Abstract
Introduction
MRI bone surface area and femoral bone shape (B-score) measures have been employed as quantitative endpoints in DMOAD clinical trials. Computerized Tomography (CT) imaging is more commonly used for 3D visualization of bony anatomy due to its high bone-soft tissue contrast. We aimed to compare CT and MRI assessments of 3D imaging biomarkers.
Methods
We used baseline and 24-month image data from the IMI-APPROACH 2-year prospective cohort study. Femur and tibia were automatically segmented using active appearance models, a machine-learning method, to measure 3D bone shape, area and 3D joint space width (3DJSW). Linear regression was used to test for correlation between measures. Limits of agreement and bias were tested using Bland-Altman analysis.
Results
CT-MR pairs of the same knee were available from 434 participants (78% female). B-scores from CT and MR were strongly correlated (CCC = 0.967) with minimal bias of 0.1 (SDD = 0.227). Area measures were also correlated but showed a consistent bias (MR smaller). 3DJSW showed different biases (MR larger) in both lateral and medial compartments.
Discussion
The strong correlation and small B-score bias suggests that B-score may be measured reliably using either modality. It is likely that the bone surface identified using MR and CT will be at slightly different positions within the bone/cartilage boundary. The negative bone area bias suggests the MR bone boundary is inside the CT boundary producing smaller areas for MR, consistent with the positive 3DJSW bias. The lateral-medial 3DJSW difference is possibly due to a difference in knee pose during acquisition (extended for CT, flexed for MR).
Trial registration
Supplementary Information
The online version contains supplementary material available at 10.1186/s12891-023-06187-2.
Keywords: Osteoarthritis, B-score, Biomarker, Machine learning, MRI, CT
Background
Osteoarthritis (OA) of the knee is a serious disease resulting in pain, loss of function and reduced quality of life; it is a leading cause of disability among older adults [1–3]. The pathophysiology of OA involves multiple tissues, with deterioration of both cartilage and bone considered integral to the OA process [4, 5]. The primary structural assessment of OA is often based on radiographs in which pathologic changes such as osteophytes are taken as the first signs of disease. Other bony changes (sclerosis and deformation of the bone contour) may be visualized at later stages of the disease and are included with osteophytes in the semiquantitative Kellgren- Lawrence grade (KLG) [6], the most commonly-used OA radiographic scoring system. However, radiography is relatively insensitive in detecting earlier changes and is imprecise due to its dependence on both acquisition method and reader [7].
The use of Magnetic Resonance Imaging (MRI) in the study of OA has not only enabled a more thorough understanding of the anatomy and pathology of the OA joint in three-dimensions, it also provides a detailed visualization of multiple tissues with excellent soft-tissue contrast. Several MRI studies using semiquantitative scoring systems have demonstrated pathology associated with OA in knees with normal radiographs. The presence of MRI-detected osteophytes has been shown to occur in 74% of knees with normal radiographs and the prevalence of “any abnormality” has been found to be as high as 89% [8].
Quantitative assessment of MR images can provide direct measurement of cartilage and bone [9], and a number of quantitative 3D MR imaging biomarkers for the assessment of knee OA have been developed and validated using large MR image datasets [10]. MRI bone morphology changes in subchondral surface area [11, 12] and parameterized shape descriptions [13, 14] have been employed as endpoints in DMOAD clinical trials [15, 16].
Statistical shape modelling (SSM), a type of supervised machine-learning, allows for the parametrization of complex 3D anatomical shapes such as the knee [17]. The method can be employed both to describe and compare knee shape; and to automatically search for and segment a knee shape in an image, enabling the analysis of very large image datasets [18]. Measurement of bone area and shape changes from MR images using SSMs has been shown to predict radiographic onset of OA [19], is associated with radiographic structural progression [20] and discriminates knees with OA from non-OA [21].
Recently, Bowes et al. [13] introduced a method for the reduction of the 3D shape of femur to a single metric value termed the “B-score”. This statistical z-score metric compares the shape of non-OA and OA knees on a continuous linear scale with an origin at 0 (KLG 0 middle-aged knee) and a unit scale based on the standard deviation of these KLG 0 knees. The approximate observable B-score range is from −3 to +7 with advancing OA in the positive direction. The B-score has been shown to be an objective, automated assessment of OA status with clinical risk defined for current and future pain, functional limitation and Total Knee Replacement (TKR) [13].
Radiographic Joint space width (JSW) measured between the femoral and tibial margins on a weight-bearing x-ray is widely accepted as an indicator of knee joint health. Because the radiograph is a 2D projection of a 3D structure, measurements are highly dependent on the acquisition conditions, such as the position of the knee, knee flexion angle, and the alignment of the X-ray beam with the tibial plateau. Even with carefully followed protocols using customized knee positioning devices and optimal acquisition conditions, conventional radiographs can remain insensitive, inaccurate, and have poor concurrent validity for knee OA features [22]. In contrast, the measurement of 3D JSW using tomographic imaging is unencumbered by the problem of overlapping anatomy and reproducible anatomical alignment. 3D JSW therefore represents a relatively novel OA imaging biomarker that is readily available from MR images that are acquired for quantitative assessment of cartilage and bone in DMOAD trials. Measurement of 3D JSW has been previously demonstrated using weight-bearing CT images [23, 24] though this has not been performed using supine MR and CT images.
While the widespread use of MRI for clinical trials and research in OA has led to bone imaging biomarkers being developed for this modality, MR is more usually employed for soft-tissue and trabecular bone evaluation since MR imaging pulse sequences usually depict cortical bone as a signal void. Computerized Tomography (CT) imaging is more commonly used for 3D visualization of bony anatomy due to its high bone-soft tissue contrast [25]. In addition, CT images are not subject to the geometric distortion that can be caused in MR images due to magnetic field inhomogeneities [26], a potential problem that is addressed in DMOAD clinical trials by the careful and consistent positioning of the knee near the isocenter of the magnet, where the field is most homogeneous.
To test the robustness to the choice of imaging modality of the B-score as a measure of femoral cortical bone shape in DMOAD clinical trials, we compared the results of automated analysis of CT-MR image pairs acquired at baseline and 24-month timepoints. We also compared a similar bone shape z-score of the tibia, and measures of bone area from femur and tibia, and medial and lateral tibiofemoral 3DJSW.
Methods
Subject image data
We used image data from the IMI “Applied Public-Private Research enabling OsteoArthritis Clinical Headway” (APPROACH) study. APPROACH is an exploratory, European, 5-centre, 2-year prospective longitudinal cohort study. It includes clinical, imaging, biomechanical and biochemical parameters, in a cohort of 297 participants (age; 66.5 ± 7.1, female; 230 (77%), BMI; 28.1 ± 5.3) recruited primarily from prior European OA cohorts using machine learning models based on retrospective patient data to exhibit a high likelihood of radiographic JSW loss and/or knee pain over the course of the study [27]. The study has been approved by the Ethical Committees of the participating countries and has been registered in Clinicaltrials.gov (NCT03883568).
Low-dose CT images (120kVp, 220 mAs, 0.625 mm slices, 0.3 mm in-plane resolution, medium kernel) were acquired at 5 sites for 271 participants at baseline and 215 participants at 24-months. Both knees were positioned to be as straight as possible, with the feet on a wedge to produce around 15o of internal rotation at the hip. These CT images were matched with sagittal 3D WATS, 3D FFE, or 3D FLASH MR images (1.5 mm slice thickness; 0.31 mm in-plane resolution; TR 17 ms, TE 7 ms, FA 12–15° with fat-saturation or water excitation) of the single index (left or right) knee for each subject acquired using 1.5 T or 3 T MR systems. MR acquisition protocol was to rotate the leg so that the toes and patella faced upward, elevate knee by putting cushions below it so that the center of the knee is place in the center of the coil and avoid scanning the knee in a fully extended or hyperextended position. CT and MR images were acquired within a mean of 12 (median: 0, max: 162) days of each other. Additional test-retest sagittal 3D WATS, 3D FFE, or 3D FLASH MR images with repositioning were available from 37 participants at baseline (8), 6-month (16) and 24-month (13) timepoints. KLG was read centrally from standard weight-bearing x-rays taken at the screening visit [27].
Statistical shape modelling & image search
Femur and tibia bones were automatically segmented from MR images using active appearance models (AAMs), a type of SSM trained to search images, provided by Imorphics (Manchester, UK). AAMs are proven technology that can segment knee bone surfaces with sub-millimeter accuracy as has been described previously [21, 28]. AAMs were constructed using a training set, from 3D high-resolution water-excitation MR images, selected to provide examples of all stages of OA [29].
A similar AAM was constructed for the automated search of CT images with a second-stage refinement using convolutional neural network machine-learning [30]. The AAM was trained on manual segmentations of 122 CT images displaying varying KLG grades, and the CNN was trained on a separate set of 3500 pre-operative CT images from a database of CT scans acquired robotic total knee replacement surgery [31]. Segmentation model accuracy was validated using another independent set of 1097 pre-operative images and expert manual segmentations. Measurement accuracy was assessed using the mean absolute point-to-surface distance between model and manual segmentations. Mean segmentation errors were 0.12 mm (standard deviation (SD)) 0.04 mm (mm) for femora and 0.13 (0.08) mm for tibiae.
Bone shape
We constructed an ‘OA vector’, defined as the line passing through the mean shape of a population with OA (OA Group, defined as all knees with KLG ≥2 at all four time points of 0, 1, 2 and 4 years) and a population without OA (Non-OA Group, defined as those with KLG of 0 at each of the same time points). For the femur, distances along the OA vector have previously been termed ‘B-score’ [13], with the origin (B-score 0) defined as the mean shape of the Non-OA Group for each sex. The B-score has the form of a statistical z-score so that 1 unit is defined as 1 SD of the Non-OA Group along the OA vector (positive values towards the OA Group) [13]. A similar bone shape z-score was constructed on an OA-vector for the tibia for each sex.
We used the same femur and tibia OA-vectors constructed using MR images to produce both the MR and CT image bone shape results. For the femur, this was the previously described shape vector used to produce the B-score. The OA-vector for the tibia has also been previously described [12]. When analyzing a CT image, the resulting bone shape surface from the CT AAM search was searched for a best fit using a version of the MR AAM trained to fit to a predefined surface shape. This process produces an MR AAM shape instance with the correct MR model landmark points but with the shape of the CT image search result. This MR model shape instance can then be projected onto the previously defined MR OA-vectors for femur or tibia. To determine the accuracy of this step, we measured the mean of absolute point-to-surface distances between the CT AAM search result and the derived MR AAM shape instance across all available baseline and 24-month CT-MR image pairs.
Bone area
Anatomical regions of total area of subchondral bone (tAB) [32] representing medial and lateral femur (MF, LF), medial and lateral femoral trochlea (TrFMed, TrFLat) and medal and lateral tibia (MT, LT) were outlined on the mean AAM bone shape as previously described [21]. The boundaries of the MF.tAB and LF.tAB regions and trochlea grove were defined as a line on the bone corresponding to the anterior edge of the medial or lateral meniscus in the mean shape. During auto-segmentation with AAMs, these regions are automatically propagated to each bone surface, allowing for the measurement of anatomically corresponded tAB regions on the knee bone surfaces from each subject. The six regions originally defined on the surface mesh of the mean MR AAM shape were then projected to the surface mesh of mean CT AAM shape by registering the two surface meshes using Iterative Closest Point (ICP) algorithm [33].
3D joint space width
3D JSW measurements (3DJSW) were produced for all the landmark points enclosed by central regions defined on the medial and lateral tibial plateaus (Fig. 1). Each region consists of approximately 2000 landmarks and were defined on the surface mesh of the mean shape of the MR AAM as the cLT and cMT regions previously described anatomically for manual cartilage measurement [34]. These regions were then projected to the surface mesh of mean CT AAM shape by registering the two surface meshes using Iterative Closest Point (ICP) algorithm [33]. During auto-segmentation with AAMs, these regions are automatically propagated to each bone surface, allowing for the measurement of 3DJSW at anatomically corresponding positions in each subject. Measurements at each point were made between the segmented tibial and femoral mesh surfaces along a normal to the tibial surface. An average 3DJSW was then computed from the measurements at all points in the medial or lateral region.
Fig. 1.
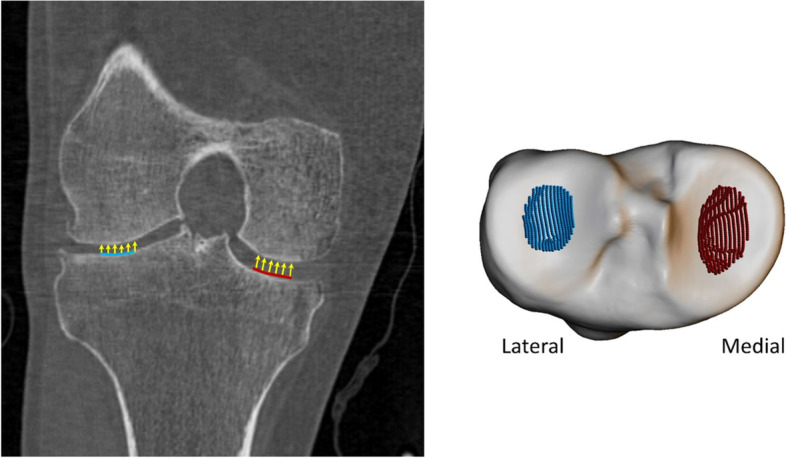
Three-dimensional measurement of central medial and lateral mean joint space width (3DJSW). The central medial region is shown in red, and the lateral region is shown in blue (right figure). The coronal view of a typical knee is shown in the left figure, which shows where these two regions are located, and how measurements are taken, normal to the 3D tibial surface
Statistical analysis
We used linear regression and Lin’s Concordance Correlation Coefficient (CCC) [35] to test for correlation between the MR and CT measures of bone shape, area and 3DJSW. Limits of agreement and systematic bias was tested using Bland-Altman analysis of these measures. Analysis was performed on both the full dataset incorporating all available KLG scores and on a limited subset incorporating only KLG 2 & 3 scores, since these would typically be chosen for inclusion in a DMOAD trial. Smallest detectable difference (SDD) of the two bone shape measures was computed from test-retest image pairs using Bland-Altman analysis. Images acquired from the same subject but at baseline or 24-month timepoints were treated as independent.
Results
Baseline and 24-month CT-MR image pairs of the same knee laterality were available from 231 and 203 participants respectively, resulting in 434 CT-MR image pairs for analysis. In the analysis set, there were 338 female knees (78%). Kellgren-Lawrence Grading for the analyzed (index) knee in these participants was KLG 0 (19%); KLG 1 (31%); KLG 2 (30%); KLG 3 (16%); KLG 4 (3%) with 5 knees ungraded (1%), providing coverage across the OA spectrum. The limited subset incorporating KLG 2 & 3 knees comprised 197 CT-MR pairs with KLG 2 (65%) and KLG 3 (35%).
Mean time between CT and MR image acquisition was 10 (median: 0 days; 95th percentile: 116) days. At least half of the MR-CT scans were acquired on the same day, and 95% were acquired within 4 months of each other, a period of time which is unlikely to result in measurable anatomical change. In general, where there was a time between acquisition of modalities, the CT acquisition came later. SDD of the B-score measure from test-retest comparison was 0.227 which is comparable to the SDD of 0.251 measured previously [13]. For the tibia z-score, SDD was 0.373. The mean (SD) of absolute point-to-surface fitting errors between the CT AAM search result and the derived MR AAM shape instance across all available baseline and 24-month CT-MR image pairs was 0.098 (0.022) mm.
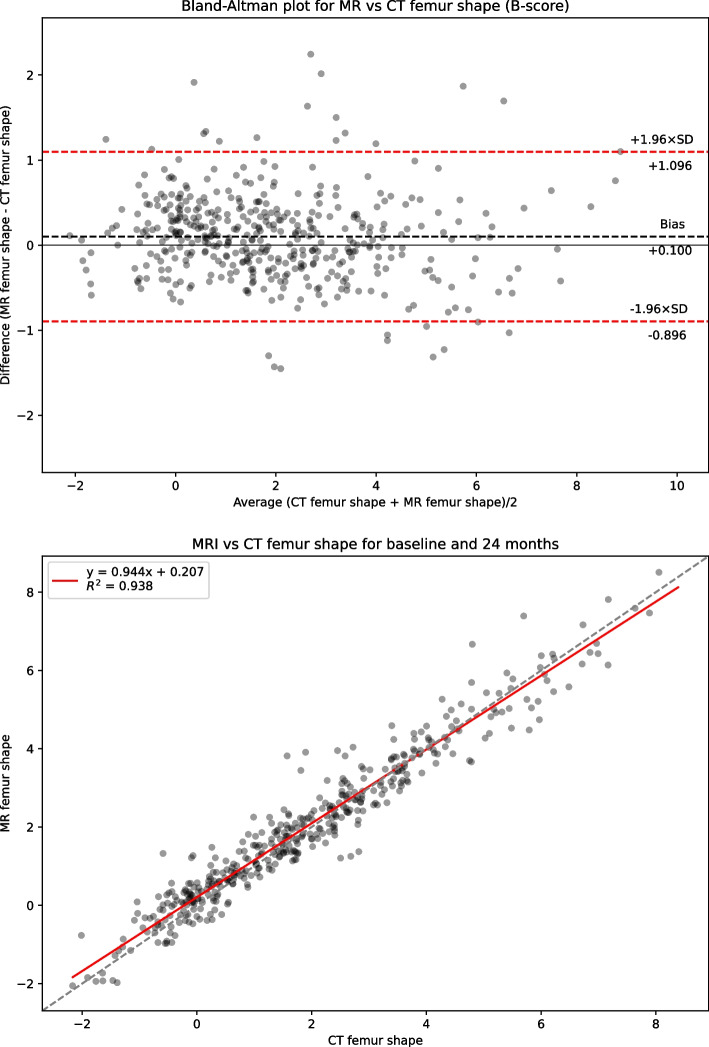
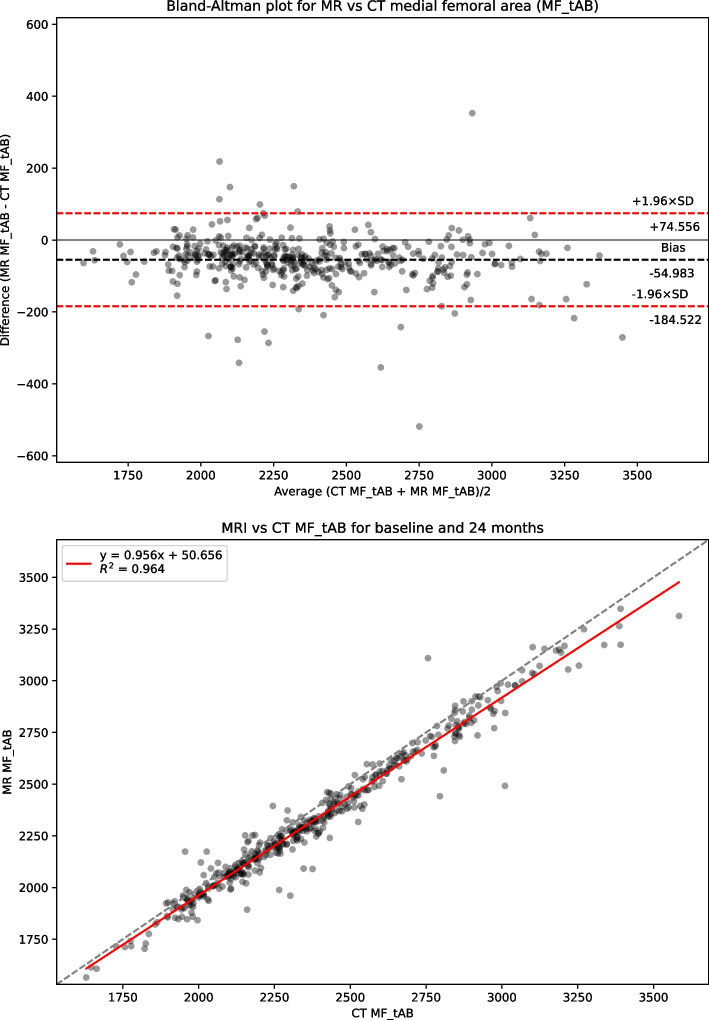
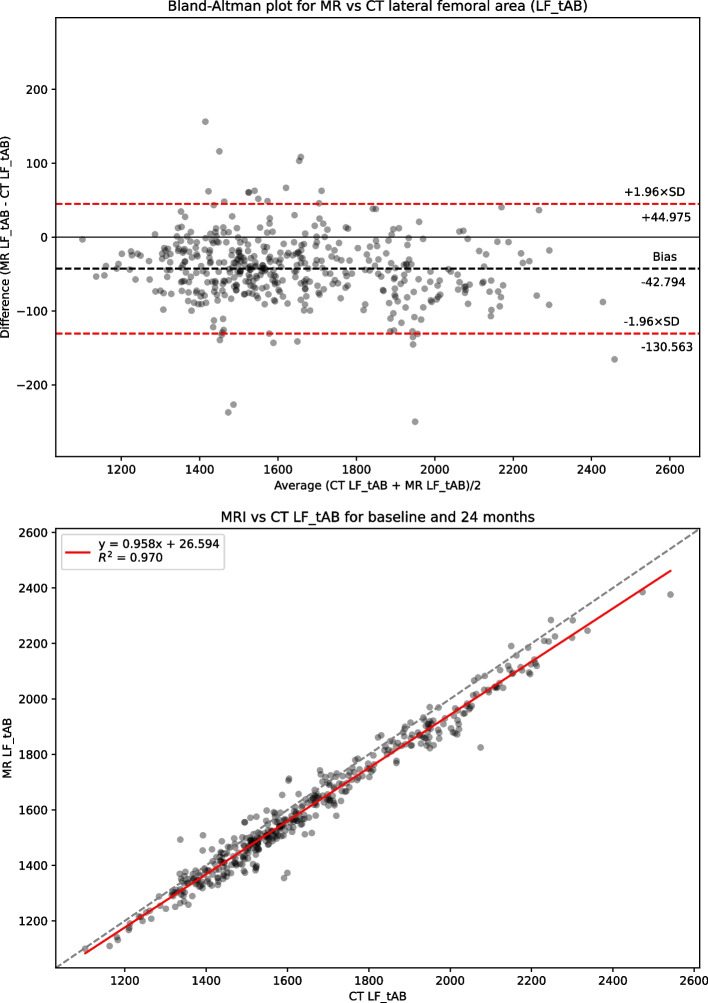
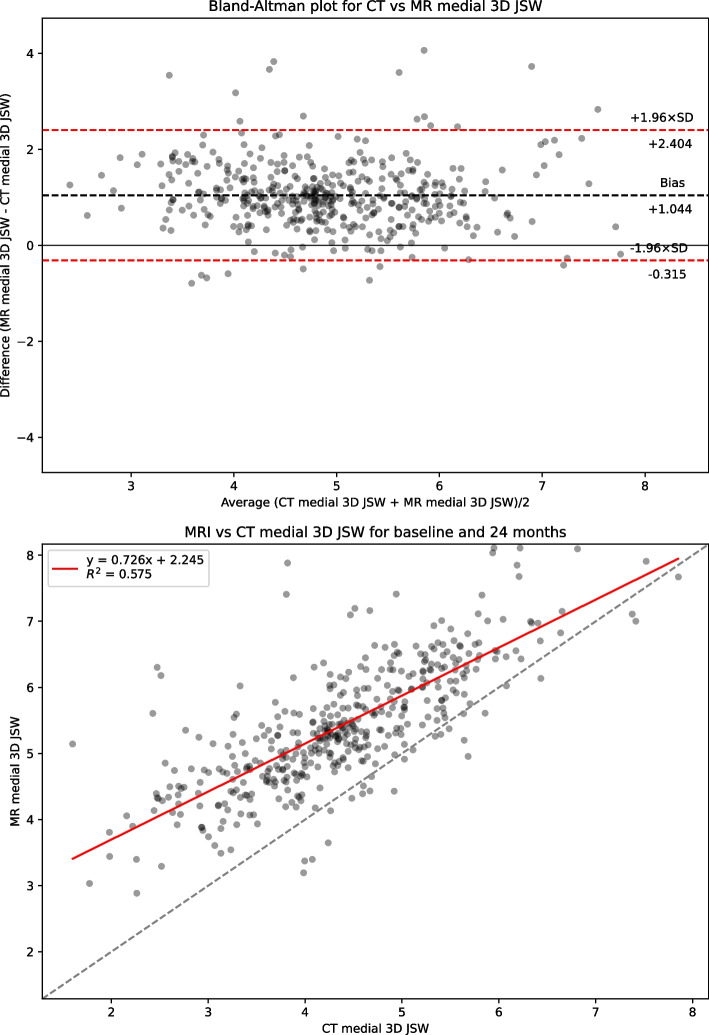
Table 1 presents summary statistics for shape, area and 3DJSW measures: Bland-Altman bias with 95% confidence intervals, Bland-Altman limits of agreement, linear regression coefficient of determination R2, and Concordance Correlation Coefficient (CCC) for the full dataset. Summary statistics for the limited KLG 2 &3 dataset is presented in supplemental Table S1. Figure 2 shows linear regression and Bland-Altman analysis for B-score. Figures 3 and 4 show linear regression and Bland-Altman analysis for bone areas MF.tAB and LF.tAB. Figures 5 and 6 show linear regression and Bland-Altman analysis for medial and lateral 3DJSW. Results for trochlea bone area, tibia shape, and tibia bone area are provided in supplemental Figs. S1-S5.
Table 1.
Bland-Altman bias (MR minus CT), limits of agreement and linear regression statistics for the comparison of various MR and CT derived measures for the full dataset (KLG 0–4)
| Measure | Bland-Altman bias [95% CI] | Bland-Altman limits of agreement | R2 | CCC | |
|---|---|---|---|---|---|
| Lower [95% CI] | Upper [95% CI] | ||||
|
Femur shape B-score |
0.100 [0.052, 0.140] |
−0.896 [−0.979, −0.813] |
1.096 [1.013, 1179] |
0.938 | 0.967 |
|
Tibia shape z-score |
−0.424 [−0.475, −0.374] |
−1.471 [−1.558, −1.384] |
0.622 [0.535, 0.709] |
0.910 | 0.924 |
|
MF.tAB (mm2) |
−54.983 [−61.218, −48.748] |
−184.522 [−195.322, −173.722] |
74.556 [63.756, 85.356] |
0.964 | 0.969 |
|
LF.tAB (mm2) |
−42.792 [−47.019, −38.569] |
−130.563 [−137.880, −123.245] |
44.975 [37.657, 52.292] |
0.970 | 0.971 |
|
MT.tAB (mm2) |
−5.858 [−9.235, −2.482] |
−75.997 [−81.84, −70.149] |
64.380 [58.432, 70.128] |
0.962 | 0.978 |
|
LT.tAB (mm2) |
−21.329 [−23.896, −18.762] |
−74.659 [−79.106, −70.213] |
32.001 [27.555, 36.447] |
0.965 | 0.968 |
|
TrFMed.tAB (mm2) |
−28.346 [−30.714, −25.978] |
−77.544 [−81.646, −73.443] |
20.853 [+16.751, +24.955] |
0.932 | 0.918 |
|
TrFLat.tAB (mm2) |
−39.387 [−43.002, −35.773] |
−114.477 [−120.738, −108.217] |
35.703 [29.442, 41.963] |
0.947 | 0.945 |
|
Medial 3DJSW (mm) |
1.044 [0.979, 1.110] |
−0.315 [−0.428, −0.202] |
2.404 [2.290, 2.517] |
0.575 | 0.635 |
|
Lateral 3DJSW (mm) |
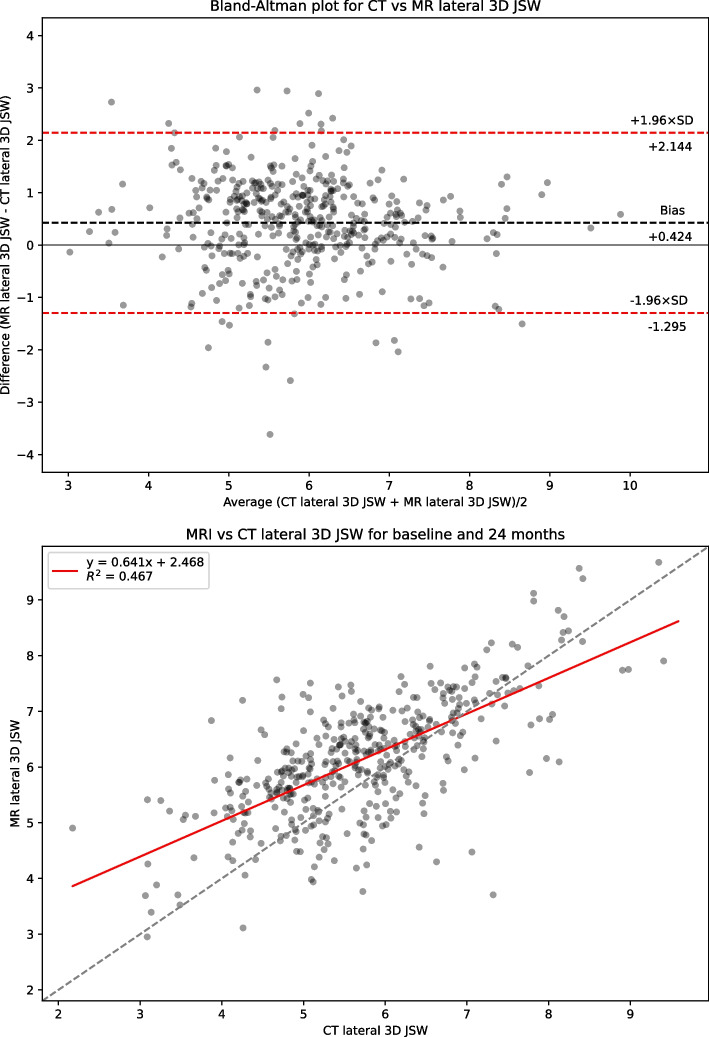
0.424 [0.342, 0.507] |
−1.295 [−1.438, −1.151] |
2.144 [2.000, 2.287] |
0.467 | 0.489 |
Fig. 2.
CT vs MRI B-score for the full dataset (KLG 0–4). Top: Bland-Altman (MR minus CT) plot; Bottom: linear regression
Fig. 3.
CT vs MRI bone area MF.tAB for the full dataset (KLG 0–4). Top: Bland-Altman (MR minus CT) plot; Bottom: linear regression
Fig. 4.
CT vs MRI bone area LF.tAB for the full dataset (KLG 0–4). Top: Bland-Altman (MR minus CT) plot; Bottom: linear regression
Fig. 5.
CT vs MRI medial 3DJSW for the full dataset (KLG 0–4). Top: Bland-Altman (MR minus CT) plot; Bottom: linear regression
Fig. 6.
CT vs MRI lateral 3DJSW. Top: Bland-Altman (MR minus CT) plot; Bottom: linear regression
B-scores measured using CT or MR images were strongly correlated (CCC = 0.967) and showed good agreement with a small MR positive bias of 0.100 [95% CI: 0.052, 0.14]. Limits of agreement (LOA) for B-score were − 0.896 and + 1.096 which is significantly higher range than the SDD of +/− 0.227. Although the tibia z-score measures were also highly correlated (CCC = 0.924), the agreement was less good with a negative bias of −0.424 [−0.475, −0.374]. All bone area measures were also highly correlated between CT and MR with a consistent negative bias (MR measures smaller). The 3DJSW measures were less well correlated and both larger were for MR than for CT but had a different bias in the lateral of 1.044 [0.979, 1.110] mm and compared to a bias of 0.424 [0.342, 0.507] mm in the medial side. Bland-Altman plots for all measures showed little or no apparent correlation and so did not indicate any association between measurement values and bias.
Discussion
B-score measures from MR or CT images were strongly correlated and showed good agreement with a relatively small bias of 0.1, suggesting that bone shape may be reliably measured using either modality. This bias could be applied to CT B-score measures as a small positive correction. However, the bias is considerably smaller than the SDD of 0.227 measured from MR images, and the linear regression model crosses the unity line indicating that this bias is positive (MR larger) for the lower B-scores and negative (CT larger) for the higher B-scores. Therefore, this small correction is probably not significant in most practical applications when measuring B-scores using CT images. In the limited subset analysis of KLG 2&3 knees, the B-score bias was reduced to 0.049, suggesting that for clinical trials incorporating such a a cohort, the potential difference obtained for the measure from the two modalities is probably not significant. In fact, this represents about 12 months of expected B-score change in a non-OA cohort [16].
The Bland-Altman limits of agreement between CT and MR measurements are relatively large compared to the SDD derived from the MR test-retest image data. This increased variability when comparing B-scores measures from CT and MR is unlikely to be due to the slightly higher sample variance in the CT measurement (4.15 compared to 3.95 for MR measurement). An observable difference in variance would produce correlation structure in the Bland-Altman plot, in which most of the big differences observed between CT and MR would occur when CT measurement is at one of the extremes of its range, since it is far less likely for MR measurement to be a nearby value; and this structure is not seen. Therefore, it is more likely that the increased variability is due to CT and MR surfaces being somewhat different due to the reconstructed CT and MR bone surfaces being somewhat different. A probable cause of this is geometric distortion by MR magnetic field inhomogeneity which would cause variability across participants and across imaging sites.
In contrast to the B-score, the tibia shape z-scores were somewhat lower from MR images, especially for the higher shape scores, with a bias of 0.424. Shape measures of the proximal tibia are somewhat more variable than those of the distal femur, with an SDD of 0.373, possibly because of the comparatively smaller surface area of this bone. Analysis of the tibia bone has been less well developed, and the reason for the bias here warrants further investigation.
CT and MR bone area measures also were all strongly correlated and all exhibited a negative bias indicating that the measures from MR images were consistently smaller than those from CT images. Because the two modalities measure different physical properties of materials, it is likely that the bone surface identified using MR and CT will be at slightly different positions within the bone/soft-tissue (cartilage) boundary. While the bone surface in an MR image will be indicated by a boundary between cortical bone with little water content, and cartilage which has a higher water content, the bone surface in a CT image is defined by the differing electron densities of cortical bone and cartilage. The consistent negative bias across all the bone area measures would indicate a MR bone boundary surface inside that of the CT boundary. This is also consistent with the larger 3DJSW measures (positive bias) from MR in comparison to CT.
In all measures, the analysis of the limited KLG 2&3 subset indicated a remarkably similar pattern and magnitude of the various statistical measures except for somewhat higher coefficient of determination and concordance correlation coefficient values in both the lateral and medial 3DJSW measurements.
Because MRI is not typically used to examine bony structures, there are few studies comparing the geometry of bone surfaces described by segmentation from CT or MR imaging. However, the use of imaging to generate 3D bone models by 3D printing for use in surgical planning and prostheses, including the design of patient-specific cutting guides and implants routinely uses CT, and the radiation dose from CT imaging can be a concern for elective surgery, prompting several studies comparing cadaveric bone model surfaces derived from CT and MR in the lower limb [36–39]. Although these studies all use different MR sequences, segmentation methodology, surface alignment algorithms, and surface comparison measures; MR and CT surfaces were found to be of comparable sub-millimeter accuracy with no significant differences in surface error. However, consistent with our results, the MR-derived surfaces were all found to be inside the CT-derived surfaces. In a direct comparison of MR and CT, Neubert et al [39] found that MR-based bone models were slightly smaller than CT-based models for all of 3 different MR sequences. A comparison of MR and CT to reference models generated by digitizing bone surfaces free of soft tissue with a mechanical contact scanner by Rathnayaka et al demonstrated that 75.8% of the surface area of MRI models underestimated the CT models [36]. Using optical scans of cleaned bones as ground truth, Broeck et al found that 3D bone models created from CT images are an overestimation of the actual bone, and MRI segmentation results in a 3D bone model that is on average an underestimation of the cleaned bone [37].
In contrast to orthopedic surgical planning, CT is not commonly employed in DMOAD trials due ionizing radiation and lack of soft tissue contrast. However, CT is more widely available than MRI and may have some advantages in clinical trials in which measurements from bone but not from bone marrow lesions or soft tissues are used as endpoints. CT images tend to be less expensive to acquire and standardization of CT image acquisition across sites in a multicenter trial should be more easily achieved than for MRI because of their lower complexity in setting acquisition protocols and lack of potential geometric distortion. CT does have an additional advantage in the determination of bone mineralization, and the measurement of bone mineral density (BMD) has been used to investigate OA-associated changes including subchondral bone trabecular remodeling [25, 40]. In addition, CT arthrography is considered the imaging reference standard for in vivo assessment of cartilage thickness [41], although this does require injection of intra-articular contrast material. Iterative reconstruction techniques are available which substantially reduces the ionizing radiation dose. Cone beam CT (CBCT) machines are becoming available that not only expose a patient to a much lower dose than conventional CT for a bilateral knee scan (~0.1 mSv or around 2 weeks of background radiation) [42], but also introduce the prospect of weight-bearing image acquisition and standardized 3DJSW measurement, which may provide more information about the disposition of the osteoarthritis knee than supine imaging [24].
The positive bias found here for both medial and lateral 3DJSW is consistent with the MR-derived surface being within the CT-derived surface, resulting in greater distance between MR femoral and tibial surfaces. However, there are substantially different biases for medial and lateral 3DJSW with the medial side being wider by around 0.6 mm. It is possible that this can be explained by differences in knee positioning during CT or MR acquisition. The APPROACH study CT acquisition protocol stipulated both knees to be as straight as possible with the feet on a wedge to produce around 15o of internal rotation at the hip. In contrast, the MR acquisition protocol was to rotate the leg so that the toes and patella faced upward, elevate knee by putting cushions below it so that the center of the knee is place in the center of the coil and avoid scanning the knee in a fully extended or hyperextended position. Therefore, the leg was imaged fully extended during a CT scan and partially flexed during an MR scan. In a supine (open-chain) knee, during last 15-20o of knee flexion, anterior tibial glide persists on the tibial medial condyle because its articular surface is longer than that on the lateral side. Prolonged anterior glide on the medial side produces external tibial rotation of around 15o, this is known as the “screw-home mechanism” [43]. This has the effect of repositioning the medial tibial region of the 3DJSW measurement (Fig. 1) more below a more anterior aspect of the medial femur, whereas the repositioning of the lateral tibial 3DJSW region will be less pronounced. This has consequences for the measurement of 3DJSW of the supine knee, and careful attention should be paid to the reproducible positioning of the knee. Further validation of non weight-bearing 3DJSW is therefore warranted.
The main limitation of this study was the lack of sufficient CT data to construct a new version of the OA-vector using shape measures derived from CT images. It would have been useful to compare this approach to the method that was used here, which was to fit the MRI AAM to the CT AAM search result surface and then project the rusting shape into the MRI OA-vector space. There was also no test-retest repositioning image data for CT available, which meant that we could not determine SDD of CT as a repeatability measure. In terms of the 3DJSW analysis, the difference in leg position during image acquisition meant that comparison of modalities is probably unreliable.
Conclusions
In conclusion, the femoral bone shape (B-score) may be reliably measured using either MR or CT with a small bias that could probably be ignored for all practical purposes. Because it is a parameterised shape measure rather than a geometric measure, the B-score has the advantage over bone area measures of being both scale-independent and robust to changes in the position of the surface boundary due to the choice of imaging modality. The 3DJSW is potentially useful in the determination of meniscal or cartilage changes from CT or MR images, but is a geometric measure and is therefore dependent upon the relative positions of the femur and the tibia during image acquisition.
Supplementary Information
Additional file 1: Table S1. Bland-Altman bias (MR minus CT), limits of agreement and linear regression statistics for the comparison of various MR and CT derived measures for the limited KLG dataset (KLG 2–3). Fig. S1. CT vs MRI bone area TrFMed.tAB for the full dataset (KLG 0–4). Top: Bland-Altman (MR minus CT) plot; Bottom: linear regression. Fig. S2. CT vs MRI bone area TrFLat.tAB for the full dataset (KLG 0–4). Top: Bland-Altman (MR minus CT) plot; Bottom: linear regression. Fig. S3. CT vs MRI tibia shape z-score for the full dataset (KLG 0–4). Top: Bland-Altman (MR minus CT) plot; Bottom: linear regression. Fig. S4. CT vs MRI bone area MT.tAB for the full dataset (KLG 0–4). Top: Bland-Altman (MR minus CT) plot; Bottom: linear regression. Fig. S5. CT vs MRI bone area LT.tAB for the full dataset (KLG 0–4). Top: Bland-Altman (MR minus CT) plot; Bottom: linear regression.
Acknowledgments
The research leading to these results has received support from the Innovative Medicines Initiative Joint Undertaking under Grant Agreement n° 115770, resources of which are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007-2013) and EFPIA companies’ in-kind contribution. See www.imi.europa.eu and www.approachproject.eu. PGC is supported in part by the UK National Institute for Health and Care Research (NIHR) Leeds Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Authors’ contributions
All authors were responsible for the conception and design of the study. AB was responsible for drafting the article. All authors provided critical revision of the article for important intellectual content. All authors were responsible for analysis and interpretation of the data and for final approval of the article and take responsibility for the integrity of the work.
Funding
The funding sources were not involved in the design, data analysis, data interpretation nor manuscript preparation.
Availability of data and materials
The data that support the findings of this study are available from the IMI-APPROACH Consortium but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the IMI-APPROACH Steering Committee.
Declarations
Ethics approval and consent to participate
The study is being conducted in compliance with the protocol, Good Clinical Practice (GCP), the Declaration of Helsinki, and the applicable ethical and legal regulatory requirements (for all countries involved), and is registered under clinicaltrials.gov nr: NCT03883568. This study involves human participants and was approved by Medisch ethische toetsingscommisscie van het UMC UtrechtID nr. 17/440. Participants gave informed consent to participate in the study before taking part.
Consent for publication
Not required.
Competing interests
AB and MB are employees of Imorphics Ltd., a wholly owned subsidiary of Stryker Corporation. PC has done consultancy or speakers bureaus for AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Eli Lilly, Galapagos, GlaxoSmithKline, Grunenthal, Merck, Novartis, Pfizer, Stryker and UCB.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alan Brett, Email: Alan.Brett@stryker.com.
Michael A. Bowes, Email: mike.bowes@stryker.com
Philip G. Conaghan, Email: p.conaghan@leeds.ac.uk
References
- 1.Guccione AA, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84(3):351–358. doi: 10.2105/AJPH.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence JS, Bremner JM, Bier F. Osteo-arthrosis. Prevalence in the population and relationship between symptoms and x-ray changes. Ann Rheum Dis. 1966;25(1):1–24. doi: 10.1136/ard.25.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.OARSI . Osteoarthritis: a serious disease, submitted to the U.S. food and drug administration. 2016. [Google Scholar]
- 4.Dieppe PA. Relationship between symptoms and structural change in osteoarthritis. what are the important targets for osteoarthritis therapy? J Rheumatol. 2004;70:50–53. [PubMed] [Google Scholar]
- 5.Glyn-Jones S, et al. Osteoarthritis. Lancet. 2015;386(9991):376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 6.Neogi T. Structural correlates of pain in osteoarthritis. Clin Exp Rheumatol. 35 Suppl 1(5):75–8 [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/28967355. [PubMed]
- 7.Guermazi A, Roemer FW, Felson DT, Brandt KD. Unresolved questions in rheumatology: motion for debate: osteoarthritis clinical trials have not identified efficacious therapies because traditional imaging outcome measures are inadequate. Arthritis Rheum. 2013;65(11):2748–2758. doi: 10.1002/art.38086. [DOI] [PubMed] [Google Scholar]
- 8.Guermazi A, et al. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: Population based observational study (Framingham Osteoarthritis Study) BMJ. 2012;345(7874):1–13. doi: 10.1136/bmj.e5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckstein F, et al. Imaging of cartilage and bone: promises and pitfalls in clinical trials of osteoarthritis. Osteoarthr Cartil. 2014;22(10):1516–1532. doi: 10.1016/j.joca.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lane NE, et al. OARSI-FDA initiative: Defining the disease state of osteoarthritis. Osteoarthr Cartil. 2011;19(5):478–482. doi: 10.1016/j.joca.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Bowes MA, Maciewicz RA, Waterton JC, Hunter DJ, Conaghan PG. Bone area provides a responsive outcome measure for bone changes in short-term knee osteoarthritis studies. J Rheumatol. 2016;43(12):2179–2182. doi: 10.3899/jrheum.151118. [DOI] [PubMed] [Google Scholar]
- 12.Hunter D, et al. Longitudinal validation of periarticular bone area and 3D shape as biomarkers for knee OA progression? Data from the FNIH OA Biomarkers Consortium. Ann Rheum Dis. 2016;75(9):1607–1614. doi: 10.1136/annrheumdis-2015-207602. [DOI] [PubMed] [Google Scholar]
- 13.Bowes MA, et al. Machine-learning, MRI bone shape and important clinical outcomes in osteoarthritis: data from the Osteoarthritis Initiative. Ann Rheum Dis. 2021;80(4):502–508. doi: 10.1136/annrheumdis-2020-217160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morales Martinez A, et al. Learning osteoarthritis imaging biomarkers from bone surface spherical encoding. Magn Reson Med. 2020:1–14. 10.1002/mrm.28251. [DOI] [PMC free article] [PubMed]
- 15.Conaghan PG, et al. Six months’ treatment with MIV-711, a novel Cathepsin K inhibitor induces osteoarthritis structure modification: results from a randomized double-blind placebo-controlled phase IIA trial. Osteoarthr Cartil. 2018;26:S25–S26. doi: 10.1016/j.joca.2018.02.066. [DOI] [Google Scholar]
- 16.McGuire D, et al. Study TPX-100-5: intra-articular TPX-100 significantly delays pathological bone shape change and stabilizes cartilage in moderate to severe bilateral knee OA. Arthritis Res Ther. 2021;23(1):242. doi: 10.1186/s13075-021-02622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heimann T, Meinzer HP. Statistical shape models for 3D medical image segmentation: A review. Med Image Anal. 2009;13(4):543–563. doi: 10.1016/j.media.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Bowes MA, Guillard GA, Vincent GR, Brett AD, Wolstenholme CBH, Conaghan PG. Precision, reliability, and responsiveness of a novel automated quantification tool for cartilage thickness: data from the osteoarthritis initiative. J Rheumatol. 2020;47(2):282–289. doi: 10.3899/jrheum.180541. [DOI] [PubMed] [Google Scholar]
- 19.Neogi T, et al. Magnetic resonance imaging-based three-dimensional bone shape of the knee predicts onset of knee osteoarthritis: data from the osteoarthritis initiative. Arthritis Rheum. 2013;65(8):2048–2058. doi: 10.1002/art.37987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barr AJ, et al. The relationship between three-dimensional knee MRI bone shape and total knee replacement—a case control study: data from the Osteoarthritis Initiative. Rheumatology. 2016;55(9):1585–1593. doi: 10.1093/rheumatology/kew191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowes MA, Vincent GR, Wolstenholme CB, Conaghan PG. A novel method for bone area measurement provides new insights into osteoarthritis and its progression. Ann Rheum Dis. 2013;74(3):519–525. doi: 10.1136/annrheumdis-2013-204052. [DOI] [PubMed] [Google Scholar]
- 22.Guermazi A, Hayashi D, Roemer FW, Felson DT. Osteoarthritis. Rheum Dis Clin N Am. 2013;39(3):567–591. doi: 10.1016/j.rdc.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Segal NA, et al. Comparison of tibiofemoral joint space width measurements from standing CT and fixed flexion radiography. J Orthop Res. 2017;35(7):1388–1395. doi: 10.1002/jor.23387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turmezei TD, et al. Quantitative three-dimensional assessment of knee joint space width from weight-bearing CT. Radiology. 2021;(9):203928. 10.1148/radiol.2021203928. [DOI] [PMC free article] [PubMed]
- 25.Bousson V, Lowitz T, Laouisset L, Engelke K, Laredo J-D. CT imaging for the investigation of subchondral bone in knee osteoarthritis. Osteoporos Int. 2012;23(S8):861–865. doi: 10.1007/s00198-012-2169-5. [DOI] [PubMed] [Google Scholar]
- 26.Koo S, Giori NJ, Gold GE, Dyrby CO, Andriacchi TP. Accuracy of 3D cartilage models generated from MR images is dependent on cartilage thickness: laser scanner based validation of in vivo cartilage. J Biomech Eng. 2009;131(12):1–13. doi: 10.1115/1.4000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Helvoort, et al. Cohort profile: The Applied Public-Private Research enabling OsteoArthritis Clinical Headway (IMI-APPROACH) study: a 2-year, European, cohort study to describe, validate and predict phenotypes of osteoarthritis using clinical, imaging and biochemical mark. BMJ Open. 2020;10(7):e035101. doi: 10.1136/bmjopen-2019-035101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams TG, et al. anatomically corresponded regional analysis of cartilage in asymptomatic and osteoarthritic knees by statistical shape modelling of the bone. IEEE Trans Med Imaging. 2010;29(8):1541–1559. doi: 10.1109/TMI.2010.2047653. [DOI] [PubMed] [Google Scholar]
- 29.Hunter DJ, et al. Can cartilage loss be detected in knee osteoarthritis (OA) patients with 3–6 months’ observation using advanced image analysis of 3T MRI? Osteoarthr Cartil. 2010;18(5):677–683. doi: 10.1016/j.joca.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ronneberger O, Fischer P, Brox T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In: Navab N, Hornegger J, Wells W, Frangi A, editors. Medical Image Computing and Computer-Assisted Intervention – MICCAI 2015. 2015. pp. 234–241. [Google Scholar]
- 31.Roche M. The MAKO robotic-arm knee arthroplasty system. Arch Orthop Trauma Surg. 2021;141(12):2043–2047. doi: 10.1007/s00402-021-04208-0. [DOI] [PubMed] [Google Scholar]
- 32.Eckstein F, et al. Proposal for a nomenclature for Magnetic Resonance Imaging based measures of articular cartilage in osteoarthritis. Osteoarthr Cartil. 2006;14(10):974–983. doi: 10.1016/j.joca.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Besl PJ, McKay ND. A method for registration of 3-D shapes. IEEE Trans Pattern Anal Mach Intell. 1992;14(2):239–256. doi: 10.1109/34.121791. [DOI] [Google Scholar]
- 34.Wirth W, Eckstein F. A Technique for Regional Analysis of Femorotibial Cartilage Thickness Based on Quantitative Magnetic Resonance Imaging. IEEE Trans Med Imaging. 2008;27(6):737–744. doi: 10.1109/TMI.2007.907323. [DOI] [PubMed] [Google Scholar]
- 35.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45(1):255–268. doi: 10.2307/2532051. [DOI] [PubMed] [Google Scholar]
- 36.Rathnayaka K, et al. Quantification of the accuracy of MRI generated 3D models of long bones compared to CT generated 3D models. Med Eng Phys. 2012;34(3):357–363. doi: 10.1016/j.medengphy.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 37.Van den Broeck, Vereecke E, Wirix-Speetjens R, Vander Sloten J. Segmentation accuracy of long bones. Med Eng Phys. 2014;36(7):949–953. doi: 10.1016/j.medengphy.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 38.Stephen JM, Calder JDF, Williams A, El Daou. Comparative accuracy of lower limb bone geometry determined using MRI, CT, and direct bone 3D models. J Orthop Res. 2020:jor.24923. 10.1002/jor.24923. [DOI] [PubMed]
- 39.Neubert A, et al. Comparison of 3D bone models of the knee joint derived from CT and 3T MR imaging. Eur J Radiol. 2017;93(May):178–184. doi: 10.1016/j.ejrad.2017.05.042. [DOI] [PubMed] [Google Scholar]
- 40.Johnston JD, Masri BA, Wilson DR. Computed tomography topographic mapping of subchondral density (CT-TOMASD) in osteoarthritic and normal knees: methodological development and preliminary findings. Osteoarthr Cartil. 2009;17(10):1319–1326. doi: 10.1016/j.joca.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 41.Roemer FW, et al. State of the Art: Imaging of Osteoarthritis—Revisited 2020. Radiology. 2020;296(1):5–21. doi: 10.1148/radiol.2020192498. [DOI] [PubMed] [Google Scholar]
- 42.Segal NA, Bergin J, Kern A, Findlay C, Anderson DD. Test–retest reliability of tibiofemoral joint space width measurements made using a low-dose standing CT scanner. Skelet Radiol. 2017;46(2):217–222. doi: 10.1007/s00256-016-2539-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim HY, Kim KJ, Yang DS, Jeung SW, Choi HG, Choy WS. Screw-home movement of the tibiofemoral joint during normal gait: three-dimensional analysis. Clin Orthop Surg. 2015;7(3):303. doi: 10.4055/cios.2015.7.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Bland-Altman bias (MR minus CT), limits of agreement and linear regression statistics for the comparison of various MR and CT derived measures for the limited KLG dataset (KLG 2–3). Fig. S1. CT vs MRI bone area TrFMed.tAB for the full dataset (KLG 0–4). Top: Bland-Altman (MR minus CT) plot; Bottom: linear regression. Fig. S2. CT vs MRI bone area TrFLat.tAB for the full dataset (KLG 0–4). Top: Bland-Altman (MR minus CT) plot; Bottom: linear regression. Fig. S3. CT vs MRI tibia shape z-score for the full dataset (KLG 0–4). Top: Bland-Altman (MR minus CT) plot; Bottom: linear regression. Fig. S4. CT vs MRI bone area MT.tAB for the full dataset (KLG 0–4). Top: Bland-Altman (MR minus CT) plot; Bottom: linear regression. Fig. S5. CT vs MRI bone area LT.tAB for the full dataset (KLG 0–4). Top: Bland-Altman (MR minus CT) plot; Bottom: linear regression.
Data Availability Statement
The data that support the findings of this study are available from the IMI-APPROACH Consortium but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the IMI-APPROACH Steering Committee.