Abstract
Aims:
Noise damage to auditory hair cells is associated with oxidative stress and mitochondrial dysfunction. This study aimed to investigate the possible effect of sestrin 2 (SESN2), an endogenous antioxidant protein, on noise-induced hearing loss (NIHL) and the underlying mechanisms.
Results:
We identified SESN2 as a protective factor against oxidative stress in NIHL through activation of Parkin-mediated mitophagy. Consistently, SESN2 expression was increased and mitophagy was induced during the early stage after a temporary threshold shift due to noise exposure or hydrogen peroxide(H2O2) stimulation; conversely, SESN2 deficiency blocked mitophagy and exacerbated acoustic trauma. Mechanistically, SESN2 interacted with Unc-51-like protein kinase 1(ULK1), promoting ULK1 protein-level stabilization by interfering with its proteasomal degradation. This stabilization is essential for mitophagy initiation, since restoring ULK1 expression in SESN2-silenced cells rescued mitophagy defects.
Innovation and Conclusion:
Our results provide novel insights regarding SESN2 as a therapeutic target against noise-induced cochlear injury, possibly through improved mitophagy. Antioxid. Redox Signal. 38, 115–136.
Keywords: sestrin 2, oxidative stress, mitophagy, noise-induced hearing loss
Innovation
Recent studies have provided evidence that autophagy protects against noise-induced hearing loss (NIHL) by attenuating oxidative stress. Our study identifies sestrin 2 (SESN2), an endogenous antioxidant protein, activation of mitophagy as a novel mechanism to protect against acoustic cochlear injury. Moreover, our data demonstrate that the molecular mechanism of SESN2 induces mitophagy in an NIHL model mediated through Unc-51-like protein kinase 1 (ULK1).
Introduction
Hearing loss caused by exposure to environmental and recreational noise is one of the most frequently occurring sensory disorders. According to the World Health Organization, the prevalence of noise-induced hearing loss (NIHL) is about 16% in the total adult population worldwide (Basner et al, 2014). NIHL is usually characterized not only by speech communication barriers, but it also brings a range of nonauditory health effects (sleep disorders, cognitive disorders, cardiovascular diseases, etc.) (Bressler et al, 2017; Münzel et al, 2018). Over the past few decades, much research has been performed to explore the mechanisms of noise-induced cochlear injury. Considerable evidence indicates that noise exposure can induce the excessive production of reactive oxygen species (ROS) in the cochlea, resulting in oxidative injury that eventually leads to auditory hair cell death through either apoptosis or necrosis (Henderson et al, 2006).
ROS are mainly produced by the mitochondria in eukaryotic cells. The mitochondria are essential for respiration and energy metabolism in most mammalian cells. Interestingly, noise exposure has been shown to induce mitochondrial function impairment by destabilizing the mitochondrial DNA, reducing the respiratory chain activity and the organelle mass, and causing morphological changes in hair cells (Park et al, 2012). In addition, exposure to loud noise reduces cochlear blood flow, leading to a decrease in the adenosine triphosphate (ATP) level (Miller et al, 2003). Therefore, a healthy mitochondrial population is critical to maintain the integrity of auditory function. Furthermore, the clearance of damaged mitochondria exerts a key role in the control of mitochondrial homeostasis. Selective mitochondrial autophagy (mitophagy) is a fundamental cellular process that eliminates damaged mitochondria through the formation of mitophagosomes and the fusion with lysosomes (Zhu et al, 2013a).
The phosphatase and tensin homolog-induced putative kinase 1 (PINK1)–E3 ubiquitin ligase Parkin-dependent pathway is one of the vital mechanisms regulating mitophagy. When the mitochondrial membrane potential is diminished, cytosolic Parkin translocates and interacts with PINK1, which is located on the outer membrane of the impaired mitochondria, and subsequently targets damaged mitochondria toward autolysosomes for elimination (Narendra et al, 2008). Increasing evidence has implicated mitophagy impairment in cancer, neurodegradation, and aging (Corti et al, 2011; Wallace, 2012). In addition, it has been reported that mitophagy is critically involved in hearing loss. Using an age-related hearing loss model, Linet al (2019) have suggested that the improved mitophagy level in senescent cells eliminates mitochondrial dysfunction, thus attenuating oxidative stress-induced cochlear damage.
A recent study also has shown that mitophagy impairment accelerates cisplatin-induced ototoxic insults. Moreover, induction of autophagy has been demonstrated to block noise-induced oxidative damage (Yuan et al, 2015). Therefore, it is necessary to further investigate the possible involvement of mitophagy in NIHL.
Autophagy/mitophagy can be induced by sestrins. Sestrins belong to an evolutionarily conserved family of stress-inducible metabolic proteins and are known to protect cells and tissues against DNA damage, oxidative stress, and hypoxia. In mammalian tissues, the sestrin protein family consists of the following three isoforms: sestrin 1 (SESN1), sestrin 2 (SESN2), and sestrin 3 (SESN3) (Lee et al, 2013). The crystal structure of the SESN2 protein reveals that it contains three domains (SESN-A, SESN-B, and SESN-C), and the oxidoreductase active site within the SESN-A domain is critical for the antioxidant activity of SESN2 (Kim et al, 2015). In addition, the inhibition of ROS by SESN2 might be elicited directly by the activation of transcription factors such as p53, nuclear factor erythroid 2-like 2, and activator protein-1, which are important regulators of various antioxidant genes, resulting in the activation of the antioxidant system (Shin et al, 2012; Zhang et al, 2013b).
Reports have revealed that a significant age-related decrease in SESN2 expression provokes an immune response in cochlear tissues (Zhang et al, 2017). Significantly fewer auditory cells were measured in SESN2-knockout cochlear explants exposed to gentamicin compared with the wild-type mice (Ebnoether et al, 2017). These results show that SESN2 plays an important role in the protection of auditory hair cells against cellular stresses. In addition, it has been demonstrated that SESN2 is responsive to various environmental stresses through regulating autophagy and mitophagy as well as improving the mitochondrial function. SESN2 promotes mitophagy by associating with Unc-51-like protein kinase 1 (ULK1) and mediating the ubiquitin-binding receptor sequestosome 1 (p62/SQSTM1) phosphorylation to trigger the translocation of p62 to damaged mitochondria (Kumar and Shaha, 2018; Ro et al, 2014).
ULK1 plays a crucial role in autophagy/mitophagy induction and has been proposed to regulate mitophagy through its posttranslational modifications, including ubiquitylation and phosphorylation, or its protein stability (Egan et al, 2011; Kim et al, 2016; Nazio et al, 2013). Despite the importance of SESN2 in many fields, little is known about its functional role in NIHL or its pathogenesis.
It has been reported that a noise exposure-induced temporary threshold shift (TTS) stimulates autophagy, while a severe permanent threshold shift due to noise conditions overwhelms autophagy. Results from our study demonstrated that TTS-noise exposure and hydrogen peroxide (H2O2) treatment induced mitophagy in auditory hair cells. We confirmed that mitophagy inhibition accelerated sensory cell damage and auditory dysfunction. Furthermore, we showed that SESN2 was upregulated with TTS-noise exposure and H2O2 treatment. Most importantly, we hypothesized that SESN2 knockdown induced oxidative stress, leading to mitochondrial damage and ultimately sensory cell death within both in vitro and in vivo models. Mechanistically, we proved that SESN2 could promote mitophagy through enhanced ULK1 levels, leading to the suppression of noise-induced oxidative stress. Taken together, our data reveal that targeting SESN2 may potentially be a great benefit for the treatment of NIHL.
Results
Noise exposure induces temporary hearing loss in C57BL/6J mice
C57BL/6J mice were used as the animal model for NIHL. In this study, we assessed the effect of 100-dB sound pressure level (SPL) noise in 5-week-old mice by measuring the auditory brain stem response (ABR), as well as the hair cell and presynaptic ribbon loss, using the protocol illustrated in Figure 1A. In this study, we first measured the auditory threshold shifts after a click stimulus and tone burst stimuli at 4, 8, 16, and 24 kHz in mice after exposure to broadband white noise at 100 dB SPL for 2 h. As shown in Figure 1B, the auditory threshold after exposure to all five frequencies increased on the first day postexposure, but the hearing threshold after exposure to the highest frequency (24 kHz) increased significantly. On the 7th day, except for the highest frequency (24 kHz), most of the frequency thresholds recovered.
FIG. 1.
Hearing threshold shifts and quantification of OHCs and ribbon synapses at 14 days postnoise exposure for the noise-exposed group and the control group. (A) An experimental design for a noise-induced TTS in C57BL/6J mice. (B) Click and pure tone-burst (4, 8, 16, and 24 kHz) ABR thresholds in C57BL/6J mice before and after noise exposure. The thresholds were significantly elevated immediately following noise exposure. On day 7 postexposure, the thresholds were significantly elevated after exposure to a tone-burst of 24 kHz. On day 14 postexposure, the thresholds were not significantly different compared with before noise exposure (n = 6). (C) Representative confocal microscopy images of myosin-VIIa-labeled sensory hair cells (red) at the apical turn, middle turn, and basal turn of control and noise-exposed mice at day 14 after noise exposure. OHC1, 2, and 3 represent three rows of OHCs. Scale bar: 10 μm. (D) Quantitative analysis of OHCs and IHCs in TTS mice after exposure to noise conditions (n = 4–5), showing no loss of hair cells. (E) Representative confocal microscopy images of CtBP2-labeled ribbon particles (green) at the apical turn, middle turn, and basal turn from the control and TTS mice at day 14 after noise exposure. Scale bar: 10 μm. (F) Quantification of CtBP2-positive puncta per IHC showing no significant reduction after noise exposure (n = 4). *p < 0.05, **p < 0.01, ***p < 0.001 by one-way ANOVA followed by Dunnett's post hoc correction in (B) and the two-tailed unpaired Student's t-test in (D, F). All data are presented as mean ± SD. For all panels, n indicates the number of biological replicates derived from independent samples. ABR, auditory brain stem response; ANOVA, analysis of variance; IF, immunofluorescence; IHC, inner hair cell; OHC, outer hair cell; SD, standard deviation; SPL, sound pressure level; TEM, transmission electron microscopy; TTS, temporary threshold shift; WB, Western blot.
On the 14th day, there were no statistically significant differences among all of the frequency thresholds, suggesting that exposure to broadband white noise at 100 dB SPL leads to a TTS only. According to the myosin-VIIα staining experiments, the auditory cells displayed a normal pattern of one row of inner hair cells (IHCs) and three rows of outer hair cells (OHCs) (Fig. 1C). Two weeks after a TTS induced by exposure to noise at 100 dB, there were no significant differences in the loss of OHCs or IHCs upon cochlear whole-mount examination (Fig. 1C, D). Also, we labeled the puncta of the presynaptic ribbons using an antibody against CtBP2. There were no significant differences in the number of CtBP2-positive puncta between the control and noise-exposed groups on postexposure day 14 (Fig. 1E, F).
These results indicate that the 100-dB noise stimulation for 2 h was not sufficient to induce changes in the morphological characteristics of cochlear hair cells.
Increased markers of oxidative stress activity in cochlear tissues and cochlear House Ear Institute-Organ of Corti 1 cells
4-Hydroxynonenal (4-HNE), a fairly stable by-product of lipid peroxidation, is a potent aldehyde that can react extensively with DNA and proteins, generating various types of adducts. The protein–HNE adducts have a documented ability to disrupt protein function, finally leading to cytotoxicity. In this study, we used 4-HNE as an oxidative stress biomarker to monitor ROS activity in the cochlea (Yamashita et al, 2004). As shown in Figure 2A, we performed immunofluorescence analyses of 4-HNE (green) in OHCs. The relative fluorescence intensity for 4-HNE was remarkably enhanced at 3 h after the noise exposure inducing a TTS (Fig. 2A, B). We then assessed the 4-HNE levels at 3 h after the noise exposure of mice in the whole mouse cochlear total protein extracts. Compared with the control group, the 4-HNE band density increased in the noise exposure group (Fig. 2C and Supplementary Fig. S8).
FIG. 2.
Noise exposure increased the production of 4-HNE in OHCs and H2O2 induced mtROS formation in HEI-OC1 cells. (A, B) The amount of immunolabeled 4-HNE (green) in OHCs stained red with phalloidin in the basal cochlear turn was significantly increased in the TTS mice compared with the controls. Scale bar: 10 μm (n = 4 in each group). (C) Immunoblots labeled with the 4-HNE antibody revealed that the ratio of the 4-HNE band density between the control and TTS mice was significantly different. GAPDH was used as a control in the WB assays (n = 3 for each group). (D) Dose- and time-dependent cell viability induced by H2O2 in HEI-OC1 cells. Left panel: cells were treated with the designated concentration of H2O2 for 12 h; right panel: cells were treated with 1 mM H2O2 for the designated periods of time (n = 4 for each group). (E, F) The level of mtROS accumulation in HEI-OC1 cells (red) was examined at 12 h after H2O2 exposure. H2O2 treatment increased mtROS accumulation in HEI-OC1 cells. Scale bar: 50 μm (n = 5 in each group). **p < 0.01, ***p < 0.001by one-way ANOVA followed by Tukey's post hoc correction in (D). **p < 0.01 by the two-tailed unpaired Student's t-test in (B, F). All data are presented as mean ± SD. For all panels, n indicates the number of biological replicates derived from independent samples. 4-HNE, 4-hydroxynonenal; H2O2, hydrogen peroxide; HEI-OC1, cochlear House Ear Institute-Organ of Corti 1; mtROS, mitochondrial reactive oxygen species.
H2O2 is commonly used to induce ROS generation in cochlear House Ear Institute-Organ of Corti 1 (HEI-OC1) cells. To some extent, the H2O2-induced cell death pattern is similar as the noise-induced hair cell death (Wu et al, 2020). However, the excess production of ROS causes cellular damage. As shown in Figure 2D, HEI-OC1 cells exposed to different concentrations of H2O2 for 3, 6, 12, and 24 h showed concentration- and time-dependent cell viability. For cells exposed to 1 mM H2O2 for 12 h, their viability was significantly reduced to 26% (Fig. 2D, left panel). In the time-course study, the cells treated with 1 mM H2O2 demonstrated a remarkable reduction in cell viability (Fig. 2D, right panel).
To evaluate the mitochondrial reactive oxygen species (mtROS) levels in HEI-OC1 cells treated with H2O2, we used the mitochondrial superoxide indicator MitoSOX red. The data demonstrate that treatment with 1 mM H2O2 significantly enhanced the mtROS levels compared with the normal control group (Fig. 2E, F).
Mitophagy was induced both in vivo and in vitro
To examine the status of mitophagy in the cochlea, the levels of mitophagy-related proteins, colocalization of mitochondria with microtubule-associated protein 1A/1B-light chain 3 (LC3), or lysosome-associated membrane glycoprotein 1 (LAMP1), as well as the mitochondrial ultrastructure changes, were checked after noise exposure. Western blot assays revealed that noise exposure induced a time-dependent increase in PINK1 and Parkin levels in the cochlea (Fig. 3A and Supplementary Fig. S3A and Supplementary Fig. S9). An increasing protein expression of LC3-II, an autophagosome marker, was observed after noise exposure in the cochlea, reaching the highest expression at 3 h (Fig. 3A and Supplementary Fig. S3A). Meanwhile, we also checked the level of mitophagy in HEI-OC1 cells treated with 1 mM H2O2 for 0, 3, 6, 12, and 24 h. Increasing PINK1, Parkin, and LC3-II protein expression levels were observed after H2O2 exposure in the HEI-OC1 cells (Fig. 3B and Supplementary Fig. S3B and Supplementary Fig. S10).
FIG. 3.
Increased mitophagy in the organ of Corti and HEI-OC1 cells. (A) Representative mitochondrial autophagy-related protein expression levels of Parkin, PINK1, p62, LC3 II, and TOMM20 as assessed by WB analyses in the cochleae of the control and noise-exposed groups at various time points. GAPDH was used as a control (n = 3–4 mice/group). (B) Example bands from WB analysis to determine the protein levels of Parkin, PINK1, p62, LC3 II, and TOMM20 required for mitophagy in HEI-OC1 cells treated with 1 mM H2O2 for various times. GAPDH was used as a control (n = 3–4 experiments). (C) Colocalization analysis of autophagosomes (LC3, green) with mitochondria (TOMM20, red) to detect mitophagy in OHCs of 5-week-old mice at 3 h after a noise-induced TTS (n = 3 mice/group). Scale bar: 10 μm. (D) Coimmunostaining analysis of lysosomes (LAMP1, red) with mitochondria (TOMM20, green) to detect mitophagy in OHCs of 5-week-old mice at 3 h after noise exposure (n = 3 mice/group). Scale bar: 10 μm. (E) Typical examples of coimmunostaining showing mitophagy of LC3 (red) and TOMM20 (green) in HEI-OC1 cells after H2O2 treatment for 12 h (n = 3 experiments). Scale bar: 10 μm. (F) IF staining of LAMP1 (red) and TOMM20 (green) showing that their colocalization (yellow, the overlap color) was significantly increased in HEI-OC1 cells after H2O2 treatment for 12 h (n = 3 experiments). Scale bar: 10 μm. (G) Representative TEM images of OHCs showing morphological changes after noise exposure or treatment with the specific mitophagy-inducing agent (CCCP; a positive control) compared with the controls (n = 3 experiments). Scale bar: 500 nm. (H) Quantification of the results in (G). (I) Representative TEM images illustrating the formation of autophagosome-containing mitochondria of HEI-OC1 cells treated with 1 mM H2O2 for 12 h or 10 μM CCCP for 12 h (n = 3 experiments). (J) Quantification of the results in (I). Arrows, mitophagosomes; G, Golgi body; M, mitochondria; N, nucleus; scale bar: 500 nm. For all panels, n indicates the number of biological replicates derived from independent samples. *p < 0.05, **p < 0.01 by one-way ANOVA followed by Tukey's post hoc correction in (H, J). All data are presented as mean ± SD. For all panels, n indicates the number of biological replicates derived from independent samples. CCCP, carbonyl cyanide 3-chlorophenyl-hydrazone; LAMP1, lysosome-associated membrane glycoprotein 1; LC3, microtubule-associated protein 1A/1B-light chain 3; PINK1, phosphatase and tensin homolog-induced putative kinase 1; TOMM20, translocase of outer mitochondrial membrane 20.
However, the protein expression of the autophagy substrate p62 was downregulated by noise or H2O2 compared with the control group (Fig. 3A, B and Supplementary Fig. S3A, B). The expression trend of LC3-II was the reverse of that of p62, indicating the integrity of mitophagic flux. The expression level of translocase of outer mitochondrial membrane 20 (TOMM20), a marker of mitochondria, was decreased both in vitro and in vivo, suggesting the clearance of damaged mitochondria (Fig. 3A, B and Supplementary Fig. S3A, B).
To further confirm the effects of noise on mitophagy, the change in mitophagosomes and mitophagolysosomes was evaluated at 3 h after noise exposure using immunofluorescence assays. Mitophagosomes (yellow fluorescence) were investigated by observing the colocalization of LC3 (green fluorescence) with TOMM20 (red fluorescence). The intensity of yellow fluorescence was significantly increased in the OHCs of TTS mice compared with that of the control mice (Fig. 3C). The fusion of lysosomes with mitochondria can be used to monitor mitophagy by observing the colocalization of LAMP1 (a marker of lysosomes) with TOMM20. Our results show that their colocalization was significantly increased in OHCs of TTS mice compared with that in the control mice (Fig. 3D). Consistent with the results from the TTS mice, the numbers of autophagosome-containing mitochondria and lysosome-containing mitochondria increased after the HEI-OC1 cells were treated with 1 mM H2O2 for 12 h (Fig. 3E, F).
Transmission electron microscopy analysis of the cochlea revealed mitochondrial autophagosome formation (Fig. 3G,H, arrow). We also observed a large amount of mitochondrial autophagosomes in HEI-OC1 cells (Fig. 3I,J). Carbonyl cyanide 3-chlorophenyl-hydrazone (CCCP), an inducer of mitochondrial fission and fragmentation, served as a positive control for mitophagy activation (Supplementary Fig. S1A).
Bafilomycin A1 (Baf A1) is a V-ATPase inhibitor that blocks the degradation of autophagosomes by inhibiting autophagolysosome formation, and this allows us to monitor autophagosome biogenesis (Klionsky et al, 2021). The autophagic flux assay, which processed LC3-II protein accumulation in response to inhibition of the autophagosome–lysosome fusion phase, is a reliable methodology for detecting a complete autophagy process. The experimental design is shown in Supplementary Figure S1B. Western blotting and confocal imaging showed that LC3B puncta were more abundant and that the LC3-II protein expression levels were increased in the Noise + Baf A1 group compared with in the Baf-alone group or noise-alone group (Supplementary Fig. S4A–D and Supplementary Fig. S17), indicating that autophagosome synthesis in OHCs was enhanced by noise exposure even when Baf A1 blocks autophagosome degradation.
Similarly, H2O2-treated HEI-OC1 cells in the presence of Baf A1 showed a further increase in the LC3-II protein expression level and the numbers of LC3B puncta (Supplementary Fig. S4E–H and Supplementary Fig. S17). These data indicate that increased mitophagy was induced in the auditory cells after treatment with noise or H2O2.
Mitophagy inhibition exacerbated NIHL and oxidative injury in the mouse cochlea
Mitochondrial division inhibitor-1 (Mdivi-1), an inhibitor of mitochondrial fission, is a tool to evaluate mitophagy. Although Mdivi-1 is characterized as a chemical inhibitor of dynamin-related protein 1 (Drp1), studies have reported the effect of Mdivi-1 on the inhibition of mitophagy activity (Vazquez-Martin et al, 2012; Zhang et al, 2013a). We evaluated mitophagy in the mice cochlea pretreatment with 50 mg/kg of Mdivi-1 before noise treatment (Supplementary Fig. S1C). We observed that treatment with Mdivi-1 reduced the level of Parkin and PINK1 proteins, indicative of mitophagy, exposed to noise relative to noise-exposed cochlea treated with vehicle alone (Supplementary Fig. S5A, B and Supplementary Fig. S18). Consistent with this inhibitory effect on mitophagy, Mdivi-1 inhibited the increase in LC3 protein and restored a noise-induced decline of p62 observed after noise treatment (Supplementary Fig. S5A, B and Supplementary Fig. S18).
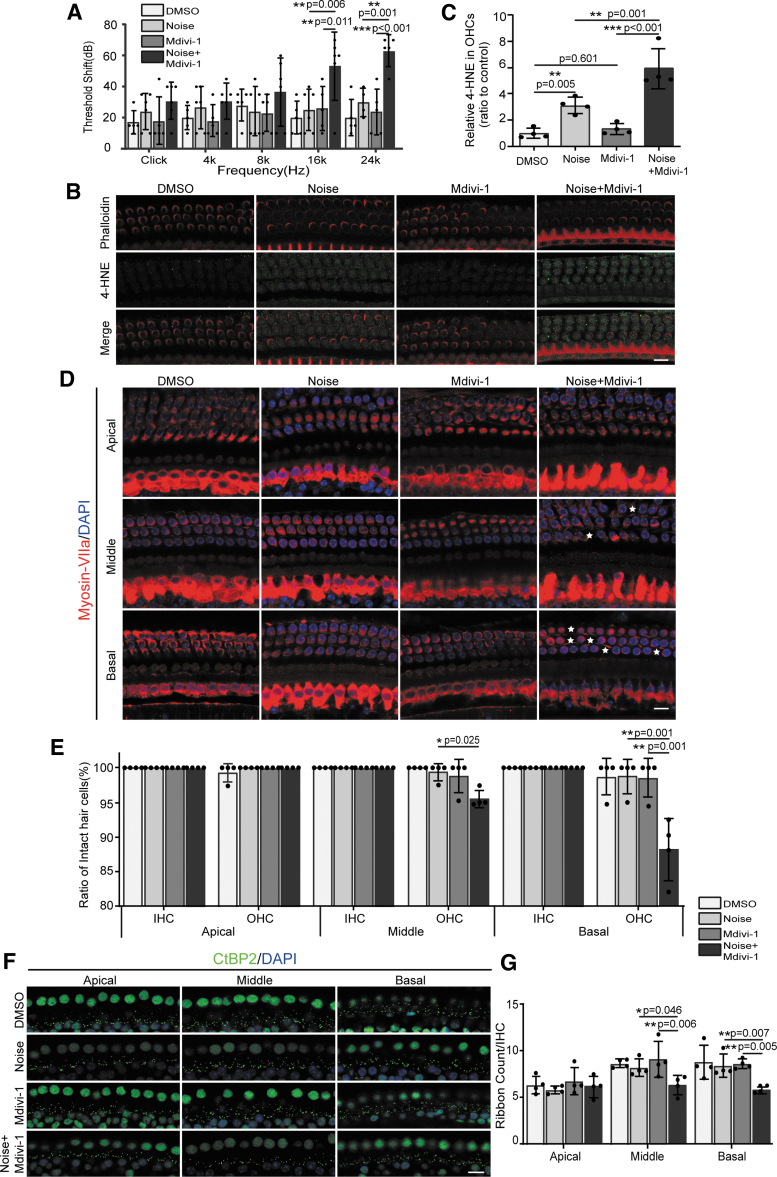
It is well documented that autophagy protects against NIHL by attenuating oxidative stress (Yuan et al, 2015). Therefore, we examined whether mitophagy is beneficial for hearing function in the cochlea of C57BL/6J mice after noise exposure. We examined the changes of the audiometric threshold, 4-HNE level, OHCs, and synapse counts for the TTS groups after Mdivi-1 pretreatment (Supplementary Fig. S1C). Treatment with Mdivi-1 before noise exposure significantly increased the ABR thresholds after exposure to both 16 and 24 kHz, but not after click, 4, or 8 kHz, at 14 days after noise exposure compared with the dimethyl sulfoxide(DMSO)-treated groups (Fig. 4A). Quantification of 4-HNE immunofluorescence in OHCs confirmed significant improvement of the noise-increased 4-HNE levels (Fig. 4B, C) after treatment with Mdivi-1.
FIG. 4.
Treatment with mitophagy inhibitor (Mdivi-1) increases noise-induced 4-HNE production, auditory threshold shifts, OHC loss, and ribbon synapse loss. (A) Click and pure tone-burst (4, 8, 16, and 24 kHz) ABR thresholds in noise-exposed mice pretreated with Mdivi-1 or DMSO (control) at 2 weeks after noise exposure. Mdivi-1 treatment enhanced the auditory threshold shifts at pure tone bursts of 16 and 24 kHz in the treated mice compared with the vehicle-treated mice (n = 4–6). (B) Immunolabeled 4-HNE (green) in OHCs with phalloidin staining (red) at 3 h after noise exposure was stronger with Mdivi-1 treatment. Scale bar: 10 μm. (C) Treatment with the mitophagy inhibitor Mdivi-1 increased the 4-HNE levels in the OHCs of the TTS mice (n = 4). (D) Representative confocal images of myosin-VII-labeled sensory hair cells (red) at the apical turn, middle turn, and basal turn from noise-exposed mice pretreated with Mdivi-1 or DMSO. Asterisks indicate OHC loss. Scale bar: 10 μm. (E) Quantitative analysis of OHCs showing significant loss of OHCs at the middle and basal turns after DMSO or Mdivi-1 treatment in the TTS mice. Loss of IHCs was not significantly different between these four groups (n = 4). (F) Representative confocal images of CtBP2-labeled ribbon particles (green) at the apical turn, middle turn, and basal turn from noise-exposed mice pretreated with Mdivi-1 or DMSO. Scale bar: 10 μm. (G) Treatment with Mdivi-1 increased noise-induced ribbon particle loss (n = 4). *p < 0.05, **p < 0.01, ***p < 0.001 by one-way ANOVA followed by Tukey's post hoc correction in (A, C, E, G). Data are presented as mean ± SD. For all panels, n indicates the number of biological replicates derived from independent samples. DMSO, dimethyl sulfoxide; Mdivi-1, mitochondrial division inhibitor-1.
Moreover, treatment with Mdivi-1 significantly reduced the number of OHCs in the middle and basal turns relative to those observed in vehicle-treated OHCs (Fig. 4D, E). Meanwhile, Mdivi-1 treatment also reduced the noise-induced ribbon synapse losses in the TTS mice (Fig. 4F, G). All IHCs remained intact after Mdivi-1treatment (Fig. 4D, E). The above results confirm that activation of mitophagy plays a vital role in the protection of auditory cells against noise-induced damage.
Upregulation of SESN2 expression in response to oxidative injury in vivo and in vitro
Previous studies have reported that the SESN2 protein is capable of functioning as an antioxidant and is responsible for decreasing the accumulation of ROS and participating in the autophagy process (Budanov et al, 2004; Luo et al, 2020). In this study, we assessed the protein levels of SESN2 in TTS mice and H2O2-treated HEI-OC1 cells. As shown in Figure 5A and B and Supplementary Fig. S11, noise exposure induced a time-dependent increase in the levels of SESN2 until 1 day after exposure, followed by a decline. Additional immunofluorescence assays showed that noise exposure time dependently enhanced SESN2 protein expression in TTS mice (Fig. 5C, D).
FIG. 5.
SESN2 expression is increased after exposure to noise or H2O2. (A) The SESN2 protein level in the cochlea was analyzed by WB at the indicated time points following noise exposure in the TTS mice. GAPDH was used as the loading control (n = 4). (B) Quantification of the results in (A). (C, D) Representative IF images of SESN2 in the OHCs of control and TTS mice. The relative expression of SESN2 was detected, and the mean ± SD values of four independent experiments are presented. Scale bar: 10 μm. (E) The protein expression level of SESN2 in HEI-OC1 cells was measured by immunoblotting following stimulation with 1 mM H2O2 for the indicated times. GAPDH was used as the loading control (n = 3). (F) Quantification of the results in (E). (G, H) Representative IF images of SESN2 expression in HEI-OC1 cells treated with 1 mM H2O2 for various times. The quantification of SESN2 protein per cell was performed by ImageJ software (n = 6). Scale bar: 50 μm. *p < 0.05, **p < 0.01, ***p < 0.001 by one-way ANOVA followed by Tukey's post hoc correction in (B, D, F) and Dunnett's post hoc correction in (H). Error bars in the graph represent the mean ± SD. For all panels, n indicates the number of biological replicates derived from independent samples. SESN2, sestrin2.
We further validated the expression changes of SESN2 in vitro. HEI-OC1 cells were incubated with 1 mM H2O2 for different times (0, 3, 6, 12, and 24 h). After treatment for 3 h, H2O2 induced a significant upregulation of SESN2 protein expression in a time-dependent manner (6 and 12 h) (Fig. 5E, F and Supplementary Fig. S11). The protein level of SESN2 was further confirmed by immunofluorescence analyses in H2O2-treated HEI-OC1 cells (Fig. 5G, H).Taken together, these findings demonstrate the activation of SESN2 by a noise-induced TTS or H2O2 treatment in the cochlea of mice and HEI-OC1 cells, respectively.
SESN2 deficiency exacerbates damage to hair cells including synaptic ribbons, leading to a permanent threshold shift
Since noise exposure caused a remarkable increase in the SESN2 protein level, we sought to explore the functional role of SESN2 in noise-induced cochlear damage. Following knockdown of SESN2 using three different short hairpin RNAs (shRNAs), we verified that SESN2 was effectively knocked down by shRNA#2 in HEI-OC1 cells (Fig. 6A). The schedule for lentivirus injection through canalostomy in mice and the following treatment are illustrated in Figure 6B. Negative control (short hairpin RNAs targeting control [shCtrl]) and shRNA targeting SESN2 (shSESN2) were, respectively, administered into the posterior semicircular canal of a mouse ear before noise exposure. SESN2 was successfully silenced in OHCs following shSESN2 transfection (Fig. 6C).
FIG. 6.
SESN2 knockdown exacerbated 4-HNEproduction, auditory threshold shifts, OHC loss, and ribbon synapse loss induced by noise exposure in mice. (A) HEI-OC1 cells were treated with shSESN2 and shCtrl. One of the three SESN2-interfering shRNAs, shSESN2#2, was found to decrease SESN2 protein expression the most efficiently. (B) The time line of the experiment. ShSESN2 or shCtrl was injected into the PSC. Two weeks after lentivirus injection, the mice were exposed to 100-dB-SPL white noise. Mice were tested and sacrificed at 3 h or 14 days after noise exposure. (C) Cochlear samples were stained with SESN2. SESN2 immunoreactivity was reduced in the SESN2 knockdown group (n = 3). Scale bar: 30 μm. (D) ABR thresholds were tested at 14 days after noise exposure in the TTS mice. SESN2 knockdown enhanced the threshold shifts at a tone burst stimulus of 16 or 24 kHz after noise exposure (n = 6). (E, F) Images of 4-HNE in OHCs at the basal turn from all groups of mice at 3 h after noise exposure. Scale bar: 10 μm. The 4-HNE level was significantly increased in the TTS-plus-SESN2-knockdown group (n = 4). (G, H) Images of myosin-VIIa-labeled hair cells at cochlear turns from all groups of mice at 14 days after noise exposure. Asterisks indicate OHC loss. Scale bar: 10 μm. The percentages of the remaining OHCs were significantly lower at the middle and basal turns in the TTS-plus-SESN2-knockdown group. The number of IHCs was not significantly different across all groups (n = 3–4). (I, J) Images of CtBP2-labeled ribbon particles at cochlear turns from all groups of mice at 2 weeks after noise exposure. Scale bar: 10 μm. The number of CtBP2-positive ribbon puncta per IHC was significantly different between the SESN2-knockdown and control groups for the middle and basal turns (n = 4). **p < 0.01, ***p < 0.001by one-way ANOVA followed by Dunnett's post hoc correction in (D, F). *p < 0.05, **p < 0.01 by the two-tailed unpaired Student's t-test in (H, J). Values are the mean ± SD from all experiments. PSC, posterior semicircular canal; shCtrl, short hairpin RNA targeting control; shRNA, short hairpin RNA; shSESN2, short hairpin RNA targeting SESN2.
The auditory threshold data show that knockdown of SESN2 aggravated hearing loss at 16 and 24 kHz after noise exposure in mice at 14 days compared with the negative control groups (Fig. 6D).The accumulation of 4-HNE was examined by immunofluorescence staining assays performed within 3 h postnoise exposure. We observed that the 4-HNE levels were increased in the noise exposure groups, and this effect was strengthened by SESN2 knockdown (Fig. 6E, F). To further investigate noise-induced cochlear damage, OHC loss in all three cochlear turns was evaluated in three to four cochleae from each group. In the middle and basal turns, the numbers of remaining OHCs in the SESN2-knockdown mice were significantly lower than those in the shCtrl mice after the noise exposure (Fig. 6G, H). However, there was no loss of IHCs.
Based on the representative immunofluorescence staining graph of CtBP2 and the statistical results, SESN2 knockdown obviously increased the loss of noise-induced ribbon synapses in the cochlear middle and basal turns (Fig. 6I, J).
Knockdown of SESN2 increases mtROS levels, enhances depolarization of mitochondrial membrane and H2O2-induced apoptosis in vitro
Using SESN2-specific shRNA, we successfully lowered the expression of SESN2 protein in HEI-OC1 cells (Fig. 6A). Next, we investigated the effects of SESN2 on mitochondrial function in HEI-OC1 cells through the MitoSOX™-red assay, Mito Probe™ 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide(JC-1) assay, and terminal deoxynucleotidyl transferase dUTP nick end labeling(TUNEL) assay.
As shown in Figure 7A and B, knockdown of SESN2 in HEI-OC1 cells significantly increased H2O2-induced mtROS production. Depolarization of the mitochondrial membrane potential is direct evidence of mitochondrial damage, which can be quantified by a decrease in the red/green fluorescent signal ratio. Compared with shCtrl-transduced controls with or without H2O2 treatment, SESN2 silencing significantly decreased the red/green ratio in HEI-OC1 cells treated with H2O2 (Fig. 7C, D). In addition, quantitative analysis of the TUNEL staining results showed that knockdown of SESN2 in HEI-OC1 cells greatly increased the number of TUNEL-stained cells (Fig. 7E, F). These findings demonstrate that SESN2 protects auditory cells from apoptotic cell death and mitochondrial damage.
FIG. 7.
SESN2 knockdown aggravated H2O2-induced mtROS accumulation, mitochondrial membrane depolarization, and apoptotic cell death in HEI-OC1 cells. HEI-OC1 cells were treated with 1 mM H2O2 for 12 h in these experiments. (A, B) The level of mtROS accumulation (red) was analyzed by MitoSOX-red staining. H2O2 treatment increased mtROS levels, and SESN2 knockdown aggravated this effect (n = 5 per group). Scale bar: 50 μm. (C, D) The mitochondrial membrane potential was analyzed by a fluorescence JC-1 assay. A decrease in the red/green ratio indicates mitochondrial membrane depolarization. Knockdown of SESN2 aggravated the decrease in the red/green ratio in H2O2-treated cells (n = 5 per group). Scale bar: 50 μm. (E, F) Representative photomicrographs of apoptotic cells (blue plus green) in HEI-OC1 cells measured by the TUNEL assay. SESN2 knockdown increased cell death (n = 6 per group). Scale bar: 50 μm. *p < 0.05, **p < 0.01, ***p < 0.001 by one-way ANOVA followed by Tukey's post hoc correction in (F) and by Dunnett's post hoc correction in (B, D). All data are expressed as the mean ± SD. For all panels, n indicates the number of biological replicates derived from independent samples. JC-1, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imida carbocyanine iodide; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
Mitophagy after noise exposure or H2O2 treatment is weakened by SESN2 knockdown
To determine whether SESN2 regulates the occurrence of mitophagy in vivo and in vitro, we first detected changes of mitophagy-related proteins. Western blot assays showed that the PINK1 and Parkin protein levels were decreased in the cochlea of mice with SESN2 knockdown, although there are no statistically significant changes in the Parkin and PINK1 protein levels (Fig. 8A and Supplementary Fig. S6A and Supplementary Fig. S12). In accordance with our hypothesis, a noise-induced TTS upregulated the LC3-II protein level and downregulated the p62 protein level. Moreover, SESN2 knockdown notably ablated the changes in the LC3-II and p62 protein expression pattern induced by noise exposure. In addition, SESN2 depletion greatly increased the TTS-mediated accumulation of TOMM20 (Fig. 8A and Supplementary Fig. S6A and Supplementary Fig. S12). Furthermore, fluorescence imaging showed that SESN2 knockdown significantly inhibited LC3 colocalization to the mitochondria in the OHCs of TTS mice compared with the control group (Fig. 8C).
FIG. 8.
Silencing of SESN2 by shRNAs blocked mitophagy triggered by noise and H2O2. (A) At 3 h after noise exposure, the protein expression of Parkin, PINK1, p62, LC3 II, and TOMM20 was examined by WB analysis in SESN2-deficient (shSESN2) and control (shCtrl) mouse cochleae. GAPDH was used as the internal control (n = 3 animals/group). (B) HEI-OC1 cells transfected with control or SESN2 shRNA were treated with H2O2 (1 mM) for 12 h and subjected to WB analysis for mitochondrial autophagy-related proteins (Parkin, PINK1, p62, LC3 II, and TOMM20). GAPDH was used as the internal control (n = 3 experiments). (C) Typical examples of colocalization of LC3 and TOMM20 to detect mitophagy in mice, with or without noise exposure for 3 h and injection of the null vector or SESN2-knockdown lentivirus (n = 3 animals/group). Scale bar: 10 μm. (D) Colocalization analysis of LC3 with TOMM20 to detect mitophagy in HEI-OC1 cells, with or without H2O2 treatment for 12 h and transfection of null vector or SESN2-knockdown lentivirus (n = 3 experiments). Scale bar: 10 μm. For all panels, n indicates the number of biological replicates derived from independent samples.
In addition, we also examined the colocalization between Parkin and PINK1 in OHCs after knockdown of SESN2 by lentivirus. As shown in Supplementary Figure S7, the colocalization of Parkin with PINK1 was significantly increased in OHCs by noise, while these effects were retarded by SESN2 knockdown.
Following knockdown of SESN2 using specific shRNA#2, we successfully lowered the protein level of SESN2 and blocked SESN2 upregulation induced by H2O2 (Figs. 6A and 8B and Supplementary Fig. S6B). The in vitro results showed that H2O2 treatment upregulated the PINK1 and Parkin protein levels, while these effects were retarded by SESN2 knockdown. In addition, SESN2 silencing significantly blocked the H2O2-induced upregulation of LC3-II expression, while it markedly increased the H2O2-induced accumulation of p62. Moreover, SENS2 knockdown resulted in a higher TOMM20 level in HEI-OC1 cells (Fig. 8B and Supplementary Fig. S6B and Supplementary Fig. S13). Next, the colocalization of LC3 and mitochondria was used to characterize the changes of mitophagy. Fluorescence imaging revealed that the overlay between LC3 and TOMM20 was increased by H2O2 treatment and was weakened by knockdown of SESN2 (Fig. 8D).
The consistent in vivo and in vitro results demonstrated that SESN2 silencing contributed to mitophagy impairment. These results reveal that activation of SESN2 plays an essential role in mitophagy induction both in vivo and in vitro.
SESN2 induces autophagic activity for mitophagy by increasing the ULK1 protein level
To further explore the molecular mechanism of SESN2-promoted mitophagy, the protein expression ULK1, a pivotal regulator of autophagy-mediated clearance of mitochondria, was evaluated (Kundu et al, 2008). As shown in Figure 9A, the protein expression of ULK1 time dependently increased with that of SESN2 in H2O2-stimulated HEI-OC1 cells, implying a possible relationship between ULK1 and SESN2 protein expression. To examine the relationship between SESN2 and ULK1, we first investigated whether SESN2 interacts with ULK1. We immunoprecipitated SESN2 from H2O2-stimulated HEI-OC1 cells using an irrelevant IgG antibody as a control. SESN2 and ULK1 formed a protein complex in H2O2-stimulated HEI-OC1 cells (Fig. 9B), as revealed by a coimmunoprecipitation experiment. Next, we tested the biochemical consequence of the SESN2–ULK1 interaction. In HEI-OC1 cells, H2O2 stimulation resulted in a higher ULK1 protein level, and this effect was retarded by SESN2 knockdown (Fig. 9C and Supplementary Fig. S14).
FIG. 9.
SESN2 regulated mitochondrial autophagy through ULK1 protein. (A) The ULK1 protein level in H2O2-stimulated HEI-OC1 cells at the indicated time points was measured by immunoblotting. GAPDH was used as the loading control (n = 3). (B) HEI-OC1 cells were grown in H2O2-containing medium for 12 h. Protein extracts were immunoprecipitated with the anti-SESN2 antibody or rabbit immunoglobulin as a negative control (IgG; n = 3). (C) SESN2-knockdown and negative control cells were treated with or without 1 mM H2O2 and immunoblotted for ULK1 (n = 3). (D) HEI-OC1 cells transfected with the indicated lentiviral SESN2 shRNA were collected, and protein lysates were subjected to immunoblotting with the indicated antibodies (n = 3). (E) The protein levels of ULK1, SESN2, and GAPDH were analyzed from HEI-OC1 cells treated withLv-SESN2 or control (vector; n = 3–5). (F, G) HEI-OC1 cells expressing SESN2-shRNA or ctrl-shRNA were treated with 25 μM CHX, a protein synthesis inhibitor, for the indicated times. ULK1, SESN2, and GAPDH were analyzed by Western blotting (F). The amount of ULK1 was graphed (G). (n = 3–4). (H) SESN2-knockdown and control HEI-OC1 cells were treated with or without 20 μM MG132, a proteasomal inhibitor, for 6 h and subjected to immunoblotting for ULK1, SESN2, and GAPDH (n = 3). (I) Protein extracts from HEI-OC1 cells infected with shSESN2 and stably reconstituted with the lentiviral Flag-ULK1 vector were analyzed by WB analysis with the indicated antibodies. (J) HEI-OC1 cells stably expressing Flag-ULK1 and shSESN2 were treated with H2O2 (1 mM) for 12 h. Representative images of LC3 colocalization with TOMM20. Scale bar: 10 μm (n = 3). (K, L) HEI-OC1 cells were infected with shSESN2 and stably reconstituted with lentiviral Flag-ULK1 vector. The histogram represents the level of mtROS accumulation (red) after the cells were cultured in the presence of H2O2 for 12 h (n = 6). Scale bar: 10 μm. ***p < 0.001 by one-way ANOVA followed by Tukey's post hoc correction in (L). *p < 0.05 by the two-tailed unpaired Student's t-test in (G). Measurement data were expressed as the mean ± SD. For all panels, n indicates the number of biological replicates derived from independent samples. CHX, cycloheximide; Lv-SESN2, lentivirus containing SESN2; ULK1, Unc-51-like protein kinase 1.
In addition, silencing of SESN2 significantly reduced the basal levels of ULK1 expression in HEI-OC1 cells (Fig. 9D). However, SESN2 overexpression notably restored the expression level of ULK1 (Fig. 9E and Supplementary Fig. S15).
To test whether SESN2 directly regulates the protein stability of ULK1, we measured the ULK1 protein half-life in SESN2-knockdown and control HEI-OC1 cells using the protein synthesis inhibitor cycloheximide. The half-life of ULK1 was markedly reduced in SESN2-silenced cells, and the ULK1 turnover rate decreased from 16 to <8 h (Fig. 9F, G). ULK1 has been suggested to degrade through the proteosomal pathway (Joo et al, 2011). Therefore, we tested whether ULK1 degradation in SESN2-silenced cells could be attributed to increased ULK1proteolysis.As shown in Figure 9H and Supplementary Fig. S16, treatment with the proteasomal inhibitor MG132 completely restored the ULK1 level in SESN2-silenced cells, suggesting that depletion of SESN2 accelerated proteasomal degradation of ULK1.
Our present results strongly suggest that SESN2 is involved in the regulation of ULK1 stability. To verify that ULK1 exerts a specific effect on defective autophagosome formation in SESN2-silenced cells, we performed a rescue experiment by transfecting ULK1-overexpressing lentivirus into SESN2-silenced HEI-OC1 cells, after which ULK1 expression was validated (Fig. 9I and Supplementary Fig. S15). Reconstitution with ULK1 profoundly increased the overlay between LC3 and TOMM20 (Fig. 9J) as well as decreased the level of mtROS (Fig. 9K, L). These results indicate that SESN2 acts on ULK1 to regulate autophagic activity for mitophagy.
Discussion
Although the involvement of impaired mitochondrial bioenergetics in the pathogenesis of NIHL has been well documented, the role of mitophagy in this disease process has not been described previously. In this study, the following results were obtained: (1) increased mitophagy was observed in OHCs and HEI-OC1 cells after noise exposure-induced TTS and H2O2 treatment, respectively; (2) SESN2 expression was increased in the auditory cells after noise exposure-induced TTS and H2O2 stimulation, respectively; (3) SESN2 efficiently attenuated oxidative damage and hearing loss by promoting mitophagy both in vivo and in vitro by interacting with ULK1 to maintain the ULK1 protein levels in the cytoplasm; and (4) by avoiding its proteasomal degradation that is required for mitophagy induction, SESN2 promoted selective autophagy (Fig. 10). Taken together, we established that SESN2 exerts a protective effect under noise conditions partially by ULK1-dependent mitophagy activation.
FIG. 10.
Schematic diagram illustrating the protective role of SESN2. In auditory cells, the levels of oxidative stress and SESN2 protein were increased after a noise-induced TTS in mice and H2O2 treatment of cells. Increased SESN2 levels induced mitophagy activation by upregulating the expression of ULK1, a regulator of autophagy-mediated clearance of mitochondria, thereby inducing the degradation of damaged mitochondria and leading to the suppression of oxidative damage and auditory hair cell apoptosis. Suppressing mitophagy by adding Mdivi-1 (a mitophagy inhibitor) observably exacerbated cell damage. Furthermore, SESN2 knockdown reduced ULK1 expression and disrupted the process of mitophagy, increased oxidative stress, and impaired mitochondrial accumulation, which might gradually induce cell apoptosis and noise-induced hearing loss. ROS, reactive oxygen species.
OHCs are involved in the process that amplifies movements of the cochlear basilar membrane, contributing to improve hearing sensitivity. The ribbon synapse is responsible for both the fast response to transient signals and the long-lasting response to persistent stimuli. OHCs and ribbon synapses (mainly in IHCs) are vulnerable to insult in mammalian models of NIHL studied to date (Kujawa and Liberman, 2009; Lin et al, 2011). In addition, NIHL and cochlear injury are mainly dependent on the intensity of the noise exposure and the duration of the exposure (Fernandez et al, 2020). Our findings demonstrated that noise exposure at a relatively low intensity results in only a TTS in mice without cochlear damage, in agreement with an earlier report (Wang et al, 2015).
It is worth noting that a noise exposure of 100 dB SPL created significant hearing loss at 1 day after the exposure, while the threshold shift remained stable for 3 days, revealing acute hearing function damage. A widely accepted mediator of noise-induced damage is the excess ROS formation (Böttger and Schacht, 2013; Hu et al, 2006; Yamashita et al, 2005). Damaging ROS comprise the superoxide (O2•−), hydroxyl radical (•OH), singlet oxygen (1O2), and H2O2 (Giorgio et al, 2007; Liochev, 2013). Ohinata et al (2000) found evidence of increased lipid peroxidation in the cochlea after noise exposure. Lipid peroxidation consists of a chain reaction through which ROS and free radicals can break down lipid molecules, including cell membrane and, in turn, cell damage and cell death.
The 4-HNE is the most representative by-product of lipid peroxidation in cells. ROS-induced oxidative stress is related to the mechanisms of cochlear damage. Noise-induced ROS production in the cochlea follows a base-to-apex gradient (Yamane et al, 1995).Our data demonstrate that the formation of increased ROS in the basal turns of the cochlea is in line with a previous study showing that free radicals are increased after a noise-induced TTS in the cochlear lateral wall (Cheng et al, 2008). It has been reported that the generation of ROS persists for several days after an intense noise exposure (Ohlemiller et al, 1999). Therefore, temporary changes in the hearing threshold after noise exposure may be attributable to metabolic overstimulation in this study.
A study by Wang et al (2015) has shown that a maximal reduction of cochlear ribbon synapses appears on the 4th day and that synaptic repair is complete on the 14th day after noise exposure. Normal hearing depends on faithful synaptic transmission at the cochlear ribbon synapses (mainly in IHCs). Hence, the loss of ribbon synapses may be another reason for hearing loss at the early stage after a TTS due to noise exposure.
Mitochondria are essential for energy metabolism, ROS generation, and calcium homeostasis, and they are also key regulators of essential signal transduction pathways (Scorrano, 2009). A growing amount of evidence has demonstrated that mitochondrial dysfunction is not only associated with central neurodegeneration but also contributes to the pathogenesis of sensorineural hearing loss, including age-related hearing loss, NIHL, and ototoxic drug-induced hearing loss (Böttger and Schacht, 2013; Corti et al, 2011). Strikingly, mitophagy plays a primary role in maintaining mitochondrial function and homeostasis by eliminating dysfunctional mitochondria (Ding and Yin, 2012). An increasing number of studies indicate that mitophagy is involved in oxidative stress as a mechanism of cell survival. Moreover, overexposure to noise triggers peroxisome proliferation in sensory hair cells and primary auditory neurons (Defourny et al, 2019; Delmaghani et al, 2015).
In response to noise exposure, pejvakin, a peroxisome-associated protein, directly mediates selective autophagic degradation of peroxisomes (pexophagy) to protect the cochlear hair cells against noise-induced damage (Defourny et al, 2019). Since peroxisomes and mitochondria both exhibit similar clearance mechanisms (Anding and Baehrecke, 2017; Lismont et al, 2015), it is essential to investigate whether mitophagy has a protective effect against noise-induced damage. In this study, we showed the activation of mitophagy in the cochlea of TTS mice as well as in H2O2-stimulated HEI-OC1 cells, protecting against NIHL and hair cell death. Reduction of mitophagy with the inhibitor Mdivi-1 enhanced 4-HNE formation, it consequently aggravated the loss of OHCs and ribbons (mainly in IHCs), resulting in hearing loss. However, the role of mitophagy in diseases remains debatable.
Recently, it has been revealed that doxorubicin treatment-enhanced mitophagy can increase cardiac dysfunction. In addition, inhibition of mitophagy by Mdivi-1 has been shown to attenuate activation of mitophagy and doxorubicin-induced cardiotoxicity (Yin et al, 2018). These results indicate that mitophagy is a two-edged sword and may play distinct functional roles in different diseases.
SESN2, an important modulator of cellular homeostasis, is ubiquitously expressed throughout the organ of Corti. Regulation of SESN2 protein levels is also a defense mechanism in several cells and tissues. SESN2 protects cells from stress-induced apoptosis through enhancement of its expression level (Luo et al, 2020; Pasha et al, 2017). Moreover, the loss of SESN2 exacerbates age-related sensory cell degeneration in cochlear tissues and enhances susceptibility to gentamicin-induced sensory hair cell death (Ebnoether et al, 2017; Zhang et al, 2017). In this study, we demonstrated that the expression of SESN2 is upregulated in hair cells, whereas inactivation of SESN2 potentiates noise-induced sensory cell damage and hearing loss, in agreement with a previous study showing that SESN2 inhibition promotes 1-methyl-4-phenyl-pyridine ion-induced oxidative stress and cellular injury (Zhang et al, 2013b). Our study suggests that SESN2 plays a protective role against noise-induced oxidative damage.
Recently, SESN2 has been reported to promote selective autophagy, which results in the elimination of ubiquitinated protein aggregates and damaged mitochondria (Johansen and Lamark, 2011). Therefore, it was of particular interest to see whether mitophagy is triggered by SESN2, given that mitophagy induction was observed in this study. The formation of LC3-positive mitophagosomes in response to noise exposure and H2O2 stimulation was partially inhibited by shRNA-mediated silencing of SESN2. Our findings suggested that mitophagy is, at least partially, regulated by SESN2 in OHCs and HEI-OC1 cells. Furthermore, Wang et al(2019) have reported that inhibition of mitophagy in a doxorubicin-induced cardiomyopathy model is reversed by overexpression of SESN2. SESN2 mediates the migration of Parkin to damaged mitochondria in response to CCCP treatment of HeLa cells (Kumar and Shaha, 2018).
These findings are consistent with our study, indicating the central role of SESN2 expression during TTS- or H2O2-induced mitophagy in auditory hair cells. Interestingly, the expression level in SESN2 and other key proteins between the immunolabeled OHCs and immunoblots using whole cochlear tissue homogenates is inconsistent, and we speculate that this is due to the presence of multiple cell types in the homogenates, such as spiral ganglion neurons, stria vascularis, and supporting cells, which masked the changes occurring only in OHCs.
ULK1 is proposed to be a mediator of autophagy and mitophagy (Alers et al, 2012). It has been reported that stabilization of the basal ULK1 level is required for autophagy initiation (Jiao et al, 2015; Kim et al, 2018a). In this study, we found that SESN2 initiated the mitophagy process by associating with ULK1 and interfering with the proteasomal degradation of ULK1 to regulate the steady-state levels of ULK1. Therefore, it seems reasonable to speculate that the SESN2–ULK1–mitophagy axis may provide a survival advantage under oxidative stress conditions. However, Kim et al have reported that an endogenous interaction between SESN2 and ULK1 was not observed in the mitophagy activation process of wild-type, bone marrow-derived macrophages upon stimulation with lipopolysaccharide and ATP. Their results suggest that the SESN2-promoted conservation of ULK1 protein levels may not be due to its association with ULK1 (Kim et al, 2016).
In addition, Ro et al (2014) have demonstrated that SESN2 may dominate the ULK1 activity through its physical association, to facilitate mitophagic degradation in HEK293 cells. Moreover, the interaction between ULK1 and p32 (a chaperone-like protein) is critical for maintaining the stable levels and activity of ULK1 to regulate starvation-induced mitophagic flux (Jiao et al, 2015). Therefore, these reports suggest that the relationship between SESN2 and ULK1 may alter, depending on the experimental conditions and the cell type. Although the results of the present study suggest that modulation of SESN2/ULK1 signaling induces mitophagy and protects auditory hair cells against oxidative stress, the precise mechanism involved in this process has not been clearly elucidated. For instance, grancalcin, a novel regulator of imatinib resistance, stabilizes and activates ULK1 by facilitating Lys63-linked ubiquitination of ULK1, resulting in autophagy induction in chronic myeloid leukemia patients (Han and Korm, 2019).
In addition, it has been reported that ULK1 is involved in mitophagy through its phosphorylation level under viral infection conditions (Egan et al, 2011). Thus, it would be of interest to explore whether SESN2 regulates the stability of ULK1 by modulating posttranslational modifications of ULK1 in NIHL in a further investigation.
In addition, the HEI-OC1 cell line, which displays several biomarkers of sensory hair cells, is a mouse cell line similar to hair cells that has been widely used in some ototoxicity studies (Chen et al, 2020; Li et al, 2019; Lin et al, 2019). To some extent, the increased level of ROS after H2O2 treatment of HEI-OC1 cells is similar as in OHCs after noise exposure. In agreement with our in vivo data, our in vitro study using direct administration of H2O2 suggests that mtROS formation decreases the cell viability, and these effects were positively correlated with the expression level of SENS2.
In conclusion, we report that SESN2 induces mitophagy through ULK1 stability. Therefore, mitophagy serves as a mechanism of cell survival during noise-induced cell damage. Although there were some limitations in this study, the role that we uncovered for SESN2 in hearing loss and cell viability after noise exposure may provide a potential novel therapeutic strategy for the treatment of NIHL.
Materials and Methods
Reagents and antibodies
Mdivi-1 (M0199), CCCP (C2759), and DMSO (RNBH8337) were purchased from Sigma-Aldrich (St. Louis, MO). Baf A1 (T6740) was purchased from TargetMol (Boston, MA). MG-132(HY-13259) and puromycin (HY-B1743A) were purchased from MedChemExpress (Monmouth Junction, NJ). Cycloheximide (S7418) was purchased from Selleck Chemicals (Houston, TX). The antibodies used for Western blot, immunofluorescence, or coimmunoprecipitation analysis are described in Supplementary Table S1 with the appropriate dilutions.
Animal
Five-week-old male C57BL/6J mice were purchased from the Animal Center at Xuzhou Medical University (Xuzhou, China). All mice were acclimated for 1 week before the experiments in pathogen-free conditions at room temperature under a standard 12-h light/dark cycle, and food and water were available ad libitum. All animal experiments were performed in compliance with the protocols approved by the Ethics Committee of the Experimental Animal Center at Xuzhou Medical University (accreditation number of the laboratory: 202101A027).
Drug administration and viral injection procedure
The doses and time points of drug administration were the same as those described previously (Li et al, 2018). CCCP, Mdivi-1, and Baf A1 were dissolved in DMSO as a stock solution (100 mg/mL) and stored at −20°C until use. Before injection into the animals, the stock solutions of CCCP, Mdivi-1, and Baf A1 were diluted with sterile saline and gently sonicated for 20 s to produce a homogenous suspension. The mice received intraperitoneal (i.p.) injections with drugs at the following doses: CCCP (5 mg/kg), Mdivi-1 (50 mg/kg), and Baf A1 (1 mg/kg). Different drugs have their treatment time in animals, as illustrated in Supplementary Figure S1.
To minimize damage to the inner ear function due to some local drug delivery strategies, canalostomy was used to deliver viral vectors into the murine inner ear in our study (Supplementary Fig. S2). Three-week-old mice were anesthetized by i.p. injection of 3% sodium pentobarbital (1 mL/kg). A small hole was made in the middle portion of the posterior semicircular canal, and 2.0 μL of lentivirus solution containing SESN2 shRNA or control shRNA (2.43 × 108 TU/mL, 1.21 × 109 TU/mL) was injected at a rate of 90 nL/min using a Picospritzer III pressure microinjection system (Picospritzer III; Parker Hannifin Corporation) based on conditions reported in the literature (Guo et al, 2018). After the injection, the mice were placed on an electric pad (∼37°C) to recover. The mice were housed for at least 1 week before testing and processing.
Noise exposure
Animals were exposed to noise in the same soundproof room as reported previously (Zhao et al, 2021). In brief, 5-week-old mice were exposed to broadband white noise (6.3–20 kHz) under anesthesia at an SPL of 100 dB for 2 h to induce a TTS. The noise was generated by an Audio Noise Generator (Randomness and Integrity Services), amplified by a power amplifier (MF-1201 MOSTET; ATech) and calibrated with a sound-level meter (2250L; Brüel & Kjær, Nærum, Denmark) to ensure uniformity of the sound field. Under anesthesia, control mice were placed in silence within the same booth for 2 h.
Auditory brain stem response
The ABR was assessed before noise exposure and drug delivery to guarantee normal hearing, and it was reassessed at 1, 3, 7, and 14 days after noise exposure to evaluate hearing loss. The mice were anesthetized with 3% sodium pentobarbital, and the body temperature was maintained near 37°C with a heating plate. The Tucker Davis Technology (TDT, RX6, Alachua, FL) System III was used to present stimuli and record signals, as described previously (Li et al, 2019). Tone burst stimuli at 4, 8, 16, and 24 kHz, as well as a click stimulus, were generated, and ABR waveforms were recorded at decreasing sound intensities at 10-dB intervals starting at 90 dB, until a threshold was reached (the lowest stimulus level that just elicited a positive wave). The ABR thresholds were determined for each frequency by ABR wave I.
Cell culture and treatment
The establishment and characterization of the HEI-OC1 cells have been described previously (Kalinec et al, 2003). The cells were cultured in complete Dulbecco's modified Eagle's medium (11995065; Gibco) containing 10% fetal bovine serum (10099-141; Gibco) at 33°C and 10% CO2. For cell viability analysis, cells were grown to 70%–80% confluency and were then treated with or without H2O2 at concentrations of 0.2, 0.5, 1, and 2 mM for the indicated times. For in vitro oxidative stress test analysis, HEI-OC1 cells were exposed to 1 mM H2O2 for the indicated time. CCCP (10 μM) was added to confirm the role of H2O2 in mitophagy induction. Cells were treated with Baf A1 (100 nM) for 24 h in the presence or absence of H2O2.
In vitro transfection
Lentivirus containing SESN2 and the fusion gene ULK1 was designed and synthesized by Obio Technology Corporation (Shanghai, China) and GeneChem Corporation (Shanghai, China). HEI-OC1 cells were seeded in six-well dishes (2 × 104 cells/well) and infected with the SESN2 recombinant lentivirus or empty vector as a control. After incubation for 4 days, the protein expression of SESN2 was examined by Western blotting. Cells with stable SESN2 silencing and control cells were selected with puromycin (20 μg/mL) for 7 days. HEI-OC1 cells with stable knockdown of SESN2 were infected with the lentiviral flag-ULK1 vector. Lysates were immunoblotted with the anti-ULK1 antibody.
Cell viability assay
Cell viability was measured using a Cell Counting Kit-8 (CCK-8; VC5001; KeyGen Biotechnology, Nanjing, China), according to the user manual. HEI-OC1 cells were plated into each well of a 96-well plate in sextuplicate. At the indicated time after treatment, cells were incubated with the CCK-8 solution (100 μL/mL of medium) at 37°C for 2 h. The absorbance was measured at 450 nm using a microplate reader (BioTek China, Beijing, China).
Assessment of TUNEL staining
A one-step TUNEL apoptosis assay kit (MA0223; Meilun Biotechnology, Dalian, China) was used to detect DNA fragmentation, according to the manufacturer's instructions. Briefly, after fixing with 4% paraformaldehyde, the cells were permeabilized for 10 min (0.3% Triton X-100/phosphate-buffered saline [PBS]) and were treated with the TUNEL reaction mixture at 37°C for 1 h. The TUNEL-positive cells (blue plus green) and TUNEL-negative cells (only blue) were visualized by fluorescence microscopy (20 × objective; Olympus), and then, six microscopic fields of each group were randomly selected to assess apoptosis.
Detection of mtROS
The mtROS levels were detected using the MitoSOX Red Mitochondrial Superoxide Indicator (M36008; Thermo Fisher Scientific). Briefly, the cells were incubated with MitoSOX reagent (5 μM) for 10 min at 37°C. The stained cells were then washed with PBS three times and incubated with 2 μg/mL of Hoechst blue (H3570; Thermo Fisher Scientific) for nuclear staining. The stained cells were observed under a laser confocal microscope (Leica STELLARIS 5). For each group, six microscopic fields were randomly selected to assess the mtROS levels.
Detection of mitochondrial membrane potential
Mitochondrial membrane potentials were measured with JC-1(KGA6-04; KeyGen Biotechnology). HEI-OC1cells were incubated with a JC-1 working solution for 20 min at 37°C in the dark, according to the manufacturer's instructions. The cells were observed under a 20 × objective on a laser confocal microscope (Leica STELLARIS 5). To determine whether the mitochondrial membrane potential was damaged, the ratio of red-to-green fluorescence was calculated.
Immunofluorescence assay
The animals were euthanized, and the scala was isolated and fixed overnight in 4% paraformaldehyde. After decalcification, the cochlea was then carefully dissected, as described previously (Zhu et al, 2013b). The basilar membrane, dissected from the cochlea, was divided into three turns (apex, middle, and base). For the cochlear cryosections, some cochlear tissues were decalcified in 10% EDTA solution, embedded in optimum cutting temperature compound (Sakura), and cryosectioned at a thickness of 8 μm for immunohistochemistry. The HEI-OC1 cells were fixed in 100% cold ethyl alcohol at −20°C for 15 min.
The basilar membranes and cochlear sections were permeabilized with 0.3% Triton X-100/PBS (PBST).The specimens were blocked with a blocking buffer (10% goat serum + PBST) for 1 h, followed by incubation with primary antibodies at 4°C overnight. The next day, samples were incubated for 2 h with Alexa flour 488 or 594 secondary antibodies (1:400) and counterstained with the Phalloidin-iFluor 555 Reagent (1:500). The specimens were then washed with PBS, and the nuclei were stained with DAPI. Immunolabeled images were taken using a laser confocal microscope (Leica STELLARIS 5).
For 4-HNE immunofluorescence, one image from the basal turn of each cochlea from four mice taken by fluorescence microscopy was collected using a 63 × magnification lens under same z-stack conditions. Relative fluorescence intensity of 4-HNE in OHCs was quantified with the ImageJ software. In addition, we manually counted the number of OHCs on surface preparation from confocal images, and then, the ratios of total fluorescence intensity to OHCs were calculated. The intensity of relative fluorescence was quantified by normalizing the ratio of average fluorescence of noise-exposed OHCs to that from unexposed controls.
The procedure used to describe the proportion of intact OHCs and the number of synaptic ribbons from surface preparations has been described previously (Kim et al, 2018b; Li et al, 2019). A sample size at least of four ears from different animals was used for each group. Images from the base-to-apex gradient of the cochlear epithelia were captured using a 63 lens on the Leica microscope. We manually counted the numbers of intact OHCs separately in three cochlear turns, and then divided by the total number of OHCs in each turn. In each turn of the cochlea, we counted the number of synaptic ribbons corresponding to ∼12 IHCs and then divided by the total number of IHCs.
Western blot analysis
The Western blot assays were performed, as described previously (Zhuang et al, 2018). After the designated treatment, the whole cochlear tissues and cultured cells were lysed in a radioimmunoprecipitation assay buffer supplemented with the protease inhibitor cocktail (KGP602; KeyGen Biotechnology). The total protein concentration was determined by a bicinchoninic acid protein assay kit (P0010S; Beyotime Biotechnology). Protein samples (40 μg) were separated by 8%–12.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane (EMd Millipore), and incubated with primary antibodies overnight at 4°C. After washing and incubation with a fluorescently labeled secondary antibody (1:1500; Sigma-Aldrich; A7650; Merck KGaA), the protein bands were visualized by the Odyssey Infrared Imaging System (LI-COR Biosciences, Waltham, MA).
GAPDH was used as a sample loading control. Protein of cochleae and cells from the different groups were separated and electrotransferred with identical solutions and processed in parallel. All targeted proteins and internal loading controls were detected and verified within the same linear range.
Coimmunoprecipitation
To assess the formation of SESN2 and ULK1 complexes, coimmunoprecipitation assays were performed. HEI-OC1 cells were lysed in immunoprecipitation lysis buffer (P0013; Beyotime Biotechnology). After determination of the protein concentration, equal quantities of proteins (1000 μg) from each sample were incubated with 2 μg of SESN2 antibody at 4°C overnight, followed by incubation with protein A/G magnetic beads (HY-K0202; MedChemExpress) for 6 h on a rotary wheel, after which the pellets were collected by a magnetic rack. The pellets were washed four times with ice-cold lysis buffer, and then, the immune complexes were eluted in the same volume of 1 × loading buffer that was boiled at 100°C for 5 min. Subsequently, Western blot assays were performed with the anti-SESN2 or anti-ULK1 antibody.
Transmission electron microscopy
The harvested cochleae from the control, CCCP treatment, and 3 h after noise exposure groups were fixed in 2.5% glutaraldehyde overnight. The cochleae were dissected under a microscope and then were divided into small blocks of ∼1 × 1 × 1 mm. The HEI-OC1 cells from the control, CCCP treatment, and 12 h after 1 mM H2O2 treatment groups were collected and fixed in 2.5% glutaraldehyde overnight.
The tissue samples and cells were postfixed in 1% osmium tetroxide for 2.5 h, dehydrated through a graded alcohol series, and then embedded in plastic resin. To enable assessment of the whole cochlear spiral, 1-μm-thick sections were taken at each location and stained with toluidine blue for light microscopy. Ultrathin sections at a thickness of 70 nm were prepared, and the material was observed with a Tecnai G2 T12 transmission electron microscope (FEI) to reveal the ultrastructure of the hair cells.
Statistical analyses
All statistical analyses were performed using SPSS software (version 19.0; SPSS, Inc., Chicago, IL), and the results were visualized using GraphPad 8.0 software (San Diego, CA) for Windows. Values are expressed as the mean ± standard deviation. Statistical analyses were performed by using the two-tailed unpaired Student's t-test for pairwise comparisons or one-way repeated measures analysis of variance with post hoc comparison for multiple comparisons. A p value of <0.05 was considered to indicate a statistically significant difference. The electronic laboratory notebook was not used.
Supplementary Material
Abbreviations Used
- 4-HNE
4-hydroxynonenal
- ABR
auditory brain stem response
- ANOVA
analysis of variance
- ATP
adenosine triphosphate
- Baf A1
bafilomycin A1
- CCCP
carbonyl cyanide 3-chlorophenyl-hydrazone
- CCK-8
Cell Counting Kit-8
- CHX
cycloheximide
- DMSO
dimethyl sulfoxide
- H2O2
hydrogen peroxide
- HEI-OC1
cochlear House Ear Institute-Organ of Corti 1
- IF
immunofluorescence
- IHC
inner hair cell
- i.p.
intraperitoneal
- JC-1
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imida carbocyanine iodide
- LAMP1
lysosome-associated membrane glycoprotein 1
- LC3
microtubule-associated protein 1A/1B-light chain 3
- Lv-SESN2
lentivirus containing SESN2
- Mdivi-1
mitochondrial division inhibitor-1
- mtROS
mitochondrial reactive oxygen species
- NIHL
noise-induced hearing loss
- OHC
outer hair cell
- PBS
phosphate-buffered saline
- PBST
Triton X-100/phosphate-buffered saline
- PINK1
phosphatase and tensin homolog-induced putative kinase 1
- PSC
posterior semicircular canal
- ROS
reactive oxygen species
- SD
standard deviation
- SESN2
Sestrin 2
- shCtrl
short hairpin RNAs targeting control
- shRNAs
short hairpin RNAs
- shSESN2
short hairpin RNAs targeting SESN2
- SPL
sound pressure level
- TEM
transmission electron microscopy
- TOMM20
translocase of outer mitochondrial membrane 20
- TTS
temporary threshold shift
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- ULK1
Unc-51-like protein kinase 1
- WB
Western blot
Authors' Contributions
Y.L., S.L., and L.W.: Designed the study, performed the experiments, analyzed the data, and wrote the article. T.W., M.L., D.D., Y.C., C.W., X.L., S.Z., Z.Z., L.Z., M.C., and M.L.: Helped design the experiments, provided technical assistance and mouse models, analyzed the data, translated literature, and polished the article. T.L., X.S., and Y.Q.: Conceived, designed, and supervised this study, and managed collaborations and funding. All the authors critically read the article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was supported by grants from the National Nature Science Foundation of China (Grant No.81800916); the Special Fund for Clinical Scientific Research of Jieping Wu Foundation (No. 320.6750.18327); the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (Grant No.18KJA320011); the Nature Science Foundation of Xuzhou (Grant No.KC20177), and the Jiangsu Provincial University Fund (Grant No.19KJA560002).
Supplementary Material
References
- Alers S, Löffler AS, Wesselborg S, et al. The incredible ULKs. Cell Commun Signal 2012;10(1):7; doi: 10.1186/1478-811x-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anding AL, Baehrecke EH. Cleaning house: Selective autophagy of organelles. Dev Cell 2017;41(1):10–22; doi: 10.1016/j.devcel.2017.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basner M, Babisch W, Davis A, et al. Auditory and non-auditory effects of noise on health. Lancet 2014;383(9925):1325–1332; doi: 10.1016/S0140-6736(13)61613-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttger EC, Schacht J. The mitochondrion: A perpetrator of acquired hearing loss. Hear Res 2013;303:12–19; doi: 10.1016/j.heares.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler S, Goldberg H, Shinn-Cunningham B. Sensory coding and cognitive processing of sound in Veterans with blast exposure. Hear Res 2017;349:98–110; doi: 10.1016/j.heares.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budanov AV, Sablina AA, Feinstein E, et al. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science 2004;304(5670):596–600; doi: 10.1126/science.1095569. [DOI] [PubMed] [Google Scholar]
- Chen GD, Daszynski DM, Ding D, et al. Novel oral multifunctional antioxidant prevents noise-induced hearing loss and hair cell loss. Hear Res 2020;388:107880; doi: 10.1016/j.heares.2019.107880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng PW, Liu SH, Young YH, et al. Protection from noise-induced temporary threshold shift by D-methionine is associated with preservation of ATPase activities. Ear Hear 2008;29(1):65–75; doi: 10.1097/AUD.0b013e31815d635b. [DOI] [PubMed] [Google Scholar]
- Corti O, Lesage S, Brice A. What genetics tells us about the causes and mechanisms of Parkinson's disease. Physiol Rev 2011;91(4):1161–1218; doi: 10.1152/physrev.00022.2010. [DOI] [PubMed] [Google Scholar]
- Defourny J, Aghaie A, Perfettini I, et al. Pejvakin-mediated pexophagy protects auditory hair cells against noise-induced damage. Proc Natl Acad Sci USA 2019;116(16):8010–8017; doi: 10.1073/pnas.1821844116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmaghani S, Defourny J, Aghaie A, et al. Hypervulnerability to sound exposure through impaired adaptive proliferation of peroxisomes. Cell 2015;163(4):894–906; doi: 10.1016/j.cell.2015.10.023. [DOI] [PubMed] [Google Scholar]
- Ding WX, Yin XM. Mitophagy: Mechanisms, pathophysiological roles, and analysis. Biol Chem 2012;393(7):547–564; doi: 10.1515/hsz-2012-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebnoether E, Ramseier A, Cortada M, et al. Sesn2 gene ablation enhances susceptibility to gentamicin-induced hair cell death via modulation of AMPK/mTOR signaling. Cell Death Discov 2017;3:17024; doi: 10.1038/cddiscovery.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan DF, Shackelford DB, Mihaylova MM, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 2011;331(6016):456–461; doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez KA, Guo D, Micucci S, et al. Noise-induced cochlear synaptopathy with and without sensory cell loss. Neuroscience 2020;427:43–57; doi: 10.1016/j.neuroscience.2019.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio M, Trinei M, Migliaccio E, et al. Hydrogen peroxide: A metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Cell Biol 2007;8(9):722–728; doi: 10.1038/nrm2240. [DOI] [PubMed] [Google Scholar]
- Guo JY, He L, Qu TF,et al. Canalostomy as a surgical approach to local drug delivery into the inner ears of adult and neonatal mice. J Vis Exp 2018;135:57351; doi: 10.3791/57351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SH, Korm S.. GCA links TRAF6-ULK1-dependent autophagy activation in resistant chronic myeloid leukemia. Autophagy 2019;15(12):2076–2090; doi: 10.1080/15548627.2019.1596492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson D, Bielefeld EC, Harris KC, et al. The role of oxidative stress in noise-induced hearing loss. Ear Hear 2006;27(1):1–19; doi: 10.1097/01.aud.0000191942.36672.f3. [DOI] [PubMed] [Google Scholar]
- Hu BH, Henderson D, Nicotera TM. Extremely rapid induction of outer hair cell apoptosis in the chinchilla cochlea following exposure to impulse noise. Hear Res 2006;211(1–2):16–25; doi: 10.1016/j.heares.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Jiao H, Su GQ, Dong W, et al. Chaperone-like protein p32 regulates ULK1 stability and autophagy. Cell Death Differ 2015;22(11):1812–1823; doi: 10.1038/cdd.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen T, Lamark T. Selective autophagy mediated by autophagic adapter proteins. Autophagy 2011;7(3):279–296; doi: 10.4161/auto.7.3.14487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo JH, Dorsey FC, Joshi A, et al. Hsp90-Cdc37 chaperone complex regulates Ulk1-and Atg13-mediated mitophagy. Mol Cell 2011;43(4):572–585; doi: 10.1016/j.molcel.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinec GM, Webster P, Lim DJ, et al. A cochlear cell line as an in vitro system for drug ototoxicity screening. Audiol Neurootol 2003;8(4):177–189; doi: 10.1159/000071059. [DOI] [PubMed] [Google Scholar]
- Kim H, An S, Ro SH, et al. Janus-faced Sestrin2 controls ROS and mTOR signalling through two separate functional domains. Nat Commun 2015;6:10025; doi: 10.1038/ncomms10025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Seo D, Kim SJ, et al. The deubiquitinating enzyme USP20 stabilizes ULK1 and promotes autophagy initiation. EMBO Rep 2018a;19(4):e44378; doi: 10.15252/embr.201744378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Bae SH, Ryu JC, et al. SESN2/sestrin2 suppresses sepsis by inducing mitophagy and inhibiting NLRP3 activation in macrophages. Autophagy 2016;12(8):1272–1291; doi: 10.1080/15548627.2016.1183081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Jung G, Kim S, et al. Novel peptide vaccine GV1001 rescues hearing in kanamycin/furosemide-treated mice. Front Cell Neurosci 2018b;12:3;doi: 10.3389/fncel.2018.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Abdel-Aziz AK, Abdelfatah S, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy 2021;17(1):1–382; doi: 10.1080/15548627.2020.1797280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: Cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci 2009;29(45):14077–14085; doi: 10.1523/jneurosci.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Shaha C. SESN2 facilitates mitophagy by helping Parkin translocation through ULK1 mediated Beclin1 phosphorylation. Sci Rep 2018;8(1):615; doi: 10.1038/s41598-017-19102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu M, Lindsten T, Yang CY, et al. Ulk1 plays a critical role in the autophagic clearance of mitochondria and ribosomes during reticulocyte maturation. Blood 2008;112(4):1493–1502; doi: 10.1182/blood-2008-02-137398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Budanov AV, Karin M. Sestrins orchestrate cellular metabolism to attenuate aging. Cell Metab 2013;18(6):792–801; doi: 10.1016/j.cmet.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Bing D, Wang S, et al. Sleep deprivation modifies noise-induced cochlear injury related to the stress hormone and autophagy in female mice. Front Neurosci 2019;13:1297; doi: 10.3389/fnins.2019.01297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ma Y, Song L, et al. SIRT3 deficiency exacerbates p53/Parkin-mediated mitophagy inhibition and promotes mitochondrial dysfunction: Implication for aged hearts. Int JMol Med 2018;41(6):3517–3526; doi: 10.3892/ijmm.2018.3555. [DOI] [PubMed] [Google Scholar]
- Lin H, Xiong H, Su Z, et al. Inhibition of DRP-1-dependent mitophagy promotes cochlea hair cell senescence and exacerbates age-related hearing loss. Front Cell Neurosci 2019;13:550; doi: 10.3389/fncel.2019.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HW, Furman AC, Kujawa SG, et al. Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift. J Assoc for Res Otolaryngol 2011;12(5):605–616; doi: 10.1007/s10162-011-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LiochevSI. Reactive oxygen species and the free radical theory of aging. Free Radic Biol Med 2013;60:1–4; doi: 10.1016/j.freeradbiomed.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Lismont C, Nordgren M, Van Veldhoven PP, et al. Redox interplay between mitochondria and peroxisomes. Front Cell Dev Biol 2015;3:35; doi: 10.3389/fcell.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Wu J, Qiao L, et al. Sestrin 2 attenuates sepsis-associated encephalopathy through the promotion of autophagy in hippocampal neurons. J Cell Mol Med 2020;24(12):6634–6643; doi: 10.1111/jcmm.15313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JM, Brown JN, Schacht J. 8-Iso-prostaglandin F(2alpha), a product of noise exposure, reduces inner ear blood flow. Audiol Neurootol 2003;8(4):207–221; doi: 10.1159/000071061. [DOI] [PubMed] [Google Scholar]
- Münzel T, Sørensen M, Schmidt F, et al. The adverse effects of environmental noise exposure on oxidative stress and cardiovascular risk.Antioxid Redox Signal 2018;28(9):873–908; doi: 10.1089/ars.2017.7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D, Tanaka A, Suen DF, et al. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 2008;183(5):795–803; doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazio F, Strappazzon F, Antonioli M, et al. mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat Cell Biol 2013;15(4):406–416; doi: 10.1038/ncb2708. [DOI] [PubMed] [Google Scholar]
- Ohinata Y, Miller JM, Altschuler RA, et al. Intense noise induces formation of vasoactive lipid peroxidation products in the cochlea. Brain Res 2000;878(1–2):163–173; doi: 10.1016/s0006-8993(00)02733-5. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Wright JS, Dugan LL. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol Neurootol 1999;4(5):229–236; doi: 10.1159/000013846. [DOI] [PubMed] [Google Scholar]
- Park MH, Lee HS, Song JJ, et al. Increased activity of mitochondrial respiratory chain complex in noise-damaged rat cochlea. Acta Otolaryngol 2012;132(Suppl 1):S134–S141; doi: 10.3109/00016489.2012.659755. [DOI] [PubMed] [Google Scholar]
- Pasha M, Eid AH, Eid AA, et al. Sestrin2 as a novel biomarker and therapeutic target for various diseases. Oxid Med Cell Longev 2017;2017:3296294; doi: 10.1155/2017/3296294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro SH, Semple IA, Park H, et al. Sestrin2 promotes Unc-51-like kinase 1 mediated phosphorylation of p62/sequestosome-1. FEBS J 2014;281(17):3816–3827; doi: 10.1111/febs.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorrano L. Opening the doors to cytochrome c: Changes in mitochondrial shape and apoptosis. Int JBiochem Cell Biol 2009;41(10):1875–1883; doi: 10.1016/j.biocel.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Shin BY, Jin SH, Cho IJ, et al. Nrf2-ARE pathway regulates induction of Sestrin-2 expression. Free Radic Biol Med 2012;53(4):834–841; doi: 10.1016/j.freeradbiomed.2012.06.026. [DOI] [PubMed] [Google Scholar]
- Vazquez-Martin A, Cufi S, Corominas-Faja B, et al. Mitochondrial fusion by pharmacological manipulation impedes somatic cell reprogramming to pluripotency: New insight into the role of mitophagy in cell stemness. Aging 2012;4(6):393–401; doi: 10.18632/aging.100465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. Mitochondria and cancer. Nat RevCancer 2012;12(10):685–698; doi: 10.1038/nrc3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhao N, Yan K, et al. Inner hair cell ribbon synapse plasticity might be molecular basis of temporary hearing threshold shifts in mice. Int J Clin Exp Patho 2015;8(7):8680–8691. PMID: . [PMC free article] [PubMed] [Google Scholar]
- Wang P, Wang L, Lu J, et al. SESN2 protects against doxorubicin-induced cardiomyopathy via rescuing mitophagy and improving mitochondrial function. J Mol Cell Cardiol 2019;133:125–137; doi: 10.1016/j.yjmcc.2019.06.005. [DOI] [PubMed] [Google Scholar]
- Wu F, Xiong H, Sha S. Noise-induced loss of sensory hair cells is mediated by ROS/AMPKα pathway. Redox Biol 2020;29:101406; doi: 10.1016/j.redox.2019.101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane H, Nakai Y, Takayama M, et al. Appearance of free radicals in the guinea pig inner ear after noise-induced acoustic trauma. Eur Arch Otorhinolaryngol 1995;252(8):504–508; doi: 10.1007/bf02114761. [DOI] [PubMed] [Google Scholar]
- Yamashita D, Jiang HY, Le Prell CG, et al. Post-exposure treatment attenuates noise-induced hearing loss. Neuroscience 2005;134(2):633–642; doi: 10.1016/j.neuroscience.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Yamashita D, Jiang HY, Schacht J, et al. Delayed production of free radicals following noise exposure. Brain Res 2004;1019(1–2):201–209; doi: 10.1016/j.brainres.2004.05.104. [DOI] [PubMed] [Google Scholar]
- Yin J, Guo J, Zhang Q, et al. Doxorubicin-induced mitophagy and mitochondrial damage is associated with dysregulation of the PINK1/parkin pathway. Toxicol In Vitro 2018;51:1–10; doi: 10.1016/j.tiv.2018.05.001. [DOI] [PubMed] [Google Scholar]
- Yuan H, Wang X, Hill K, et al. Autophagy attenuates noise-induced hearing loss by reducing oxidative stress. Antioxid Redox Signal 2015;22(15):1308–1324; doi: 10.1089/ars.2014.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Sun W, Li J, et al. Loss of sestrin 2 potentiates the early onset of age-related sensory cell degeneration in the cochlea. Neuroscience 2017;361:179–191; doi: 10.1016/j.neuroscience.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yan H, Yuan Y, et al. Cerebral ischemia-reperfusion-induced autophagy protects against neuronal injury by mitochondrial clearance. Autophagy 2013a;9(9):1321–1333; doi: 10.4161/auto.25132. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Wu XQ, Deng R, et al. Upregulation of sestrin 2 expression via JNK pathway activation contributes to autophagy induction in cancer cells. Cell Signal 2013b;25(1):150–158; doi: 10.1016/j.cellsig.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Han Z, Naveena K, et al. ROS-responsive nanoparticle as a berberine carrier for OHC-targeted therapy of noise-induced hearing loss.ACS Appl Mater Interfaces 2021;13(6):7102–7114; doi: 10.1021/acsami.0c21151. [DOI] [PubMed] [Google Scholar]
- Zhu J, Wang KZ, Chu CT. After the banquet: Mitochondrial biogenesis, mitophagy, and cell survival. Autophagy 2013a;9(11):1663–1676; doi: 10.4161/auto.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Liang C, Chen J, et al. Active cochlear amplification is dependent on supporting cell gap junctions. Nat Commun 2013b;4:1786; doi: 10.1038/ncomms2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang W, Li T, Wang C, et al. Berberine exerts antioxidant effects via protection of spiral ganglion cells against cytomegalovirus-induced apoptosis. Free Radic Biol Med 2018;121:127–135; doi: 10.1016/j.freeradbiomed.2018.04.575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.