Abstract
Objective:
For comatose survivors of out-of-hospital cardiac arrest (OHCA), current guidelines recommend targeted temperature management (TTM) with a goal temperature of 32°C – 36°C for at least 24 hours. We examined adherence to temperature targets, quantified as time-in-therapeutic range (TTR), and association of TTR with survival and neurologic outcomes.
Methods:
We conducted a retrospective cohort study of the Resuscitation Outcomes Consortium-Continuous Chest Compressions trial, including adults with OHCA who underwent TTM for >12 hours. We imputed continuous temperatures between consecutive temperature measurements using the linear interpolation method and calculated TTR for multiple target temperatures. The association of TTR with survival to hospital discharge and favorable neurological outcome was evaluated using hierarchical regression models.
Main Results:
Among 2,637 patients (mean age 62.3 years, 29.9% female), the median duration of TTR for TTM between 32°C – 36°C was 23 (IQR: 21–24) hours with a median time outside therapeutic range of 0.9 (IQR: 0.0 – 4.2) hours. In risk-adjusted analyses, there was no association of TTR of 32°C – 36°C with overall survival (OR 1.00 [95% CI, 0.90 – 1.10]) or favorable neurologic outcome (1.02 [95% CI, 0.90 – 1.14]). However, in assessments of TTR 33°C – 36°C, there was a significant association with favorable neurologic survival (OR 1.12 [1.01 – 1.25]) but not overall survival (OR 1.04 [0.94 – 1.15]).
Conclusions:
Among patients with OHCA who underwent TTM, we found variability in adherence to guideline-recommended treatment targets. Higher TTR was not associated with overall survival, but for certain temperature thresholds, TTR was associated with favorable neurologic outcome.
BACKGROUND
Only 1 in 10 patients with an out-of-hospital cardiac arrest (OHCA) survives to hospital discharge, and 1 in 5 of those have a poor neurological outcome.1 Targeted temperature management (TTM) is recommended by international guidelines to reduce hypoxic-ischemic brain injury in patients who remain unable to follow commands post-arrest.2,3 This recommendation is based on initial randomized clinical trials which found that maintaining a target temperature of 33°C was associated with higher survival and favorable neurologic outcomes compared to a target temperature of 37°C.4,5 Favorable survival and neurologic outcomes with TTM were initially described in OHCA patients with a shockable rhythm.4,5 Subsequently, the HYPERION trial included patients with non-shockable rhythm suffering either in or out-of-hospital cardiac arrest and found that the use of TTM in this broad patient population was associated with favorable neurologic outcomes, although no survival benefit was observed.6
In contrast, the recently published TTM2 trial, which represents the largest and most contemporary evaluation of TTM, found no significant difference in 6-month survival or neurologic disability between patients randomized to a temperature target of 33°C compared with targeted normothermia, defined as maintaining a target temperature of ≤ 37.5°C.7 These findings extended observations from the TTM trial that similarly found no difference in outcomes between temperatures targets of 33°C and 36°C.8 Given these recent findings, questions remain about the clinical utility of therapeutic hypothermia and currently recommended temperature targets for improving patient outcomes.
The effect of TTM quality on patient outcomes outside of TTM-specific clinical trials has not previously been assessed. We hypothesized that there is variation in adherence to TTM guidelines at both the patient and institution level, as assessed by time in therapeutic range (TTR) for a given target temperature. Using data from the Resuscitation Outcomes Consortium Continuous Cardiac Compressions (ROC-CCC) trial, we evaluated the quality of TTM through a novel metric of TTR and performed an exploratory analysis to assess the association of TTR with both overall survival and a favorable neurologic outcome at hospital discharge.
METHODS
Data Source
The ROC-CCC was a large, randomized controlled trial that included 8 regional sites and 114 emergency medical services (EMS) agencies across North America which enrolled patients with a non-traumatic OHCA from 2011–2015.9 The sites and EMS services were divided into 49 clusters for randomization, representing a set of EMS agencies serving the same set of hospitals in a regional site. Detailed methods regarding these data are described elsewhere.9,10 Briefly, exclusion criteria included pregnancy, incarceration, arrest witnessed by EMS personnel, and asphyxiation as the primary cause of arrest. Patients were randomly assigned to receive continuous versus interrupted chest compressions, with mortality and neurologic disability as the primary study outcomes. The trial showed no difference in these outcomes between the intervention groups.
A subset of the patients included in the ROC-CCC trial across the study arms underwent TTM in accordance with practice guidelines recommending therapeutic hypothermia for patients unable to follow commands post-arrest. The TTM documentation protocol is described in detail elsewhere.9 Use of TTM was not required or encouraged by the trial protocol and cooling parameters were at the discretion of treating clinicians. Patients who underwent cooling could have treatment initiated in the field by EMS or at the hospital. Cooling could be performed via intra or extravascular methods and temperatures were recorded during the cooling period. Temperatures collected during the first 24 hours after initiation of cooling were defined as the ‘cooling phase’ of TTM in the study protocol. Time achieve therapeutic temperature was defined as the interval after first ROSC to first recorded temperature < 36°C following initiation of TTM. The data used in the study are available from the National Heart, Lung, and Blood Institute’s Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) with an approved proposal.
Study Population and Covariates
All patients enrolled in ROC-CCC who underwent TTM with 12 or more hours of cooling time and at least 4 available temperature measurements within the first 12 hours were included. Temperature measures were obtained based on local protocols.
We considered each variable in the ROC-CCC dataset and included those which were deemed clinically relevant or were previously used in studies of resuscitation outcomes.11–13 A full list of included variables can be found in eTable 1.
A shockable rhythm was defined as initial rhythm of ventricular fibrillation or pulseless ventricular tachycardia; pulseless electrical activity, asystole, or unknown rhythm were considered non-shockable. We excluded variables with greater than 10% missing values from the risk-adjustment models. Time to first return of spontaneous circulation (ROSC) was included despite being missing in 10.9% of patients given the known clinical importance of the metric. Of note, race was not recorded in most patients (1,777 or 65.9%) and was excluded as a study covariate. The remaining variables with missingness less than 10% were imputed using a non-parametric random forest method.14
Study Exposure: Time in Therapeutic Range (TTR)
TTR was calculated using a linear interpolation method for a temperature range of 32°C – 36°C in accordance with guideline recommendations.2,15 This approach is analogous to the TTR for anticoagulation with warfarin.16 Briefly, the approach assumes that temperature changed linearly between two consecutive measures. We parametrized time between temperature measures in completed hours, and TTR was calculated as the proportion of the time within a specified temperature range. An example of the TTR calculation for temperature range 32°C – 36°C is shown in eFig. 1. Similarly, TTR was calculated for additional temperature ranges: (a) 33°C – 36°C, and those corresponding to avoidance of (b) severe hypothermia (T < 32°C or T < 33°C), and (c) hyperthermia (T > 37°C).
Study Outcomes
The study outcomes were survival to hospital discharge and favorable neurologic outcome at time of discharge. Neurological outcomes were assessed using the modified Rankin scale, which is scored from 0 to 6, with 0 representing no symptoms and 6 representing death.17 Favorable neurologic outcome at the time of hospital discharge was defined as modified Rankin scale score of 0 – 3.
Statistical Analysis
Each trial participant was associated with a cluster corresponding to the EMS service that brought them to the hospital. These clusters were used to define site-level differences in patient care and outcomes. Data were analyzed at both the patient and randomization cluster level.
Patients were divided into tertiles based on TTR and patient characteristics were compared across tertiles. The highest tertile included all patients with TTR equal to 100%; the remainder of patients were then divided evenly into the lowest and middle tertile. Differences between patient tertiles were assessed by Jonckheere-Terpstra test for continuous variables and Cochran-Armitage test for categorical variables.
To evaluate cluster-level variation, TTR was also dichotomized into >90% versus <90% for each patient, and the proportion of patients with a TTR greater than 90% was calculated for each cluster. A hierarchical logistic regression model with randomization cluster as the random effect was used to derive the median odds ratio, which quantifies the odds that patients with similar clinical characteristics would differ in TTR between two randomly selected clusters.18
To evaluate the association between TTR and patient survival, we constructed a hierarchical logistic regression model with either survival or favorable neurologic status at hospital discharge as the outcome, randomization cluster as the random effect and patient-level fixed effects.19 TTR was modeled as a continuous variable as a fixed effect of interest. Other patient-level covariates included in the model were age, sex, shockable versus non-shockable initial rhythm, public versus private location of the arrest, residential status of the patient, presence of a witness, loss of pulse after achieving ROSC, time from initial call to EMS initiation of CPR, time to 1st ROSC, time to ED arrival, presence of STEMI on arrival, initial temperature, initial pH, use of insulin during first 48 hours of hospitalization, use of anticonvulsants at any time, use of pressors during first 72 hours of hospitalization, and use of mechanical circulatory support at any time.
We performed a sensitivity analysis in which variables representing post-arrest management were excluded from the model, as these variables could be indirectly associated with the quality of TTM. We performed a second analysis including the total duration of TTM as a covariate in the full multivariable model. Finally, we re-calculated TTR using initial temperature, rather than temperature at time of TTM initiation, with time of arrival to the emergency department as time zero in the linear interpolation.
All predictor variables were standardized to a mean of zero and standard deviation of one. The association of TTR odds ratios represent change in likelihood of survival or favorable neurologic outcome at time of hospital discharge per standard deviation increase in TTR. Ninety-five percent confidence intervals for effect estimates were calculated using the Wald test.19
To assess the association between cluster-level TTR and outcomes, we calculated risk-standardized survival for each cluster using hierarchical logistic regression, where randomization cluster was a random effect and patient arrest characteristics were included as fixed effects. All patient level variables described above were included with the exception of TTR. The relationship between risk-standardized survival and mean TTR for each cluster was assessed using Spearman’s correlation.
Analyses were conducted using R 4.0, and all statistical tests were two sided with a level of significance set at 0.05. The Yale Institutional Review Board reviewed the study, ‘Quality of Care in Out-of-Hospital Cardiac Arrest’ on 9/2/2021 (approval number 2000029749) and waived informed consent as the study represented secondary analyses of deidentified data. Procedures were followed in accordance with the ethical standards of the Yale Institutional Review Board.
RESULTS
There were 3,457 patients in ROC-CCC who underwent TTM and had at least one recorded temperature. Of these, 2,637 patients underwent TTM at least 12 hours and had at least four temperature measurements (eFig. 2 in the Online Supplement). The mean age of patients was 62.3 (SD ± 15.2) years, 784 (29.7%) were women, 1,468 (55.7%) had VF/VT as the first recorded pulseless rhythm, and the other 1,158 (43.9%) had PEA, asystole, or an undefined non-shockable rhythm (n = 11 had no documented rhythm).
Time-in-Therapeutic Range
A total of 1,061 (40.2%) of patients who received TTM survived to hospital discharge, with 868 (32.9%) surviving with favorable neurological status. The median initial temperature was 35.8°C (IQR 34.8 – 36.4°C). During the hypothermia period, the median recorded temperature was 33.3°C (IQR 32.9 – 34.5°C) and ranged between 25.1°C and 40.6°C. Overall, 33,218 of 38,878 (85.4%) temperature measures were between 32°C and 36°C (Fig. 1A). Patients had a median of 13 (IQR 12 – 18) temperature measurements, with a median of 2.0 (IQR 1.0 – 2.0) hours between recorded temperatures (Fig. 1B). A subset of 2,304 (87%) patients had data available on time to initiate hypothermia. Among these, the median time from first ROSC to initiation of cooling was 91 (IQR 43 – 206) minutes. Using < 36°C as the temperature target, the median time to first recorded therapeutic temperature was 321 (IQR 185 – 522) minutes after ROSC among the 2,228 patients who reached this temperature threshold. There was substantial variation among the randomization clusters in time to initiate cooling and achieve target temperature (eFig. 3).
Figure 1: Temperature Measurement Distribution, Time Between Temperature Measurements, Duration of Hypothermia Period.
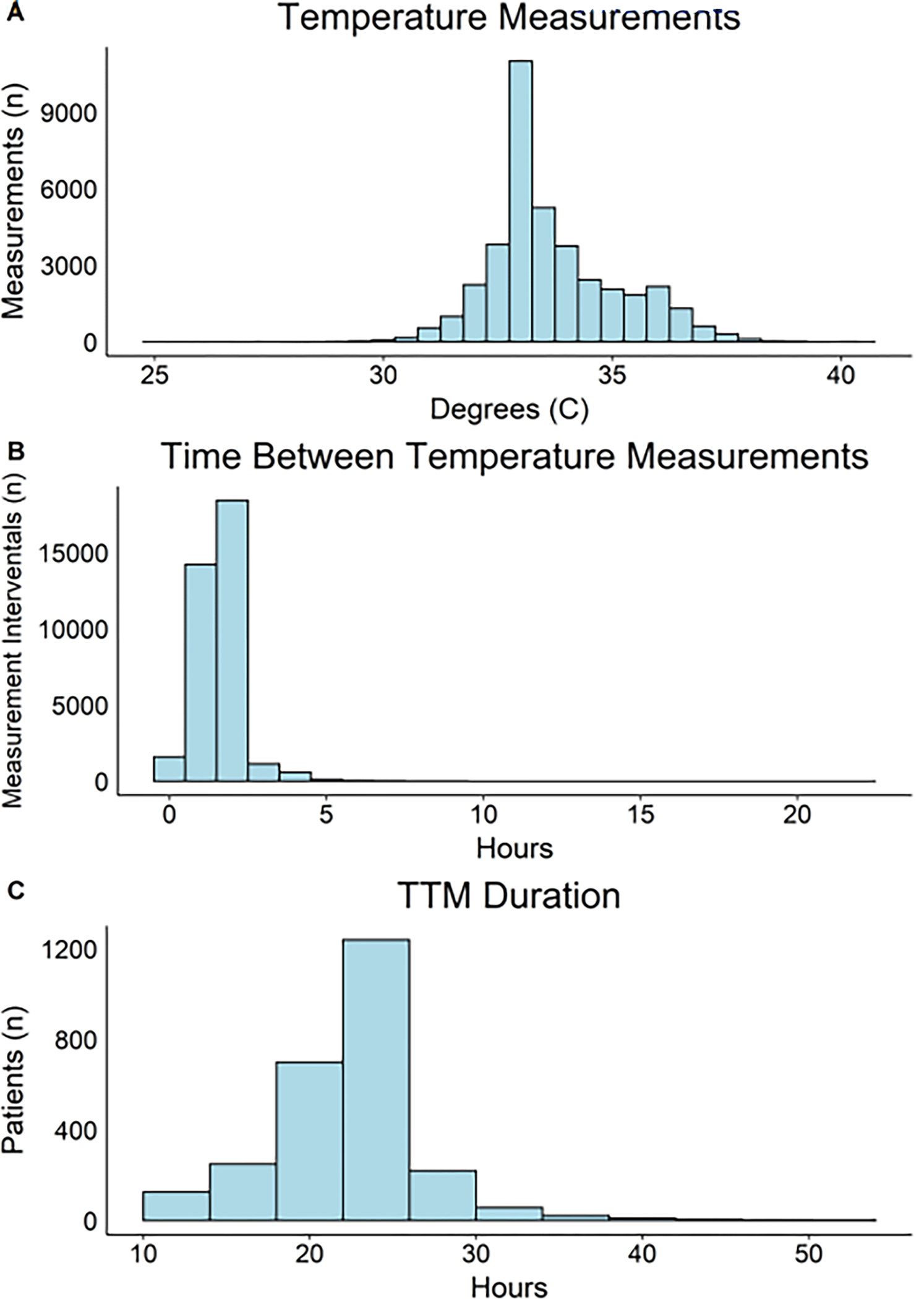
The median duration of therapeutic hypothermia was 23 (IQR: 21–24) hours (Fig. 1C). For a TTR of 32°C - 36°C, the median time outside therapeutic range was 0.9 (IQR: 0.0 – 4.2) hours, with a median TTR of 96% (IQR 81% – 100%). For temperature range of 32°C - 36°C, 36.7% (n=968) of participants had a TTR that was <90% of the cooling period, 18.4% (n=495) had a TTR <90% for temperature range of >32°C (avoiding over-cooling), and 5.4% (n=145) had a TTR <90% for temperature range of <37°C (avoiding under-cooling). TTR was lower for temperature range 33°C – 36°C and > 33°C, with 82.2% (n=2,168) and 72.0% (n=1,899) of patients having TTR <90%, respectively. Density plots for each TTR goal are shown in eFig. 4.
Patients in the highest tertile of TTR were older, had a longer time to first ROSC, were more likely to have loss of pulse after first ROSC, require insulin administration, and less likely to survive to hospital discharge (Table 1).
Table 1:
Participant characteristics.
| Tertile 1 (n=809) | Tertile 2 (n=809) | Tertile 3 (n=1019) | P for Trend* | |
|---|---|---|---|---|
|
| ||||
| Median (IQR) | ||||
| TTR 32°C – 36°C (%) | 68.0 (45.9 – 79.1) | 94.5 (90.9 – 97.3) | 100.0 (100.0 – 100.0) | - |
| TTR > 32°C (%) | 90.0 (74.6 – 100) | 99.4 (94.5 – 100.0) | 100.0 (100.0 – 100.0) | - |
| TTR < 37°C (%) | 100.0 (95.8 – 100.0) | 100.0 (100.0 – 100.0) | 100.0 (100.0 – 100.0) | - |
| Time to initiate cooling (min.) † | 104.9 (42.0 – 226.0) | 88.7 (46.0 – 193.0) | 86.0 (44.0 – 199.8) | 0.952 |
| Time to reach 36°C (min.) † | 388.1 (198.9 – 659.9) | 320.0 (195.0 – 498.0) | 282.6 (168.5 – 460.7) | 0.999 |
|
| ||||
| Mean (SD) | ||||
| Age (years) | 61.3 (16.1) | 62.5 (14.6) | 62.9 (14.9) | 0.010 |
| Time to EMS initiating CPR (min.) | 7.8 (3.1) | 7.8 (2.9) | 8.0 (3.3) | 0.199 |
| Time to first ROSC (min.) | 21.0 (7.7) | 20.9 (8.1) | 22.0 (8.4) | 0.006 |
| Time to ED arrival (min.) | 44.7 (12.7) | 44.1 (13.5) | 45.5 (14.4) | 0.425 |
| pH on arrival | 7.20 (0.16) | 7.20 (0.15) | 7.18 (0.17) | 0.999 |
| Temperature on arrival (C) | 35.6 (1.4) | 35.7 (1.3) | 35.3 (1.3) | 0.999 |
|
| ||||
| N (%) | ||||
| Male Sex | 574 (71.0%) | 575 (71.1%) | 704 (69.1%) | 0.516 |
| Shockable Rhythm | 452 (55.9%) | 489 (60.4%) | 530 (52.0%) | 0.151 |
| STEMI | 58 (7.2%) | 53 (6.6%) | 67 (6.6%) | 0.850 |
| Public Arrest Location | 235 (29.0%) | 242 (29.9%) | 266 (26.1%) | 0.539 |
| Witnessed Arrest | 539 (66.6%) | 544 (67.2%) | 668 (65.6%) | 0.507 |
| Pulse lost after first ROSC | 223 (27.6%) | 225 (27.8%) | 344 (33.8%) | 0.021 |
| Insulin use during first 48 hrs. | 408 (50.4%) | 432 (53.4%) | 615 (60.4%) | <0.001 |
| Anti-convulsant use | 557 (68.9%) | 535 (66.1%) | 663 (65.1%) | 0.050 |
| Pressor support during first 72 hrs. | 644 (79.6%) | 630 (77.9%) | 806 (79.1%) | 0.648 |
| Mechanical circulatory support | 31 (3.8%) | 51 (6.3%) | 62 (6.1%) | 0.121 |
| Survival to discharge | 345 (42.6%) | 349 (43.1%) | 367 (36.0%) | 0.014 |
Patients were divided into tertiles based on their TTR for a goal range of 32°C – 36°C. The highest tertile included all patients with 100% TTR for 32°C – 36°C. TTR values are reported as median (interquartile range). Patient characteristics are reported as mean (standard deviation) for continuous variables and percentages for categorical variables.
For patient-level factors, p-values for trend were assessed with the Jonckheer-Terpstra test for continuous variables and the Cochran-Armitage test for categorical variables.
Data available for n=2288 patients (n=713 for tertile 1, n=713 for tertile 2, and n=862 for tertile 3)
Variation in TTR across clusters
There were 49 randomization clusters, with a median 49 (range 13 – 125) patients per cluster. There was modest variation in TTR among clusters; for a temperature range of 32°C – 36°C, the median TTR ranged from 81% to 100%. For temperature target of > 32°C, median TTR ranged from 97% - 100% (100% at 45/49 clusters); for temperature < 37°C, median TTR was 100% at every cluster. Variation was higher for temperature ranges 33°C – 36°C and > 33°C, with median TTR ranging from 47% - 83% and 44% - 100% for these temperature range across clusters.
For temperature range 32°C – 36°C, the percentage of patients with TTR > 90% ranged from 34% at the lowest cluster to 91% at the highest cluster (Figure 2). The median odds ratio for patients achieving a TTR >90% of the cooling period was 1.74 for a temperature range of 32°C - 36°C, 1.91 for a temperature range of >32°C, 1.73 for a temperature range of <37°C, 1.65 for a temperature range of 33°C - 36°C, and 1.84 for a temperature range of >33°C. This indicates that the odds for two similar patients achieving TTR>90% varied by at least 65% at two randomly chosen clusters, regardless of the chosen temperature target.
Figure 2: Percentage of patients with TTR above 90% by randomization cluster.
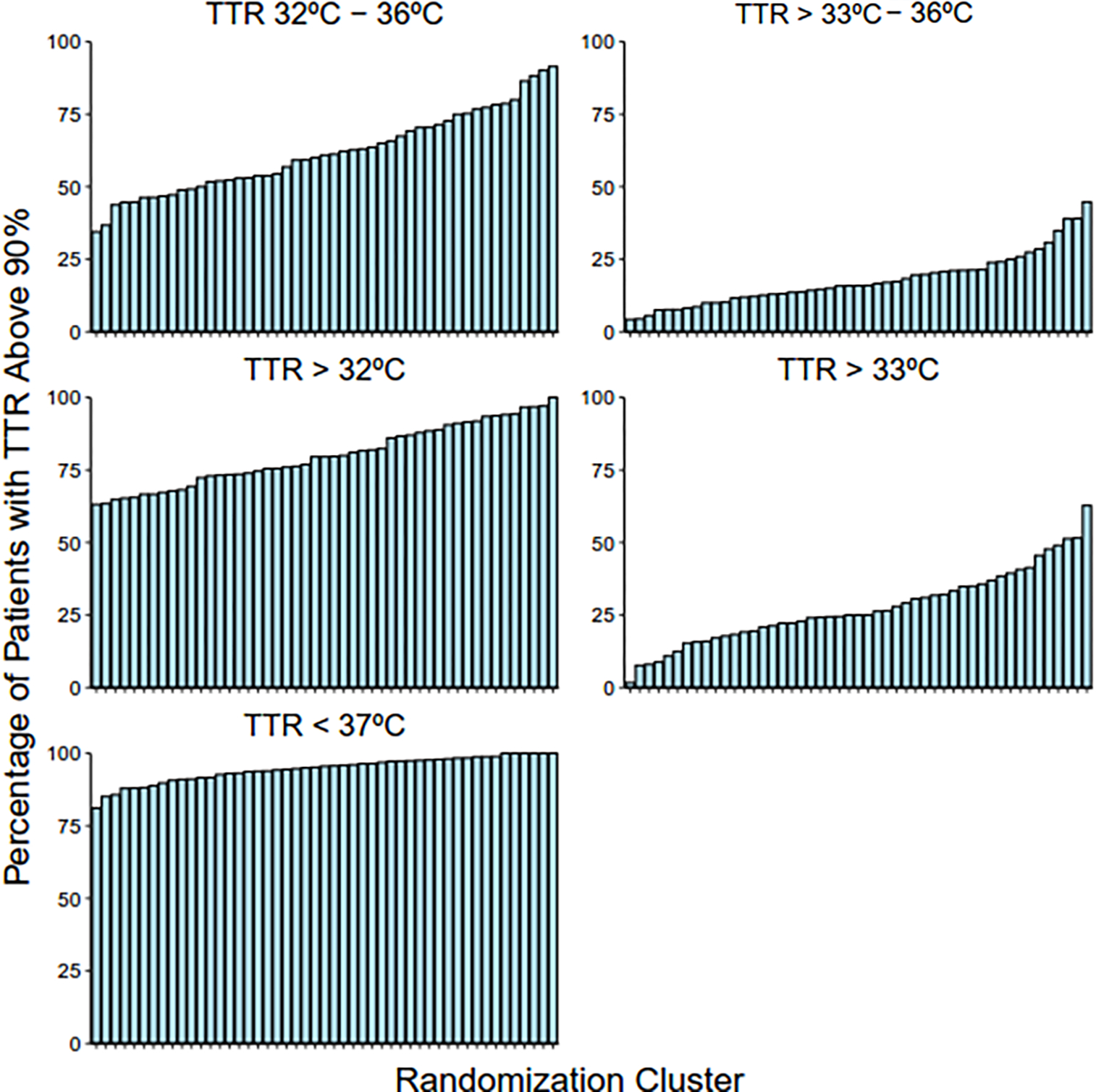
TTR for temperature ranges 32°C – 36°C, >32°C, and <37°C were calculated for each patient. Patients were categorized by TTR less than or greater than 90%.
Association of TTR with Outcomes
The association of TTR with survival and neurologic outcome at hospital discharge is shown in Table 2. In risk-adjusted analyses, a higher TTR for any of the assessed target temperatures was not significantly associated with survival to discharge. However, a one standard deviation increase in TTR for the target temperatures of 33°C – 36°C and TTR >33°C were both associated with 12% greater odds of favorable neurologic outcome (OR 1.12 [1.01 – 1.25] and 1.12 [1.00 – 1.26], respectively). This association was unchanged when including duration of TTM (eTable 2) or exluding post-arrest management variables (eTable 3). In sensitivity analyses that broadened the temperature measures to include the temperature on arrival to the emergency department as the initial temperature, TTR was not associated with survival or neurologic disability for any of the target temperature targets (eTable 4).
Table 2:
Association between time in therapeutic range and survival outcomes.
|
Survival to Discharge
|
||
| Univariable Odds Ratio | Multivariable Odds Ratio | |
| TTR 32°C – 36°C | 0.95 (0.88 – 1.03) | 1.00 (0.90 – 1.10) |
| TTR 33°C – 36°C | 1.14 (1.05 – 1.23)* | 1.04 (0.94 – 1.15) |
| TTR > 32°C | 1.14 (1.05 – 1.24)* | 1.02 (0.92 – 1.14) |
| TTR > 33°C | 1.31 (1.21 – 1.43)* | 1.08 (0.97 – 1.20) |
| TTR < 37°C | 0.91 (0.86 – 1.01) | 1.03 (0.94 – 1.14) |
|
| ||
|
Favorable Neurologic Outcome
|
||
| Univariate Odds Ratiox | Multivariate Odds Ratio | |
| TTR 32°C – 36°C | 0.97 (0.89 – 1.06) | 1.02 (0.92 – 1.14) |
| TTR 33°C – 36°C | 1.18 (1.08 – 1.28)* | 1.12 (1.01 – 1.25)* |
| TTR > 32°C | 1.14 (1.04 – 1.25)* | 1.01 (0.90 – 1.14) |
| TTR > 33°C | 1.33 (1.22 – 1.46)* | 1.12 (1.00 – 1.26)* |
| TTR < 37°C | 0.95 (0.87 – 1.03) | 1.06 (0.95 – 1.17) |
Odds ratios for survival to hospital discharge were calculated for each TTR category using logistic regression models. TTR was modeled as a continuous variable. An odds ratio > 1 indicates that higher TTR was associated with a higher likelihood of survival to discharge or favorable neurologic outcome. Favorable neurologic outcome was defined as a modified Rankin score of < 4.
p-value < 0.05
DISCUSSION
In a post-hoc secondary analysis of a large clinical trial that enrolled patients with OHCA in multiple sites across North America, we found adherence to current TTM guideline recommendations was generally high. However, over a third of participants spent more than 10% of the time outside the therapeutic range for the recommended temperature target of 32°C – 36°C, with only modest difference if the target temperature ranges were defined at different thresholds (>32°C, >33°C, 33°C – 36°C, or <37°C). Notably, there was significant regional variation in TTR, measured across clusters included in the study. There was a positive association between the quality of hypothermia, as measured by TTR, with favorable neurologic outcome at hospital discharge for targeting a temperature of 33°C to 36°C or maintaining a temperature of >33°C, but this association was not observed for a broader, and more conventional definition of TTM target range of 32°C – 36°C. There was no association of greater TTR for any target temperature range with survival at hospital discharge among patients who received TTM in the multivariable analysis.
In this secondary analysis of a large, multicenter study of out-of-hospital cardiac arrest, we examined TTR as a post-hoc metric for the quality of maintaining temperature targets in TTM. It has been previously shown that there is underuse of TTM with variation for similar patients across hospitals, but this prior analysis did not examine individual-level variation in TTM.20 In this analysis of the ROC-CCC clinical trial data, we found that overall TTR was high with a mean of 85.4%. Although the quality of TTM on achieving target temperature was generally good, we observed significant variability in TTR across patients and trial clusters. We leveraged this variation in TTR and found that the extent of variation in TTR was associated with a favorable neurologic prognosis at hospital discharge for temperature targets 33°C – 36°C and maintaining a temperature >33°C, but not across broader definitions of therapeutic hypothermia that included a lower temperature (32°C – 36°C). The observations suggest that since quantifiable differences in compliance to some temperature targets are associated with differences in neurological outcomes, there may be value in rigorously assessing whether some patients achieving deleterious temperatures negates the broader benefit from TTM.
These observations may have important implications. A recent clinical trial – the TTM2 trial – failed to replicate a benefit from the use of TTM in the current era. TTM2 is the largest trial evaluating hypothermia in cardiac arrest and found that targeting a temperature of 33°C compared with normothermia (less than 37.5°C) in comatose cardiac arrest survivors did not improve survival or neurologic outcomes at six months. However, the TTM2 trial contrasts with studies that found improved outcomes with neurologic disability.4–8 The inconsistencies in benefit of TTM may be related to unmeasured differences in cooling protocols used at different institutions. The current study addresses a key knowledge gap. While we did not find a consistent relationship between compliance with certain temperature goals in TTM and survival, we found evidence of better neurologic outcomes with certain temperature targets.
Our study has several limitations. First, this was a post-hoc analysis of a clinical trial that was not designed to test the effectiveness of TTM, and the TTR measurement used was not defined at the time data collection occurred. However, the null trial captured key information on temperature measurements during hypothermia and thereby represents one of the largest multicenter registries of OHCA with detailed information on temperature measurements. Second, while we pursued broad risk adjustment and explicitly accounted for cluster variation, with the included features being discriminative of risk of death (AUROC 0.85 for patient-level features in logistic regression), we cannot exclude unmeasured confounders that attenuate the effect of TTR on outcomes. We also did not have access to data regarding neuroprognostication or reasons that life sustaining treatment was withdrawn. However, we were able to adjust for arrest and post-arrest care characteristics not typically available in OHCA registries (e.g., initial pH and temperature, mechanical support, vasopressors post-arrest). Third, most patients included in the study had a high TTR, and a vast majority averted hyperthermia. These factors could have limited our ability to detect smaller differences in outcomes. It also is possible that TTM delivered in routine care may vary much more widely than that observed in the ROC-CCC trial, the data source for this study. Finally, to maintain statistical power in this exploratory analysis we did not adjust our findings for multiple comparisons. Where we to have performed this adjustment, the association of TTR 33°C – 36°C and > 33°C with neurologic outcome would not be statistically significant.
Conclusion
Among patients with OHCA who underwent TTM, we found variability in adherence to guideline-recommended treatment targets. Higher TTR was not associated with overall survival, but for certain temperature thresholds, TTR was associated with favorable neurologic outcome.
Supplementary Material
Funding
This study was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under the award K23HL153775. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest
Dr Khera receives support from the Doris Duke Charitable Foundation (under award, 2022060). He also receives research support, through Yale, from Bristol-Myers Squibb. He is a coinventor of U.S. Provisional Patent Applications 63/177,117 and 63/346,610, unrelated to current work. He is also a founder of Evidence2Health, a precision health platform to improve evidence-based cardiovascular care. The other authors have no relevant disclosures.
CRediT Author Statement
Kevin M Wheelock: Methodology, Software, Formal Analysis, Writing – Original Draft, Writing – Review & Editing, Visualization
Paul S Chan: Conceptualization, Methodology, Writing – Original Draft, Writing – Review & Editing
Lian Chen: Methodology, Software, Formal Analysis, Visualization
James A de Lemos: Conceptualization, Methodology, Writing – Review & Editing
P Elliott Miller: Methodology, Writing – Review & Editing
Brahmajee K Nallamothu: Conceptualization, Methodology, Writing – Review & Editing
Saket Girotra: Conceptualization, Methodology, Writing – Review & Editing
Rohan Khera: Conceptualization, Methodology, Resources, Writing – Original Draft, Writing – Review & Editing, Supervision, Funding Acquisition
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panchal AR, Bartos JA, Cabañas JG, et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142:S366–S468. [DOI] [PubMed] [Google Scholar]
- 3.Nolan JP, Soar J, Cariou A, et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines for Post-resuscitation Care 2015: Section 5 of the European Resuscitation Council Guidelines for Resuscitation 2015. Resuscitation. 2015;95:202–22. [DOI] [PubMed] [Google Scholar]
- 4.Hypothermia After Cardiac Arrest Study Group. Mild Therapeutic Hypothermia to Improve the Neurologic Outcome after Cardiac Arrest. N Engl J Med. 2002;346:549–56. [DOI] [PubMed] [Google Scholar]
- 5.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–63. [DOI] [PubMed] [Google Scholar]
- 6.Lascarrou JB, Merdji H, Le Gouge A, et al. Targeted Temperature Management for Cardiac Arrest with Nonshockable Rhythm. N Engl J Med. 2019;381:2327–37. [DOI] [PubMed] [Google Scholar]
- 7.Dankiewicz J, Cronberg T, Lilja G, et al. Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest. N Engl J Med. 2021;384:2283–94. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen N, Wetterslev J, Cronberg T, et al. Targeted Temperature Management at 33°C versus 36°C after Cardiac Arrest. N Engl J Med. 2013;369:2197–206. [DOI] [PubMed] [Google Scholar]
- 9.Brown SP, Wang H, Aufderheide TP, et al. A Randomized Trial of Continuous Versus Interrupted Chest Compressions in Out-of-Hospital Cardiac Arrest: Rationale for and Design of the Resuscitation Outcomes Consortium CCC Trial. Am Heart J. 2015;169:334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nichol G, Leroux B, Wang H, et al. Trial of Continuous or Interrupted Chest Compressions during CPR. N Engl J Med. 2015;373:2203–14. [DOI] [PubMed] [Google Scholar]
- 11.Khera R, Chan PS, Donnino M, Girotra S. Hospital Variation in Time to Epinephrine for Nonshockable In-Hospital Cardiac Arrest. Circulation. 2016;134:2105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan PS, Berg RA, Tang Y, Curtis LH, Spertus JA, for the American Heart Association’s Get With the Guidelines–Resuscitation Investigators. Association Between Therapeutic Hypothermia and Survival After In-Hospital Cardiac Arrest. JAMA. 2016;316:1375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasson C, Rogers MAM, Dahl J, Kellermann AL. Predictors of Survival From Out-of-Hospital Cardiac Arrest. Circ Cardiovasc Qual Outcomes. 2010;3:63–81. [DOI] [PubMed] [Google Scholar]
- 14.Stekhoven DJ, Bühlmann P. MissForest—non-parametric missing value imputation for mixed-type data. Bioinformatics. 2012;28:112–18. [DOI] [PubMed] [Google Scholar]
- 15.Donnino MW, Andersen LW, Berg KM, et al. Temperature Management After Cardiac Arrest. Circulation. 2015;132:2448–56. [DOI] [PubMed] [Google Scholar]
- 16.Amin A, Deitelzweig S, Jing Y, et al. Estimation of the impact of warfarin’s time-in-therapeutic range on stroke and major bleeding rates and its influence on the medical cost avoidance associated with novel oral anticoagulant use-learnings from ARISTOTLE, ROCKET-AF, and RE-LY trials. J Thromb Thrombolysis. 2014;38:150–9. [DOI] [PubMed] [Google Scholar]
- 17.Rankin J Cerebral Vascular Accidents in Patients over the Age of 60: II. Prognosis. Scott Med J. 1957;2:200–15. [DOI] [PubMed] [Google Scholar]
- 18.Merlo J, Chaix B, Ohlsson H, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006;60:290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 20.Khera R, Humbert A, Leroux B, et al. Hospital Variation in the Utilization and Implementation of Targeted Temperature Management in Out-of-Hospital Cardiac Arrest. Circ Cardiovasc Qual Outcomes. 2018;11:e004829. [DOI] [PubMed] [Google Scholar]
- 21.Busch M, Soreide E, Lossius HM, Lexow K, Dickstein K. Rapid implementation of therapeutic hypothermia in comatose out-of-hospital cardiac arrest survivors. Acta Anaesthesiol Scand. 2006;50:1277–83. [DOI] [PubMed] [Google Scholar]
- 22.Oddo M, Schaller MD, Feihl F, Ribordy V, Liaudet L. From evidence to clinical practice: Effective implementation of therapeutic hypothermia to improve patient outcome after cardiac arrest*: Crit Care Med. 2006;34:1865–73. [DOI] [PubMed] [Google Scholar]
- 23.Friberg H, Nielsen N. Hypothermia after Cardiac Arrest: Lessons Learned from National Registries. J Neurotrauma. 2009;26:365–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


