Abstract
Drugs of abuse including cannabis and inhalants impair risk/reward decision making. Cannabis use is often concurrent with inhalant intoxication, yet preclinical studies investigating the role of endocannabinoids in inhalant misuse are limited. To address this gap in the literature we used the well-validated probabilistic discounting task to assess risk/reward decision making in rodents following combinations of toluene vapor (a common inhalant) and manipulations of cannabinoid receptor type 1 (CB1R) signaling. As reported previously, acute exposure to toluene vapor disrupted behavioral flexibility during probabilistic discounting. Systemic administration of the CB1R inverse agonist AM281 did not prevent toluene-induced alterations in risky choices, but did independently reduce win-stay behavior, increase choice latency, and increase omissions. Toluene-induced deficits in probabilistic discounting are thought to involve impaired medial prefrontal cortex (mPFC) activity. As we previously reported that some of toluene’s inhibitory effects on glutamatergic signaling in the mPFC are endocannabinoid-dependent, we tested the hypothesis that mPFC CB1R activity mediates toluene-induced deficits in discounting. However, bilateral injection of the CB1R inverse agonist AM251 prior to toluene vapor exposure had no effect on toluene-induced changes in risk behavior. In a final set of experiments, we injected the CB1R inverse agonist AM251 (5 and 50 ng), the CB1R agonist WIN55,212-2 (50 ng and 500 ng), or vehicle into the mPFC prior to testing. While mPFC CB1R stimulation did not affect any of the measures tested, the CB1R inverse agonist caused a dose-dependent reduction in win-stay behavior without altering any other measures. Together, these studies indicate that toluene-induced deficits in probabilistic discounting are largely distinct from CB1R-dependent effects, that include decreased effectiveness of positive reinforcement (mPFC CB1Rs), decision making speed, and task engagement (non-mPFC CB1Rs).
Keywords: inhalant, gambling, endocannabinoid, rat, prelimbic cortex, AM 251, AM 281, WIN 55, 212-2
Introduction
Abused substances impair risky decision making in humans (Lane et al., 2005; Euser et al., 2011; Buelow and Suhr, 2014) and rodents (Floresco and Whelan, 2009; Mitchell and Blankenship, 2011). Preclinical studies on the effects of drugs of abuse on decision making have focused primarily on stimulants and alcohol. However, recent work from our laboratory has shown that toluene, a volatile organic solvent and abused inhalant, also perturbs flexible risk/reward decision making and associated reward-related activity in the prefrontal cortex (PFC; Braunscheidel et al., 2019).
Interestingly, some of the inhibitory effects of toluene on glutamatergic signaling are mediated by enhanced endocannabinoid/cannabinoid receptor type 1 receptor (CB1R) activity in regions of the brain that are important for decision making. For example, a brief exposure to toluene produces a long-lasting endocannabinoid/CB1R-dependent depression in AMPA-mediated synaptic currents in deep-layer pyramidal neurons in the prelimbic PFC (Beckley and Woodward, 2011) and those generated in D2 but not D1 medium spiny neurons in the nucleus accumbens (Beckley et al., 2016). Beyond these reports, preclinical studies investigating the interaction between inhalants and endocannabinoids are limited.
Research on the modulation of CB1Rs in the context of cost/benefit decision making are important for developing informed health and safety policies. Relaxed legislation on cannabis restrictions is leading to greater consumption of delta9-tetrahydrocannabinol-containing products in adults (Carliner et al., 2017) and cannabis is the most common illicit intoxicant observed in ambulance attendees involving inhalant misuse (Crossin et al., 2018). Given the comorbities of cannabis use with drug use disorders, mood disorders, and anxiety disorders (Stinson et al., 2006), it is not surprising that CB1R receptor expression is widespread in regions that are important for decision making such as the prefrontal cortex (Eggan et al., 2010), hippocampus (Davies et al., 2002), and striatum (Julian et al., 2003; Pickel et al., 2004) for review, see (Hu and Mackie, 2015). Enhancing CB1R signaling disrupts higher cognitive functions including risky decision making in humans (Lane and Cherek, 2002; Lane et al., 2005; Anderson et al., 2010). However, these effects have not been reproduced in two separate preclinical models: a rat gambling task (Ferland et al., 2018) or one involving punishment-based risk taking (Freels et al., 2020). Here we use a third, well-validated rodent model of risk/reward decision making, the probabilistic discounting task (St Onge and Floresco, 2009; St Onge et al., 2012; Braunscheidel et al., 2019) to test the hypotheses that CB1R antagonism is sufficient to modulate risky decision making and that toluene-induced impairments depend on CB1R signaling.
Interestingly, impairments in behavioral flexibility caused by toluene mimic those observed by inactivation of the mPFC (St Onge and Floresco, 2010), and exposure to toluene vapor disrupts mPFC pyramidal activity during contingency updating and monitoring (Braunscheidel et al., 2019). As mentioned above, results from our previous electrophysiology recordings in the mPFC show that ex vivo toluene application reduced AMPA-mediated excitatory signaling via activation of presynaptic CB1Rs (Beckley and Woodward, 2011). While mPFC CB1R signaling is involved in fear and anxiety-related decision making (Draycott et al., 2014; Schneider et al., 2015), their role in risk/reward decision making have not been explored. To address this gap in the literature we studied the effect of local pharmacological mPFC CB1R manipulation on probabilistic discounting. Finally, we test the hypothesis that toluene-induced impairments depend on mPFC CB1R signaling. Our results provide evidence for the involvement of CB1R signaling during probabilistic discounting that is unrelated to toluene-induced deficits in risk/reward decision making.
Materials and Methods
Subjects
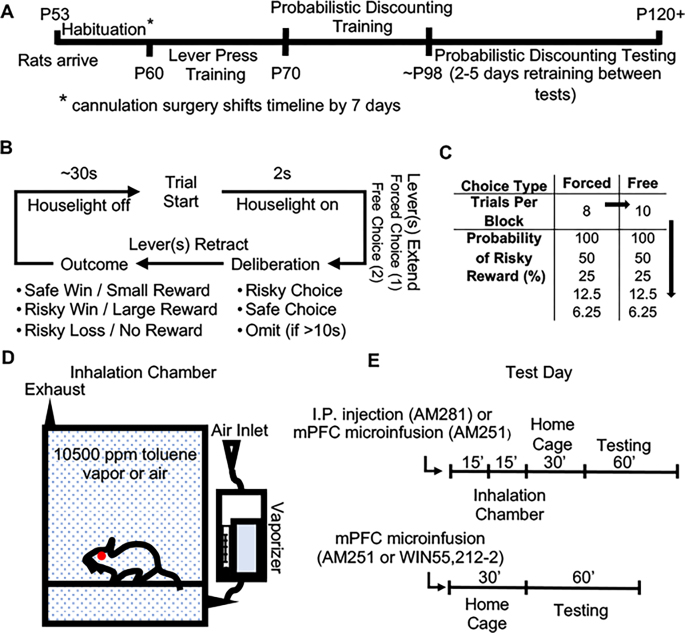
Fifty-eight male Sprague-Dawley rats (post-natal day (P) 53 on arrival; Envigo RMS, Indianapolis, IN) were pair-housed in polypropylene cages on a reverse light cycle (lights off at 0900) in a climate-controlled room with food and water delivered ad libitum. At approximately P60–70, rats were food restricted to maintain 85–90% of their free feeding weight (weight at time of final testing (300–400 g). Figure 1A details the experimental timeline for all rodents. All procedures were performed in compliance with Medical University of South Carolina IACUC protocols.
Figure 1. Probabilistic discounting training and test design.
(A) Experimental timeline and corresponding rat age. (B) Flow chart detailing a single trial of the probabilistic discounting task. (C) Breakdown of the 10 different probability blocks within the probabilistic discounting task with odds presented in descending order. (D) Inhalation chamber schematic. (E) Test day progression for the two toluene exposure experiments (top) and two mPFC CB1R modulation experiments (bottom).
Surgeries
A subset of 30 animals underwent stereotaxic surgery ~1 week after arrival during which deep anesthesia was achieved via an isoflurane vaporizer (Penlon; 1 L/min, 5% induction, 2–3% maintenance). Bilateral guide cannula (Plastics One) were implanted above the prelimbic mPFC (± 0.6ML, +2.95 AP, −2.85DV from Bregma) and microinfusion tips extended 1 mm from the guide cannula for a final injection location of −3.85 DV from Bregma. Cefazolin (165 mg/kg, SC) was injected post-operation to limit infection. Animals were also injected with carprofen (2.5 mg/kg, SC) analgesia pre- and post-operation. Carprofen injections were continued once daily for the three days following surgery in compliance with Medical University of South Carolina IACUC protocols.
Lever Press Training
Lever press training occurred over the course of 1–2 weeks as previously described (Braunscheidel et al 2019). In brief, rats were habituated to a reward of 20% sweetened condensed milk (SCM), by giving them free access to 10 ml SCM for two days prior to operant training. Over the course of two phases, rats (P60–70) were trained to lever press in operant chambers (Med Associates, St. Albans, VT) for SCM delivered to a central feeding well via a pump-activated syringe. Phase 1 (2–5 days; 30 min sessions) began with one lever (left or right, pseudo-randomly assigned) reinforced with 45 μl SCM on an FR1 schedule. Upon meeting criteria (50 presses for 2 consecutive days), the presented lever was switched, and rats were tested to criteria before moving on to phase 2. Phase 2 (6–7 days; 60-minute sessions) consisted of 90 trials separated by 35s. Each session began with an illuminated house light and 2s later, the left or right lever extended in a pseudo-random order. When pressed, the lever retracted, and 45 μl SCM was delivered on 75% of trials. If a lever was not pressed within 20s, it retracted, and the trial was recorded as an omission. Following completion of two consecutive days with less than 10 omissions per session, the time to omission was reduced to 10s. When rats met criteria again, the lever reward probability reduced to 50%. When rats met criteria a third time, a side preference test was performed. Briefly, for each of 60 trials, both levers extended simultaneously and were reinforced on an FR1 schedule. A trial concluded when two presses occurred, which resulted in lever retraction for 20s. The preferred side was defined as the side that a rat pressed first most often across trials. Rodents then began training in the probabilistic discounting task.
Probabilistic Discounting
A probabilistic discounting procedure used to assess risk/reward decision making in rodents as previously described (St Onge and Floresco, 2009; Braunscheidel et al., 2019). This two-lever choice task (Fig. 1B) consists of a “safe” lever that delivered a small reward (30 μl SCM) 100% of the time and a “risky” lever that delivered a large reward (90 μl SCM) with varying probability of reinforcement. The risky lever was assigned to the non-preferred lever position as determined by the side preference test. Each session consisted of 90 trials separated into 5 blocks and each block started with 8 forced-choice trials that set the probability of reinforcement for the following 10 free-choice trials (Fig. 1C). The probability of obtaining a large reward was varied from high-to-low with the following probabilities: 100%, 50%, 25%, 12.5%, 6.25%. Each trial lasted 35s and began with an illuminated house light and 2s later levers extended into the chamber. A press on either lever caused both of them retract and turned the house light off. On rewarded trials, reward was delivered to the central feeding well. These “wins” were paired with a discriminative cue: a flashing light above the food well to indicate whether the reward was small or large (safe win: 2 pulses, 0.35s pulse width, 0.5 Hz; risky win: 5 pulses, 0.35s pulse width, 0.5 Hz). On non-reinforced “loss” trials, no cue light was provided. If a lever was not pressed in 10s, it was recorded as an omission and the houselights were extinguished for 25s. Following ~20 days of training (5–6 days per week), rats exhibited stable responding (two-way ANOVA on three consecutive testing days yields no block × day interaction or main effect of day, p > 0.1) and were subjected to drug tests.
Systemic drug administration and inhalation chamber treatments
In some studies, rats received an intra-peritoneal (i.p.) injection of the CB1R inverse agonist AM251 (2 mg/kg; Tocris Bioscience) or vehicle (DMSO) 15 min prior to gas exposures in a 30×30×30 cm inhalation chamber (Fig. 1D, Plas Labs, Lansing, MI). We previously reported that this dose of AM251 reduced perseverative errors in a rat set-shifting task (Hill et al., 2006). Rats then were then returned to their home cage for 30 min prior to task performance (Fig. 1E).
Rats were exposed for 15 minutes to either air or toluene vapor (10,500 ppm; confirmed with a portable toluene gas detector, DOD Technologies, Cary, IL) delivered via a sevoflurane vaporizer (Penlon Limited; flow rate 4L/min) as previously described (Braunscheidel et al., 2017, 2019; Wayman and Woodward, 2018). At this dose of toluene, rats exhibit lethargy after ~10 minutes of exposure and were nearly immobile after 15 minutes. Rats fully regained ambulation following approximately 15 minutes of recovery in the home cage (unpublished observations). These four treatments (AM251 + air, AM251 + toluene, vehicle + air, vehicle + toluene) were administered in a within-subject counter-balanced design with 2–5 days of retraining between tests such that pre-test day performance was equivalent (two-way ANOVA yields no main effect of day, p > 0.1).
Microinfusions
Three separate cohorts of rats were used in microinfusion studies where animals received a series of microinfusions (300 nl over 1 min) into the prelimbic mPFC using a within-subject, counter-balanced design. The first cohort of animals received two doses of the CB1R inverse agonist AM251 (5 and 50 ng) and vehicle (3% DMSO, 3% Tween80, PBS). A second group of animals received two doses of CB1R agonist WIN55,212-2 (50 ng and 500 ng; Tocris Bioscience) and vehicle (3% DMSO, 3% Tween80, PBS). Microinjections were administered 30 min before testing (Fig. 1 E). These doses were selected as they have been shown to alter fear-related behaviors when infused into the mPFC (Laviolette and Grace, 2006). In the third cohort, 50 ng AM251 or vehicle was administered via the cannula 15 min prior to toluene exposure (i.e. 1hr prior to task performance, see Fig. 1E). Rats were given 2–5 days of retraining between tests such that pre-test day performance was equivalent (two-way ANOVA yields no main effect of day, p > 0.1). This design has been validated elsewhere for addressing the neuropharmacology of probabilistic discounting (Stopper et al., 2013).
Statistics
The primary dependent variable of the probabilistic discounting test was risky lever preference, expressed as the proportion of risky choices (number risky lever presses/total lever presses) during each of the five probability blocks separated by likelihood of a rewarded risky lever press. Additional performance variables including omissions, latency to choice, win-stay (number of risky choices following a risky win/total number of risky wins), and lose-shift (number of shifts to the safe lever following a risky loss/total number of risky losses) were also recorded. Only free-choice trials were considered in the behavioral analyses. Choice data obtained from the microinfusion experiments were analyzed with a two-way repeated measures ANOVA with treatment and probability block as factors. Win-stay, lose-shift, and omissions were analyzed with a one-way ANOVA with appropriate multiple comparisons. The systemic AM281 studies were analyzed with a three-way ANOVA with i.p. treatment, inhalation treatment, and probability block as factors. Win-stay, lose-shift, and omissions were analyzed with a 2-way ANOVA with appropriate post hoc multiple comparisons. All statistics and graphing was performed with Prism 8 software (Graphpad Software San Diego, CA) using the recommended tests (Tukey’s, Dunnett’s Sidak’s) for post-hoc comparisons.
Results
Systemic reduction in CB1R activity alters probabilistic discounting performance independently from acute toluene effects:
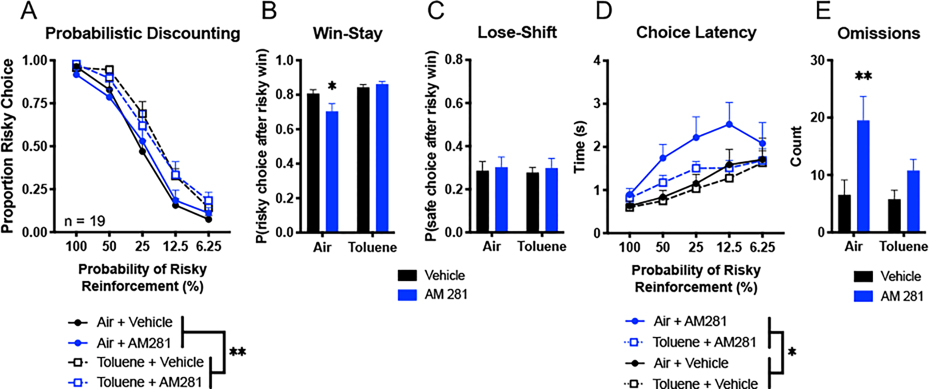
Previous studies in our laboratory have shown that acute toluene exposure impairs flexible adjustments in choice biases during probabilistic discounting (Braunscheidel et al., 2019). In this experiment, we sought to determine whether this effect may be modulated by CB1R activity. Figure 2A shows risk preference during probabilistic discounting behavior in well-trained Sprague-Dawley rats following four treatments: 2 mg/kg AM281 + air inhalation, vehicle + air inhalation, 2 mg/kg AM281 + toluene inhalation, and vehicle + toluene inhalation. Analysis of the choice data revealed that toluene exposure again impaired shifts in choice biases manifesting as increased risky choice under these conditions, as evidenced by a main effect of toluene (F(1, 18) = 14.58, p = 0.0013). Post hoc two-way ANOVA of these data collapsed across all toluene/air treatments revealed that toluene exposure increased risky choice specifically during times of high uncertainty (50%, 25%, and 12.5% blocks, all t(38) > 2.86, p < 0.024) but not during blocks where there was relatively little uncertainty of obtaining the larger reward (100% and 6.25% blocks, both t(38) < 1.78, p > 0.33). However, there was no interaction between the factors (three-way ANOVA, toluene × AM281 × probability block, F(4, 72) = 0.85, p = 0.50) or any combination of two factors (toluene × AM281 F(1, 18) = 0.12, p = 0.74; toluene × probability block F(4, 72) = 1.96, p = 0.11; AM281 × probability block F(4, 72) = 0.55, p = 0.70). Furthermore, AM281 did not appear to alter choice during probabilistic discounting (main effect, F(1, 18) = 0.0020, p = 0.97).
Figure 2. Systemic CB1R inverse agonism alters probabilistic discounting performance independently from acute toluene effects.
Well-trained rats were treated with a combination of injections (2 mg/kg AM281 or vehicle, i.p.) and vapor exposure (toluene or air) prior to task performance. (A) Proportion of risky choice within each probability block across treatments. (B, C) Choice strategies employed across all trials. Win-stay (B) indicates choice of risky lever after risky win while lose-shift (C) indicates choice of safe lever after risky loss. (D) Time to choice selection within each probability block. (E) Omissions across all trials indicate no lever press within the 10 s trial period. Data shown are mean + SEM; all n = 19; three-way ANOVA main effects, *p < 0.05, **p < 0.01; Tukey’s post hoc, *p < 0.05, **p < 0.01.
Subsequent trial-by-trial analysis of the choice data were conduced to examine how the outcomes of risky choices influenced subsequent choice. Figure 2B illustrates the effect of recent positive reinforcement on choice strategy, measured as the probability of choosing the risky lever following a risky win (“win-stay”). Two-way ANOVA revealed an increase in win-stay strategy in toluene treated animals, (main effect, F(1, 18) = 13.18, p = 0.0020) with a significant interaction between toluene and AM281 treatments (F(1, 18) = 7.00, p = 0.017) driven by a reduction in win-stay behavior by AM281 relatively to vehicle treated air exposed animals (Tukey’s post hoc, AM281 vs vehicle, q(14) = 4.48, p = 0.025). Thus, even though CB1R stimulation did not alter overall choice levels, it reduced the influence of recently rewarded risky choices on subsequent choices. In contrast, the effect of recent negative feedback sensitivity on choice strategy, measured as the probability of choosing the safe lever following a risky loss (“lose-shift”; Fig 2C) did not differ across treatment conditions (F(1, 18) < 0.28, p > 0.60). Taken together, the fact that AM281 did not alter the effects of toluene on in probabilistic discounting suggests that the alterations in decision making induced by this inhalant are not mediated by increased systemic CB1R activation.
Choice latency and omission data may reflect decision speed, impulsivity and/or general motivation to lever press for reward. Analysis of the choice data partitioned across blocks yielded no interaction between the factors (three-way ANOVA, toluene × AM281 × risk preference, F(4, 72) = 0.42, p = 0.79) or any combination of two factors (toluene × AM281 F(1, 18) = 1.37, p = 0.26; toluene × risk, F(4, 72) = 1.07, p = 0.38; AM281 × risk, F(4, 72) = 1.10, p =0.38) on choice latency (Fig 2D). On the other hand, whereas toluene did not impact choice latency (main effect, F(1, 18) = 2.45, p = 0.14), it was increased by AM281 (main effect, F(1, 18) = 7.36, p = 0.014). Post hoc two-way ANOVA of these data collapsed across all AM281/vehicle treatments reveals that these differences occur during times of high uncertainty (50%, 25%, and 12.5% blocks, all t(38) > 3.0, p < 0.015) and not when the potential outcome of a risky choice was more certain (100% and 6.25% blocks, both t(38) < 1.24, p > 0.70). AM281-treated animals also had more omissions than their vehicle-treated counterparts (Fig. 2E, two-way ANOVA, main effect of AM281, F(1, 18) = 13.20, p = 0.0020) following air treatment (Tukey’s post hoc, AM281 vs vehicle, q(18) = 5.77, p = 0.0036), but not after toluene exposure. Taken together, these data suggest that AM281 causes task disengagement or delayed decision making speeds, especially during times of increased difficulty or uncertainty.
Toluene induced deficits in probabilistic discounting are not prevented by reduced mPFC CB1R activity
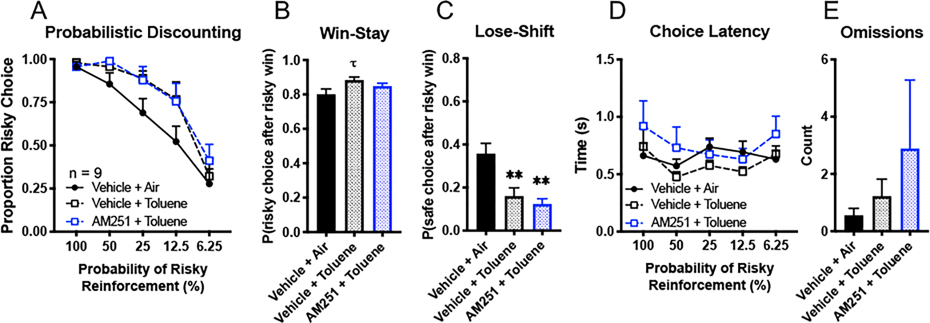
In the next set of experiments, we trained a separate cohort of animals and specifically targeted mPFC CB1Rs via bilateral microinfusion of AM251 into the prelimbic cortex. In keeping with the findings following systemic administration of this compound, intra-PFC infusion of AM251 (50 ng) did not prevent the shift in risk preference following toluene treatment (Fig. 3A, two-way ANOVA, treatment × probability block, F(8, 64) = 0.70, p = 0.69; main effect of treatment, F(2, 16) = 2.15, p = 0.15; Sidak’s post hoc comparing AM251 + toluene to vehicle + toluene, all t(64) < 0.93, p > 0.73). With respect to reward and negative feedback sensitivity, there was a strong trend for toluene to increase win-stay behavior relative to air-treatment although this did not reach statistical significance (Figure 3B; F(2,16) = 3.04, p = 0.076.). Figure 3C shows the effect of these treatments on lose-shift behavior. Surprisingly, toluene decreased this metric compared to the vehicle + air control (one-way ANOVA, F(2,16) = 10.58, p = 0.0012), regardless of whether rats were treated with AM251 (Tukey’s post hoc, both q(16) > 5.09, p < 0.0064). Pretreament with AM251 did not alter toluene’s effect on lose-shift behavior (q(18) = 9.45, p = 0.76). Finally, these treatments had no effect on choice latency (Fig. 3D, two-way ANOVA, probability block × treatment, F(8, 64) = 1.17, p = 0.33; main effect of treatment, F(2, 16) = 1.10, p = 0.36) or omissions (Fig. 3E, one-way ANOVA, F(2, 16) = 0.78, p = 0.48). These data suggest that antagonizing CB1Rs within the prefrontal cortex does not block toluene-induced deficits in probabilistic discounting.
Figure 3. Toluene-induced impairments in probabilistic discounting does not depend on mPFC CB1R signaling.
Using a within-subject design, well-trained rats were given the following treatments prior to task performance across three test days: vehicle mPFC microinjection + air exposure, vehicle mPFC microinjection + toluene, or 50 ng AM251 mPFC microinjection + toluene. (A) Proportion of risky choice within each probability block across treatments. (B, C) Choice strategies employed across all trials. Win-stay (B) indicates choice of risky lever after risky win while lose-shift (C) indicates choice of safe lever after risky loss. (D) Time to choice selection within each probability block. (E) Omissions across all trials indicate no lever press within the 10 s trial period. Data shown are mean + SEM; all n = 9; *p < 0.05; Tukey’s post hoc, τp = 0.063, *p < 0.05, **p < 0.01.
Effect of mPFC CB1R modulation on probabilistic discounting
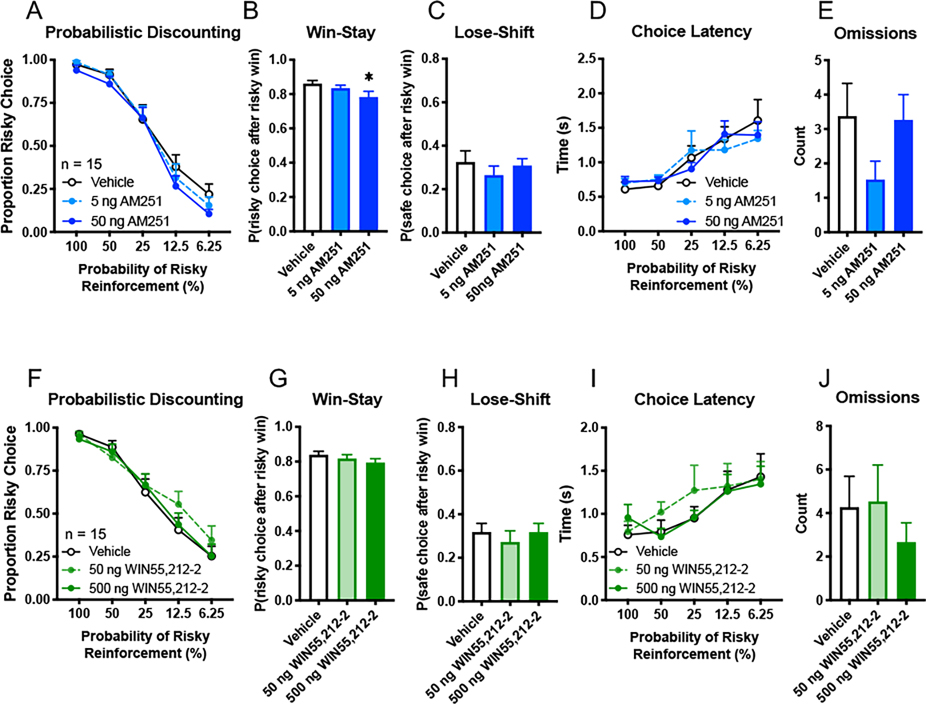
Although toluene-mediated alterations in risk behavior were not significantly altered by CB1R antagonists, these compounds alone produced selective effects on measures of probabilistic discounting. To further address this, a new cohort of animals were microinfused with different doses of AM251 (5 ng, 50 ng) or vehicle directly into the prelimbic cortex prior to probabilistic discounting test sessions. These treatments did not alter risk preference (Fig. 4A, two-way ANOVA, probability block × AM251, F(8, 112) = 0.41, p = 0.91; main effect of AM251, F(2,28) = 1.20, p = 0.32) while the high dose of AM251 decreased win-stay behavior (Fig. 4B, one-way ANOVA, F(2, 28) = 4.00, p = 0.030 and Dunnett’s post hoc, 50 ng AM251 vs vehicle, q(28) = 2.78, p = 0.018), consistent with its effect when administered systemically. Lose-shift behavior was unaffected by mPFC AM251 (Fig. 4C, one-way ANOVA, F(2, 28) = 0.60, p = 0.55) as was choice latency (Fig. 4D, two-way ANOVA, probability block × AM251, F(8, 112) = 0.76, p = 0.64; main effect of AM251, F(2,28) = 0.027, p =0.97) and omissions (Fig. 4E, one-way ANOVA, F(2, 28) = 3.11, p = 0.060). These data suggest that while mPFC CB1R signaling may promote win-stay behavior during probabilistic discounting, intra-PFC infusions of AM251 did not recapitulate its actions on choice latency and omissions following systemic injection suggesting that other brain regions mediate these effects.
Figure 4. Effects of mPFC CB1R manipulation on probabilistic discounting.
Using a within-subjects design and two separate cohorts of animals, a CB1R inverse agonist AM251 (5 ng, 50 ng; A-E), CB1R agonist WIN55,212-2 (50 ng, 500 ng; F-J) or vehicle was bilaterally microinfused into the mPFC of well-trained rats prior to task performance. (A, F) Proportion of risky choice within each probability block across treatments. (B, C, G, H) Choice strategies employed across all trials. Win-stay (B, G) indicates choice of risky lever after risky win while lose-shift (C, H) indicates choice of safe lever after risky loss. (D, I) Time to choice selection within each probability block. (E, J) Omissions across all trials indicate no lever press within the 10 s trial period. Data shown are mean + SEM; all n = 15; Dunnett’s post hoc, *p < 0.05.
In a final set of studies, we trained a new cohort of animals and tested whether intra-mPFC infusion of the CB1R agonist WIN55,212-2 affects task performance. In separate test sessions, animals were infused with vehicle and two doses of WIN55,212-2 (50 ng and 500 ng) 30 min prior to probabilistic discounting. Neither dose of WIN55,212-2 affected risk preference (Fig. 4F, two-way ANOVA, probability block × treatment, F(8, 112) = 0.79, p = 0.61; main effect of treatment, F(2, 28) = 0.48, p = 0.62), win-stay (Fig. 4G, one-way ANOVA, F(2, 28) = 1.40, p = 0.26), lose-shift (Fig. 4H, F(2, 28) = 0.47, p = 0.63), choice latency (Fig. 4I, two-way ANOVA, probability block × treatment, F(8, 112) = 0.53, p = 0.83; main effect of treatment F(2, 28), p = 0.56), or omissions (Fig. 4J, one-way ANOVA, F(2, 28) = 0.78, p = 0.47). Thus, stimulating CB1R receptors in the mPFC does not appear to alter risk/reward decision making or other motivational measures in the probabilistic discounting task.
Discussion
Toluene-induced impairments in probabilistic discounting do not depend on systemic or mPFC CB1R inverse agonism.
Previous studies in our laboratory have shown that acute toluene exposure impairs flexible risk/reward decision making during a probabilistic discounting task and this effect is associated with changes in mPFC neural activity (Braunscheidel et al., 2019). CB1Rs are highly expressed in the mPFC (Marsicano and Lutz, 1999; Eggan et al., 2010) and toluene inhibits glutamatergic synaptic activity in neurons from the mPFC and other brain regions via endocannabinoid/CB1R signalling (Beckley and Woodward, 2011; Beckley et al., 2016). Interestingly, a previous study showed that pharmacological inactivation of CB1Rs reduced choice perseveration during a deterministic decision making task (Hill et al., 2006). Here, we test the hypothesis that toluene-induced impairments in probabilistic discounting are mediated by enhanced endocannabinoid/CB1R signaling. To test this, we treated rats with CB1R inverse agonists prior to exposure to air or toluene vapor. Replicating our previous findings, toluene vapor increased risk preference during a probabilistic discounting task when reward probabilities were initially high and then decreased over a session (Braunscheidel et al., 2019). Counter to our hypothesis however, this effect was not mitigated by co-treatment with systemic or intra-mPFC infusions of CB1R inverse agonists.
An analysis of the trial-by-trial choice strategies during the task suggests that reducing CB1R activity either systemically or within the mPFC may mitigate the expected toluene-induced increases in win-stay behavior, a marker of sensitivity to recent positive reinforcement. However, this effect was independent of toluene treatment, as a reduction in win-stay behavior was also observed in animals given the CB1R antagonist alone. There were also no interactions between toluene and CB1R treatment on lose-shift behavior, an indicator of recent negative reinforcement. Likewise, no interaction between toluene and CB1R treatment were detected in choice latency or number of omissions, markers of decision impulsivity, processing speeds and/or task engagement. These findings suggest that toluene-induced impairments in probabilistic discounting do not depend on enhanced mPFC endocannabinoid signaling. Instead, toluene’s direct inhibition of NMDA receptors (Cruz et al., 2000; Beckley and Woodward, 2011) may perturb mPFC functions required for flexible decision making (St Onge and Floresco, 2010; Braunscheidel et al., 2019). An additional alternative possibility is that toluene specifically impairs activity of mPFC projections to the basolateral amygdala, as disruption of this connection causes effects on probabilistic discounting similar to those observed following bilateral mPFC inactivations (St Onge et al., 2012; Jenni et al., 2017). In support of this idea, results from our preliminary studies show that optical stimulation of channelrhodopsin-2 expressing mPFC terminals evokes AMPA-mediated EPSCs in BLA neurons that are blocked by a CB1R antagonist (Braunscheidel, 2020). Future studies can extend these findings by using intra-BLA injections of CB1R selective agents to identify the role of this areas in mediating toluene’s effects on risk/reward decision making.
Effect of systemic CB1R inverse agonism on probabilistic discounting
In the present study, systemic AM281 administration did not affect risky choice during probabilistic discounting. This finding is consistent with reports showing that systemic administration of the CB1R inverse agonist rimonabant does not change risky decision making in the rodent gambling task (Ferland et al., 2018) or probabilistic discounting under threat of shock (Freels et al., 2020). However, in the present study, AM281 treatments were not completely without effect, as analysis of trial-by-trial choice strategies suggests that it reduces the effect of recent positive enforcement on upcoming choice selection without affecting the influence of non-rewarded choices on action selection. The lack of effect of reducing CB1R activity on flexible choice is perhaps surprising given that treatment with the same dose of AM281 reduces perseverative errors during strategy set shifting (Hill et al., 2006) that may be thought of as a failure in lose-shift decision making. However, the optimal choice following a loss trial varies drastically between the two tasks. For example, returning to a choice on the risky lever that is not rewarded during the 50% probability block in the discounting task is still the theoretically optimal selection, whereas perseverating during a simple strategy shift where rewards are always deterministic is suboptimal. Moreover, the effects of AM281 on set-shifting in the Hill et al. study were tested after a single shift, whereas in the present study, rats were well-trained to adjust choice biases as reward probabilities changed. These critical differences in task design are important when interpreting the effect of systemic CB1R inverse agonism on choice behavior. Nevertheless, it is possible that CB1R activity may play a more prominent role in modulating flexible action selection when outcomes are deterministic rather than probabilistic.
Systemic CB1R inverse agonism increased choice latency and omissions in the current study, possibly reflecting a reduction in task engagement and/or reduced executive processing speeds. This idea is consistent with the observation that the CB1 receptor antagonist rimonabant decreased premature responding in the five-choice serial reaction time test (Pattij et al., 2007; Wiskerke et al., 2011) and reduced motivation to obtain food and drug rewards absent reward consumption (Solinas and Goldberg, 2005). Alternatively, these results might reflect a generalized reduction in food motivation caused by reduced CB1R activity (Freedland et al., 2000; Thornton-Jones et al., 2005; McReynolds et al., 2016). However, this would not explain why increases in choice latency in the present study emerged exclusively in the middle of the task, during times of high difficulty (50% – 12.5% probability blocks) while being absent in the beginning and end of the task, when choices are clear (100% and 6.25% probability blocks). Furthermore, reducing food motivation with pre-feeding does alter choice during probabilistic discounting (St Onge and Floresco 2009), whereas AM281 did not affect this measure in the present study. ln addition, treatment with this drug did not alter choice latency or omissions on a rodent gambling task (Ferland et al., 2018, Gueye et al., 2016). Notably, in that task, probabilities associated with four different rewards remain static over the session, whereas in the probabilistic discounting protocol used in the present study, probabilities of obtaining the larger reward are volatile. The extra cognitive load required to monitor changes in reward probabilisties might be better suited to elicit latency effects caused by CB1R inverse agonism. Further, reductions in intake caused by CB1R inverse agonism did not extend to water consumption (Verty et al., 2004; Gardner and Mallet, 2006), that may be more comparable to the liquid reinforcer used in the present studies.
Freels et al (2020) did not observe changes in choice latency following systemic treatment with a CB1R inverse agonist in a risky decision making task, where choice of a larger reward was associated with increasing probabilities of foot shock. Divergent findings between probabilistic discounting with and without physical punishment are not uncommon, as the tasks employ overlapping, but not identical neurocircuitry (Winstanley and Floresco, 2016). Further, differences exist in the specificity and mechanism of action of rimonabant, the CB1R inverse agonist used by Freels et al, and AM281, the compound used in the current studies (Pertwee, 2006). Taken together, these results suggest an important role of CB1Rs in regulating decision making speed and/or task engagement during probabilistic discounting.
A final caveat to the current studies is that only a single dose of AM281 was tested (2 mg/kg). This dose was based on findings by Hill and colleagues (2006) showing that it reduces perseverative responding during strategy set shifting. Interestingly, these authors also reported that a higher dose (5 mg/kg) had no effect suggesting an inverted-U dose response curve to this compound. Thus, although not tested in the present study, systemic CB1R stimulation might be protective against toluene-induced deficits in risk decision making at a dose between 2 and 5 mg/kg.
Future studies are required to identify the locus of the CB1R-mediated effects on choice latency and omission deficits observed here. Of particular interest might be the nucleus accumbens, given the unique expression profile of CB1Rs in this region (Pickel et al., 2004) and findings showing that pharmacological inactivation of the nucleus accumbens (or a mPFC-accumbens disconnection) also increases choice latency without affecting overall risk preference (Stopper and Floresco, 2011; St Onge et al., 2012).
Modulation of mPFC CB1R signaling during probabilistic discounting
CB1Rs are abundantly expressed in the PFC (Marsicano and Lutz, 1999; Eggan et al., 2010) and mPFC CB1R inverse agonism impairs certain fear-related memories in rodents (Laviolette and Grace, 2006; Lin et al., 2009; Kuhnert et al., 2013). Moreover, the mPFC plays a critical role in facilitating flexible adjustments in choice biases (St Onge and Floresco, 2010). Yet, very little is known about how diminishing mPFC CB1R signaling may affect appetitive decision making. Here we found that mPFC CB1R inverse agonism is sufficient to mediate the observed reduction in positive reinforcement on biasing future choice seen in the systemic CB1R applications, although this effect was not sufficient to cause significant changes in risky choice.
Unlike silencing mPFC CB1R signaling, intra-mPFC infusion of a CB1R agonist did not affect any of the task parameters measured during probabilistic discounting. These results are a bit surprising given that acute administration of the CB1R partial agonist delta9-tetrahydrocannabinol in humans impairs behavioral flexibility (Lane and Cherek, 2002; Anderson et al., 2010), increases gambling risk preference, and decreases lose-shift behavior (Lane et al., 2005). Furthermore, enhancing mPFC CB1R signaling in adult rats via viral-mediated receptor over-expression impaired reversal learning in the attentional set shifting task (Klugmann et al., 2011) and direct CB1R agonists interfere with memory recall and extinction learning in rodents (Kuhnert et al., 2013). However, in line with our results, a recent preclinical study showed that systemic treatment with a CB1R agonist did not alter risk preference during a rodent gambling task, although it may promote more appropriate responding in risk preferring individuals (Gueye et al., 2016; Ferland et al., 2018). Ferland and colleagues argue that their task perhaps did not engage the circuitry impaired by delta9-tetrahydrocannabinol administration due to the static probabilities used in their task. While the studies herein certainly would engage such circuitry, it appears that enhanced mPFC CB1R activity is not sufficient to drive abnormal risk preference. Nevertheless, the finding that these treatments blunted the impact that rewarded risky choices have on subsequent choice, suggests that targeting CB1R may be a potential strategy for mitigating the effects of maladaptive pleasure seeking (e.g. gambling addiction treatment, substance use relapse prevention, OCD treatment) on decision making. Additional studies are needed to evaluate this idea.
Conclusion
The studies herein investigate an endocannabinoid-mediated mechanism for the behavioral flexibility deficits caused by acute exposure to the abused inhalant toluene. We found that systemic or local mPFC CB1R inhibition did not mitigate the impairments caused by toluene. However, the data suggest that mPFC CB1R signaling is necessary for normal integration of recent positive reinforcement on future decisions, and that non-mPFC CB1R signaling is likely important for maintaining decision making speeds and task engagement during risky decision making. To our knowledge, these studies are the first to investigate the role of mPFC CB1R inverse agonism in the context of risk/reward decision making and could help inform future studies on disorders marked by maladaptive pleasure seeking.
Funding
This work was supported by the NIH National Institute on Drug Abuse: F31 DA045485 (K.M.B.), R01 DA013951 (J.J.W.), T32 DA007288 (J. F. McGinty), by the National Institute of Alcohol Abuse and Alcoholism via a Collaborative Research on Addiction supplement to T32 AA007474 (J.J.W.), and by a Project Grant from the Canadian Institutes of Health Research, PJT-162444 (S.B.F.).
Footnotes
Conflict of Interest Statement
On behalf of all authors, the corresponding states that there is no conflict of interest.
Disclosure
The authors declare no competing financial interests.
References
- Anderson BM, Rizzo M, Block RI, Pearlson GD, O’Leary DS (2010) Sex, drugs, and cognition: Effects of marijuana. J Psychoactive Drugs 42:413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckley JT, Randall PK, Smith RJ, Hughes BA, Kalivas PW, Woodward JJ (2016) Phenotype-dependent inhibition of glutamatergic transmission on nucleus accumbens medium spiny neurons by the abused inhalant toluene. Addict Biol 21:530–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckley JT, Woodward JJ (2011) The abused inhalant toluene differentially modulates excitatory and inhibitory synaptic transmission in deep-layer neurons of the medial prefrontal cortex. Neuropsychopharmacology 36:1531–1542 Available at: 10.1038/npp.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunscheidel KM, Gass JT, Mulholland PJ, Floresco SB, Woodward JJ (2017) Persistent cognitive and morphological alterations induced by repeated exposure of adolescent rats to the abused inhalant toluene. Neurobiol Learn Mem 144:136–146 Available at: 10.1016/j.nlm.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunscheidel KM, Okas MP, Hoffman M, Mulholland PJ, Floresco SB, Woodward JJ (2019) The Abused Inhalant Toluene Impairs Medial Prefrontal Cortex Activity and Risk/Reward Decision-Making during a Probabilistic Discounting Task. J Neurosci 39:9207–9220 Available at: http://www.jneurosci.org/lookup/doi/10.1523/JNEUROSCI.1674-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buelow MT, Suhr JA (2014) Risky decision making in smoking and nonsmoking college students: examination of Iowa Gambling Task performance by deck type selections. Appl Neuropsychol Child 3:38–44. [DOI] [PubMed] [Google Scholar]
- Cabral GA, Ferreira GA, Jamerson MJ (2015) Endocannabinoids and the Immune System in Health and Disease. In: Endocannabinoids (Pertwee RG, ed), pp 185–211. Cham: Springer International Publishing. Available at: 10.1007/978-3-319-20825-1_6. [DOI] [PubMed] [Google Scholar]
- Carliner H, Brown QL, Sarvet AL, Hasin DS (2017) Cannabis use, attitudes, and legal status in the U.S.: A review. Prev Med (Baltim) 104:13–23 Available at: 10.1016/j.ypmed.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossin R, Scott D, Witt KG, Duncan JR, Smith K, Lubman DI (2018) Acute harms associated with inhalant misuse: Co-morbidities and trends relative to age and gender among ambulance attendees. Drug Alcohol Depend 190:46–53 Available at: 10.1016/j.drugalcdep.2018.05.026. [DOI] [PubMed] [Google Scholar]
- Cruz SL, Balster RL, Woodward JJ (2000) Effects of volatile solvents on recombinant N-methyly-D-aspartate receptors expressed in Xenopus oocytes. Br J Pharmacol 131:1303–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SN, Pertwee RG, Riedel G (2002) Functions of cannabinoid receptors in the hippocampus. Neuropharmacology 42:993–1007. [DOI] [PubMed] [Google Scholar]
- Draycott B, Loureiro M, Ahmad T, Tan H, Zunder J, Laviolette SR (2014) Cannabinoid Transmission in the Prefrontal Cortex Bi-Phasically Controls Emotional Memory Formation via Functional Interactions with the Ventral Tegmental Area. J Neurosci 34:13096–13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggan SM, Stoyak SR, Verrico CD, Lewis DA (2010) Cannabinoid CB1 receptor immunoreactivity in the prefrontal cortex: Comparison of schizophrenia and major depressive disorder. Neuropsychopharmacology 35:2060–2071 Available at: 10.1038/npp.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euser AS, Van Meel CS, Snelleman M, Franken IHA (2011) Acute effects of alcohol on feedback processing and outcome evaluation during risky decision-making: An ERP study. Psychopharmacology (Berl) 217:111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferland JMN, Carr MR, Lee AM, Hoogeland ME, Winstanley CA, Pattij T (2018) Examination of the effects of cannabinoid ligands on decision making in a rat gambling task. Pharmacol Biochem Behav 170:87–97 Available at: 10.1016/j.pbb.2018.05.012. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Whelan JM (2009). Perturbations in different forms of cost/benefit decision making induced by repeated amphetamine exposure. Psychopharmacology (Berl) 205(2): 189–201. [DOI] [PubMed] [Google Scholar]
- Freedland CS, Poston JS, Porrino LJ (2000) Effects of SR141716A, a central cannabinoid receptor antagonist, on food-maintained responding. Pharmacol Biochem Behav 67:265–270. [DOI] [PubMed] [Google Scholar]
- Freels TG, Liley AE, Gabriel DBK, Simon NW (2020) Impact of Cannabinoid Type 1 Receptor Modulation on Risky Decision-Making. bioRxiv Anim Behav Cogn Available at: http://biorxiv.org/cgi/content/short/2020.03.13.990721v1?rss=1&utm_source=researcher_app&utm_medium=referral&utm_campaign=RESR_MRKT_Researcher_inbound. [Google Scholar]
- Gardner A, Mallet PE (2006) Suppression of feeding, drinking, and locomotion by a putative cannabinoid receptor “silent antagonist.” Eur J Pharmacol 530:103–106. [DOI] [PubMed] [Google Scholar]
- Gueye AB, Trigo JM, Vemuri KV., Makriyannis, Le Foll B (2016) Effects of various cannabinoid ligands on choice behaviour in a rat model of gambling. Behav Pharmacol 27:258–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Froese LM, Morrish AC, Sun JC, Floresco SB (2006) Alterations in behavioral flexibility by cannabinoid CB1 receptor agonists and antagonists. Psychopharmacology (Berl) 187:245–259. [DOI] [PubMed] [Google Scholar]
- Hu SS-J, Mackie K (2015) Distribution of the Endocannabinoid System in the Central Nervous System. In: Endocannabinoids (Pertwee RG, ed), pp 59–93. Cham: Springer International Publishing. Available at: 10.1007/978-3-319-20825-1_3. [DOI] [PubMed] [Google Scholar]
- Jenni NL, Larkin JD, Floresco SB (2017) Prefrontal Dopamine D 1 and D 2 Receptors Regulate Dissociable Aspects of Decision Making via Distinct Ventral Striatal and Amygdalar Circuits. J Neurosci 37:6200–6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian MD, Martin AB, Cuellar B, Rodriguez De Fonseca F, Navarro M, Moratalla R, Garcia-Segura LM (2003) Neuroanatomical relationship between type 1 cannabinoid receptors and dopaminergic systems in the rat basal ganglia. Neuroscience 119:309–318. [DOI] [PubMed] [Google Scholar]
- Klugmann M, Goepfrich A, Friemel CM, Schneider M (2011) AAV-mediated overexpression of the CB1 receptor in the mPFC of adult rats alters cognitive flexibility, social behavior, and emotional reactivity. Front Behav Neurosci 5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnert S, Meyer C, Koch M (2013) Involvement of cannabinoid receptors in the amygdala and prefrontal cortex of rats in fear learning, consolidation, retrieval and extinction. Behav Brain Res 250:274–284 Available at: 10.1016/j.bbr.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Kulkarni-Narla A, Brown DR (2000) Localization of CB1-cannabinoid receptor immunoreactivity in the porcine enteric nervous system. Cell Tissue Res 302:73–80. [DOI] [PubMed] [Google Scholar]
- Lane SD, Cherek DR (2002) Marijuana effects on sensitivity to reinforcement in humans. Neuropsychopharmacology 26:520–529. [DOI] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Tcheremissine OV., Lieving LM, Pietras CJ (2005) Acute marijuana effects on human risk taking. Neuropsychopharmacology 30:800–809. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA (2006) Cannabinoids Potentiate Emotional Learning Plasticity in Neurons of the Medial Prefrontal Cortex through Basolateral Amygdala Inputs. J Neurosci 26:6458–6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Su CL, Gean PW (2009) The role of prefrontal cortex CB1 receptors in the modulation of fear memory. Cereb Cortex 19:165–175. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Lutz B (1999) Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci 11:4213–4225. [DOI] [PubMed] [Google Scholar]
- McReynolds JR, Doncheck EM, Vranjkovic O, Ganzman GS, Baker DA, Hillard CJ, Mantsch JR (2016) CB1 receptor antagonism blocks stress-potentiated reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 233:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MR, Blankenship AL (2011) Effects of acute administration of nicotine, amphetamine, diazepam, morphine, and ethanol on risky decision making. Psychopharmacol 218:703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattij T, Janssen MCW, Schepers I, González-Cuevas G, De Vries TJ, Schoffelmeer ANM (2007) Effects of the cannabinoid CB1 receptor antagonist rimonabant on distinct measures of impulsive behavior in rats. Psychopharmacology (Berl) 193:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG (2006) The pharmacology of cannabinoid receptors and their ligands: An overview. Int J Obes 30:S13–S18. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Kash TL, Rodríguez JJ, Mackie K (2004) Compartment-specific localization of cannabinoid 1 (CB1) and μ-opioid receptors in rat nucleus accumbens. Neuroscience 127:101–112. [DOI] [PubMed] [Google Scholar]
- Schneider M, Kasanetz F, Lynch DL, Friemel XCM, Lassalle XO, Hurst DP, Steindel F, Monory XK, Scha C, Miederer I, Leweke XFM, Schreckenberger M, Lutz B, Reggio PH, Manzoni OJ, Spanagel R (2015) Enhanced Functional Activity of the Cannabinoid Type-1 Receptor Mediates Adolescent Behavior. 35:13975–13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR (2005) Motivational effects of cannabinoids and opioids on food reinforcement depend on simultaneous activation of cannabinoid and opioid systems. Neuropsychopharmacology 30:2035–2045. [DOI] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB (2009) Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology 34:681–697. [DOI] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB (2010) Prefrontal cortical contribution to risk-based decision making. Cereb Cortex 20:1816–1828. [DOI] [PubMed] [Google Scholar]
- St Onge JR, Stopper CM, Zahm DS, Floresco SB (2012) Separate Prefrontal-Subcortical Circuits Mediate Different Components of Risk-Based Decision Making. J Neurosci 32:2886–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson FS, Ruan WJ, Pickering R, Grant BF (2006) Cannabis use disorders in the USA: Prevalence, correlates and co-morbidity. Psychol Med 36:1447–1460. [DOI] [PubMed] [Google Scholar]
- Stopper CM, Floresco SB (2011) Contributions of the nucleus accumbens and its subregions to different aspects of risk-based decision making. Cogn Affect Behav Neurosci 11:97–112. [DOI] [PubMed] [Google Scholar]
- Stopper CM, Khayambashi S, Floresco SB (2013) Receptor-Specific Modulation of Risk-Based Decision Making by Nucleus Accumbens Dopamine. Neuropsychopharmacology 38:715–728 Available at: 10.1038/npp.2012.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton-Jones ZD, Vickers SP, Clifton PG (2005) The cannabinoid CB1 receptor antagonist SR141716A reduces appetitive and consummatory responses for food. Psychopharmacology (Berl) 179:452–460. [DOI] [PubMed] [Google Scholar]
- Verty ANA, McFarlane JR, McGregor IS, Mallet PE (2004) Evidence for an interaction between CB1 cannabinoid and oxytocin receptors in food and water intake. Neuropharmacology 47:593–603. [DOI] [PubMed] [Google Scholar]
- Wayman WN, Woodward JJ (2018) Chemogenetic Excitation of Accumbens-Projecting Infralimbic Cortical Neurons Blocks Toluene-Induced Conditioned Place Preference. J Neurosci 38:1462–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Floresco SB (2016). Deciphering Decision Making: Variation in Animal Models of Effort- and Uncertainty-Based Choice Reveals Distinct Neural Circuitries Underlying Core Cognitive Processes. J Neurosci 36(48): 12069–12079. PMC6601981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskerke J, Stoop N, Schetters D, Schoffelmeer ANM, Pattij T (2011) Cannabinoid CB1 receptor activation mediates the opposing effects of amphetamine on impulsive action and impulsive choice. PLoS One 6. [DOI] [PMC free article] [PubMed] [Google Scholar]