Abstract
Objective:
The GH and IGF-1 axis is a candidate disease-modifying target in nonalcoholic fatty liver disease (NAFLD) given its lipolytic, anti-inflammatory and anti-fibrotic properties. IGF-1 receptor (IGF-1R) and GH receptor (GHR) expression in adult, human hepatic tissue is not well understood across the spectrum of NAFLD severity. Therefore, we sought to investigate hepatic IGF-1R and GHR expression in subjects with NAFLD utilizing gene expression analysis (GEA) and immunohistochemistry (IHC).
Design:
GEA (n=318) and IHC (n=30) cohorts were identified from the Massachusetts General Hospital NAFLD Tissue Repository. GEA subjects were categorized based on histopathology as normal liver histology (NLH), steatosis only (Steatosis), nonalcoholic steatohepatitis (NASH) without fibrosis (NASH F0), and NASH with fibrosis (NASH F1-4) with GEA by the Nanostring nCounter assay. IHC subjects were matched for age, body mass index (BMI), sex, and diabetic status across three groups (n=10 each): NLH, Steatosis, and NASH with fibrosis (NASH F1-3). IHC for IGF-1R, IGF-1 and GHR was performed on formalin-fixed, paraffin-embedded hepatic tissue samples.
Results:
IGF-1R gene expression did not differ across NAFLD severity while IGF-1 gene expression decreased with increasing NAFLD severity, including when controlled for BMI and age. GHR expression did not differ by severity of NAFLD based on GEA or IHC.
Conclusions:
IGF-1R and GHR expression levels were not significantly different across NAFLD disease severity. However, expression of IGF-1 was lower with increasing severity of NAFLD. These findings implicate the GH/IGF-1 axis as a potential target in the treatment of NAFLD and NASH.
Keywords: Insulin-like growth factor-1 (IGF-1), IGF-1 receptor, growth hormone (GH), GH receptor, Nonalcoholic fatty liver disease (NAFLD), Nonalcoholic steatohepatitis (NASH), immunohistochemistry, gene expression analysis
Introduction
Nonalcoholic fatty liver disease (NAFLD) is present in 80% of individuals with obesity in the United States (1). Nonalcoholic steatohepatitis (NASH) is the progressive form of the disease characterized by inflammation and cell death, and NASH cirrhosis is predicted to be the most common indication for liver transplantation in the near future (2–4). While insulin resistance, oxidative stress and inflammation have all been implicated as mechanisms contributing to NAFLD and NASH, their pathogenesis is only partially understood, and no FDA-approved treatments currently exist (3).
The GH and IGF-1 axis is a candidate disease-modifying target in NAFLD and NASH because of its lipolytic, anti-inflammatory and anti-fibrotic properties (5,6). GH is secreted from the pituitary and acts through GH receptors (GHR) on hepatocytes to produce insulin-like growth factor-1 (IGF-1) (7). Obesity is a state of relative GH and IGF-1 deficiency, with GH production reduced to 25% of normal and concomitant moderate reductions in IGF-1 (5,6), although one study noted a potentially compensatory increase in hepatic GH sensitivity in this population (8). Animal models (9–15) and clinical data in patients with severe GH deficiency (16–18) are consistent with an etiopathologic role of relative GH and IGF-1 deficiency in NAFLD/NASH.
However, the mechanisms of the impact of GH and IGF-1 on hepatic steatosis, inflammation and fibrosis in NAFLD and NASH have not been fully elucidated, particularly in humans. GH has well known lipolytic actions in humans and has been shown to decrease visceral adipose tissue in pituitary patients with severe GH deficiency (19–21) and in otherwise healthy individuals with overweight/obesity (22,23). The GH receptor is expressed on normal human hepatocytes and signals through the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathways. Multiple mouse models have demonstrated that hepatocyte-specific knockouts of the GH receptor or components of the JAK-STAT signaling pathway lead to the NAFLD phenotype (10,13,14).
In contrast, the IGF-1R is not expressed in normal, healthy adult rat, mouse (24), or human hepatocytes (25,26). However, the IGF-1 receptor has been shown to be expressed in hepatocytes in the presence of liver disease, including in mice after hepatectomy (25) and adults with chronic hepatitis C (26). Hepatic IGF-1 receptor expression was demonstrated in children with NAFLD, and expression was increased with fibrosis progression, although predominantly in hepatic stellate cells (27). No study has examined the expression of the IGF-1 receptor or GH receptor in adult, human hepatic tissue, particularly hepatocytes, across the spectrum of NAFLD and NASH.
In this study, we sought to investigate IGF-1 receptor and GH receptor physiology in patients with NAFLD and NASH, with quantification of hepatic gene expression and localization of receptor expression by immunohistochemistry. We hypothesized that expression of hepatic IGF-1 receptor and GH receptor would be increased with worsening NAFLD disease severity given the potentially positive effects of GH and IGF-1 on hepatic steatosis, fibrosis and hepatocyte regeneration. We additionally hypothesized that IGF-1 receptor would be expressed specifically on hepatocytes of human subjects in the setting of hepatic injury due to NAFLD/NASH.
Methods
Study approval and informed consent
The present study was approved by the Partners Human Research Committee as a secondary use of the NAFLD repository. All subjects signed informed consent to participate in the NAFLD repository.
Study Cohort
Subjects were identified from the Massachusetts General Hospital (MGH) NAFLD Repository, a prospective study of consented and enrolled patients with suspected or diagnosed NAFLD from the MGH Gastroenterology Unit and MGH Weight Center. Subjects with history of other chronic liver disease or drug-induced NAFLD/NASH due to tamoxifen, oral steroids or methotrexate were excluded. Excess hepatic tissue samples were obtained during routine percutaneous liver biopsy or routine wedge liver biopsy performed during bariatric surgery. One part of the specimen was immediately flash frozen and stored at −80 degrees Celsius for future gene expression analysis. An additional section of each biopsy was formalin fixed and paraffin embedded (FFPE) for histologic evaluation and future immunohistochemistry analysis.
Liver biopsy assessment
Liver biopsies were reviewed by a single blinded hepatopathologist (RM) per the NASH Clinical Research Network (NASH CRN) criteria (28). H&E stained slides were used to assess steatosis grade (0 = <5%; 1 = 5–33%; 2 = 33–66%; 3 >66%), lobular inflammation (foci/200x; 0 = none; 1 = <2; 2 = 2–4; 3 = >4 foci), hepatocyte ballooning (0 = no ballooning; 1 = few; 2 = many), NAFLD activity score (NAS) (assigned from 0–8 as a sum of the steatosis, lobular inflammation and hepatocyte ballooning grades). NASH was defined by the predominance of zone 3 macrovesicular steatosis, hepatocyte ballooning grade ≥1, and lobular inflammation grade ≥1 (presence of ≥1 foci per 200x field) as defined by the NASH Clinical Research Network (NASH CRN). Masson’s trichrome stained slides were used to assess fibrosis, according to the modified Brunt stage 0–4, with 4 representing cirrhosis (28). Subjects for the immunohistochemistry cohort were initially selected based on a clinical pathology read which was then verified using the blinded NASH CRN read as described above.
Subject Identification and Characterization
For the gene expression cohort, 318 individuals with paired histologic analysis and available flash frozen tissue were identified as previously published (29). Subjects were categorized into the following histologically defined subgroups: normal liver histology (NLH), Steatosis, NASH without fibrosis (NASH F0) and NASH with fibrosis (NASH F1–4).
For the immunohistochemistry cohort, 30 adult subjects who had adequate archived FFPE hepatic tissue samples available were identified. Subjects were selected from three distinct histological groups: normal liver histology (NLH), steatosis only without inflammation, hepatocyte ballooning or fibrosis (Steatosis) and NASH with fibrosis stage 2–3 (NASH F2–3). Subjects were matched across groups of three for BMI (± 6 kg/m2), age (± 6 years), sex and menopausal status, so that each matched triplet contained a subject with NLH, Steatosis and NASH F2–3.
Gene expression analysis
RNA extraction was performed as previously described using the following kits: miRNeasy Mini Kit (Qiagen) for flash-frozen samples or RNA-later liver or High Pure FFPET RNA Isolation Kit (Roche Life Science) for Formalin-Fixed Paraffin-Embedded (FFPE) Tissue. An Agilent 2010 bioanalyzer (Santa Clara, CA) was used to assess RNA quality and quantity with RIN numbers from 1.5–9 appropriate for the nCounter platform (29). We quantified gene expression in liver samples using a NanoString probeset as previously published that included GH receptor, IGF-1 receptor and IGF-1. GH expression was not available in this NanoString probeset.
Immunohistochemistry
Immunohistochemistry (IHC) was performed on FFPE hepatic tissue samples for expression of IGF-1 receptor and GH receptor. Anti-IGF-1 receptor antibodies that would cross-react with the insulin receptor, which has a similar homology, were avoided. Three IGF-1 receptor antibodies underwent attempted validation using human breast cancer tissue samples (D4O6W, Cell Signaling, Danvers, MA, USA; Ab131476, Abcam, Cambridge, MA, USA and LS-B2905, LSBio, Seattle, WA, USA). One antibody was successfully validated (D4O6W, Cell Signaling) and utilized to stain all subject slides [dilution 1:50, antigen heat mediated retrieval (Biocare Medical), manual staining]. Two additional anti-IGF-1 receptor antibodies failed validation (Ab131476 [Abcam] for absent staining on breast cancer control samples and LS-B2905 [LSBio] for nonspecific staining on breast cancer control samples). One anti-GH receptor antibody was successfully validated using normal hepatic tissue (HPA045339, Atlas, Sweden) and was then utilized to stain all subject slides (dilution 1:25, antigen heat mediated retrieval [Biocare Medical], manual staining). Results were systematically reviewed by a single pathologist (RM) with blinded assessment of all samples for staining intensity and zonal distribution. Staining intensity was categorized as either weak, moderate or strong and zonality was categorized as centrilobular (zone 3>1), absent or peri-portal (zone 1>3).
Statistical analysis
Continuous variables are reported as mean ± SD and categorical variables as n (%), unless specified otherwise. JMP Pro Statistical Database Software (Version 12; SAS Institute, Cary, NC) was used to perform all IHC analyses and R was used to perform Nanostring data analysis. Gene expression data were not normally distributed in all groups, and therefore, a generalized linear model (GLM) approach for non-normal data was utilized to determine differences in gene expression (30). This approach has been utilized in order to address biologic variability in RNAseq analyses (31). Specifically, the GLM function in R was utilized with a binomial family parameter for binary logistic regression of non-normally distributed data, and these models were adjusted for age and BMI.
Results
Gene expression cohort clinical characteristics
Clinical characteristics of the gene expression cohort were previously published and are presented in Table 1 (29). In brief, the cohort (n=318) was 76.4% (n=243) female with a mean BMI of 47±7 kg/m2 and mean age of 44±12 years. The subjects were divided into four histologic groups, including NLH (n=76), Steatosis (n=88), NASH F0 (n=72) and NASH F1–4 (n=82). Mean age and BMI did not differ across groups.
Table 1:
Gene Expression Analysis Cohort Clinical Characteristics
| Normal Liver Histology n=76 | Steatosis n=88 | NASH F0 n=72 | NASH F1–F4 n=82 | Overall n=318 | P-value | |
|---|---|---|---|---|---|---|
| Age, years | 41.6±11.5 | 45.2±11.7 | 43.9±12.2 | 45.1±12.9 | 44.0±12.1 | 0.146 |
| BMI (kg/m2) | 45.7±6.6 | 46.1±7.2 | 47.4±7.6 | 47.9±7.3 | 46.8±7.2 | 0.169 |
| Female, n (%) | 65 (85.5) | 65 (73.9) | 59 (81.9) | 54 (65.9) | 243 (76.4) | 0.018 |
| Race, n (%) | ||||||
| Black or African American | 36 (47.4) | 27 (30.7) | 22 (30.6) | 13 (15.9) | 98 (30.8) | 0.014 |
| White | 36 (47.4) | 58 (65.9) | 49 (68.1) | 68 (82.9) | 211 (66.4) | |
| Other | 1 (13) | 1 (11) | 0 (0.0) | 1 (12) | 3 (0.9) | |
| Unknown | 3 (3.9) | 2 (2.2) | 1 (14) | 0 (0.0) | 6 (1.9) | |
| Ethnicity, n (%) | ||||||
| Hispanic | 4 (5.3) | 6 (6.8) | 3 (4.2) | 6 (7.3) | 19 (6.0) | 0.011 |
| Diabetes, n (%) | 6 (7.9) | 20 (22.7) | 21 (29.2) | 46 (56.1) | 93 (29.2) | <0.001 |
| Dyslipidemia, n (%) | 17 (22.4) | 27 (31.0) | 27 (37.5) | 44 (53.7) | 115 (36.3) | <0.001 |
| ALT (U/L) | 25±2 | 29±2 | 32±2 | 42±2 | 32±2 | <0.001 |
| AST (U/L) | 18±2 | 20±2 | 19±2 | 28±2 | 21±2 | <0.001 |
| Creatinine (mg/dL) | 0.86±0.48 | 0.73±0.17 | 0.78±0.15 | 0.83±0.22 | 0.80±0.29 | 0.428 |
Values for categorical variables are expressed as n (percentage) and continuous variables are presented in the form of mean±SD. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; HDL, high-density lipoprotein; NASH, nonalcoholic steatohepatitis. Data adapted from Subudhi et al., Hepatology Communications, 2021.
Gene expression results
Gene expression analysis demonstrated that there was no difference in IGF-1 receptor (Fig. 1A) or GH receptor gene expression (Fig. 1B) between NLH, Steatosis, NASH F0 and NASH F1–4 in multivariable models controlled for BMI and age. However, consistent with prior literature (32), IGF-1 gene expression decreased across disease stages (Fig. 1C). A sensitivity analysis was performed excluding subjects with cirrhosis (NASH F4, n=3), and the results of these analyses were unchanged.
Figure 1:
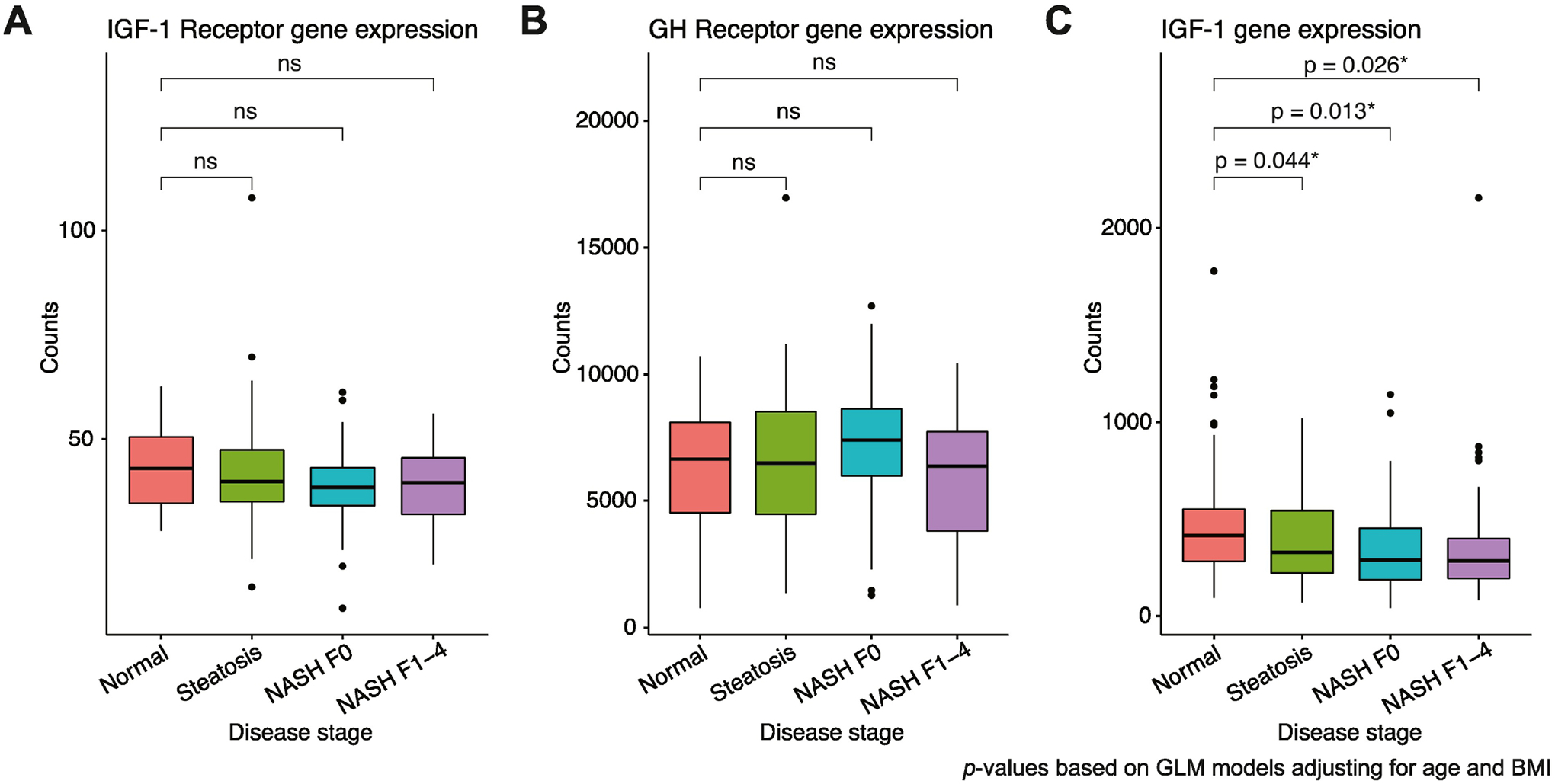
Gene expression analysis for (A) IGF-1 receptor, (B) GH receptor and (C) IGF-1 across worsening stages of NAFLD/NASH. Raw data are plotted. P-values based on generalized linear models adjusted for age and BMI. Box plot lower and upper hinges correspond to the 25th and 75th percentiles, respectively. Lower whisker extends to the smallest value at most 1.5 times the interquartile range and upper whisker extends to the highest value no further than 1.5 times the interquartile range. Data points beyond the whiskers are plotted as individual outliers.
Gene expression by sex and presumed menopausal status
GH receptor gene expression was significantly higher in women versus men across all disease severity groups (Fig. 2B). When broken down by age as a proxy of menopausal status, women <50 years (presumed premenopausal) had significantly higher expression of GH receptor compared to men only but not compared to women ≥50 years (presumed postmenopausal) (Fig. 2E); there was no difference in GH receptor expression between presumed postmenopausal women and men (Fig. 2E).
Figure 2:
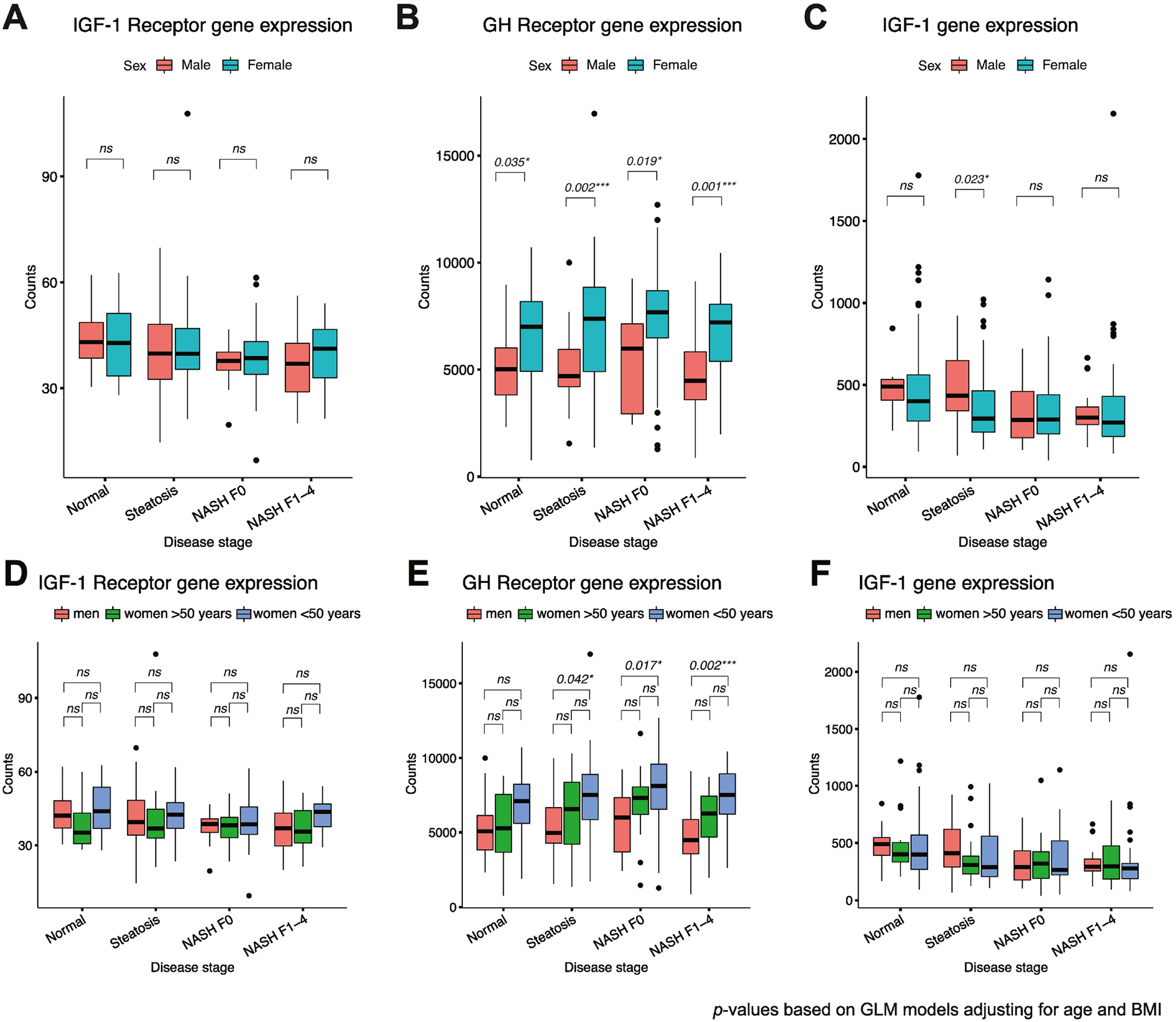
(A) IGF-1 receptor, (B) GH receptor and (C) IGF-1 gene expression by sex and presumed menopausal status (premenopausal <50 years and postmenopausal ≥50 years). Box plot lower and upper hinges correspond to the 25th and 75th percentiles, respectively. Lower whisker extends to the smallest value at most 1.5 times the interquartile range and upper whisker extends to the highest value no further than 1.5 times the interquartile range. Data points beyond the whiskers are plotted as individual outliers.
In contrast, there were no significant differences in IGF-1 receptor (Fig. 2A and 2D) or IGF-1 expression (Figs. 2C and 2F) between men and women, including when classified by presumed menopausal status, with the exception of higher IGF-1 gene expression in men versus all women in the Steatosis group only (Fig. 2C).
Immunohistochemistry cohort clinical characteristics
The mean age for the immunohistochemistry cohort was 57±8 years and mean BMI was 44±6 kg/m2. Cohort characteristics and liver histology by group are reported in Table 2. Mean (±SD) BMI, age, sex and diabetes status did not differ across the NLH, steatosis and NASH F2–3 groups, per study design. Of note, one subject assigned to the NLH group based on clinical pathologic read was found to have mild liver fat based on subsequent blinded pathological review but was included in the NLH group for analysis based on the a priori intention to analyze matching categorization.
Table 2:
Immunohistochemistry Cohort Demographics and GH Receptor Staining
| NLH n=10 | Steatosis n=10 | NASH n=10 | P-value | |
|---|---|---|---|---|
| Cohort Characteristics | ||||
| Age, years (mean±SD) | 59±5 | 56±9 | 55±8 | NS |
| BMI (kg/m2) | 43±7 | 46±5 | 45±7 | NS |
| Male, n (%) | 5 (50) | 5 (50) | 5 (50) | NS |
| Race | ||||
| White, n (%) | 10 (100) | 8 (80) | 9 (90) | NS |
| Other, n (%) | 0 (0) | 2 (20) | 1 (10) | |
| Hispanic Ethnicity, n (%) | 0 (0) | 0 (0) | 1 (10) | NS |
| Diabetes, n (%) | 10 (100) | 10 (100) | 10 (100) | NS |
| NAFLD Activity Score (NAS)# | 0.1±0.3a | 1.1±0.3b | 5.9±1.0c | <0.0001 |
| Fibrosis stage | 0±0a | 0±0a | 2.4±0.5b | <0.0001 |
| Immunohistochemistry Results | ||||
| GH Receptor Staining Intensity | ||||
| Low (weak) | 4 | 7 | 4 | NS |
| High (moderate-strong) | 6 | 3 | 6 | NS |
| GH Receptor Zonality | ||||
| Centrilobular (Z3>1) | 7 | 4 | 7 | NS |
| Absent | 2 | 6 | 3 | NS |
| Peri-portal (Z1>3) | 1 | 0 | 0 | NS |
NLH NAFLD Activity Score (NAS) was >0 due to one NLH subject being classified as having mild liver fat based on blinded assessment post group assignment. Superscript letters indicate statistical difference across different letters. Abbreviations: NLH, normal liver histology and BMI, body mass index.
Mean NAFLD Activity Score (NAS) was higher in the NASH versus Steatosis and NLH groups, per study design. Mean NAS score for the NASH group was 5.9±1.0 (range 0–8), consistent with steatohepatitis.
IGF-1 receptor staining in NAFLD/NASH
Despite adequate validation of the Cell Signaling anti-IGF-1 receptor antibody with expected staining of breast cancer control tissue, there was no staining of the hepatic tissue across any subject group. Confirmatory testing was attempted with two additional anti-IGF-1 receptor antibodies that both failed validation as described in the methods.
GH receptor staining in NAFLD/NASH
GH receptor staining was localized to hepatocytes based on morphology. There was no significant difference between GH receptor staining intensity or zonal distribution between the three groups (Table 2).
Discussion
We hypothesized that the degree of expression of both IGF-1 receptor and GH receptor would directly correlate with the severity of NAFLD/NASH. Contrary to our hypothesis, IGF-1 receptor and GH receptor expression by gene expression analysis remained unchanged across the spectrum of disease severity in NAFLD/NASH. GH receptor expression also appeared to be estrogen dependent when analyzed by sex and menopausal status, with higher GH receptor expression in presumed premenopausal women. These data suggest that IGF-1 receptors and GH receptors remain a potentially actionable target for novel therapeutics in this disease state, and that the interaction between estrogen and GH may play an important role in mediating sex differences in NAFLD and NASH.
We additionally hypothesized that IGF-1R would be pathologically expressed specifically on hepatocytes of patients with liver damage due to NAFLD/NASH. We were unable to demonstrate IGF-1R expression in hepatocytes or hepatic tissue of individuals with NAFLD and NASH by immunohistochemistry, findings that were likely limited by technical factors, as gene expression analysis did demonstrate consistent IGF-1 receptor expression across all severity of NAFLD. In contrast, immunohistochemistry methods were able to successfully demonstrate robust GH receptor staining localized to human hepatocytes, with intensity and zonality that was not affected by the severity of NAFLD or NASH.
Sex differences in GH receptor gene expression are of particular interest, as GH receptor expression was higher in premenopausal women (presumed by age) with high estrogen exposure compared to men. However, no differences were seen in IGF-1 gene expression by sex or menopausal status. These data are consistent with animal models demonstrating higher levels of hepatic GH receptor binding sites in females versus males as well as increased expression of hepatic GH receptor in rats with estrogen exposure (33–35). Estrogen is known to induce hepatic GH resistance by inhibiting GH receptor signaling via the JAK/STAT pathway (36), although IGF-1 levels and age-adjusted normal ranges do not differ between men and women (37). Therefore, these data may suggest that GH receptor is potentially upregulated in the presence of estrogen in order to maintain similar serum IGF-1 levels in women as in men. This is of particular interest given that premenopausal women are relatively protected from the development of NAFLD (38), particularly until menopause (39), and that postmenopausal women in particular have a higher risk of NASH and advanced fibrosis as compared to men (38,40,41). It is possible that GH regulation of lipolysis and IGF-1 production may be somewhat uncoupled, with endogenous estrogen inducing GH resistance with respect to IGF-1 production but not lipolysis (42). Further investigation of the interaction between estrogen and the GH axis as a contributor to sex differences in the development of NAFLD and progression to NASH is needed to define this complex pathophysiology.
This study also demonstrated that hepatic IGF-1 gene expression significantly decreased with increasing histologic severity of NAFLD, including when controlled for age and BMI. While we were unable to obtain serum IGF-1 levels to correlate with hepatic IGF-1 gene expression, data from the current study are concordant with our findings from a prior cohort that hepatocyte ballooning, lobular inflammation, NASH and fibrosis were associated with lower serum IGF-1 levels (32).
Improved understanding of the mechanisms and impact of GH and IGF-1 on hepatic steatosis, inflammation and fibrosis could lead to additional insights into the pathophysiology of this multifactorial disease process. However, teasing apart the specific effects of GH and IGF-1 is challenging, as interruption of GH signaling leads to a concurrent decline in IGF-1 levels and reconstitution of functional GH signaling leads to a concurrent restoration of IGF-1. GH has known lipolytic and anti-inflammatory effects, and disruption of GH signaling pathways in preclinical models lead to hepatic steatosis (9–15) as well as inflammation (9,43) and fibrosis (9). While IGF-1 is not lipolytic, it improves insulin sensitivity, and there are preclinical and clinical data that implicate IGF-1 in hepatic regeneration and fibrosis reduction. However, it is not known whether these regenerative effects are mediated through hepatocytes, hepatic stellate cells or both. Overexpression of IGF-1 receptor in hepatic stellate cells has been shown to enhance hepatic regeneration in cirrhotic rats by reducing stellate cell activation and decreasing fibrogenesis (44). The IGF-1 receptor is not expressed in normal hepatocytes in adult mice (44) or in humans (25,45). However, hepatocyte IGF-1 receptor expression has been demonstrated in the presence of liver damage, suggestive of a compensatory regenerative response. IGF-1 receptor expression has been demonstrated in mouse hepatocytes post injury by hepatectomy (25), and a hepatocyte specific IGF-1 knockout mouse demonstrated reduced regeneration with corresponding reduction in the IGF-1 receptor, IRS-1/ERK signaling pathway (45). Adults with chronic hepatitis C have increased hepatic and hepatocyte expression of IGF-1 receptor mRNA that normalizes after virologic response to treatment (26). Finally, Alisi et al. were able to demonstrate higher hepatic IGF-1R expression with increasing levels of fibrosis in a pediatric cohort, although this may have predominantly been due to an increased expression of IGF-1 receptor specifically in hepatic stellate cells (27). We were unable to make conclusions about IGF-1R expression in hepatocytes in the setting of NAFLD and NASH from this investigation due to technical factors, as no staining was seen in any hepatic tissue even when utilizing an anti-IGF-1R antibody that had been successfully validated in breast cancer tissue.
Finally, it is important to acknowledge the newly proposed nomenclature of Metabolic Associated Fatty Liver Disease (MAFLD), which is defined as fatty liver with associated with one of the following: 1) overweight/obesity, 2) diabetes or 3) evidence of metabolic dysfunction (46,47). This MAFLD nomenclature seeks to provide an alternative to the term nonalcoholic fatty liver disease, which is a diagnosis of exclusion that also carries the stigmata of being defined by extent of alcohol use. The MAFLD definition sets up a scaffolding by which patients with fatty liver can be further characterized and studied, both in clinical care and research. In fact, all subjects in this study meet criteria of hepatic steatosis with overweight/obesity, and thus could be characterized as having MAFLD. Perhaps in the future, characterization of the GH/IGF-1 axis could be utilized as a biomarker to identify a novel subtype of MAFLD associated with this relative deficiency of the endogenous hormone axis.
This study was limited by a small sample size in the immunohistochemistry cohort, which was limited by our goal of strict matching on age, sex, BMI and diabetes status. However, the sample size was robust in the gene expression analysis cohort. Additionally, we were unable to localize IGF-1 receptor expression, despite attempting to utilize three different anti-IGF-1 antibody receptors. In the gene expression analysis cohort, we were unable to quantify GH gene expression, which was not included in our Nanostring nCounter gene panel. We additionally did not have estradiol levels and/or specific information regarding menopausal status, thus used aged as a proxy for this analysis.
In conclusion, our study is the first to demonstrate preserved expression of the IGF-1R and GHR despite worsening severity of NAFLD and NASH in adults. Additionally, this is the first study in humans to demonstrate that women, and particularly premenopausal women, have higher levels of hepatic GHR expression as compared to men, which possibly underlies sex differences in NAFLD and NASH. Additional research is needed regarding the contribution of the GH/IGF-1 axis to the pathophysiology of NAFLD and NASH as well as the potential impact of GH administration on hepatic endpoints in this highly prevalent and morbid disease.
HIGHLIGHTS.
GH and IGF-1 have been implicated in the pathogenesis of NAFLD
Lower IGF-1 expression is associated with more severe NAFLD
Expression of IGF-1 and GH receptors is unchanged with increasing severity of NAFLD
The GH/IGF-1 axis is a potential therapeutic target in NAFLD
Funding Statement:
This work was conducted with support from the following National Institutes of Health grants: K23 DK113220 (LED), P30 DK046200 (LED Pilot Award), K24DK109940 (MAB) and the Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health. The Massachusetts General Hospital Research Scholars Program supported RTC. German Research Foundation supported HKD (DR 1161/1-1) and LMB (BA 596 7175/1-1).
Conflict of Interest Statement:
KEC serves on the scientific advisory board for Novo Nordisk, Theratechnologies and Bristol Myers Squibb (BMS) and has received grant funding from Boehringer-Ingelheim, BMS and Novartis. LED has received drug donation from Pfizer by investigator-initiated request. DWG is a consultant to Boston Scientific, Ethicon J&J Medical Devices and Medtronic and serves on the medical advisory board for Boston Scientific, and New View Surgical. All other authors have no conflicts of interest to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28(1):155–161. [DOI] [PubMed] [Google Scholar]
- 2.Wree A, Broderick L, Canbay A, Hoffman HM, Feldstein AE. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol. 2013;10(11):627–636. [DOI] [PubMed] [Google Scholar]
- 3.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Am J Gastroenterol. 2012;107(6):811–826. [DOI] [PubMed] [Google Scholar]
- 4.Charlton M Cirrhosis and liver failure in nonalcoholic fatty liver disease: Molehill or mountain? Hepatology. 2008;47(5):1431–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Utz AL, Yamamoto A, Hemphill L, Miller KK. Growth hormone deficiency by growth hormone releasing hormone-arginine testing criteria predicts increased cardiovascular risk markers in normal young overweight and obese women. J Clin Endocrinol Metab. 2008;93(7):2507–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaves VE, Junior FM, Bertolini GL. The metabolic effects of growth hormone in adipose tissue. Endocrine. 2013;44(2):293–302. [DOI] [PubMed] [Google Scholar]
- 7.Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev. 2008;29(5):535–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gleeson HK, Lissett CA, Shalet SM. Insulin-like growth factor-I response to a single bolus of growth hormone is increased in obesity. J Clin Endocrinol Metab. 2005;90(2):1061–1067. [DOI] [PubMed] [Google Scholar]
- 9.Nishizawa H, Takahashi M, Fukuoka H, Iguchi G, Kitazawa R, Takahashi Y. GH-independent IGF-I action is essential to prevent the development of nonalcoholic steatohepatitis in a GH-deficient rat model. Biochem Biophys Res Commun. 2012;423(2):295–300. [DOI] [PubMed] [Google Scholar]
- 10.Sos BC, Harris C, Nordstrom SM, et al. Abrogation of growth hormone secretion rescues fatty liver in mice with hepatocyte-specific deletion of JAK2. The Journal of clinical investigation. 2011;121(4):1412–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cordoba-Chacon J, Majumdar N, List EO, et al. Growth hormone inhibits hepatic de novo lipogenesis in adult mice. Diabetes. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nordstrom SM, Tran JL, Sos BC, Wagner KU, Weiss EJ. Disruption of JAK2 in adipocytes impairs lipolysis and improves fatty liver in mice with elevated GH. Mol Endocrinol. 2013;27(8):1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan Y, Menon RK, Cohen P, et al. Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. The Journal of biological chemistry. 2009;284(30):19937–19944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barclay JL, Nelson CN, Ishikawa M, et al. GH-dependent STAT5 signaling plays an important role in hepatic lipid metabolism. Endocrinology. 2011;152(1):181–192. [DOI] [PubMed] [Google Scholar]
- 15.Collin de l’Hortet A, Zerrad-Saadi A, Prip-Buus C, et al. GH administration rescues fatty liver regeneration impairment by restoring GH/EGFR pathway deficiency. Endocrinology. 2014;155(7):2545–2554. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto R, Fukuoka H, Iguchi G, et al. Long-term effects of growth hormone replacement therapy on liver function in adult patients with growth hormone deficiency. Growth Horm IGF Res. 2014;24(5):174–179. [DOI] [PubMed] [Google Scholar]
- 17.Nishizawa H, Iguchi G, Murawaki A, et al. Nonalcoholic fatty liver disease in adult hypopituitary patients with GH deficiency and the impact of GH replacement therapy. European journal of endocrinology / European Federation of Endocrine Societies. 2012;167(1):67–74. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi Y, Iida K, Takahashi K, et al. Growth hormone reverses nonalcoholic steatohepatitis in a patient with adult growth hormone deficiency. Gastroenterology. 2007;132(3):938–943. [DOI] [PubMed] [Google Scholar]
- 19.Beauregard C, Utz AL, Schaub AE, et al. Growth hormone decreases visceral fat and improves cardiovascular risk markers in women with hypopituitarism: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2008;93(6):2063–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snel YE, Doerga ME, Brummer RM, Zelissen PM, Koppeschaar HP. Magnetic resonance imaging-assessed adipose tissue and serum lipid and insulin concentrations in growth hormone-deficient adults. Effect of growth hormone replacement. Arterioscler Thromb Vasc Biol. 1995;15(10):1543–1548. [DOI] [PubMed] [Google Scholar]
- 21.Bengtsson BA, Brummer RJ, Eden S, Rosen T, Sjostrom L. Effects of growth hormone on fat mass and fat distribution. Acta Paediatr Suppl. 1992;383:62–65; discussion 66. [PubMed] [Google Scholar]
- 22.Bredella MA, Lin E, Brick DJ, et al. Effects of GH in women with abdominal adiposity: a 6-month randomized, double-blind, placebo-controlled trial. Eur J Endocrinol. 2012;166(4):601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bredella MA, Gerweck AV, Lin E, et al. Effects of GH on body composition and cardiovascular risk markers in young men with abdominal obesity. J Clin Endocrinol Metab. 2013;98(9):3864–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massague J, Czech MP. The subunit structures of two distinct receptors for insulin-like growth factors I and II and their relationship to the insulin receptor. J Biol Chem. 1982;257(9):5038–5045. [PubMed] [Google Scholar]
- 25.Caro JF, Poulos J, Ittoop O, Pories WJ, Flickinger EG, Sinha MK. Insulin-like growth factor I binding in hepatocytes from human liver, human hepatoma, and normal, regenerating, and fetal rat liver. J Clin Invest. 1998;81(4):976–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stefano JT, Correa-Giannella ML, Ribeiro CM, et al. Increased hepatic expression of insulin-like growth factor-I receptor in chronic hepatitis C. World J Gastroenterol. 2006;12(24):3821–3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alisi A, Pampanini V, De Stefanis C, et al. Expression of insulin-like growth factor I and its receptor in the liver of children with biopsy-proven NAFLD. PLoS One. 2018;13(7):e0201566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. [DOI] [PubMed] [Google Scholar]
- 29.Subudhi S, Drescher HK, Dichtel LE, et al. Distinct Hepatic Gene-Expression Patterns of NAFLD in Patients With Obesity. Hepatol Commun. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartoszek K The New Statistics with R: an Introduction for Biologists. — By Andy Hector. Systematic Biology. 2015;64(6):1125–1128. [Google Scholar]
- 31.McCarthy DJ, Chen Y, Smyth GK. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic acids research. 2012;40(10):4288–4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dichtel LE, Corey KE, Misdraji J, et al. The Association Between IGF-1 Levels and the Histologic Severity of Nonalcoholic Fatty Liver Disease. Clin Transl Gastroenterol. 2017;8(1):e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennett PA, Levy A, Carmignac DF, Robinson IC, Lightman SL. Differential regulation of the growth hormone receptor gene: effects of dexamethasone and estradiol. Endocrinology. 1996;137(9):3891–3896. [DOI] [PubMed] [Google Scholar]
- 34.Maes M, De Hertogh R, Watrin-Granger P, Ketelslegers JM. Ontogeny of liver somatotropic and lactogenic binding sites in male and female rats. Endocrinology. 1983;113(4):1325–1332. [DOI] [PubMed] [Google Scholar]
- 35.Baxter RC, Bryson JM, Turtle JR. Somatogenic receptors of rat liver: regulation by insulin. Endocrinology. 1980;107(4):1176–1181. [DOI] [PubMed] [Google Scholar]
- 36.Leung K-C, Johannsson G, Leong GM, Ho KKY. Estrogen Regulation of Growth Hormone Action. Endocrine Reviews. 2004;25(5):693–721. [DOI] [PubMed] [Google Scholar]
- 37.Span JP, Pieters GF, Sweep CG, Hermus AR, Smals AG. Gender difference in insulin-like growth factor I response to growth hormone (GH) treatment in GH-deficient adults: role of sex hormone replacement. J Clin Endocrinol Metab. 2000;85(3):1121–1125. [DOI] [PubMed] [Google Scholar]
- 38.Balakrishnan M, Patel P, Dunn-Valadez S, et al. Women Have a Lower Risk of Nonalcoholic Fatty Liver Disease but a Higher Risk of Progression vs Men: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2021;19(1):61–71.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gutierrez-Grobe Y, Ponciano-Rodríguez G, Ramos MH, Uribe M, Méndez-Sánchez N. Prevalence of non alcoholic fatty liver disease in premenopausal, posmenopausal and polycystic ovary syndrome women. The role of estrogens. Ann Hepatol. 2010;9(4):402–409. [PubMed] [Google Scholar]
- 40.Klair JS, Yang JD, Abdelmalek MF, et al. A longer duration of estrogen deficiency increases fibrosis risk among postmenopausal women with nonalcoholic fatty liver disease. Hepatology. 2016;64(1):85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsuo K, Gualtieri MR, Cahoon SS, et al. Surgical menopause and increased risk of nonalcoholic fatty liver disease in endometrial cancer. Menopause. 2016;23(2):189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneider G, Kirschner MA, Berkowitz R, Ertel NH. Increased estrogen production in obese men. J Clin Endocrinol Metab. 1979;48(4):633–638. [DOI] [PubMed] [Google Scholar]
- 43.Sarmento-Cabral A, Del Rio-Moreno M, Vazquez-Borrego MC, et al. GH directly inhibits steatosis and liver injury in a sex-dependent and IGF1-independent manner. J Endocrinol. 2021;248(1):31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanz S, Pucilowska JB, Liu S, et al. Expression of insulin-like growth factor I by activated hepatic stellate cells reduces fibrogenesis and enhances regeneration after liver injury. Gut. 54(1):134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Desbois-Mouthon C, Wendum D, Cadoret A, et al. Hepatocyte proliferation during liver regeneration is impaired in mice with liver-specific IGF-1R knockout. Faseb j. 2006;20(6):773–775. [DOI] [PubMed] [Google Scholar]
- 46.Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. Journal of Hepatology. 2020;73(1):202–209. [DOI] [PubMed] [Google Scholar]
- 47.Eslam M, Sanyal AJ, George J, et al. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158(7):1999–2014.e1991. [DOI] [PubMed] [Google Scholar]


