURE 1.
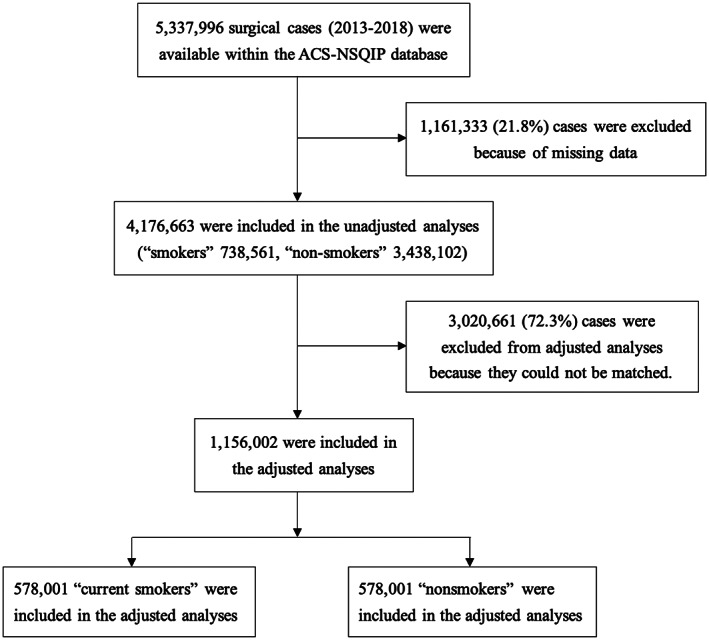
Flow of patients from initial identification in the database through to final cohort. ACS‐NSQIP, The American College of Surgeons National Surgical Quality Improvement Program
Patients were stratified based on their smoking status as current smokers or nonsmokers. Current smokers were defined as patients who self‐reported smoking cigarettes within 1 year before surgery. Outcome variables included postoperative complications (wound complications, ventilator>48 hours, reintubation), length of hospital stay, and in‐hospital mortality. Wound complications include SSI and wound disruption within 30 days after the primary procedure. SSIs were defined as a patient with at least one instance of a superficial SSI, deep SSI, or organ space SSI.
2.3. Statistical analysis
We used propensity score analysis to control for confounding factors in this study. Propensity score matching was applied to match several parameters to make the two groups more comparable. 19 For the primary analysis, we determined the relationship between smoking and postoperative morbidity after propensity score matching current smokers to nonsmokers with the available confounding variables (sex, age, American Society of Anesthesiologists (ASA), preoperative functional status, race, body mass index (BMI), medical conditions, preoperative steroid use, surgical specialty, types of anaesthesia, operation time and wound classification). Matched cohort analysis was performed by using a 1:1 (nonsmoker: smoker) greedy nearest neighbour matching without replacement. To optimise matching for selective variables, we used a calliper width of 0.1 standard deviations of the logit of the propensity score. Therefore, the closest match between subjects from each cohort (based on propensity score) and patients (who were within the calliper range) was matched together. The propensity score for each group was estimated using a logistic model based on the baseline variables. An absolute standardised difference of <0.1 for each baseline factor was considered a negligible difference between matched groups, which implies there was balance and good quality matching. 19
Odds ratios (ORs) and 95% confidence intervals (CIs) for wound complications, in‐hospital mortality, ventilator >48 hours, and reintubation for matched current smokers and nonsmokers were analysed with multivariable logistic regression models adjusted for sex, age, ASA, functional status, race, BMI, medical conditions, surgical specialty, wound classification, type of anaesthesia, and operation time. The length of hospital stay was analysed using paired t tests. All P values were 2‐sided, and a P value <.05 was considered significant for the propensity score matching analysis.
The association between smoking status and postoperative wound complications was further explored by stratified analysis, and adjusted ORs with 95% CIs were calculated in the multivariate logistic regressions by sex, age, duration of surgery, and anaesthetic techniques. SAS software (version 9.4, SAS Institute Inc., Cary, North Carolina) was used for data analyses.
3. RESULTS
There were 5 337 996 surgical cases identified within the ACS NSQIP database during 2013 to 2018. We excluded 21.8% of patients with missing data. Among the remaining 4 176 663 patients, 738 561 (17.7%) were current smokers and 3 438 102 (82.3%) were nonsmokers. We successfully matched 578 001 current smokers and 578 001 nonsmokers for a total of 1 156 002 patients. Figure 1 shows details of the numbers of exclusions.
Table 1 summarises the baseline data of each cohort (nonsmokers and smokers). Baseline variables, including sex, age, ASA classification, functional status, race, BMI, comorbidities, surgical specialties, wound classification, anaesthesia technique, and operation time were different between two groups (p value <.01) except that disseminated cancer before surgery (p value .1095) was comparable between the two groups. Smokers had higher proportions of ASA class III and IV, chronic obstructive pulmonary disease (COPD), ventilator dependency, congestive heart failure (CHF), bleeding disorders, acute renal failure, and wounds with higher contamination levels than nonsmokers.
TABLE 1.
Characteristics of smokers and nonsmokers before matching
| Non‐smoker (N = 3 438 102) | Smoker (N = 738 561) | ||||
|---|---|---|---|---|---|
| n | (%) | n | (%) | P‐value | |
| Gender | <.0001 | ||||
| Female | 1 989 602 | (57.9) | 367 644 | (49.8) | |
| Male | 1 448 500 | (42.1) | 370 917 | (50.2) | |
| Age, years | <.0001 | ||||
| 20‐29 | 167 026 | (4.9) | 47 079 | (6.4) | |
| 30‐39 | 271 569 | (7.9) | 83 258 | (11.3) | |
| 40‐49 | 435 781 | (12.7) | 122 394 | (16.6) | |
| 50‐59 | 657 254 | (19.1) | 205 016 | (27.8) | |
| 60‐69 | 864 608 | (25.2) | 181 065 | (24.5) | |
| 70‐79 | 680 470 | (19.8) | 82 083 | (11.1) | |
| ≥80 | 361 394 | (10.5) | 17 666 | (2.4) | |
| ASA physical status | <.0001 | ||||
| I | 206 577 | (6.0) | 19 872 | (2.7) | |
| II | 1 472 463 | (42.8) | 286 437 | (38.8) | |
| III | 1 535 166 | (44.7) | 363 816 | (49.3) | |
| IV | 218 593 | (6.4) | 66 372 | (9.0) | |
| V | 5303 | (0.2) | 2064 | (0.3) | |
| Functional status prior to surgery | <.0001 | ||||
| Independent | 3 331 971 | (96.9) | 719 138 | (97.4) | |
| Dependent | 106 131 | (3.1) | 19 423 | (2.6) | |
| Race | <.0001 | ||||
| Asian | 104 535 | (3.0) | 10 237 | (1.4) | |
| Black | 339 066 | (9.9) | 98 489 | (13.3) | |
| Other/unknown | 485 885 | (14.1) | 92 924 | (12.6) | |
| White | 2 508 616 | (73.0) | 536 911 | (72.7) | |
| BMI | <.0001 | ||||
| <18.5 | 51 655 | (1.5) | 25 960 | (8.3) | |
| 18.5‐24.9 | 777 999 | (22.6) | 213 738 | (28.9) | |
| 25‐29.9 | 1 067 718 | (31.1) | 219 895 | (29.8) | |
| 30‐34.9 | 756 430 | (22.0) | 144 413 | (19.6) | |
| 35‐39.9 | 411 987 | (12.0) | 73 626 | (10.0) | |
| >=40 | 372 313 | (10.8) | 60 929 | (8.3) | |
| Medical conditions | |||||
| Hypertension | 1 760 483 | (51.2) | 343 150 | (46.5) | <.0001 |
| Diabetes | 620 768 | (18.1) | 121 649 | (16.5) | <.0001 |
| COPD | 125 168 | (3.6) | 86 292 | (11.7) | <.0001 |
| Ventilator dependent | 10 136 | (0.3) | 3877 | (0.5) | <.0001 |
| CHF | 34 184 | (1.0) | 8262 | (1.1) | <.0001 |
| Disseminated cancer | 93 291 | (2.7) | 20 287 | (2.8) | .1095 |
| Bleeding disorders | 154 969 | (4.5) | 44 311 | (6.0) | <.0001 |
| Dialysis | 52 500 | (1.5) | 10 509 | (1.4) | <.0001 |
| Acute renal failure | 13 334 | (0.4) | 3394 | (0.5) | <.0001 |
| Steroid use | 146 133 | (4.3) | 26 922 | (3.7) | <.0001 |
| Surgical specialty | <.0001 | ||||
| Cardiac surgery | 17 822 | (0.5) | 4443 | (0.6) | |
| General surgery | 1 599 183 | (46.5) | 334 228 | (45.3) | |
| Gynaecology | 249 211 | (7.3) | 43 395 | (5.9) | |
| Neurosurgery | 189 245 | (5.5) | 52 154 | (7.1) | |
| Orthopaedics | 795 346 | (23.1) | 125 315 | (17.0) | |
| Otolaryngology | 64 051 | (1.9) | 15 443 | (2.1) | |
| Plastics | 76 482 | (2.2) | 10 893 | (1.5) | |
| Thoracic | 42 668 | (1.2) | 16 586 | (2.3) | |
| Urology | 216 202 | (6.3) | 41 693 | (5.7) | |
| Vascular | 187 892 | (5.5) | 94 411 | (12.8) | |
| Wound classification | <.0001 | ||||
| Clean | 1 874 424 | (54.5) | 396 204 | (53.7) | |
| Clean/contaminated | 1 167 239 | (34.0) | 229 838 | (31.1) | |
| Contaminated | 224 660 | (6.5) | 53 906 | (7.3) | |
| Dirty/infected | 171 779 | (5.0) | 58 613 | (7.9) | |
| Types of anaesthesia | <.0001 | ||||
| General | 3 031 846 | (88.2) | 679 661 | (92.0) | |
| Other | 406 256 | (11.8) | 58 900 | (8.0) | |
| Operation time (h) | <.0001 | ||||
| <2 | 2 260 547 | (65.8) | 466 965 | (63.2) | |
| 2‐4 | 869 634 | (25.3) | 193 589 | (26.2) | |
| >=4 | 307 921 | (9.0) | 78 007 | (10.6) | |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease.
The characteristics of smokers and nonsmokers after propensity score matching are summarised in Table 2. Our sample had 48.2% male patients. There were 43.8% patients with ASA class II and 48.1% patients with ASA class III. Regarding functional status prior to surgery, most cases were independent (99.0%). Among these patients, 39.7% cases had a BMI above 30. Regarding medical conditions, 44.3% of the population had a history of hypertension, and 14.4% of patients had a history of diabetes. General surgery accounted for 45.6% of all surgical specialties. Most patients (92.4%) underwent general anaesthesia.
TABLE 2.
Characteristics of smokers and nonsmokers after matching
| Non‐smoker (N = 578 001) | Smoker (N = 578 001) | ||||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ASD | |
| Sex | |||||
| Female | 299 215 | (51.8) | 299 215 | (51.8) | .00 |
| Male | 278 786 | (48.2) | 278 786 | (48.2) | .00 |
| Age, years | |||||
| 20‐29 | 36 948 | (6.4) | 36 948 | (6.4) | 0.00 |
| 30‐39 | 67 674 | (11.7) | 67 674 | (11.7) | 0.00 |
| 40‐49 | 98 358 | (17.0) | 98 358 | (17.0) | 0.00 |
| 50‐59 | 155 131 | (26.8) | 155 131 | (26.8) | 0.00 |
| 60‐69 | 140 590 | (24.3) | 140 590 | (24.3) | 0.00 |
| 70‐79 | 65 826 | (11.4) | 65 826 | (11.4) | 0.00 |
| ≥80 | 13 474 | (2.3) | 13 474 | (2.3) | 0.00 |
| ASA physical status | |||||
| I | 16 675 | (2.9) | 16 675 | (2.9) | 0.00 |
| II | 252 972 | (43.8) | 252 972 | (43.8) | 0.00 |
| III | 277 918 | (48.1) | 277 918 | (48.1) | 0.00 |
| IV | 30 312 | (5.2) | 30 312 | (5.2) | 0.00 |
| V | 124 | (0.0) | 124 | (0.0) | 0.00 |
| Functional status prior to surgery | |||||
| Independent | 572 353 | (99.0) | 572 353 | (99.0) | 0.00 |
| Dependent | 5648 | (1.0) | 5648 | (1.0) | 0.00 |
| Race | |||||
| Asian | 7519 | (1.3) | 7519 | (1.3) | 0.00 |
| Black | 70 206 | (12.2) | 70 206 | (12.2) | 0.00 |
| Other/unknown | 72 821 | (12.6) | 72 821 | (12.6) | 0.00 |
| White | 427 455 | (74.0) | 427 455 | (74.0) | 0.00 |
| BMI | |||||
| <18.5 | 11 850 | (2.1) | 11 850 | (2.1) | 0.00 |
| 18.5‐24.9 | 157 118 | (27.2) | 157 118 | (27.2) | 0.00 |
| 25‐29.9 | 179 456 | (31.1) | 179 456 | (31.1) | 0.00 |
| 30‐34.9 | 119 536 | (20.7) | 119 536 | (20.7) | 0.00 |
| 35‐39.9 | 60 350 | (10.4) | 60 350 | (10.4) | 0.00 |
| >=40 | 49 691 | (8.6) | 49 691 | (8.6) | 0.00 |
| Medical conditions | |||||
| Hypertension | 258 317 | (44.7) | 253 446 | (43.9) | 0.01688 |
| Diabetes | 84 548 | (14.6) | 82 024 | (14.2) | 0.01187 |
| COPD | 41 417 | (7.2) | 41 446 | (7.2) | 0.00020 |
| Ventilator dependent | 244 | (0.0) | 304 | (0.1) | 0.00319 |
| CHF | 2588 | (0.5) | 3234 | (0.6) | 0.01273 |
| Disseminated cancer | 12 866 | (2.2) | 12 978 | (2.3) | 0.00121 |
| Bleeding disorders | 21 886 | (3.8) | 27 756 | (4.8) | 0.04981 |
| Dialysis | 3628 | (0.6) | 3640 | (0.6) | 0.00020 |
| Acute renal failure | 620 | (0.1) | 791 | (0.1) | 0.00654 |
| Steroid | 15 915 | (2.8) | 15 946 | (2.8) | 0.00028 |
| Surgical specialty | |||||
| Cardiac surgery | 2907 | (0.5) | 2907 | (0.5) | 0.00 |
| General surgery | 263 388 | (45.6) | 263 388 | (45.6) | 0.00 |
| Gynaecology | 40 432 | (7.0) | 40 432 | (7.0) | 0.00 |
| Neurosurgery | 44 745 | (7.7) | 44 745 | (7.7) | 0.00 |
| Orthopaedics | 107 017 | (18.5) | 107 017 | (18.5) | 0.00 |
| Otolaryngology | 11 896 | (2.1) | 11 896 | (2.1) | 0.00 |
| Plastics | 7486 | (1.3) | 7486 | (1.3) | 0.00 |
| Thoracic | 10 952 | (1.9) | 10 952 | (1.9) | 0.00 |
| Urology | 36 082 | (6.2) | 36 082 | (6.2) | 0.00 |
| Vascular | 53 096 | (9.2) | 53 096 | (9.2) | 0.00 |
| Wound classification | |||||
| Clean | 327 777 | (56.7) | 327 777 | (56.7) | 0.00 |
| Clean/contaminated | 199 815 | (34.6) | 199 815 | (34.6) | 0.00 |
| Contaminated | 33 688 | (5.8) | 33 688 | (5.8) | 0.00 |
| Dirty/infected | 16 721 | (2.9) | 16 721 | (2.9) | 0.00 |
| Types of anaesthesia | |||||
| General anaesthesia | 533 845 | (92.4) | 533 845 | (92.4) | 0.00 |
| Other | 44 156 | (7.6) | 44 156 | (7.6) | 0.00 |
| Operation time (h) | |||||
| <2 | 367 465 | (63.6) | 367 465 | (63.6) | 0.00 |
| 2‐4 | 152 741 | (26.4) | 152 741 | (26.4) | 0.00 |
| >=4 | 57 795 | (10.0) | 57 795 | (10.0) | 0.00 |
Abbreviations: ASA, American Society of Anesthesiologists; ASD, absolute standardised difference; BMI, body mass index; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease.
Table 3 shows the risk of postoperative outcomes with current smoking from the propensity score matching analysis. Smoking was associated with a significantly increased risk of postoperative SSI (OR 1.31, 95% CI 1.28‐1.34) and wound disruption (OR 1.65, 95% CI 1.56‐1.75). Current smokers had higher risk of in‐hospital mortality (OR 1.13, 95% CI 1.07‐1.19) and pulmonary complications, including reintubation (OR 1.47, 95% CI 1.40‐1.54) and ventilator >48 hours (OR, 1.44; 95% CI, 1.37‐1.52), than nonsmokers. The length of hospital stays was significantly higher among current smokers than nonsmokers (P < .0001).
TABLE 3.
Risk of postoperative mortality and wound complications in patients with current smoking
| Non‐smoker (N = 578 001) | Smoker (N = 578 001) | Outcome risk | ||||
|---|---|---|---|---|---|---|
| Postoperative outcomes | Events | % | Event | % | OR | (95% CI) a |
| In‐hospital mortality | 2705 | 0.4 | 3067 | 0.5 | 1.13 | (1.07‐1.19) |
| SSI | 14 887 | 2.6 | 19 262 | 3.3 | 1.31 | (1.28‐1.34) |
| Superficial SSI | 7610 | 1.3 | 10 228 | 1.8 | 1.35 | (1.31‐1.39) |
| Deep SSI | 1886 | 0.3 | 2892 | 0.5 | 1.53 | (1.45‐1.63) |
| Organ space SSI | 5819 | 1.0 | 6770 | 1.2 | 1.17 | (1.13‐1.21) |
| Wound disruption | 1784 | 0.3 | 2944 | 0.5 | 1.65 | (1.56‐1.75) |
| Ventilator >48 hours | 2516 | 0.4 | 3577 | 0.6 | 1.44 | (1.37‐1.52) |
| Reintubation | 3136 | 0.5 | 4552 | 0.8 | 1.47 | (1.40‐1.54) |
| Length of hospital stay, days b | 2.88 ± 4.85 | 3.04 ± 4.96 | p < .0001 | |||
Abbreviations: CI, confidence interval; OR, odds ratio; SSI, surgical site infection (superficial, deep, and organ space).
Adjusted for all covariates listed in Table 2.
Mean ± SD.
Table 4 presents the risk of postoperative wound complications associated with current smoking and stratification by sex, age, duration of surgery, and anaesthetic technique. Current smokers were associated with a higher risk of postoperative wound complications in both the male (OR, 1.27; 95% CI, 1.23‐1.31) and female groups (OR, 1.40; 95% CI, 1.36‐1.44), and the increased risk was more prominent in the female group. Current smokers were related to an increased risk of postoperative wound complications in all age groups. The positive relationship between current smokers and major postoperative wound complications was apparent regardless of the duration of surgery or anaesthetic technique.
TABLE 4.
Stratified analysis for the risk of postoperative wound complications associated with current smoking
| Wound complications | ||||||
|---|---|---|---|---|---|---|
| n | Events | Rate, % | OR | (95% CI) | ||
| Male | Control | 278 786 | 8077 | 2.9 | 1.00 | |
| Current smoking | 278 786 | 10 116 | 3.6 | 1.27 | (1.23‐1.31) | |
| Female | Control | 299 215 | 7946 | 2.7 | 1.00 | |
| Current smoking | 299 215 | 10 932 | 3.7 | 1.40 | (1.36‐1.44) | |
| Age, 20‐29 years | Control | 36 948 | 618 | 1.7 | 1.00 | |
| Current smoking | 36 948 | 825 | 2.2 | 1.35 | (1.21‐1.50) | |
| Age, 30‐39 years | Control | 67 674 | 1608 | 2.4 | 1.00 | |
| Current smoking | 67 674 | 2102 | 3.1 | 1.32 | (1.24‐1.41) | |
| Age, 40‐49 years | Control | 98 358 | 2703 | 2.8 | 1.00 | |
| Current smoking | 98 358 | 3796 | 3.9 | 1.43 | (1.36‐1.51) | |
| Age, 50‐59 years | Control | 155 131 | 4521 | 2.9 | 1.00 | |
| Current smoking | 155 131 | 6305 | 4.1 | 1.43 | (1.37‐1.48) | |
| Age, 60‐69 years | Control | 140 590 | 4325 | 3.1 | 1.00 | |
| Current smoking | 140 590 | 5457 | 3.9 | 1.28 | (1.23‐1.34) | |
| Age, ≥70 years | Control | 79 300 | 2248 | 2.8 | 1.00 | |
| Current smoking | 79 300 | 2563 | 3.2 | 1.15 | (1.08‐1.22) | |
| <2 hours of surgery | Control | 367 465 | 5268 | 1.4 | 1.00 | |
| Current smoking | 367 465 | 7345 | 2.0 | 1.40 | (1.35‐1.45) | |
| 2‐4 hours of surgery | Control | 152 741 | 5683 | 3.7 | 1.00 | |
| Current smoking | 152 741 | 7608 | 5.0 | 1.36 | (1.32‐1.41) | |
| ≥4 hours of surgery | Control | 57 795 | 5072 | 8.8 | 1.00 | |
| Current smoking | 57 795 | 6095 | 10.6 | 1.23 | (1.18‐1.28) | |
| General anaesthesia | Control | 533 845 | 15 575 | 2.9 | 1.00 | |
| Current smoking | 533 845 | 20 425 | 3.8 | 1.33 | (1.31‐1.36) | |
| Other anaesthesia | Control | 44 156 | 448 | 1.0 | 1.00 | |
| Current smoking | 44 156 | 623 | 1.4 | 1.41 | (1.24‐1.59) | |
Note: Wound complications included surgical site infection (superficial, deep, and organ space) and wound disruption.
Abbreviation: OR, odds ratio.
4. DISCUSSION
In recent years, there have been expanding studies about the adverse impact of smoking on wound healing. 1 , 2 , 20 , 21 , 22 , 23 Wound complications, such as SSI and surgical wound dehiscence, are associated with an increased risk of unplanned reoperation, readmission, and extended length of stay. 24 , 25 , 26 Wound complications will also impact patient quality of life and health care budgets. 27 Most importantly, this behavioural risk factor may be preventable. 23 Our study adds to existing evidence and improves our understanding of healing complications and major complications in smoking surgical cases according to the recent NSQIP database. Among the surgical cases in the database, approximately 17.7% were current smokers, which declined from 26.5% between 2005 and 2008 and was larger than the 15.5% in 2016 among U.S. adults. 1 , 28 The results of this propensity score matching study showed a higher risk of wound complications and pulmonary complications along with an increased risk of in‐hospital mortality and extended length of hospital stays in current smokers.
We found that current smokers who underwent surgery had approximately 30% increased odds of developing SSI and 65% increased odds of developing wound disruption. Prior studies have consistently found that current smokers have an increased risk of wound complications in nonspecific operations. A meta‐analysis by Grønkjær et al, which includes 107 studies found that smokers undergoing surgery had significantly increased odds of wound complications over nonsmokers. 2 Turan et al, used the NSQIP dataset from 2005 to 2008 to survey the association between smoking status and perioperative outcomes. Current smokers had significantly higher odds of wound disruption and superficial and deep incisional infections. 1 An observational, matched case‐control study by Nolan et al found that current smoking status was related to higher odds of SSI. 22 Our data are consistent with these findings, and the benefits of using NSQIP data include a recent and satisfactory number of surgical cases, modifications for probable confounders, and the use of a clear definition of smoking status.
The findings that current smokers have a higher risk of SSI and wound disruption can be explained by the pathophysiological mechanisms related to the toxic effects and oxidative destruction induced by smoking. 29 Regarding the mechanisms of increasing risk of wound disruption, cigarette smoke consists of a complex mixture of compounds, and the primary toxins related to delayed wound healing are nicotine and carbon monoxide. 30 , 31 , 32 Carbon monoxide has a greater affinity for haemoglobin than the affinity of oxygen for haemoglobin. Its presence negatively affects oxygen delivery to the tissues. Nicotine stimulates nicotinic acetylcholine receptors and triggers the secretion of neurotransmitters, such as serotonin, dopamine, noradrenaline, adrenaline, vasopressin, and serotonin. 33 These complex compounds have been proven to impact the wound healing course and tissue oxygenation. 29 , 34 Tissue hypoxia is one of the crucial mechanisms through which cigarette smoking disrupts wound healing. Oxygen is vital for the entire wound healing process, including cell migration to wound sites, bactericidal mechanisms, angiogenesis, and collagen metabolism. 35 , 36 , 37 In addition, nicotine‐mediated vasoconstriction can temporarily reduce tissue blood flow. Some wounds with a vulnerable blood supply, such as intestinal anastomoses, tissue flaps, and ischemic tissues (eg, peripheral artery disease), may be susceptible to blood flow reduction caused by smoking. A systemic review by Sørensen LT demonstrated that smoking has an extensive impact on all phases of wound healing. 29
The potential mechanisms of cigarette smoking on wound infection are multifactorial. 29 , 38 Impeded blood flow caused by a potent vasoconstrictor, nicotine, will cause tissue hypoxia and acidosis. 39 , 40 Hypoxia will decrease the ability of neutrophils and macrophages to oxidative kill pathogens. 41 , 42 , 43 Prolonged acidosis increases the risk of infection. 44 In our study, smokers had a higher risk of deep SSI (OR 1.53, 95% CI 1.45‐1.63) than superficial SSI (OR 1.35, 95% CI 1.31‐1.39). However, there is not enough evidence to prove the mechanism of the association between smoking and SSI. 22 In a retrospective study of NSQIP dataset in 2011, current smoking was a risk factor for both superficial and deep/organ‐space SSI but also varied in terms of magnitude and significance. 45 Therefore, different SSIs may have divergent disease processes, and cigarettes may have distinct impacts on them.
In addition to wound complications, our results show that smokers have an increased risk of pulmonary complications (ventilator >48 hours and reintubation). Smoking impedes the innate defence system of the lung, including damaging mucus transport, aggravating mucus production, and diminishing macrophage function. 46 These findings are consistent with previous studies, which identify smoking as a risk factor for postoperative pulmonary complications. 1 , 47 , 48 However, lifetime exposure to smoking is now considered more influential on the rate of postoperative pulmonary complications than current smoking status. 49
This study using propensity score matching and multivariable logistic regression adds validity to the results. Propensity score matching techniques can control confounding variables by constructing propensity scores based on selected clinical characteristics and matching subjects with similar propensity score values. Our selected variables included in the matching method were based on clinical factors that can influence wound healing or SSI. After matching, multivariate logistic regression techniques were used to diminish the risk of confounding other variables on the outcomes of concern.
Other than a traditional cigarette, it is worth noting that e‐cigarettes, which are alternative nicotine options, are gaining popularity. The content and adverse effects of e‐cigarettes are less well known. The major constituents of e‐cigarettes include nicotine, glycerol, propylene glycol, and other flavourings. There is limited evidence comparing perioperative complications in e‐cigarette consumers to traditional cigarette consumers. A case report by Fracol et al described an e‐cigarette user who had significant mastectomy skin flap necrosis after a bilateral mastectomy. 34 Despite insufficient evidence, data suggest that e‐cigarettes may have a significant adverse effect on wound healing. 50 , 51 , 52 Further investigations are needed to validate the effects of e‐cigarettes on perioperative outcomes.
Although one strength of our study is its large sample size, our study has limitations. First, we could not identify the data on the duration of smoking cessation or pack‐years of smoking from the NSQIP, which made it difficult to measure the transitory effect of smoking cessation or smoking status on postoperative outcomes. For example, patients who had quit smoking within 1 year of surgery were defined as current smokers by the NSQIP. This will cause underestimation of the smoking effect on postoperative complications. Second, there were residual confounders that were not adjusted for. Factors affecting surgical‐wound healing include individual characteristics (aging, malnutrition, immobilisation, diabetes, chronic renal failure, obesity, alcoholism, and jaundice), anaesthetic characteristics (tissue perfusion, normovolaemia, body temperature, concentration of inspired oxygen, and analgesia), and surgical characteristics (site, duration, and complexity of surgery, suturing quality, hematoma, and prophylactic antibiotics). 53 Some of these factors were included in our propensity matching, but there were still other uncorrected factors. Third, propensity score matching still carries bias, as it cannot balance all selected factors, including duration and the severity of comorbidities. 54 Studies with more corrected confounding factors or prospective, randomised trials are needed to verify our results and provide adequate evidence of wound complications.
5. CONCLUSION
Smoking status is related to increased perioperative risk for wound complications following major surgical procedures. The current literature review has shown that smoking harms wound healing. Our study adds to existing evidence and improves our understanding of healing complications in smoking surgical cases. Wound complications are associated with other adverse outcomes and have a significant impact on patient quality of life and health care budgets. Therefore, patients who smoke should be informed about the potentially increased risks of complications before surgery. Our results encourage smoking cessation prior to surgery, although the data do not allow us to evaluate the effects of smoking cessation.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
This study is based on data obtained from the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database. This study was supported in part by Taiwan's Ministry of Science and Technology (MOST109‐2221‐E‐038‐003‐MY2).
Fan Chiang Y‐H, Lee Y‐W, Lam F, Liao C‐C, Chang C‐C, Lin C‐S. Smoking increases the risk of postoperative wound complications: A propensity score‐matched cohort study. Int Wound J. 2023;20(2):391‐402. doi: 10.1111/iwj.13887
Chuen‐Chau Chang has equal contribution with the corresponding author.
Funding information Taiwan's Ministry of Science and Technology, Grant/Award Number: MOST109‐2221‐E‐038‐003‐MY2; American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP)
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from The American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP). Restrictions apply to the availability of these data, which were used under license for this study.
REFERENCES
- 1. Turan A, Mascha EJ, Roberman D, et al. Smoking and perioperative outcomes. Anesthesiology. 2011;114(4):837‐846. [DOI] [PubMed] [Google Scholar]
- 2. Grønkjær M, Eliasen M, Skov‐Ettrup LS, et al. Preoperative smoking status and postoperative complications: a systematic review and meta‐analysis. Ann Surg. 2014;259(1):52‐71. [DOI] [PubMed] [Google Scholar]
- 3. Agrawal S, Ingrande J, Said ET, Gabriel RA. The association of preoperative smoking with postoperative outcomes in patients undergoing total hip arthroplasty. J Arthroplast. 2021;36(3):1029‐1034. [DOI] [PubMed] [Google Scholar]
- 4. Alverdy JC, Prachand V. Smoking and postoperative surgical site infection: where there's smoke, there's fire. JAMA Surg. 2017;152(5):484. [DOI] [PubMed] [Google Scholar]
- 5. Warner DO. Feasibility of tobacco interventions in anesthesiology practices: a pilot study. Anesthesiology. 2009;110(6):1223‐1228. [DOI] [PubMed] [Google Scholar]
- 6. Wong J, An D, Urman RD, et al. Society for perioperative assessment and quality improvement (SPAQI) consensus statement on perioperative smoking cessation. Anesth Analg. 2020;131(3):955‐968. [DOI] [PubMed] [Google Scholar]
- 7. Wong J, Lam DP, Abrishami A, Chan MT, Chung F. Short‐term preoperative smoking cessation and postoperative complications: a systematic review and meta‐analysis. Can J Anaesth. 2012;59(3):268‐279. [DOI] [PubMed] [Google Scholar]
- 8. Janis JE, Harrison B. Wound healing: part I. Basic science. Plast Reconstr Surg. 2016;138(3 Suppl):9s‐17s. [DOI] [PubMed] [Google Scholar]
- 9. Sørensen LT, Hørby J, Friis E, Pilsgaard B, Jørgensen T. Smoking as a risk factor for wound healing and infection in breast cancer surgery. Eur J Surg Oncol. 2002;28(8):815‐820. [DOI] [PubMed] [Google Scholar]
- 10. Myles PS, Iacono GA, Hunt JO, et al. Risk of respiratory complications and wound infection in patients undergoing ambulatory surgery: smokers versus nonsmokers. Anesthesiology. 2002;97(4):842‐847. [DOI] [PubMed] [Google Scholar]
- 11. Gajdos C, Hawn MT, Campagna EJ, Henderson WG, Singh JA, Houston T. Adverse effects of smoking on postoperative outcomes in cancer patients. Ann Surg Oncol. 2012;19(5):1430‐1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sharma A, Deeb AP, Iannuzzi JC, Rickles AS, Monson JR, Fleming FJ. Tobacco smoking and postoperative outcomes after colorectal surgery. Ann Surg. 2013;258(2):296‐300. [DOI] [PubMed] [Google Scholar]
- 13. Karamanos E, Saad N, Smith KE, Patnaik R, Wang HT, Cromack D. Not all flaps are created equal: assessing the impact of active smoking in muscle‐only versus perforator flaps for patients undergoing nonelective extremity‐free tissue transfer‐a case control study. Microsurgery. 2021;41(6):513‐521. [DOI] [PubMed] [Google Scholar]
- 14. Ehrl D, Heidekrueger PI, Ninkovic M, Broer PN. Effect of preoperative medical status on microsurgical free flap reconstructions: a matched cohort analysis of 969 cases. J Reconstr Microsurg. 2018;34(3):170‐175. [DOI] [PubMed] [Google Scholar]
- 15. Giebe S, Hofmann A, Brux M, et al. Comparative study of the effects of cigarette smoke versus next generation tobacco and nicotine product extracts on endothelial function. Redox Biol. 2021;47:102150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Raval MV, Pawlik TM. Practical guide to surgical data sets: National Surgical Quality Improvement Program (NSQIP) and pediatric NSQIP. JAMA Surg. 2018;153(8):764‐765. [DOI] [PubMed] [Google Scholar]
- 17. Khuri SF. The NSQIP: a new frontier in surgery. Surgery. 2005;138(5):837‐843. [DOI] [PubMed] [Google Scholar]
- 18. Agha R, Abdall‐Razak A, Crossley E, Dowlut N, Iosifidis C, Mathew G. STROCSS 2019 guideline: strengthening the reporting of cohort studies in surgery. Int J Surg. 2019;72:156‐165. [DOI] [PubMed] [Google Scholar]
- 19. Staffa SJ, Zurakowski D. Five steps to successfully implement and evaluate propensity score matching in clinical research studies. Anesth Analg. 2018;127(4):1066‐1073. [DOI] [PubMed] [Google Scholar]
- 20. Goltsman D, Munabi NCO, Ascherman JA. The association between smoking and plastic surgery outcomes in 40,465 patients: an analysis of the American College of Surgeons National Surgical Quality Improvement Program Data Sets. Plast Reconstr Surg. 2017;139(2):503‐511. [DOI] [PubMed] [Google Scholar]
- 21. Schmid M, Sood A, Campbell L, et al. Impact of smoking on perioperative outcomes after major surgery. Am J Surg. 2015;210(2):221‐9.e6. [DOI] [PubMed] [Google Scholar]
- 22. Nolan MB, Martin DP, Thompson R, Schroeder DR, Hanson AC, Warner DO. Association between smoking status, preoperative exhaled carbon monoxide levels, and postoperative surgical site infection in patients undergoing elective surgery. JAMA Surg. 2017;152(5):476‐483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sørensen LT. Wound healing and infection in surgery. The clinical impact of smoking and smoking cessation: a systematic review and meta‐analysis. Arch Surg. 2012;147(4):373‐383. [DOI] [PubMed] [Google Scholar]
- 24. Sandy‐Hodgetts K. Surgical wound complications: a 21st century problem? J Wound Care. 2019;28(10):645. [DOI] [PubMed] [Google Scholar]
- 25. Merkow RP, Ju MH, Chung JW, et al. Underlying reasons associated with hospital readmission following surgery in the United States. JAMA. 2015;313(5):483‐495. [DOI] [PubMed] [Google Scholar]
- 26. Berríos‐Torres SI, Umscheid CA, Bratzler DW, et al. Centers for Disease Control and Prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017;152(8):784‐791. [DOI] [PubMed] [Google Scholar]
- 27. Turner MC, Migaly J. Surgical site infection: the clinical and economic impact. Clin Colon Rectal Surg. 2019;32(3):157‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jamal APE, Gentzke AS, et al. Current cigarette smoking among adults — United States, 2016. Morb Mortal Wkly Rep. 2018;67:53‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sørensen LT. Wound healing and infection in surgery: the pathophysiological impact of smoking, smoking cessation, and nicotine replacement therapy: a systematic review. Ann Surg. 2012;255(6):1069‐1079. [DOI] [PubMed] [Google Scholar]
- 30. Ahn C, Mulligan P, Salcido RS. Smoking‐the bane of wound healing: biomedical interventions and social influences. Adv Skin Wound Care. 2008;21(5):227‐236; quiz 37‐8. [DOI] [PubMed] [Google Scholar]
- 31. McDaniel JC, Browning KK. Smoking, chronic wound healing, and implications for evidence‐based practice. J Wound Ostomy Continence Nurs. 2014;41(5):415; quiz E1‐2‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Warner DO. Perioperative abstinence from cigarettes: physiologic and clinical consequences. Anesthesiology. 2006;104(2):356‐367. [DOI] [PubMed] [Google Scholar]
- 33. Carrick MA, Robson JM, Thomas C. Smoking and anaesthesia. BJA Educ. 2019;19(1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fracol M, Dorfman R, Janes L, et al. The surgical impact of E‐cigarettes: a case report and review of the current literature. Arch Plast Surg. 2017;44(6):477‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schreml S, Szeimies RM, Prantl L, Karrer S, Landthaler M, Babilas P. Oxygen in acute and chronic wound healing. Br J Dermatol. 2010;163(2):257‐268. [DOI] [PubMed] [Google Scholar]
- 36. Heeschen C, Jang JJ, Weis M, et al. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med. 2001;7(7):833‐839. [DOI] [PubMed] [Google Scholar]
- 37. Yang GP, Longaker MT. Abstinence from smoking reduces incisional wound infection: a randomized, controlled trial. Ann Surg. 2003;238(1):6‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sorensen LT, Karlsmark T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Ann Surg. 2003;238(1):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sørensen LT, Jørgensen S, Petersen LJ, et al. Acute effects of nicotine and smoking on blood flow, tissue oxygen, and aerobe metabolism of the skin and subcutis. J Surg Res. 2009;152(2):224‐230. [DOI] [PubMed] [Google Scholar]
- 40. Jensen JA, Goodson WH, Hopf HW, Hunt TK. Cigarette smoking decreases tissue oxygen. Arch Surg. 1991;126(9):1131‐1134. [DOI] [PubMed] [Google Scholar]
- 41. Younis I. Role of oxygen in wound healing. J Wound Care. 2020;29(Sup5b):S4‐s10. [DOI] [PubMed] [Google Scholar]
- 42. Babior BM. Oxygen‐dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978;298(12):659‐668. [DOI] [PubMed] [Google Scholar]
- 43. Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med. 2004;164(20):2206‐2216. [DOI] [PubMed] [Google Scholar]
- 44. Allen DB, Maguire JJ, Mahdavian M, et al. Wound hypoxia and acidosis limit neutrophil bacterial killing mechanisms. Arch Surg. 1997;132(9):991‐996. [DOI] [PubMed] [Google Scholar]
- 45. Lawson EH, Hall BL, Ko CY. Risk factors for superficial vs deep/organ‐space surgical site infections: implications for quality improvement initiatives. JAMA Surg. 2013;148(9):849‐858. [DOI] [PubMed] [Google Scholar]
- 46. Centers for Disease C, Prevention, National Center for Chronic Disease P, Health P, Office on S, Health . Publications and reports of the surgeon general. How Tobacco Smoke Causes Disease: the Biology and Behavioral Basis for Smoking‐Attributable Disease: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US). 2010. [PubMed]
- 47. Smetana GW, Lawrence VA, Cornell JE. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144(8):581‐595. [DOI] [PubMed] [Google Scholar]
- 48. Miskovic A, Lumb AB. Postoperative pulmonary complications. Br J Anaesth. 2017;118(3):317‐334. [DOI] [PubMed] [Google Scholar]
- 49. Canet J, Gallart L, Gomar C, et al. Prediction of postoperative pulmonary complications in a population‐based surgical cohort. Anesthesiology. 2010;113(6):1338‐1350. [DOI] [PubMed] [Google Scholar]
- 50. Famiglietti A, Memoli JW, Khaitan PG. Are electronic cigarettes and vaping effective tools for smoking cessation? Limited evidence on surgical outcomes: a narrative review. J Thorac Dis. 2021;13(1):384‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Troiano C, Jaleel Z, Spiegel JH. Association of electronic cigarette vaping and cigarette smoking with decreased random flap viability in rats. JAMA Facial Plast Surg. 2019;21(1):5‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rau AS, Reinikovaite V, Schmidt EP, Taraseviciene‐Stewart L, Deleyiannis FW. Electronic cigarettes are as toxic to skin flap survival as tobacco cigarettes. Ann Plast Surg. 2017;79(1):86‐91. [DOI] [PubMed] [Google Scholar]
- 53. Buggy D. Can anaesthetic management influence surgical‐wound healing? Lancet. 2000;356(9227):355‐357. [DOI] [PubMed] [Google Scholar]
- 54. Reiffel JA. Propensity score matching: the ‘Devil is in the Details’ where more may be hidden than you know. Am J Med. 2020;133(2):178‐181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from The American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP). Restrictions apply to the availability of these data, which were used under license for this study.


