Abstract
PURPOSE
Despite frequent use of self-expanding stents (SES) in treating obstructive arterial lesions in Takayasu arteritis (TA), spontaneous delayed stent expansion (SDSE) in TA remains unstudied. This study aimed primarily to document and quantify SDSE and secondarily to determine factors that might be associated with this process.
METHODS
Consecutive TA patients with obstructive arterial lesions undergoing routine percutaneous intervention involving SES use (sized 1 : 1 with normal vessel diameter but dilated only to 4 mm/5 mm) were recruited prospectively. Final stent diameters obtained were measured at 1 cm intervals along the length of the stent using fluoroscopic images and an indwelling marker catheter. At angiographic follow-up, stent diameters were measured again in identical fashion. Interval change in stent diameter at each point was averaged for each stent. In a small sub-study, intravascular ultrasound was used at follow-up to obtain potential mechanistic insights.
RESULTS
Seventeen TA patients (age 33 ± 13 years, 15 female) had 22 arterial obstructive lesions (16 occlusions and 18 subclavian) treated with 1 SES each. Follow-up obtained in all patients after 8.7 ± 3.8 months (range: 3-18 months) showed interval increase in mean stent diameter of 1.6 ± 0.5 mm, range 0.7-2.8 mm (P < .001); 36% of stents achieved 100% of the nominal diameter at follow-up, while 90% of stents achieved ≥90%. The degree of SDSE did not correlate with the segment of artery stented or with TA disease activity at baseline. Intravascular ultrasound in 4 lesions showed that SDSE was associated with positive medial-adventitial remodelling and that neointimal hyperplasia occurs concurrently, causing in-stent luminal narrowing.
CONCLUSION
SDSE, to diameters equal or close to nominal, occurs in all stenotic TA lesions treated with SES. The degree of SDSE does not correlate with the segment of artery stented or with TA disease activity at baseline. Preliminary results suggest that the mechanism by which SDSE is accommodated by the arterial wall is by positive medial-adventitial remodelling.
Main points
Self-expanding bare metal stents are commonly used in treating long obstructive arterial lesions in Takayasu arteritis.
Spontaneous delayed stent expansion, to diameters at or close to nominal, occurs in all stenotic lesions treated with bare self-expanding stents in Takayasu arteritis.
Preliminary results suggest that the mechanism by which spontaneous delayed stent expansion is accommodated by the arterial wall is by positive medial-adventitial remodelling.
Takayasu arteritis (TA) is a chronic, immune-mediated, inflammatory disease that primarily affects large- and medium-sized vessels, such as the aorta and its main branches.1 It usually has an early age of onset, is predominantly seen in females, and has a worldwide distribution.2 Inflammation of the vessel wall in TA commonly causes progressive narrowing of the vessel (stenosis or occlusion) which may lead to distal ischemia and related symptoms such as claudication or dizziness.3 Percutaneous interventions are being increasingly used to treat obstructive lesions in TA, often with deployment of stents following balloon dilatation of the lesion.4-6 Self-expanding bare metal stents are advantageous when the lesion length is long and in areas that may be subject to external compressive forces such as in the neck or limbs.7 An unreported phenomenon that we have noted at follow-up after deployment of self-expanding stents in obstructive lesions in TA is spontaneous expansion of the stent over time to reach its nominal diameter. This study aims primarily to document and quantify this phenomenon of spontaneous delayed stent expansion (SDSE) and secondarily to determine factors that might be associated with this process.
Methods
Patients
Patients who met both the American College of Rheumatology8 and the modified Ishikawa (clinical) criteria9 for the diagnosis of TA were prospectively and consecutively recruited into the study immediately after undergoing routine percutaneous intervention involving deployment of a self-expanding stent for obstructive lesions in the subclavian, common carotid or iliac artery. Patients were recruited irrespective of age, sex, disease activity, extent of disease, or need for intervention in other vessels. The study was approved by the institutional review board (decision number IRB 10896/October 3, 2017). Written informed consent was obtained routinely for angiography and intervention and specifically for participation in the study.
Disease activity and immunosuppressive therapy
Disease (arteritis) was classified as active if erythrocyte sedimentation rate was >20 mm at 1 h, and C-reactive protein was >6.0 mg/L, smouldering if 1 of the 2 was above this threshold and inactive if neither was above this threshold. All patients received long-term immunosuppressive therapy, usually a combination of mycophenolate and deflazacort, under the supervision of a rheumatologist. Dosage of medication was titrated based on disease activity and continued for several years. In most cases, immunosuppressive therapy was started well before the planned intervention, while in a few, it was started concomitantly.
Interventional treatment protocol
For long obstructive lesions, most often a 2-stage intervention was performed to minimize the risk of vessel rupture (Figure 1). In the initial intervention, the lesion was crossed or recanalized, dilated to only 4 or 5 mm (less than the estimated normal vessel diameter), and a self-expanding stent was deployed (sized 1 : 1 with the estimated normal vessel diameter). Since the distal normal vessel is often small due to chronic under-filling, stent diameters 1 or 2 mm larger are usually selected. The length of stent chosen was the least that could cover the lesion and reach the normal segment on both sides. At follow-up, planned after a 6- to 12-month interval, if there was >50% luminal diameter stenosis, balloon angioplasty was performed with the balloon sized 1 : 1 with the estimated normal vessel diameter; this was usually supplemented by drug-coated balloon application to limit intimal hyperplasia and restenosis.10,11 The 2-stage interventional strategy was based on the observation that even though the initial intervention in TA may result in restenosis,12-14 with repeated balloon dilatations (mean 1.6 sittings for subclavian lesions), sustained patency can be achieved in 87% of lesions.15 If subclavian artery stenting resulted in ostial narrowing of vertebral arteries that were flowing anterogradely at baseline, balloon angioplasty or stenting of the vertebral artery through the struts of the subclavian stent was performed to restore normal flow.
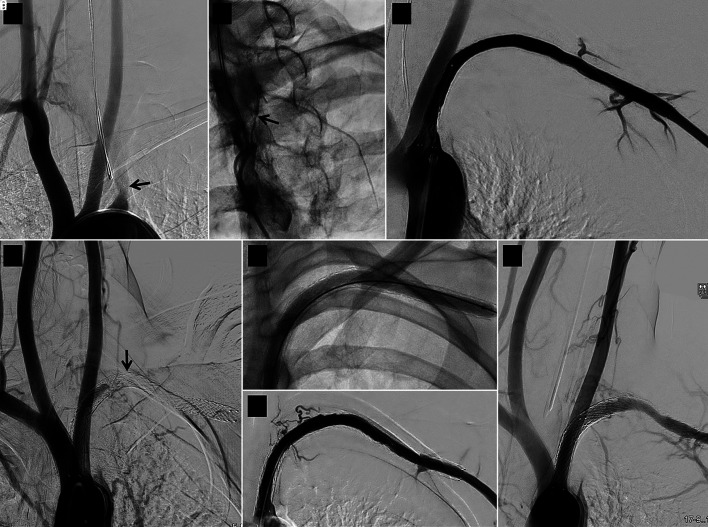
Figure 1.
Serial angiographic images of a case relating to stent 16 in the study. (a) Baseline image showing long left subclavian artery occlusion starting in the proximal segment (arrow). (b) A narrow track (arrow) is seen leading into the occlusion on wedge angiography. (c) Good immediate result after recanalization, 5 mm diameter balloon dilatation and implantation of a 7 × 120 mm self-expanding stent. (d) Follow-up angiogram 6 months later showing stent occlusion (arrow); this was treated by a second intervention that included 6 mm diameter balloon dilatation (e) achieving a good result immediately (f) and 11 months after the second intervention (g).
Study protocol
At the end of the stenting procedure, a centimeter marker catheter was introduced over an indwelling guidewire and positioned within the stent; fluoroscopic images were acquired in a view orthogonal to the vessel trajectory. Stent diameters were measured at 1 cm intervals along its entire length using imaging software (InstaPACS; Meddiff Technologies), and the mean was determined (Figure 2). Stent diameters were measured again during angiographic follow-up using the same technique, at the same locations, before any further intervention was carried out (Figure 3). The difference in mean stent diameter from baseline to follow-up was calculated. The subclavian artery was divided into 4 segments (shown in Figure 2b) to analyze potential regional variations in response. In a small sub-study, intravascular ultrasound was used at follow-up to obtain potential insights into the mechanism of stent expansion.
Figure 2.

(a) Measurement of post-implantation left subclavian artery stent diameters (stent 16 in the study) using imaging software. The software read the distance between the last 2 adjacent centimeter markers (extreme right) as 1.2 cm and so the measured stent diameters (in green boxes) had to be corrected by a factor of 0.833 to yield the correct stent diameters (marked in yellow). (b) Left subclavian artery angiogram (obtained immediately after a second intervention) with the 4 subclavian artery segments used in the study demarcated: 1—ostioproximal (first 1 cm), 2—beyond segment 1 and up to vertebral artery, 3—beyond segment 2 and up to first rib, 4—beyond first rib (axillary artery); the stent (stent 7 in the study) is deployed in segments 2, 3, and 4. For the right subclavian artery, segment 1 was the innominate artery, segment 2 between the right common carotid and right vertebral artery origins, and segments 3 and 4 similar to the left side.
Figure 3.
Spontaneous expansion of a left subclavian artery self-expanding stent of nominal diameter 7 mm (stent 16 in the study) over 6 months. (a) Baseline diameter measurements at 2, 6, and 10 cm from the distal end of the stent (which is on the right side of the image) immediately after stent deployment. (b) Corresponding stent diameter measurements at 6-month follow-up. The stent has expanded to its nominal diameter.
Statistics
Quantitative variables were summarized using mean and standard deviation for normally distributed variables. Paired/unpaired t-test was used to determine significance of differences between means. Standard errors of sample means and 95% CIs of the difference in means were determined. For all the analyses, 5% level of significance was considered significant. Statistical Package for the Social Sciences software (IBM SPSS Statistics version 21; International Business Machines Corporation) was used for data analysis. Factors that could potentially be associated with SDSE such as disease activity and anatomic location were assessed at follow-up.
Results
Seventeen TA patients (age 32 ± 13 years, range: 12-54 years, 15 female) had 22 arterial obstructive lesions (18 subclavian, 3 common carotid, and 1 external iliac artery) treated by percutaneous intervention involving deployment of 1 self-expanding stent in each lesion (Table 1). Of these 22 lesions, 16 were occlusions (13 subclavian, 2 carotid, and 1 iliac), and most were symptomatic (claudication 15, dizziness/blurring of vision 3, and stroke 1). The subclavian lesions typically involved multiple segments of the artery. The average stent length was 110 ± 50 mm. The nominal diameter of the stents (as specified by the manufacturer) ranged from 6 to 8 mm. The aorto-ostial part of the vessel (3 left common carotid and 8 left subclavian arteries) was included in the stented segment in 11 lesions and the axillary artery in 13 subclavian lesions. The disease was inactive at the time of first intervention in patients with 11/22 lesions (50%), smouldering in 9/22 (41%), and active in 2/22 (9%); patients with 17/22 lesions were already on long-term immunosuppressive therapy, and in the remainder, it was started at the time of first intervention. In the 2 patients with active disease, immunosuppressive therapy had been given for a year and inflammatory markers had reduced from earlier levels. The interventional procedures were successful in the short term in all lesions, achieving <50% residual stenosis without major complications (including arterial rupture and cerebrovascular accidents) and with relief of reversible symptoms. Four of 5 vertebral arteries that were flowing anterogradely at baseline and were covered by the subclavian artery stent had significant ostial narrowing that was resolved by balloon angioplasty in 3 and stenting in 1.
Table 1.
Procedure details
| Stent no. |
Patient age (y) and sex |
Disease activity# | Vessel stented |
Occlusion | Self-expanding stent used | Stent size: diameter × length (mm) |
Vessel ostium covered by stent |
Subclavian artery segments‡ covered by stent |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||||||
| 1 | 25 F | Smouldering | LSA | No | Epic | 7 × 120 |
No | − | − | + | + |
| 2 | 18 F | Inactive | LCCA | Yes | Epic | 7 × 100 |
Yes | Not applicable | |||
| 3 | 18 F | Inactive | LSA | Yes | Epic | 8 × 100 |
Yes | + | + | + | − |
| 4 | 23 F | Active | LSA | Yes | Epic | 7 × 60 |
Yes | + | + | + | − |
| 5 | 48 F | Inactive | RSA | No | Absolute | 7 × 100 |
No | − | + | + | + |
| 6 | 48 F | Inactive | LSA | Yes | Absolute | 8 × 100 |
No | − | + | + | + |
| 7 | 35 F | Smouldering | LSA | Yes | Epic | 7 × 100 |
Yes | − | + | + | + |
| 8 | 29 F | Inactive | LSA | Yes | Protégé | 6 × 200 |
No | − | − | + | + |
| 9 | 23 F | Inactive | RSA | Yes | Epic | 6 × 80 |
No | − | − | − | + |
| 10 | 23 F | Inactive | REIA | Yes | Epic | 8 × 120 |
No | Not Applicable | |||
| 11 | 27 F | Inactive | LSA | No | Protégé | 6 × 200 |
Yes | + | + | + | + |
| 12 | 54 F | Smouldering | LSA | Yes | Protégé | 7 × 100 |
Yes | − | + | + | + |
| 13 | 54 F | Inactive | RSA | Yes | Epic | 7 × 40 |
Yes | − | + | + | − |
| 14 | 49 F | Smouldering | LCCA | Yes | Epic | 8 × 40 |
Yes | Not applicable | |||
| 15 | 15 F | Active | LSA | Yes | Zilver | 6 × 200 |
Yes | + | + | + | + |
| 16 | 21 M | Inactive | LSA | Yes | Epic | 7 × 120 |
Yes | + | + | + | + |
| 17 | 50 M | Smouldering | LSA | Yes | Epic | 7 × 60 |
Yes | + | + | + | − |
| 18 | 35 F | Smouldering | LSA | Yes | Zilver | 6 × 200 |
Yes | + | + | + | + |
| 19 | 33 F | Smouldering | RSA | No | Epic | 7 × 120 |
No | − | − | − | + |
| 20 | 33 F | Smouldering | LCCA | No | Epic | 7 × 80 |
Yes | Not Applicable | |||
| 21 | 49 F | Smouldering | RSA | No | Epic | 7 × 80 |
No | − | − | − | + |
| 22 | 12 F | Inactive | LSA | Yes | Epic | 7 × 80 |
Yes | + | + | + | − |
Self-expanding stents: Epic (Boston Scientific); Absolute, Absolute Pro (Abbott Vascular); Protégé, Protege EverFlex (Medtronic); Zilver, Zilver Flex (Cook medical). #Disease (arteritis) was classified as active if the erythrocyte sedimentation rate was >20 mm at 1 h and C-reactive protein was >6.0 mg/L, smouldering if 1 of the 2 was above this threshold and inactive if neither was above this threshold. ‡Subclavian artery segments are defined in Figure 2b. F, female; M, male; LSA, left subclavian artery; LCCA, left common carotid artery; RSA, right subclavian artery; REIA, right external iliac artery.
Angiographic follow-up obtained in all patients after 8.7 ± 3.8 months (range: 3-18 months) showed interval increase in mean stent diameter of 1.6 ± 0.5 mm, range 0.7-2.8 mm (P < .001; Table 2); 36% of stents achieved 100% of the nominal diameter, and 90% of stents achieved ≥90% of the nominal diameter at follow-up. Intravascular ultrasound in 3 patients (4 lesions) at follow-up showed that SDSE was associated with positive medial-adventitial remodelling and that stent expansion did not occur by extrusion of stent struts through the arterial wall; concomitant neointimal hyperplasia within the stent resulted in luminal narrowing (Figure 4). Factors potentially affecting the degree of SDSE were analyzed (Figure 5). In left subclavian and left common carotid arteries, the degree of SDSE was not significantly different between stented aorto-ostial segments and the remaining segments (P = .31). The axillary artery (segment 4 in Figure 2b) showed slightly more SDSE (1.64 mm) compared to the other segments (1.26 mm), though this did not achieve statistical significance (P = .060). Disease activity (categorized into active/smouldering versus inactive) did not correlate with the degree of SDSE (P = .66). Lesions with >50% luminal narrowing at the first follow-up (17/22) successfully underwent a second percutaneous intervention as planned in the interventional treatment protocol; 3 lesions required additional stent deployment. All repeat interventions were immediately successful, uncomplicated, and usually involved 1-day hospitalization. After further follow-up, sustained stent patency was seen in 18/22 lesions (82%) while 4/22 (18%) had restenosis again (Table 2); the latter 4 lesions were subjected to a third percutaneous intervention (done in a similar fashion to the second) whose follow-up is awaited.
Table 2.
Stent expansion and outcome at follow-up
| Stent no. | Proportion of NSD reached at implant | Duration of follow-up | First follow-up | Intervention (PI-2) at first follow-up | Residual stenosis after PI-2 | Duration of further follow-up | ISR at last follow-up | ||
|---|---|---|---|---|---|---|---|---|---|
| Proportion of NSD reached | Mean ↑ in stent diameter | Luminal diameter stenosis | |||||||
| % | months | % | mm | % | % | months | |||
| 1 | 67 | 18 | 97 | 2.1 | 100 | BA, DCB | 20 | 14 | No |
| 2 | 64 | 7 | 98 | 2.4 | 84 | BA, DCB | <5 | 25 | No |
| 3 | 56 | 7 | 92 | 2.8 | 87 | BA, DCB | <5 | 25 | No |
| 4 | 87 | 7 | 97 | 0.7 | 100 | BA, DCB | 14 | 5 | No |
| 5 | 70 | 9 | 87 | 1.2 | 100 | BA, DCB | 33 | 18 | No |
| 6 | 63 | 9 | 79 | 1.3 | 85 | BA, DCB, stent | <5 | 18 | No |
| 7 | 59 | 6 | 92 | 2.4 | 100 | BA | 33 | 9 | Yes |
| 8 | 72 | 9 | 100 | 1.7 | 61 | BA, DCB | 39 | 6 | No |
| 9 | 68 | 12 | 90 | 1.3 | 40 | BA, DCB | 21 | 10 | No |
| 10 | 68 | 12 | 92 | 2.0 | 11 | None | NA | 10 | No |
| 11 | 75 | 6 | 100 | 1.5 | 91 | BA, DCB | 25 | 13 | Yes |
| 12 | 80 | 12 | 100 | 1.4 | 12 | None | NA | 11 | No |
| 13 | 80 | 6 | 100 | 1.4 | <5 | None | NA | 17 | No |
| 14 | 80 | 10 | 100 | 1.6 | 30 | None | NA | 14 | No |
| 15 | 70 | 3 | 95 | 1.5 | 76 | BA, DCB | 27 | 8 | No |
| 16 | 81 | 6 | 100 | 1.3 | 100 | BA, DCB, stent | <5 | 11 | No |
| 17 | 71 | 5 | 100 | 2.0 | 88 | BA, DCB | 38 | 7 | Yes |
| 18 | 70 | 5 | 96 | 1.6 | 72 | BA, DCB | 15 | 8 | No |
| 19 | 69 | 6 | 90 | 1.5 | 100 | BA, DCB | 8 | 3 | No |
| 20 | 71 | 6 | 95 | 1.7 | 62 | BA, stent | <5 | 3 | No |
| 21 | 86 | 10 | 100 | 1.0 | 100 | BA, DCB | 25 | 8 | Yes |
| 22 | 72 | 10 | 94 | 1.5 | 36 | None | NA | 0 | No |
| Mean | 71 | 8.7 | 95 | 1.6 | 70 | - | - | 11 | - |
NSD, nominal stent diameter; ↑, increase; PI-2, second percutaneous intervention; BA, balloon angioplasty; DCB, drug-coated balloon application; NA, not applicable; ISR, in-stent restenosis (luminal diameter stenosis >50%).
Figure 4.
Follow-up intravascular ultrasound images of left subclavian arteries in which self-expanding bare nitinol stents were deployed 6 months earlier. (a) Stent 11 in the study, nominal diameter 6 mm. (b) Stent 12 in the study, nominal diameter 7 mm. Both stents have expanded to the nominal diameter (red arrows), but neointimal hyperplasia (yellow arrows) has considerably reduced the lumen (black space in the center of each image) in the first. The medial and adventitial layers of the vessel wall lie external to the stent. The distance between adjacent fine blue marker dots is 1 mm.
Figure 5.
Bar chart illustrating the extent of spontaneous delayed stent expansion seen in the study and the relationship of 3 different factors with the degree of mean stent expansion. Error bars represent standard errors of sample means. CI indicates 95% confidence intervals of the difference in means.
Discussion
The primary objective of this study was to document and quantify the phenomenon of spontaneous delayed expansion of self-expanding stents in obstructive lesions in TA. The outward expansive force exerted by self-expanding stents that have not yet attained their nominal diameter is likely to be responsible for this phenomenon. Though SDSE may be anticipated intuitively, we could not find any previous report documenting or studying this phenomenon. Intravascular ultrasound showed that SDSE was associated with positive medial-adventitial remodelling and that the mechanism of stent expansion was not by extrusion of stent struts through the wall of the artery; however, this was demonstrated in a small sub-study that was not the primary objective and needs to be confirmed in a larger study. The high percentage of the nominal diameter achieved by the self-expanding stents over time in this study, irrespective of TA disease activity and vessel segment stented, indicates adaptability of the vessel wall to accommodate stent expansion despite the fibrotic nature of arterial lesions seen in TA.16 Stent expansion, positive medial-adventitial remodelling, and intimal hyperplasia were seen as early as 3 months after the initial intervention in this study, indicating that these processes happen rapidly. It should be noted that SDSE results in increase in cross-sectional area of the stent, while intimal hyperplasia results in reduction in the patent luminal area within the stent.
Awareness of the phenomenon of SDSE gives interventionists the option of not having to dilate self-expanding stents to the reference diameter of the vessel during the first intervention of obstructive lesions in TA, which reduces the risk of arterial rupture. Typically balloon dilatation to only 4 or 5 mm is carried out in the first intervention in arteries that are 6 or 7 mm in reference diameter. If subsequent interventions are required, dilatation using balloons of the same diameter as the reference vessel diameter can be safely carried out (without risk of rupture) because the stent has already expanded to its nominal diameter (which was selected to be equal to the reference vessel diameter) and the media-adventitia of the artery has positively remodelled. The obstructing intimal hyperplasia is compressed in the process, and patent luminal diameter is increased.
Positive vessel remodelling is a common phenomenon, the growth of the internal mammary artery after coronary artery bypass grafting and that of an arteriovenous shunt after dialysis fistula creation being well-known examples.17 Glagov et al.18 showed that in atherosclerotic human coronary arteries, the media-adventitia remodels positively as the plaque burden grows, resulting in no luminal area compromise until the lesion reachs 40% area stenosis. Positive vessel remodelling induced by drugs eluted by coronary stents19 is thought to be a major cause of delayed stent mal-apposition and very late stent thrombosis20; however, in most patients, there is concurrent neointimal hyperplasia caused by foreign body reaction to stents which prevents delayed stent mal-apposition.17 It should be noted that coronary stents are balloon-expandable, are usually dilated to the estimated normal vessel diameter at the time of deployment, and do not enlarge spontaneously any further. The situation in the patients in the present study was dissimilar in that the stents were self-expanding, were dilated to a diameter less than the vessel diameter at the time of deployment, and were not drug-eluting; also, the underlying pathology (TA), the vessel involved (medium-sized arteries), and lesion length (mean stent length 11 cm) were different.
Restenosis is a common accompaniment of percutaneous intervention (both plain balloon and stent-supported angioplasty) in TA,12,13,21-24 with some studies reporting rates above 70%.12,13 At our center, we perform repeat balloon angioplasty in restenotic lesions, usually supplemented with drug-coated balloon application which has shown promising early results.10-11 Most restenotic lesions require only 1 or 2 additional procedures before sustained success is obtained.15,25,26 With each repeat intervention in TA, the cumulative sustained vessel patency rate increases, reaching 87% for all vessels considered together in a series of 401 TA patients undergoing percutaneous interventions.15 This is comparable to the 82% sustained stent patency obtained in this study after 1 or 2 interventions. Repeat interventions usually are simple percutaneous procedures requiring just a day in hospital; importantly, balloons can be safely sized 1 : 1 with the estimated normal vessel diameter with low risk of rupture at this stage because the stent is now fully expanded.25,26
The subclavian artery has some features that could predispose lesions here to restenose after percutaneous intervention in TA: lesions here are usually long occlusions, the artery is subject to movement and external forces (akin to the superficial femoral artery), and is the most involved vessel in TA. The frequent involvement of the subclavian artery in TA makes it the cornerstone of diagnostic criteria in TA,8,9 and this selectively higher involvement and tendency to restenose15 could potentially be related to distinct toll-like receptor profile in this macrovascular territory that imposes a vessel-specific risk.27
Limitations of this study include the facts that only a moderate number of patients and lesions were studied and that it was a single-center study. The distribution of lesions was skewed toward the subclavian artery, and few carotid or iliac lesions were studied. Intravascular ultrasound was used only within a small sub-study due to cost issues and should ideally have been used in all lesions; however, this was not the primary objective of the study.
Conclusion
SDSE, to diameters at or close to nominal, occurs in all stenotic TA lesions treated with self-expanding stents. The degree of SDSE does not correlate with the segment of artery stented or with TA disease activity at baseline. Positive medial-adventitial remodelling appears to be the mechanism by which delayed stent expansion is accommodated by the arterial wall, but this finding needs to be confirmed by larger studies.
Footnotes
Conflict of interest disclosure The authors declared no conflicts of interest.
References
- 1. Kerr GS. Takayasu’s arteritis. Rheum Dis Clin North Am. 1995;21(4):1041 1058. 10.1016/S0889-857X(21)00484-1) [DOI] [PubMed] [Google Scholar]
- 2. Onen F, Akkoc N. Epidemiology of Takayasu arteritis. Presse Med. 2017;46(7-8 Pt 2):e197 e203. 10.1016/j.lpm.2017.05.034) [DOI] [PubMed] [Google Scholar]
- 3. Numano F, Okawara M, Inomata H, Kobayashi Y. Takayasu’s arteritis. Lancet. 2000;356(9234):1023 1025. 10.1016/S0140-6736(00)02701-X) [DOI] [PubMed] [Google Scholar]
- 4. Min PK, Park S, Jung JH.et al. Endovascular therapy combined with immunosuppressive treatment for occlusive arterial disease in patients with Takayasu’s arteritis. J Endovasc Ther. 2005;12(1):28 34. 10.1583/12-01-04-1329.1) [DOI] [PubMed] [Google Scholar]
- 5. Gülcü A, Gezer NS, Akar S, Akkoç N, Önen F, Göktay AY. Long-term follow-up of endovascular repair in the management of arterial stenosis caused by Takayasu’s arteritis. Ann Vasc Surg. 2017;42:93 100. 10.1016/j.avsg.2016.10.066) [DOI] [PubMed] [Google Scholar]
- 6. Che W, Xiong H, Jiang X.et al. Stenting for middle aortic syndrome caused by Takayasu arteritis-immediate and long-term outcomes. Catheter Cardiovasc Interv. 2018;91(S1):623 631. 10.1002/ccd.27492) [DOI] [PubMed] [Google Scholar]
- 7. Mueller DK. Peripheral vascular stent insertion. Medscape. 2020. Available at: https://emedicine.medscape.com/article/1839716-overview#a5. Accessed November 2020. [Google Scholar]
- 8. Arend WP, Michel BA, Bloch DA.et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33(8):1129 1134. 10.1002/art.1780330811) [DOI] [PubMed] [Google Scholar]
- 9. Spacek M, Zimolova P, Veselka J. Takayasu arteritis: use of drug-eluting stent and balloon to treat recurring carotid restenosis. J Invasive Cardiol. 2012;24(9):E190 E192. [PubMed] [Google Scholar]
- 10. Yamamoto T, Shirai K, Okamura K, Urata H. Two years efficacy of paclitaxel-coated balloon dilation for in-stent renal artery restenosis due to Takayasu arteritis. Am J Case Rep. 2019;20:1089 1093. 10.12659/AJCR.916105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sharma BK, Jain S, Suri S, Numano F. Diagnostic criteria for Takayasu arteritis. Int J Cardiol. 1996;54(suppl):S141 S147. 10.1016/s0167-5273(96)88783-3) [DOI] [PubMed] [Google Scholar]
- 12. Maksimowicz-McKinnon K, Clark TM, Hoffman GS. Limitations of therapy and a guarded prognosis in an American cohort of Takayasu arteritis patients. Arthritis Rheum. 2007;56(3):1000 1009. 10.1002/art.22404) [DOI] [PubMed] [Google Scholar]
- 13. Cong XL, Dai SM, Feng X.et al. Takayasu’s arteritis: clinical features and outcomes of 125 patients in China. Clin Rheumatol. 2010;29(9):973 981. 10.1007/s10067-010-1496-1) [DOI] [PubMed] [Google Scholar]
- 14. Keser G, Aksu K, Direskeneli H. Takayasu arteritis: an update. Turk J Med Sci. 2018;48(4):681 697. 10.3906/sag-1804-136) [DOI] [PubMed] [Google Scholar]
- 15. Joseph G, Danda D. Outcome of 1516 percutaneous interventions in 401 patients with Takayasu arteritis – a single-center experience from South India. Presse Med. 2013;42(4):721. 10.1016/j.lpm.2013.02.163) [DOI] [Google Scholar]
- 16. Vaideeswar P, Deshpande JR. Pathology of Takayasu arteritis: a brief review. Ann Pediatr Cardiol. 2013;6(1):52 58. 10.4103/0974-2069.107235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kleber FX, Schulz A, Köln P. Positive vessel remodelling. Eur Med J. 2018;3:111 115. Available at: https://www.emjreviews.com/cardiology/article/positive-vessel-remodelling/. Accessed November 2020. [Google Scholar]
- 18. Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316(22):1371 1375. 10.1056/NEJM198705283162204) [DOI] [PubMed] [Google Scholar]
- 19. Hong MK, Mintz GS, Lee CW.et al. Late stent malapposition after drug-eluting stent implantation: an intravascular ultrasound analysis with long-term follow-up. Circulation. 2006;113(3):414 419. 10.1161/CIRCULATIONAHA.105.563403) [DOI] [PubMed] [Google Scholar]
- 20. Cook S, Wenaweser P, Togni M.et al. Incomplete stent apposition and very late stent thrombosis after drug-eluting stent implantation. Circulation. 2007;115(18):2426 2434. 10.1161/CIRCULATIONAHA.106.658237) [DOI] [PubMed] [Google Scholar]
- 21. Kim YW, Kim DI, Park YJ.et al. Surgical bypass vs endovascular treatment for patients with supra-aortic arterial occlusive disease due to Takayasu arteritis. J Vasc Surg. 2012;55(3):693 700. 10.1016/j.jvs.2011.09.051) [DOI] [PubMed] [Google Scholar]
- 22. Saadoun D, Lambert M, Mirault T.et al. Retrospective analysis of surgery versus endovascular intervention in Takayasu arteritis: a multicenter experience. Circulation. 2012;125(6):813 819. 10.1161/CIRCULATIONAHA.111.058032) [DOI] [PubMed] [Google Scholar]
- 23. Ham SW, Kumar SR, Wang BR, Rowe VL, Weaver FA. Late outcomes of endovascular and open revascularization for nonatherosclerotic renal artery disease. Arch Surg. 2010;145(9):832 839. 10.1001/archsurg.2010.183) [DOI] [PubMed] [Google Scholar]
- 24. Wu X, Duan HY, Gu YQ.et al. Surgical treatment of brachiocephalic vessel involvement in Takayasu’s arteritis. Chin Med J (Engl). 2010;123(9):1122 1126. [PubMed] [Google Scholar]
- 25. Lee BB, Laredo J, Neville R, Villavicencio JL. Endovascular management of Takayasu arteritis: is it a durable option? Vascular. 2009;17(3):138 146. 10.2310/6670.2009.00012) [DOI] [PubMed] [Google Scholar]
- 26. Joseph G. L49. Percutaneous interventions in Takayasu arteritis. Presse Med. 2013;42(4 Pt 2):635 637. 10.1016/j.lpm.2013.01.045) [DOI] [PubMed] [Google Scholar]
- 27. Pryshchep O, Ma-Krupa W, Younge BR, Goronzy JJ, Weyand CM. Vessel-specific toll-like receptor profiles in human medium and large arteries. Circulation. 2008;118(12):1276 1284. 10.1161/CIRCULATIONAHA.108.789172) [DOI] [PMC free article] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a