Abstract
In this article, starting with the recognition that iodine is essential for normal thyroid function and is a component of thyroid hormone (TH) molecules, we discuss the many seminal observations and discoveries that have led to identification of various pathways of TH metabolism and their potential roles in TH economy and action. We then recount evidence that TH metabolism participates in maintaining the appropriate content of active hormone in a TH-responsive tissue or cell. Thus, metabolism of the TH is not merely a means by which it is degraded and eliminated from the body, but an essential component of an intricate system by which the thyroid exerts its multiple regulatory effects on almost all organs and tissues. The article ends with a summary of the current concepts and some outstanding questions that are awaiting answers.
Keywords: deiodinases, thyroxine, thyroxine glucuronide, thyroxine sulfate, triiodothyronine
Introduction
In 1820, Coindet reported that the newly discovered element, iodine, could be used successfully to treat endemic goiter, suggesting that it is important for normal functioning of the thyroid gland (1). In 1874, Gull published the symptoms of a disease that he described as a cretinoid state supervening in adult life in women (2), and in 1878, Ord proposed the term myxedema for this disease and noted that it was associated with atrophy of the thyroid (3).
Five years later, J and A Reverdin described the clinical picture following 21 thyroidectomies and called it “myxoedème opératoire” (4,5), and Kocher reported the results of 200 thyroidectomies performed by himself and other surgeons in Switzerland and Germany. He termed the resulting condition, which resembled that of myxedema, cachexia strumipriva (6).
In 1888, a committee that had been established to consider these conditions reported their conclusion that myxedema, cretinism, and the conditions that resulted from total thyroidectomy resulted from impaired thyroid function (7). In 1890, Bettencourt R and Serrano J-A reported rapid improvement in a patient with myxedema who had received a graft of sheep thyroid (8), and a year later, Murray showed that myxedema could be successfully treated with daily injections of sheep thyroid extract (9).
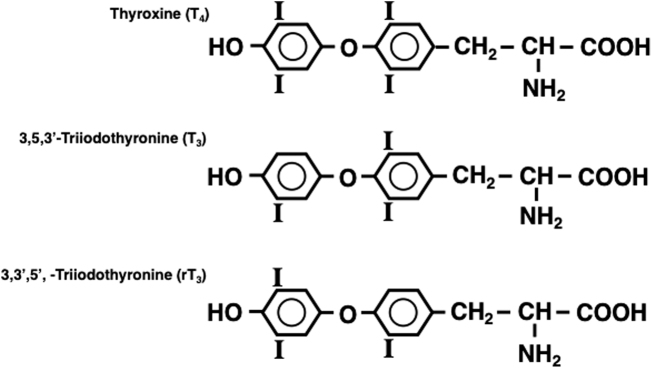
Then in 1896, Baumann reported that the thyroid gland contained significant amounts of iodine, contained predominantly in a protein fraction that on hydrolysis yielded a substance that ameliorated symptoms of myxedema in women and thyroidectomy in animals (10,11). Chemical purification of an active principle from the thyroid, which contained 65% iodine, was achieved by Kendall in 1914 and he named it thyroxin (12). Harington determined that the compound is a p-hydroxyphenyl ether of tyrosine, with iodine atoms located in the 3,5,3′ and 5′ positions, and he renamed it thyroxine (T4) (13) (Fig. 1).
FIG. 1.
Chemical structures of Thyroxine (T4), 3,5,3′-triiodothyronine (T3) and 3,3′,5′-triiodothyronine (reverseT3, rT3).
Although it was then evident that most of the biological activity of the thyroid was due to T4, it was not clear whether it was also the circulating hormone. In fact, it was not until 1948 that Taurog and Chaikoff, using [131I]T4, found that in normal animals, most (if not all) of the organic iodine in the circulation consisted of T4 reversibly bound to plasma proteins (14). This was unequivocally confirmed by Laidlaw, using the newly developed technique of paper chromatographic analysis (15).
Studies of T4 Metabolism In Vivo
In the 1940s, the availability of radioactive iodine and paper chromatography opened up a new era of thyroid hormone (TH) research, including studies of T4 metabolism. Gross and Leblond reported that 2 hours following injection of [131I]T4 into rats, up to 50% of the injected radioactivity was located in the liver, bile, and gastrointestinal tract, and after 24 hours, as much as 80% was located in the feces as [131I]T4, while 10% appeared in the urine as [131I]iodide (16).
Taurog et al. demonstrated that majority of the organic iodine in bile was T4-glucuronide (T4G), a compound formed by conjugation of glucuronic acid (GA) with the phenolic hydroxyl group of T4 (17). T4 was also present in bile in the form of a sulfate ester (T4S) (18). The conjugated forms were shown to be readily hydrolyzed in the intestine and thus T4 was excreted in feces in the unconjugated form (17,18). Initially it was felt that this metabolic pathway was solely a means by which the liver eliminated excess T4 from the body. However, Albert and Keating, using a physiological level of [131I]T4, demonstrated the presence of enterohepatic circulation of T4, as indicated by the finding that the rate of secretion of radioactivity into the bile was much higher than its rate of excretion in the feces (19). The proportion of hormone resorbed from the intestine and the relative fractions of hormonal iodine excreted in urine and feces were found to vary widely among species (20).
Many derivatives of T4, including 3,5,3′-triiodothyronine (T3) and 3,3′5′-triiodothyronine (rT3) (Fig. 1), and the acetic acid derivatives of both T4 and T3 (tetrac, TA4, and triac, TA3), can also undergo conjugation with GA and esterification with sulfate. Several studies indicate that whereas T4 appears to conjugate more readily with GA in the liver, T3 is esterified primarily with sulfate (21).
In rats given exogenous T4, the clearance rate of T4 through the hepatic/fecal route steadily increases as the dose of T4 is raised, suggesting that the process participates in regulation of the serum T4 level (20). T4G is also formed and sequestered in the kidney, and it has been suggested that this organ provides an additional mechanism for regulating the circulating T4 level (20).
TH also can undergo oxidative deamination and decarboxylation. In 1954, Jouan et al. reported the presence of triac in the kidneys of rats given [131I]T3 (22), and Albright et al. demonstrated the formation of both Tetrac and Triac from the parent hormones in rat kidney slices (23). Both analogs are formed from endogenous T4 and T3 in the liver and kidney (24).
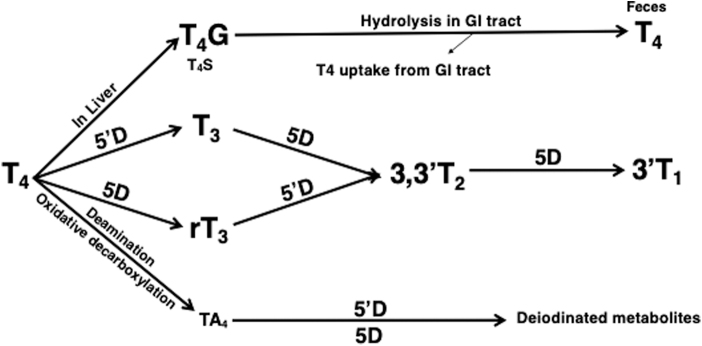
However, although the acetic acid analogs have significant thyromimetic activity, the importance of this metabolic pathway and the physiological role of endogenous acetic acid analogs were unknown at the time (21). The pathways of T4 metabolism recognized by the end of the 1970s are shown in Figure 2.
FIG. 2.
Pathways of metabolism and excretion of T4 identified by 1980. 5′D, 5′-deiodination; 5D, 5-deiodination; NB, significant amounts of T4G are also formed and stored in the kidneys; T4G, T4 glucuronide; T4S, T4 sulfate; TA4, tetrac tetraiodothyroacetic acid.
The Role of T4 in TH Action
Although by the 1950s it was recognized that T4 is quantitatively the major iodinated compound secreted by the thyroid and present in circulation, there was some doubt that it was the active form of the TH. The observations that T4 had a long latent period of action when administered in vivo and had no significant effect when studied in tissues in vitro suggested that it had to be metabolized in peripheral tissues to an active form.
This concept was substantiated when T3 was identified in the thyroid and circulation by Gross and Pitt-Rivers. They showed that T3 was more potent, and its action more rapid, than T4 and hypothesized that T3 was the active form of the hormone and T4 was its precursor (25). However, the necessary proof was elusive. As early as 1955, Pitt-Rivers pointed out that although rats responded more rapidly to T3 than to T4, the response was still measured in hours and injected [131I]T3 disappeared more rapidly from the body than did [131I]T4. Furthermore, T3 also appeared to be inactive when added to tissue preparations in vitro.
Arguably, the more significant issue was that, despite several attempts, the presence of [131I]T3 following injection of [131I]T4 into athyreotic humans could not be unequivocally demonstrated. Although in 1955 Pitt-Rivers and Stanbury reported the presence of [131I]T3 in circulation of patients given [131I]T4 (26), Stanbury became concerned that the reported data yielded curves for the appearance of labeled T3 and failed to conform to the theory that T3 was formed from T4 by a simple precursor–product relationship. There was also considerable overlap of T4 and T3 on the chromatograms. He repeated the study in six patients using a solvent system that provided excellent separation of the two hormones and found no evidence of any T4 to T3 conversion (27).
Attempts to detect T4 to T3 conversion in vitro were also largely unsuccessful. Although conversion of [131I]T4 to [131I]T3 in rat kidney slices was reported (28,29), the findings could not be confirmed (23), and many other groups were unable to demonstrate [131I]T3 generation from [131I]T4 in a variety of tissues despite the fact that deiodination of T4, as evidenced by the release of [131I]iodide, clearly occurred (21).
At this point, concern was growing regarding the physiological significance of studies of T4 deiodination in vitro. There were many reports describing the presence in tissues, particularly in broken cell preparations, of T4 deiodinating systems that were heat stable and thus unlikely to be mediated by an enzyme. Furthermore, several compounds were not only found to stimulate deiodination, including hydrogen peroxide, flavin compounds, ferrous ions, and ascorbic acid, but most of the effects were also obtained in heat-treated tissue preparations.
In all these studies, the reaction product was invariably inorganic iodide, but no T3 was generated. These diverse studies, the possible mechanisms involved, and their physiological significance are considered in a 1963 review (21).
By the mid-1960s, it was felt that the results obtained in studies of T4 deiodination in vitro were of questionable physiological importance, and investigators focused on studies in vivo, in particular the isotopic equilibrium technique developed by Van Middlesworth (30). This involved giving rats a daily injection of [131I]T4. Once the daily output of radioactivity in urine and feces became constant, the model could be used to test the effects of conditions or substances on the urinary output of [131I]iodide as a measure of T4 deiodination. For example, Van Middlesworth used this technique to demonstrate that 6-n-propyl-2-thiouracil (PTU) partially inhibited the deiodination of T4. However, it was not possible to determine from these studies whether deiodination is a process essential for TH action or merely one by which it is degraded, and no evidence of [131I]T3 generation was obtained.
The answer finally came in the early 1970s as a result of two critical findings. First, in 1970, Braverman et al. demonstrated unequivocally that T4 to T3 conversion occurs in athyreotic humans (31), and Sterling et al. confirmed this by demonstrating that [14C]T3 was present in euthyroid human subjects injected with [14C]T4. In humans, up to a third of the T4 that was metabolized was converted to T3 (32), and in rats, at least 20% of the total body extrathyroidal T3 was derived from T4 (33). These convincing findings laid to rest a major inconsistency in the theory that T3 was the active form of the hormone, namely, the previous failures to demonstrate T4 to T3 conversion.
The second finding was the discovery in 1972 of TH receptors (TRs) by Oppenheimer et al., who demonstrated that they are located in the nucleus and have much higher affinity for T3 than for T4 (34). Furthermore, the majority of iodothyronine bound to TRs in rat liver and kidney was T3 (35).
Together, these findings provided convincing evidence that the majority of TH action is initiated by T3 rather than T4. Thus, 5′-deiodination (5′D) is an activating process and therefore an essential component of TH action.
Identification and Characterization of Deiodinases
Now that the importance of 5′D for production of T3 in peripheral tissues was established, interest turned to the identification and characterization of the specific enzyme(s) involved. Three reviews documenting the progress made in this area by Leonard and Visser (1986), Bianco et al. (2002), and St Germain et al. (2009) are available (36–38).
As discussed above, earlier attempts to study these enzymes in vitro had been repeatedly unsuccessful. However, this problem was resolved in 1976 when Visser et al. reported that thiol groups were essential cofactors for deiodination in vitro, and in the presence of dithiothreitol, subcellular fractions of rat liver readily converted T4 to T3 (39).
It was soon established that there are two enzymes that catalyze the conversion of T4 to T3, the type 1 and 2 deiodinases (D1 and D2). D1 and D2 were distinguished initially because D1, in contrast to D2, was inhibited by PTU (40). D1 is a dual-purpose enzyme. In addition to its ability to activate T4 by converting it to T3 by 5′D, it can also inactivate iodothyronines by inner-ring or 5-deiodination (5D), in particular after they have undergone esterification with sulfate. Thus, whereas D1 is capable of deiodinating unconjugated T4 by 5′D or 5D with comparable efficiency, after sulfation of T4 to T4S, 5′D by D1 is essentially blocked and T4S is subjected to 5D by D1 to generate the inactive metabolite, rT3S (41). It is notable that the preferred substrate of D1 for 5′D is not T4, but rT3. D1 activity is increased in hyperthyroidism and decreased in hypothyroidism. Its expression is most abundant in the kidney, liver, and thyroid but it is also expressed at a low level in other tissues (36–38).
D2 catalyzes only 5′D and its Kd for T4 is in the nanomolar range, approximately three orders of magnitude lower than that of D1 for T4. In contrast to D1, the substrate preference of D2 for T4 is greater than that for rT3 and its expression is increased in hypothyroidism. D2 activity is expressed at low levels in many tissues, but most abundantly in the pituitary, brain, and brown fat (BAT) (36–38).
A third deiodinase (type 3 deiodinase [D3]), first demonstrated in cultured monkey hepatocarcinoma cells (42), catalyzes only 5D and inactivates both T4 and T3. D3 activity is expressed at high levels in the placenta and pregnant uterus and at lower levels in fetal and neonatal tissues, in particular the brain. In nonpregnant adults, it is found predominantly in the brain and skin (36–38).
Evidence concerning the role of 5′D in peripheral tissues was also accumulating from studies in vivo. In 1979, Larsen et al. demonstrated that inhibition of intrapituitary 5′D activity prevented the acute suppression of thyrotropin release by T4 in hypothyroid rats, indicating that conversion to T3 is essential for the action of T4 in this tissue (43). The following year it was shown that T3 produced from T4 by 5′D supplies much of the endogenous T3 in rat brain, including that it was located on the nuclear TRs (44,45).
It was also found that T3 derived from T4 by 5′D is exchanged with the circulating T3 (46). [131]rT3 was also found in brain tissue following injection of [131I]T4, suggesting that D3 may play a role in TH economy in this organ (47). Further evidence for this concept was provided by Kaplan and Yaskoski (48) who measured 5′D and 5D in areas of rat brain, starting at birth. It was notable that in each area studied, 5D was highest at, or soon after, birth and then decreased, whereas 5′D was relatively low at birth and then increased markedly over the neonatal period, a time when considerable maturation of the brain occurs.
Additional evidence for the role of 5′D came from studies in BAT. In 1985, Silva and Larsen demonstrated that in rats exposed to cold, 5′D activity in BAT was enhanced and this was associated with an increase in both local and plasma T3 content (49). Local conversion of T4 to T3 by 5′D activity was found to be critical for thermogenesis in BAT (50). It was now clearly evident that in addition to the control of TH secretion by the thyroid gland, TH economy and presumably action are also regulated, at least in part, by both 5′D and 5D in peripheral tissues.
By the mid-1980s, it was widely accepted that a major role of D1 is to generate T3 for circulation, whereas that of D2 is to generate T3 from T4 for local use within the same cell or tissue. T3 generated by D2 can also contribute to the circulating T3 (49). However, the precise roles of each of the three deiodinases in vivo, particularly in tissues that express more than one deiodinase, remained to be established.
One approach to defining the role of a deiodinase is to study animal models rendered completely deficient in its activity. Unfortunately, no compound had been found that could make animals completely deficient in a single deiodinase. However, cloning of cDNAs for the three deiodinases provided a novel and more specific way to achieve this goal.
Cloning the Deiodinases
In 1991, Berry et al. (51) used expression cloning in the Xenopus oocyte to isolate a cDNA for D1 from a rat liver cDNA library. The kinetic properties of the protein expressed in transient assay systems, tissue distribution of its messenger RNA, and changes in its level with thyroid status confirmed its identity. They also found that the mRNA for D1 contains in its coding region a UGA codon that codes for selenocysteine, a rare amino acid essential for full activity of the enzyme; enzyme activity was markedly reduced when the selenocysteine is replaced by cysteine, which has sulfur instead of selenium (SEC) in its structure (52). Reduced D1 activity and abnormal TH metabolism in humans exhibiting a mutation in the SEC insertion sequence-binding protein 2 have been reported (53).
D3 was the next deiodinase to be cloned. The group of Dr. Donald D. Brown (Carnegie Institution for Science) isolated a cDNA from a Xenopus laevis tail tissue cDNA library, which exhibited some homology to the mammalian D1 cDNA. A collaboration with the laboratories of Galton and St Germain resulted in the finding that this cDNA coded for a deiodinase with properties characteristic of D3 (54). The Xenopus cDNA was used as a probe to isolate D3 cDNAs from other species.
D2 was also first cloned from amphibian tissue by Davey et al. (55) and then used as a probe to clone a D2 cDNA from rat tissues. D2 and D3 were also shown to have selenocysteine at their active sites.
Studies in Mice Rendered Deiodinase Deficient by Targeted Disruption of the Corresponding Gene
With the sequences of cDNAs for the deiodinases now available, Schneider et al. were able to create mice deficient in D2 (D2KO) (56), D1 (D1KO) (57), and both D1 and D2 (D1/D2KO) (58), and a D3KO mouse was created by Hernandez et al (59). Surprisingly, none of the three 5′D-deficient mouse models exhibited any appreciable gross phenotype. Growth was essentially normal, reproductive capacity was seemingly unimpaired, and the serum T3 level was normal in all three models. However, the serum T4 level was significantly elevated in D1KO and D2KO mice and by almost twofold in double D1/D2KO mice (56–58), and detailed studies in individual organs revealed many tissue-specific phenotypes, most notable in the D2-deficient mice. In these mice, the phenotype included pituitary resistance to TH (56,58), reduced tissue T3 content and altered gene expression in the brain (60), impaired hearing (61), impaired BAT thermogenesis (62), and impaired bone strength and mineralization (63).
Analysis of the D1KO phenotype revealed that serum rT3 was markedly elevated (57), an abnormality also noted in two patients who had a mutation in the DIO1 gene (64). It has been estimated that in humans, the T4 secreted by the thyroid generates rT3 and T3 in approximately equal amounts (65). These findings, together with the knowledge that rT3 is the preferred substrate for D1, suggest that a significant fraction of rT3 normally undergoes 5′D by D1, rather than being excreted as rT3 in the feces, a mechanism that would conserve iodine should conditions warrant it. Although the serum level of T4 was elevated, the levels of T3 and TSH were unchanged, as were several indices of peripheral thyroid status, suggesting that D1 is not essential for maintenance of a normal T3 level in this species (57). However, D1 deficiency resulted in a marked decrease in excretion of iodine derived from TH, whereas excretion of iodothyronines in feces was markedly increased (57).
The D3KO mouse exhibited a much more marked gross phenotype than those of D1 and D2KO mice, and a list of both general and tissue-specific phenotypic changes can be found in a recent review (66). They include growth retardation, impaired fertility, neonatal thyrotoxicosis, adult hypothyroidism, impaired brain development and function, and impaired hearing and vision (66,67). Unlike in the D1 and D2KO mice, D3KO mice exhibit considerable perinatal lethality. Since D3 is expressed at high levels in both the placenta and uterus, this is likely due, at least in part, to exposure of fetuses to toxic levels of TH derived from the mother (66,67).
A Major Role for TH Metabolism by D2 and D3 in the Regulation of Tissue T3 Content and Action
Information obtained from the D2 and D3-deficient mice, together with the known expression profiles of D2 and D3 activities in some tissues, has provided unequivocal evidence that both these enzymes play a major role in determining the intracellular T3 content and hence action in peripheral tissues. The coordinated interplay of these two enzyme activities appears to be critically important during development, as demonstrated by several groups, in particular those of Drs. Bianco, Forrest, and Hernandez.
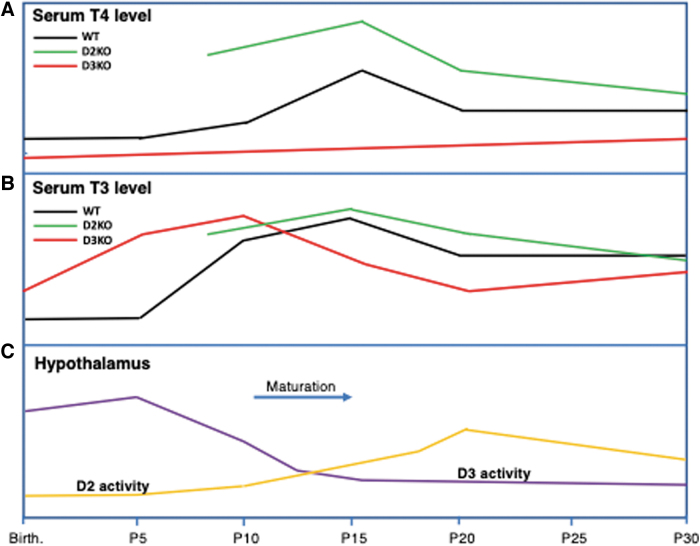
Contributions in this regard are detailed and referenced in a recent review (66). In this review, the data obtained by Hernandez (59,66) on the maturing hypothalamic/pituitary/thyroid (HPT) axis are used as a representative example of this concept. In both the D2KO and D3KO mice, maturation of the HPT axis is impaired. Figure 3 shows the general profiles, relative to each other, of the circulating T4 (panel A) and T3 (panel B) levels in wild-type (WT), D2KO, and D3KO mice between birth and postpartum day 30 (P30). Panel C shows the profiles of D2 and D3 activities in the hypothalamus of WT mice during the same period. In rodents, maturation of the HPT axis, the time when it reaches its set point, occurs at approximately 2 weeks of life. It is at this age that the circulating T4 and T3 levels, which are relatively low at birth, reach their peaks. In the hypothalamus, D3 activity, which is highest shortly after birth, starts to decrease steadily during neonatal life, reaching a low plateau by P20. In contrast, hypothalamic D2 activity is low at birth, but increases steadily and its peak largely coincides with those of the serum TH levels (Fig. 3). These activity profiles indicate a scenario whereby in the first few days of life, intrahypothalamic T3 content is maintained at an appropriately low level by the presence of relatively high D3 activity and minimal D2 activity, until the T3-dependent maturation period approaches, when the level of D3 activity declines and that of D2 increases, changes that enable an increase in intrahypothalamic T3 content.
FIG. 3.
Profiles of serum T4 levels (A) and T3 levels (B) in WT, D2KO, and D3KO mice between birth and P30. Profiles of D2 and D3 activities during the same period (C). D2, type 2 deiodinase; D3, type 3 deiodinase; P30, postpartum day 30; WT, wild-type.
The importance of this delicate interplay of two enzymes becomes evident when either of them is absent. Thus, in the D3KO mouse, the serum T3 level is markedly elevated in the first few days after birth and this results in elevation of brain T3 content and TH-responsive gene expression in the hypothalamus (59). The outcome of this T3 toxicity is that the set point of the HPT axis is impaired, as indicated by subsequent hypothyroidism. In the absence of D2 activity, hypothalamic T3 content is reduced and T4 content is elevated at P15, resulting in partial resistance of the HPT axis to the negative feedback effect of T4 (56,60). It is notable that a combined D3 and D2 deficiency results in partial rescue of the HPT axis impairment (68).
Current Conclusions and Unanswered Questions
To summarize the evolution of knowledge concerning TH metabolism, a timeline of the major discoveries is shown in Table 1. Perhaps the most important thing we have learned in the last 50 decades is that maintenance of a euthyroid state in humans and animals is dependent not only on regulation of the rate of secretion of TH from the thyroid gland but also on a delicate interplay among the various metabolic processes to which the hormones are subjected in peripheral tissues. These processes, together with specific TH transporters that facilitate the entry of hormones into cells (69), participate in ensuring that the appropriate amount of active hormone is maintained within a given tissue or cell. Thus, both D2 and D3 participate in regulating the intracellular content of the more active TH, T3, the former by generating it from T4 and the latter by converting both T4 and T3 to inactive metabolites.
Table 1.
Timeline of Advances That Have Contributed to Our Understanding of Thyroid Hormone Metabolism and Its Physiological Importance
| Year | Report | Reference |
|---|---|---|
| 1820 | Iodine is shown to be important for normal function of the thyroid gland | (1) |
| 1878 | The syndrome called myxedema is noted to be associated with thyroid atrophy | (3) |
| 1883 | Thyroidectomy is shown to result in cachexia strumipriva, which resembled myxedema | (6) |
| 1890 | Symptoms of myxedema are shown to be alleviated by a graft of sheep thyroid | (8) |
| 1896 | Symptoms of myxedema ameliorated with an iodinated substance isolated from the thyroid | (10,11) |
| 1914 | An active compound, thyroxin, containing 65% iodine is purified from the thyroid | (12) |
| 1926 | The active compound is synthesized, its structure is determined, and it is renamed thyroxine | (13) |
| 1947 | [131I] iodide derived by deiodination of injected [131I]T4 is found in rat urine | (16) |
| 1947 | Significant radioactivity is found in the liver, bile, and GI tract after injection of [131I]T4 | (16) |
| 1948 | The main circulating iodinated compound is demonstrated to be T4 | (14) |
| 1952 | Conjugate of T4 with GA is detected in bile | (17) |
| 1952 | The enterohepatic circulation of T4 is demonstrated | (19) |
| 1952 | T3 is identified in thyroid and plasma | (25) |
| 1956 | TH shown to undergo oxidative deamination to form acetic acid analogs in vivo | (22) |
| 1960 | Conjugate of T4 with sulfate is detected in plasma | (18) |
| 1970 | 5′D of T4 to T3 is demonstrated in athyreotic humans | (31,32) |
| 1972 | TH nuclear receptors that have a higher affinity for T3 than T4 are discovered | (34) |
| 1977 | 5D of TH is demonstrated in cultured monkey hepatocarcinoma cells | (41) |
| 1979 | First report indicating that 5′D of T4 is critical for its physiological action | (43) |
| 1982 | Evidence suggesting that there are two mechanisms for 5′D of TH is reported | (40) |
| 1991 | A cDNA for D1 is cloned, and the enzyme is shown to contain selenocysteine at its active site that is necessary for full enzyme activity | (51,52) |
| 1994 | A cDNA for D3 is cloned and the deiodinase shown to be a selenoprotein | (54) |
| 1995 | A cDNA for D2 is cloned and the deiodinase shown to be a selenoprotein | (55) |
| 2001 | A mouse completely deficient in D2 is created by targeted disruption of its gene | (56) |
| 2002 | Identification of human DIO2 SNP associated with impaired glucose metabolism | (71) |
| 2004 | 3-Iodothyronamine is shown to be an endogenous active derivative of the thyroid hormone | (70) |
| 2006 | Mice completely deficient in D1 or D3 are created by targeted disruption of their genes | (57,59) |
| 2021 | Mutations in the human DIO1 gene associated with abnormal TH metabolism are reported | (66) |
5′D, 5′-deiodination; 5D, 5-deiodination; D1, type 1 deiodinase; D2, type 2 deiodinase; D3, type 3 deiodinase; GA, glucuronic acid; GI, gastrointestinal; SNP, single-nucleotide polymorphism; T3, 3,5,3′-triiodothyronine; T4, thyroxine; TH, thyroid hormone.
It also appears that a major role for D1 is the 5′D of rT3, a process that serves to conserve hormonal iodine. However, there are still many questions left unanswered. We do not know the extent to which D1 is involved in converting T4 to T3 or to what extent the circulating T3 comprises T3 generated in peripheral tissues by D1 and D2 and T3 secreted by the thyroid.
Other outstanding questions include the physiological role and significance of sulfate conjugation of T4 and T3 as it affects their mode of deiodination; the role of the liver and the enterohepatic circulation in regulating circulating TH levels; the role of T4G that is stored in the liver and kidney, whether T4 has genomic actions that differ from those of T3; and to what extent other iodinated derivatives of T4 and T3, including tetrac, triac, 3,5-T2, and thyronamines (70), have physiological significance with respect to TH economy and biological effects.
Authors' Contributions
V.A.G. researched the literature and wrote the initial draft of the article. A.H. critiqued the draft and provided significant input to the last section, which comprised some of his work.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
V.A.G. did not receive any funding. A.H. was supported, in part, by National Institutes of Health grants, DK095908 and MH096050.
References
- 1. Coindet JF 1820. Discovery of a new cure for goiter [Découverte d'un nouveau remède contre le goître]. Ann Chim Phys Paris 15:49–59. [Google Scholar]
- 2. Gull WW 1874. On a cretinoid state supervening in adult life women. Trans Clin Soc Lond 7:180–185. [Google Scholar]
- 3. Ord WM 1978 On myxoedema, a term proposed to be applied to an essential condition in the “cretinoid” affection occasionally observed in middle-aged women.” Med Chir Trans 61:57–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reverdin J 1982. Accidents following total ablation goiter [Accidents consécutifs à l'ablation totale du goitre]. Rev Méd Suisse Romande 2:539. [Google Scholar]
- 5. Reverdin JL, Reverdin A. 1883. Note on twenty-two goiter operations [Note sur vingt-deux opérations de goitre]. Rev Med Sui Rom 3:169–198, 233–278, 309–364. [Google Scholar]
- 6. Kocher T 1883. About goiter extirpation and its consequences [Ueber Kropfexstirpation und ihre Folgen]. Arch Klin Chir 29:254–337. [Google Scholar]
- 7. Report of a committee of the Clinical Society of London nominated December 14th 1888, to investigate the subject of myxoedema. Trans Clin Soc Lond 21(Supplement). [Google Scholar]
- 8. Bettencourt R, Serrano J-A A case of myxedema treated by the hypodermic transplant of the thyroid body of a sheep. 1890. [Un cas de myxoedème traité par la greffe hypodermique du corps thyroïde d'un mouton 1890]. La Sem Méd 10:294. [Google Scholar]
- 9. Murray GR 1891. Note on the treatment of myxoedema by hypodermic injections of an extract of thyroid gland of sheep. Brit Med J 1606:796–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baumann E 1896. About the normal occurrence of iodine in the animal body [Über das normale Vorkommen von Jod im Thierkörper]. Ztschr Phys Chem 21:319–321. [Google Scholar]
- 11. Baumann E Über das Thyrojodin 1896. Munch Med Wschr 43:309. [Google Scholar]
- 12. Kendall EC 1915. The isolation in crystalline form of the compound containing iodine which occurs in the thyroid gland. J Am Med Assoc 64:2042–2043. [Google Scholar]
- 13. Harington CR 1926. Thyroxine: its biosynthesis and its immunochemistry. Proc R Soc Lond B 132:223–238. [Google Scholar]
- 14. Taurog A, Chaikoff IL. 1948. The nature of the circulating hormone. J Biol Chem 176:639–656. [PubMed] [Google Scholar]
- 15. Laidlaw JC 1949. Nature of the circulating thyroid hormone. Nature 164:927–928. [DOI] [PubMed] [Google Scholar]
- 16. Gross J, Leblond CP. 1947. Distribution of a large dose of thyroxine labeled with radioiodine in the organs of the rat. J Biol Chem 71:309–320. [Google Scholar]
- 17. Taurog A, Briggs FN, Chaikoff IL. 1952. I131-labeled 1-thyroxine. II. Nature of the excretion product in bile. J Biol Chem 194:655–688. [PubMed] [Google Scholar]
- 18. Michel R, de Gregorio, Lobo LC, Varrone S. 1960. On the presence in rat plasma of the sulfoconjugate of thyroxin (ST4) after injection of low doses of radioactive iodine. C R Seances Soc Biol Fil 1654:1153–1156. [PubMed] [Google Scholar]
- 19. Albert A, Keating FR. 1952. The Role of the gastrointestinal tract, including the liver in the metabolism of radiothyroxine. Endocrinology 51:427–443. [DOI] [PubMed] [Google Scholar]
- 20. Galton VA 1969 The physiological role of thyroid hormone metabolism. In: James VHT (eds) Recent Advances in Endocrinology. Eighth edition. Churchill Press, England, pp 181–206. [Google Scholar]
- 21. Ingbar SH, Galton VA. 1963. Thyroid. Ann Rev Physiol 25:361–380. [DOI] [PubMed] [Google Scholar]
- 22. Jouan P, Michel R, Roche J, Wolf W. 1956. The recovery of 3:5:3’ -triiodothyroacetic acid and 3:3’ -diiodothyronine from rat kidney after injection of 3:5:3’ triiodothyronine. Endocrinology 59:425–432. [DOI] [PubMed] [Google Scholar]
- 23. Albright EC, Lardy HA, Larson FC, et al. 1957. Enzymatic conversion of thyroxine to tetraiodothyroacetic acid and of triiodothyronine to triiodothyroacetic acid. J Biol Chem 224:387–397. [PubMed] [Google Scholar]
- 24. Galton VA, Pitt-Rivers R. 1959. Thyroid hormone metabolism in the kidney. Biochem J 72:314–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gross J, Pitt-Rivers R. 1952. Physiological activity of 3,5,3-triiodothyronine. Lancet 259:593–594. [DOI] [PubMed] [Google Scholar]
- 26. Pitt-Rivers R, Stanbury JB, Rapp B. 1955. Conversion of thyroxine to 3,5,3 -triiodothyronine in vivo. J Clin Endocrinol Metab 15:616–620. [DOI] [PubMed] [Google Scholar]
- 27. Lassiter WE, Stanbury JB. 1958. The in vivo conversion of thyroxine to 3,5/,3’ -triiodothyronine. J Clin Endocrinol Metab 18:903–906. [DOI] [PubMed] [Google Scholar]
- 28. Albright EC, Larson FC, Tust RH. 1954. In vitro conversion of thyroxine to triiodothyronine by kidney slices. Proc Soc Expt Biol Med 86:37–140. [DOI] [PubMed] [Google Scholar]
- 29. Albright EC, Larsen FC. 1959. Metabolism of L-thyroxine by human tissue slices. J Clin Invest 38:1899–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jones SL, van Middlesworth L. 1960. Normal 131I L-thyroxine metabolism in the presence of potassium perchlorate and interrupted by propylthiouracil. Endocrinology 67:855–861. [DOI] [PubMed] [Google Scholar]
- 31. Braverman LE, Ingbar SH, Sterling K. 1970. Conversion of thyroxine to triiodothyronine in athyreotic human subjects. J Clin Invest 49:55–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sterling K, Brenner MA, Newman ES. 1970. Conversion of thyroxine to triiodothyronine in normal human subjects. Science 169:1099–1100. [DOI] [PubMed] [Google Scholar]
- 33. Schwartz HL, Surks MI, Oppenheimer JH. 1972. Quantitation of extrathyroidal conversion of l-thyroxine to 3,5,30-triiodo-L-thyronine in the rat. J Clin Invest 50:1124–1130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oppenheimer JH, Koerner D, Schwartz HL, et al. 1972. Specific nuclear triiodothyronine binding sites in rat liver and kidney. J Clin Endocrinol Metab 35:330–333. [DOI] [PubMed] [Google Scholar]
- 35. Surks MI, Oppenheimer JH. 1977. Concentration of L-thyroxine and L-triiodothyronine specifically bound to nuclear receptors in rat liver and kidney. J Clin Invest 60:555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leonard JL, Visser TJ 1986 Biochemistry of deiodination. In: Hennemann G (ed) Thyroid Hormone Metabolism. Marcel Dekker, New York, pp 189–229. [Google Scholar]
- 37. Bianco AC, Salvatore D, Gereben B, et al. 2002. Biochemistry, cellular and molecular biology, and physiological roles of the Iodothyronine selenodeiodinases. Endo Rev 23:38–89. [DOI] [PubMed] [Google Scholar]
- 38. St Germain DL, Galton VA, Hernandez A. 2009. Minireview: defining the roles of the Iodothyronine deiodinases: Current concepts and challenges. Endocrinology 150:1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Visser TJ, Van Der Does-Tobe I, Docter R, Hennemann G. 1976. Subcellular localization of a rat liver enzyme converting thyroxine into triiodothyronine and possible involvement of essential thiol groups. Biochem J 157:479–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Visser TJ, Leonard JL, Kaplan MM, et al. 1982. Kinetic evidence suggesting two mechanisms for iodothyronine 5'-deiodination in rat cerebral cortex. Proc Natl Acad Sci 79:5080–5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu S-Y, Green WL, Huang W-S, et al. 2005. Alternate pathways of thyroid hormone metabolism. Thyroid 15:943–958. [DOI] [PubMed] [Google Scholar]
- 42. Sorimachi K, Robbins J. 1977. Metabolism of thyroid hormones by cultured monkey hepatocarcinoma cells: nonphenolic ring deiodination and sulfation. J Biol Chem 252:4458–4463. [PubMed] [Google Scholar]
- 43. Larsen PR, Dick TE, Markovitz BP, et al. 1979. Inhibition of intrapituitary thyroxine to 3.5.3'-triiodothyronine conversion prevents the acute suppression of thyrotropin release by thyroxine in hypothyroid rats. J Clin Invest 64:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Crantz FR, Larsen PR. 1980. Rapid thyroxine to 3,5,3'-triiodothyronine conversion and nuclear 3,5,3'-triiodothyronine binding in rat cerebral cortex and cerebellum. J Clin Invest 65:935–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vigouroux E, Clos J, Legrand J. 1979. Uptake and metablism of exogenous and endogenous thyroxine in the brain of young rats. Horm Metab Res 11:228–232. [DOI] [PubMed] [Google Scholar]
- 46. Obregon MJ, Roelfsema F, Morreale de Escobar G, et al. 1979. Exchange of triiodothyronine derived from thyroxine with circulating triiodothyronine as studied in the rat. Clin Endocrinol 10:305–315. [DOI] [PubMed] [Google Scholar]
- 47. Dratman MB, Crutchfield FL. 1978. Synaptosomal (125I)triiodothyronine after intravenous (125I)thyroxine. Am J Physiol 235:E638–E647. [DOI] [PubMed] [Google Scholar]
- 48. Kaplan MM, Yaskoski KA. 1981. Maturational Patterns of lodothyronine Phenolic and tyrosyl ring deiodinase activities in rat cerebrum, cerebellum, and hypothalamus. J Clin Invest 67:1208–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Silva JE, Larsen PR. 1985. Potential of brown adipose tissue type II thyroxine 5'-deiodinase as a local and systemic source of triiodothyronine in rats. J Clin Invest 76:2296–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bianco AC, Silva JE. 1987. Intracellular conversion of thyroxine to triiodothyronine is required for optimal thermogenic function of brown adipose tissue. J Clin Invest 79:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Berry MJ, Banu L, Larsen PR. 1991. Type I iodothyronine deiodinase is a selenocysteine-containing enzyme. Nature 349:438–440. [DOI] [PubMed] [Google Scholar]
- 52. Berry MJ, Kieffer JD, Harney JW, et al. 1991. Selenocysteine confers the biochemical properties characteristic of the type I iodothyronine deiodinase. J Biol Chem 266:14155–14158. [PubMed] [Google Scholar]
- 53. Dumitrescu AM, Liao XH, Abdullah MS, et al. 2005. Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat Genet 37:1247–1252. [DOI] [PubMed] [Google Scholar]
- 54. St Germain DL, Schwartzman R, Croteau W, et al. 1994. A thyroid hormone regulated gene in Xenopus laevis encodes a type III iodothyronine 5-deiodinase. Proc Natl Acad Sci 91:7767–7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Davey JC, Becker KB, Schneider M J, et al. 1995. Cloning of a cDNA for the type II iodothyronine deiodinase. J Biol Chem 270:26786–26789. [DOI] [PubMed] [Google Scholar]
- 56. Schneider MJ, Fiering SN, Pallud SE, et al. 2001. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol Endocrinol 15:2137–2148. [DOI] [PubMed] [Google Scholar]
- 57. Schneider MJ, Fiering SN, Thai B, et al. 2006. Targeted disruption of the type1 selenodeiodinase gene (Dio1) results in marked changes in thyroid hormone economy in mice. Endocrinology 147:580–589. [DOI] [PubMed] [Google Scholar]
- 58. Galton VA, Schneider MJ, Clark AS, et al. 2009. Life without thyroxine to 3,5,3'-triiodothyronine conversion: studies in mice devoid of the 5'-deiodinases. Endocrinology 150:2957–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hernandez A, Martinez ME, Fiering S, et al. 2006. Type 3 deiodinase is critical for the maturation and function of the thyroid axis. J Clin Invest 116:476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Galton VA, Wood WT, St Germain EA, et al. 2007. Thyroid hormone homeostasis and action in the type 2 deiodinase-deficient rodent brain during development. Endocrinology 148:3080–3088. [DOI] [PubMed] [Google Scholar]
- 61. Ng L, Goodyear RJ, Woods CA, et al. 2004. Hearing loss and retarded cochlear development in mice lacking type 2 iodothyronine deiodinase. Proc Soc Natl Acad Sci 101:3474–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. de Jesus LA, Carvalho SD, Ribeiro MO, et al. 2001. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest 108:1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bassett JH, Boyde A, Howell PG, et al. 2010. Optimal bone strength and mineralization requires the type 2 iodothyronine deiodinase in osteoblasts. Proc Natl Acad Sci 107:7604–7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Franca MM, German A, Fernandez GW, et al. 2021. Human type1 iodothyronine deiodinase (DIO1) mutations cause abnormal thyroid hormone metabolism. Thyroid 31:202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Engler D, Burger AG. 1984. The deiodination of the iodothyronines and of their derivatives in man. Endocrine Rev 5:151–184. [DOI] [PubMed] [Google Scholar]
- 66. Hernandez A, Martinez ME, Ng L, et al. 2021. Thyroid hormone deiodinases: dynamic switches in developmental transitions. Endocrinology 108:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ng L, Hernandez A, He W. 2009. A protective role for type 3 deiodinase, a thyroid hormone-inactivating enzyme, in cochlear development and auditory function. Endocrinology 150:1952–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Galton VA, de Waard E, Parlow AF, et al. 2014. Life without the iodothyronine deiodinases. Endocrinology 155:4081–4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Friesema EC, Jansen J, Milici C, et al. 2007. Thyroid hormone transporters. Vitam Horm 70:137–167. [DOI] [PubMed] [Google Scholar]
- 70. Scanlan TS, Suchland KL, Hart ME, et al. 2004. 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nat Med 10:638–642. [DOI] [PubMed] [Google Scholar]
- 71. Mentuccia D, Proietti-Pannunzi L, Tanner K, et al. 2002. Association between a novel variant of the human type 2 deiodinase gene Thr92Ala and insulin resistance. Evidence of interaction with the Trp64Arg variant of the β-3-adrenergic receptor. Diabetes 51:880–883. [DOI] [PubMed] [Google Scholar]