Abstract
The potential of human monocytes to mediate the clearance of Bordetella pertussis infection was examined. Bacteria expressing green fluorescent protein were incubated with adherent peripheral blood monocytes, and phagocytosis was quantified by using fluorescence microscopy. Monocytes internalized only a small percentage of the adherent bacteria. Surface-associated Bvg-regulated virulence factors, including adenylate cyclase toxin and filamentous hemagglutinin, did not affect attachment or phagocytosis. However, 1-h pretreatment with purified pertussis toxin inhibited the ability of monocytes to internalize wild-type bacteria. Mutations affecting the terminal trisaccharide of lipopolysaccharide resulted in reduced internalization without affecting adherence of bacteria to monocytes. Opsonization with human serum played only a modest role in promoting phagocytosis. The viability of internalized bacteria was determined by colony counts following treatment with polymyxin B and gentamicin. Less than 1% of internalized bacteria remained viable. These results suggest that pertussis toxin plays a role in the evasion of monocyte phagocytosis and that these cells represent a potential mediator of the clearance of B. pertussis infection.
The gram-negative bacterium Bordetella pertussis, the causative agent of whooping cough, is transmitted via an airborne route, usually by respiratory droplets expelled by an infected individual during a coughing paroxysm. The bacteria colonize the ciliated cells of the human upper respiratory tract. Even in a nonimmunized host, B. pertussis will encounter a number of innate immune defenses. One of the earliest immune responses is the recruitment of phagocytic cells. In particular, neutrophils and monocytes (progenitors of macrophages) cross from the bloodstream into the respiratory mucosa in response to infection. Once present, these cells are capable of binding to and internalizing invading bacterial cells. Internalization is often followed by a respiratory burst, which can potentially kill the internalized microorganisms. In vivo studies in mice suggest phagocytic cells can play an important role in clearing B. pertussis infection (21, 34). One study demonstrated that, following challenge with live B. pertussis organisms, there was a dramatic increase in the numbers of neutrophils and lymphocytes present in the lungs of both unimmunized mice and mice previously immunized with whole-cell pertussis vaccine (30). In mice immunized with an acellular vaccine, an influx of macrophages was observed in response to infection (30).
Previous work has demonstrated that, although capable of readily binding B. pertussis, human neutrophils are inefficient at internalizing the bacteria. All of the bacteria were found associated with the neutrophils, but only about 15% were actually phagocytosed (27, 42). This inefficiency, and not an inability to kill internalized bacteria, makes this defense only minimally effective against B. pertussis. In fact, once internalized, only about 1.7% of the phagocytosed bacteria survived after 2 h (27). Specific virulence factors were found to affect phagocytosis by neutrophils. Attachment was mediated by filamentous hemagglutinin (FHA); however, attachment via opsonizing antibody, not FHA, was essential for internalization (42). Additionally, it was found that the B. pertussis adenylate cyclase toxin was important in crippling the phagocytic functions of neutrophils, since internalization only occurred in the presence of neutralizing antibodies to adenylate cyclase toxin (41) when an adenylate cyclase toxin mutant was used (42) or when adenylate cyclase toxin was inactivated by treatment with fluorescein isothiocyanate (FITC) (39).
Contrary to this clear picture of phagocytosis of B. pertussis by human neutrophils, a comparable model has not been developed for monocytes and macrophages. Monocytes are among the first responders to a B. pertussis infection and represent cells in the earliest stages of differentiation into macrophages. Although there are a number of differences between the two cell types, the two possess a number of common receptors and signaling pathways. Shortly after adherence, monocytes begin to mature into macrophages, and the distinction between the two cell types (adherent monocytes and macrophages) becomes less clear.
Initial reports suggested that B. pertussis is capable of surviving within monocytes and macrophages. One study used endogenous complement to kill extracellular B. pertussis (15). B. pertussis is only susceptible to antibody-dependent killing by complement (14), and the reported survival was possibly due to a failure of serum from donors with a different immune status to kill all of the extracellular bacteria. All other published reports have used antibiotics to kill extracellular bacteria. Intracellular phenotypic modulation within macrophages has been suggested to be a bacterial adaptation to enhance intracellular survival (28, 29). However, a 2 log drop in bacterial viability was reported (28, 29), and these studies focused on the 1% of the viable bacteria recovered following phagocytosis, not the 99% that were killed by the macrophages. In another study, significant numbers of viable intracellular Bordetella bronchiseptica organisms were recovered from macrophages after 4 days of incubation, but B. pertussis was eliminated by about 24 h (4). Thus, the only study to report significant long-term intracellular survival of B. pertussis was the study that used a highly variable reagent, complement plus antibody, instead of antibiotics to kill the extracellular bacteria.
It is likely that phagocytosis by macrophages could play an important role in clearing infections by B. pertussis. Previous studies have reported a role for FHA, pertactin, and fimbriae in influencing attachment and phagocytosis by monocytes (17–19, 21, 23). In these studies B. pertussis was added to adherent monocytes in culture and centrifugation was not used to promote contact between the bacteria and the monocytes. B. pertussis in suspension does not settle significantly, even over several hours (27). Random collisions must bring the suspended bacteria into contact with the monocytes, and association is most likely to be maintained with bacteria that mediate their own adherence or adhere via the Fc receptors on antibodies, leading to increased phagocytosis of strains that mediate their own adherence (27). However in the human body, monocytes are motile and are capable of pursuing bacteria. Centrifugation has been shown to enhance contact between B. pertussis and adherent cells in culture and eliminate the bias towards phagocytosis of adherent bacteria (27), and it may more accurately reflect events in vivo.
We examined the ability of adherent human monocytes to internalize and kill B. pertussis bacteria expressing different virulence factors. In this study, increased phagocytosis of strains expressing adhesins and opsonized bacteria was observed for bacteria in suspension but not when the bacteria were centrifuged to promote association with the monocytes. Only pertussis toxin and lipopolysaccharide (LPS) influenced phagocytosis by monocytes when centrifugation was used to enhance contact. The ability of the bacteria to survive following internalization was also examined, and less than 1% of the internalized bacteria remained viable.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Plasmids and bacterial strains used in this study are described in Table 1. B. pertussis strains expressing green fluorescent protein (GFP) were grown in Stainer Scholte (SS) broth on Bordet Gengou (BG) agar (Difco, Detroit, Mich.) plates for 24 h as described previously (11). Briefly, strains were suspended in 7 ml of SS broth containing the appropriate selective antibiotics to an optical density at 600 nm (OD600) of approximately 0.1, overlaid on BG agar plates containing 15% defibrinated sheep's blood (Colorado Serum Company, Denver, Colo.) and appropriate antibiotics, and incubated at 37°C for 24 h.
TABLE 1.
Bacterial plasmids and strains used in this study
| Plasmid or strain | Phenotype | Reference |
|---|---|---|
| Plasmids | ||
| pGB5P1 | Kanr; high-level expression of GFP from B. pertussis constitutive promoter | 39 |
| pCW505 | Kanr; Genr; pBBRMCS-2 expressing GFP under B. pertussis promoter | 42 |
| pCW382-6 | Kanr; Cmr; pBBRMCS-1 expressing GFP under B. pertussis promoter | This study |
| Strains | ||
| WT BP338(pGB5P1) | Wild type; Nalr, Kanr; Tohama I | 43 |
| Bvg mutant BP347(pCW382-6) | Bvg mutant, avirulent BP338; Nalr, Kanr, Cmr | 43 |
| FHA mutant BP353(pCW382-6) | BP338 with transposon insertion in FHA operon; FHA- and fimbriae-deficient; Nalr, Kanr, Cmr | 43 |
| Cyc mutant BP3183(pCW382-6) | Cyc, BP338 with transposon insertion in adenylate cyclase toxin gene; Nalr, Kanr, Cmr | 44 |
| LPS MLT7(pCW505) | BP338 with transposon insertion in LPS operon; Nalr, Kanr, Genr | 37 |
| WT BP536(pCW505) | Wild type; Tohama I; Nalr, Smr, Kanr; band A and band B LPS | 2 |
| WlbD WlbD(pCW382-6) | BP536ΔwlbD::Kan; Nalr, Kanr, Smr, Cmr; LPS mutant, band B and aberrant LPS | 2, 3 |
| WlbG WIbG(pCW382-6) | BP536ΔwlbG::Kan; Nalr, Kanr, Smr, Cmr; LPS mutant, band B LPS | 2, 3 |
| WlbH WIbH(pCW382-6) | BP536ΔwlbH::Kan; Nalr, Kanr, Smr, Cmr; LPS mutant, band B and intermediate band LPS | 2, 3 |
| WlbL WIbL(pCW382-6) | BP536ΔwlbL::Kan; Nalr, Kanr, Smr, Cmr; LPS mutant, band B LPS | 2, 3 |
Antibiotics were used at the following concentrations: gentamicin sulfate, 30 μg/ml; nalidixic acid, 30 μg/ml; kanamycin, 30 μg/ml; chloramphenicol, 15 μg/ml.
Antibody opsonization.
Antibody was obtained from whole human serum pooled from six adult volunteers. The pooled serum had antibodies specific to B. pertussis, as determined by Western blotting, and the ability to mediate antibody-dependent complement killing (data not shown). Pooled serum was incubated for 30 min at 56°C to heat-inactivate the complement. Bacteria were pelleted by centrifugation and resuspended in 15 μl of serum and incubated for 15 min at 37°C prior to addition to monocyte cultures.
Adherent monocytes.
Monocytes were isolated from healthy adult volunteers. Blood was collected by venipuncture, combined with 0.11 ml of 3.8% sodium citrate per ml of blood to prevent coagulation, and centrifuged for 20 min at 100 × g to remove plasma. Total white blood cells were separated from the remaining whole blood by incubation for 1 h with a final concentration of 0.6% dextran in 0.9% saline. Peripheral blood mononuclear cells were isolated from this layer by centrifugation over Ficoll-Paque (Amersham Pharmacia, Uppsala, Sweden) gradients at 450 × g for 25 min. Isolated mononuclear cells were further purified on Percoll gradients, formed by centrifuging 7 ml of Percoll (Sigma, St. Louis, Mo.) and 6 ml of 2× phosphate-buffered saline (PBS) at 27,000 × g for 40 min. Mononuclear cells were layered on the gradient and centrifuged for 20 min at 1,000 × g. Following centrifugation four bands were observed, and the second band from the top contained mostly monocytes, as had been reported previously (38). Monocytes were suspended at 6 × 106 cells per ml in Hanks' buffered salt solution (BioWhittaker, Rockland, Md.) buffered with 10 mM HEPES (Sigma) (H-H), and autologous serum (0.001%). One milliliter was plated in each well of a sterile 24-well tissue culture dish containing glass coverslips. Plates were incubated for 1 h at 37°C, 5% CO2 for 1 h to allow adherence.
Phagocytosis assay.
To determine the number of bacteria phagocytosed by human monocytes, we used an assay similar to that previously described for human neutrophils (39). Bacteria expressing GFP were grown in SS on BG, washed, and resuspended to an OD600 of approximately 1.0 in H-H. This bacterial suspension (15 μl, or approximately 3 × 107 cells) was diluted to 400 μl and added to each well containing adherent monocytes to achieve a multiplicity of infection (MOI) of approximately 5.
For all experiments, except where indicated, the plates were then centrifuged for 5 min at 640 × g to facilitate contact between bacteria and adherent monocytes. Plates were incubated for 1 h at 37°C, 5% CO2 to allow phagocytosis to occur. Wells were washed several times with H-H to remove any nonadherent bacterial cells. Cultures were stained for 10 min with 0.5 ml of a 0.05-mg/ml solution of ethidium bromide in H-H. Wells were washed twice to remove any residual ethidium bromide and fixed overnight at 4°C in a 0.01 M phosphate buffer–1% paraformaldehyde fixative. Coverslips were washed, mounted on microscope slides with 5% glycerol in PBS, and sealed using clear nail varnish. Slides were observed by using fluorescence microscopy. Previous studies have shown that in the short duration of the staining, internalized bacteria expressing GFP resist staining with ethidium bromide and appear green, while noninternalized, adherent bacteria stain orange (39). The numbers of adherent and internalized bacteria were counted for 100 monocytes per coverslip, performed in triplicate, with at least three independent repetitions. Statistical analysis was performed using the paired Student t test.
Pertussis toxin pretreatment.
Monocytes were isolated and allowed to adhere as described above. Adherent monocytes were incubated with purified pertussis toxin or purified B-oligomer (List Biologicals, Campbell, Calif.) for 1 h at 37°C, 5% CO2. After this incubation, bacterial suspensions were added and phagocytosis assays were performed as described above.
Intracellular survival assay.
Assays similar to those previously used for human neutrophils were performed to assess the intracellular survival of B. pertussis (27). Phagocytosis was allowed to occur for 1 h as described above, followed by incubation with 300 μg of gentamicin/ml and 100 μg of polymyxin B/ml for 1 h. These antibiotics are unable to penetrate the eukaryotic cell membrane and will kill adherent extracellular, but not internalized, bacterial cells (12). Following this incubation, wells were washed thoroughly with H-H to remove the antibiotics. Wells were then aspirated to remove medium and 1 ml of sterile, distilled H2O was added to each well. Following this osmotic lysis of the monocytes, wells were scraped thoroughly with a rubber policeman. Serial 10-fold dilutions of the well contents were made in PBS (pH 7.4) and plated on BG agar. Plates were incubated at 37°C for 5 days and CFU were determined. Wells lacking monocytes were treated identically to serve as an antibiotic killing control.
RESULTS
Effects of centrifugation on attachment and phagocytosis.
Previous reports have suggested that B. pertussis cells in suspension do not settle significantly over a period of a few hours, and this can affect phagocytosis (27). To assess the effect of centrifugation on phagocytosis by monocytes, duplicate plates of monocytes were prepared. One plate was centrifuged for 5 min at 640 × g to facilitate contact between bacteria and monocytes, while the control plate was left at room temperature. Following centrifugation, phagocytosis was allowed to proceed at 37°C.
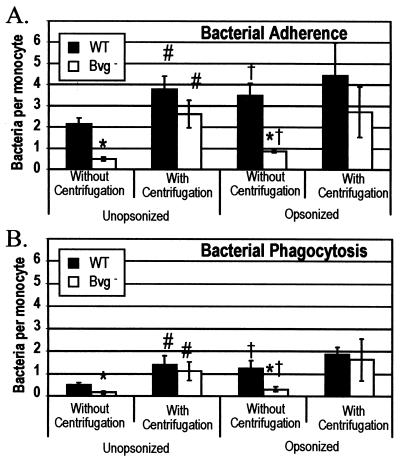
Two strains were characterized: the wild type, BP338, and the Bvg mutant strain, BP347. The BvgAS locus is responsible for regulating the expression of a number of virulence factors, including pertussis toxin, adenylate cyclase toxin, FHA, pertactin, and fimbriae. The transposon insertion in the BvgAS locus in BP347 results in an avirulent strain of B. pertussis that fails to express any of the Bvg-regulated virulence factors (43). In the absence of centrifugation, monocytes had a statistically significant reduction in the ability to bind the Bvg mutant strain compared to that of the wild type (Fig. 1A). Centrifugation increased the adherence of the wild type and Bvg mutant to comparable levels.
FIG. 1.
Effects of centrifugation on adherence (A) and phagocytosis (B) of B. pertussis cells by human monocytes. Bacterial suspensions were added at an MOI of 5 to adherent monocytes on glass coverslips. Plates were either centrifuged at 640 × g for 5 min or allowed to sit at room temperature for 5 min and then incubated for 1 h at 37°C. The numbers of adherent and internalized bacteria were determined by fluorescence microscopy. WT, BP338; Bvg − BP347. Each bar represents the mean ± standard error of the mean of three independent experiments performed in triplicate. ∗, significantly different from parent wild type (P < 0.05); #, significantly different from results without centrifugation (P < 0.05); †, significantly different from unopsonized bacteria (P < 0.05).
Trends for phagocytosis paralleled those for attachment. In the noncentrifuged samples, the Bvg mutant was internalized in lower numbers than was the wild-type strain (Fig. 1B). When samples were centrifuged, no significant difference in phagocytosis was observed between the two strains. The bacteria were added at an MOI of approximately 5, and after centrifugation all of the bacteria added to the culture appeared to be associated with the monocytes, in either the adherent or intracellular pool.
Opsonization increased both attachment and internalization in the noncentrifuged samples but had little effect on the centrifuged samples. In all subsequent experiments, plates were centrifuged as described above to facilitate contact between monocytes and bacteria.
Effects of virulence determinants and LPS on phagocytosis.
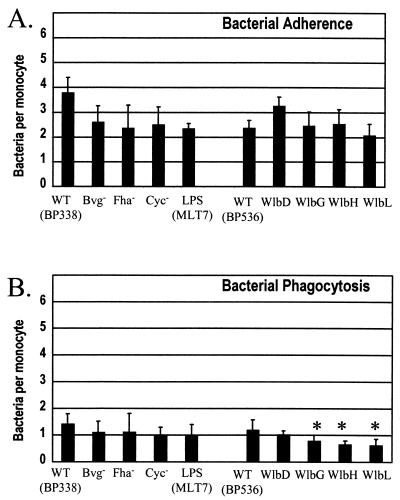
Adherence and phagocytosis of the wild type and mutants lacking specific virulence factors were assessed (Fig. 2). Contrary to results observed in neutrophils (42), the Bvg-regulated virulence factors FHA and adenylate cyclase toxin did not influence the adherence or phagocytosis of B. pertussis by human monocytes (Fig. 2). However, a small but statistically significant decrease in phagocytosis was seen for three of the five LPS mutants: WlbG, WlbH, and WlbL (Fig. 2B). Similar results were observed when experiments were repeated using an MOI of 20 (data not shown).
FIG. 2.
Effects of virulence factors on adherence (A) and phagocytosis (B) of B. pertussis. Bacteria were added to adherent monocytes, centrifugation was performed to promote contact, and phagocytosis was allowed to occur for 1 h. One hundred monocytes were examined by fluorescence microscopy to determine the number of bacteria attached to (A) and internalized by (B) each monocyte. Strains are defined in Table 1. Data were analyzed by using Student's t test. Bars represent the mean + standard error of the mean of four independent experiments performed in triplicate. ∗, significantly different from parental wild type (P < 0.05).
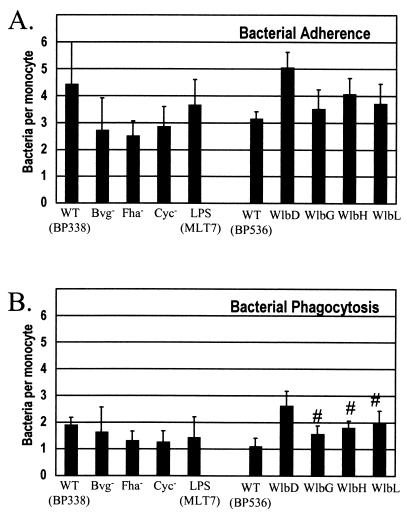
Antibody opsonization had little effect on adherence or phagocytosis of the wild type and Bvg-regulated mutant strains (Fig. 3). However, opsonization resulted in increased phagocytosis of the wlb mutant strains, which were internalized at wild-type levels (Fig. 3).
FIG. 3.
Effects of antibody opsonization on adherence (A) and phagocytosis (B) of B. pertussis. Bacteria were suspended in 15 μl of pooled human serum and incubated for 15 min at 37°C. Bacteria were then added to adherent monocytes, centrifugation was performed to promote contact, and phagocytosis was allowed to occur for 1 h. One hundred monocytes were examined by fluorescence microscopy to determine the number of bacteria attached to (A) and internalized by (B) each monocyte. Data were analyzed by using Student's t test. Bars represent the mean + standard error of the mean of four independent experiments performed in triplicate. #, significantly different from unopsonized bacteria results shown in Fig. 2 (P < 0.05).
Effects of purified pertussis toxin on phagocytosis.
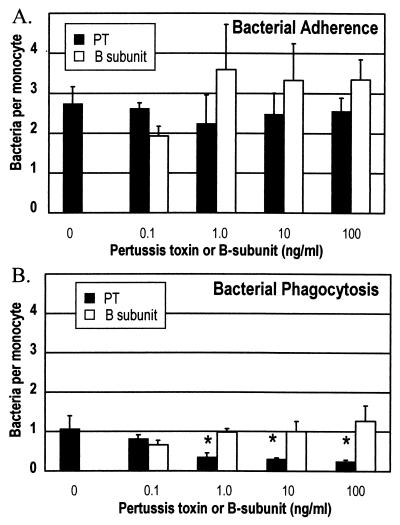
Pertussis toxin is secreted by B. pertussis and moves through host cells to the Golgi complex by using the retrograde transport system (13). In in vitro experiments using cultured mammalian cell lines, intoxication required approximately 1 h (13). It would be difficult to observe pertussis toxin effects in the short-term phagocytosis assays, especially if the bacteria must first secrete the toxin. To overcome these problems, we pretreated the monocytes with pertussis toxin for 1 h prior to addition of bacteria. Pertussis toxin pretreatment had no effect on the adherence of wild-type B. pertussis, BP338, to human monocytes (Fig. 4A). However, incubation with pertussis toxin at 1.0 ng/ml or greater caused a decrease in the phagocytic abilities of the monocytes (Fig. 4B). Treatment with purified B-subunit caused no significant change in phagocytosis, implying that the inhibition requires the catalytic activity of the toxin. In these studies, bacteria were added at an MOI of 20. No differences in adherence or uptake following pretreatment of monocytes with pertussis toxin occurred when experiments were conducted at an MOI of 5 (data not shown). At an MOI of 5 very few bacteria are internalized, and it is difficult to discern differences when there are only a small number of events.
FIG. 4.
Effects of pertussis toxin (PT) pretreatment on adherence (A) and phagocytosis (B) of wild-type B. pertussis BP338 cells by human monocytes. Adherent monocytes were treated with increasing concentrations of purified pertussis toxin (dark bars) or pertussis toxin B-subunit (open bars) for 1 h prior to addition of bacteria. Plates were centrifuged to facilitate contact and phagocytosis was allowed to occur for 1 h. One hundred monocytes were examined by fluorescence microscopy to determine the number of bacteria attached to (A) and internalized by (B) each monocyte. Data were analyzed by using Student's t test. Bars represent the mean + standard error of the mean of four independent experiments performed in triplicate. ∗, significantly different from no-pertussis toxin control (P < 0.05).
Survival of wild-type B. pertussis in human monocytes.
Several early reports suggested that B. pertussis was able to survive inside human monocytes and macrophages (15, 20, 29). However, other reports have suggested that intracellular survival is only transient (4, 24) and that B. pertussis kills monocytes and macrophages by inducing apoptosis (1, 5, 35). None of these studies quantified bacterial survival, so we examined survival as a function of the number of bacteria internalized. Antibiotic treatment with polymyxin B and gentamicin was used to eliminate any adherent bacteria, which are bound tightly to the surface of the monocytes and cannot be removed by washing. These antibiotics do not penetrate mammalian cells and will not affect the viability of internalized bacteria. To determine the extent of killing, control wells with no monocytes plated in them were also inoculated with B. pertussis cultures.
Overall, less than 1% of the phagocytosed bacteria could be recovered following lysis of the monocytes (Table 2). For the wild type, Bvg mutant, and WlbL mutant, this number is similar to that in antibiotic control wells lacking monocytes, and the number of bacteria that have survived within the monocytes cannot be distinguished from adherent cells that escaped antibiotic treatment. For one of the two LPS mutant strains tested, WlbG, intracellular survival was similar to that of the wild type but was statistically greater than that in the antibiotic killing control, since WlbG appears to be more sensitive to the polymyxin B and gentamicin treatment. Only 0.02% of the internalized WlbG survived, suggesting that killing is quite efficient.
TABLE 2.
Survival of B. pertussis in human monocytes
| Condition | Survival (%) of strain
|
|||
|---|---|---|---|---|
| Wild type | Bvg mutant | WIbG | WIbL | |
| Phagocytoseda | (6.5 ± 1.6) × 106 | (3.4 ± 0.8) × 106 | (4.3 ± 1.1) × 106 | (3.8 ± 1.6) × 106 |
| Viable intracellularb | (3.8 ± 1.4) × 103 (0.06) | (2.9 ± 1.6) × 103 (0.09) | (9.4 ± 2.4) × 102∗ (0.02) | (4.8 ± 2.1) × 103 (0.13) |
| Antibiotic controlc | (1.8 ± 0.4) × 103 (0.03) | (1.5 ± 1.3) × 104 (0.44) | (1.8 ± 0.8) × 100∗ (<0.01) | (4.2 ± 3.9) × 103 (0.11) |
Wells were inoculated with approximately 3 × 107 B. pertussis bacteria to reach an estimated MOI of 5. Values represent the number of bacteria internalized by monocytes ± the standard error (SE), calculated based on the number observed for 100 monocytes.
CFU ± SE recovered after incubation with monocytes and treatment with gentamicin and polymyxin B.
CFU ± SE recovered following treatment with antibiotics in the absence of monocytes. ∗ Significantly (P < 0.05) different from wild type.
DISCUSSION
Monocytes/macrophages and neutrophils represent two arms of the innate immune response. It seems reasonable that these two cell types would use different mechanisms to combat invading microorganisms. The differences observed between the present study and previous studies with neutrophils (27, 41, 42) confirm the diversity of the phagocytic defenses, and B. pertussis appears to have successfully evolved a means of evading both types of phagocytic cells. Two virulence factors were shown to influence adherence and phagocytosis of B. pertussis by human neutrophils (41, 42). Strains lacking FHA expression showed a marked reduction in adherence, and adenylate cyclase toxin blocked phagocytosis of bacteria. Based upon these studies, a model of how B. pertussis evades phagocytosis by human neutrophils was developed. Adherence via FHA appears to allow for attachment with little risk of phagocytosis due to a failure to activate signaling mechanisms in neutrophils. If attachment occurs via opsonizing antibodies rather than FHA, adenylate cyclase toxin cripples the phagocytic abilities of neutrophils.
Interestingly, phagocytosis of B. pertussis by monocytes was not different for mutants lacking FHA, adenylate cyclase toxin, or a Bvg mutant lacking all virulence factors. Several studies have demonstrated that FHA and adenylate cyclase toxin exert toxic effects on monocytes and macrophages, including an ability to promote apoptosis (1, 5, 35); however, toxic effects were only observed several hours after treatment. Based on our studies, these virulence factors do not alter the short-term phagocytic abilities of monocytes; however, it is likely that they contribute to the long-term inhibition of monocytes and macrophages that has been observed in response to B. pertussis infection (5, 10, 16, 24, 25).
A role for FHA, pertactin, and fimbriae in influencing attachment and phagocytosis by monocytes has been reported in previous studies (17–19, 21, 23). While we did not characterize a mutant lacking only pertactin, characterization of the Bvg mutant, lacking all adhesins reported to affect phagocytosis, should reflect the role of this adhesin on phagocytosis. The FHA mutant used in this study is unable to express both FHA and fimbriae. We did not observe any of these mutants to be different from the wild type, and the disparity with previous reports likely resulted from technical differences. In several studies, the bacteria were chemically labeled with FITC under highly basic conditions (pH 9.0) to allow visualization by fluorescence microscopy (17–20, 23). FITC covalently binds to the α-amino groups at the N terminus or the ɛ-amino groups of lysines on proteins that are exposed on the bacterial surface. FITC labeling has been shown to inactivate the adenylate cyclase toxin (39) and is likely to modify other virulence factors as well, making it difficult to be sure that the behavior of FITC-labeled bacteria reflects the behavior of unlabeled bacteria. In this study, we used B. pertussis expressing GFP in the cytoplasm. GFP does not affect the growth of the bacteria or alter expression of virulence factors (39–42).
The studies which reported a role for the adhesins in promoting phagocytosis did not use centrifugation to promote contact between B. pertussis and the phagocytes (17–20). We also observed decreased uptake of a Bvg mutant compared to that of the wild-type strain in the absence of centrifugation (Fig. 1) but not when centrifugation was used to promote association. Similarly, opsonization promoted phagocytosis of bacteria in the absence of centrifugation but had no effect in the presence of centrifugation (Fig. 1). In the human body, monocytes are motile and are capable of pursuing bacteria. In culture, adherent monocytes cannot pursue the bacteria, and contact is most likely to be maintained with bacteria that mediate their own adherence or adhere via the Fc receptors on antibodies. Centrifugation promotes association between the bacteria and monocytes and appears to eliminate the bias toward adherent bacteria. We feel these experimental conditions more closely parallel events in vivo.
LPS appears to influence phagocytosis of B. pertussis by human monocytes, even when centrifugation is used to promote close contact with the bacteria. B. pertussis has an unusually short LPS with a single nonrepeating trisaccharide moiety comprising the O-antigen (6, 8, 36). Five mutants with different deficiencies in production of the trisaccharide were characterized, and three mutants, WlbG, WlbL, and WlbH, showed a statistically significant decrease in internalization compared to the parental strain in the absence of antibody. The WlbG and WlbL mutants lack the trisaccharide (2, 3), while the WlbH mutant only adds the first two sugars of the trisaccharide (2). The susceptibilities of the WlbD and MLT7 mutants to phagocytosis were indistinguishable from that of the wild type in the presence or absence of antibody. The LPS of the WlbD mutant is larger than wild-type LPS and possibly has more than three terminal sugars. The nature of the mutation in MLT7 is unknown, but this mutant synthesizes an LPS that appears to contain only the first sugar of the trisaccharide (2). It is interesting that LPS mutants should be more resistant to phagocytosis than the wild type, although the observed effect was very modest. Monocytes possess specific receptors for LPS. Human toll-like receptor 4 is one of the many phagocytic receptor types exposed on the surface of human monocytes and macrophages. This class of receptor has been shown to specifically signal in response to LPS (9, 26), and it is likely that this receptor family plays a role in the internalization of B. pertussis.
Pertussis toxin is likely to be a key mechanism by which B. pertussis resists phagocytosis by monocytes and macrophages. Pertussis toxin has been shown to inhibit chemotaxis and migration of monocytes to the site of infection (31–33). Furthermore, pertussis toxin has been implicated in inhibiting a number of activities of human phagocytic cells, including phagocytosis and induction of nitric oxide synthesis (22, 45). Thus, in addition to preventing migration to the site of infection, pertussis toxin limits the phagocytic potential of monocytes in the airway. We found that pretreatment of monocytes with pertussis toxin at concentrations as low as 1.0 ng/ml significantly reduced phagocytosis of wild-type BP338. The B-subunit alone was without effect, suggesting a role for ADP ribosylation. The failure to see differences between the wild-type strain and Bvg mutant, which fails to produce pertussis toxin, is likely due to the lag time for pertussis toxin to reach and modify its intracellular target (13). One-hour phagocytosis assays do not allow sufficient time for pertussis toxin activity to be observed.
Opsonization with pooled sera from adult volunteers in this study did not promote phagocytosis when the bacteria were brought into close association with the monocytes by centrifugation. In studies with neutrophils, opsonization did not promote phagocytosis unless neutralizing antibodies to adenylate cyclase toxin were present (41). Opsonization with postimmunization sera from adult participants in a pertussis acellular vaccine study also failed to improve phagocytosis of B. pertussis by neutrophils (40); however, the acellular vaccines do not include the adenylate cyclase toxin as an antigen. Pertussis toxin appears to be the only factor hindering phagocytosis by monocytes. The role of specific antibodies, in particular neutralizing antibodies to pertussis toxin, in promoting phagocytosis by monocytes will be examined in future studies.
Previous reports have suggested that B. pertussis is capable of surviving in monocytes and macrophages (7, 15, 20). Quantitative assessment of intracellular survival in this study suggests that less than 1% of the internalized bacteria survive following phagocytosis, suggesting that monocytes could contribute to clearance of B. pertussis. However, phagocytosis by monocytes does not appear to be very efficient, and a greater understanding of the mechanism by which B. pertussis resists phagocytosis by monocytes is needed.
ACKNOWLEDGMENTS
This study was supported by grant RO1 AI45715 to A.A.W. L.M.S. was the recipient of a University Distinguished Graduate Assistantship from the University of Cincinnati.
REFERENCES
- 1.Abramson T, Kedem H, Relman D A. Proinflammatory and proapoptotic activities associated with Bordetella pertussis filamentous hemagglutinin. Infect Immun. 2001;69:2650–2658. doi: 10.1128/IAI.69.4.2650-2658.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen A, Maskell D. The identification, cloning and mutagenesis of a genetic locus required for lipopolysaccharide biosynthesis in Bordetella pertussis. Mol Microbiol. 1996;19:37–52. doi: 10.1046/j.1365-2958.1996.354877.x. [DOI] [PubMed] [Google Scholar]
- 3.Allen A G, Thomas R M, Cadisch J T, Maskell D J. Molecular and functional analysis of the lipopolysaccharide biosynthesis locus wlb from Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Mol Microbiol. 1998;29:27–38. doi: 10.1046/j.1365-2958.1998.00878.x. [DOI] [PubMed] [Google Scholar]
- 4.Banemann A, Gross R. Phase variation affects long-term survival of Bordetella bronchiseptica in professional phagocytes. Infect Immun. 1997;65:3469–3473. doi: 10.1128/iai.65.8.3469-3473.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boschwitz J S, Batanghari J W, Kedem H, Relman D A. Bordetella pertussis infection of human monocytes inhibits antigen-dependent CD4 T cell proliferation. J Infect Dis. 1997;176:678–686. doi: 10.1086/514090. [DOI] [PubMed] [Google Scholar]
- 6.Brodeur B R, Martin D, Hamel J, Shahin R D, Laferriere C. Antigenic analysis of the saccharide moiety of the lipooligosaccharide of Bordetella pertussis. Semin Immunopathol. 1993;15:205–215. doi: 10.1007/BF00201101. [DOI] [PubMed] [Google Scholar]
- 7.Bromberg K, Tannis G, Steiner P. Detection of Bordetella pertussis associated with the alveolar macrophages of children with human immunodeficiency virus infection. Infect Immun. 1991;59:4715–4719. doi: 10.1128/iai.59.12.4715-4719.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caroff M, Brisson J, Martin A, Karibian D. Structure of the Bordetella pertussis 1414 endotoxin. FEBS Lett. 2000;477:8–14. doi: 10.1016/s0014-5793(00)01720-8. [DOI] [PubMed] [Google Scholar]
- 9.Chow J C, Young D W, Golenbock D T, Christ W J, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 10.Confer D L, Eaton J W. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science. 1982;217:948–950. doi: 10.1126/science.6287574. [DOI] [PubMed] [Google Scholar]
- 11.Craig-Mylius K A, Weiss A A. Antibacterial agents and release of periplasmic pertussis toxin from Bordetella pertussis. Antimicrob Agents Chemother. 2000;44:1383–1386. doi: 10.1128/aac.44.5.1383-1386.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drevets D A, Canono B P, Leenen P J, Campbell P A. Gentamicin kills intracellular Listeria monocytogenes. Infect Immun. 1994;62:2222–2228. doi: 10.1128/iai.62.6.2222-2228.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.el Baya A, Linnemann R, von Olleschik-Elbheim L, Robenek H, Schmidt M A. Endocytosis and retrograde transport of pertussis toxin to the Golgi complex as a prerequisite for cellular intoxication. Eur J Cell Biol. 1997;73:40–48. [PubMed] [Google Scholar]
- 14.Fernandez R C, Weiss A A. Cloning and sequencing of a Bordetella pertussis serum resistance locus. Infect Immun. 1994;62:4727–4738. doi: 10.1128/iai.62.11.4727-4738.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman R L, Nordensson K, Wilson L, Akporiaye E T, Yocum D E. Uptake and intracellular survival of Bordetella pertussis in human macrophages. Infect Immun. 1992;60:4578–4585. doi: 10.1128/iai.60.11.4578-4585.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gueirard P, Druilhe A, Pretolani M, Guiso N. Role of adenylate cyclase-hemolysin in alveolar macrophage apoptosis during Bordetella pertussis infection in vivo. Infect Immun. 1998;66:1718–1725. doi: 10.1128/iai.66.4.1718-1725.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hazenbos W L, Geuijen C A B, van den Berg M, Mooi F R, van Furth R. Bordetella pertussis fimbriae bind to human monocytes via the minor fimbrial subunit FimD. J Infect Dis. 1995;171:924–929. doi: 10.1093/infdis/171.4.924. [DOI] [PubMed] [Google Scholar]
- 18.Hazenbos W L, van den Berg B M, Geuijen C W, Mooi F R, van Furth R. Binding of FimD on Bordetella pertussis to very late antigen-5 on monocytes activates complement receptor type 3 via protein tyrosine kinases. J Immunol. 1995;155:3972–3978. [PubMed] [Google Scholar]
- 19.Hazenbos W L, van den Berg B M, van't Wout J W, Mooi F R, van Furth R. Virulence factors determine attachment and ingestion of nonopsonized and opsonized Bordetella pertussis by human monocytes. Infect Immun. 1994;62:4818–4824. doi: 10.1128/iai.62.11.4818-4824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hellwig S M, Hazenbos W L, van de Winkel J G, Mooi F R. Evidence for an intracellular niche for Bordetella pertussis in broncho-alveolar lavage cells of mice. FEMS Immunol Med Microbiol. 1999;26:203–207. doi: 10.1111/j.1574-695X.1999.tb01391.x. [DOI] [PubMed] [Google Scholar]
- 21.Hellwig S M, van Oirschot H F, Hazenbos W L, van Spriel A B, Mooi F R, van de Winkel J G. Targeting to Fcgamma receptors, but not CR3 (CD11b/CD18), increases clearance of Bordetella pertussis. J Infect Dis. 2001;183:871–879. doi: 10.1086/319266. [DOI] [PubMed] [Google Scholar]
- 22.Hiemstra P S, Annema A, Schippers E F, van Furth R. Pertussis toxin partially inhibits phagocytosis of immunoglobulin G-opsonized Staphylococcus aureus by human granulocytes but does not affect intracellular killing. Infect Immun. 1992;60:202–205. doi: 10.1128/iai.60.1.202-205.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishibashi Y, Claus S, Relman D A. Bordetella pertussis filamentous hemagglutinin interacts with a leukocyte signal transduction complex and stimulates bacterial adherence to monocyte CR3 (CD11b/CD18) J Exp Med. 1994;180:1225–1233. doi: 10.1084/jem.180.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khelef N, Guiso N. Induction of macrophage apoptosis by Bordetella pertussis adenylate cyclase-hemolysin. FEMS Microbiol Lett. 1995;134:27–32. doi: 10.1111/j.1574-6968.1995.tb07909.x. [DOI] [PubMed] [Google Scholar]
- 25.Khelef N, Zychlinsky A, Guiso N. Bordetella pertussis induces apoptosis in macrophages: role of adenylate cyclase-hemolysin. Infect Immun. 1993;61:4064–4071. doi: 10.1128/iai.61.10.4064-4071.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirschning C J, Wesche H, Merrill Ayres T, Rothe M. Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med. 1998;188:2091–2097. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenz D H, Weingart C L, Weiss A A. Phagocytosed Bordetella pertussis fails to survive in human neutrophils. Infect Immun. 2000;68:956–959. doi: 10.1128/iai.68.2.956-959.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masure H R. The adenylate cyclase toxin contributes to the survival of Bordetella pertussis within human macrophages. Microb Pathog. 1993;14:253–260. doi: 10.1006/mpat.1993.1025. [DOI] [PubMed] [Google Scholar]
- 29.Masure H R. Modulation of adenylate cyclase toxin production as Bordetella pertussis enters human macrophages. Proc Natl Acad Sci USA. 1992;89:6521–6525. doi: 10.1073/pnas.89.14.6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGuirk P, Mills K H. A regulatory role for interleukin 4 in differential inflammatory responses in the lung following infection of mice primed with Th1- or Th2-inducing pertussis vaccines. Infect Immun. 2000;68:1383–1390. doi: 10.1128/iai.68.3.1383-1390.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meade B D, Kind P D, Ewell J B, McGrath P P, Manclark C R. In vitro inhibition of murine macrophage migration by Bordetella pertussis lymphocytosis-promoting factor. Infect Immun. 1984;45:718–725. doi: 10.1128/iai.45.3.718-725.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meade B D, Kind P D, Manclark C R. Altered mononuclear phagocyte function in mice treated with the lymphocytosis promoting factor of Bordetella pertussis. Dev Biol Stand. 1985;61:63–74. [PubMed] [Google Scholar]
- 33.Meade B D, Kind P D, Manclark C R. Lymphocytosis-promoting factor of Bordetella pertussis alters mononuclear phagocyte circulation and response to inflammation. Infect Immun. 1984;46:733–739. doi: 10.1128/iai.46.3.733-739.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mills K H, Barnard A, Watkins J, Redhead K. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect Immun. 1993;61:399–410. doi: 10.1128/iai.61.2.399-410.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Njamkepo E, Pinot F, Francois D, Guiso N, Polla B S, Bachelet M. Adaptive responses of human monocytes infected by Bordetella pertussis: the role of adenylate cyclase hemolysin. J Cell Physiol. 2000;183:91–99. doi: 10.1002/(SICI)1097-4652(200004)183:1<91::AID-JCP11>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 36.Peppler M S. Two physically and serologically distinct lipopolysaccharide profiles in strains of Bordetella pertussis and their phenotype variants. Infect Immun. 1984;43:224–232. doi: 10.1128/iai.43.1.224-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turcotte M L, Martin D, Brodeur B R, Peppler M S. Tn5-induced lipopolysaccharide mutations in Bordetella pertussis that affect outer membrane function. Microbiology. 1997;143:2381–2394. doi: 10.1099/00221287-143-7-2381. [DOI] [PubMed] [Google Scholar]
- 38.Weiner R S, Mason R R. Subfractionation of human blood monocyte subsets with Percoll. Exp Hematol. 1984;12:800–804. [PubMed] [Google Scholar]
- 39.Weingart C L, Broitman-Maduro G, Dean G, Newman S, Peppler M, Weiss A A. Fluorescent labels influence phagocytosis of Bordetella pertussis by human neutrophils. Infect Immun. 1999;67:4264–4267. doi: 10.1128/iai.67.8.4264-4267.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weingart C L, Keitel W A, Edwards K M, Weiss A A. Characterization of bactericidal immune responses following vaccination with acellular pertussis vaccines in adults. Infect Immun. 2000;68:7175–7179. doi: 10.1128/iai.68.12.7175-7179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weingart C L, Mobberley-Schuman P S, Hewlett E L, Gray M C, Weiss A A. Neutralizing antibodies to adenylate cyclase toxin promote phagocytosis of Bordetella pertussis by human neutrophils. Infect Immun. 2000;68:7152–7155. doi: 10.1128/iai.68.12.7152-7155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weingart C L, Weiss A A. Bordetella pertussis virulence factors affect phagocytosis by human neutrophils. Infect Immun. 2000;68:1735–1739. doi: 10.1128/iai.68.3.1735-1739.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiss A A, Hewlett E L, Myers G A, Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983;42:33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiss A A, Melton A R, Walker K E, Andraos-Selim C, Meidl J J. Use of the promoter fusion transposon Tn5 lac to identify mutations in Bordetella pertussis vir-regulated genes. Infect Immun. 1989;57:2674–2682. doi: 10.1128/iai.57.9.2674-2682.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xing D K, Canthaboo C, Corbel M J. Effect of pertussis toxin on the induction of nitric oxide synthesis in murine macrophages and on protection in vivo. Vaccine. 2000;18:2110–2119. doi: 10.1016/s0264-410x(99)00562-9. [DOI] [PubMed] [Google Scholar]