Abstract
Objectives:
Switching from combustible tobacco cigarettes to electronic cigarettes (e-cigs) may or may not help smokers to reduce cigarette consumption and toxicant exposure. This pilot study investigated the effects of asking smokers to switch to e-cigs for 6 weeks on smoking, exhaled carbon monoxide (CO) concentration, dependence, and motivation to quit smoking.
Methods:
Non-treatment seeking daily smokers (n = 18) were given free e-cigs and instructed to use them instead of smoking cigarettes for 6 weeks. Smokers were assessed at baseline, weekly for 6 weeks, and at 8 and 10 weeks for cigarettes/day, e-cig use, CO, cigarette dependence, and Contemplation Ladder.
Results:
All participants completed 6 weeks; 17 completed 10 weeks. At Week 6, cigarettes/day were reduced by two-thirds and CO by 45% from baseline (p’s < .001), with reductions maintained at Week 10 (p’s < .005). Cigarette dependence scores were a third lower at Weeks 6 (p < .002) and 10 (p < .001) than at baseline. Contemplation Ladder scores were higher at Weeks 6 and 10 (p’s < .001) than at baseline. All these statistical effect sizes were large. At Week 6, number of reasons not to use e-cigs increased (p < .011).
Conclusions:
Results show preliminary evidence for beneficial effects of short-term switching to e-cigs by non-treatment seeking smokers in terms of reduced smoke toxicant exposure and cigarette dependence, and increased motivation to quit, all maintained at least 4 weeks after free e-cigs were no longer provided.
1. Introduction
Electronic cigarettes (e-cigs) vaporize a solution containing nicotine, water, propylene glycol and flavorings. While considered by many to be a lower-risk cigarette substitute that may reduce deaths from smoking (Cahn & Siegel, 2011; Etter, 2014; Fagerström & Bridgman, 2014), the long-term health effects of e-cigs are unclear (USDHHS, 2016). Most e-cig users continue cigarette use (dual users) (Kalkhoran & Glantz, 2016), with reductions in smoking commonly reported (e.g., Adriaens, Van Gucht, Declerck, & Baeyens, 2014; Dawkins, Turner, Roberts, & Soar, 2013; Nides, Leischow, Bhatter, & Simmons, 2014). Across treatment trials, regular intensive use of e-cigs facilitates smoking reductions or cessation (Glasser et al., 2017). The fact that there is insufficient objective information about the health effects, effects on tobacco dependence, and efficacy of these products as used over time in the natural environment has led to ethical debates on the risks versus benefits of switching from cigarettes to e-cigs, resulting in an international call for research on this topic (Etter, Bullen, Flouris, & Laugesen, 2011; Farsalinos and Polosa, 2014; USDHHS, 2016). Given the many smokers unable to quit smoking, effects of using e-cigs with adequate nicotine delivery on smoking and health risks in needs further evaluation in well-controlled prospective studies (Glasser et al., 2017).
Toxicant exposure, cigarette dependence, and motivation to quit smoking are three areas that e-cig use could affect due to reduced cigarette use. While brief e-cig use did not affect expired carbon monoxide (CO) concentration in one study (Vansickel, Cobb, Weaver, & Eissenberg, 2010) and decreased CO concentration in another (Adriaens et al., 2014), instructing 20 smokers to completely switch to e-cigs for 2 weeks (50% complied) produced a 75% reduction in exhaled CO (Goniewicz et al., 2017). We found no studies on the effects on cigarette dependence of switching to e-cigs. Use of e-cigs for at least one week increased readiness to quit smoking in one study (Wagener et al., 2014). Effects on CO, dependence, and motivation need to be studied in adult daily smokers, having the smokers switch to daily e-cig use for some weeks, and using a motivational measure with a broader range of choices.
Evidence about the effects of switching to e-cigs on changes in motives for using or discontinuing e-cigs would also be useful. Surveys indicate that e-cig use among smokers is motivated by the choice of flavors (e.g., Audrain-McGovern, Strasser, & Wileyto, 2016) and by beliefs that e-cigs are far less harmful than cigarettes (Coleman et al., 2016), aid in quitting or reducing cigarette consumption (Piñeiro et al., 2016), and relieve withdrawal in smoke-free workplaces (Dawkins et al., 2013; Etter, 2010; Etter & Bullen, 2011; Li, Bullen, Newcombe, Walker, & Walton, 2013; McNeill, Brose, Calder, & Hitchman, 2015; Zezima, 2009). When people discontinued regular use of e-cigs, they reported they did not like the feel of e-cigs or that e-cigs did not help reduce cravings (Simonavicius, McNeill, Arnott, & Brose, 2017).
This pilot study recruited daily moderate smokers to switch from cigarettes to e-cigs for 6 weeks, with 4 weeks of follow-up. Preliminary evidence was sought about the ability of non-treatment seeking smokers to switch to e-cig use, given that dual use is common when using e-cigs (e.g., Simonavicius et al., 2017). Second, effects of switching to e-cigs on reducing CO concentration, reducing cigarette dependence, and increasing motivation for quitting smoking were investigated. Third, effects of switching to e-cigs on reasons to use or not to use e-cigs were investigated. Fourth, we investigated whether either rebound effects or continued beneficial effects would occur 4 weeks later in terms of cigarettes per day, CO concentration or cigarette dependence.
2. Methods
2.1. Design and overview
A single-group pre-post design was used in which daily smokers were asked to use e-cigs instead of smoking for 6-weeks. Procedures were approved by the Brown University Institutional Review Board (IRB).
2.2. Participants
The participants were recruited from the community using flyers and ads saying: “E-cig study for smokers. Are you a smoker who doesn’t vape? A research study is being conducted on effects of using electronic cigarettes for 6 weeks May earn up to $330.” Inclusionary criteria were: (a) Smoked 10+ cigarettes/day for past 6 months and CO > 8 ppm; (b) thinking about quitting smoking but not seeking treatment (score of 4–8 on the Contemplation Ladder; Biener & Abrams, 1991); (c) breath alcohol < 0.02 g% and negative urine drug screen (other than marijuana) on day of informed consent and baseline assessment; (d) 18–65 years old; (e) if female, report reliable birth control, menopause or past hysterectomy. Exclusionary criteria were: (a) currently quitting smoking, used any smoking cessation products or e-cigs in the past 30 days, used non-cigarette tobacco > 2 days in past 30 days, daily use of marijuana, weekly use of marijuana mixed with tobacco, any other illicit drugs in the past 30 days; (b) medications that could reduce smoking (naltrexone, buprenorphine, acamprosate, anti-seizure medications, disulfiram); (c) medical contraindications for inhaled nicotine (e.g., asthma, unstable angina, pregnancy, breastfeeding, etc.); (d) unstable medical or psychiatric conditions (requires current regular medical visits, or self-reported hallucinations or delusions, or not stabilized on psychotropic medications [i.e., anti-depressant, anti-anxiety or anti-manic medications changed within past 4 weeks]).
2.3. Screening and informed consent
Telephone screening questions determined eligibility for in-person informed consent. After consent, research staff assessed all eligibility criteria using self-report and the following tests. The urine drug screens used On Trak® test-strip cups. Over-the counter pregnancy tests assessed pregnancy. Breath alcohol was tested with Alco Sensor IV® by Intoximeters. If eligible, baseline assessment was conducted.
2.4. Experimental Period Procedures
2.4.1. E-cigs
We used Smoktech cartomizers (dual coil 1.5 Ω, size XL), and eGo batteries (3.3 V, 1100 mAh). We filled cartomizers with 1 ml e-liquid (18 mg/ml nicotine) each week with participants’ choice of four flavors (tobacco, menthol, chocolate dessert, and mixed fruit flavors; the types of flavors most commonly chosen by consumers, per the manufacturer). E-liquid was manufactured under controlled conditions and tested to ensure a specific nicotine concentration by Avail Sciences (Richmond, VA). This e-cig provides acceptable nicotine delivery in users trained in the optimal puffing method (Lopez et al., 2016; Talih et al., 2015). Participants received enough cartomizers to cover 150% of their usual nicotine ingestion, in case of increases due to free e-cigs.
2.4.2. Instructions and training
Participants were told to use e-cigs every day whenever they would usually smoke a cigarette, and to take at least 5 puffs a day (to ensure some use). Participants were trained in how to use e-cigs effectively (long slow inhalations), with a follow-up training session after 2 days of use. They were asked to refrain from other nicotine and tobacco but were told there was no penalty for such use and that it was crucial that they report such use to us. We did not insist on no smoking since dual use is common (Etter & Bullen, 2011, 2014) so needs assessing.
2.4.3. Weekly meetings
Participants met with a research interviewer weekly for assessment and problem-solving around using e-cigs. Participants returned all unused cartomizers to the laboratory each week for counting, to validate self-reports of e-cig use. Participants were then issued new cartomizers and batteries.
2.4.4. Follow-up
At study weeks 8 and 10, we assessed cigarettes/day, e-cig cartomizers/week, cigarette dependence, CO concentration, and motivation to quit smoking.
2.4.5. Participant stipend
At in-person screening, ineligible participants were paid $25. Eligible participants received $40 for baseline, $25/session at weeks 1–6, and $50 at weeks 8 and 10. A completion bonus of $5 per assessment was given for completing assessments after baseline within 4 days after the due date and on returning used and unused e-cig cartridges, and, at Week 6, returning the batteries. The total possible compensation was $330 via debit card. The IRB considered this consistent with community standards for participant time and effort.
2.5. Assessments
2.5.1. Schedule
Assessments were at baseline, weekly for 6 weeks, and at weeks 8 and 10.
2.5.2. Assessment measures and methods
Participant diaries were used to aid recall of cartomizers used/week (per Nides et al., 2014) and compared to number of empty cartomizers returned each week.
2.5.3. Baseline
Age, race, ethnicity, history of cigarette and e-cig use, and the measures (below).
2.5.4. Smoking-related measures
Number of cigarettes/day for past 30 days at baseline and each week during the 10 weeks was assessed by Time Line Follow Back (TLFB; Brown et al., 1998). Exhaled CO concentration was assessed at every assessment using EC50 Micro III Smokerlyzer® (Bedfont Scientific Ltd). Abstinence reports were confirmed with a CO < 6 ppm. Fagerström Test for Cigarette Dependence (FTCD; Fagerström, 2012; Heatherton, Koslowski, Frecker, & Fagerström, 1991) and Contemplation Ladder were given at baseline and Weeks 6 and 10.
2.5.5. E-cig beliefs
Perceived benefits and harms of e-cigs were assessed at baseline and 6 weeks. The checklist items were derived from Etter (2010); Etter and Bullen (2011); Ayers, Ribisl, and Brownstein (2011). Perceived benefits of using e-cigs (titled “Reasons to Use E-cigs”) asked “If you were to choose to use electronic cigarettes on your own, which of the following are reasons why you would you use them?” followed by 24 items such as: help me cut down on smoking, help me quit smoking, use in smoke-free places, less harmful to own health, less harmful to family or friends, keep from bothering other people, cheaper, less risk of cancer, reduce coughing, reduce tar in lungs, improve breathing, smell less bad (hands/clothes; breath; bad odors), improve ability to taste and smell, they taste good, they feel good when inhaling, reduce cravings, reduce withdrawal, get as much or more nicotine as from smoking, sleep better. Perceived harms of using e-cigs (titled “Reasons Not to Use E-cigs”) asked “Which of the following do you believe to be true about effects of using electronic cigarettes?” The 19 items included toxicity (contains chemicals which are not safe, toxic substances), health effects (causes lung problems, cancers, shortness of breath, wet or dry cough), sensory unpleasantness (burns my throat, dry mouth/throat, tastes bad, causes headache/nausea/dizziness/weight gain), ineffective (still be addicted, not enough nicotine, hard to adjust dose of nicotine, still have cravings, won’t help quit smoking, go back to smoking if stop using these).
2.5.6. E-cigarette Evaluation Questionnaire
We developed eight rating scales to assess satisfaction with using e-cigs, asked at 6 weeks. Two questions, rated on 5-point fully anchored scales, were what level of nicotine did participants think was in the e-cigarettes compared to their usual cigarettes (from 1, very low amount, to 5, very high amount) and how did the e-cigarettes affect their urges to smoke cigarettes (from 1, decreased my urges a lot, to 5, increased my urges a lot). The other 6 questions (see Table 3 for items), adapted from the Cigarette Evaluation Scale (Westman, Levin, & Rose, 1992), were rated on fully anchored scales from 1, not at all, to 7, extremely.
Table 3.
Evaluation of e-cig experience at end of 6 weeks of use.
| E-cigarette Evaluation Questionnaire item | M (SD) | Range |
|---|---|---|
| Amount of nicotine in e-cigs versus usual cigarettesa | 2.69 (1.08) | 1–5 |
| Effect of e-cigs on urge to smoke cigarettesb | 1.75 (0.58) | 1–5 |
| Was using the e-cigarettes satisfying?c | 4.56 (1.82) | 1–7 |
| Did the e-cigarettes taste good?c | 4.19 (2.01) | 1–7 |
| Enjoy the sensations in your throat and chest?c | 3.44 (2.06) | 1–7 |
| Did using e-cigarettes calm you down?c | 4.00 (1.75) | 1–7 |
| Immediately reduce your craving for cigarettes?c | 4.06 (1.69) | 1–7 |
| Did you enjoy using the e-cigarettes?c | 4.50 (1.75) | 1–7 |
Rated from 1, very low amount of nicotine, to 3, average/moderate amount of nicotine, to 5, very high amount of nicotine.
Rated from 1, decreased my urges a lot, to 3, no effect on my urges to 5, increased my urges a lot.
Rated from 1, not at all, to 4, moderately, to 7, extremely
2.6. Data analysis methods
Assumptions of normality were checked; no variable used in analyses was significantly skewed. For changes from baseline to Week 6 and Week 10, Generalized Estimating Equations (GEE; Zeger & Liang, 1986) models were fit, with the Wald statistic used for significance testing. Significant time effects were followed up with planned paired t-tests of baseline to each later time point, with effect size d calculated. Paired t-tests were used for change in number of reasons to use or not use e-cigs. The power (Cohen, 1988) was 0.65 to detect the lowest medium effect size (d = 0.50), and 0.95 to detect a large effect size (d = 0.80). The number of people with confirmed 7-day point-prevalence abstinence or with ≥50% reduction in cigarettes/day, and responses to the E-cigarette Evaluation Questionnaire were reported descriptively.
3. Results
3.1. Participant enrollment and retention
Of 64 who responded to ads, 31 ruled out by telephone, 4 failed to show, 9 ruled out in person. Of 20 who signed consent and completed baseline, 1 withdrew immediately (illness), and one was chosen a priori to refine our procedures (omitted from analyses). Therefore, 18 participants started the experimental period. Completion rates were 100% (n = 18) at 6 weeks and 94% (n = 17) at 8 and 10 weeks.
Participants were 39% male, 94% White, 6% Black, 11% Hispanic, with mean (SD) age of 45.1 (7.8), median income between $10,000 and $19,999; 12 (67%) smoked menthol cigarettes. See Table 1 for other baseline data.
Table 1.
Dependent variables at baseline, 6 weeks (end of experimental period), and at 8 and 10 week follow ups.
| Smoking-related measures | Baseline M (SD) | 6 Weeks M (SD) | 8 Weeks M (SD) | 10 weeks M (SD) |
|---|---|---|---|---|
| Number of cartomizers/week | 0 | 4.6 (5.4) | 3.1 (5.3) | 3.2 (6.0) |
| Cigarettes/day past 7 days | 19.6 (8.2) | 6.7 (7.9) | 8.3 (8.6) | 8.8 (8.3) |
| Expired CO concentration (ppm) | 17.33 (6.11) | 9.67 (6.98) | 11.65 (6.56) | 11.25 (6.38) |
| FTCD | 5.94 (1.96) | 3.89 (2.78) | – | 3.50 (3.08) |
| Contemplation Ladder | 5.17 (0.99) | 7.17 (1.58) | – | 6.94 (1.75) |
| Reasons to use e-cigs | 20.8 (2.0) | 19.9 (4.7) | – | – |
| Reasons not to use e-cigs | 4.11 (3.20) | 7.11 (3.79) | – | – |
Note: FTCD means Fagerström Test for Cigarette Dependence, CO means carbon monoxide, ppm means parts per million.
3.2. Amount of e-cig use
Number of e-cig cartomizers used per week was M (SD) = 3.4 (3.8) in Week 1 and reached its maximum by Week 4 (M = 4.6 (5.3)), staying at this level for Week 5 (M = 4.5 (5.0)) and Week 6 (M = 4.6 (5.4)). Participants continued to report using cartomizers after we stopped supplying them (see Table 1). One or two people each week chose only tobacco flavored e-liquid, M = 33% used only menthol flavor, M = 20% used only fruit flavor, and M = 33% used only chocolate dessert flavors; after the first 2 weeks, another M = 22% used two or more flavors each week.
3.3. Change in smoking-related measures
3.3.1. Missing data
Analyses were based on data from 18 people at Week 6 and 17 at Week 10, except for FTCD for which n = 16 at Week 10.
3.3.2. Cigarettes/day
The model was significant (see Tables 1–2, Fig. 1). Cigarettes/day was significantly lower at Weeks 6 and 10 than baseline, with large statistical effect sizes. Inspecting weekly reports, cigarettes/day dropped in Week 1 to M = 7.9 (8.2) and reached its lowest value at Week 6. Confirmed 7-day cigarette abstinence was seen for one person at Week 2 and 3, and for two people at Weeks 6, 8 and 10. The number of participants with ≥50% reduction in cigarettes per day was 11 (61%) at Weeks 6 and 8, and 9 (53%) at Week 10.
Table 2.
Analyses of smoking outcomes by time for baseline to 6 and 10 weeks for smoking and motivation outcomes, with results of planned comparisons (baseline to 6 weeks and baseline to 10 weeks). Results of paired t-tests (baseline to 6-weeks) for reasons to use or not use e-cigarettes.
| Smoking-related measures | GEE Model | Baseline to 6 Wks | Baseline to 10 Wks | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | Wald (1) | p | t(17) | d | p | t(16)a | d | p | |
| Cigarettes/day past 7 days | − 10.86 | 2.22 | 23.85 | .0001 | 6.95 | 1.64 | .001 | 4.62 | 1.12 | .005 |
| Expired CO concentration (ppm) | − 6.23 | 1.82 | 11.79 | .001 | 4.02 | 0.95 | .001 | 3.39 | 0.82 | .004 |
| FTCD | − 2.51 | 0.57 | 19.66 | .0001 | 3.73 | 0.88 | .002 | 4.12 | 1.03 | .001 |
| Contemplation Ladder | 1.78 | 0.39 | 20.75 | .0001 | 5.53 | 1.31 | .001 | 4.34 | 1.05 | .001 |
| Reasons to use e-cigs | < 1 | 0.20 | .415 | – | – | – | ||||
| Reasons not to use e-cigs | 2.84 | 0.67 | .011 | – | – | – | ||||
Note: FTCD means Fagerström Test for Cigarette Dependence, CO means carbon monoxide, ppm means parts per million, SE means standard error.
Note: For FTCD, df = 15 at Week 10.
Fig. 1.
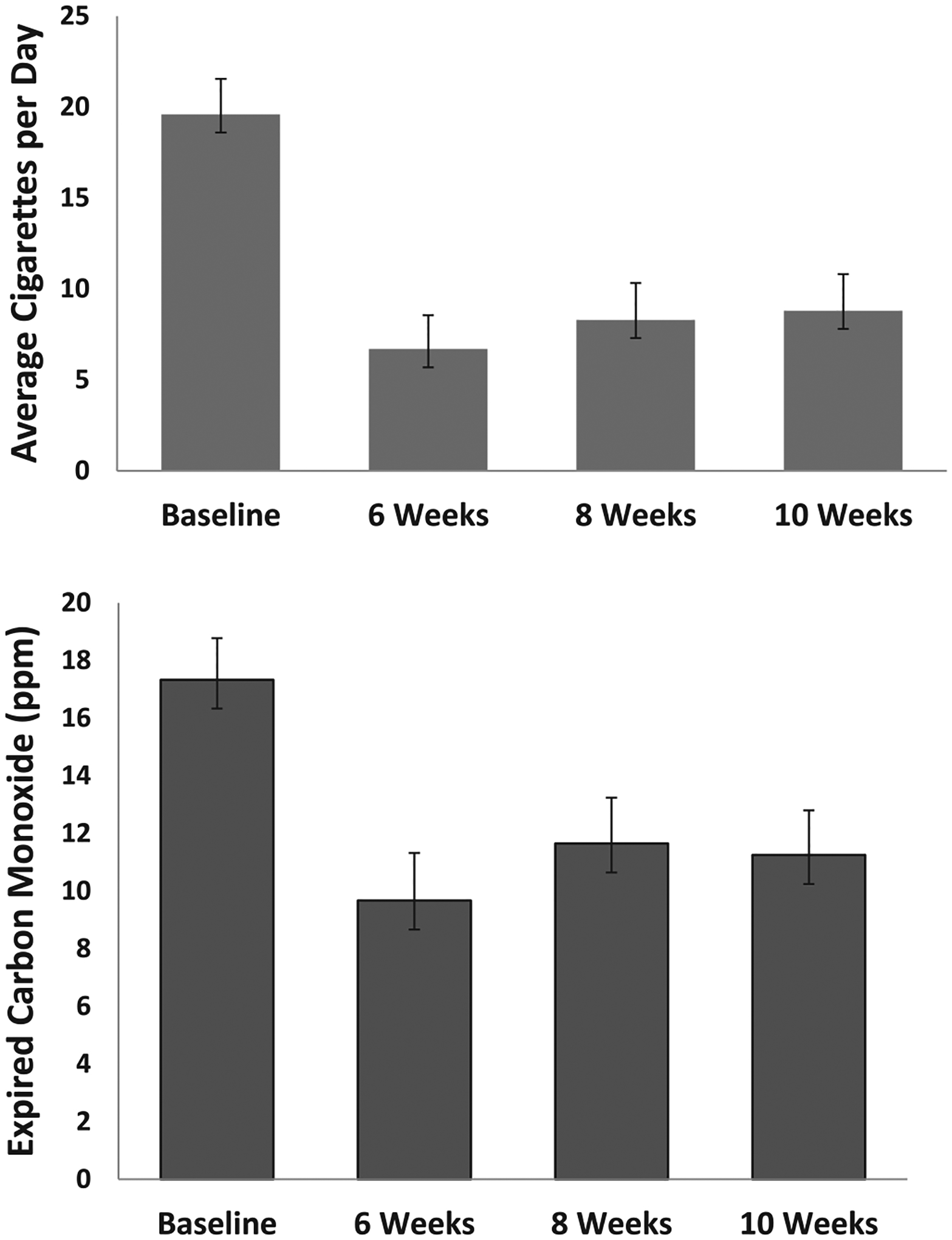
Upper: Cigarettes per day across time. Standard error bars are shown. Change from baseline was analyzed at 6 and 10 weeks, *p < .005; ** p < .001. Lower: Expired carbon monoxide (CO) across time. Standard error bars are shown. PPM means parts per million. Change from baseline was analyzed at 6 and 10 weeks, * p < .005, ** p < .001.
3.3.3. CO, dependence, and motivation to quit smoking
The GEE models were significant (see Tables 1–2, Figs. 1–2). Expired CO concentration was significantly lower at Weeks 6, 8 and 10 than baseline, with medium to large effect sizes. FTCD scores were significantly lower at Weeks 6 and 10 than baseline, with large effect sizes. Contemplation Ladder scores were significantly higher at Weeks 6 and 10 than baseline, with large effect sizes.
Fig. 2.
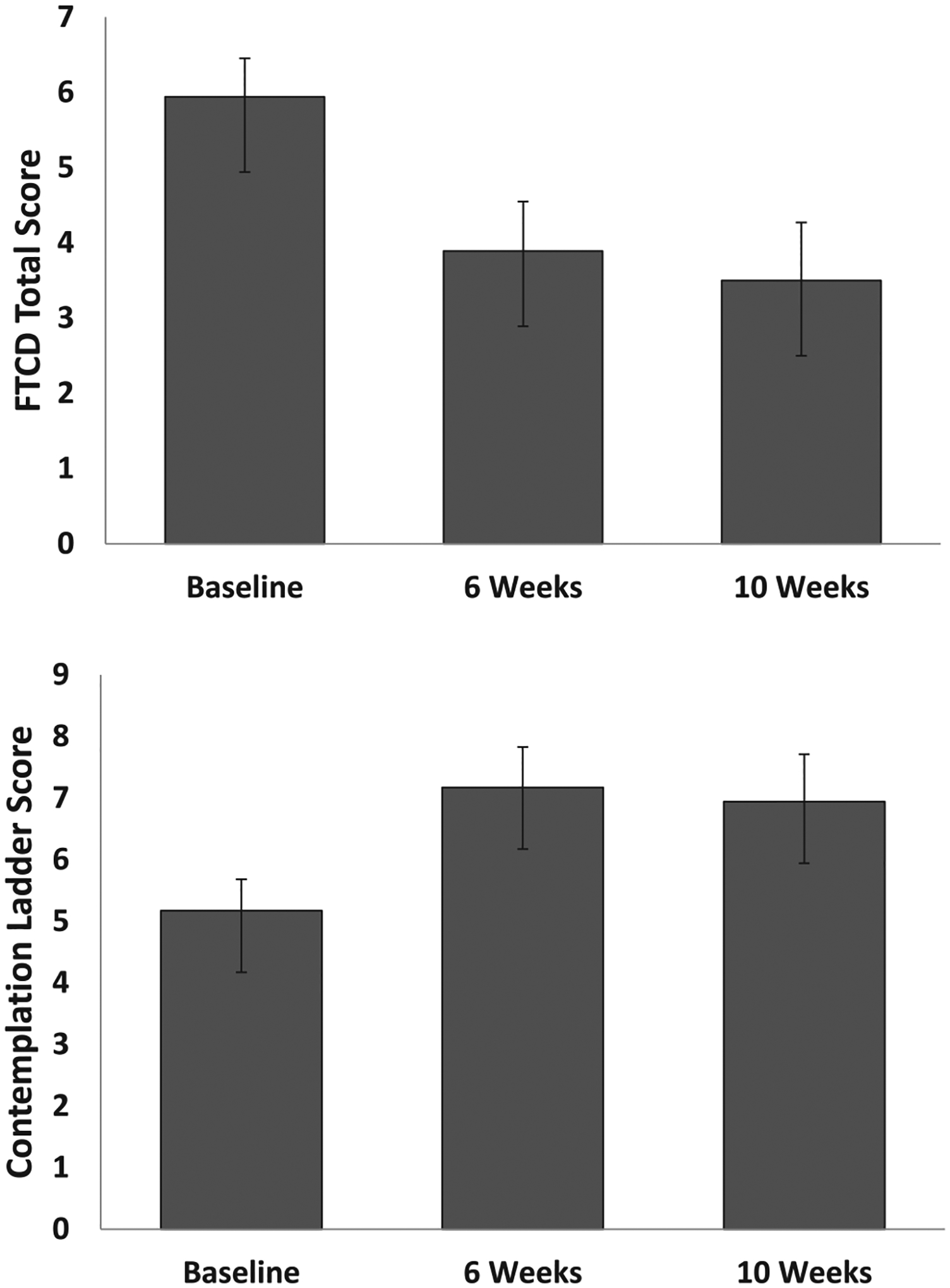
Upper: Fagerström Test for Cigarette Dependence (FTCD) score across time. Standard error bars are shown. For change from baseline, *p < .002; ** p < .001. Lower: Contemplation Ladder score across time. Standard error bars are shown. For change from baseline, *p < .001.
3.4. Perceived reasons to use and evaluation of e-cigs
3.4.1. Reasons to use or not use e-cigs
Endorsed number of reasons not to use e-cigs increased significantly from baseline to 6 Weeks, unlike number of reasons to use e-cigs (see Tables 1–2, Fig. 3). The reasons not to use that had the greatest increases in number of participants endorsing them (≥4 additional participants) were four experiential ones (dry cough, wet cough, burn my throat, and dry mouth or throat); and three knowledge-based ones (vapors are not safe, vapor causes lung problems, still get nicotine so stay addicted).
Fig. 3.
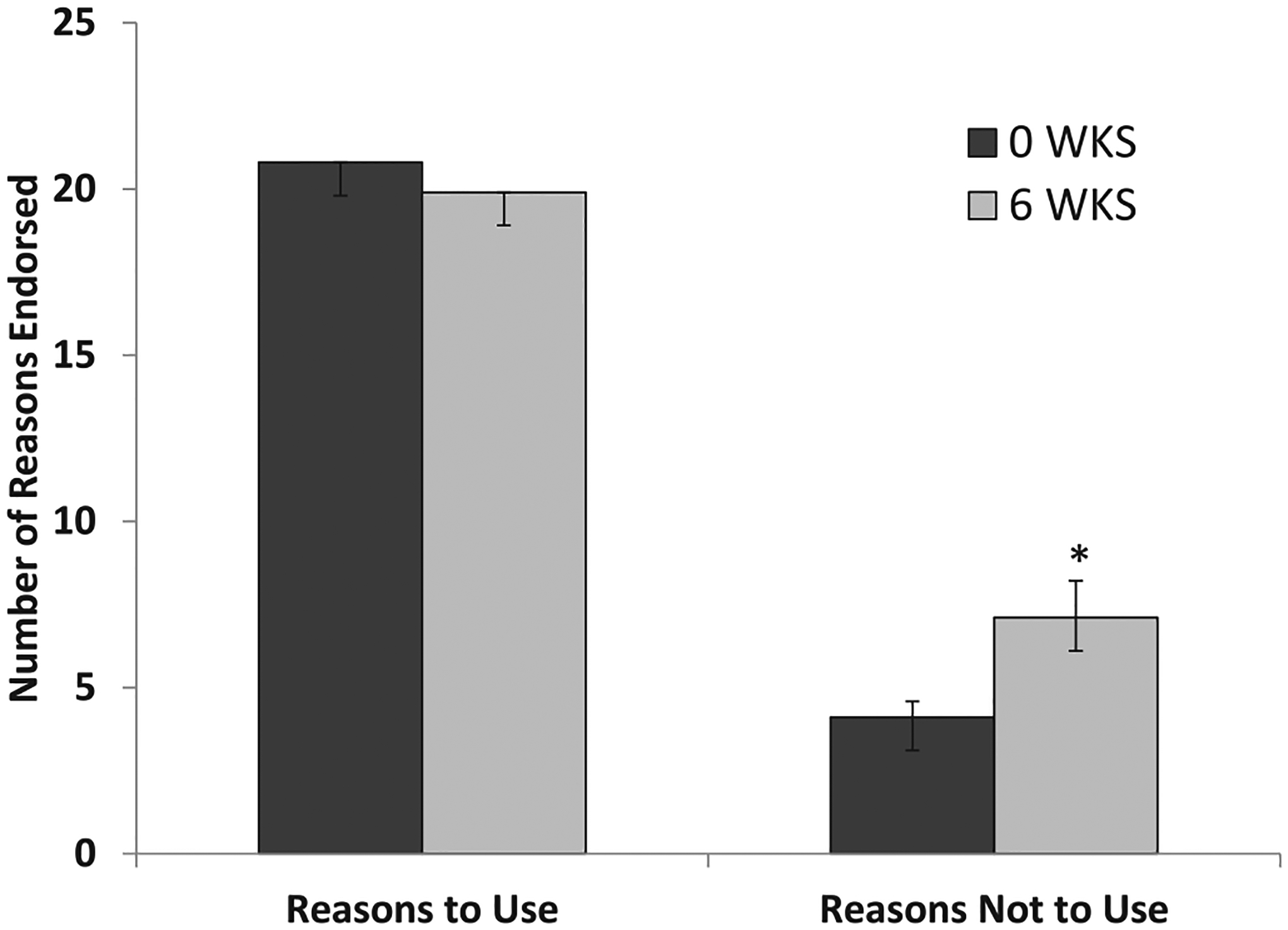
Reasons to Use or Not to Use E-cigs: For change from baseline, *p < .011
3.4.2. E-cigarette Evaluation Questionnaire
Participants reported that the nicotine level of e-cigs was low to average compared to cigarettes and that the e-cigs decreased their urges to smoke moderately (see Table 3). While on average participants reported little enjoyment from the sensations of using e-cigs, they reported that the e-cigs tasted moderately good, calmed them down moderately, and were moderately to highly satisfying and enjoyable to use.
4. Discussion
Results indicate that smokers who do not currently use e-cigs can be recruited and retained in a research study in which they are paid to switch to free e-cigs for 6 weeks. The fact that they maintained or increased e-cig use over the 6-week period indicates that as smokers became accustomed to this novel e-cig product, they increasingly replaced smoked cigarettes with e-cigs. Furthermore, participants continued using over 3 cartomizers/week during the four weeks after we stopped supplying these, thus showing an interest in continued use despite needing to purchase their own e-cigs.
Effects of being encouraged to use free e-cigs were shown on reduction in number of cigarettes/day smoked, with large effect sizes, with resulting reductions in mean expired CO concentration, indicating less exposure to toxicants emitted from combustible cigarette. Parallel to these changes were reductions in cigarette dependence and increased motivation to quit smoking, also with large statistical effect sizes. All of these changes still showed large statistical effects 4 weeks after we stopped supplying e-cigs. Cigarettes/day at 6 weeks were reduced by two thirds from baseline on average, with > 50% of participants reducing their cigarettes/day by 50% or greater at all time points, indicating that the reductions were substantial for many. Thus, the results show promise for reducing harmful exposure to combustible tobacco and reducing cigarette dependence when smokers are encouraged to switch to e-cigs and provided an effective product. These data are important for improving understanding of some potential benefits of e-cigs for cigarette smokers, given the ethical debate about e-cig use. The monetary compensation, necessary to ensure full retention in assessments so as to test the effects of e-cigs when they are actually used, means this approach would not be used this way for a clinical intervention. However, there was no monetary penalty for using cigarettes or discontinuing e-cigs, and payments were not contingent on changes in CO, dependence or motivation, so these changes were not a direct result of financial contingencies. Furthermore, continued changes during the follow-up period, when only attendance at assessments was compensated, were not due to the payments.
Unexpectedly, the 6-week period of e-cig use did not result in participants reporting any increase in perceived benefit or number of reasons to use e-cigs, but instead resulted in participants reporting more reasons not to use e-cigs. Still, they endorsed about 20 out of 24 reasons to use e-cigs (almost a ceiling effect) and only 7 out of 19 reasons not to use them after 6 weeks of use, so on balance had more reasons to use than not use them. The increase in concerns about the harmful effects of e-cigs parallels recent increases in these concerns seen in the US population (Majeed et al., 2017). Furthermore, participants reported only moderately enjoying the sensations of vaping, and found e-cigs only moderately effective at reducing cravings to smoke. Possibly the choice of flavors was a factor in the enjoyment. Future work needs to investigate the predictive relationship, if any, between positive and negative e-cig expectancies and actual use of e-cigs.
Limitations of this research include the small number of participants, the single urban site, only 6 weeks of e-cig use, large payments for participation, and having only 18 mg/ml nicotine liquid. However, the results showed that this type of research is feasible so a larger future study could investigate effects on biomarkers of toxicity and health. Results showed promise for effects of e-cig use on smoking reduction, dependence and interest in quitting smoking in this non-treatment-seeking group. The work needs to be repeated with a large sample size, a control group of participants not asked to switch, and biological measures of exposure to tobacco-related toxicants.
5. Conclusions
This pilot study suggests that non-treatment-seeking smokers can reduce cigarette consumption with short-term e-cig use, with more than half showing reductions of 50% or more in cigarettes per day. Beneficial effects included reduced exposure to cigarette-emitted toxicants (i.e., CO concentration), reduced dependence on cigarettes, and increased motivation to quit, all maintained for at least 4 weeks after free e-cigs were no longer provided. Given known benefits of smoking reductions (Begh, Lindson-Hawley, & Aveyard, 2015), e-cigs could assist with such reductions. One strength of this pilot study is that it involved a combination of e-cig device and nicotine liquid that is demonstrably effective at delivering nicotine when users are trained in effective puffing methods (Lopez et al., 2016). Future work also would benefit from the standardized inclusion of product combinations that have a well-characterized nicotine delivery profile.
HIGHLIGHTS.
Smokers were asked to switch from cigarettes to electronic cigarettes for 6 weeks.
After 6 weeks, their cigarettes per day was reduced by two-thirds and was still low 4 weeks later.
After 6 weeks, their exhaled carbon monoxide levels were 45% lower and were still low 4 weeks later.
Their dependence on cigarettes decreased by a third and stayed low 4 weeks later.
Switching to e-cigarettes might reduce some types of harm from smoking.
Acknowledgements
Preliminary results were presented at the 2017 meeting of the Society for Research on Nicotine and Tobacco, Florence, Italy, March 2017. Appreciation is expressed to Suzanne Sales for conducting the statistical analyses.
Role of funding sources
This work was supported by a Research Excellence Award from Brown University’s Center for Alcohol and Addiction Studies. The funding source had no further role in study design, in the collection, analysis and interpretation of the data, in the writing of the report, or in the decision to submit the paper for publication. Dr. Eissenberg’s effort was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number P50DA036105. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration or the Brown University Center for Alcohol and Addiction Studies.
Footnotes
Conflict of Interest
Dr. Eissenberg is a paid consultant in litigation against the tobacco industry and is named on a patent application for a device that measures the puffing behavior of electronic cigarette users. There are no other interests to declare.
References
- Adriaens K, Van Gucht D, Declerck P, & Baeyens F (2014). Effectiveness of the electronic cigarette: An eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. International Journal of Environmental Research and Public Health, 11, 11220–11248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Strasser AA, & Wileyto EP (2016). The impact of flavoring on the rewarding and reinforcing value of e-cigarettes with nicotine among young adult smokers. Drug and Alcohol Dependence, 166, 263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers JW, Ribisl KM, & Brownstein JS (2011). Tracking the rise in popularity of electronic nicotine delivery systems (electronic cigarettes) using search query surveillance. American Journal of Preventive Medicine, 40, 448–453. [DOI] [PubMed] [Google Scholar]
- Begh R, Lindson-Hawley N, & Aveyard P (2015). Does reduced smoking if you can’t stop make any difference? BMC Medicine, 13, 257. Published on-line 10.1186/s12916-015-0505-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biener L, & Abrams DB (1991). The contemplation ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychology, 10, 360–365. [DOI] [PubMed] [Google Scholar]
- Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, & Miller IW (1998). Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behaviors, 12, 101–112. [Google Scholar]
- Cahn Z, & Siegel M (2011). Electronic cigarettes as a harm reduction strategy for tobacco control: A step forward or a repeat of past mistakes? Journal of Public Health Policy, 32, 16–31. [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences (2nd Ed). Hillsdale, NJ: L. Erlbaum Associates. [Google Scholar]
- Coleman BN, Johnson SE, Tessman GK, Tworek C, Alexander J, Dickinson DM, … Green KM (2016). “It’s not smoke. It’s not tar. It’s not 4000 chemicals. Case closed.” exploring attitude, beliefs, and perceived social norms of e-cigarette use among adult users. Drug and Alcohol Dependence, 159, 80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins L, Turner J, Roberts A, & Soar K (2013). ‘Vaping’ profiles and preferences: An online survey of electronic cigarette users. Addiction, 108, 1115–1125. [DOI] [PubMed] [Google Scholar]
- Etter JF (2010). Electronic cigarettes: A survey of users. BioMed Central Public Health, 10, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter JF (2014). E-cigarettes: An innovation that can obsolete cigarettes. Summer: The Addictions Newsletter; 12–13. [Google Scholar]
- Etter JF, & Bullen C (2011). Electronic cigarettes: Users profile, utilization, satisfaction and perceived efficacy. Addiction, 106, 2017–2028. [DOI] [PubMed] [Google Scholar]
- Etter JF, & Bullen C (2014). A longitudinal study of electronic cigarette users. Addictive Behaviors, 39, 491–494. [DOI] [PubMed] [Google Scholar]
- Etter JF, Bullen C, Flouris AD, & Laugesen M (2011). Electronic nicotine delivery systems: A research agenda. Tobacco Control, 20, 243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerström KO (2012). Determinants of tobacco use and renaming the FTND to the Fagerström Test of Cigarette Dependence. Nicotine & Tobacco Research, 14, 75–78. [DOI] [PubMed] [Google Scholar]
- Fagerström KO, & Bridgman K (2014). Tobacco harm reduction: The need for new products that can compete with cigarettes. Addictive Behaviors, 39, 502–511. [DOI] [PubMed] [Google Scholar]
- Farsalinos KE, & Polosa R (2014). Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitute: A systematic review. Therapeutic Advances in Drug Safety, 5, 67–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser AM, Collins L, Pearson JL, Abudayyeh J, Niaura RS, Abrams DB, & Villanti AC (2017). Overview of electronic nicotine delivery systems: A systematic review. American Journal of Preventive Medicine, 52, e33–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz ML, Gawron M, Smith DM, Peng M, Jacob P III, & Benowitz NL (2017). Exposure to nicotine and selected toxicants in cigarettes smokers who switched to electronic cigarettes: A longitudinal within-subjects observational study. Nicotine & Tobacco Research, 19, 160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Koslowski LT, Frecker RC, & Fagerström KO (1991). The Fagerström test for nicotine dependence: A revision of the Fagerström tolerance questionnaire. British Journal of Addiction, 86, 1119–1127. [DOI] [PubMed] [Google Scholar]
- Kalkhoran S, & Glantz SA (2016). E-cigarettes and smoking cessation in real-world and clinical settings: A systematic review and meta-analysis. The Lancet Respiratory Medicine, 4, 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Bullen C, Newcombe R, Walker N, & Walton D (2013). The use and acceptability of electronic cigarettes among New Zealand smokers. New Zealand Medical Journal, 126, 48–57. [PubMed] [Google Scholar]
- Lopez AA, Hiler MM, Soule EK, Ramôa CP, Karaoghlanian NV, Lipato T, … Eissenberg T (2016). Effects of electronic cigarette liquid nicotine concentration on plasma nicotine and puff topography in tobacco cigarette smokers: A preliminary report. Nicotine & Tobacco Research, 18, 730–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed BA, Weaver SR, Gregory KR, Whitney CF, Slovic P, Pechacek TF, & Eriksen MP (2017). Changing perceptions of harm of e-cigarettes among U.S. adults, 2012–2015. American Journal of Preventive Medicine, 52, 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill A, Brose LS, Calder R, & Hitchman SC (2015). E-cigarettes: An evidence update. A report commissioned by Public Health England. London: Public Health England. [Google Scholar]
- Nides MA, Leischow SJ, Bhatter M, & Simmons M (2014). Nicotine blood levels and short-term smoking reduction with an electronic nicotine delivery system. American Journal of Health Behavior, 38, 265–274. [DOI] [PubMed] [Google Scholar]
- Piñeiro B, Correa JB, Simmons VN, Harrell PT, Menzie NS, Unrod M, … Brandon TH (2016). Gender differences in use and expectancies of e-cigarettes: Online survey results. Addictive Behaviors, 52, 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonavicius E, McNeill A, Arnott D, & Brose LS (2017). What factors are associated with current smokers using or stopping e-cigarette use? Drug and Alcohol Dependence, 173, 139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talih S, Balhas Z, Eissenberg T, Salman R, Karaoghlanian N, El Hellani A, … Shihadeh A (2015). Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: Measurements and model predictions. Nicotine & Tobacco Research, 17, 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Health and Human Service (USDHHS) (2016). E-cigarette use among youth and young adults: A report of the Surgeon General. Rockville, MD: Public Health Service. [Google Scholar]
- Vansickel AR, Cobb CO, Weaver MF, & Eissenberg T (2010). A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: Nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiology, Biomarkers & Prevention, 19, 1945–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagener TL, Meier E, Hale JJ, Oliver ER, Warner EL, Driskill LM, … Foster S (2014). Pilot investigation of changes in readiness and confidence to quit smoking after e-cigarette experimentation and 1 week of use. Nicotine & Tobacco Research, 16, 108–114. [DOI] [PubMed] [Google Scholar]
- Westman EC, Levin ED, & Rose JE (1992). Smoking while wearing the nicotine patch: Is smoking satisfying or harmful? Clinical Research, 40, 871A. [Google Scholar]
- Zeger SL, & Liang K-Y (1986). Longitudinal data analysis for discrete and continuous outcomes. Biometrics, 42, 121–130. [PubMed] [Google Scholar]
- Zezima K (2009). Cigarettes without smoke or regulations. June 1, 2009New York: New York Times. http://www.nytimes.com/2009/06/02/us/02cigarette.html. [Google Scholar]


