Abstract
At least three processes determine whether information we encounter is attended to or ignored. First, attentional capture occurs when attention is drawn automatically by “bottom up” processes, to distinctive, salient, rewarding, or unexpected stimuli when they enter our sensory field. Second, “top down” attentional control can direct cognitive processing towards goal-relevant targets. Third, selection history, operates through repeated exposure to a stimulus, particularly when associated with reward. Attentional control is measured using tasks that require subjects to selectively attend to goal-relevant stimuli in the face of distractions. In the Eriksen flanker task, human participants report which direction a centrally placed arrow is facing, while ignoring “flanking” arrows that may point in the opposite direction. Attentional control is evident to the extent that performance reflects only the direction of the central arrow. We describe four experiments in which we systematically assessed attentional control in rhesus monkeys using a flanker task. In Experiment 1, monkeys responded according to the identity of a central target, and accuracy and latency varied systematically with manipulations of flanking stimuli, validating our adaptation of the Eriksen flanker task. We then tested for converging evidence of attentional control across three experiments in which flanker performance was modulated by the distance separating targets from flankers (Experiment 2), luminance differences (Experiment 3), and differences in associative value (Experiment 4). The approach described is a new and reliable measure of attentional control in rhesus monkeys that can be applied to a wide range of situations with freely behaving animals.
Keywords: Attentional Control, Rhesus Monkeys, Flanker Task
Organisms are constantly bombarded by stimuli. Most are irrelevant, but some are critical for survival. There are at least three processes that determine whether particular stimuli are attended to or ignored. First, top-down attentional control directs cognitive processing to goal-relevant information. You exercise attentional control when deliberatively searching for a particular pen on top of your cluttered desk. Comparative studies have reported attentional control across a variety of animal species with diverse neurological structure, such as amphibians, birds, fish, and mammals—including human and nonhuman primates (Knudsen, 2018; Krauzlis et al., 2018). Attentional control likely first evolved because it provided an advantage in prey detection or predator avoidance. With increased neurological complexity, attentional control became more plastic and better able to handle a wider range of sensory domains (Krauzlis et al., 2018). Attentional control facilitates an array of nonhuman primate behaviors including social behaviors, foraging, and problem solving (Beran & Hopkins, 2018; Franzen & Myers, 1973; Genovesio et al., 2014; Myers et al., 1973; Sayers & Menzel, 2012).
The second process, attentional capture, is a bottom-up process that automatically draws attention to distinctive, salient, rewarding, or unexpected stimuli when they enter the sensory field (Anderson et al., 2011; Corbetta & Shulman, 2002; Desimone & Duncan, 1995; Theeuwes, 1992, 2004, 2010). The sirens and flashing lights on emergency vehicles make use of attentional capture to ensure that drivers who might be focusing elsewhere attend to the emergency vehicle. Attentional capture may either reinforce or compete with attentional control. To the extent that the pen you are searching for stands out in terms of shape or color, attentional capture and control work together. To the extent that flashing lights on a passing ambulance draw your attention away from reading at your desk at night, attentional control is compromised by attentional capture (Gaspelin et al., 2015; Theeuwes, 2004). Attentional capture has been reported in a wide variety of species (Buschman & Miller, 2007; Cai & Dent, 2020; Rochais et al., 2017) and is likely involved in a wide range of animal behaviors such as to hunting and foraging (Ben-Tov et al., 2015) as well as predator detection (Lipp, 2006; Shibasaki & Kawai, 2009).
The third influence, selection history, operates through repeated exposure to a stimulus, particularly when associated with reward. Rewarded exposure biases attention toward that stimulus in future encounters—even after the reward has stopped (Awh et al., 2012; Failing & Theeuwes, 2018; Theeuwes, 2019). Stimuli that gain attentional bias through selection history need not be inherently salient or goal-relevant to gain control over attention (Theeuwes, 2019). This influence of reward on attention may be evident, for example, when attention is captured by a favorite song from high school playing quietly in the background at a store.
Attentional control is assessed by measuring how well a subject can selectively attend to relevant information and ignore distractions. In the classic flanker task (Fig. 1a, Colegate et al., 1973; Eriksen & Hoffman, 1972, 1973; Ridderinkhof et al., 2021), participants view a horizontal array of arrows and are instructed to identify which direction the central-most arrow is facing. Human participants respond significantly faster when the target and flanking arrows are congruent, facing the same direction, compared to when the target and flanking arrows are incongruent, facing in opposite directions (Eriksen & Eriksen, 1974). The extent to which attentional control is challenged by flanking stimuli can result from two causes. Response conflict refers to the fact that the flankers direct a response opposite that indicated by the central arrow, and that response must be suppressed. Perceptual conflict refers to the fact that the stimuli flanking the central arrow have perceptual features that draw attention away from the central target. When the target is flanked by neutral stimuli other than arrows, removing the response incongruency, participants show an intermediate response latency (Eriksen & Eriksen, 1974). Thus, perceptual conflict experienced in both incongruent and neutral trials tax attentional resources and draw attention away from the target. Incongruent trials are particularly challenging because incongruent flankers additionally cause response conflict (Servant & Logan, 2019; Verbruggen et al., 2006).
Fig. 1.
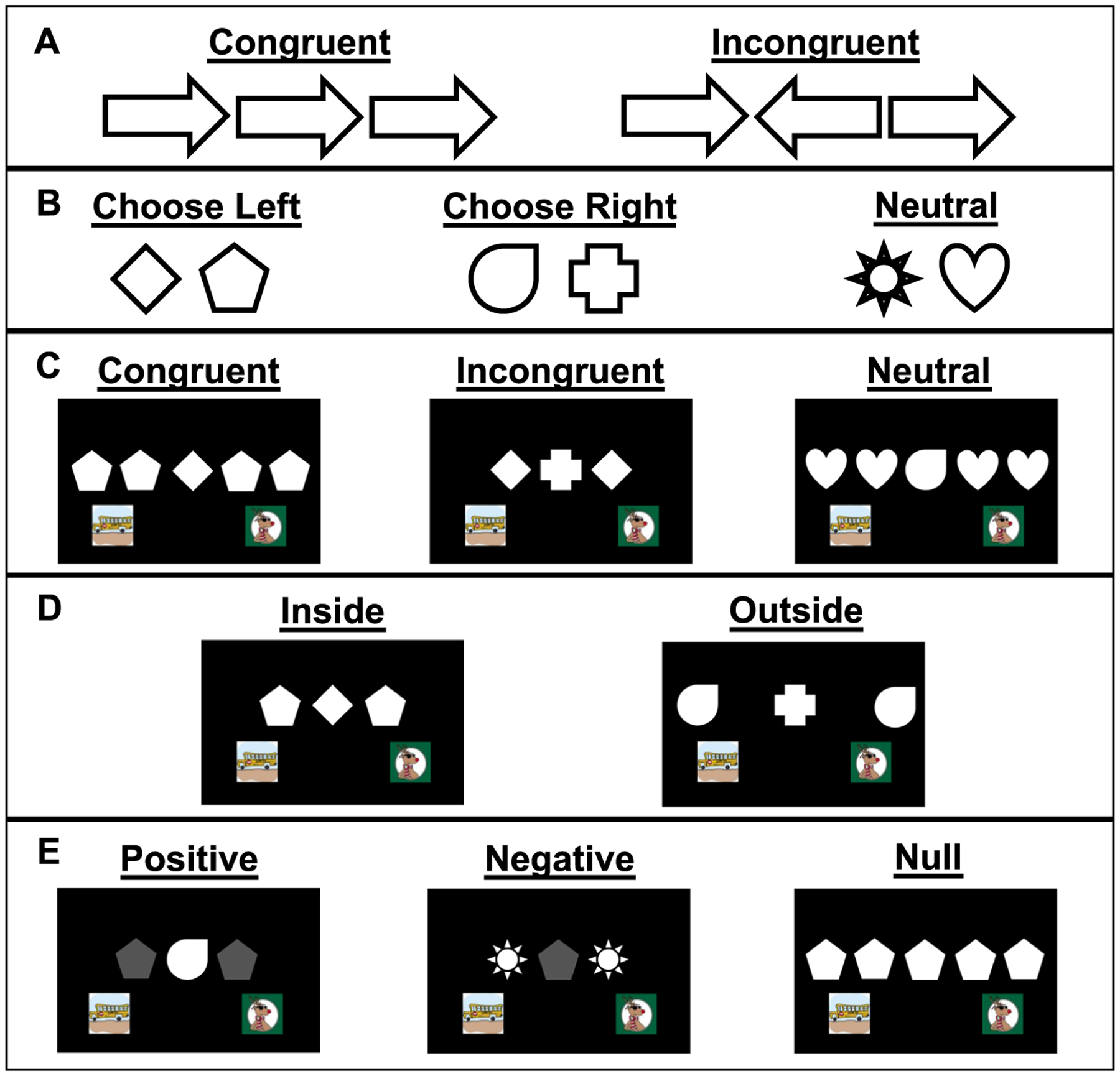
Flanker task test types. a example of stimuli used with humans in the classic Erickson flanker test. Tests with monkeys were initiated by monkeys touching a green start square (not shown). b Monkeys were trained to associate centrally placed diamond or pentagon stimuli with a response to the lower left bus image; responses to the lower right reindeer image were correct if the central stimulus was a tear drop or a cross. Eight neutral stimuli (two shown here) were not associated with either a left or right response and never appeared as a central target stimulus. Any images “flanking” the central images were to be ignored. c The three trial types that appeared in Experiment 1. d Examples of inside and outside trials presented in Experiment 2. e The three types of luminance configurations used in Experiment 3. The null condition also included trials in which all stimuli were dim
There are only a few reports of performance by nonhuman animals in flanker tasks, and the reported results are mixed. In one study, two rhesus monkeys were tested on a modified flanker task using letter stimuli. One of the two showed significantly faster responses to congruent relative to incongruent trials, and both showed significantly faster responses on neutral compared to either congruent or incongruent trials (Beran et al., 2003). In another study, a subset of rhesus monkeys—those that were not at ceiling performance—showed comparatively better accuracy on congruent relative to incongruent trials, but the relationship with respect to response time was less clear (Bramlett-Parker & Washburn, 2016). In another study, comparatively better accuracy and faster responding on congruent relative to incongruent trials was reported among 16 mice tested on an analog flanker task. Flanker performance was also sensitive to the contrast of flanking stimuli, and mice performed better when flanking stimuli had comparatively weaker contrast than the target (You & Mysore, 2020).
There is a need to develop and validate tests that measure attentional control that can be implemented across a variety of research settings. Specifically, the study of attentional control in nonhuman primates has overwhelmingly relied on cognitive tasks that measure saccadic eye movements, which can only realistically be implemented in laboratory settings (Cole et al., 2009). Technological and methodological advances have made it possible to study primate cognition in semi-natural settings (Fagot et al., 2013; Gazes et al., 2013), making nonhuman primate research uniquely positioned to address novel questions related to attentional control, such as selection history and attentional control over the lifetime of an animal, or the effect of chronic psychosocial stress on the development of attentional control. The flanker task may be appropriate for use across research settings given the simplicity of the task and the minimal resources required to implement it.
We describe four experiments in which we systematically assessed attentional control in rhesus monkeys using a flanker task. In Experiment 1, we tested whether attentional control was taxed by the presence of flanking stimuli, assessing the validity of our adaptation of the human flanker task. We then tested for converging evidence of attentional control across three experiments that assessed whether flanker performance was modulated by the spatial separation of targets and flankers (Experiment 2), luminance differences (Experiment 3), and differences in reinforcement history (Experiment 4).
Experiment 1
We tested whether incongruent and neutral flanking stimuli would impair performance consistent with both response and perceptual competition. We hypothesized that if stimulus incongruency taxes attentional resources in rhesus monkeys, then monkeys would be significantly less accurate and slower to respond on incongruent trials relative to congruent trials. Furthermore, we hypothesized that if both response conflict and perceptual conflict contribute to this effect, performance on neutral trials would fall between that on congruent and incongruent trials. Because monkeys do not know how to interpret arrows, we trained them to make specific responses to arbitrary images.
Procedure
Subjects
We tested seven adult male rhesus monkeys (mean age: 7.8 years old) that had extensive experience with touchscreen cognitive testing. Monkeys were pair housed and had visual contact with additional conspecifics. Monkeys were tested in their home cages and separated only during testing hours. Monkeys were tested for up to 8 hours each day, six days a week. Monkeys had ad libitum access to water, received daily fruit or vegetable enrichment, and received their daily caloric intake through a combination of nutritionally balanced reinforcement pellets earned in testing, and monkey biscuits fed at the end of the day.
Apparatus
Our computerized testing systems consisted of a touch-sensitive LCD monitor (Elo TouchSystems, Menlo Park, Ca) and two food dispensers (Med Associates Inc., St. Albans, VT), controlled by custom programs written in Visual Studio 2013 (Microsoft Corporation) run on laptop computers.
Flanker Training and Testing Procedures
Monkeys progressed through a series of 5 training phases and 1 test phase.
Training Phase 1
Trials were initiated by touching a green start square twice. A centrally placed shape appeared with two choice stimuli in the lower left and right corners of the screen. The central shape was either a white diamond or tear-shaped stimulus, and these two stimuli were presented equally often, according to a pseudorandom schedule (Fig. 1b). If the central stimulus was a diamond, monkeys were reinforced for selecting the left choice, which was always an image of a cartoon school bus. If the central stimulus was the tear shape, monkeys were reinforced for selecting the right stimulus, which was always a cartoon image of a Reindeer. Correct choices resulted in positive audio feedback, a food pellet reward, and a 3 second inter-trial interval. Incorrect choices resulted in negative audio feedback, a 6 second time-out, and repetition of the trial. If the monkey erred a second time, only the correct choice appeared on the third try, ensuring that monkeys selected that response. Each session consisted of 100 trials, and monkeys progressed to Phase 2 after achieving 90% accuracy or better in 2 consecutive sessions, not counting repeated trials following errors.
Training Phase 2
Training phase 2 was identical to Phase 1 except that monkeys were trained with two new shapes, a white pentagon and white cross. Monkeys were reinforced for selecting the left choice if the shape was a pentagon, and right choice for cross (Fig. 1b).
Training Phase 3
Training phase 3 was identical to training phases 1 and 2, except that any of the 4 stimuli from the previous 2 phases could appear in the central location on a given trial.
Training Phase 4
Each trial, 2 or 4 neutral stimuli flanked the target. There were a total of 8 neutral stimuli that were used according to a pseudorandom schedule (Fig. 1b). The same performance criterion was used to move monkeys to the final training phase.
Training Phase 5
In the final training phase, congruent and incongruent flankers were introduced (Fig. 1c). On congruent trials, the central stimulus and flanking stimuli were associated with the same response. On incongruent trials, the central stimulus and flanking stimuli were associated with opposite responses. On neutral trials the flanking stimuli were not associated with any response. Each 100-trial session consisted of 40 congruent trials, 40 incongruent trials, and 20 neutral trials. Once monkeys were 80% accurate or better for 2 consecutive sessions, they progressed to test.
Flanker Test Phase
The test phase was identical to training phase 5, however, no correction procedure was used. When monkeys erred, they received negative auditory feedback, a 6 second delay, and then could initiate the next trial. Monkeys received 14 sessions.
Data Preparation
Here and in the experiments that follow, accuracy was determined by calculating the proportion of trials where the monkeys were correct, and latency was determined by calculating the median latency for correct trials only. Median latency was used, rather than mean latency, to correct for positive skew in reaction time data. Proportion correct scores were arcsine transformed prior to analyses to better approximate normality (Aron & Aron, 1999). Repeated measures Analysis of Variance tests were performed using R Studio (R Core Team, 2020) the ez package (Lawrence, 2016).
Results & Discussion
Monkeys were most accurate on congruent trials, less accurate on neutral trials, and least accurate on incongruent trials (Fig. 2top: F(2,12) = 35.528, p < .001, η2G = 0.856). Follow-up TukeyHSD pairwise comparisons showed that all three conditions were significantly different from one another following adjustment for multiple comparisons (Congruent vs Incongruent: p = .002; Congruent vs Neutral: p = .017; Neutral vs Incongruent: p = .002). Thus, attentional control was present and was negatively impacted by both response and perceptual conflict, based on the predicted response pattern and similarity to human responding.
Fig. 2.
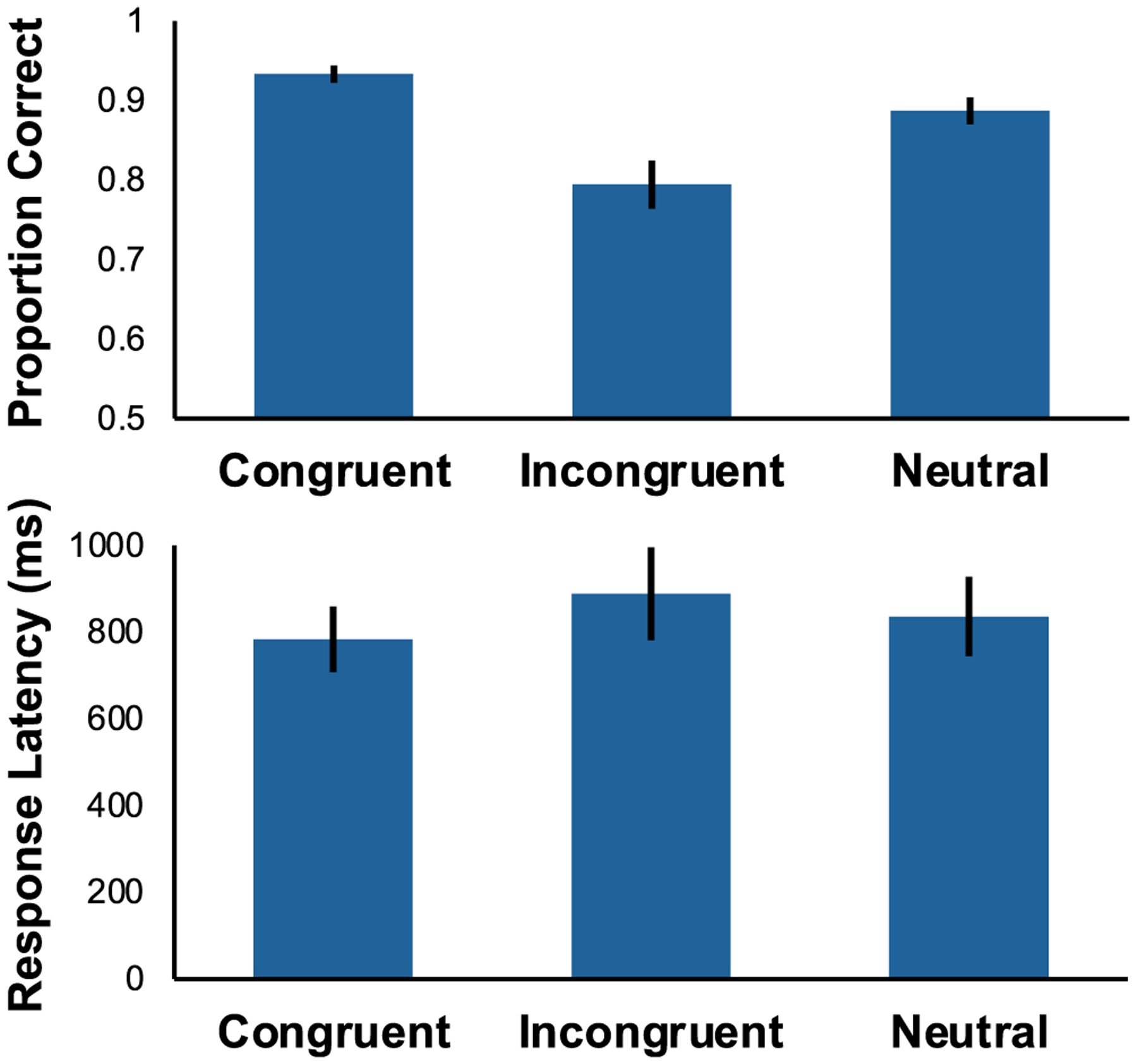
Accuracy (top) and response latency (bottom) when targets were flanked by congruent, incongruent, and neutral flankers. Error bars reflect standard error of the mean (SEM). Monkeys were most accurate on congruent trials, least accurate on incongruent trials, and intermediate on neutral trials. There was a significant main effect of latency, but the pairwise differences across conditions were not significant
Monkeys responded most slowly on incongruent trials, but this difference was small (Fig. 2bottom: F(2,12) = 6.380, p = .013, η2G = 0.515). Follow-up TukeyHSD pairwise comparisons found no significant pairwise differences between any of the three conditions following adjustment for multiple comparisons (Congruent vs Incongruent: p = .101, Congruent vs Neutral: p = .091, Incongruent vs Neutral: p = .329).
The characteristic flanker effect in human is that participants respond more slowly on incongruent trials relative to congruent or neutral trials (Kopp et al., 1996; Verbruggen et al., 2006). However, when a response time limit is imposed, conditions differ in accuracy rather than latency (Rinkenauer et al., 2004), indicating that participants trade-off accuracy and speed, favoring accuracy unless there is a time demand. Monkeys showed the strongest effects for accuracy, suggesting that they favor speed over accuracy or are relatively impulsive compared to humans. It is not uncommon for monkeys to only show accuracy differences where humans usually show speed differences. For example, monkeys made more errors rather than slowing down in cognitive control tasks (attention network test: Bramlett-Parker & Washburn, 2016; stroop: Washburn, 1994; stop signal: Godlove et al., 2011).
The findings from Experiment 1, in addition to the two previous flanker studies in monkeys, provide initial evidence that attentional control is necessary for flanker performance in rhesus monkeys (Beran et al., 2003; Bramlett-Parker & Washburn, 2016). However, similar findings across studies using similar procedures does not, alone, prove that humans and nonhumans rely on a common mechanism in a given task. It is possible for animals to mirror human performance on a cognitive task while relying on a functionally distinct cognitive system, or a different strategy. To further test whether our flanker task measures attentional control, we carried out additional experiments. If attentional control is measured by flanker performance in rhesus monkeys, then flanker performance should vary in predictable ways when we systematically alter demands on attentional control. One way to alter these demands is to increase the distance between target and flanking stimuli. Presumably the further distractors are from the center of the focus of attention, the less they distract.
Increasing the distance between target and flanking stimuli improved flanker performance in humans (Eriksen & Eriksen, 1974; Eriksen & Hoffman, 1972). Moving flanking stimuli away from the center of the screen, where subjects are presumably foveating, should reduce the extent to which flankers engage attention. We assessed whether the spacing between target and flanking stimuli affects the degree to which attentional control is necessary for flanker performance in monkeys.
Experiment 2
If flanker performance depends on attentional control in rhesus monkeys, then a larger distance between target and flanking stimuli should produce more accurate and faster performance. Furthermore, given that attentional control is necessary for successful performance on incongruent but not congruent trials, we hypothesized that the effect of spatial distance would be larger for incongruent trials than for neutral or congruent trials.
Procedure
Subjects
We tested six adult male rhesus monkeys (mean age: 9.8 years old) that had extensive experience with touchscreen cognitive testing. Five of the seven monkeys used in Experiment 1 were used again in Experiment 2. Two of the seven became unavailable due to reassignment outside our laboratory. An additional monkey that had been trained in a pilot version of Experiment 1 with slightly modified procedures from that described here was not included in the analysis of Experiment 1, but joined the study cohort with Experiment 2, yielding a total of 6 subjects. All monkeys were pair housed and had visual contact with conspecifics. Testing and feeding conditions were identical to Experiment 1.
Apparatus
The same equipment was used as in Experiment 1.
Flanker Training and Test Procedures
There were 2 training phases and 1 test phase.
Training Phase 1
The procedure was identical to Experiment 1 training phase 1 except we reduced the number of trials per session from 100 to 96.
Training Phase 2
Each trial, 2 neutral stimuli were presented at the outermost flanking positions within the presentation array to adapt monkeys to this novel test array configuration. A space of 212 pixels (5.61cm) separated the edges of the target and neutral images. Each session consisted of 96 trials, and monkeys progressed to the test phase after achieving 90% accuracy or better in 2 consecutive sessions.
Test Phase
Flanking stimuli were either incongruent, neutral, or congruent relative to the target, and flankers were placed either adjacent to or away from the target (Fig. 1d). When presented in the inner position, the inner most edges of flanking stimuli were 9 pixels (0.24cm) away from the outer edges of the target. When presented in the outer position, the inner most edges of the flanking stimuli were 212 pixels (5.61cm) away from the outer edges of the target. Each session consisted of 96 trials. Incongruent, neutral, and congruent trials were presented equally often, with half being close to the central stimulus and half distant, yielding 16 trials per session for each of the 6 trial type combinations. Monkeys completed 25 sessions.
Results & Discussion
Monkeys were more accurate when flankers were positioned farther away than close (Fig. 3top: F(1,5) = 16.662, p = 0.009, η2G = 0.426). Consistent with the findings from Experiment 1, accuracy varied as a function of flanker type (F(2,10) = 9.621, p = 0.004, η2G = 0.509). Follow-up TukeyHSD pairwise comparisons found a significant difference between congruent and incongruent conditions only following adjustment for multiple comparisons (p = 0.017). The interaction between position and type of flanking stimuli was significant (F(2,10) = 17.096, p < 0.001, η2G = 0.449), indicating that incongruent stimuli had the greatest effect when positioned close to the target. Pairwise comparisons found that monkeys were less accurate on incongruent flanker trials where the flankers were placed close to the target (Incongruent Inside vs Congruent Inside: p = 0.005; Incongruent Inside vs Congruent Outside: p = 0.015; Incongruent Inside vs Incongruent Outside: p = 0.027; Incongruent Inside vs Neutral Outside: p = 0.013).
Fig. 3.
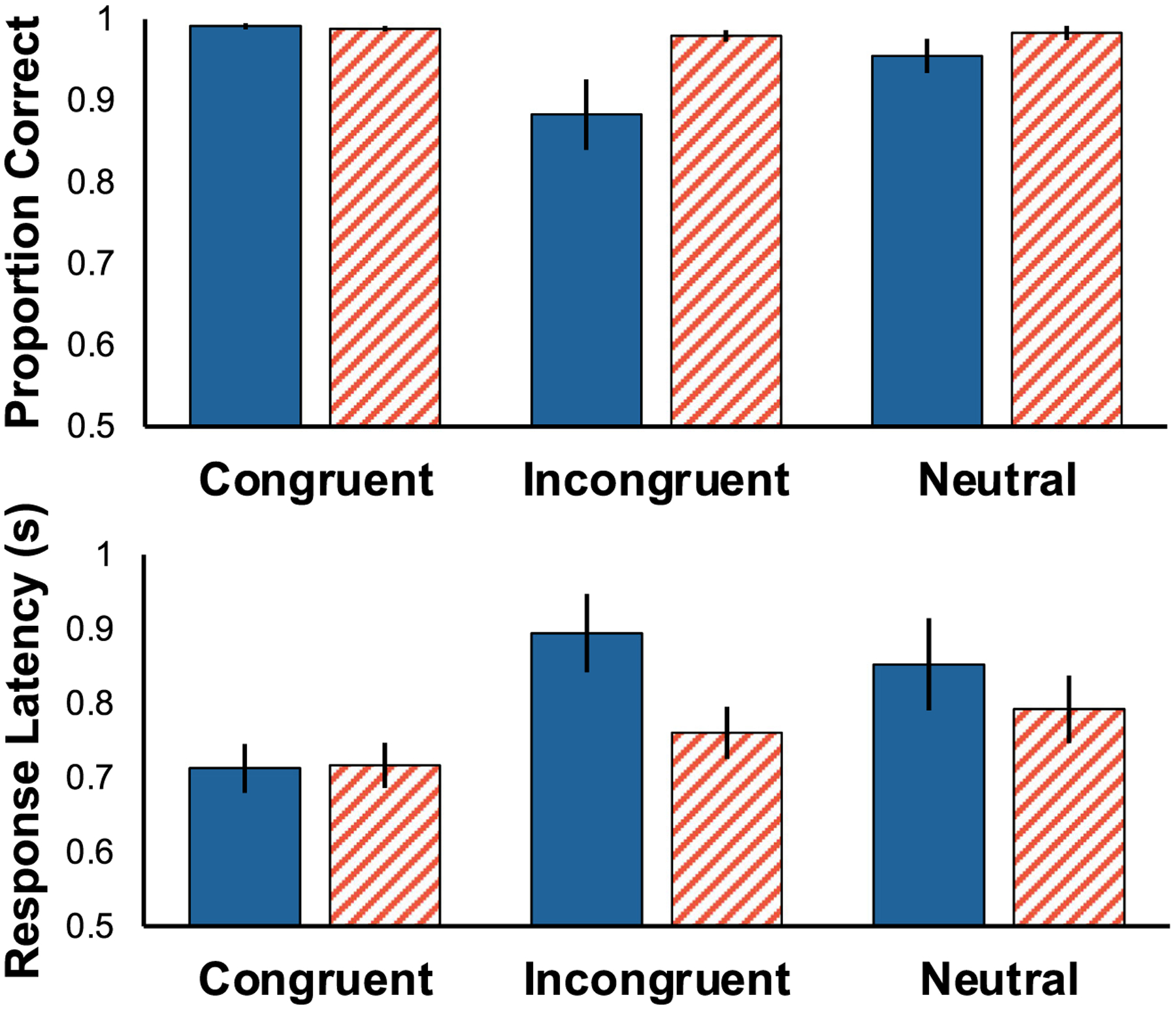
Accuracy (top) and response latency (bottom) as a function of both flanker position and flanker congruency. Error bars reflect standard error of the mean (SEM). Performance was significantly worse for inside trials (blue, solid) compared to outside trials (red slanted) and this effect was particularly pronounced for incongruent trials
Monkeys responded more quickly when flankers were positioned farther away than close (Fig. 3bottom: F(1,5) = 32.439, p = 0.002, η2G = 0.391). Consistent with the findings from Experiment 1, response times differed significantly as a function of flanker type (F(2,10) = 11.928, p = 0.002, η2G = 0.632). Follow-up TukeyHSD pairwise comparisons found a significant difference between congruent and incongruent conditions (p = .042), a marginal difference between congruent and neutral (p = 0.055), and no difference between neutral and incongruent (p = 0.992). Incongruent stimuli slowed responding most when close to the target (interaction of Position and Flanker Type: F(2,10) = 14.017, p = 0.001, η2G = 0.335). Follow up tests revealed no significant pair-wise differences.
The results from Experiment 2 provide converging evidence that attentional control is important for flanker performance in rhesus monkeys. The proximity of target and flanking stimuli impacted performance to varying degrees depending on the type of trial. For congruent trials, the distance between target and flanking stimuli had essentially no effect on performance. For incongruent trials, the distance between target and flanking stimuli had a pronounced effect on performance. In contrast to Experiment 1, monkeys did not exhibit an intermediate accuracy effect on neutral trials overall, this perhaps due to a smaller valence effect when averaged across inside and outside trials. Thus, these results support the hypothesis that it is the extent to which flankers compete for attention that determines their effects on performance. In the next experiment we further evaluated whether our flanker task measures attentional control by using another manipulation likely to affect the extent to which attention is captured, visual contrast.
In visual search paradigms, including a distracting stimulus that differs from all other stimuli by some task-irrelevant feature increases the latency to find a target (Theeuwes, 1991, 2004; Turatto & Galfano, 2000). For instance, objects with relatively greater contrast from their surroundings are more visually salient, and increasing the luminance of target or distractor stimuli shortens or increases visual search time, respectively (Spehar & Owens, 2012). In Experiment 3, we tested whether flanker performance was sensitive to variations in luminance among target and flanking stimuli.
Experiment 3
In Experiments 1 and 2, performance varied as a function of demands on attentional control. In Experiment 3, we tested whether inherently salient stimuli would affect flanker performance through attentional capture. Specifically, we modulated the relative luminance of target and flanking stimuli such that on some trials the target contrasted more with the background than did flanking stimuli, and on other trials flanking stimuli contrasted more with the background than did the target. On yet other trials, target and flanking stimuli contrasted equally with the background. If our task measures attentional control, then performance should vary as a function of the extent of attentional capture produced by the three luminance conditions. Specifically, performance should be best when the target stimulus contrasts most with the background, compared to flanking stimuli. Moreover, the effect of luminance should be more pronounced in incongruent trials compared to congruent or neutral trials.
Procedure
Subjects
Subjects and testing conditions were identical to Experiment 2.
Apparatus
Equipment was identical to Experiment 2.
Flanker Training and Test Procedures
There were 2 training phases and 1 test phase.
Training Phase 1
This was identical to Training Phase 1 in Experiment 2.
Training Phase 2
Three luminance conditions were introduced: positive, in which the target was bright and flanking stimuli were dim; negative, in which the target was dim and flankers were bright; and null, in which target and flanking stimuli were all bright or all dim (Fig. 1e). Positive, negative, null bright, and null dim trials were presented equally often. Bright stimuli had RGB values of 255, 255, 255 and dim stimuli had RGB values of 84, 84, 84. Targets were flanked by either two or four stimuli, all of which shared the same luminance value. Each session consisted of 96 trials, and monkeys completed training phase 2 by achieving 80% accuracy or better for 2 consecutive sessions.
Test Phase
The procedure used in the test phase was identical to training phase 2. Monkeys completed 25 test sessions.
Results & Discussion
The effect of luminance on accuracy was more pronounced on trials that tested for attentional control than on trials that did not, indicated by a significant luminance by flanker type interaction (Fig. 4top: F(4,20) = 3.325, p = 0.032, η2G = 0.122). Follow up tests confirmed that accuracy on negative incongruent trials, where flankers were brighter than the target and attentional control demands were highest, was significantly worse than most other conditions (NegI vs NegC: p = 0.001, NegI vs NullC: p = 0.002, NegI vs PosC: p = 0.002, NegI vs PosN: p = 0.024). Flanker type significantly affected accuracy (F(2,10) = 34.372, p < 0.001, η2G = 0.449), with monkeys being significantly more accurate on congruent than incongruent trials (p < 0.001). There was not a main effect of luminance on accuracy (F(2,10) = 3.529, p = 0.069, η2G = 0.321).
Fig. 4.
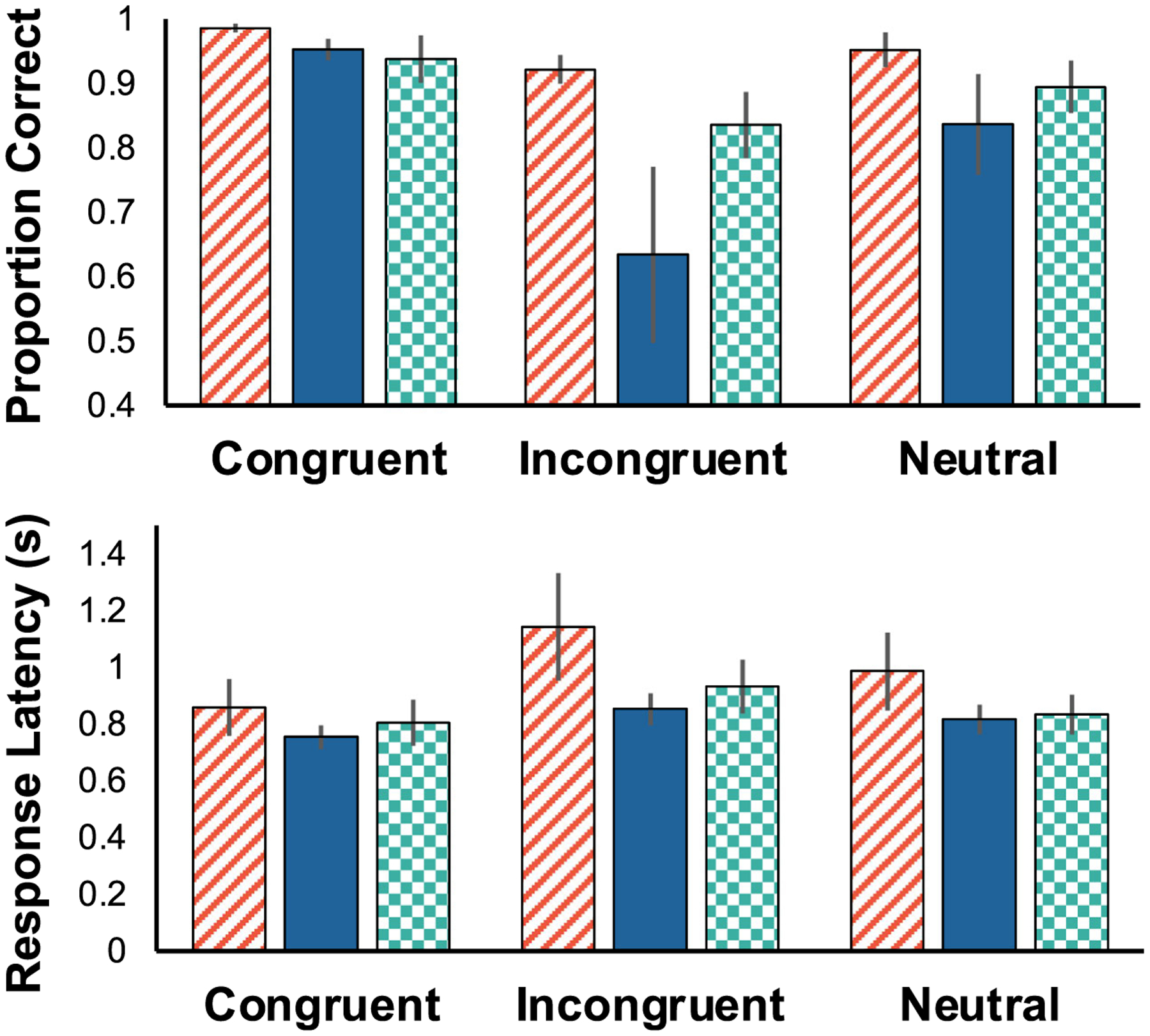
Accuracy (top) and latency (bottom) as a function of luminance condition and flanker valence. Positive (red slanted), negative (blue solid), and null (green checkered) luminance configurations are shown. Error bars reflect standard error of the mean (SEM). Accuracy varied as a function of both luminance and flanker valence, with performance being notably worse during incongruent and negative luminance trials
The speed with which monkeys completed flanker trials was unaffected by the relative luminance of target and flanking stimuli (Fig. 4bottom: F(2,10) = 2.920, p = 0.100, η2G = 0.284). There was a significant main effect of flanker type on response latency (F(2,10) = 9.888, p = 0.004, η2G = 0.233), however follow-up TukeyHSD pairwise comparisons found no significant differences between conditions. The difference between congruent and incongruent trials approached significance (p = 0.069). There was no interaction between luminance and flanker valence (F(4,20) = 2.293, p = 0.095, η2G = 0.069).
The results from Experiment 3 provide further evidence that attentional control supports flanker performance in rhesus monkeys. Monkeys were less accurate on trials on which the luminance contrast was lower for target than flanking stimuli, particularly on incongruent trials. Luminance contrast alone, independent of flanker type, had no effect on response latency.
We often think of the capacity of stimuli to capture attention in terms of perceptual properties, such as a brightness or loudness. However, stimuli that are rewarding or threatening can also capture attention irrespective of their sensory properties. Such value driven attentional capture, requires that subjects have previously had reinforcing experiences with initially neutral stimuli (Anderson et al., 2011). In Experiment 4, we tested whether flanker performance varied as a function of the reinforcement histories of target and flanking stimuli.
Experiment 4
In Experiments 2 and 3 we tested whether physical changes in flankers affected the extent to which they captured attention. In Experiment 4 we investigated whether associative value also acts to capture attention, even when physical features of stimuli are matched. We hypothesized that if stimuli paired with comparatively higher reward are more likely to capture attention, then monkeys will show superior flanker performance on trials where high reward stimuli are targets compared to flankers. Moreover, this effect might be more pronounced on incongruent trials compared to congruent trials.
Procedure
Subjects
Subjects and testing conditions were identical to Experiment 3.
Apparatus
Testing equipment was identical to Experiment 3.
Training and Test Procedures
There were 2 training phases and 1 test phase.
Training Phase 1
Training phase 1 was identical to Training Phase 1 in Experiment 3, except that correct responses were associated with different rewards depending on which stimulus was the target. If a diamond or tear shape was the target, monkeys were rewarded with one pellet for a correct response. If a pentagon or cross was the target, monkeys were rewarded with three pellets for a correct response.
Training Phase 2
Three value configurations were used: positive, in which the target was a high value stimulus and flankers were a low value; negative, in which the target was a low value stimulus and flankers were a high value; or null, in which the target and flankers were both low or both high value stimuli. Each session consisted of 96 trials, and monkeys completed training phase 2 by achieving 80% accuracy or better in 2 consecutive sessions.
Test Phase
The procedure used in the test phase was identical to training phase 2. The differential reinforcement of the four stimuli was maintained throughout the test phase to prevent changes in associative value. Monkeys completed 25 sessions.
Data Preparation
Test trials were categorized into three categories: positive, in which the target was a high value stimulus and flankers were low value stimuli; null, in which target and flanking stimuli were either all high or low value stimuli; and negative, in which the target was a low value stimulus and flanking stimuli were high value stimuli. Trials with neutral flankers were not included in the analysis because they could not be meaningfully compared with congruent and incongruent trials. This is because neutral trials contained only two stimulus value configurations, with high or low value targets flanked by neutral stimuli that could not be assigned associative values.
Results & Discussion
The configuration of high and low value stimuli affected accuracy equally across congruent and incongruent trials. There was a main effect of value configuration on accuracy (Fig. 5top: F(2,10) = 7.441, p = 0.010, η2G = 0.415), and follow up tests found significantly more accurate responses on positive trials compared to negative trials (p = 0.007). Monkeys were significantly more accurate on congruent trials compared to incongruent trials (F(1,5) = 52.481, p < 0.001, η2G = 0.655). However, the interaction between flanker type and value configuration was not significant (F(2,10) = 1.088, p = 0.373, η2G = 0.068), indicating that positive, null, and negative value configurations affected accuracy equally, regardless of whether stimuli were congruent or incongruent. It is surprising that all flanker valence conditions, including congruent trials, were affected by reinforcement history, given that there is no response conflict during congruent trials, thus it is unclear why accuracy would be sensitive to different value configurations.
Fig. 5.
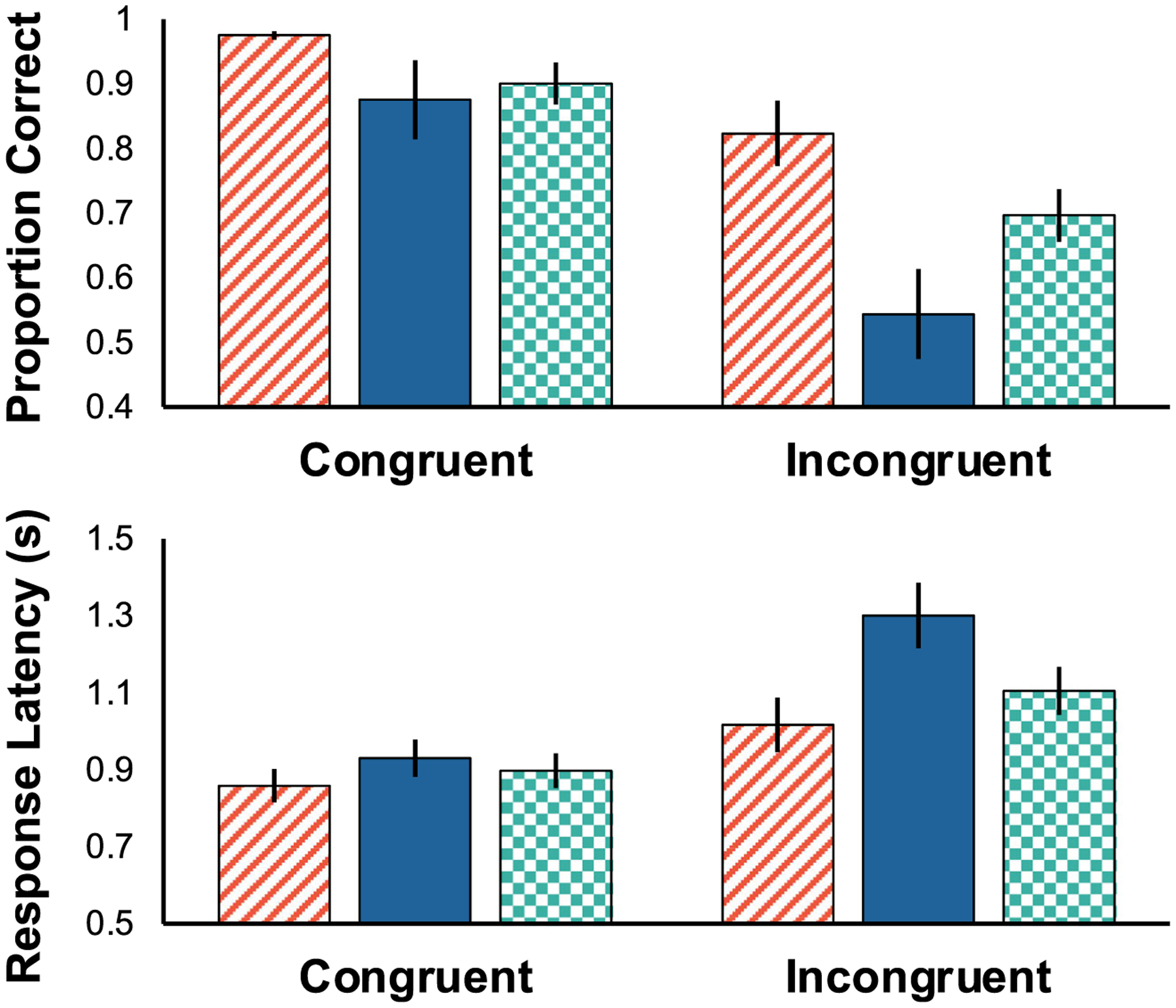
Accuracy (top) and latency (bottom) as a function of value configuration and congruency. Error bars reflect standard error of the mean (SEM). Monkeys were more accurate and responded more quickly to positive value configurations (red slanted) compared to negative configurations (blue solid). Null configurations (green checkered) did not differ from either positive or negative. For latency only, the effect of value configuration was particularly pronounced on incongruent trials
Monkeys were slower to respond when trials contained a low value target and high value flankers compared to the opposite configuration—and this effect was particularly pronounced for incongruent trials relative to congruent. Specifically, there was a significant main effect of associative value on latency (Fig. 5bottom: F(2,10) = 14.783, p = 0.001, η2G = 0.343), and follow-up TukeyHSD pairwise comparisons revealed significantly faster responses for positive configurations than negative configurations following adjustment (p = 0.037). Monkeys were also significantly faster to respond on congruent trials relative to incongruent trials (F(1,5) = 10.118, p = 0.024, η2G = 0.592). Finally, there was a significant interaction between value configuration and congruency (F(2,10) = 9.731, p = 0.004, η2G = 0.167), and follow up tests revealed that negative incongruent trials were significantly different from all congruent configurations (Negative Incongruent [NeI] vs NeC: p = 0.006; NeI vs NuC: p < 0.001; NeI vs PosC: p < 0.001).
The results from Experiment 4 provide further converging evidence for attentional control as a critical process supporting flanker performance in rhesus monkeys. Stimuli with comparatively rich reinforcement histories engaged attention and had a pronounced impact on both accuracy and latency. When flanking stimuli had comparatively rich reinforcement histories compared to target stimuli, monkeys were slower and less accurate. When flanking stimuli had comparatively low associative value relative to target stimuli, performance was faster and more accurate. With respect to latency, performance differences between these two configurations was larger for incongruent trials than congruent trials. Because we changed only reinforcement history and not the perceptual properties of the stimuli in this experiment, it is likely that the impairments in performance we observed as a result of associative value differences result solely from response conflict, not perceptual conflict. However, because we could not include associatively equivalent neutral flankers in the analysis, we cannot directly evaluate whether or not associative differences might produce perceptual differences as a result of learned salience.
General Discussion
Our four experiments provide converging evidence that the flanker task measures attentional control in rhesus monkeys. In Experiment 1, we tested whether stimulus incongruency affected attentional control. Monkeys were significantly less accurate and slower on incongruent trials relative to congruent trials. The fact that the effect of neutral stimuli fell between incongruent and congruent indicated that there were two causes of interference, both response conflict and perceptual conflict (Eriksen & Eriksen, 1974). Experiments 2–4 then documented further properties of monkey attention with this procedure. In Experiment 2 we found that monkeys were faster and more accurate when target and flanking stimuli were spaced apart rather than adjacent to each another, and the effect of spacing on accuracy was particularly pronounced for incongruent trials. In Experiment 3 we found that accuracy was worst when low luminance targets were placed next to high luminance incongruent flankers. Finally, in Experiment 4, we found that monkeys were more accurate and responded most quickly when target stimuli had richer reinforcement histories than flankers, and that responses were slowest when low value targets were flanked by high value distractors.
Attentional control is a foundational process that supports a variety of human capacities, such as visual working memory (Astle & Scerif, 2011) and target finding (Eimer, 2014). Although studies of nonhuman primates have been integral for advancing scientific knowledge about the neural mechanisms of attentional control (Desimone & Duncan, 1995; Miller & Cohen, 2001), we know comparatively less about what roles attentional control plays in natural nonhuman primate behavior, and what ecological and social factors affect it (Morin et al., 2019). We can now evaluate nonhuman primate cognition in naturalistic environments (Fagot & Paleressompoulle, 2009; Gazes et al., 2013), and we can extend this ability to the study of attention with appropriate tasks like the one described here. The flanker task is well suited for application in naturalistic environments given that animals need not be restrained, trials are rapid and independent from one another, and the rate of reinforcement is comparatively high. The flanker task has been and continues to be used as a tool for measuring attention in human subjects, and thus, provides a framework for future comparative research on nonhuman attention (Erb et al., 2021; Ridderinkhof et al., 2021).
Naturalistic settings afford opportunities to address previously unanswerable questions about nonhuman attention. These questions include the effects of social status, stress, and reinforcement history on attention. As demonstrated in Experiment 4, stimuli associated with different levels of reward differentially affect flanker performance. Such an effect can be most reliably studied in an experimental setting where the entire history of reward with particular stimuli can be controlled. In humans, previously neutral stimuli that undergo reward training continue to capture attention when used as irrelevant distractors as long as 7–9 months after rewarded training stops (Anderson & Yantis, 2013). Rhesus monkeys afford a unique opportunity to study the extent to which the effects of selection history persist across the lifetime of an animal, as well as how relative control by reinforcement history is modified during times of chronic stress or cognitive senescence. Technological developments now allow researchers to track and manipulate diet and cognitive performance across virtually the entire lifetime of rhesus monkeys, thereby creating an opportunity to study the effects of diet on cognition (Gazes et al., 2013; Wilson et al., 2008).
The nonhuman flanker analog described in this study can be readily implemented in naturalistic environments, affording the opportunity to investigate attentional control in a variety of contexts. Rhesus monkeys live in large social groups organized into linear dominance hierarchies (Vessey, 1984). In naturalistic settings, social dominance is a pronounced psychosocial stressor, where subordinate individuals exhibit a physiological profile that mirrors humans exposed to chronic psychosocial stress, including blunted stress response to physiological and psychosocial stressors, impaired gluco-corticoid negative feedback, and overall higher hair cortisol concentrations relative to dominant monkeys (Michopoulos, Higgins, et al., 2012a; Michopoulos, Reding, et al., 2012b; Qin et al., 2013). Chronic stress, particularly when experienced early in life, may have a pronounced negative effect on attentional control, and has been shown to increase susceptibility to attentional capture of threatening and emotional stimuli in both children and rodents (Cohen et al., 2013; Tottenham et al., 2010, 2011). Rhesus monkeys who experience early life stress have also shown modified responses to threatening social stimuli compared to controls in a dot-probe task (Morin et al., 2019). Little work has been done to evaluate whether social status in group-housed rhesus monkeys predicts cognitive performance on cognitive control tasks. The nonhuman flanker analog described in this manuscript may afford the opportunity to evaluate whether social status or stress modulate attentional control in rhesus monkeys.
We report a set of converging experiments showing that rhesus monkeys rely on attentional control in a flanker task. We found that performance varied with flanker congruency, the distance between targets and flankers, differences in luminance, and differences in associative value. Most studies of attentional control in nonhuman primates have depended on measures of saccadic eye movements, which can only realistically be implemented in laboratory settings (Cole et al., 2009). The work described in this paper presents a validated alternative measure of attentional control in rhesus monkeys which may be more readily applied across both laboratory and naturalistic settings. The procedures described in this study may be readily applied to other nonhuman animals, providing opportunity to determine the extent to which these findings generalize to other species.
The data and materials for all experiments may be made available from the authors upon request. None of these experiments were preregistered.
Author Note
We acknowledge support from the National Science Foundation (BCS-1632477; BCS-1946767) and from the National Institutes of Health’s Office of the Director, Office of Research Infrastructure Programs, P51OD011132. We are grateful to Tara Dove-VanWormer for help testing monkeys. The authors declare no conflicts of interest.
References
- Anderson BA, Yantis S (2013) Persistence of value-driven attentional capture. Journal of Experimental Psychology: Human Perception and Performance, 39(1), 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, Yantis S (2011) Value-driven attentional capture. Proceedings of the National Academy of Sciences of the United States of America 108(25): 10367–10371. 10.1073/pnas.1104047108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A, Aron EN (1999) Statistics for psychology. Prentice-Hall, Inc. [Google Scholar]
- Astle DE, Scerif G (2011) Interactions between attention and visual short-term memory (VSTM): What can be learnt from individual and developmental differences?. Neuropsychologia, 49(6), 1435–1445. [DOI] [PubMed] [Google Scholar]
- Awh E, Belopolsky AV, Theeuwes J (2012) Top-down versus bottom-up attentional control: A failed theoretical dichotomy. Trends in Cognitive Sciences, 16(8), 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Tov M, Donchin O, Ben-Shahar O, Segev R (2015) Pop-out in visual search of moving targets in the archer fish. Nature Communications, 6(1), 1–11. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Hopkins WD (2018) Self-control in chimpanzees relates to general intelligence. Current Biology 28(4): 574–579. 10.1016/j.cub.2017.12.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Washburn DA, Kleinman S (2003, November) Attention Network Task (ANT) performance by children and rhesus monkeys. In 44th Annual Meeting of the Psychonomic Society, Vancouver, BC. [Google Scholar]
- Bramlett-Parker J, Washburn DA (2016) Can Rhesus Monkeys Learn Executive Attention? Behavioral Science 6(2): 11. 10.3390/bs6020011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK (2007) Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science, 315(5820), 1860–1862. [DOI] [PubMed] [Google Scholar]
- Cai H, Dent ML (2020) Attention capture in birds performing an auditory streaming task. PLoS One, 15(6), e0235420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MM, Jing D, Yang RR, Tottenham N, Lee FS, Casey BJ (2013) Early-life stress has persistent effects on amygdala function and development in mice and humans. P Natl A Sci 110(45): 18274–18278. 10.1073/pnas.1310163110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Yeung N, Freiwald WA, Botvinick M (2009) Cingulate cortex: diverging data from humans and monkeys. Trends in Neurosciences 32(11): 566–574. 10.1016/j.tins.2009.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colegate RL, Hoffman JE, Eriksen CW (1973) Selective encoding from multielement visual displays. Perception & Psychophysics 14(2): 217–224. 10.3758/BF03212380 [DOI] [Google Scholar]
- Corbetta M, Shulman GL (2002) Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews. Neuroscience 3(3): 201–215. 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J (1995) Neural mechanisms of selective visual attention. Annual Review of Neuroscience, 18(1): 193–222. [DOI] [PubMed] [Google Scholar]
- Eimer M (2014) The neural basis of attentional control in visual search. Trends in Cognitive Sciences, 18(10), 526–535. [DOI] [PubMed] [Google Scholar]
- Erb CD, Smith KA, Moher J (2021) Tracking continuities in the flanker task: From continuous flow to movement trajectories. Attention, Perception, & Psychophysics, 83(2), 731–747. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW (1974) Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics 16(1): 143–149. 10.3758/BF03203267 [DOI] [Google Scholar]
- Eriksen CW, Hoffman JE (1972) Temporal and spatial characteristics of selective encoding from visual displays. Perception & Psychophysics 12(2): 201–204. [Google Scholar]
- Eriksen CW, Hoffman JE (1973) The extent of processing of noise elements during selective encoding from visual displays. Perception & Psychophysics 14(1): 155–160. 10.3758/BF03198630 [DOI] [Google Scholar]
- Fagot J, Paleressompoulle D (2009) Automatic testing of cognitive performance in baboons maintained in social groups. Behavior Research Methods, 41(2), 396–404. [DOI] [PubMed] [Google Scholar]
- Fagot J, Bonté E, Hopkins WD (2013) Age-dependant behavioral strategies in a visual search task in baboons (Papio papio) and their relation to inhibitory control. Journal of Comparative Psychology 127(2): 194–201. 10.1037/a0026385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Failing M, Theeuwes J (2018) Selection history: How reward modulates selectivity of visual attention. Psychonomic Bulletin & Review, 25(2), 514–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen EA, Myers RE (1973) Neural control of social behavior: prefrontal and anterior temporal cortex. Neuropsychologia 11(2): 141–157. [DOI] [PubMed] [Google Scholar]
- Gaspelin N, Margett-Jordan T, Ruthruff E (2015) Susceptible to distraction: Children lack top-down control over spatial attention capture. Psychonomic Bulletin & Review, 22(2), 461–468. [DOI] [PubMed] [Google Scholar]
- Gazes RP, Brown EK, Basile BM, Hampton RR (2013) Automated cognitive testing of monkeys in social groups yields results comparable to individual laboratory-based testing. Animal Cognition 16: 445–458. 10.1007/s10071-012-0585-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovesio A, Wise SP, Passingham RE (2014) Prefrontal–parietal function: from foraging to foresight. Trends in Cognitive Sciences 18(2): 72–81. 10.1016/j.tics.2013.11.007 [DOI] [PubMed] [Google Scholar]
- Godlove DC, Emeric EE, Segovis CM, Young MS, Schall JD, Woodman GF (2011) Event-related potentials elicited by errors during the stop-signal task. I. Macaque monkeys. The Journal of Neuroscience 31(44): 15640–15649. doi: 10.1523/JNEUROSCI.3349-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI (2018) Neural circuits that mediate selective attention: a comparative perspective. Trends in Neurosciences 41(11): 789–805. 10.1016/j.tins.2018.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp B, Rist F, Mattler UWE (1996) N200 in the flanker task as a neurobehavioral tool for investigating executive control. Psychophysiology 33(3): 282–294. 10.1111/j.1469-8986.1996.tb00425.x [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Bogadhi AR, Herman JP, Bollimunta A (2018) Selective attention without a neocortex. Cortex 102: 161–175. 10.1016/j.cortex.2017.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MA (2016) ez: Easy Analysis and Visualization of Factorial Experiments. R package version 4.4–0. https://CRAN.R-project.org/package=ez
- Lipp OV (2006) Of snakes and flowers: Does preferential detection of pictures of fear-relevant animals in visual search reflect on fear-relevance?. Emotion, 6(2), 296. [DOI] [PubMed] [Google Scholar]
- Michopoulos V, Higgins M, Toufexis D, Wilson ME (2012a) Social subordination produces distinct stress-related phenotypes in female rhesus monkeys. Psychoneuroendocrinology 37(7): 1071–1085. 10.1016/j.psyneuen.2011.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Reding KM, Wilson ME, Toufexis D (2012b) Social subordination impairs hypothalamic–pituitary–adrenal function in female rhesus monkeys. Hormones and Behavior 62(4): 389–399. 10.1016/j.yhbeh.2012.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD (2001) An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24(1), 167–202. [DOI] [PubMed] [Google Scholar]
- Morin EL, Howell BR, Meyer JS, Sanchez MM (2019) Effects of early maternal care on adolescent attention bias to threat in nonhuman primates. Developmental Cognitive Neuroscience 38: 100643. 10.1016/j.dcn.2019.100643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RE, Swett C, Miller M (1973) Loss of social group affinity following prefrontal lesions in free-ranging macaques. Brain Research 64: 257–269. 10.1016/0006-8993(73)90182-0 [DOI] [PubMed] [Google Scholar]
- Qin Dong-Dong, Rizak Joshua D, Feng Xiao-Li, Chu Xun-Xun, Yang Shang-Chuan, Li Chun-Lu, Lv Long-Bao, Ma Yuan-Ye, Hu Xin-Tian (2013) Social rank and cortisol among female rhesus macaques (Macaca mulatta). Zoological Research 34(E2): E42–E49. : 10.3724/SP.J.1141.2013.E02E42 [DOI] [PubMed] [Google Scholar]
- R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Version 4.0.2 URL https://www.R-project.org/ [Google Scholar]
- Ridderinkhof KR, Wylie SA, van den Wildenberg WP, Bashore TR, van der Molen MW (2021) The arrow of time: Advancing insights into action control from the arrow version of the Eriksen flanker task. Attention, Perception, & Psychophysics, 83(2), 700–721. 10.3758/s13414-020-02167- [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkenauer G, Osman A, Ulrich R, Müller-Gethmann H, Mattes S (2004) On the locus of speed-accuracy trade-off in reaction time: inferences from the lateralized readiness potential. Journal of Experimental Psychology. General 133(2): 261–282. 10.1037/0096-3445.133.2.261 [DOI] [PubMed] [Google Scholar]
- Rochais C, Henry S, Hausberger M (2017) Spontaneous attention-capture by auditory distractors as predictor of distractibility: a study of domestic horses (Equus caballus). Scientific Reports, 7(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers K, Menzel CR (2012) Memory and foraging theory: chimpanzee utilization of optimality heuristics in the rank-order recovery of hidden foods. Animal Behaviour 84(4): 795–803. 10.1016/j.anbehav.2012.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servant M, Logan GD (2019) Dynamics of attentional focusing in the Eriksen flanker task. Attention, Perception, & Psychophysics, 81(8), 2710–2721. 10.3758/s13414-019-01796-3 [DOI] [PubMed] [Google Scholar]
- Shibasaki M, Kawai N (2009) Rapid detection of snakes by Japanese monkeys (Macaca fuscata): an evolutionarily predisposed visual system. Journal of Comparative Psychology, 123(2), 131. [DOI] [PubMed] [Google Scholar]
- Spehar B, Owens C (2012) When do luminance changes capture attention? Attention, Perception, & Psychophysics 74(4): 674–690. 10.3758/s13414-011-0266-8 [DOI] [PubMed] [Google Scholar]
- Theeuwes J (1991) Cross-dimensional perceptual selectivity. Perception & Psychophysics 50(2): 184–193. 10.3758/BF03212219 [DOI] [PubMed] [Google Scholar]
- Theeuwes J (1992) Perceptual selectivity for color and form. Perception & Psychophysics 51(6): 599–606. 10.3758/BF03211656 [DOI] [PubMed] [Google Scholar]
- Theeuwes J (2004) Top-down search strategies cannot override attentional capture. Psychonomic Bulletin & Review 11(1): 65–70. 10.3758/BF03206462 [DOI] [PubMed] [Google Scholar]
- Theeuwes J (2010) Top–down and bottom–up control of visual selection. Acta Psychologica 135(2): 77–99. 10.1016/j.actpsy.2010.02.006 [DOI] [PubMed] [Google Scholar]
- Theeuwes J (2019) Goal-driven, stimulus-driven, and history-driven selection. Current Opinion in Psychology, 29, 97–101. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, et al. (2010) Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental Science 13(1): 46–61. 10.1111/j.1467-7687.2009.00852.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ (2011) Elevated amygdala response to faces following early deprivation. Developmental Science 14(2): 190–204. 10.1111/j.1467-7687.2010.00971.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turatto M, Galfano G (2000) Color, form and luminance capture attention in visual search. Vision Research 40(13): 1639–1643. 10.1016/S0042-6989(00)00061-4 [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Notebaert W, Liefooghe B, Vandierendonck A (2006) Stimulus-and response-conflict-induced cognitive control in the flanker task. Psychonomic Bulletin & Review 13(2): 328–333. 10.3758/BF03193852 [DOI] [PubMed] [Google Scholar]
- Vessey SH (1984) Dominance among rhesus monkeys. Political Psychology 5(4): 623–628. 10.2307/3791232 [DOI] [Google Scholar]
- Washburn DA (1994) Stroop-like effects for monkeys and humans: Processing speed or strength of association? Psychological Science 5(6): 375–379. 10.1111/j.1467-9280.1994.tb00288. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Fisher J, Fischer A, Lee V, Harris RB, Bartness TJ (2008) Quantifying food intake in socially housed monkeys: social status effects on caloric consumption. Physiology & Behavior, 94(4), 586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You WK, Mysore SP (2020) Endogenous and exogenous control of visuospatial selective attention in freely behaving mice. Nature Communications, 11(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]


