Abstract
Significance:
A burgeoning literature has attributed varied physiological effects to hydrogen sulfide (H2S), which is a product of eukaryotic sulfur amino acid metabolism. Protein persulfidation represents a major focus of studies elucidating the mechanism underlying H2S signaling. On the contrary, the capacity of H2S to induce reductive stress by targeting the electron transport chain (ETC) and signal by reprogramming redox metabolism has only recently begun to be elucidated.
Recent Advances:
In contrast to the nonspecific reaction of H2S with oxidized cysteines to form protein persulfides, its inhibition of complex IV represents a specific mechanism of action. Studies on the dual impact of H2S as an ETC substrate and an inhibitor have led to the exciting discovery of ETC plasticity and the use of fumarate as a terminal electron acceptor. H2S oxidation combined with complex IV targeting generates mitochondrial reductive stress, which is signaled through the metabolic network, leading to increased aerobic glycolysis, glutamine-dependent reductive carboxylation, and lipogenesis.
Critical Issues:
Insights into H2S-induced metabolic reprogramming are ushering in a paradigm shift for understanding the mechanism of its cellular action. It will be critical to reevaluate the physiological effects of H2S, for example, cytoprotection against ischemia-reperfusion injury, through the framework of metabolic reprogramming and ETC remodeling by H2S.
Future Directions:
The metabolic ramifications of H2S in other cellular compartments, for example, the endoplasmic reticulum and the nucleus, as well as the intersections between hypoxia and H2S signaling are important future directions that merit elucidation. Antioxid. Redox Signal. 38, 57–67.
Keywords: metabolism, mitochondria, redox metabolism, electron transport chain, reductive stress
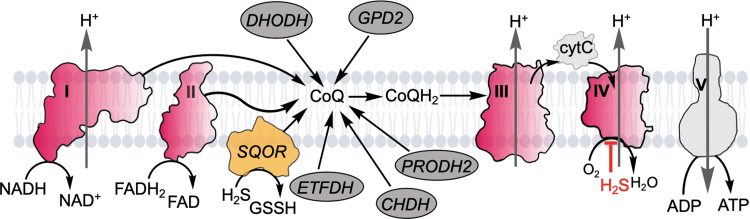
A myriad of signaling modules maintain metabolic homeostasis and orchestrate cellular responses to environmental cues such as O2 and nutrient fluctuations. Mitochondria, as hubs of carbon and energy metabolism, are important constituents of signaling networks. Central to oxidative metabolism, mitochondria recycle the reducing equivalents in NADH and FADH2 that are captured during catabolism, and funnel them to the lipidic electron acceptor, coenzyme Q (CoQ or ubiquinone), generating CoQH2 (or ubiquinol). Several mitochondrial dehydrogenases intersect at the CoQ pool, a redox node that influences amino acid, lipid, nucleotide, choline, and sulfide metabolic pathways (Fig. 1). Of the various CoQ users, complex I (NADH:ubiquinone oxidoreductase) and complex II (succinate dehydrogenase) are components of the electron transport chain (ETC). CoQH2 transfers electrons to complex III (cytochrome bc1), which in turn reduces cytochrome c, and subsequently, complex IV (cytochrome c oxidase).
FIG. 1.
Intersection of H2S and mitochondrial energy metabolism. CoQ is an electron acceptor that is used by several enzymes including DHODH, GPD2, ETFDH, CHDH, PRODH2, and SQOR, which oxidizes H2S to GSSH. In the canonical operation of the ETC, CoQH2 is recycled by complex III. Both H2S oxidation and its inhibition of complex IV lead to a reductive shift in the CoQ pool that is propagated upstream to the NADH/NAD+ and FADH2/FAD pools. CHDH, choline dehydrogenase; CoQ, coenzyme Q; cytC, cytochrome C; DHODH, dihydroorotate dehydrogenase; ETC, electron transport chain; ETFDH, electron transfer flavoprotein quinone oxidoreductase; FAD, flavin adenine dinucleotide; GPD2, glycerol 3-phosphate dehydrogenase; GSSH, glutathione persulfide; H2S, hydrogen sulfide; PRODH2, proline dehydrogenase 2; SQOR, sulfide quinone oxidoreductase.
In the canonical operation of the ETC, O2 serves as a terminal electron acceptor and is converted to H2O at complex IV. In a recently described noncanonical route, CoQH2 transfers electrons to complex II, and fumarate serves as the terminal electron acceptor (39, 78). Electron transfer at complexes I, III, and IV is coupled to proton translocation across the inner mitochondrial membrane, generating a proton motive force that drives ATP synthesis by complex V and powers other functions such as transport.
A decrease in the O2 concentration triggers a major signaling response that is initiated by stabilization of HIF1, the hypoxia inducible factor, which regulates the expression of numerous target genes (72). Hydrogen sulfide (H2S) is another modulator of redox metabolism whose primary target is the ETC (38). H2S is both an ETC substrate (22) and an inhibitor (60), and recent studies have revealed that H2S induces reductive stress with wide-ranging metabolic effects that fan out from the mitochondrion (38). At H2S concentrations that inhibit complex IV and therefore access to O2 as a terminal electron acceptor, cells exploit a fork in the ETC, using fumarate as an alternate electron acceptor (39).
In this noncanonical operation of the ETC, electron flow is redirected away from complex III to complex II, leading to CoQ regeneration, which is supported by fumarate that is sourced from different pathways. A similar use of the fork in the ETC is observed under hypoxic conditions (78). Some of the metabolic consequences of H2S-induced reductive stress include enhanced aerobic glycolysis (84), reductive carboxylation of glutamine (48), and lipid biogenesis (8). The emerging metabolic paradigm for H2S signaling, which ripples out from the ETC, is the focus of this review.
Enzymology of H2S Homeostasis: Production and Oxidation
Sulfide production
H2S is a product of mammalian sulfur metabolism and is derived from the amino acids, cysteine and homocysteine (Fig. 2). While the canonical reactions catalyzed by cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) in the transsulfuration pathway generate cysteine, substrate promiscuity and lax substrate specificity also support H2S synthesis by both enzymes (3, 30, 31). At cellular concentrations of substrates, the dominant route for H2S synthesis by CBS is via the β-elimination of cysteine and homocysteine, and by CSE, via the α,β-elimination of cysteine (10, 76). Both CBS and CSE are PLP enzymes (74), while CBS additionally harbors a heme cofactor (75), which makes it susceptible to gas regulation by •NO and CO (66, 80). The transsulfuration pathway enzymes also utilize cysteine (CBS, CSE) (25, 90) and homocysteine (CSE) (90) as substrates, generating the corresponding persulfides.
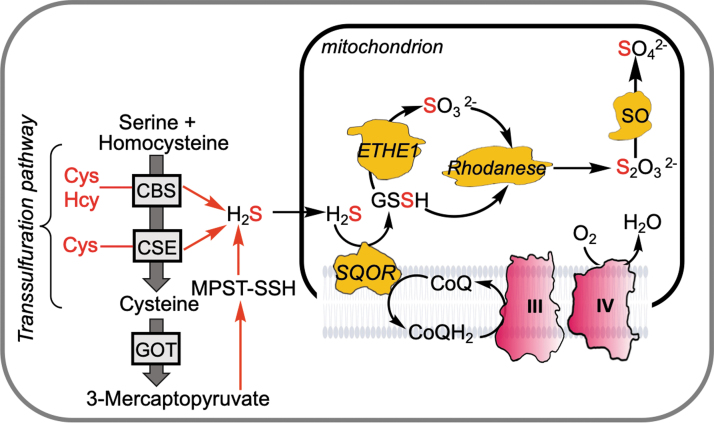
FIG. 2.
Sulfide production and oxidation pathways. H2S is derived (red arrows) from cysteine and/or homocysteine in reactions catalyzed by CBS and CSE, or from 3-mercaptopyuvate in a reaction catalyzed by MPST, which releases H2S from a persulfide intermediate (MPST-SSH). The cysteine-generating canonical reactions catalyzed by CBS and CSE constitute the transsulfuration pathway. H2S is oxidized in the mitochondrion to either thiosulfate or sulfate. The oxidation pathway comprises SQOR, ETHE1 (a persulfide dioxygenase), rhodanese (a thiosulfate sulfur transferase), and SO. CBS, cystathionine β-synthase; CSE, cystathionine γ-lyase; GOT, glutamate-oxaloacetate transaminase or cysteine aminotransferase; MPST, mercaptopyruvate sulfurtransferase; SO, sulfite oxidase.
Mercaptopyruvate sulfurtransferase (MPST) uses 3-mercaptopyruvate, which is a product of the transamination of cysteine catalyzed by glutamate-oxaloacetate transaminase (GOT), a component of the malate–aspartate shuttle (59, 92). The immediate product of the MPST reaction is an enzyme-bound persulfide, which can be transferred to a thiophilic acceptor such as thioredoxin or cysteine, from which H2S is subsequently released. Interestingly, reduced thioredoxin at physiologically relevant concentrations increases the KM for 3-mercaptopyruvate, effectively inhibiting MPST activity (91). Under oxidizing conditions, this inhibition is alleviated (91), which might represent a mechanism for regulating MPST-dependent persulfidation of alternate targets. While CBS and CSE reside in the cytoplasm, the MPST1 and MPST2 isoforms are located in the cytoplasm and mitochondrion, respectively (91).
Given the multitude of reactions catalyzed by CBS (76) and CSE (10), it is important to understand how the transsulfuration pathway meets cellular demands for cysteine versus H2S. CBS is a major regulatory hub; it is inhibited by sumoylation (1, 34), CO (7, 66, 81), •NO (80), and nitrite (21), but is activated by AdoMet (18) and by glutathionylation (63). Furthermore, some of the allosteric effectors interact as exemplified by the observation that AdoMet increases the affinity of ferrous CBS for •NO (twofold) and CO (fivefold) and thereby sensitizes the enzyme to inhibition by these gas regulators (83).
A novel heme-dependent switch in CBS can flip the operating preference of the transsulfuration pathway from cysteine to H2S synthesis (33). Under basal conditions or upon AdoMet activation of ferric CBS, the predominant flux is toward cysteine synthesis. On the contrary, conditions that induce CO or •NO synthesis and trigger H2S-signaling such as endoplasmic reticulum stress (15) inhibit CBS. With reduced competition from the CBS product, cystathionine, CSE switches to cysteine-dependent H2S synthesis (33). In addition, elevated homocysteine due to CBS deficiency or inhibition can stimulate CSE-dependent H2S biogenesis (10). The implications of metabolic track switching by the transsulfuration pathway enzymes are potentially significant. Since compromised endoplasmic reticulum function is a significant contributing factor in the development of cardiovascular, neurodegenerative, neoplastic, and metabolic diseases (6), the track switching model suggests that H2S-dependent signaling cascades might be perturbed in these complex diseases.
Global suppression of protein synthesis via the integrated stress response or due to mTORC1 inhibition induces translation of the activating transcription factor ATF4, eliciting a defensive response. CSE expression is upregulated by ATF4, which in turn increases H2S production and is a component of the ATF4-dependent stress and antiaging response (15, 79). On the contrary, 1,25-dihydroxyvitamin D3 regulates CBS and increases transsulfuration pathway activity in preosteoblastic cells; the effects on H2S production were not studied (37).
Sulfide oxidation
A mitochondrial resident sulfide oxidation pathway clears H2S (23, 49) and shields the ETC from respiratory poisoning (Fig. 2) (48). Sulfide quinone oxidoreductase (SQOR) catalyzes the committing step in this pathway converting H2S to glutathione persulfide (GSSH) (42, 56) and transfers electrons to CoQ via an flavin adenine dinucleotide cofactor (43). The next step in the pathway results in the conversion of GSSH to sulfite in a reaction catalyzed by the persulfide dioxygenase, ETHE1 (29, 32). Rhodanese catalyzes a sulfur transfer reaction from GSSH to sulfite forming thiosulfate (47), or sulfite can be further oxidized to sulfate in cells harboring sulfite oxidase (36). SQOR has an unusual cysteine trisulfide redox cofactor (27, 45) that confers an ∼105-fold rate enhancement of the sulfide addition reaction over a cysteine disulfide (44).
The sulfane sulfur can be transferred to a variety of acceptors other than GSH, including coenzyme A and methanethiol, forming the corresponding persulfides or it can be transferred to sulfite forming thiosulfate (28, 42). GSSH is, however, predicted to be the dominant product at physiologically relevant concentrations of acceptors (41, 42).
The sulfide oxidation pathway enzymes exhibit apical localization in colonic crypts (48), likely representing a host adaptation to high H2S exposure from microbial metabolism at the host–microbiota interface (53). Colorectal cancer tissues exhibit elevated levels of the sulfide oxidation pathway enzymes compared with normal margins (48). Sulfide preconditioning increases hypoxia tolerance in male mice via upregulation of SQOR in the brain; the higher expression level of SQOR in the brain of female mice is correlated with increased tolerance to hypoxia (54). A neuron-specific increase in SQOR expression confers resistance to hypoxia and to ischemic brain injury (54). The mechanism underlying the hypoxic increase in sulfide, that is, through increased synthesis and/or decreased oxidation, remains to be established. An SQOR inhibitor ST1, with an IC50 value of 29 nM, is competitive with respect to CoQ, and cardioprotective in a mouse model of heart failure with reduced ejection fraction (26).
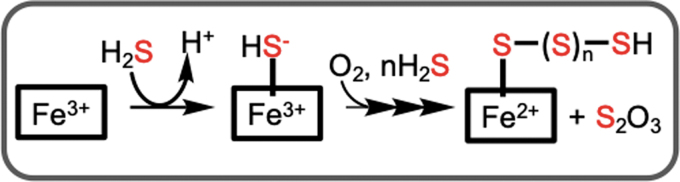
In addition to the sulfide oxidation pathway, H2S can also be oxidized by ferric heme proteins, including hemoglobin (86, 87), myoglobin (5), neuroglobin (70), and cytochrome c (85). Under aerobic conditions, the initially formed ferric sulfide is oxidized to a mixture of thiosulfate and ferrous heme bound polysulfides (Fig. 3). This mode of H2S clearance might be important in red blood cells, which lack mitochondria and in cells with low SQOR expression.
FIG. 3.
Scheme outlining the mechanism of sulfide oxidation to thiosulfate and polysulfides catalyzed by ferric hemeproteins.
Complex I and SQOR compete for the CoQ pool
With its dependence on CoQ as an electron acceptor, SQOR is potentially impacted by and influences other CoQ users including complex I. Complex I is a quantitatively significant competitor for the CoQ pool, feeding electrons from NADH oxidation to the ETC. Thus, pharmacological inhibition by rotenone or genetic ablation of complex I (by NDUFS3 knockdown) increases the cellular efficiency of H2S clearance (39). Consistent with a competitive relationship, ectopic expression of the water-forming NADH oxidase LbNOX (82) in the mitochondria, but not in the cytoplasm, accelerates H2S oxidation by dissipating the NADH/NAD+ pool (39). Similarly, depletion of the mitochondrial NADPH pool by TPNOX (12) enhances H2S clearance, while the nicotinamide nucleotide transhydrogenase (NNT) inhibitor, 4-chloro-7-nitrobenzofurazan chloride, attenuates the effect of TPNOX (39).
These effects can be explained by the activity of NNT, an electrogenic mitochondrial inner membrane transhydrogenase that converts NADH and NADP+ to NAD+ and NADPH, respectively. Under pathophysiological conditions, NNT catalyzes the reverse reaction, using NADPH to support NADH and ATP generation, but in so doing depletes cellular antioxidant capacity (62).
Complex II reversal supports fumarate-dependent H2S oxidation
In addition to their capacity for endogenous H2S synthesis, colonic epithelial cells are exposed to inhibitory concentrations of exogenous H2S that is derived from gut microbial metabolism and can range from ∼0.2 to 2.4 mM (14, 53). We have recently discovered that when complex IV is inhibited, H2S oxidation is sustained via a redox cycle between SQOR and complex II, operating in reverse (39). Under these conditions, fumarate is utilized as a terminal electron acceptor, leading to succinate accumulation, while CoQH2 is oxidized to CoQ, enabling H2S oxidation (39). When CoQ is limiting, an alternative route for electron transfer from SQOR is to O2, generating H2O2 (39). The electron transfer rate from reduced SQOR to O2 is, however, ∼1000-fold lower than to CoQ, and therefore unlikely to be significant if complex II is available to recycle CoQH2.
On the contrary, in cells where complex II and/or fumarate are limiting, electron transfer from SQOR to O2 could represent a route for sulfide oxidation and a source of reactive oxygen species (ROS) production.
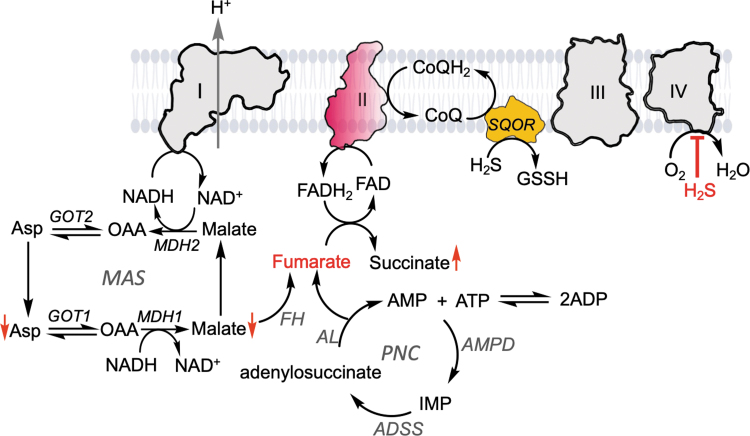
Fumarate is derived from both the purine nucleotide cycle and the malate–aspartate shuttle (Fig. 4) (39). The enzymes in the purine nucleotide cycle, adenylosuccinate synthetase, adenylosuccinate lyase, and adenosine monophosphate (AMP) deaminase, are found in most tissues. AMP deaminase hydrolyzes AMP into inosine monophosphate and ammonia. Adenylosuccinate synthetase catalyzes the condensation of inosine 5′-monophosphate and aspartate to adenylosuccinate in the presence of guanosine triphosphate. Adenylosuccinate lyase cleaves the C-N bond of adenylosuccinate, yielding AMP and fumarate, and it also catalyzes the cleavage of 1-(5-phosphoribosyl)-4-(N-succinocarboxamide)-5-aminoimidazole to 1-(5-phosphoribosyl)-5-amino-4-imidazolecarboxiamide and fumarate, in the de novo purine biosynthetic pathway. The purine nucleotide cycle is important for ATP generation during intense exercise in muscles and the regeneration of Krebs cycle intermediates (51).
FIG. 4.
Fumarate serves as a terminal electron acceptor for H2S oxidation. When complex IV is inhibited (e.g., by high H2S concentrations), O2 cannot be used as a terminal electron acceptor and a new redox cycle between SQOR and complex II recycles CoQH2. The fumarate used to support complex II reversal is derived from the MAS and the PNC. Fumarate hydratase, adenylosuccinate synthetase, adenylosuccinate lyase, and adenosine monophosphate deaminase are fumarate hydratase, adenylosuccinate synthetase, adenylosuccinate lyase, and AMP deaminase, respectively. The up and down red arrows indicate metabolites that increase and decrease, respectively, in H2S-treated cells. AMP, adenosine monophosphate; MAS, malate–aspartate shuttle; PNC, purine nucleotide cycle.
The malate–aspartate shuttle translocates reducing equivalents from the cytoplasm to the mitochondrion for oxidative phosphorylation (Fig. 4). The cytoplasmic malate dehydrogenase (MDH)1 oxidizes NADH to NAD+ as it reduces oxaloacetate to malate, which is transferred across the inner mitochondrial membrane using the electroneutral and reversible α-ketoglutarate/malate carrier. The mitochondrial isoform of MDH2 catalyzes the reverse reaction, generating NADH and oxaloacetate, which is converted via GOT2 to aspartate. The latter enters the cytoplasm via the electrogenic aspartate/glutamate carrier that powers aspartate efflux to the cytoplasm and glutamate influx to the mitochondrion by proton translocation from the inner mitochondrial membrane space.
Finally, cytoplasmic GOT1 converts aspartate to oxaloacetate, completing the shuttle. Knockdown of GOT1 but not GOT2 increased the efficiency of sulfide clearance, suggesting that oxaloacetate in the cytosolic arm of the malate–aspartate shuttle is diverted toward fumarate synthesis (39, 48). In addition to its generation by GOT1, cytosolic oxaloacetate is also derived from citrate cleavage catalyzed by ATP-citrate lyase, as described below.
Metabolomic analysis of the human colorectal adenocarcinoma HT29 cells, reveal that 1 h after (100 μM) H2S treatment, malate, aspartate, glutamate, and α-ketoglutarate levels are down, while succinate levels are up compared with untreated controls (48). Reduced malate levels could reflect its conversion to fumarate catalyzed by fumarate hydratase (Fig. 4), which in turn supports reverse complex II activity and leads to the observed accumulation of succinate. Since succinate acts as competitive inhibitor of α-ketoglutarate-dependent dioxygenases, H2S-induced succinate accumulation could impact histone and DNA methylation linking H2S metabolism to epigenetic regulation (77). Succinate accumulation could also promote protein succinylation, a posttranslational modification, which reportedly increases complex II activity and could further promote H2S clearance (64).
Physiological Implications of H2S-Mediated Complex II Reversal
Upregulation of endogenous H2S synthesis or provision of exogenous H2S is cytoprotective against ischemic injury if administered during the reperfusion phase [reviewed in Nicholson and Calvert (61)]. We posit that complex II reversal by H2S is the underlying mechanism of the cytoprotective effect. The twin effects of H2S on complex II reversal and complex IV inhibition would be predicted to lower the mitochondrial membrane potential and CoQH2 buildup, which are drivers of complex I reversal and ROS formation and injury during reperfusion (11, 67). The relevance of this proposed mechanism for the cardioprotective effects of H2S warrants testing.
Complex II reversal and the use of fumarate as the terminal electron acceptor are also observed under hypoxic conditions (78). Metabolic labeling studies reveal that organs such as the kidney, liver, and brain constitutively use fumarate as a terminal electron acceptor, while other organs such as the white adipose tissue and heart resort to fumarate oxidation under conditions of exercise stress. In tissues such as the lung, gastrocnemius muscle, pancreas, and thymus on the contrray, succinate oxidation appears to be the dominant reaction catalyzed by complex II.
In addition to H2S oxidation, CoQH2 recycling by fumarate reduction also supports the activity of other CoQ users, notably dihydroorotate dehydrogenase, thus supporting de novo pyrimidine synthesis (78). The mechanisms by which CoQH2 oxidation at complex III versus complex II is regulated are not understood. Modulation of the O2 affinity and/or the O2 reduction rate at complex IV by the gas regulators H2S and •NO has been postulated to be an important mechanism for partitioning electron flow at the ETC fork, and remains to be more completely elucidated (4).
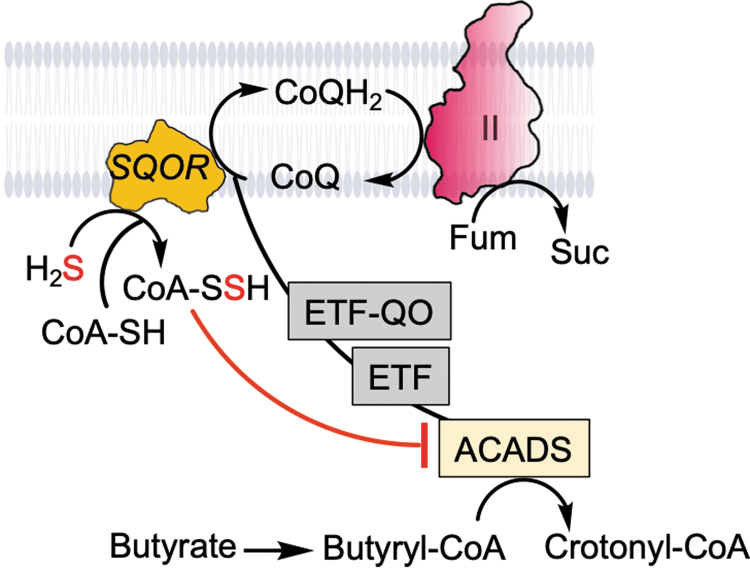
Colonocytes are adapted to tolerate exposure to toxic levels of H2S and avert its build up by expressing high levels of the sulfide oxidation pathway enzymes (40, 48). Butyrate is a major energy source for colonocytes, and acyl-CoA dehydrogenase (ACADS) catalyzes the oxidation of butyryl-CoA to crotonyl-CoA, transferring electrons to CoQ via the ETF/ETF-QO redox protein pair (Fig. 5). In the backdrop of high luminal H2S exposure, the CoQ pool can become limiting and the observed increase in butyryl-CoA and decrease in crotonyl-CoA levels are consistent with ACADS inhibition (2, 68). We have demonstrated that SQOR catalyzes the synthesis of coenzyme A persulfide (CoA-SSH) (45), which is a tight-binding inhibitor of ACADS (73). We posit that inhibition of ACADS by SQOR-derived CoA-SSH represents a strategy for prioritizing sulfide detoxification. Whether this strategy is additionally facilitated by complex II reversal (Fig. 5) remains to be tested.
FIG. 5.
Model showing interactions between H2S and butyrate oxidation and complex II reversal. SQOR is promiscuous and can use CoA as a sulfane sulfur acceptor, forming CoA-SSH, which inhibits ACADS. The latter oxidizes butyryl-CoA, which is a major fuel source for colonocytes. Inhibition of ACADS by CoA-SSH relieves competition for the CoQ pool and allows prioritization of H2S oxidation. CoQ recycling through complex II reversal further supports SQOR-mediated H2S oxidation. Fum and Suc denote fumarate and succinate, respectively. ACADS, acyl-CoA dehydrogenase.
Increased colonization of sulfate reducing bacteria and higher fecal sulfide is reportedly associated with ulcerative colitis (65, 69), suggesting the involvement of H2S in intestinal bowel disease pathogenesis possibly via dysregulated butyrate oxidation.
A Metabolic Paradigm for H2S Signaling
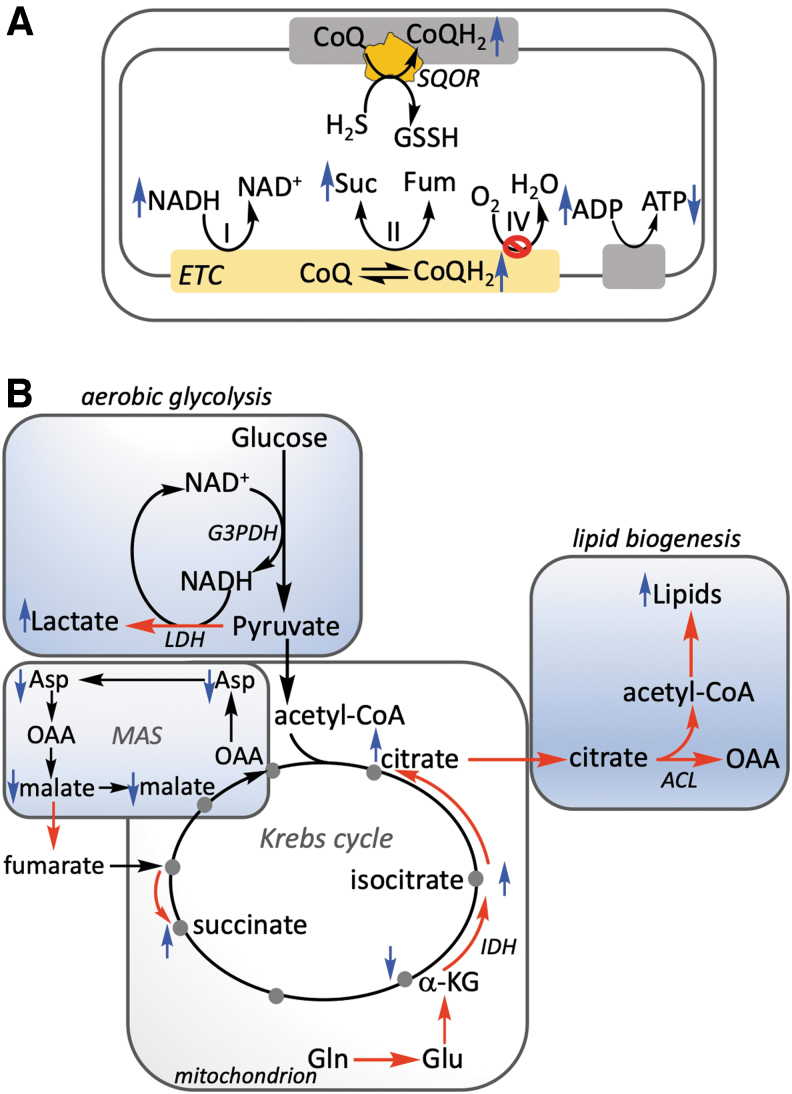
The prevalent view of how H2S signals is through reactive persulfides and polysulfides, leading to posttranslational modifications on accessible cysteines (35, 58). The issues with the lack of specificity of this mechanism and the challenges with rigorously establishing a physiologically relevant and quantitatively significant change in the persulfidation status of target proteins have been reviewed (38). In contrast to protein per- and polysulfidation, complex IV inhibition is a specific and a physiologically established target of H2S action. Its consequent impact on mitochondrial bioenergetics and cell metabolism has recently begun to be elucidated. Pleiotropic effects ensue upstream of complex IV in the ETC following inhibition by H2S, which includes a reductive shift in the electron carriers (i.e., CoQH2/CoQ and NADH/NAD+) (Fig. 6A), which in turn affect redox reactions that are dependent on them (Fig. 6B).
FIG. 6.
Metabolic effects of H2S. (A) H2S oxidation by SQOR leads to CoQH2 generation, which combined with H2S inhibition of complex IV results in a reductive shift upstream in the NADH/NAD+ pool, reversal of complex II, and decreased ATP synthesis. The observed and predicted changes (up or down) in metabolites are depicted by the blue arrows. (B) Perturbations in mitochondrial bioenergetics by H2S have pleiotropic effects on central carbon and energy metabolism that are depicted by the red arrows. The metabolic adaptations include enhanced aerobic glycolysis (leading to glucose carbon being secreted as lactate), glutamine-dependent reductive carboxylation, and increased lipid biogenesis. H2S-induced reductive stress also affects the MAS, which furnishes fumarate to support complex II reversal and leads to aspartate insufficiency, limiting proliferation. The blue up and down arrows indicate metabolites whose levels increase and decrease, respectively, in response to H2S treatment. ACL, ATP-citrate lyase; G3PDH, glyceraldehyde 3-phosphate dehydrogenase; IDH, isocitrate dehydrogenase; LDH, lactate dehydrogenase.
Consistent with this model, pharmacological inhibition or genetic ablation of complex I increases the efficacy of H2S oxidation (39), presumably by decreasing competition for CoQ. Genetic ablation of SQOR, on the contrary, sensitizes cells to sulfide-dependent inhibition of respiration (48), enhances the reductive shift in the NAD+ pool in response to H2S (48, 54), and creates an electron acceptor insufficiency (48). These changes in turn lead to uridine and aspartate becoming limiting for cell proliferation, which can be relieved by exogenous uridine and either aspartate or pyruvate (48), which upon reduction to lactate increases NAD+ availability (Fig. 6B).
H2S enhances aerobic glycolysis
Glycolysis and oxidative phosphorylation are tightly regulated to meet cellular demands for NAD+ to support oxidative metabolism, ATP, and macromolecular precursor synthesis (52). While complex II reversal recycles CoQH2, the contributions of complexes III and IV to proton motive force generation are precluded by this noncanonical mode of ETC operation, impacting the transmembrane potential and ATP synthesis. A decrease in ATP and an increase in ADP levels are observed in H2S-treated cells (Fig. 6A), which are associated with an increase in aerobic glycolysis (84). Thus, glucose carbons are predominantly diverted to lactate, which sets up a redox neutral cycle between glyceraldehyde 3-phosphate dehydrogenase in the glycolytic pathway and lactate dehydrogenase, and leads to NADH recycling that is independent of the malate–aspartate cycle and the ETC (Fig. 6B).
Since SQOR knockdown enhances the sensitivity of cells to respiratory inhibition by H2S, upregulation of aerobic glycolysis occurs at a lower concentration (50 μM) of sulfide compared with scrambled controls (100 μM). In contrast, ETHE1 knockdown does not sensitize cells to H2S-triggered upregulation of aerobic glycolysis (84).
Although the cytoplasmic and mitochondrial NADH pools are interconnected via the malate–aspartate shuttle, the effect of H2S on aerobic glycolysis is sensitive only to the mitochondrial NADH/NAD+ ratio (84). Thus, while dissipation of the mitochondrial NADH pool increases basal aerobic glycolysis, these cells do not show a further increase in glucose consumption in response to H2S. GOT1 or GOT2 knockdowns have no effect on H2S-stimulated aerobic glycolysis, which is consistent with an H2S-induced disabling of the intercompartmental malate–aspartate shuttle as a carrier of reducing equivalents (48, 84). Specifically, H2S-induced reductive stress with a consequent shortage of mitochondrial NAD+ would limit the MDH2-dependent conversion of malate to oxaloacetate. Furthermore, the reductive redirection within the Krebs cycle leading to increased conversion of α-ketoglutarate to citrate would also decrease oxaloacetate availability for the GOT2-dependent synthesis of mitochondrial aspartate.
Finally, a drop in the transmembrane potential would adversely impact aspartate export, which is coupled to glutamate and proton import into the mitochondrion. These changes in the mitochondrial arm of the shuttle are predicted to fan out to the cytoplasm via the decreased availability of aspartate for GOT1-dependent synthesis of oxaloacetate and its diversion to fumarate via MDH1 and fumarate hydratase (Fig. 4).
Several physiological effects have been associated with H2S-mediated enhancement of aerobic glycolysis, including the proangiogenic migratory behavior of endothelial cells (50). In this, and a previous study (24), endogenous H2S biogenesis was enhanced by dietary, specifically sulfur amino acid, restriction, which led to the ATF4-dependent upregulation of CSE and to H2S synthesis, and conferred protection against hepatic ischemia-reperfusion injury. Increased H2S-induced glucose consumption has also been associated with inhibition of cardiomyocyte hypertrophy (46). CSE knockdown in human umbilical vein endothelial cells reportedly enhanced glycolysis and increased the release of antiangiogenic factors, soluble Fms-like tyrosine kinase-1 and endoglin that are associated with preeclampsia (71).
However, the possibility that a compensatory increase in CBS expression in CSE knockdown cells contributed to increased rather than decreased H2S production was not examined. Increased glycolysis at low concentrations of H2S was concluded to underlie the observed increase in ATP concentrations in murine red blood cells; however, glucose consumption was not measured, and the mechanism underlying the ETC-independent upregulation of glycolysis was not addressed in this study (89).
H2S stimulates glutamine-dependent reductive carboxylation and lipogenesis
Mitochondrial electron acceptor insufficiency induced by H2S promotes reductive carboxylation of glutamine-derived α-ketoglutarate to citrate (Fig. 6B). Sulfide exposure of HT29 cells labeled with [U-13C]-labeled glutamine increased the fraction of the m + 5 isotopolog of citrate, consistent with its generation via reductive carboxylation, which leads to the retention of all five glutamine carbons (48). Reductive carboxylation is upregulated during growth factor-dependent (nonmalignant) and growth factor-independent (malignant) cell proliferation (13, 57) when the ETC operation is impaired due to disease-associated mitochondrial DNA mutations (9), or hypoxia (55, 88).
H2S promoted the utilization of glutamine-derived carbon for lipid biogenesis in a panel of malignant and nonmalignant cell lines (HT29, HCEC, HCT116, J774, 143BWT, and 143CytB), but not in the human hepatocellular carcinoma cell line HepG2 (8). The magnitude of the H2S-dependent increase in [14C]-glutamine incorporation into lipids varied from ∼1.1- to 5-fold under normoxic (20% O2) conditions (8). Hypoxic culture (2% O2) also increased [14C]-glutamine incorporation into lipids in these cell lines except for HepG2, which exhibited no change, and J774, which exhibited significantly reduced radiolabel incorporation. Hypoxia, however, blunted the H2S-stimulated incorporation of glutamine into lipids except in the human osteosarcoma cell line 143B, which exhibited an ∼15-fold increase at 2% versus an ∼5-fold increase in lipid labeling at 20% O2 (8).
A possible rationale for increased lipid biogenesis is that it counters H2S-induced reductive stress by consuming reducing equivalents. Isocitrate dehydrogenase 1 and 2 (IDH1 and IDH2) uses NADPH to carboxylate α-ketoglutarate, while 14 NADPH equivalents are used for the synthesis of a single equivalent of palmitate, a precursor of many lipids. Lipidomic analysis revealed that H2S elicits numerous changes in lipid profiles including an increase in triacylglycerides and a decrease in phosphatidylcholine. Sulfide did not increase the flux of glucose into the pentose phosphate pathway in malignant (HT29) or nonmalignant (HCEC) colonocytes. Thus, the NADPH pool that fuels lipid biosynthesis could be derived from mitochondrial NADH via the activity of NNT (19). This is consistent with the observation that targeted dissipation of the mitochondrial but not the cytoplasmic NAD(P)H pool significantly decreased the level of H2S-stimulated labeling of lipids from glutamine (8).
Abbreviations Used
- ACADS
acyl-CoA dehydrogenase
- ACL
ATP-citrate lyase
- AdoMet
S-adenosylmethionine
- AMP
adenosine monophosphate
- ATF4
activating transcription factor 4
- CBS
cystathionine β-synthase
- CHDH
choline dehydrogenase
- CoA-SSH
coenzyme A persulfide
- CoQ
coenzyme Q
- CSE
cystathionine γ-lyase
- cytC
cytochrome C
- DHODH
dihydroorotate dehydrogenase
- ETC
electron transport chain
- ETFDH
electron transfer flavoprotein quinone oxidoreductase
- ETHE1
persulfide dioxygenase
- FAD
flavin adenine dinucleotide
- G3PDH
glyceraldehyde 3-phosphate dehydrogenase
- GOT1/2
glutamate-oxaloacetate transaminase 1 or 2
- GPD2
glycerol 3-phosphate dehydrogenase
- GSH
glutathione
- GSSH
glutathione persulfide
- H2S
hydrogen sulfide
- IDH1/2
isocitrate dehydrogenase 1 or 2
- LDH
lactate dehydrogenase
- MDH1/2
malate dehydrogenase 1 or 2
- MPST
mercaptopyruvate sulfurtransferase
- NNT
nicotinamide nucleotide transhydrogenase
- PRODH2
proline dehydrogenase 2
- ROS
reactive oxygen species
- SQOR
sulfide quinone oxidoreductase
Summary
To understand the chemical biology underlying the varied physiological effects attributed to H2S, it is imperative to identify plausible and specific biological targets that can mediate specific cellular responses. While the field has been focused on persulfide modifications of cysteine thiols [reviewed in Filipovic et al. (17)] and while these posttranslational modifications undoubtedly exist in the proteome (16, 20), a quantitatively significant change in persulfidation levels in a target protein that is associated with a functional change in the cellular milieu remains to be demonstrated [reviewed in Kumar and Banerjee (38)]. In contrast, the ability of H2S to target the ETC, a central bioenergetic highway that has widespread ramifications on redox metabolism, represents a plausible molecular mechanism by which sulfide signals. Recent studies on the metabolic reprogramming elicited by H2S, which originates in the mitochondrion, are leading to a paradigmatic shift in our understanding of how H2S signals. These studies are revealing a central role for reductive stress signaling and have led to the exciting discovery of plasticity in the eukaryotic ETC, allowing the use of fumarate to support oxidative metabolism when complex IV activity is stalled (39). Additional metabolic impacts of H2S in other cellular compartments, particularly the endoplasmic reticulum and the nucleus, represent areas that are ripe for investigation.
Funding Information
This work was supported, in part, by grants from the National Institutes of Health (GM130183 to Ruma Banerjee and F32GM140694 to David Hanna) and the American Heart Association (826245 to Roshan Kumar).
References
- 1. Agrawal N and Banerjee R. Human polycomb 2 protein is a SUMO E3 ligase and alleviates substrate-induced inhibition of cystathionine beta-synthase sumoylation. PLoS One 3: e4032, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Babidge W, Millard S, and Roediger W. Sulfides impair short chain fatty acid beta-oxidation at acyl-CoA dehydrogenase level in colonocytes: implications for ulcerative colitis. Mol Cell Biochem 181: 117–124, 1998. [DOI] [PubMed] [Google Scholar]
- 3. Banerjee R. Catalytic promiscuity and heme-dependent redox regulation of H2S synthesis. Curr Opin Chem Biol 37: 115–121, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Banerjee R and Kumar R.. Gas regulation of complex II reversal via electron shunting to fumarate in the mammalian ETC. Trends Bioc Sci 2022. [Epub ahead of print]; DOI: 10.1016/j.tibs.2022.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bostelaar T, Vitvitsky V, Kumutima J, Lewis BE, Yadav PK, Brunold TC, Filipovic M, Lehnert N, Stemmler TL, and Banerjee R. Hydrogen sulfide oxidation by myoglobin. J Am Chem Soc 138: 8476–8488, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cao SS and Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal 21: 396–413, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carballal S, Cuevasanta E, Marmisolle I, Kabil O, Gherasim C, Ballou DP, Banerjee R, and Alvarez B. Kinetics of reversible reductive carbonylation of heme in human cystathionine beta-synthase. Biochemistry 52: 4553–4562, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carballal S, Vitvitsky V, Kumar R, Hanna DA, Libiad M, Gupta A, Jones JW, and Banerjee R. Hydrogen sulfide stimulates lipid biogenesis from glutamine that is dependent on the mitochondrial NAD(P)H pool. J Biol Chem 292: 100950, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen Q, Kirk K, Shurubor YI, Zhao D, Arreguin AJ, Shahi I, Valsecchi F, Primiano G, Calder EL, Carelli V, Denton TT, Beal MF, Gross SS, Manfredi G, and D'Aurelio M. Rewiring of glutamine metabolism is a bioenergetic adaptation of human cells with mitochondrial DNA mutations. Cell Metab 27: 1007–1025.e5, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chiku T, Padovani D, Zhu W, Singh S, Vitvitsky V, and Banerjee R. H2S biogenesis by human cystathionine γ-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J Biol Chem 284: 11601–11612, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord ENJ, Smith AC, Eyassu F, Shirley R, Hu CH, Dare AJ, James AM, Rogatti S, Hartley RC, Eaton S, Costa ASH, Brookes PS, Davidson SM, Duchen MR, Saeb-Parsy K, Shattock MJ, Robinson AJ, Work LM, Frezza C, Krieg T, and Murphy MP. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515: 431–435, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cracan V, Titov DV, Shen H, Grabarek Z, and Mootha VK. A genetically encoded tool for manipulation of NADP(+)/NADPH in living cells. Nat Chem Biol 13: 1088–1095, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DeBerardinis RJ, Lum JJ, Hatzivassiliou G, and Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 7: 11–20, 2008. [DOI] [PubMed] [Google Scholar]
- 14. Deplancke B, Finster K, Graham WV, Collier CT, Thurmond JE, and Gaskins HR. Gastrointestinal and microbial responses to sulfate-supplemented drinking water in mice. Exp Biol Med (Maywood) 228: 424–433, 2003. [DOI] [PubMed] [Google Scholar]
- 15. Dickhout JG, Carlisle RE, Jerome DE, Mohammed-Ali Z, Jiang H, Yang G, Mani S, Garg SK, Banerjee R, Kaufman RJ, Maclean KN, Wang R, and Austin RC. Integrated stress response modulates cellular redox state via induction of cystathionine gamma-lyase: cross-talk between integrated stress response and thiol metabolism. J Biol Chem 287: 7603–7614, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doka E, Pader I, Biro A, Johansson K, Cheng Q, Ballago K, Prigge JR, Pastor-Flores D, Dick TP, Schmidt EE, Arner ES, and Nagy P. A novel persulfide detection method reveals protein persulfide- and polysulfide-reducing functions of thioredoxin and glutathione systems. Sci Adv 2: e1500968, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Filipovic MR, Zivanovic J, Alvarez B, and Banerjee R. Chemical biology of H2S signaling through persulfidation. Chem Rev 118: 1253–1337, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Finkelstein JD, Kyle WE, Martin JL, and Pick AM. Activation of cystathionine synthase by adenosylmethionine and adenosylethionine. Biochem Biophys Res Commun 66: 81–87, 1975. [DOI] [PubMed] [Google Scholar]
- 19. Gameiro PA, Laviolette LA, Kelleher JK, Iliopoulos O, and Stephanopoulos G. Cofactor balance by nicotinamide nucleotide transhydrogenase (NNT) coordinates reductive carboxylation and glucose catabolism in the tricarboxylic acid (TCA) cycle. J Biol Chem 288: 12967–12977, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gao XH, Krokowski D, Guan BJ, Bederman I, Majumder M, Parisien M, Diatchenko L, Kabil O, Willard B, Banerjee R, Wang B, Bebek G, Evans CR, Fox PL, Gerson SL, Hoppel C, Liu M, Arvan P, and Hatzoglou M. Quantitative H2S-mediated protein sulfhydration reveals metabolic reprogramming during the Integrated Stress Response. Elife 4: e10067, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gherasim C, Yadav PK, Kabil O, Niu WN, and Banerjee R. Nitrite reductase activity and inhibition of H2S biogenesis by human cystathionine beta-synthase. PLoS One 9: e85544, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goubern M, Andriamihaja M, Nubel T, Blachier F, and Bouillaud F. Sulfide, the first inorganic substrate for human cells. FASEB J 21: 1699–1706, 2007. [DOI] [PubMed] [Google Scholar]
- 23. Hildebrandt TM and Grieshaber MK. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J 275: 3352–3361, 2008. [DOI] [PubMed] [Google Scholar]
- 24. Hine C, Harputlugil E, Zhang Y, Ruckenstuhl C, Lee BC, Brace L, Longchamp A, Trevino-Villarreal JH, Mejia P, Ozaki CK, Wang R, Gladyshev VN, Madeo F, Mair WB, and Mitchell JR. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell 160: 132–144, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ida T, Sawa T, Ihara H, Tsuchiya Y, Watanabe Y, Kumagai Y, Suematsu M, Motohashi H, Fujii S, Matsunaga T, Yamamoto M, Ono K, Devarie-Baez NO, Xian M, Fukuto JM, and Akaike T. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox. signaling. Proc Natl Acad Sci U S A 111: 7606–7611, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jackson MR, Cox KD, Baugh SDP, Wakeen L, Rashad AA, Lam PYS, Polyak B, and Jorns MS.. Discovery of a first-in-class inhibitor of sulfide:quinone oxidoreductase that protects against adverse cardiac remodeling and heart failure. Cardiovasc Res 2021. [Epub ahead of print]; DOI: 10.1093/cvr/cvab206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jackson MR, Loll PJ, and Jorns MS. X-Ray Structure of human sulfide:quinone oxidoreductase: insights into the mechanism of mitochondrial hydrogen sulfide oxidation. Structure 27: 794–805.e4, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jackson MR, Melideo SL, and Jorns MS. Human sulfide:quinone oxidoreductase catalyzes the first step in hydrogen sulfide metabolism and produces a sulfane sulfur metabolite. Biochemistry 51: 6804–6815, 2012. [DOI] [PubMed] [Google Scholar]
- 29. Kabil O and Banerjee R. Characterization of patient mutations in human persulfide dioxygenase (ETHE1) Involved in H2S catabolism. J Biol Chem 287: 44561–44567, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kabil O and Banerjee R. Enzymology of H2S biogenesis, decay and signaling. Antioxid Redox Signal 20: 770–782, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kabil O, Motl N, and Banerjee R. H2S and its role in redox signaling. Biochim Biophys Acta 1844: 1355–1366, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kabil O, Motl N, Strack M, Seravalli J, Metzler-Nolte N, and Banerjee R. Mechanism-based inhibition of human persulfide dioxygenase by gamma-glutamyl-homocysteinyl-glycine. J Biol Chem 293: 12429–12439, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kabil O, Yadav V, and Banerjee R. Heme-dependent metabolite switching regulates H2S synthesis in response to ER stress. J Biol Chem 291: 16418–16423, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kabil O, Zhou Y, and Banerjee R. Human cystathionine beta-synthase is a target for sumoylation. Biochemistry 45: 13528–13536, 2006. [DOI] [PubMed] [Google Scholar]
- 35. Kimura H. Signaling of hydrogen sulfide and polysulfides. Antioxid Redox Signal 22: 347–349, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kisker C, Schindelin H, Pacheco A, Wehbi WA, Garrett RM, Rajagopalan KV, Enemark JH, and Rees DC. Molecular basis of sulfite oxidase deficiency from the structure of sulfite oxidase. Cell 91: 973–983, 1997. [DOI] [PubMed] [Google Scholar]
- 37. Kriebitzsch C, Verlinden L, Eelen G, van Schoor NM, Swart K, Lips P, Meyer MB, Pike JW, Boonen S, Carlberg C, Vitvitsky V, Bouillon R, Banerjee R, and Verstuyf A. 1,25-dihydroxyvitamin D3 influences cellular homocysteine levels in murine preosteoblastic MC3T3-E1 cells by direct regulation of cystathionine. beta-synthase. J Bone Miner Res 26: 2991–3000, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kumar R and Banerjee R. Regulation of the redox metabolome and thiol proteome by hydrogen sulfide. Crit Rev Biochem Mol Biol 56: 221–235, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kumar R, Landry AP, Guha A, Vitvitsky V, Lee HJ, Seike K, Reddy P, Lyssiotis CA, and Banerjee R. A redox cycle with complex II prioritizes sulfide quinone oxidoreductase-dependent H2S oxidation. J Biol Chem 298: 101435, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lagoutte E, Mimoun S, Andriamihaja M, Chaumontet C, Blachier F, and Bouillaud F. Oxidation of hydrogen sulfide remains a priority in mammalian cells and causes reverse electron transfer in colonocytes. Biochim Biophys Acta 1797: 1500–1511, 2010. [DOI] [PubMed] [Google Scholar]
- 41. Landry AP, Ballou DP, and Banerjee R. H2S oxidation by nanodisc-embedded human sulfide quinone oxidoreductase. J Biol Chem 292: 11641–11649, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Landry AP, Ballou DP, and Banerjee R. Modulation of catalytic promiscuity during hydrogen sulfide oxidation. ACS Chem Biol 13: 1651–1658, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Landry AP, Ballou DP, and Banerjee R. Hydrogen sulfide oxidation by sulfide quinone oxidoreductase. Chembiochem 22: 949–960, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Landry AP, Moon S, Bonanata J, Cho US, Coitino EL, and Banerjee R. Dismantling and rebuilding the trisulfide cofactor demonstrates Its essential role in human sulfide quinone. oxidoreductase. J Am Chem Soc 142: 14295–14306, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Landry AP, Moon S, Kim H, Yadav PK, Guha A, Cho US, and Banerjee R. A catalytic trisulfide in human sulfide quinone oxidoreductase catalyzes coenzyme A persulfide synthesis and inhibits butyrate oxidation. Cell Chem Biol 26: 1515–1525.e4, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liang M, Jin S, Wu DD, Wang MJ, and Zhu YC. Hydrogen sulfide improves glucose metabolism and prevents hypertrophy in cardiomyocytes. Nitric Oxide 46: 114–122, 2015. [DOI] [PubMed] [Google Scholar]
- 47. Libiad M, Sriraman A, and Banerjee R. Polymorphic variants of human rhodanese exhibit differences in thermal stability and sulfur transfer kinetics. J Biol Chem 290: 23579–23588, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Libiad M, Vitvitsky V, Bostelaar T, Bak DW, Lee HJ, Sakamoto N, Fearon E, Lyssiotis CA, Weerapana E, and Banerjee R. Hydrogen sulfide perturbs mitochondrial bioenergetics and triggers metabolic reprogramming in colon cells. J Biol Chem 294: 12077–12090, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Libiad M, Yadav PK, Vitvitsky V, Martinov M, and Banerjee R. Organization of the human mitochondrial sulfide oxidation pathway. J Biol Chem 289: 30901–30910, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Longchamp A, Mirabella T, Arduini A, MacArthur MR, Das A, Trevino-Villarreal JH, Hine C, Ben-Sahra I, Knudsen NH, Brace LE, Reynolds J, Mejia P, Tao M, Sharma G, Wang R, Corpataux JM, Haefliger JA, Ahn KH, Lee CH, Manning BD, Sinclair DA, Chen CS, Ozaki CK, and Mitchell JR. Amino acid restriction triggers angiogenesis via GCN2/ATF4 regulation of VEGF and H2S production. Cell 173: 117–129 e14, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lowenstein J and Tornheim K. Ammonia production in muscle: the purine nucleotide cycle. Science 171: 397–400, 1971. [DOI] [PubMed] [Google Scholar]
- 52. Luengo A, Li Z, Gui DY, Sullivan LB, Zagorulya M, Do BT, Ferreira R, Naamati A, Ali A, Lewis CA, Thomas CJ, Spranger S, Matheson NJ, and Vander Heiden MG. Increased demand for NAD(+) relative to ATP drives aerobic glycolysis. Mol Cell 81: 691–707.e6, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Macfarlane GT, Gibson GR, and Cummings JH. Comparison of fermentation reactions in different regions of the human colon. J Appl Bacteriol 72: 57–64, 1992. [DOI] [PubMed] [Google Scholar]
- 54. Marutani E, Morita M, Hirai S, Kai S, Grange RMH, Miyazaki Y, Nagashima F, Traeger L, Magliocca A, Ida T, Matsunaga T, Flicker DR, Corman B, Mori N, Yamazaki Y, Batten A, Li R, Tanaka T, Ikeda T, Nakagawa A, Atochin DN, Ihara H, Olenchock BA, Shen X, Nishida M, Hanaoka K, Kevil CG, Xian M, Bloch DB, Akaike T, Hindle AG, Motohashi H, and Ichinose F. Sulfide catabolism ameliorates hypoxic brain injury. Nat Commun 12: 3108, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L, Kelleher JK, Vander Heiden MG, Iliopoulos O, and Stephanopoulos G. Reductive glutamine metabolism by IDH1 mediates lipogenesis under. hypoxia. Nature 481: 380–384, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mishanina TV, Yadav PK, Ballou DP, and Banerjee R. Transient kinetic analysis of hydrogen sulfide oxidation catalyzed by human sulfide quinone oxidoreductase. J Biol Chem 290: 25072–25080, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mullen AR, Wheaton WW, Jin ES, Chen PH, Sullivan LB, Cheng T, Yang Y, Linehan WM, Chandel NS, and DeBerardinis RJ. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 481: 385–388, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, and Snyder SH. H2S signals through protein S-sulfhydration. Sci Signal 2: ra72, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nagahara N and Sawada N. The mercaptopyruvate pathway in cysteine catabolism: a physiologic role and related disease of the multifunctional 3-mercaptopyruvate sulfurtransferase. Curr Med Chem 13: 1219–1230, 2006. [DOI] [PubMed] [Google Scholar]
- 60. Nicholls P and Kim JK. Sulphide as an inhibitor and electron donor for the cytochrome c oxidase system. Can J Biochem 60: 613–623, 1982. [DOI] [PubMed] [Google Scholar]
- 61. Nicholson CK and Calvert JW. Hydrogen sulfide and ischemia-reperfusion injury. Pharmacol Res 62: 289–297, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nickel AG, von Hardenberg A, Hohl M, Loffler JR, Kohlhaas M, Becker J, Reil JC, Kazakov A, Bonnekoh J, Stadelmaier M, Puhl SL, Wagner M, Bogeski I, Cortassa S, Kappl R, Pasieka B, Lafontaine M, Lancaster CR, Blacker TS, Hall AR, Duchen MR, Kastner L, Lipp P, Zeller T, Muller C, Knopp A, Laufs U, Bohm M, Hoth M, and Maack C. Reversal of mitochondrial transhydrogenase causes oxidative stress in heart failure. Cell Metab 22: 472–484, 2015. [DOI] [PubMed] [Google Scholar]
- 63. Niu WN, Yadav PK, Adamec J, and Banerjee R. S-glutathionylation enhances human cystathionine beta-synthase activity under oxidative stress conditions. Antioxid Redox Signal 22: 350–361, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Park J, Chen Y, Tishkoff DX, Peng C, Tan M, Dai L, Xie Z, Zhang Y, Zwaans BM, Skinner ME, Lombard DB, and Zhao Y. SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol Cell 50: 919–930, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pitcher MC, Beatty ER, and Cummings JH. The contribution of sulphate reducing bacteria and 5-aminosalicylic acid to faecal sulphide in patients with ulcerative colitis. Gut 46: 64–72, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Puranik M, Weeks CL, Lahaye D, Kabil O, Taoka S, Nielsen SB, Groves JT, Banerjee R, and Spiro TG. Dynamics of carbon monoxide binding to cystathionine beta-synthase. J Biol Chem 281: 13433–13438, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Robb EL, Hall AR, Prime TA, Eaton S, Szibor M, Viscomi C, James AM, and Murphy MP. Control of mitochondrial superoxide production by reverse electron transport at complex I. J Biol Chem 293: 9869–9879, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Roediger WE, Duncan A, Kapaniris O, and Millard S. Sulphide impairment of substrate oxidation in rat colonocytes: a biochemical basis for ulcerative colitis? Clin Sci (Lond) 85: 623–627, 1993. [DOI] [PubMed] [Google Scholar]
- 69. Rowan FE, Docherty NG, Coffey JC, and O'Connell PR. Sulphate-reducing bacteria and hydrogen sulphide in the aetiology of ulcerative colitis. Br J Surg 96: 151–158, 2009. [DOI] [PubMed] [Google Scholar]
- 70. Ruetz M, Kumutima J, Lewis BE, Filipovic MR, Lehnert N, Stemmler TL, and Banerjee R. A distal ligand mutes the interaction of hydrogen sulfide with human neuroglobin. J Biol Chem 292: 6512–6528, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sanchez-Aranguren LC, Ahmad S, Dias IHK, Alzahrani FA, Rezai H, Wang K, and Ahmed A. Bioenergetic effects of hydrogen sulfide suppress soluble Flt-1 and soluble endoglin in cystathionine gamma-lyase compromised endothelial cells. Sci Rep 10: 15810, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol 13: 167–171, 2001. [DOI] [PubMed] [Google Scholar]
- 73. Shaw L and Engel PC. CoA-persulphide: a possible in vivo inhibitor of mammalian short-chain acyl-CoA dehydrogenase. Biochim Biophys Acta 919: 171–174, 1987. [DOI] [PubMed] [Google Scholar]
- 74. Singh S and Banerjee R. PLP-dependent H2S biogenesis. Biochim Biophys Acta 1814: 1518–1527, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Singh S, Madzelan P, and Banerjee R. Properties of an unusual heme cofactor in PLP-dependent cystathionine. beta-synthase. Nat Prod Rep 24: 631–639, 2007. [DOI] [PubMed] [Google Scholar]
- 76. Singh S, Padovani D, Leslie RA, Chiku T, and Banerjee R. Relative contributions of cystathionine beta-synthase and gamma-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J Biol Chem 284: 22457–22466, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Smith EH, Janknecht R, and Maher LJ, 3rd. Succinate inhibition of alpha-ketoglutarate-dependent enzymes in a yeast model of paraganglioma. Hum Mol Genet 16: 3136–3148, 2007. [DOI] [PubMed] [Google Scholar]
- 78. Spinelli JB, Rosen PC, Sprenger HG, Puszynska AM, Mann JL, Roessler JM, Cangelosi AL, Henne A, Condon KJ, Zhang T, Kunchok T, Lewis CA, Chandel NS, and Sabatini DM. Fumarate is a terminal electron acceptor in the mammalian electron transport chain. Science 374: 1227–1237, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Statzer C, Meng J, Venz R, Bland M, Robida-Stubbs S, Patel K, Petrovic D, Emsley R, Liu P, Morantte I, Haynes C, Mair WB, Longchamp A, Filipovic MR, Blackwell TK, and Ewald CY. ATF-4 and hydrogen sulfide signalling mediate longevity in response to inhibition of translation or mTORC1. Nat Commun 13: 967, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Taoka S and Banerjee R. Characterization of NO binding to human cystathionine beta-synthase: possible implications of the effects of CO and NO binding to the human enzyme. J Inorg Biochem 87: 245–251, 2001. [DOI] [PubMed] [Google Scholar]
- 81. Taoka S, West M, and Banerjee R. Characterization of the heme and pyridoxal phosphate cofactors of human cystathionine beta-synthase reveals nonequivalent active sites. Biochemistry 38: 2738–2744, 1999. [DOI] [PubMed] [Google Scholar]
- 82. Titov DV, Cracan V, Goodman RP, Peng J, Grabarek Z, and Mootha VK. Complementation of mitochondrial electron transport chain by manipulation of the NAD+/NADH ratio. Science 352: 231–235, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Vicente JB, Colaco HG, Sarti P, Leandro P, and Giuffre A. S-Adenosyl-l-methionine modulates CO and NO* binding to the human H2S-generating enzyme cystathionine. beta-synthase. J Biol Chem 291: 572–581, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Vitvitsky V, Kumar R, Libiad M, Maebius A, Landry A, and Banerjee R.. The mitochondrial NADH pool is involved in hydrogen sulfide signaling and stimulation of aerobic glycolysis. J Biol Chem 100736, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Vitvitsky V, Miljkovic JL, Bostelaar T, Adhikari B, Yadav PK, Steiger AK, Torregrossa R, Pluth MD, Whiteman M, Banerjee R, and Filipovic MR. Cytochrome c reduction by H2S potentiates sulfide signaling. ACS Chem Biol 13: 2300–2307, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Vitvitsky V, Yadav PK, An S, Seravalli J, Cho US, and Banerjee R. Structural and mechanistic insights into hemoglobin-catalyzed hydrogen sulfide oxidation and the fate of polysulfide products. J Biol Chem 292: 5584–5592, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Vitvitsky V, Yadav PK, Kurthen A, and Banerjee R. Sulfide oxidation by a noncanonical pathway in red blood cells generates thiosulfate and polysulfides. J Biol Chem 290: 8310–8320, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wise DR, Ward PS, Shay JE, Cross JR, Gruber JJ, Sachdeva UM, Platt JM, DeMatteo RG, Simon MC, and Thompson CB. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci U S A 108: 19611–19616, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wondimu ET, Zhang Q, Jin Z, Fu M, Torregrossa R, Whiteman M, Yang G, Wu L, and Wang R. Effect of hydrogen sulfide on glycolysis-based energy production in mouse erythrocytes. J Cell Physiol 237: 763–773, 2022. [DOI] [PubMed] [Google Scholar]
- 90. Yadav PK, Martinov M, Vitvitsky V, Seravalli J, Wedmann R, Filipovic MR, and Banerjee R. Biosynthesis and reactivity of cysteine persulfides in signaling. J Am Chem Soc 138: 289–299, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yadav PK, Vitvitsky V, Carballal S, Seravalli J, and Banerjee R. Thioredoxin regulates human mercaptopyruvate sulfurtransferase at physiologically-relevant concentrations. J Biol Chem 295: 6299–6311, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yadav PK, Yamada K, Chiku T, Koutmos M, and Banerjee R. Structure and kinetic analysis of H2S production by human mercaptopyruvate sulfurtransferase. J Biol Chem 288: 20002–20013, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]