Abstract
Leptospira interrogans is a mammalian pathogen which must adapt to a range of new environmental conditions including temperature change when it infects new hosts. In vitro studies of organisms cultured at 30°C and shifted to 37°C for 5 to 7 days have confirmed that synthesis of several proteins involved in equine infection is regulated in response to temperature change (J. E. Nally, J. F. Timoney, and B. Stevenson, Infect. Immun. 69:400–404, 2001). In order to specifically identify antigenic proteins upregulated at 37°C, groups of three ponies were immunized with organisms shifted to 37°C for 5 to 7 days or with organisms maintained at 30°C. A lambda ZAP II genomic DNA library was screened with the pool of antisera to organisms shifted to 37°C. Clones reactive with this pool but unreactive with the pool of pony antisera to organisms cultured at 30°C were selected for further analysis. Sequence analysis of the first two clones identified open reading frames for proteins designated Qlp42 and Hsp15. Qlp42 is predicted to be an outer membrane lipoprotein. Its synthesis was upregulated when cultures were shifted from 30 to 37°C and downregulated when cultures were shifted from 37 to 30°C. Although the predicted molecular mass of Qlp42 is 39.8 kDa for the mature protein, Qlp42-specific equine antiserum was reactive with two bands of 30 and 29.5 kDa. Hsp15 is a stress response protein and a member of the Hsp20/α-crystallin family. PCR detected homologues of qlp42 and hsp15 in pathogenic serovars of L. interrogans but not in the nonpathogenic Leptospira biflexa. Enzyme-linked immunosorbent assays of antibody in convalescent sera from mares naturally infected with L. interrogans suggest that Qlp42 is expressed during leptospiral infection.
Leptospirosis, a worldwide zoonotic disease caused by pathogenic species of the spirochete genus Leptospira, is regarded as a reemerging infectious disease (18). Most human outbreaks are confined to developing tropical and subtropical countries, but more recently, outbreaks have been documented in temperate countries such as the United States (5, 18). Reservoirs of infection include chronically infected wild or domesticated carrier animals, and transmission is effected directly from contaminated urine or indirectly by entry from environments that permit leptospiral survival. Maintenance of leptospires in chronically infected wildlife and domestic livestock makes control of the disease difficult and results in significant economic losses in cattle, pigs, and horses. Leptospires are a significant cause of equine abortion in central Kentucky, and equine fetuses are a convenient source of material for pathogenesis and immunological studies (3).
Transmission of leptospires requires that the bacteria adapt to and survive in a range of different environmental conditions such as those encountered in the ecosystem and tissues and organs of newly infected hosts. Recent studies have shown that a shift in culture temperature from 30 to 37°C, similar to that encountered during infection of a host from an environmental source, is associated with the differential synthesis of several proteins of the outer membrane, periplasm, and cytoplasm (20). Further, several of these temperature-regulated proteins are immunogenic and react with sera from naturally infected convalescent mares, indicating a potentially significant role in the host-pathogen interaction during infection. In this study, we specifically identified immunogenic proteins that were upregulated after temperature shift from 30 to 37°C, as such proteins are likely to be expressed during natural mammalian infection and thus provide insights into the infection mechanisms of Leptospira interrogans.
MATERIALS AND METHODS
Leptospira isolates.
An isolate of L. interrogans, kindly provided by Mike Donahue (Livestock Disease Diagnostic Center, University of Kentucky, Lexington), was cultured from the kidney of an aborted equine fetus in 1997. This was subsequently plated on Johnson-Harris bovine serum albumin-Tween 80 medium (12) (Bovuminar PLM-5 Microbiological Medium; Intergen, Purchase, N.Y.) solidified with 1% Noble agar, and a single colony was grown for further study. The clonal isolate, JEN4, was typed as L. interrogans serovar pomona type kennewicki by Carole Bolin (National Animal Disease Center, Ames, Iowa). JEN4 was maintained in liquid culture in darkness at 30°C (unless otherwise indicated) and routinely passaged every few weeks. Temperature shift studies were done as previously described (20). A panel of reference Leptospira strains comprising Icterohaemorrhagiae serovar copenhageni and serovars Canicola, Grippotyphosa, Hardjo, Pomona, and Bratislava (Table 1) was kindly provided by Barbara Smith (Livestock Disease Diagnostic Center) and maintained at 30°C as described above. The nonpathogenic Leptospira biflexa was obtained from The National Veterinary Services Laboratories, Ames, Iowa.
TABLE 1.
Leptospira strains
| Species | Serogroup | Serovar | Strain |
|---|---|---|---|
| L. interrogans (pathogenic) | Australis | bratislava | Jez Bratislava |
| Canicola | canicola | Hond Utrech IV | |
| Grippotyphosa | grippotyphosa | Andaman | |
| Sejroe | hardjo | Hardjoprajitno | |
| Icterohaemorrhagiae | copenhageni | M 20 | |
| Pomona | pomona | pomona | |
| kennewicki | JEN4 | ||
| L. biflexa (nonpathogenic) | Biflexa | biflexa | codice |
Gel electrophoresis and immunoblotting.
Organisms were cultured at either 30 or 37°C until mid-logarithmic phase (5 to 7 days) and harvested by centrifugation at 15,000 × g for 10 min at 4°C. Cell pellets were washed twice in phosphate-buffered saline (PBS), resuspended in PBS, and lysed by boiling for 10 min, and protein concentrations were determined by the bicinchoninic acid assay (BCA protein assay kit; Pierce, Rockford, Ill.). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed using the discontinuous buffer system as described by Laemmli (15) using 12% acrylamide gels. Samples for electrophoresis were mixed with an equal volume of 2× sample loading buffer (125 mM Tris-Cl, 4% SDS, 2% glycerol, 1% β-mercaptoethanol, and 0.5% bromophenol blue) and boiled for 5 min before loading. Electrophoresis was carried out in an X-Cell SureLock Mini-Cell (Invitrogen, Carlsbad, Calif.) for 2 h at 125 V in Tris-glycine running buffer (25 mM Tris, 192 mM glycine, 0.1% SDS, pH 8.3). Proteins were visualized by soaking polyacrylamide gels in Coomassie blue stain (0.25% Coomassie brilliant blue, 50% methanol, 20% acetic acid) for 1 h and destaining overnight in several washes of destaining solution (45% methanol, 10% acetic acid). Proteins were also transferred to nitrocellulose membranes (0.22-μm pore size; Schleicher & Schuell, Keene, N.H.) and blocked with 5% (wt/vol) nonfat dry milk in PBS–0.05% Tween 20 (PBS-T). Membranes were individually incubated with antisera raised against LipL36 (D. Haake, University of California at Los Angeles) (9), Qlp42, or Hsp15 (this study) followed by incubation with either horseradish peroxidase–goat anti-rabbit immunoglobulin G conjugate or protein G conjugated to horseradish peroxidase (Zymed, South San Francisco, Calif.). Bound conjugate was detected by using 10 mg of 4-chloro-1-naphthol (Sigma, St. Louis, Mo.) dissolved in 5 ml of methanol–25 ml of PBS–50 μl of 30% hydrogen peroxide for approximately 10 min followed by washing in distilled H2O.
Preparation of equine antisera against whole leptospiral organisms.
Two separate bacterins were prepared from cultures of L. interrogans serovar pomona type kennewicki grown at 30°C (Lik30) or grown at 30°C and then shifted to 37°C (Lik37) as previously described (20). Cultures were harvested at the same bacterial density at mid-logarithmic phase by centrifugation and washed twice in PBS. Cultures were killed by overnight freezing at −20°C and, following thawing, checked for viability by dark-field microscopy. Two groups of three 2-year-old Welsh ponies were each immunized with a bacterin comprised of 350 μg of protein of Leptospira and 25% aluminum hydroxide (Alhydrogel; Accurate Chemical & Scientific Corp., Westbury, N.Y.) at a final volume of 1 ml in PBS. Ponies were immunized subcutaneously and received a booster vaccination 2 weeks later of 180 μg of Leptospira and 25% aluminum hydroxide at a final volume of 1 ml in PBS. Four weeks after the initial vaccination, serum was harvested by jugular venipuncture.
Immunological screening of a lambda ZAP II library.
A lambda ZAP II genomic DNA library (Stratagene, La Jolla, Calif.) containing 3- to 5-kb fragments of L. interrogans serovar pomona type kennewicki DNA digested with Tsp509I (13) was screened to identify phage expressing gene products reactive with a pool of antisera from ponies vaccinated with bacterin prepared from Leptospira cultured at 37°C. The titers of the phage library were determined on Escherichia coli XL-1 MRF′, and the phage were plated on Luria-Bertani agar plates per the manufacturer's instructions. Plaques were transferred to duplicate nitrocellulose disks and immunoblotted with Lik37 antiserum (1:400). Reactive plaques were rescreened until all gave a positive signal with the antiserum. A secondary screening was performed with a pool of antisera from those ponies vaccinated with bacterin prepared from Leptospira cultured at 30°C (Lik30 antiserum) to identify plaques reactive with Lik37 antiserum but not with Lik30 antiserum. Selected plaques were recovered for in vivo excision of the pSK(−) phagemid from the lambda ZAP II vector containing the cloned inserts and maintained in E. coli SOLR (Stratagene).
DNA sequence determination of cloned inserts.
Two pSK(−) plasmids expressing cloned inserts in E. coli SOLR were selected for DNA sequencing. Plasmid DNA was isolated using the QIAprep Spin Miniprep kit (Qiagen, Valencia, Calif.) and sequenced using the T7 and reverse primers in a commercial DNA sequencing facility (Davis Sequencing LLC, Davis, Calif.). Sequences were viewed using Chromas 1.61 (Technelysium Pty Ltd., Helensville, Queensland, Australia). The remaining cloned insert DNA sequence was determined using specific primers designed from a previously determined sequence. Analysis of overlapping DNA sequences was performed using DNASIS (Hitachi Software Engineering Co., Ltd., San Francisco, Calif.).
Nucleic acid and amino acid analysis.
Nucleotide sequences were aligned and analyzed using DNASIS. The National Center for Biotechnology Information (NCBI) databases were used to search for homologous sequences using Basic Local Alignment Search Tool (BLAST) programs (1). Secondary structure predictions were performed using PEPTIDESTRUCTURE and PLOTSTRUCTURE programs with The University of Wisconsin Genetics Computer Group package. Multiple sequence alignments were generated using ClustalW (25) and BioNavigator by eBioinformatics Pty. Ltd. Sequence analysis using profile hidden Markov models (profile HMMs) was performed using HMMPFAM (1a; S. Eddy, HMMER user's guide, 1998) and BioNavigator.
Amino acid sequence determinations.
Both of the recombinant proteins of Leptospira expressed in E. coli SOLR were purified from the periplasm as previously described (13). Bacteria were grown overnight in Luria-Bertani medium (5 ml) at 37°C, harvested by centrifugation, and frozen at −20°C. Cells were resuspended in 1.5 ml of Tris-sucrose solution (50 mM Tris-HCl [pH 7.5], 10% sucrose) and 150 μl of lysis solution (0.3 M spermidine-HCl, 2 M NaCl, 10% sucrose, pH 7.5) which had been prewarmed to 37°C. After thawing, the pH was adjusted to 7.5 with 2 M Tris, and 1 mg of lysozyme was added. Cells were incubated for 1 h at 4°C, heated in a 37°C water bath for 4 min, and then placed on ice. The lysate was centrifuged, and the supernatant containing recombinant proteins was retained. Proteins were subjected to SDS-PAGE and transferred electrophoretically to an Immobilon-P membrane (Millipore, Bedford, Mass.). The recombinant proteins were detected by staining with Ponceau S (0.5% [wt/vol] Ponceau S in 1% acetic acid) and excised, and microsequence analysis was performed on a model 477A pulse liquid-phase sequencer (Applied BioSystems) by Carol Beach at the University of Kentucky Macromolecular Synthesis Laboratory.
Isolation of leptospiral genomic DNA.
Ten milliliters of stationary-phase culture was harvested by centrifugation and resuspended in 400 μl of TE buffer (10 mM Tris-Cl [pH 8.0], 1 mM EDTA) containing 0.5% SDS and 100 μg of proteinase K per ml for 1 h at 37°C. NaCl was added to a final concentration of 1 M. Samples were then extracted with an equal volume of phenol-chloroform-isoamyl alcohol (25:24:1). The aqueous phase was removed and extracted with chloroform-isoamyl alcohol (24:1). DNA was precipitated from the aqueous phase with 2 volumes of 95% ethanol at −20°C for 1 h. After centrifugation, DNA pellets were washed in 70% ethanol for 5 min, recentrifuged, and air dried before being resuspended in 30 μl of H2O.
Production of His-tagged recombinant proteins.
Forward and reverse primers, incorporating a XhoI and a BamHI restriction enzyme site, respectively, were designed using Primer 2 (Scientific & Educational Software, 1991) for subcloning of qlp42 and hsp15 into the pET-15b expression system (Novagen, Madison, Wis.). The pET-15b vector facilitates expression of Qlp42 and Hsp15 with an N-terminal His tag sequence in E. coli BL21. Forward and reverse primers for qlp42 were designated bpNF and bpNR, respectively. The forward primer, bpNF (5′-CCGCTCGAGAGTTCCAAGGCAGCCGCTACTA-3′), was designed so that the recombinant His-tagged protein would not include the predicted 21-amino-acid signal peptide. The reverse primer, bpNR (5′-CCGGATCCATCATATAGGCGGCAATTAG-3′), was designed such that the predicted rho-independent terminator would also be included in the PCR-amplified product. Forward and reverse primers for hsp15 were designated LepCFXho (5′-CCGCTCGAGAACCAACTAACAACGATTAGG-3′) and LepCRBam (5′-GGATCCGGTAAAGATGAACTCGCCGAC-3′), respectively. Both qlp42 and hsp15 were amplified from genomic DNA of JEN4 and expressed from pET-15b in E. coli BL21. Expression of Qlp42 and Hsp15 was induced in mid-logarithmic growth-phase E. coli BL21 (optical density of 0.6 at 600 nm) with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside), and cultures were harvested after 3 h. His-tagged proteins were purified with Talon metal affinity resin per the manufacturer's instructions (Clontech, Palo Alto, Calif.).
Both qlp42 and hsp15 were amplified by PCR from genomic DNA of Leptospira with primers bpNF and bpNR and primers LepCFXho and LepCRBam, respectively. DNA was denatured at 92°C for 2 min before 30 cycles of 92°C at 1 min, 56°C at 1 min, and 72°C for 1.5 min. A final extension run of 5 min at 72°C concluded the reaction. A concentration of 2 mM MgCl2 was used.
Preparation of equine antisera against His-tagged recombinant proteins.
Ponies were immunized subcutaneously with 500 μg of recombinant protein in PBS, resuspended in 30% aluminum hydroxide (Alhydrogel) in a final volume of 2 ml. Ponies were boosted 2 weeks later with another 500 μg, and serum was harvested 5 weeks after initial immunization by jugular venipuncture.
ELISA.
Sera from convalescent mares which had aborted due to naturally acquired infection with pathogenic Leptospira were kindly provided by Barbara Smith (Livestock Disease Diagnostic Center). Negative control serum was obtained from ponies with no known exposure or indications of previous infection with Leptospira. A checkerboard enzyme-linked immunosorbent assay (ELISA) was performed to determine optimal coating concentrations of antigen. Flexible 96-well flat-bottomed polystyrene MicroTest III assay plates (Falcon, Becton Dickinson, Oxnard, Calif.) were coated with 100 μl of a 2-μg/ml recombinant Qlp42 or Hsp15 resuspended in coating buffer (0.1 M carbonate bicarbonate, pH 9.2) overnight at 4°C. After being washed in PBS-T (PBS, 0.05% Tween 20), plates were blocked for 1 h at 37°C with 100 μl of 2% nonfat dried milk in PBS-T. Serum (100 μl) was then added in triplicate wells for 1 h at 37°C. Bound immunoglobulin G was detected by incubation with 100 μl of horseradish peroxidase–protein G (Zymed) for 1 h at 37°C. Plates were developed with 10 mg of O-phenylenediamine (Sigma) in 15 ml of 0.1 M citrate phosphate buffer (24.3 ml of 0.1 M citric acid and 25.7 ml of 0.2 M Na2HPO4 · 2H2O at a final volume of 100 ml, pH 5.0) and 50 μl of H2O2. The reaction was terminated by the addition of 2 M H2SO4, and ELISA plates were read at 490 nm. Wells with no coating antigen were used as blanks.
MAT.
The microscopic agglutination test (MAT) is the standard diagnostic serology test to diagnose leptospiral infection and was carried out in the Livestock Disease Diagnostic Center by Barbara Smith according to the National Veterinary Service Laboratory guidelines (presented at the 91st Annual Meeting of the U.S. Animal Health Association, 1987).
Nucleotide sequence accession numbers.
The nucleotide sequences of qlp42 and hsp15 have been deposited in the GenBank database under accession no. AF320329 and AF320330, respectively.
RESULTS
Immunological screening of a lambda ZAP II library.
Screening of 100,000 plaques of a lambda ZAP II genomic DNA library of L. interrogans with a pool of sera from ponies immunized with Lik37 identified 14 reactive phages. These were rescreened with a pool of sera from ponies immunized with Lik30, resulting in the isolation of five phages which were reactive with Lik37 but not with Lik30 antisera. This mode of screening greatly simplified and enhanced detection of clones expressing proteins upregulated at 37°C. Selected phage were recovered for in vivo excision of the pSK(−) phagemid from the lambda ZAP II vector containing the cloned inserts and were maintained in E. coli SOLR. Two clones were selected for further analysis.
Sequence analysis of clone 1.
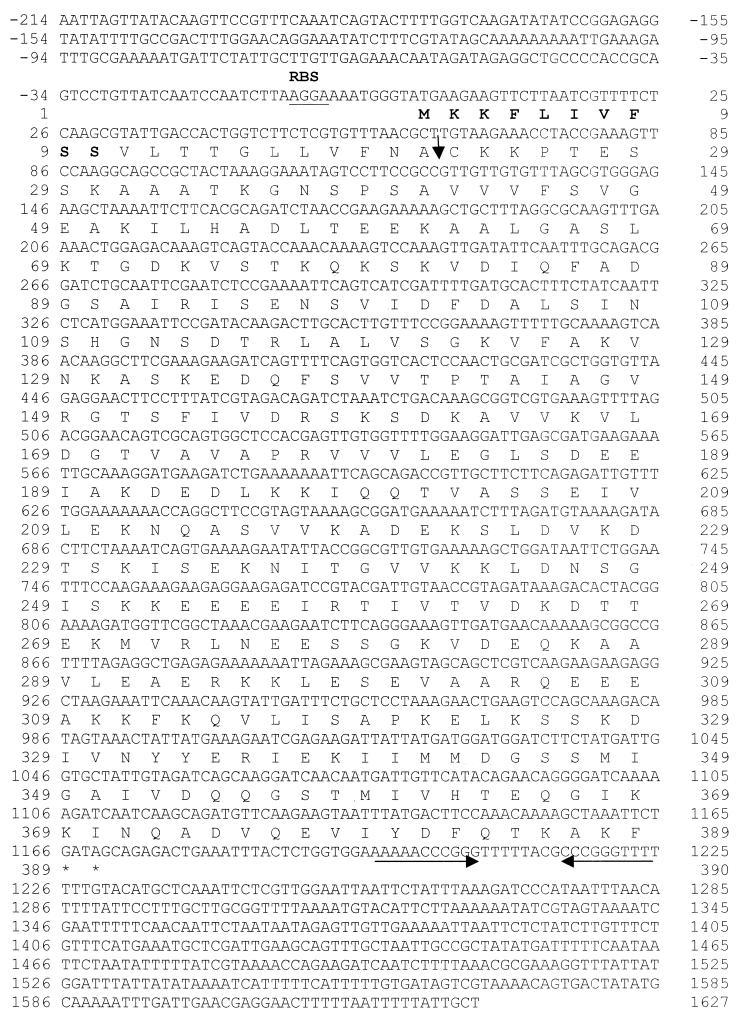
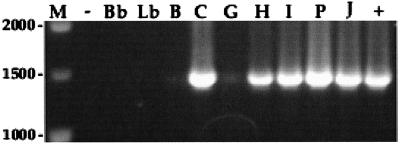
Clone 1 contained an insert of 3,343 bp which was found to encode a putative membrane lipoprotein, designated Qlp42. The structural gene qlp42 comprises 1,167 bases encoding 388 amino acids (Fig. 1), with a G+C base content of 38.96%. A potential ribosome-binding site (AGGA) is present 8 bp upstream from the initiation codon. As described previously for lipoproteins (8), the deduced amino acid sequence begins with a 21-residue signal peptide that has a positively charged amino-terminal region (lysine at positions 2 and 3) followed by a central hydrophobic core, amino acids 4 to 18, and a potential carboxy-terminal lipoprotein signal peptidase cleavage site, Phe-Asn-Ala-Cys. Lipoprotein precursors are predicted to have a β-turn secondary structure immediately following the cleavage site at the +2 or +3 position, also evident in secondary structure predictions for Qlp42 (data not shown), which contains a potential turn structure amino acid in proline at the +4 position after cleavage. Expression of qlp42 from excised phage was confirmed by N-terminal amino acid microsequencing (Fig. 1). Amino acid sequence obtained from the recombinant protein showed that the signal sequence was not processed in E. coli. The Qlp42 protein has a predicted molecular mass of 42 kDa (39.8 kDa after cleavage of the signal peptide) and an isoelectric point after cleavage of 6.1. Homology searches with the deduced amino acid sequence using the standard protein-protein BLASTp program against the NCBI protein databases revealed some homologies with other proteins in the database. Amino acids 41 to 174 showed 31% identity (49% positives, E = 7e-06) with a hypothetical protein, PA4035, from Pseudomonas aeruginosa (accession no. G83141). Similarly, amino acids 62 to 172 showed 33% identity (51% positives, E = 8e-06) with a hypothetical protein from Deinococcus radiodurans (accession no. C75373). No significant homologies to genes in the genomes of Borrelia burgdorferi or Treponema pallidum were identified. PCR was performed with primers bpNF and bpNR to detect qlp42 in other selected serovars of L. interrogans. An amplified fragment of 1,395 bp indicated the presence of qlp42 in all L. interrogans serovars examined. However, only a faint amplified DNA band was observed for serovars bratislava and grippotyphosa. No amplification of qlp42 was apparent in the nonpathogenic L. biflexa serovar biflexa or in the Lyme disease spirochete B. burgdorferi (Fig. 2).
FIG. 1.
Nucleotide and deduced amino acid sequences of qlp42. The amino acid sequence confirmed by microsequence analysis is highlighted in boldface. A potential upstream ribosomal binding site (RBS) is underlined. The putative lipoprotein signal peptidase cleavage site is indicated by a vertical arrow. Stop codons are indicated by asterisks. An inverted repeat, which may function as a rho-independent transcription terminator, is indicated by horizontal arrows.
FIG. 2.
Detection of qlp42 by PCR using primers bpNF and bpNR. An amplified fragment of 1,395 bp suggested conservation among pathogenic L. interrogans serovars. Only a faint signal was amplified from lanes B and G. Lane M; DNA ladder (size indicated in base pairs); lane −, negative control; lane Bb, B. burgdorferi; lane Lb, nonpathogenic L. biflexa; lane B, L. interrogans serovar bratislava; lane C, serovar canicola; lane G, serovar grippotyphosa; lane H, serovar hardjo; lane I, serogroup Icterohaemorrhagiae serovar copenhageni; lane P, serovar pomona; lane J, JEN4; lane +, positive control.
Sequence analysis of clone 2.
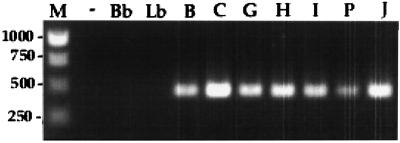
Clone 2 contained an insert of 3,205 bp that was found to encode a putative stress response protein and designated hsp15. The hsp15 structural gene comprises 393 bases encoding 130 amino acids (Fig. 3) with a G+C base content of 35.87%. Expression of hsp15 from excised phage was confirmed by N-terminal amino acid microsequencing. A potential ribosome-binding site (AGGA) is present 8 bp upstream from the initiation codon. Hsp15 has a predicted molecular mass of 15 kDa and an isoelectric point of 5.48. Homology searches with the deduced amino acid sequence using the standard protein-protein BLASTp program against the NCBI protein databases revealed significant homologies with other proteins in the database. More specifically, use of, profile HMMs indicated that Hsp15 contained a highly conserved consensus sequence common to the Hsp20/α-crystallin family (score, 81.4; E value, 3.3e-21) (Fig. 4). No homologies to genes in the genomes of B. burgdorferi or T. pallidum were identified. PCR was performed with primers LepCFXho and LepCRBam primers to determine conservation of hsp15 in other serovars of L. interrogans. An amplified fragment of 468 bp indicated conserved hsp15 sequence in all serovars of L. interrogans but not in the nonpathogenic L. biflexa serovar biflexa and the Lyme disease spirochete B. burgdorferi (Fig. 5).
FIG. 3.
Nucleotide and deduced amino acid sequences of hsp15. The amino acid sequence confirmed by microsequence analysis is highlighted in boldface. A potential upstream ribosomal binding site (RBS) is underlined. The location of the TAA stop codon is indicated by an asterisk.
FIG. 4.
Alignment of Hsp15 with the primary structural consensus sequence of the Hsp20/α-crystallin family as identified using profile HMMs. The most highly conserved residues in the conserved region of the HMM profiles are indicated by capital letters. Lowercase letters signify a lower level of conservation. Plus signs indicate the lowest level of conservation.
FIG. 5.
Detection of hsp15 among pathogenic L. interrogans serovars by PCR using primers LepCFXho and LepCRBam. Lane M, DNA ladder (size indicated in base pairs); lane −, negative control; lane Bb, B. burgdorferi; lane Lb, nonpathogenic L. biflexa; lane B, L. interrogans serovar bratislava; lane C, serovar canicola; lane G, serovar grippotyphosa; lane H, serovar hardjo; lane I, serogroup Icterohaemorrhagiae serovar copenhageni; lane P, serovar pomona; lane J, positive control JEN4. The amplified fragments were approximately 468 bp.
Qlp42 is upregulated after temperature shift from 30 to 37°C.
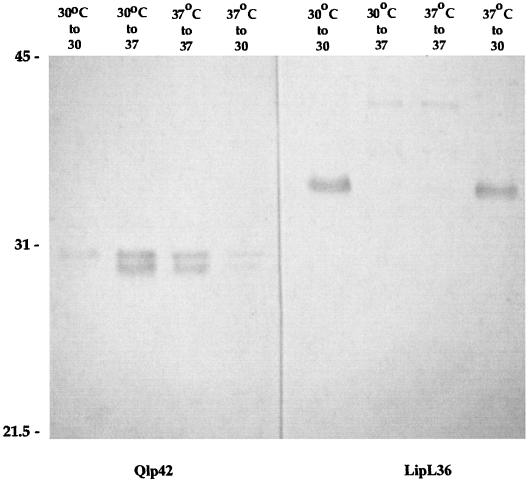
Lysates of JEN4 cultured at both 30 and 37°C were separated by SDS-PAGE and transferred to nitrocellulose for analysis by immunoblotting with Qlp42-specific horse antisera (Fig. 6). Qlp42 was upregulated when cultures were shifted from 30 to 37°C, in contrast to LipL36, the expression of which was switched off. However, although the predicted molecular mass of Qlp42 is 39.8 kDa without the signal peptide, antiserum to recombinant Qlp42 was reactive with two bands with relative molecular masses of 30 and 29.5 kDa. Recombinant Qlp42 runs at the predicted molecular mass (data noto shown). Further, the antiserum was slightly reactive with a 30-kDa band in lysates at 30°C.
FIG. 6.
Immunoblots of JEN4 grown at 30°C, grown at 30°C and shifted to 37°C, grown at 37°C and maintained at 37°C, or grown at 37°C and shifted to 30°C. Following SDS-PAGE, lysates were immunoblotted with Qlp42- and LipL36-specific antisera. Molecular mass markers (in kilodaltons) are indicated on the left.
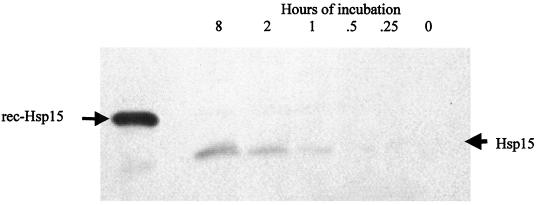
Temperature regulation of Hsp15.
Hsp15-specific antiserum failed to react with any bands from whole-cell lysates of Leptospira cultured at 30 and 37°C for 5 to 7 days (data not shown). Therefore, immunoblot analysis was performed to determine whether expression of Hsp15 would be induced within minutes of temperature shift of culture from 30 to 37°C, as is typical of other heat shock proteins (Fig. 7) (23). Hsp15-specific antiserum reacted with a 15-kDa band that was expressed from 1 to 8 h.
FIG. 7.
Whole-cell Leptospira lysates (JEN4) shifted from 30 to 37°C for different times (hours) and blotted with Hsp15-specific antiserum. rec, recombinant.
Qlp42- and Hsp15-specific antibodies in sera of convalescent mares.
ELISAs were performed to determine whether antibodies reactive with recombinant Qlp42 and Hsp15 were produced by naturally infected horses. Both Qlp42 and Hsp15 were amplified from genomic DNA of JEN4 and expressed from pET-15b in E. coli BL21. Recombinant His-tagged Qlp42 consists of 384 amino acids with a predicted molecular mass of 41.65 kDa. Recombinant His-tagged Hsp15 consists of 163 amino acids with a predicted molecular mass of 18.76 kDa. Sera of convalescent mares were assayed at 1:100 and 1:1,000 (Table 2). Levels of Qlp42-specific antibodies were high in six mares (M1, M2, M3, M4, M6, and M11), low in two mares (M7 and M9), and undetectable in three mares (M5, M8, and M10). All mares had lower levels of antibodies against Hsp15 than against Qlp42.
TABLE 2.
MAT titers and Qlp42- and Hsp15-specific antibody levels (ELISA) of naturally infected convalescent mares' sera
| Mare | MAT titera to serovar pomona | ELISA optical density for antibody to protein at dilution
|
||
|---|---|---|---|---|
| Qlp42 (1:100) | Qlp42 (1:1,000) | Hsp15 (1:100) | ||
| Negative control | 0 | 0.34 | 0.04 | 0.03 |
| M1 | 25,600 | 2.14 | 0.78 | 0.54 |
| M2 | 409,600 | 1.95 | 0.30 | 0.32 |
| M3 | 102,400 | 2.08 | 0.38 | 0.34 |
| M4 | 102,400 | 1.90 | 0.58 | 0.57 |
| M5 | 102,400 | 0.47 | 0.12 | 0.27 |
| M6 | 409,600 | 1.77 | 0.39 | 0.48 |
| M7 | 204,800 | 1.04 | 0.20 | 0.33 |
| M8 | 409,600 | 0.60 | 0.09 | 0.33 |
| M9 | 204,800 | 0.90 | 0.18 | 0.31 |
| M10 | 51,200 | 0.61 | 0.12 | 0.35 |
| M11 | 102,400 | 1.24 | 0.25 | 0.65 |
Reciprocal of dilution at endpoint.
DISCUSSION
Use of the double antibody screen proved to be a simple yet effective method of detecting clones of a genomic DNA library expressing proteins that were upregulated at 37°C. The genes for two such novel leptospiral proteins were identified, characterized, and designated qlp42 and hsp15.
Sequence analysis of qlp42 identified a gene encoding a putative lipoprotein of 388 amino acids. Although genes for partially homologous proteins were found in the database, these genes were identified during genomic sequencing of the opportunistic pathogen P. aeruginosa (24) and the radiation-resistant bacterium D. radiodurans (26) and are hypothetical proteins based on sequence analysis but with no characterized function with respect to pathogenesis and protective immunogenicity. There were no significant homologies with the genomes of either of the spirochetes B. burgdorferi and T. pallidum. Lipoproteins are common in spirochetes (6, 7), and the finding of a cysteine residue after a signal peptide suggests that a protein is lipidated (8). Sequence analysis of Qlp42 identified a 21-amino-acid signal peptide followed by a cysteine residue, indicating that it may also be a lipoprotein (10). A potential lipoprotein signal peptidase II cleavage site is provided in Phe-Asn-Ala-Cys, which deviates somewhat from the typical E. coli lipobox of Leu-X-Y-Cys. However, phenylalanine has been found in place of leucine in several other spirochetal proteins for which there is experimental evidence of lipidation, including OspC and OppA-II of B. burgdorferi, Vsp33 of Borrelia hermsii, SmpA of Brachyspira hyodysenteriae, and TpN29-35 of T. pallidum (8).
The pathogenic serovars of L. interrogans considered to be especially important in human and animal infections include serogroup Icterohaemorrhagiae serovar copenhageni and serovars pomona, bratislava, canicola, grippotyphosa, and hardjo. PCR confirmed that sequences similar to that of Qlp42 occur among these serovars, although only a faintly visible amplified product was observed for serovars bratislava and grippotyphosa. Differences in nucleotide sequence complementary to primers may account for the poor amplification, since the same result was obtained using different concentrations and preparations of DNA. In contrast, no PCR products were amplified from the nonpathogenic L. biflexa or the pathogenic B. burgdorferi.
Profile HMMs are statistical models of the primary structure consensus of a sequence family (S. Eddy, HMMER user's guide, 1998). These algorithms have identified a consensus sequence in Hsp15 indicating that it is a member of the Hsp20/α-crystallin family of proteins. Prokaryotic and eukaryotic organisms respond to heat shock or other environmental stress by inducing the synthesis of proteins collectively known as heat shock proteins. The principal heat shock proteins with chaperone activity, i.e., those that protect newly made proteins from misfolding, belong to five conserved classes: HSP100, HSP90, HSP70, HSP60, and the small heat shock proteins (sHSPs) (14) The sHSPs are a family of proteins with an average molecular mass of 20 kDa that can form large multimeric structures and have a wide range of cellular functions, including endowing cells with thermotolerance in vivo and being able to act as molecular chaperones in vitro (2, 16). They accomplish this by forming stable complexes with folding intermediates of their protein substrates (4, 17). The crystal structure of an sHSP from the hyperthermophilic archaeon Methanococcus jannaschii has been reported previously (14). Hsp15 is the first sHSP of the Hsp20/α-crystallin family described for spirochetes. No significant homologies were found in the complete genome sequence of B. burgdorferi or T. pallidum. It is reasonable to assume that Hsp15 has the same functional capabilities as other sHSPs and so may also function by forming stable complexes with folding intermediates. Expression of Hsp15 in response to stress signals may be especially important in Leptospira, for the stable expression of a downstream cascade of proteins required to adapt to conditions encountered upon host infection. The predicted molecular mass of 15 kDa for Hsp15 corresponds in size to a protein of the same molecular mass induced within 15 min after heat shock of L. interrogans from 30 to 37°C (23). PCR confirmed that sequences similar to hsp15 occur among other serovars of L. interrogans. In contrast, no PCR products were amplified from the nonpathogenic L. biflexa or the Lyme disease spirochete B. burgdorferi. Although PCR does not preclude the presence of homologous sequences in the saprophytic L. biflexa, the lack of a conserved amplified product may partially explain both the inability to grow in vitro at 37°C and the lack of virulence. It is interesting to note that conserved secondary structure domains are the basis for inclusion of α-crystallins and sHSPs in the same protein family. The α-crystallins are major lens structural proteins of the vertebrate eye and have functions comparable to those of sHSPs (11, 19). Although it is tempting to speculate that Hsp15 may be a cross-reactive antigen hypothetically implicated in human and equine recurrent uveitis (21, 22), the absence of Hsp15 specific antibodies in uveitic eye fluids argues to the contrary (unpublished observations). The presence of qlp42 and hsp15 in pathogenic serovars of Leptospira suggests a role in the adaptive response of pathogenic leptospires to higher temperatures, as encountered during infection of a suitable host.
Antiserum specific for recombinant Qlp42 confirmed that Qlp42 is expressed in JEN4. Further, expression of Qlp42 is upregulated when organisms are shifted from 30 to 37°C. Immunoblot analysis also confirmed that expression of Qlp42 is maintained at 37°C, and as cultures are shifted from 37 to 30°C, expression is downregulated. This is in direct contrast to expression of LipL36, which is switched off at 37°C and switched on at 30°C. Interestingly, Qlp42-specific antiserum reacted with more than one band in whole-cell lysates of L. interrogans (JEN4) shifted to 37°C. These may be modified forms of the same antigen or may be different cross-reactive antigens. Further, the two reactive bands of ∼30 and 29.5 kDa differed significantly from the predicted molecular mass of 39.8 kDa for the mature Qlp42 protein. The reason for this is unclear, but it may be due to several possible pre- and posttranslational modifications. A similar observation was also noted with specific antiserum against a recently cloned leptospiral protein, Lk90 (accession no. U95056) (13).
Antiserum to Hsp15 was unreactive with components of Leptospira whole-cell lysates shifted from 30 to 37°C for 5 to 7 days. However, as for other heat shock proteins, expression of Hsp15 was detected within hours of temperature shift, suggesting that Hsp15 may be an important constituent in the early stages of adaptation to the higher body temperature of the host.
Many spirochetal proteins are differentially expressed, presumably for the purpose of adapting to different environmental conditions (8). The results of this study show that temperature affects expression of a range of characterized leptospiral proteins. Specifically, LipL36 is switched off in response to temperature change from 30 to 37°C, while Qlp42 and Hsp15 are switched on. These examples will be useful as models for study of the mechanism of temperature regulation, such as identification of the upstream nucleotide motifs required for upregulation or downregulation of gene products in response to environmental signals.
Are Qlp42 and Hsp15 expressed in vivo? Of 11 convalescent mares, 6 had high levels of Qlp42-specific antibody, 2 had low levels, and 3 were negative (Table 2), suggesting expression of Qlp42 in the majority of infected equines. In the case of Hsp15, the sera of all mares were positive for specific antibody compared to the negative controls, although the lower antibody levels suggest that Hsp15 may be less immunogenic or restricted in its expression in the host.
Finally, the study also validates use of double antibody screening to identify antigenic constituents of Leptospira expressed in vivo and is a means to identify other unknown proteins expressed in different environments. In addition to identifying upregulated proteins, the method could be adapted to identify proteins downregulated during temperature change or proteins regulated in response to pH, etc. Ultimately, characterization and identification of all thermoregulated proteins of Leptospira and elucidation of the mechanisms that regulate temperature-dependent protein synthesis will undoubtedly provide important insights into the pathogenesis of leptospirosis and identify as-yet-undiscovered targets of protective immune responses.
ACKNOWLEDGMENTS
This work was funded by the Keeneland Association. J. E. Nally was funded by a Maxwell and Muriel Gluck Fellowship in Equine Studies.
We thank Mike Donahue for providing the Leptospira isolate, Carole Bolin for typing of the isolate, Barbara Smith for providing the reference panel of Leptospira isolates and convalescent equine sera, and David Haake for providing LipL36-specific antisera. We thank John Walker and Brian Stevenson for critical review and helpful discussions in preparation of the manuscript.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 1a.Bateman A, Birney E, Durbin R, Eddy S R, Howe K L, Sonnhammer E L L. The Pfam protein families database. Nucleic Acids Res. 2000;28:263–266. doi: 10.1093/nar/28.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang Z, Primm T P, Jakana J, Lee I H, Serysheva I, Chiu W, Gilbert H F, Quiocho F A. Mycobacterium tuberculosis 16-kDa antigen (Hsp16.3) functions as an oligomeric structure in vitro to suppress thermal aggregation. J Biol Chem. 1996;271:7218–7223. [PubMed] [Google Scholar]
- 3.Donahue J M. Equine leptospirosis. Equine Dis Q. 2001;9:5. [Google Scholar]
- 4.Ehrnsperger M, Graber S, Gaestel M, Buchner J. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J. 1997;16:221–229. doi: 10.1093/emboj/16.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faine S, Adler B, Bolin C, Perolat P. Leptospira and leptospirosis. 2nd ed. Melbourne, Australia: MediSci; 1999. [Google Scholar]
- 6.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams M D, Gocayne J, Venter J C, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 7.Fraser C M, Norris S J, Weinstock G M, White O, Sutton G G, Dodson R, Gwinn M, Hickey E K, Clayton R, Ketchum K A, Sodergren E, Hardham J M, McLeod M P, Salzberg S, Peterson J, Khalak H, Richardson D, Howell J K, Chidambaram M, Utterback T, McDonald L, Artiach P, Bowman C, Cotton M D, Venter J C, et al. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science. 1998;281:375–388. doi: 10.1126/science.281.5375.375. [DOI] [PubMed] [Google Scholar]
- 8.Haake D A. Spirochaetal lipoproteins and pathogenesis. Microbiology. 2000;146(Part 7):1491–1504. doi: 10.1099/00221287-146-7-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haake D A, Martinich C, Summers T A, Shang E S, Pruetz J D, McCoy A M, Mazel M K, Bolin C A. Characterization of leptospiral outer membrane lipoprotein LipL36: downregulation associated with late-log-phase growth and mammalian infection. Infect Immun. 1998;66:1579–1587. doi: 10.1128/iai.66.4.1579-1587.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashi S, Wu H C. Lipoproteins in bacteria. J Bioenerg Biomembr. 1990;22:451–471. doi: 10.1007/BF00763177. [DOI] [PubMed] [Google Scholar]
- 11.Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson R C, Harris V G. Differentiation of pathogenic and saprophytic leptospires. I. Growth at low temperatures. J Bacteriol. 1967;94:27–31. doi: 10.1128/jb.94.1.27-31.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jusuf S D. Proteins of Leptospira interrogans serovar pomona type kennewicki reactive with serum antibodies of aborting mares and their fetuses. Doctoral dissertation. Lexington: University of Kentucky; 1997. [Google Scholar]
- 14.Kim K K, Kim R, Kim S H. Crystal structure of a small heat-shock protein. Nature. 1998;394:595–599. doi: 10.1038/29106. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Lee G J, Pokala N, Vierling E. Structure and in vitro molecular chaperone activity of cytosolic small heat shock proteins from pea. J Biol Chem. 1995;270:10432–10438. doi: 10.1074/jbc.270.18.10432. [DOI] [PubMed] [Google Scholar]
- 17.Lee G J, Roseman A M, Saibil H R, Vierling E. A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J. 1997;16:659–671. doi: 10.1093/emboj/16.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levett P N. Leptospirosis. Clin Microbiol Rev. 2001;14:296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merck K B, Groenen P J, Voorter C E, de Haard-Hoekman W A, Horwitz J, Bloemendal H, de Jong W W. Structural and functional similarities of bovine alpha-crystallin and mouse small heat-shock protein. A family of chaperones. J Biol Chem. 1993;268:1046–1052. [PubMed] [Google Scholar]
- 20.Nally J E, Timoney J F, Stevenson B. Temperature-regulated protein synthesis by Leptospira interrogans. Infect Immun. 2001;69:400–404. doi: 10.1128/IAI.69.1.400-404.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parma A E, Cerone S I, Sansinanea S A. Biochemical analysis by SDS-PAGE and western blotting of the antigenic relationship between Leptospira and equine ocular tissues. Vet Immunol Immunopathol. 1992;33:179–185. doi: 10.1016/0165-2427(92)90045-r. [DOI] [PubMed] [Google Scholar]
- 22.Parma A E, Santisteban C G, Villalba J S, Bowden R A. Experimental demonstration of an antigenic relationship between Leptospira and equine cornea. Vet Immunol Immunopathol. 1985;10:215–224. doi: 10.1016/0165-2427(85)90048-0. [DOI] [PubMed] [Google Scholar]
- 23.Stamm L V, Gherardini F C, Parrish E A, Moomaw C R. Heat shock response of spirochetes. Infect Immun. 1991;59:1572–1575. doi: 10.1128/iai.59.4.1572-1575.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stover C K, Pham X Q, Erwin A L, Mizoguchi S D, Warrener P, Hickey M J, Brinkman F S, Hufnagle W O, Kowalik D J, Lagrou M, Garber R L, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody L L, Coulter S N, Folger K R, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong G K, Wu Z, Paulsen I T. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 25.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White O, Eisen J A, Heidelberg J F, Hickey E K, Peterson J D, Dodson R J, Haft D H, Gwinn M L, Nelson W C, Richardson D L, Moffat K S, Qin H, Jiang L, Pamphile W, Crosby M, Shen M, Vamathevan J J, Lam P, McDonald L, Utterback T, Zalewski C, Makarova K S, Aravind L, Daly M J, Fraser C M, et al. Genome sequence of the radioresistant bacterium Deinococcus radiodurans R1. Science. 1999;286:1571–1577. doi: 10.1126/science.286.5444.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]