Abstract
Objective:
Trichorhinophalangeal syndrome is a rare autosomal dominant disorder characterized by distinctive craniofacial and skeletal abnormalities. This study aimed to delineate the trichorhinophalangeal syndrome phenotype and to compare the clinical and molecular findings between trichorhinophalangeal syndrome types I and II.
Materials and Methods:
A total of 22 trichorhinophalangeal syndrome patients aged 0.9-45 years from 17 families were enrolled. Nineteen patients were diagnosed with trichorhinophalangeal syndrome I and 3 with trichorhinophalangeal syndrome II. Genetic analyses were made by TRPS1 sequencing and/or chromosomal microarray analyses.
Results
: A novel frameshift variant (c.531_532del), a known missense variant, and whole-gene deletions were the pathogenic TRPS1 variants detected in trichorhinophalangeal syndrome I. Three trichorhinophalangeal syndrome II patients had large deletions with variable breakpoints involving the TRPS1-EXT1 interval. All patients had the typical craniofacial findings of trichorhinophalangeal syndrome such as a pear-shaped nose, long philtrum, and thin upper lip, as well as cone-shaped epiphyses. Sparse hair and eyebrows (20/22), short metacarpals and metatarsals (20/22), and small hands (19/22) were common. While craniofacial and limb abnormalities were similar in trichorhinophalangeal syndrome I and II, 3 of 19 trichorhinophalangeal syndrome I patients had mild, and 2 of 3 trichorhinophalangeal syndrome II patients had severe intellectual disability. Three trichorhinophalangeal syndrome II patients including the patient with the EXT1 deletion beginning from exon 2 had exostoses. In trichorhinophalangeal syndrome II, although microdeletion sizes and facial or skeletal features were not correlated, patients with larger deletions had severe intellectual disability.
Conclusion:
This study has expanded the existing knowledge on the phenotype–genotype spectrum in trichorhinophalangeal syndrome. We suggest including the EXT1 gene partially in the minimal critical region for trichorhinophalangeal syndrome II.
Keywords: Trichorhinophalangeal syndrome, TRPS1, brachydactyly, cone-shaped epiphyses, exostosis
What is already known on this topic?
Trichorhinophalangeal syndrome (TRPS) is a rare autosomal dominant disease characterized by distinctive facial, ectodermal, and skeletal malformations. Trichorhinophalangeal syndrome I is the classical form caused by the haploinsufficiency of TRPS1. Trichorhinophalangeal syndrome II is a contiguous deletion syndrome that involves TRPS1 and EXT1, causing additional findings such as exostoses and intellectual impairment.
What this study adds to this topic?
Typical facial findings and cone-shaped epiphyses were constant findings in TRPS I and II. Severe intellectual disability and exostoses were distinguishable features of TRPS II from TRPS I. Moreover, we suggest that the EXT1 gene should be included in the minimal critical region for TRPS II, beginning from exon 2.
Introduction
Trichorhinophalangeal syndrome (TRPS) is a rare autosomal dominant disease characterized by distinctive facial findings including a bulbous nose with underdeveloped alae, long philtrum, thin upper lip, protruding ears, and ectodermal characteristics such as sparse and slowly growing hair, sparse eyebrows, and dystrophic nails. In addition, patients present skeletal abnormalities, such as mild to severe brachydactyly with cone-shaped epiphyses at the phalanges, and short stature.1-4 Trichorhinophalangeal syndrome type I (TRPS I, OMIM 604386) is the classical form caused by heterozygous pathogenic TRPS1 variants or whole-gene deletions. Trichorhinophalangeal syndrome type II (TRPS II or Langer–Giedion syndrome, OMIM 150230), which is characterized by multiple exostoses and mild-to-moderate intellectual disability, is a contiguous gene deletion syndrome caused by a deletion in the chromosome 8q23.3-q24.11 region, spanning the TRPS1 and EXT1 genes.4 Trichorhinophalangeal syndrome III (OMIM 190351), which is associated with more severe short stature and severe brachydactyly due to short metacarpals, is currently considered within the phenotypic spectrum of TRPS I.2,4
TRPS1 encodes TRPS1, a polypeptide of 1281 amino acids that regulates chondrocyte proliferation and apoptosis through Stat3 signaling as a nuclear transcription factor.5 The TRPS1 gene is involved in craniofacial development, epithelial/mesenchymal cell interactions in developing hair follicles, and chondrocyte differentiation in the growth plate.6-8 The phenotype seen in TRPS II is typically dominated by the deletion of TRPS1 and EXT1, and the deletion of EXT1 is responsible for multiple exostoses. Moreover, it has been suggested that additional clinical features may be present in large deletions involving more genes outside the TRPS1-EXT1 interval.3
The aim of this study is to delineate the clinical spectrum of a Turkish TRPS cohort, to compare the clinical and molecular findings between patients diagnosed with TRPS types I and II, and to investigate the genotype–phenotype relationship by comparing molecular analysis results.
Materials and Methods
Ethical Compliance
Each patient or the parents of the patient gave their written informed consent according to the International Ethical Guidelines and Declaration of Helsinki for molecular analyses as well as for the publication of clinical findings, patient images, and molecular data. The study was approved by the Ethics Committee of Cerrahpasa Medical Faculty (approval date/number: December 03, 2020/158702).
Patients
This study included 22 patients from 17 families followed up by our department with clinical diagnoses of TRPS I and TRPS II. The clinical diagnosis of TRPS I was made based on distinctive facial characteristics (a bulbous nose with underdeveloped alae, long philtrum, thin upper lip, protruding ears), ectodermal manifestations (sparse hair and eyebrows, dystrophic nails), and skeletal features (short stature, brachydactyly, ulnar or radial deviation of the fingers, cone-shaped epiphyses at the phalanges, hip dysplasia).1,2,4 The diagnosis of TRPS II was established with the presence of multiple exostoses in addition to the clinical findings observed in TRPS I.4 Demographic data of the patients including gender, age, and family history were noted. The measurements of height, weight, and head circumference, as well as the clinical data including facial, ectodermal, skeletal abnormalities, and radiological findings of the patient cohort were evaluated for the study. The Denver developmental screening test II (DDST-II) was performed to detect neuromotor retardation in terms of personal-social skills, speech, and fine and coarse motor abilities. The developmental quotient (DQ) was calculated by dividing the developmental age by chronologic age.
Genetic Studies
Cytogenetic analyses were performed on peripheral blood lymphocytes from the patients. Blood samples were harvested and treated by conventional cytogenetic methods. Chromosomes were analyzed by standard Giemsa Trypsin G-banding. Chromosomal microarray analysis using the HumanCytoSNP-12 BeadChip array (Illumina Inc., San Diego, Calif, USA) was performed according to the manufacturer’s instructions. This array contains approximately 300 000 single nucleotide polymorphism markers per sample, spanning the entire genome with an average probe spacing of 72 kb. The B-allele frequency and log R ratio data were analyzed with KaryoStudio software (Illumina Inc). The genomic positions were determined using GRCh38/hg38, UCSC Genome Browser.
Genomic DNA was extracted from the blood samples of the patients using standard techniques after obtaining informed consents from the patients or their parents. TRPS1 (NM_014112.5) primers for all exons and exon-intron flanking regions were designed. Sanger sequencing reaction was performed on capillary electrophoresis (ABI 3500, Applied Biosystems, Foster City, Calif, USA), and BLAST searches were performed against public databases for the annotations of the disclosed variants.
Variant interpretations were made according to the American College of Medical Genetics and Genomics practice guidelines.9 Bioinformatics tools (PolyPhen2, SIFT, Mutation Taster, DANN) and electronic data (dbSNP, ExAC, 1000G, ClinVar, Varsome, HGMD Professional version, DGV, DECIPHER) were used to identify the variant pathogenicity.
Statistical Analysis
Statistical analysis was performed with the International Business Machines’ Statistical Package for Social Sciences statistical software (SPSS v.21.0 for Mac OS X). Continuous data are presented as median (range), and count data are presented as number (n) and percentage (%). Due to the small number of patients, the data were mainly evaluated as descriptive.
Results
Clinical Findings
The clinical features of the cohort are summarized in Table 1. The median age of the study cohort at the first examination was 12 years and included 11 males and 11 females. While 3 unrelated patients (P) (P1, P2, and P6) were diagnosed with TRPS II, 4 of the 14 families diagnosed with TRPS I were familial.
Table 1.
Summary of the Clinical Characteristics of the Total TRPS Cohort
| Case | Diagnosis | Sex | Age (*/**) | Height (SDS) | OFC (SDS) | Sparse Hair | Thick Eyebrows | Pear-Shaped Nose with Underdeveloped Alae | Long Philtrum | Thin Upper Lip | Prominent Ears | Small Hands/Short Metacarpals | Dystrophic Nails | Cone-Shaped Epiphysis | Exostoses | Hip Dysplasia | DQ/IQ | Other Clinical Features |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | TRPS II | M | 2 yr. 7 mo./ 6 yr. 1 mo. |
103 cm (−2.4)** | 46 cm (−4.1)** | + | + | + | + | + | + | +/+ | − | + | + | − | DQ = 89 | |
| P2 | TRPS II | F | 1 yr. 4 mo./ 3 yr. 4 mo. |
90 cm (−1.6)** | 46 cm (−1.8)** | + | − | + | + | + | + | +/+ | − | + | + | − | DQ = 46 | Scoliosis |
| P3 | TRPS I | M | 1 yr. 3 mo./ 3 yr. 10 mo. |
97 cm (−0.9)** | 51 cm (+0.5)** | + | − | + | + | + | − | +/+ | + | + | − | − | DQ = 59 | Autism spectrum disorder |
| P4 | TRPS I | M | 11 mo./ N/A |
67 cm (−2.8)* | 45 cm (−0.8)* | + | − | + | + | + | − | +/+ | + | N/A | − | − | Normal | |
| P5 | TRPS I | F | 30 yr. 1 mo./ 32 yr. 7 mo. |
N/A | N/A | + | − | + | + | + | + | +/+ | + | + | − | − | Normal | |
| P6 | TRPS II | F | 14 yr. 3 mo./ N/A |
129 cm (−4.8)* | 48 cm (−5.3)* | + | + | + | + | + | + | −/+ | + | + | + | + | N/A (intellectual disability) | Oligodactyly on both feet |
| P7 | TRPS I | F | 11 yr. 9 mo./ 30 yr. 10 mo. |
152 cm (−1.7)** | 54 cm (−0.3)** | + | + | + | + | + | − | +/+ | + | + | − | − | Normal | |
| P8 | TRPS I | F | 11 yr./ 26 yr. 7 mo. |
126 cm (−2.4)* | 52 cm (−0.4)* | + | + | + | + | + | + | +/+ | − | + | − | − | IQ = 78 | Hypopituitarism, hearing deficit |
| P9 | TRPS I | F | 12 yr. 7 mo./ 13 yr. 6 mo. |
111 cm (−5.8)* | 46 cm (−5)* | + | − | + | + | + | − | −/− | − | + | − | − | Normal | |
| P10 | TRPS I | F | 3 yr. 7 mo./ 10 yr. 11 mo. |
158 cm (+2.1)** | 53 cm (+0.3)** | + | + | + | + | + | − | +/+ | − | + | − | − | IQ = 86 | |
| P11 | TRPS I | F | 1 yr. 2 mo./ 4 yr. 11 mo. |
109 cm (+0.2)** | 51 cm (+0.5)** | + | + | + | + | + | − | −/− | − | N/A | − | − | DQ = 76 | Scoliosis |
| P12 | TRPS I | M | 12 yr. 4 mo./ N/A |
146 cm (−0.4)* | 54 cm (+0.1)* | + | + | + | + | + | + | +/+ | + | + | − | + | Normal | |
| P13 | TRPS I | F | 22 yr. 10 mo./ N/A |
153 cm (−1.5)* | 57 cm (+2.4)* | + | − | + | + | + | − | +/+ | + | N/A | − | + | Normal | |
| P14 | TRPS I | F | 10 yr. 10 mo./ N/A |
130 cm (−1.7)* | 52 cm (−0.3)* | + | + | + | + | + | − | +/+ | + | + | − | − | Normal | |
| P15 | TRPS I | M | 5 yr. 2 mo./ 6 yr. 8 mo. |
103 cm (−3)** | 46 cm (−4.3)** | + | − | + | + | + | − | +/+ | − | N/A | − | − | DQ = 72 | Operated cleft palate, hearing deficit |
| P16 | TRPS I | M | 7 yr. 2 mo./ N/A |
112 cm (−1.9)* | 52 cm (−0.01)* | + | + | + | + | + | + | +/+ | − | + | − | + | Normal | |
| P17 | TRPS I | M | 39 yr./ N/A |
N/A | N/A | + | − | + | + | + | + | +/+ | − | + | − | + | Normal | |
| P18 | TRPS I | M | 13 yr. 11 mo./ N/A |
155 cm (−0.8)* | 52 cm (−1.6)* | − | + | + | + | + | + | +/+ | + | + | − | − | Normal | |
| P19 | TRPS I | F | 17 yr./ N/A |
146 cm (−2.5)* | N/A | + | + | + | + | + | + | +/+ | − | N/A | − | − | Normal | |
| P20 | TRPS I | M | 38 yr./ N/A |
153 cm (−3.2)* | N/A | + | + | + | + | + | + | +/+ | − | N/A | − | − | Normal | |
| P21 | TRPS I | M | 14 yr. 3 mo./ N/A |
157 cm (−0.8)* | 57 cm (+1.5)* | − | + | + | + | + | − | +/+ | + | + | − | − | Normal | Scoliosis |
| P22 | TRPS I | M | 45 yr./ N/A |
N/A | N/A | + | + | + | + | + | + | +/+ | − | N/A | − | − | Normal |
*At the first examination; **At the last examination.
DQ, development quotient; F, female; IQ, intelligence quotient; M, male; mo., months; N/A, not available; OFC, occipitofrontal circumference; SDS, standard deviation score; TRPS, trichorhinophalangeal syndrome; yr., years.
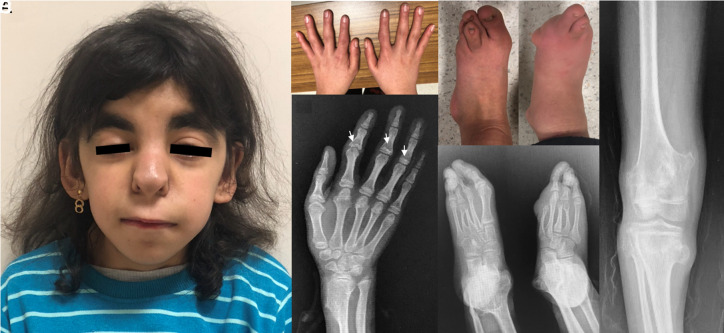
All patients in the cohort were observed to have a large nose with a broad ridge and tip and underdeveloped alae, long philtrum, and thin upper lip vermillion. Sparse and slowly growing hair (20/22), broad eyebrows (14/22), and protruding ears (12/22) were also common findings. The main findings in the limbs were short metacarpals and metatarsals (20/22), small hands (19/22), and brachydactyly (18/22). Dystrophic nails were present in 10 patients. All patients with available radiographic examination had cone-shaped epiphyses at the phalanges. One patient diagnosed with TRPS II (P6) had oligodactyly on both feet. Five patients (5/22) including P6 had marked hip dysplasia. Multiple exostoses were detected only in 3 patients with TRPS II (P1, P2, and P6). The earliest age at which exostoses were noticed was around 2 years in P1 and 3 years in P2. Only 1 TRPS II patient (P2) had persistent foramen ovale, and none of the remaining patients had a significant cardiac abnormality. The craniofacial characteristics, distal limb abnormalities, and radiological findings of P6 and P16 are shown in Figures 1 and 2, respectively.
Figure 1.
Photographs and radiological findings of P6 at 14 years of age. A long face, thick eyebrows, ptosis on the right eye, a pear-shaped nose with a broad septum and underdeveloped alae, long philtrum, thin upper lip, and protruding ears were prominent craniofacial features of the patient (A). Note the ulnar and radial deviation of the fingers and metacarpal shortening bilaterally (B), the cone-shaped epiphyses of the second to fourth middle phalanges (arrows), and the exostosis affecting the distal radius (C). Oligodactyly and dysplastic nails were visible bilaterally (D). Radiological examination of the feet (E) revealed the agenesis of the right big toe, short and irregular metatarsal bones, and multiple exostoses affecting both ankles. Note shorter third and fourth metatarsal bones and a missing toe distal to these bones. Multiple exostoses can be seen in the distal femur, proximal tibia, and fibula (F).
Figure 2.
Photographs and hand x-ray findings of P16 at 7 years of age. Sparse and slowly growing hair, broad eyebrows, a bulbous nose with underdeveloped alae, long philtrum, thin upper lip, and protruding ears were prominent craniofacial findings of the patient (A). Note the small hands with short metacarpals and brachydactyly (B, C), and cone-shaped epiphyses of the second to fourth proximal phalanges (arrows).
Patients’ median standard deviation score (SDS) of the occipitofrontal circumference at their last examination was −0.3, but the SDS values were in a broad range from −5.3 to 2.4. From the total cohort, 2 patients with TRPS I (P9 and P15) and 2 patients with TRPS II (P1 and P6) had microcephaly that did not correlate with intellectual development. Patients’ median SDS of height was −1.7 and the values were also in a heterogeneous distribution (range: −5.8 to 2.1). Four patients with microcephaly (P1, P6, P9, and P15) also had short stature, in addition, short stature was observed in 4 further patients (P4, P8, P19, and P20).
Intellectual disability in the total cohort was observed in 5 patients including 3 TRPS I (P3, P8, and P11) and 2 TRPS II patients (P2, P6). The intellectual disability observed in TRPS I was mild in 2 patients (P8 and P11) and mild-to-moderate in 1 patient (P3) with autism spectrum disorder. The most prominent feature of the developmental delay in P3 was the delay in language development, and he was unable to speak a word at 3 years and 10 months of age at his last examination, and DDST-II showed a DQ of 59. The intelligence of 1 patient with TRPS II (P1) was appropriate to his age according to the clinical assessment, while severe intellectual disability was observed in 2 patients with TRPS II (P2 and P6).
Genetic Studies
Molecular studies: The results of the genetic analyses of the patients are summarized in Table 2. Trichorhinophalangeal syndrome I patients carrying TRPS1 variants were 1 patient (P7) with a novel frameshift variant (c.531_532del; p.Gly178AlafsTer34) and 2 patients from 1 family (P19 and P20) with a known missense variant (c.2794G>A; p.Ala932Thr). No pathogenic variants were found in 3 clinically diagnosed TRPS I patients (P8, P11, and P12) by TRPS1 sequencing, and chromosomal microarray analysis was planned for these patients to investigate large deletions involving the TRPS1 gene.
Table 2.
The Results of Genetic Analyses of the TRPS Cohort
| Proband Code | Diagnosis | Family History | Microarray | TRPS1 Sequencing | Variant Type | Variant Pathogenicity | Karyotype Analysis |
|---|---|---|---|---|---|---|---|
| P1 | TRPS II | De novo | arr[GRCh38] 8q23.3q24.12 (113028744-118670887)x1 | N/A | Large deletion (over 5.6 Mb) | Pathogenic | 46,XY |
| P2 | TRPS II | De novo | arr[GRCh38] 8q23.3q24.12 (112473987-125160640)x1 | N/A | Large deletion (over 12.6 Mb) | Pathogenic | 46,XX,del(8)(q23.3→q24.13 |
| P3, P4, P5 | TRPS I | Maternal inheritance | arr[GRCh38]8q23.3 (113788073-116309018)x1 | Not found | Large deletion (over 2.5 Mb) | Pathogenic | N/A |
| P6 | TRPS II | De novo | arr[GRCh38]8q23.1q24.11 (107382797-117861338)x1 | N/A | Large deletion (over 10 Mb) | Pathogenic | N/A |
| P7 | TRPS I | De novo | N/A | c.531_532del (p.Gly178AlafsTer34) | Frameshift | Pathogenic | 46,XX |
| P8 | TRPS I | De novo | N/A | Not found | – | – | 46,XX |
| P9 | TRPS I | De novo | N/A | N/A | – | – | 46,XX |
| P10 | TRPS I | De novo | N/A | N/A | – | – | 46,XX |
| P11 | TRPS I | De novo | N/A | Not found | – | – | 46,XX |
| P12 | TRPS I | De novo | N/A | Not found | – | – | N/A |
| P13 | TRPS I | De novo | N/A | N/A | – | – | 46,XX |
| P14 | TRPS I | De novo | N/A | N/A | – | – | N/A |
| P15 | TRPS I | De novo | N/A | N/A | – | – | 46,XY |
| P16, P17 | TRPS I | Paternal inheritance | N/A | N/A | – | – | 46,XY (in P16) |
| P18 | TRPS I | De novo | N/A | N/A | – | – | N/A |
| P19, P20 | TRPS I | Paternal inheritance | N/A | c.2794G>A (p.Ala932Thr) | Missense | Pathogenic | N/A |
| P21, P22 | TRPS I | Paternal inheritance | N/A | N/A | – | – | N/A |
Mb, megabase; N/A, not available; TRPS, trichorhinophalangeal syndrome.
Chromosomal and chromosomal microarray analyses: Karyotype analyses in our cohort were normal except for P2. P1, P2, and P6 were diagnosed with TRPS II and had large deletions (over 5.6, 12.6, and 10.40 megabase (Mb), respectively) with variable breakpoints involving TRPS1 and EXT1. Three patients from 1 family (P3, P4, and P5) with an 8q23.3 deletion over 2.5 Mb involving the TRPS1 gene were diagnosed with TRPS I.
Comparison of the Findings Between Trichorhinophalangeal Syndrome I and II
The typical craniofacial findings of TRPS and cone-shaped epiphyses at the phalanges were constant findings that did not differ between patients with TRPS I and II. Severe intellectual disability (P2 and P6) and microcephaly (P1 and P6) were each present in two-thirds of TRPS II patients, whereas 3 of 19 TRPS I patients (P3, P8, and P11) had mild intellectual disability and 2 of 14 TRPS I cases (P9 and P15) had microcephaly. While 2 of 3 TRPS II cases (P1 and P6) had short stature, a height below the third centile was present in 6 of 16 TRPS I patients (P4, P8, P9, P15, P19, and P20). In addition to intellectual disability, microcephaly, and short stature, which were relatively more common in TRPS II, the distinguishing feature of TRPS II from TRPS I was the presence of multiple exostoses detected in all patients with TRPS II (P1, P2, and P6).
Discussion
The diagnosis of TRPS I is established with the typical craniofacial features, ectodermal manifestations, distal limb abnormalities, and radiographic findings of cone-shaped epiphyses at the phalanges. In addition to the clinical findings observed in TRPS I, TRPS II is characterized by multiple exostoses and an increased risk of intellectual disability.1,2,4 The typical craniofacial features of TRPS were fine and sparse hair, thick and broad eyebrows, a pear-shaped nose with underdeveloped alae, a long philtrum with thin upper vermillion, and prominent ears.4 The nose in TRPS is the most distinctive facial finding and has been described as a pear-shaped or a large nose with a broad ridge and tip but a narrow bridge with underdeveloped alae.1,4 The sparse and slowly growing hair, long philtrum, thin upper lip, and a large nose with a broad ridge and tip with underdeveloped alae were detected in all our patients. There was no difference in the facial features between patients with TRPS I and TRPS II compatible with the literature.1 Cone-shaped epiphysis of the middle and proximal phalanges were present in our total cohort, while generalized shortening of the phalangeal, metacarpal, and metatarsal bones was also a common skeletal finding. Consistent with the literature, thin and dystrophic nails were present in about half of the cohort.4
Since the phenotype in TRPS is often distinct, genetic analyses have been used to confirm the clinical diagnosis, and molecular confirmation can also be helpful in the presence of a mild or atypical clinical presentation.4 While the haploinsufficiency of TRPS1 due to pathogenic variants or whole-gene deletions causes TRPS I, contiguous 8q23.3-q24.11 deletions involving the TRPS1 and EXT1 genes cause TRPS II.3,4 TRPS1 variants detected in our patients with TRPS I were 2 intragenic pathogenic variants and TRPS1 deletions. Furthermore, 3 TRPS II patients had large deletions with variable sizes and breakpoints encompassing the TRPS1-EXT1 interval. Correspondingly, Maas et al (2015)1 reported that the size of contiguous gene deletions in TRPS II varies significantly and there are no common breakpoints. Chromosomal abnormalities causing a functional disturbance of the TRPS1 gene have been rarely reported in patients with TRPS.4,10 No chromosomal abnormality was detected in the karyotype analysis of any of our patients, except for P2 with the TRPS II phenotype.
The phenotype in TRPS III, described with severe short stature and brachydactyly, has been considered as a part of the TRPS I phenotype, within the most severe end of the clinical spectrum.2,11 This hypothesis may be supported by the intrafamilial phenotypic variability seen in familial cases in which the same pathogenic variant causes both TRPS I and TRPS III phenotypes.1,12 Missense pathogenic TRPS1 variants in exon 6, encoding a presumptive GATA DNA-binding zinc-finger domain, have been associated with the severe form of TRPS I.1,2 On the other hand, missense variants located outside this domain have been reported to cause a mild-to-moderate disease phenotype.13,14 The missense pathogenic TRPS1 variant identified in our study was also in exon 6. Two related patients (P19 and P20) carrying this variant shared the clinical findings including marked craniofacial findings, severe brachydactyly, and short stature, which were consistent with the findings of previously reported patients with the same pathogenic variant.1,15 Although most patients with nonsense pathogenic TRPS1 variants have been associated with the mild TRPS I phenotype, marked variability in disease severity has also been described.1,2 A frameshift pathogenic variant in exon 4 responsible for both TRPS I and TRPS III phenotypes has been reported in patients from 1 family.12 In our TRPS I patient with the novel frameshift TRPS1 variant in exon 3, distinct facial features and distal limb abnormalities in the absence of short stature were observed. Three further TRPS I patients from 1 family with the same 8q23.3 deletion involving TRPS1 were observed to have similar facial features, while their linear growth and intellectual development were not at the same level.
In 3 TRPS II patients from our cohort, no correlation was found between linear growth and the size of the detected microdeletion. Except for P2, who had the largest deleted segment, 2 TRPS II patients had microcephaly. The severity of microcephaly did not correlate with the size of the deleted region. Two patients with TRPS II (P2 and P6) had severe intellectual disability, the severity of which appeared to be related to the size of the microdeletion. Genotype–phenotype studies in TRPS II have shown no correlation between the size of the deleted segment and the severity of disease manifestations.16,17 Correspondingly, among the 3 TRPS II patients in our cohort, P6 had the second largest deletion despite having the most severe phenotype with severe microcephaly, developmental delay, short stature, and oligodactyly. Of our 3 patients with TRPS II, 2 patients had deletions of the entire EXT1 gene, and 1 patient (P6) had a deletion in EXT1 beginning from the second exon. On the other hand, exon 1 deletions of the EXT1 gene have been described previously in patients with multiple osteochondromas, and it has been suggested that this region may be a hotspot region.18,19 A very recent genotype–phenotype study reported that the minimal critical region responsible for the classical phenotypic features of TRPS II is a region of approximately 3 Mb in size, encompassing the TRPS1-EXT1 interval.17 However, with the addition of our TRPS II patient with the partial EXT1 deletion, the minimal critical region for TRPS II has proven to be smaller, since the clinical findings in this patient occurred due to the haploinsufficiency of the EXT1 gene partially rather than the entire gene.
Mild-to-moderate intellectual disability has been reported in two-thirds of TRPS II cases, whereas an increased risk of intellectual disability has not been associated with TRPS I.4 Two out of 3 TRPS II patients in our cohort had severe intellectual disability. Out of 19 TRPS I patients, 2 patients had mild, and 1 patient had mild-to-moderate intellectual disability. The TRPS I patient with mild-to-moderate intellectual disability (P3) also had autism spectrum disorder and a marked delay in his language development was observed. In addition, the fact that there was no family member diagnosed with autism spectrum disorder, including his affected brother (P4) and mother (P5), was interpreted as the coincidental occurrence of this condition. Maas et al (2015)1 postulated that intellectual disability in TRPS II could be explained by the deletion of the RAD21 gene located at 8q24.11 between TRPS1 and EXT1. Heterozygous pathogenic variants in RAD21 cause Cornelia de Lange syndrome-4 (OMIM 606462) and have been associated with a relatively mild phenotype.20, 21 However, a TRPS II patient from our cohort who had a deletion including the RAD21 gene had no intellectual disability and had none of the facial manifestations of Cornelia de Lange syndrome-4.
Reduced linear growth has frequently been reported in TRPS, especially in those with TRPS II.4,22 In our cohort, SDS of height values were in a heterogeneous distribution within both the TRPS I and TRPS II patient groups. However, the fact that short stature was more common in TRPS II rather than in TRPS I was consistent with the observation of Maas et al (2015)1 that TRPS II patients with microdeletions had more severe short stature among the TRPS cohort. About two-thirds of TRPS II and one-third of TRPS I cases in our cohort had a height below the third centile. The SDS values of occipitofrontal circumference were also in a broad range within TRPS I and TRPS II. Two patients with TRPS I (2/14) and two-thirds of patients with TRPS II had a head circumference below the third centile, and the presence or severity of microcephaly was not associated with the patients’ intellectual development.
The limitations of this study are that genetic testing was not performed in all probands, and multiplex ligation dependent probe amplification (MLPA) or chromosomal microarray analyses were not performed in patients whose sequence analysis did not show pathogenic variants.
Conclusion
In the current study, we observed that typical facial findings and cone-shaped epiphyses were constant findings that did not differ between TRPS I and TRPS II patients. The most distinguishable features of TRPS II from TRPS I were the presence of multiple exostoses, which were noticeable after 2 years of age at the earliest, and the increased frequency and severity of the intellectual disability. Short stature, microcephaly, and severe intellectual disability, the severity of which appears to be related to the size of the deleted region, were each present in two-thirds of TRPS II patients. A patient with a microdeletion including the EXT1 gene partially rather than the entire gene had the TRPS II phenotype. Trichorhinophalangeal syndrome I patients with a known missense TRPS1 variant in exon 6 had marked craniofacial findings, severe brachydactyly, and short stature, consistent with previous reports. Although TRPS is a disease with characteristic clinical and radiological features and molecular genetic testing is not usually required to make the diagnosis, molecular confirmation can be helpful, especially when the clinical presentation is mild or atypical.
Footnotes
Ethics Committee Approval: Ethical committee approval was received from the Ethics Committee of Cerrahpasa Medical Faculty (Approval No: 158702, Date: 3.12.2020).
Informed Consent: Written informed consent was obtained from each patient or the parents of the patient.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept and Design – B.T., N.G.; Supervision – B.T., Z.O.U.; Funding – B.T., Z.O.U.; Materials – B.T., N.G., Z.O.U.; Data Collection and/or Processing – A.Y.Ü., B.T., D.U.A., E.Ç.S., E.U., F.C.E., N.G., Z.O.U.; Analysis and/or Interpretation – A.Y.Ü., B.T., D.U.A., E.Ç.S., E.U., F.C.E., N.G., Z.O.U.; Literature Review – B.T., N.G.;Writing – B.T., N.G.; Critical Review – B.T., N.G., Z.O.U.
Declaration of Interests: The authors declare that they have no competing interest.
Funding: The authors declare that this study had received no financial support.
References
- 1. Maas SM, Shaw AC, Bikker H.et al. Phenotype and genotype in 103 patients with tricho-rhino-phalangeal syndrome. Eur J Med Genet. 2015;58(5):279 292. ( 10.1016/j.ejmg.2015.03.002) [DOI] [PubMed] [Google Scholar]
- 2. Lüdecke HJ, Schaper J, Meinecke P.et al. Genotypic and phenotypic spectrum in tricho-rhino-phalangeal syndrome types I and III. Am J Hum Genet. 2001;68(1):81 91. ( 10.1086/316926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lüdecke HJ, Schmidt O, Nardmann J.et al. Genes and chromosomal breakpoints in the Langer-Giedion syndrome region on human chromosome 8. Hum Genet. 1999;105(6):619 628. ( 10.1007/s004399900176) [DOI] [PubMed] [Google Scholar]
- 4. Maas S, Shaw A, Bikker H, Hennekam RCM. Trichorhinophalangeal syndrome. In: Adam MP, Mirzaa GM, Pagon RA.et al., eds. GeneReviews. Seattle (WA): University of Washington; 1993. [PubMed] [Google Scholar]
- 5. Suemoto H, Muragaki Y, Nishioka K.et al. Trps1 regulates proliferation and apoptosis of chondrocytes through Stat3 signaling. Dev Biol. 2007;312(2):572 581. ( 10.1016/j.ydbio.2007.10.001) [DOI] [PubMed] [Google Scholar]
- 6. Cho KY, Kelley BP, Monier D, Lee B, Szabo-Rogers H, Napierala D. Trps1 regulates development of craniofacial skeleton and is required for the initiation of palatal shelves fusion. Front Physiol. 2019;10:513. ( 10.3389/fphys.2019.00513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Itoh S, Kanno S, Gai Z.et al. Trps1 plays a pivotal role downstream of Gdf5 signaling in promoting chondrogenesis and apoptosis of ATDC5 cells. Genes Cells. 2008;13(4):355 363. ( 10.1111/j.1365-2443.2008.01170.x) [DOI] [PubMed] [Google Scholar]
- 8. Nishioka K, Itoh S, Suemoto H.et al. Trps1 deficiency enlarges the proliferative zone of growth plate cartilage by upregulation of Pthrp. Bone. 2008;43(1):64 71. ( 10.1016/j.bone.2008.03.009) [DOI] [PubMed] [Google Scholar]
- 9. Richards S, Aziz N, Bale S.et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405 424. ( 10.1038/gim.2015.30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. David D, Marques B, Ferreira C.et al. Co-segregation of trichorhinophalangeal syndrome with a t(8;13)(q23.3;q21.31) familial translocation that appears to increase TRPS1 gene expression. Hum Genet. 2013;132(11):1287 1299. ( 10.1007/s00439-013-1333-0) [DOI] [PubMed] [Google Scholar]
- 11. Fang X, Yang Q. A missense mutation in TRPS1 in a family with trichorhinophalangeal syndrome Type III accompanied by ankylosing spondylitis. Ann Dermatol. 2022;34(2):139 143. ( 10.5021/ad.2022.34.2.139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Itoh M, Kittaka Y, Niida Y, Saikawa Y. A novel frameshift mutation in the TRPS1 gene caused tricho-rhino-phalangeal syndrome type I and III in a Japanese family. Clin Pediatr Endocrinol. 2016;25(3):115 118. ( 10.1297/cpe.25.115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Torai R, Makino T, Mizawa M, Shimomura Y, Shimizu T. A novel missense mutation in exon 3 of the TRPS1 gene in a patient with a mild phenotype of tricho-rhino-phalangeal syndrome type 1. Eur J Dermatol. 2018;28(2):271 272. ( 10.1684/ejd.2018.3233) [DOI] [PubMed] [Google Scholar]
- 14. Rossi A, Devirgiliis V, Panasiti V.et al. Missense mutation in exon 7 of TRPS1 gene in an Italian family with a mild form of trichorhinophalangeal syndrome type I. Br J Dermatol. 2007;157(5):1021 1024. ( 10.1111/j.1365-2133.2007.08158.x) [DOI] [PubMed] [Google Scholar]
- 15. Su W, Shi X, Lin M.et al. Non-ossifying fibroma with a pathologic fracture in a 12-year-old girl with tricho-rhino-phalangeal syndrome: a case report. BMC Med Genet. 2018;19(1):211. ( 10.1186/s12881-018-0732-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schinzel A, Riegel M, Baumer A.et al. Long-term follow-up of four patients with Langer-Giedion syndrome: clinical course and complications. Am J Med Genet. 2013;161(9):2216 2225. ( 10.1002/ajmg.a.36062) [DOI] [PubMed] [Google Scholar]
- 17. Favilla BP, Burssed B, Yamashiro Coelho ÉM.et al. Minimal critical region and genes for a typical presentation of Langer-Giedion syndrome. Cytogenet Genome Res. 2022;162(1-2):46 54. ( 10.1159/000522034) [DOI] [PubMed] [Google Scholar]
- 18. Santos SCL, Rizzo IMPO, Takata RI, Speck-Martins CE, Brum JM, Sollaci C. Analysis of mutations in EXT1 and EXT2 in Brazilian patients with multiple osteochondromas. Mol Genet Genomic Med. 2018;6(3):382 392. ( 10.1002/mgg3.382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vink GR, White SJ, Gabelic S, Hogendoorn PC, Breuning MH, Bakker E. Mutation screening of EXT1 and EXT2 by direct sequence analysis and MLPA in patients with multiple osteochondromas: splice site mutations and exonic deletions account for more than half of the mutations. Eur J Hum Genet. 2005;13(4):470 474. ( 10.1038/sj.ejhg.5201343) [DOI] [PubMed] [Google Scholar]
- 20. Krab LC, Marcos-Alcalde I, Assaf M.et al. Delineation of phenotypes and genotypes related to cohesin structural protein RAD21. Hum Genet. 2020;139(5):575 592. ( 10.1007/s00439-020-02138-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deardorff MA, Wilde JJ, Albrecht M.et al. RAD21 mutations cause a human cohesinopathy. Am J Hum Genet. 2012;90(6):1014 1027. ( 10.1016/j.ajhg.2012.04.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levy-Shraga Y, Modan-Moses D, Wientroub S, Ovadia D, Zeitlin L. The effect of growth hormone treatment in a child with tricho-rhino-phalangeal syndrome: a case report and review of the literature. Eur J Med Genet. 2020;63(4):103830. ( 10.1016/j.ejmg.2019.103830) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a