Abstract
Objectives.
The development of thermosetting polymers with autonomic reparability has become an important research topic since it has the potential to benefit several fields such as biomaterials, tissue engineering, paint and coating technologies, electronics, and soft robotics. In dentistry, the development of restorative materials capable of inhibiting the propagation of microcracks caused by masticatory forces and thermal stress may represent a crucial expansion of the limited clinical lifespan of dental restorations, which is a pressing challenge. Biological systems have inspired the underlying concepts and designs of synthetic polymeric self-healing systems, and different strategies have been used to impart autonomous repair capability in polymers. In this review, the most relevant intrinsic strategies are categorized based on the reaction mechanisms. In general, these strategies rely on the incorporation of latent functionalities capable of undergoing reversible chemical bonds within the polymeric structure (chemically or compositionally tuned).
Search Strategy.
The searches were conducted in the databases Scopus, PubMed, and Google Scholar and limited to articles that were written in English and published during the last ten years. A few additional articles were included by complementing the database searches with manual review of the reference lists.
Overall Conclusions.
Although intrinsic approaches remain underexplored in dentistry, a wide variety of elegant chemistries with tremendous translational potential employed in other fields to promote autonomic repair are highlighted in this review.
Keywords: Dental polymers, self-healing biomaterials, dental resin composites, stimuliresponsive polymers, autonomous self-healing materials
Graphical Abstract

Introduction
Restorative dental materials capable of autonomously responding and healing microcracks have emerged as alternatives to prevent premature fracture and overcome the limited service life of dental restorations.1 Inspired by the healing processes of living organisms, different strategies have been designed to enable thermosetting polymers to inhibit the propagation of cracks resulting from masticatory forces and thermal stress. In general, there are 2 main categories of self-healing dental polymers: extrinsic and intrinsic.2 In the extrinsic systems, the healing agent, a low viscosity reactive compound, is kept sequestered from the organic matrix in microcontainers (microcapsules or 3-dimensional microvascular networks) and released as the propagation of the microcracks leads to the rupture of these reservoirs.2–6 In dentistry, the extrinsic microcapsule-based systems have been the most consistently used, and, at least in part, this is due to the fact that this approach does not require fundamental changes in the process used to manufacture and apply dental polymers.6 This review focuses on the less-studied intrinsic systems as they can potentially be applied in dentistry, based on the advances made in other fields.
Intrinsic self-healing systems are considered a perpetual self-healing approach due to the possibility of fully or partially repairing the initial bulk properties ad aeternum, which is not possible with extrinsic systems.7 In intrinsic self-healing systems, the polymeric network contains latent functional groups capable of reorganizing and re-forming bonds, which makes the organic matrix inherently selfhealing.8 As mentioned, this approach has been underexplored in dentistry, mainly because the high glass transition temperature (Tg) of the densely crosslinked networks required for intraoral use is at odds with the diffusion-controlled chemical group accessibility required in most of the dynamic reactions at room or body temperature. Replacing the bulk of the material with some of these chemistries would be counterproductive and might actually explain why this approach has been less commonly used in dental material applications. However, it is still possible to harness some of the elegant chemistry strategies that have been successfully used in drug delivery, organic coatings, automobile and aerospace industries, and a wide variety of other fields by, for example, localizing the self-healing moieties at the filler-matrix interface (for composites) or including these in lower Tg adhesive materials.
In this review, reversible chemical reactions used to design intrinsic self-healing polymeric systems are presented. The potential applicability of each of them to dentistry is discussed, taking into consideration predicate materials or existing biological uses, such as hydrogels, where there is overalp of mechanical and biocompatibility requirements. The aim of this review is to develop a thorough understanding of the available alternatives and build a tool kit tailored to the different clinical applications of dental polymers.
Methods & Discussion
Overview of Intrinsic Strategies
Intrinsic self-healing systems comprise latent functionalities within the polymeric structure (chemically or compositionally tuned), which form reversible chemical bonds. The healing is triggered by damage (surface tension and elastic energy of the stress source) or external stimulus (light, heat, pH, solvent).9,10 The process is characterized by a hierarchical sequence of steps initiated by the damage and followed by resting time, chain reorganization, and the maintenance of the polymer integrity during the healing kinetics, with the dimension of the damaged area playing a critical role.10 In thermoset polymers, the healing can proceed via chemical bond formation (covalent or noncovalent) or physical interaction, such as chain entanglements. Although physical entanglements are feasible, these strategies require specific conditions such as high temperatures and presence of solvents to enable sufficient polymeric chain mobility, which may represent a roadblock for their use in dental polymers. Therefore, this literature review will focus on the mechanisms involving reversible chemical bond formation.
Chemical crosslinking reactions
In this intrinsic strategy, dynamic reversible bonds are incorporated within and between the polymer chains to promote molecular network rearrangements at disrupted regions. These reversible bonds can be covalent or non-covalent (supramolecular chemistry).
Covalent
Dynamic covalent bonds encompass a wide range of re-bonding mechanisms in which reversibility and repair are achieved while maintaining net covalent linkages.11 The reversible chemistry can be either at the crosslink between polymeric chains or within the polymer backbone.12 The first strategy is called reversible macromer and is characterized by the presence of irreversible chains crosslinked by reversible covalent bonds, which can either be part of the network forming chemistry or can stay latent in the monomer backbone.13 The latter approach is classified as reversible monomer and is based on the presence of reversible crosslinks uniformly distributed throughout the network.14 The 2 strategies result in polymeric networks with different architectures and final mechanical properties.12 The 4 main reversible self-healing covalent available approaches rely on (1) exchange, (2) stable free radical-mediated reshuffle, (3) addition, and reactions with (4) heterocyclic compounds.
Reversible Exchange Reactions
In these chemical reactions, 2 reactants interchange bonds, with the specific molecular connectivity being alternated by forward and reverse reactions. There is no decrease in net connectivity or crosslink density during the transition state, and some of these reactions are triggered under mild conditions such as physiological temperatures, which makes this strategy feasible for dental polymers. This approach is being used in at least 1 commercial material already.12,15 There are several exchange reactions, the most relevant of which are shown in Figure 1.
Figure 1.
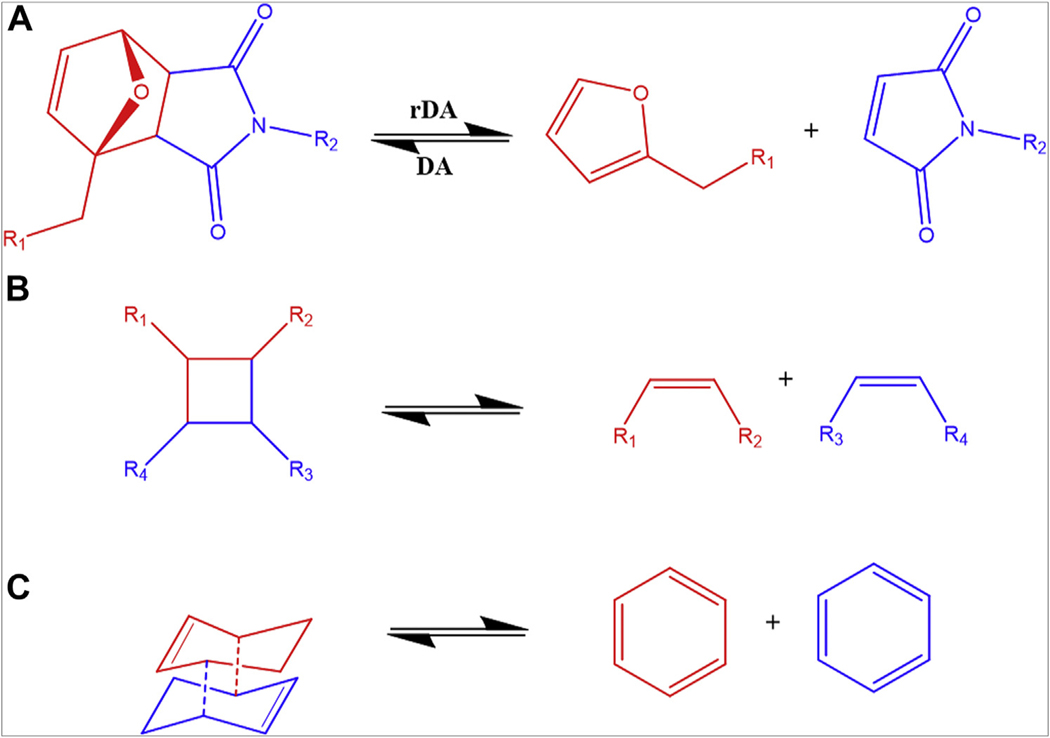
Schematic representation of chemical crosslinking covalent dynamic bonds by exchange reactions: transesterification (Diels-Alder) (A); reversible addition-fragmentation chain transfer reactions (B); disulfide bonds (C1), thiol-thiourethane (C2), thiol-thioester (C3), and thiol-disulfide (C4); dynamic urea bonds (D); and siloxane exchange (E).
Transesterification reactions.
In these chemical reactions, ester bonds exchange with alcohols, carboxylic acids, and other esters in the presence of a catalyst16 (Figure 1A). Stable vitrimer networks based on transesterification via epoxy-thiol polymerization have been reported.17 Although typical transesterification reactions require high temperatures (up to 100 ◦C), strong acids, and specific catalysts,18 the click-reaction between the epoxy and thiol functionalities at room temperature leads to the formation of a network with low Tg (10–20 °C) and reduced activation energy to trigger transesterification reactions.17 As mentioned, although it would not be practical to replace the bulk of the restorative material with this, it could be strategically localized at stress-concentrating areas, such as the filler-matrix interface.19
Reversible addition–fragmentation chain transfer reactions.
In this modality, a degenerate chain transfer agent (alkyl sulfides and trithiocarbonates [TTCs]) exchanges functionality with another similar functional group in a reversible, equilibrium-based deactivation reaction12,20 (Figure 1B). Similar technology has been used in the Filtek One Bulk Fill dental composite (3M ESPE). This system is formulated with an addition fragmentation monomer capable of cleaving and re-forming bonds via reactive fragments during polymerization at room temperature, which ultimately reduces stress,21 in a mechanism analogous to edge dislocation movement through a slip plane in metals.
Sulfur-based bonds.
Disulfide bonds are a radical-mediated reaction in which disulfide bonds (S-S) are homolytically cleaved and followed by subsequent transfer of sulfur-based radicals, which necessarily requires a deprotonated thiol. Disulfide bonds have lower activation energies than carbon bonds, which makes the mechanical scission much more likely, including at mild temperatures.22,23 In addition, since disulfide bonds are easily oxidized and reduced, the exchange reaction can be also triggered by pH variation.24 Temperature and pH variations are common in the oral cavity, and if properly engineered, these materials could actually take advantage of those conditions for activation. Furthermore, these reactions can be tuned to become light responsive12 (Figure 1C–1). Disulfide exchange reactions have been reported in thiourethane networks in the presence of bases and under heat or ultraviolet (UV) light.25 In 2020, a self-healing thermoset system composed of a waterborne polyurethane based on disulfide bonds (4-aminophenyl disulfide) was designed and showed dynamic and exchangeable states at room temperature.26
In thiol-thiourethane and thiol-yhioester reactions, pendant thiols exchange with thiols present in thiourethane27 or thioester bonds28–30 (Figures 1C–2 and 1C–3, respectively), at room temperature and in the absence of additional catalyst, as long as a slight thiol–isocyanate stoichiometric imbalance is present.27 This mechanism makes it possible for thiourethane bonds to be reprocessable and recyclable in solvated systems27 and has been explored for tuning hydrogels developed to trigger specific biological responses.29,30 Recyclable and repolymerizable photopolymer networks can be created without solvent in thiolene photopolymerization reactions.31
In the thiol-disulfide category of reversible reactions, a reduced thiol is exchanged with a disulfide, and a new thiol and a new disulfide are formed (Figure 1C–4). The thiol nucleophile attacks the electrophilic disulfide.32 A thermoset system based on this chemistry has been reported and has shown promising self-healing capability in a pH-dependent (optimal pKa = 12) reaction at mild temperatures (full exchange at 60 °C).33
As a practical example of a potential dental application, resin composites containing thiourethane additives in the organic matrix or on the filler particle surfaces showed stark reduction in polymerization stress and fracture toughness reinforcement.34–40 Although delayed gelation or vitrification through chain transfer reactions via the pendant thiols is most likely the main mechanism involved in the relief of stresses generated during the polymerization, the significant increase in toughness and more recent stress-relaxation results suggest that sulfur bond dynamics can potentially also play a role.41 Studies from other fields have shown that several associative and dissociative reversible reactions take place in thiourethane polymeric networks, such as associative thiol-thiourethane exchange,27 associative disulfide exchange,25 associative exchange trans-thio-carbamoylation,42,43 and dissociative thiol-isocyanate.27 Although in these studies lower Tg networks were used overall, for dental applications, these strategies will replace only a part of the organic matrix or be localized at the filler interface, which, as mentioned, has been shown to be feasible.41
Dynamic urea bonds.
This is a dissociation-recombination reaction, in which the urea bonds bearing a bulky group on the nitrogen atom are reversibly dissociated in amine and isocyanate moieties and are capable of subsequently reforming urea bonds.44 The bulky substituent on the urea nitrogen atom is essential to weaken the urea bond and allow the reversibility of the reaction. Otherwise, the conjugation between the lone pair of electrons in the nitrogen atom and the π electrons in the carbonyl will make the bond highly stable and irreversible.45 The use of hindered urea bonds to obtain polyurethane-urea with lower Tg makes it possible for the self-healing dynamics to be triggered at ambient conditions15 (Figure 1D). A 2020 study developed an urease-aided self-healing dental composite, which hydrolyses urea and forms calcium carbonate as a precipitate.46 In that proof-of-concept article, a disk polymer containing urease was incubated in a medium supplemented with urea and calcium chloride, and a calcium carbonate film was deposited on the disk surface. The authors postulated that this film was capable of filling superficial cracks and preventing further damage.
Siloxane exchange.
In this strategy, anionic reactive silanolate end groups react with network polymeric chains, and the crosslinks are restructured via covalent bonding between the oxygen atoms of the 2 molecules47 (Figure 1E). Silyl ether bonds incorporated into hydroxyl-containing polymeric networks allow for full reprocessability of these materials, although this still requires temperatures above 150 °C.48 However, this reaction is amendable to catalysis, which would be a needed development to allow for this to be potentially used at temperatures applicable in dentistry.
Stable Free Radical-Mediated Reshuffle Reactions
The exchange reactions described above are the foundation for these reshuffle reactions. The differential is the presence of stable, free radicals to mediate the exchange process and ultimately provide sufficient time for covalent bond re-formation.15 As the cleavage of covalent bonds in the damaged area generates free radicals, recoupling and re-formation of covalent linkages is achieved. In common exchange reactions, the free radicals are not stabilized and are highly susceptible to oxidation, leading to loss of reactivity and termination of the self-healing process. However, in the presence of stable free radicals, the recoupling process is still possible for an extended period, which allows for polymeric network rearrangement before the formation of new covalent bonds.15
TTC reshuffling.
This reaction is based on the reversible addition-fragmentation chain transfer reactions principle, with the TTC units acting as photoinitiators and undergoing reshuffling in response to a light stimulus.49 Under UV radiation, carbon-sulfur bonds generate stable free radicals that are able to exchange with neighboring TTC groups.15 Based on the dynamic covalent reshuffling of the TTC, the network is able to autorepair, including the macroscopic fusion of separate pieces (Figure 2A).49
Figure 2.
Schematic representation of chemical crosslinking covalent dynamic bonds by stable free radical-mediated reshuffle: trithiocar-bonate reshuffling (A), allyl sulfide-thiol (B), and alkoxyamine (C).
Allyl sulfilde-thiol.
In this mechanism, thiol-containing compounds exchange with the allyl sulfide functional group. The carbon-carbon double bond is attacked by a thiyl radical that is added to the backbone.50 The carbon-carbon double bond is regenerated as a new thiyl radical is formed (Figure 2B).51 This reaction has been combined with the chain transfer mechanism to develop tunable hydrogels51 and to synthesize malleable and recyclable thermoset plastics under light stimulus.52,53
Alkoxyamine.
This reaction is characterized by the reversible dissociation and formation of alkoxyamine bonds on heating. Although this is essentially an exchange reaction, the differential is the participation of stable nitroxide free radical species which are responsible for mediating the capping of the polymer chain end.54 A polystyrene network is an example of polymeric chains crosslinked by dynamic reversible alkoxyamine groups.15 The challenge in these dynamic reactions is to achieve enough chain mobility, which can be tuned by the molar ratio between styrene and nitroxide bonds and by selecting backbone structures that generally afford low Tg (Figure 2C).15
The last 3 reactions in this group, in general, require light irradiation or thermal activation, and it would be more challenging to use them in dentistry; the restricted motion of macromolecular segments would be another challenge. However, it has been shown that reassociation reactions are possible at room temperature and without requiring light activation, which can be accomplished by varying the chain length between crosslinks to generate more flexible networks or by engineering a higher compliance phase or domain within a rigid network.15 For example, alkoxyamines incorporated as crosslinkers into a linear polyurethane matrix showed macrorepairing capability starting at physiological temperatures.55 Although the challenge is the oxidation sensitivity of the free radicals, conceivably a system could be developed for dental adhesives.
Reversible Addition Reactions
In general, addition reversible reactions take place when the π bonds of 2 compounds are broken and recombined into a single compound that contains the elements present in the original reactants.
Hydrazones.
In these reversible reactions, ketones or aldehydes react with amines or hydrazides to produce hydrazones.56 The reaction is facilitated by heating or by an increase in water concentration. In the absence of water, associative addition and elimination reactions still take place and rely on the presence of pendant amine-functionalities.57,58 This chemistry has been used for hydrogel synthesis59 and may be feasible for dental adhesives or drug delivery at the adhesive hybrid layer.
Boroxine bonds.
Networks based on boronic esters and bor-oxines contain boron-oxygen reprocessable dynamic bonds.60 Three different reactions are possible: hydrolysis–dehydration (boronic acid is dehydrated yielding cyclic trimers–bor-oxides), transesterification of boronic esters by external addition diols, and direct metathesis between different boronic ester compounds.60 In the metathesis reaction, 2 hydrocarbons (alkynes, alkenes, or alkanes) are combined into 2 new hydrocarbons by the exchange of carbon-carbon bonds (triple, double, or single, respectively) in a reaction catalyzed by metals. Room temperature self-healing polymers based on dynamic-covalent boronic esters polymers have been reported in the literature.61 The reaction is triggered in the presence of water61 and has been applied to design drug delivery systems,62 which may make this approach an interesting chemistry for dental adhesives.
Cycloaddition reactions.
These are pericyclic reactions characterized by the addition of π reactants and result in the formation of a new cyclic molecule and 2 new σ bonds. The process takes place via a simultaneous series of bond-breaking and bond-making events in a single kinetic step; that is, there is only 1 transition state.63
In a thermo-cycloaddition reaction, Diels-Alder (DA) or retro-Diels-Alder (retro-DA) [2 + 4] thermoreversible reaction between a diene and a dienophile (typically an alkene) results in a 6-membered ring. In the retro-DA reactions, the diene and the dienophile are disconnected in a reaction triggered by elevated temperatures (typically above Tg) or crack formation, and subsequently, at lower temperatures, the covalent bonds are reconstructed and the damaged area is repaired.15 Examples of reversible DA or retro-DA reactions are presented in Figure 3A. Although in industrial applications, these reactions are normally realized at high temperatures, there are examples in nature, such as the natural catalysis of spirotetronate cyclase AbyU, an enzyme shown to be a bona fide natural Diels–Alderase,64 that could inspire potential dentin adhesives.
Figure 3.
Schematic representation of chemical crosslinking covalent dynamic bonds by cycloaddition reactions: Diels-Alder (DA) and retro-Diels-Alder (rDA) (A), [2 + 2] cycloaddition (B), and [4 + 4] cycloaddition (C).
In photochemical cycloaddition reactions, the chemical reactions are characterized by ring opening and closure modulated via light exposure at different wavelengths.15 There are 2 types of photochemical reversible cycloaddition reactions: [2 + 2] and [4 + 4]. In [2 + 2] cycloaddition reactions, 2 π bonds of 2 different molecules are broken, and the molecules are combined by the formation of 2 σ bonds in a 4-membered ring (eg, 2 ethenes forming cyclobutane). In [4 + 4] cycloaddition reactions, 4 π bonds of 2 different molecules are broken, and the molecules are combined in an 8-membered ring (eg, anthracene derivates). Examples of photochemical cycloaddition reactions are depicted in Figure 3B, C. Cycloaddition reactions are just 1 representative of pericyclic chemistry modality. There are 3 other types of reversible reactions based on reorganization of bonding electron pairs by way of cyclic transition states, namely: electrocyclic, sigmatropic, and ene reactions. Extending the application of photochemical cycloaddition reactions to biological settings was the motivation for the development of low-energy, long-wavelength light with high penetration, as well as methods for regulation of the photochemical process by pH control.65 In an elegant approach to synthesize a cell-laden hydrogel, the [2 + 2] cycloaddition of a halochromic system based on a styrylquinoxaline moiety was induced by green light (λ= 550 nm) and combined with a reversible pH switching mechanism to exercise fine control over the photo reactivity.65 The platform was used to synthesize a fibroblast-laden hydrogel with high cell viability (>80%) and pH-responsiveness, which conceivably could be applicable to dental adhesives.
Reaction With Heterocyclic Compounds
This approach involves the addition of heterocyclic compounds (such as oxolane and oxetane) in the polymeric network to generate stable free radicals to mediate the autorepair of the damaged area.15 Heterocyclic compounds are ring-containing molecules composed of at least 2 different types of atoms. The activation energy required to open a 4- or 5-membered heterocyclic compound is low and results in the formation of stable free radicals66 and has been successfully used to mediate the re-formation of urethane and ether linkages at room temperature under UV irradiation.66,67 Urethanes and ethers are already broadly used in dentistry, and this type of chemistry could be easily incorporated but might require, for example, on-demand repairing procedures mediated by UV irradiation chair side. Although not practical for direct restorations, this could potentially alleviate cracks induced during finishing and polishing procedures in indirect restorations.
Noncovalent (or supramolecular chemistry)
This intrinsic self-healing strategy relies in the reformation of noncovalent transient bonds in a reversible and dynamic process.68 The energy required to disrupt noncovalent, secondary intermolecular bonds (or supramolecular interactions) is from 0.5 through 40 kJ/mol, much lower that in covalent bonds—345 kJ/mol68,69 and easily achieved during load application. Similarly, these bonds are easily re-formed, depending on the proximity and dynamics of the the individual polymer chains to enable mobility of the supramolecular groups and the time required for the bond re-formation.68 There are at least 5 noncovalent intermolecular interactions to carry out the network repair:
Hydrogen Bonding
This is an intermolecular bonding interaction between the antibonding molecular orbital of a hydrogen atom and the lone pair of electrons of a strongly electronegative atom such as nitrogen, oxygen, or fluorine.70 Hydrogen bonds (H bonds) are the strongest type of noncovalent intermolecular forces. In general, the self-healing dynamic is triggered by the presence of a certain percentage of the mobile phase, and no additional stimulus is required (Figure 4A).15 The main disadvantage of this strategy is the fact that in the presence of moisture, there is competition for the formation of bonds with the molecules of water or even intramolecular H bonds,15 which can potentially inhibit the self-healing potential. Bisphenol A-glycidyl methacrylate (BisGMA) and urethane dimethacrylate are dental examples of monomer molecules with strong hydrogen bonding potential, with much evidence for toughening in composites,71 although their influence on potential repairability remains unexplored.
Figure 4.
Chemical crosslinking noncovalent dynamic bonds (Supramolecular chemistry): hydrogen bond (A), π-π stacking (B), ionic interactions (C), metal-ligand coordination bonds (D), and host-guest interactions (E).
π-π stacking (or aromatic-aromatic interaction)
This is an attractive force between the π electrons of 2 aromatic rings. An offset stack is formed due to the alignment of 1 ring with positive electrostatic potential (π electron deficient) with another ring with negative electrostatic potential (π electron rich) (Figure 4B).70 The Tg of these networks is tuned by changing the spacers and adjusting the blend composition, which makes this strategy more useful in temperatures between 50 and 100 °C.15 On stress induction, the π-π interactions are disrupted and re-formed through heating, which enables the endcapped chains to disengage, flow, and restack.9 These interactions can be present in dental networks containing aromatic compounds (BisGMA and ethoxylated bisphenol-A dimethacrylate, for example), although the molecular range of motion in noncrystalline networks, such as in composites, might make it challenging to harness any self-healing potential. However, engineered prepolymers can be envisioned to be used as low-profile additives for network reinforcement, including by the induction of controlled phase separation, where stress relaxation can be achieved by differential moduli during microdomain formation.72
Ionic Interactions
This approach is based on the presence of ionic side groups and their corresponding counter-ions within the polymer backbone forming clusters that act as physical crosslinking points and enable formation and re-formation of the network architecture9,68 (Figure 4C). With stimuli, the clusters are dissociated, and the heat generated by friction during the disruption or from an external thermal source is responsible for generating a local melt state, which leads to the fusion of the surfaces followed by there shuffling of the ionic clusters.15
Dynamic Metal-Ligand Coordination Bonds
This refers to the interaction between lone pairs of electrons of small molecules (ligands) and metal ions to form stable metal complexes (Figure 4D).70 Depending on the match between the metal ion and ligand substitutes, the noncovalent bond formed is be dynamically reversible and used in self-repairing metallo-supramolecular polymeric networks.15 The coordination centers are tunable with stimuli and can assume different configurations such as linear, highly branched, star shaped, and dendritic.15 The reversibility of these networks is related to the dissociation of the coordination bonds or formation and dissociation of ionic clusters triggered by stimuli such as light, heat, presence of ions, and pH. This approach has been shown using mussel-inspired compounds, where the reversible metal-ligand coordination bonds between catechol and iron3+ provide intrinsic self-healing properties for the material.73 In addition to iron3+, catechol seems capable of forming complexes with other metals such as copper2+, cobalt2+, nickel2+, and vanadium3+.73
Host-Guest Interactions
This interaction relies on the selective inclusion of complexation macrocyclic hosts that are able to interlock with smaller guest molecules by H bonds, π-π interactions, van der Waals forces, charge-transfer, ion-dipole, and hydrophobic interactions (Figure 4E).15,74 Triggered by redox, solvent, temperature, photo-irradiation, anions and cations, and pH stimuli, these interactions are dissociated and re-associated, leading to stress dispersion and spontaneous repair of the damaged area.15,74,75
The central advantage of the supramolecular chemistry strategy to impart repairability in thermosetting polymeric networks is the possibility of ad aeternum healing events. In addition, some of the supramolecular dynamics do not depend on external stimuli, which confers the desired autonomic component to these systems.76 On the other hand, the weak nature of these supramolecular interactions has to be critically considered to achieve a balance between healing efficiency and mechanical properties of the host polymer. One potential elegant solution is to design glasslike polymers with slower dissociation rates (Kd < 1s–1, where Kd is the dissociation constant), which produces polymers with high compression strength and the capability of fast, room temperature self-recovery.77 In a 2022 article, the dissociation rate of host-guest complexes was tuned by changing the structure of the phenyl group of the second guest to increase its hydrophobic volume.77 This modification significantly decreased the activation energy for the self-healing and holds a great promise for biomaterial applications.77 Another interesting approach, which combines covalent bond and supramolecular network-based strategies, is to incorporate low Tg dynamic networks as additives or filler particle functionalizing agents and use catalysts or stoichiometric imbalances to promote autonomic bond reprocess-ability.40,41,78 As already mentioned, this strategy has been used to incorporate thiourethane into dental resin composite formulations with high toughness and intrinsic stress-relaxation mechanisms.41 The dynamic and self-healing character of the thiourethane networks has been extensively explored in other fields,79,80 but specifically, in the case of BisGMA- and urethane dimethacrylate–containing organic matrixes in dental composites, the thio-carbamate bonds also improve hydrogen bonding, and because the low Tg, flexible oligomers are swollen by the matrix, they may provide localized intermolecular reinforcement. This is especially true for thiourethane-functionalized filler particles, since it is well know that stress concentration is more marked at the filler particle-organic matrix interface,19 as mentioned above.
Conclusions
The original goal of this review was to focus on the strategies broadly used in industry and other fields, which can potentially be applied to dental polymeric networks with autorepair capabilities. However, the challenge in incorporating self-healing components into dental materials is to ensure the level of mechanical integrity for function, which in general means Tg higher than 100 °C,81,82 flexural modulus higher than 70 MPa (International Standards Organization 4049),83 and cytocompatibility in accordance with US Food and Drug Administration standards.84 In addition, polymeric materials for dental applications should be physically and chemically stable in an aqueous environment under constant pH oscillations and multiple enzymatic activities.85 Therefore, as shown in this review, although a number of dynamic reversible chemistries are available, not all of them would be amendable to dental application, at least not without substantial engineering effort.
In addition to the dental-related challenges, several conditions related to the self-healing chemistries also need to be met. Namely, the triggering of the dynamics should be as autonomous as possible, since one of the main motivations behind the development of self-healing dental materials is to heal and inhibit the propagation of subcritical microcracks, which are initiated in the material body by masticatory forces and thermal stress. The system must also be stable in the oral environment, under quasi-constant mechanical loading, with marked oscillations in pH and temperature, and in the presence of enzymes and water. However, despite the challenges, there is a wide variety of strategies used in other fields with tremendous translational potential for dental polymers, as shown in this review. In addition, more recent approaches such as shape memory-assisted self-healing (SMASH) have emerged as a promising alternative. In SMASH systems, physical and chemical healing are synergistically combined to enable the host polymer with the intrinsic capability of healing macroscopic cracks on a molecular scale.86 In short, the shape-memory properties are manipulated to physically close the crack that is chemically healed. Although the need for thermo or light activation to trigger the healing still poses an obstacle to autonomic repairability in dental polymers, SMASH technology is an interesting approach and may be tuned to allow for clinical translation.
Despite the challenges, the advances in the development of self-healing dental polymers has been noticeable in the last 2 decades. The field is rife with opportunities inspired by strategies used in a wide variety of other fields. Although creative adaptations are still needed to make the systems compatible with dental applications and to allow for them to be autonomously triggered, elegant chemistry strategies can be used to start a new stimuli-responsive generation of dental polymers.
Funding.
This work was financially supported by grants K99-DE028876 and K02-DE025280 from the National Institute of Dental and Craniofacial Research, National Institutes of Health, awarded to A.P.F. and C.S.P., respectively.
Footnotes
Disclosure. Drs Fugolin and Pfeifer did not report any disclosures.
References
- 1.Özcan M, da Fonseca Roberti Garcia L, Volpato CAM. Bioactive materials for direct and indirect restorations: concepts and applications. Front Dent Med. 2021;2:35. 10.3389/fdmed.2021.647267 [DOI] [Google Scholar]
- 2.White SR, Sottos NR, Geubelle PH, et al. Autonomic healing of polymer composites. Nature. 2001;409(6822):794–797. 10.1038/35057232 [DOI] [PubMed] [Google Scholar]
- 3.Brown EN, Kessler MR, Sottos NR, White SR. In situ poly (urea-form-aldehyde) microencapsulation of dicyclopentadiene. J Microencapsul. 2003;20(6):719–730. 10.1080/0265204031000154160 [DOI] [PubMed] [Google Scholar]
- 4.Brown EN, White SR, Sottos NR. Microcapsule induced toughening in a self-healing polymer composite. J Mater Sci. 2004;39(5):1703–1710. 10.1023/B:JMSC.0000016173.73733.dc [DOI] [Google Scholar]
- 5.Blaiszik BJ, Kramer SLB, Olugebefola SC, Moore JS, Sottos NR,White SR. Self-healing polymers and composites. Annu Rev Mater Res. 2010;40(1):179–211. 10.1146/annurev-matsci-070909-104532 [DOI] [Google Scholar]
- 6.White SR, Blaiszik BJ, Kramer SL, Olugebefola S, Moore J,Sottos NR. Self-healing polymers and composites. Am Sci. 2011;99(5):392–399. 10.1511/2011.92.392 [DOI] [Google Scholar]
- 7.Mphahlele K, Ray SS, Kolesnikov A. Self-healing polymeric composite material design, failure analysis and future outlook: a review. Polymers. 2017;9(10):535. 10.3390/polym9100535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paolillo S, Bose RK, Santana MH, Grande AM. Intrinsic self-healing-epoxies in polymer matrix composites (PMCs) for aerospace applications. Polymers. 2021;13(2):201. 10.3390/polym13020201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urdl K, Kandelbauer A, Kern W, Muller U, Thebault M, Zikulnig-Rusch E. Self-healing of densely crosslinked thermoset polymers: a critical review. Prog Org Coat. 2017;104:232–249. 10.1016/j.porgcoat.2016.11.010 [DOI] [Google Scholar]
- 10.Garcia SJ. Effect of polymer architecture on the intrinsic self-healing character of polymers. Eur Polym J. 2014;53:118–125. 10.1016/j.eurpolymj.2014.01.026 [DOI] [Google Scholar]
- 11.Mao J, Hai Y, Ye H, You L. Adaptive covalent networks enabled bydual reactivity: the evolution of reversible covalent bonds, their molecular assemblies, and guest recognition. J Org Chem. 2020;85(8): 5351–5361. 10.1021/acs.joc.0c00051 [DOI] [PubMed] [Google Scholar]
- 12.Kloxin CJ, Bowman CN. Covalent adaptable networks: smart, reconfigurable and responsive network systems. Chem Soc Rev. 2013;42(17): 7161–7173. 10.1039/c3cs60046g [DOI] [PubMed] [Google Scholar]
- 13.Hayes W, Greenland BW. Healable Polymer Systems. Royal Society of Chemistry; 2013. [Google Scholar]
- 14.Hayes SA, Jones FR, Marshiya K, Zhang W. A self-healing thermos-setting composite material. Compos A. 2007;38(4):1116–1120. 10.1016/j.compositesa.2006.06.008 [DOI] [Google Scholar]
- 15.Urban MW. Stimuli-Responsive Materials: from Molecules to Nature Mimicking Materials Design. Royal Society of Chemistry; 2019. [Google Scholar]
- 16.Trost BM, Fleming I. Comprehensive Organic Synthesis. Vol. 5. Pergamin Press; 1991. [Google Scholar]
- 17.Gablier A, Saed MO, Terentjev EM. Rates of transesterification in epoxy–thiol vitrimers. Soft Matter. 2020;16(22):5195–5202. 10.1039/d0sm00742k [DOI] [PubMed] [Google Scholar]
- 18.Ferreira AB, Lemos Cardoso A, da Silva MJ. Tin-catalyzed esterification and transesterification reactions: a review. Int Sch Res Not. 2012;2012:1–13. 10.5402/2012/142857 [DOI] [Google Scholar]
- 19.Metın D, Tihminlioglu F, Balk˘ ose D,¨ Ulk¨ u S. The effect of interfacial¨ interactions on the mechanical properties of polypropylene/natural zeolite composites. Compos A. 2004;35(1):23–32. 10.1016/j.compositesa.2003.09.021 [DOI] [Google Scholar]
- 20.Roth PJ, Boyer C, Lowe AB, Davis TP. RAFT polymerization and thiolchemistry: a complementary pairing for implementing modern macromolecular design. Macromol Rapid Commun. 2011;32(15):1123–1143. 10.1002/marc.201100127 [DOI] [PubMed] [Google Scholar]
- 21.Park HY, Kloxin CJ, Fordney MF, Bowman CN. Stress relaxation of trithiocarbonate-dimethacrylate-based dental composites. Dent Mater. 2012;28(8):888–893. 10.1016/j.dental.2012.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang K, Jia H, Gu SY. A transparent, highly stretchable, self-healing polyurethane based on disulfide bonds. Eur Polym J. 2019;112:82–2831. 10.1016/j.eurpolymj.2018.11.005 [DOI] [Google Scholar]
- 23.Nevejans S, Ballard N, Miranda JI, Reck B, Asua JM. The underlying mechanisms for self-healing of poly(disulfide)s. Phys Chem Chem Phys. 2016;18(39):27577–27583. 10.1039/c6cp04028d [DOI] [PubMed] [Google Scholar]
- 24.Wojtecki RJ, Meador MA, Rowan SJ. Using the dynamic bond to access macroscopically responsive structurally dynamic polymers. Nat Mater. 2011;10(1):14–27. 10.1038/nmat2891 [DOI] [PubMed] [Google Scholar]
- 25.Liu Q, Liu Y, Zheng H, Li C, Zhang Y, Zhang Q. Design and development of self-repairable and recyclable crosslinked poly (thio-urethane-urethane) via enhanced aliphatic disulfide chemistry. J Polym Sci. 2020;58(8):1092–1104. 10.1002/pol.20190186 [DOI] [Google Scholar]
- 26.Zhang M, Zhao F, Xin W, Luo Y. Room-temperature self-healing and reprocessable waterborne polyurethane with dynamically exchangeable disulfide bonds. ChemistrySelect. 2020;5(15):4608–4618. 10.1002/slct.201904316 [DOI] [Google Scholar]
- 27.Li L, Chen X, Torkelson JM. Reprocessable polymer networks via thiourethane dynamic chemistry: recovery of cross-link density after recycling and proof-of-principle solvolysis leading to monomer recovery. Macromolecules. 2019;52(21):8207–8216. 10.1021/acs.macromol.9b01359 [DOI] [Google Scholar]
- 28.Worrell BT, Mavila S, Wang C, et al. A user’s guide to the thiolthioester exchange in organic media: scope, limitations, and applications in material science. Polym Chem. 2018;9(36):4523–4534. 10.1039/C8PY01031E [DOI] [Google Scholar]
- 29.Brown TE, Carberry BJ, Worrell BT, et al. Photopolymerized dynamic hydrogels with tunable viscoelastic properties through thioester exchange. Biomaterials. 2018;178:496–503. 10.1016/j.biomaterials.2018.03.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carberry BJ, Rao VV, Anseth KS. Phototunable viscoelasticity in hydrogels through thioester exchange. Ann Biomed Eng. 2020;48(7): 2053–2063. 10.1007/s10439-020-02460-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C, Goldman TM, Worrell BT, McBride MK, Alim MD, Bowman CN. Recyclable and repolymerizable thiol–X photopolymers. Mater Horiz. 2018;5(6):1042–1046. 10.1039/C8MH00724A [DOI] [Google Scholar]
- 32.Gallogly MM, Starke DW, Mieyal JJ. Mechanistic and kinetic detailsof catalysis of thiol-disulfide exchange by glutaredoxins and potential mechanisms of regulation. Antioxid Redox Signal. 2009;11(5):1059–1081. 10.1089/ars.2008.2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pepels M, Filot I, Klumperman B, Goossens H. Self-healing systems based on disulfide–thiol exchange reactions. Polym Chem. 2013;4(18): 4955–4965. 10.1039/c3py00087g [DOI] [Google Scholar]
- 34.Bacchi A, Dobson A, Ferracane JL, Consani R, Pfeifer CS. Thio-ure-thanes improve properties of dual-cured composite cements. J Dent Res. 2014;93(12):1320–1325. 10.1177/0022034514551768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bacchi A, Nelson M, Pfeifer CS. Characterization of methacrylate-based composites containing thio-urethane oligomers. Dent Mater. 2016;32(2):233–239. 10.1016/j.dental.2015.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bacchi A, Pfeifer CS. Rheological and mechanical properties and interfacial stress development of composite cements modified with thio-urethane oligomers. Dent Mater. 2016;32(8):978–986. 10.1016/j.dental.2016.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faria-e-Silva AL, dos Santos A, Tang A, Girotto EM, Pfeifer CS. Effect of thiourethane filler surface functionalization on stress, conversion and mechanical properties of restorative dental composites. Dent Mater. 2018;34(9):1351–1358. 10.1016/j.dental.2018.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faria-e-Silva AL, Pfeifer CS. Impact of thio-urethane additive and filler type on light-transmission and depth of polymerization of dental composites. Dent Mater. 2017;33(11):1274–1285. 10.1016/j.dental.2017.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fugolin AP, Costa AR, Kono E, Quirk E, Ferracane JL, Pfeifer CS. Influence of the organic matrix composition on the polymerization behavior and bulk properties of resin composites containing thiourethane-functionalized fillers. Eur Polym J. 2020;130:109664. 10.1016/j.eurpolymj.2020.109664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fugolin AP, Sundfeld D, Ferracane JL, Pfeifer CS. Toughening of dental composites with thiourethane-modified filler interfaces. Sci Rep. 2019;9(1):2286. 10.1038/s41598-019-39003-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fugolin APP, Costa AR, Lewis SH, Goulart M, Erhardt MC, Pfeifer CS. Probing stress relaxation behavior in glassy methacrylate networks containing thio-carbamate additives. J Mater Chem B. 2021;9(13):3015–3024. 10.1039/d1tb00176k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gamardella F, De la Flor S, Ramis X, Serra A. Recyclable poly (thiourethane) vitrimers with high Tg. Influence of the isocyanate structure. React Funct Polym. 2020;151:104574. 10.1016/j.reactfunctpolym.2020.104574 [DOI] [Google Scholar]
- 43.Gamardella F, Guerrero F, De la Flor S, Ramis X, Serra MA. A newclass of vitrimers based on aliphatic poly (thiourethane) networks with shape memory and permanent shape reconfiguration. Eur Polym J. 2020;122:109361. 10.1016/j.eurpolymj.2019.109361 [DOI] [Google Scholar]
- 44.Ying H, Zhang Y, Cheng J. Dynamic urea bond for the design of reversible and self-healing polymers. Nat Commun. 2014;5(1):3218. 10.1038/ncomms4218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang W, Jin Y. Dynamic Covalent Chemistry: Principles, Reactions, and Applications. John Wiley & Sons; 2017. [Google Scholar]
- 46.Seifan M, Sarabadani Z, Berenjian A. Development of an innovativeurease-aided self-healing dental composite. Catalysts. 2020;10(1):84. 10.3390/catal10010084 [DOI] [Google Scholar]
- 47.Zheng P, McCarthy TJ. A surprise from 1954: siloxane equilibrationis a simple, robust, and obvious polymer self-healing mechanism. J Am Chem Soc. 2012;134(4):2024–2027. 10.1021/ja2113257 [DOI] [PubMed] [Google Scholar]
- 48.Nishimura Y, Chung J, Muradyan H, Guan Z. Silyl ether as a robustand thermally stable dynamic covalent motif for malleable polymer design. J Am Chem Soc. 2017;139(42):14881–14884. 10.1021/jacs.7b08826 [DOI] [PubMed] [Google Scholar]
- 49.Amamoto Y, Kamada J, Otsuka H, Takahara A, Matyjaszewski K. Repeatable photoinduced self-healing of covalently cross-linked polymers through reshuffling of trithiocarbonate units. Angew Chem Int Ed Engl. 2011;50(7):1660–1663. 10.1002/anie.201003888 [DOI] [PubMed] [Google Scholar]
- 50.Park HY, Kloxin CJ, Scott TF, Bowman CN. Covalent adaptable networks as dental restorative resins: stress relaxation by addition-fragmentation chain transfer in allyl sulfide-containing resins. Dent Mater. 2010;26(10):1010–1016. 10.1016/j.dental.2010.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gandavarapu NR, Azagarsamy MA, Anseth KS. Photo-click living strategy for controlled, reversible exchange of biochemical ligands. Adv Mater. 2014;26(16):2521–2526. 10.1002/adma.201304847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scott TF, Schneider AD, Cook WD, Bowman CN. Photoinduced plasticity in cross-linked polymers. Science. 2005;308(5728):16151617. 10.1126/science.1110505 [DOI] [PubMed] [Google Scholar]
- 53.Jin Y, Lei Z, Taynton P, Huang S, Zhang W. Malleable and recyclable thermosets: the next generation of plastics. Matter. 2019;1(6):1456–1493. 10.1016/j.matt.2019.09.004 [DOI] [Google Scholar]
- 54.Otsuka H.Reorganization of polymer structures based on dynamic covalent chemistry: polymer reactions by dynamic covalent exchanges of alkoxyamine units. Polym J. 2013;45(9):879–891. 10.1038/pj.2013.17 [DOI] [Google Scholar]
- 55.Yuan C, Rong MZ, Zhang MQ. Self-healing polyurethane elastomer with thermally reversible alkoxyamines as crosslinkages. Polymer. 2014;55(7):1782–1791. 10.1016/j.polymer.2014.02.033 [DOI] [Google Scholar]
- 56.Winne JM, Leibler L, Du Prez FE. Dynamic covalent chemistry inpolymer networks: a mechanistic perspective. Polym Chem. 2019;10(45):6091–6108. 10.1039/C9PY01260E [DOI] [Google Scholar]
- 57.Taynton P, Yu K, Shoemaker RK, Jin Y, Qi HJ, Zhang W. Heat- or water-driven malleability in a highly recyclable covalent network polymer. Adv Mater. 2014;26(23):3938–3942. 10.1002/adma.201400317 [DOI] [PubMed] [Google Scholar]
- 58.Skene WG, Lehn JM. Dynamers: polyacylhydrazone reversible covalent polymers, component exchange, and constitutional diversity. Proc Natl Acad Sci U S A. 2004;101(22):8270–8275. 10.1073/pnas.0401885101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Richardson BM, Walker CJ, Macdougall LJ, et al. Viscoelasticity of hydrazone crosslinked poly (ethylene glycol) hydrogels directs chondrocyte morphology during mechanical deformation. Biomater Sci. 2020;8(14):3804–3811. 10.1039/d0bm00860e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bapat AP, Sumerlin BS, Sutti A. Bulk network polymers with dynamicB–O bonds: healable and reprocessable materials. Mater Horiz. 2020;7(3):694–714. 10.1039/C9MH01223K [DOI] [Google Scholar]
- 61.Cash JJ, Kubo T, Bapat AP, Sumerlin BS. Room-temperature self-healing polymers based on dynamic-covalent boronic esters. Macromolecules. 2015;48(7):2098–2106. 10.1021/acs.macromol.5b00210 [DOI] [Google Scholar]
- 62.Ghosh T, Biswas A, Gavel PK, Das AK. Engineered dynamic boronate ester-mediated self-healable biocompatible G-quadruplex hydrogels for sustained release of vitamins. Langmuir. 2020;36(6):1574–1584. 10.1021/acs.langmuir.9b03837 [DOI] [PubMed] [Google Scholar]
- 63.Bent SF. Chapter 5. Semiconductor surface chemistry. In: Nilsson A, Pettersson LGM, Nørskov JK, eds. Chemical Bonding at Surfaces and Interfaces. Elsevier; 2008:323–395 [Google Scholar]
- 64.Byrne MJ, Lees NR, Han LC, et al. The catalytic mechanism of anatural Diels–Alderase revealed in molecular detail. J Am Chem Soc. 2016;138(19):6095–6098. 10.1021/jacs.6b00232 [DOI] [PubMed] [Google Scholar]
- 65.Kalayci K, Frisch H, Truong VX, Barner-Kowollik C. Green light triggered [2+2] cycloaddition of halochromic styrylquinoxaline: controlling photoreactivity by pH. Nat Commun. 2020;11(1):4193. 10.1038/s41467-020-18057-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghosh B.Self-Repairable Polymeric Networks: Synthesis and Network Design. Dissertation. The University of Southern Mississippi; 2011. [Google Scholar]
- 67.Ghosh B, Chellappan KV, Urban MW. UV-initiated self-healing of oxolane–chitosan–polyurethane (OXO–CHI–PUR) networks. J Mater Chem. 2012;22(31):16104–16113. 10.1039/c2jm31126g [DOI] [Google Scholar]
- 68.Binder WH. Self-Healing Polymers: from Principles to Applications. John Wiley & Sons; 2013. [Google Scholar]
- 69.Desiraju GR. A bond by any other name. Angew Chem Int Ed Engl. 2011;50(1):52–59. 10.1002/anie.201002960 [DOI] [PubMed] [Google Scholar]
- 70.Tro NJ. Chemistry: Structure and Properties. 2nd ed. Pearson; 2017. [Google Scholar]
- 71.Lemon MT, Jones MS, Stansbury JW. Hydrogen bonding interactions in methacrylate monomers and polymers. J Biomed Mater Res A. 2007;83(3):734–746. 10.1002/jbm.a.31448 [DOI] [PubMed] [Google Scholar]
- 72.Ostanin SA, Mokeev MV, Pikhurov DV, Sakhatskii AS, Zuev VV. Interplay of structural factors in molecular dynamics of microphase-separated or microphase- mixed structures of polyurethanes revealed by solid-state NMR and dielectric spectroscopy. Chem Phys Impact. 2022;4:100066. 10.1016/j.chphi.2022.100066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li K, Tsoi JKH, Yiu CKY. The application of novel mussel-inspired compounds in dentistry. Dent Mater. 2021;37(4):655–671. 10.1016/j.dental.2021.01.005 [DOI] [PubMed] [Google Scholar]
- 74.Sinawang G, Osaki M, Takashima Y, Yamaguchi H, Harada A. Supramolecular self-healing materials from non-covalent cross-linking host-guest interactions. Chem Commun (Camb). 2020;56(32):4381–4395. 10.1039/d0cc00672f [DOI] [PubMed] [Google Scholar]
- 75.Liu S, Gong W, Yang X. Self-healing supramolecular polymers via host-guest interactions. Curr Org Chem. 2014;18(15):2010–2015. 10.2174/1385272819666140514005435 [DOI] [Google Scholar]
- 76.Thangavel G, Tan MWM, Lee PS. Advances in self-healing supramolecular soft materials and nanocomposites. Nano Converg. 2019;6(1):29. 10.1186/s40580-019-0199-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang Z, Chen X, O’Neill SJK, et al. Highly compressible glass-like supramolecular polymer networks. Nat Mater. 2022;21(1):103–109. 10.1038/s41563-021-01124-x [DOI] [PubMed] [Google Scholar]
- 78.Bacchi A, Yih JA, Platta J, Knight J, Pfeifer CS. Shrinkage/stress reduction and mechanical properties improvement in restorative composites formulated with thio-urethane oligomers. J Mech Behav Biomed Mater. 2018;78:235–240. 10.1016/j.jmbbm.2017.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Erice A, Ruiz de Luzuriaga AR, Azcune I, et al. New injectable and self-healable thermoset polythiourethane based on S-aromatic thiourethane dissociative exchange mechanism. Polymer. 2020;196:122461. 10.1016/j.polymer.2020.122461 [DOI] [Google Scholar]
- 80.Ying WB, Liu H, Gao P, et al. An anti-stress relaxation, anti-fatigue,mildew proof and self-healing poly (thiourethane-urethane) for durably stretchable electronics. Chem Eng J. 2021;420(part 2):127691. 10.1016/j.cej.2020.127691 [DOI] [Google Scholar]
- 81.Moore RJ, Watts JT, Hood JA, Burritt DJ. Intra-oral temperature evariation over 24 hours. Eur J Orthod. 1999;21(3):249–261. 10.1093/ejo/21.3.249 [DOI] [PubMed] [Google Scholar]
- 82.Rueggeberg FA, Maher FT, Kelly MT. Thermal properties of a methyl-methacrylate-based orthodontic bonding adhesive. Am J Orthod Den-tofacial Orthop. 1992;101(4):342–349. 10.1016/S0889-5406(05)80327-0 [DOI] [PubMed] [Google Scholar]
- 83.ISO 4049: 2019 dentistry—polymer-based restorative materials. International Organization for Standardization. Accessed February 26, 2022. https://www.iso.org/standard/67596.html
- 84.Analytical procedures and methods validation for drugs and biologics. US Food and Drug Administration. Accessed April 6, 2022. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/analytical-procedures-and-methods-validation-drugs-andbiologics [Google Scholar]
- 85.Apaza-Bedoya K, Bijukumar D, Benfatti CAM, et al. Chapter 4. Adverse local and systemic effect of nanoparticles released from oral and cranio-maxillofacial implants. In: Souza JCM, Hotza D, Henriques B, Boccaccini AR, eds. Nanostructured Biomaterials for Cranio-Maxillofacial and Oral Applications. Elsevier; 2018:63–79. [Google Scholar]
- 86.Alabiso W, Hron TM, Reisinger D, Bautista-Anguís DB, Schlögl S. Shape memory-assisted self-healing of dynamic thiol-acrylate networks. Polym Chem. 2021;12(39):5704–5714. 10.1039/D1PY00925G [DOI] [Google Scholar]