Abstract
Background:
Centralized chlorination of urban piped water supplies has historically contributed to major reductions in waterborne illness. In locations without effective centralized water treatment, point-of-use (POU) chlorination for households is widely promoted to improve drinking water quality and health. Realizing these health benefits requires correct, consistent, and sustained product use, but real-world evaluations have often observed low levels of use. To our knowledge, no prior reviews exist on adoption of chlorine POU products.
Objectives:
Our objectives were to identify which indicators of adoption are most often used in chlorine POU studies, summarize levels of adoption observed, understand how adoption changes over time, and determine how adoption is affected by frequency of contact between participants and study staff.
Methods:
We conducted a systematic review of household POU chlorination interventions or programs from 1990 through 2021 that reported a quantitative measure of adoption, were conducted in low- and middle-income countries, included data collection at households, and reported the intervention start date.
Results:
We identified 36 studies of household drinking water chlorination products that met prespecified eligibility criteria and extracted data from 46 chlorine intervention groups with a variety of chlorine POU products and locations. There was no consensus definition of adoption of household water treatment; the most common indicator was the proportion of household stored water samples with free chlorine residual . Among studies that reported either free or total chlorine–confirmed adoption of chlorine POU products, use was highly variable (across all chlorine intervention groups at the last time point measured in each study; range: 1.5%–100%; sample size-weighted ; unweighted ). The median follow-up duration among intervention groups was 3 months. On average, adoption declined over time and was positively associated with frequency of contact between respondents and study staff.
Discussion:
Although prior research has shown that POU chlorine products improve health when correctly and consistently used, a reliance on individual adoption for effective treatment is unlikely to lead to the widespread public health benefits historically associated with pressurized, centralized treatment of piped water supplies. https://doi.org/10.1289/EHP10839
Introduction
Chlorination of urban piped water supplies contributed to substantial declines in waterborne disease in major cities in the early 1900s.1 Today the addition of chlorine-based disinfectants is a standard step in effective municipal water treatment processes.2 For the people globally lacking access to safe and effectively treated water,3 point-of-use (POU) chlorination at the household level has been promoted as an alternative and interim strategy to realize the benefits of safe water in the absence of large-scale infrastructure.4 However, there has been debate about whether evidence supports widespread investment in household water treatment,4–6 and recent randomized controlled trials that included chlorine POU products found little to no impact on child health outcomes that have previously been linked to safe water consumption.7–9
A key difference between the systems that have historically delivered enormous public health benefits and household-level chlorination strategies is that the latter relies on individuals to implement treatment. Modeling studies have concluded that high levels of correct, consistent, and sustained use of household water treatment products are required to realize the health benefits of such treatment,10,11 and meta-analyses have confirmed that greater health benefits are associated with higher levels of adoption.12,13 Thus, an important question is whether these POU products can achieve high levels of correct and consistent use: what is often referred to as adherence to treatment or product adoption.
Substantial research efforts have been made to identify ways to increase adoption.14 Adoption of POU treatment is not uniformly reported in the literature, despite being a critical determinant of the benefits for water quality or health.10,11 It is difficult to measure and lacks a standard definition.15–17 Use is commonly based on self-report or on an indirect measure, such as observed product presence in the home.16 Chlorine POU products offer a meaningful advantage for measuring use as compared with non-chlorine POU products: the ability to measure chlorine in stored drinking water as an objective measure of current product use. In a recent large trial where adoption was measured through both self-report and residual chlorine measurement, self-reported use was higher than objectively measured use.18 A second component of adoption is exclusive consumption of treated water. However, because this is less reported in the literature, and much harder to objectively verify, we decided to focus here on product use (a lower bar).
In this systematic review, we aimed to summarize the evidence on adoption of chlorine POU products and factors associated with high levels of adoption. Our objectives were to a) identify which indicators have been used to assess adoption in chlorine POU studies, b) describe the levels of adoption and barriers to use observed across studies, c) determine trends in adoption over time, and d) assess the relationship between adoption and frequency of contact between study staff and participants.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines19 to develop a review protocol prior to beginning our search. The full protocol is available at https://osf.io/ptc3m/. Our search strategy was developed to first identify studies that included a chlorine POU product as a component of an intervention or program, recognizing that adoption is typically not considered a main outcome in household water treatment studies and therefore unlikely to be included in keywords, titles, or abstracts. The search terms for previous systematic reviews of household water treatment studies, which summarized evidence on health or water quality impacts, were used as a starting point and further refined for our purposes.12,20
We searched for “drinking water,” “potable water,” “tap water,” “household water,” or “domestic water” in combination with terms and brands associated with chlorine POU products: “chemical disinfectant,” chlorin*, chlorate, chlorite, disinfec*, hypochlorite, “sodium hypochlorite,” “calcium hypochlorite,” “sodium dichloroisocyanurate,” NaDCC, trichlor, Aquatab, Waterguard or WaterGuard, Klorin, Pur, “water quality,” “free residual chlorine,” or “free chlorine.” We additionally included the names of all countries included in the World Bank 2019 low- and lower-middle income country categories21 and limited our search to articles published after 1 January 1990. We conducted database searches in PubMed/MEDLINE, Web of Science, Global Health [CAB Abstracts and Global Health (CABI)], and Embase. The exact search term sets are included in the Supplemental Material in the section “Full Search Terms.” We also hand searched the reference sections of four prior systematic reviews of household safe water interventions to ensure all relevant studies were included.12,13,20,22 While screening full texts, we identified additional references that we screened for inclusion.
We downloaded search results from each database search and screened titles and available abstracts in Covidence systematic review software (Veritas Health Innovation). Two authors independently reviewed each title/abstract. Inter-reviewer agreement at this stage was . Subsequently, full texts of articles were collected in a Google drive folder and assessed using full eligibility criteria. Y.S.C. screened all full texts for inclusion, and were screened by authors M.T. or M.M. Y.S.C. did data extraction for all included full texts. Other authors partially replicated data extraction for of texts, and A.J.P. fully replicated this step for of texts.
Selection Criteria and Data Extraction
Eligible studies included a) a clearly described drinking water intervention or program with a chlorine POU product, including combined flocculant–disinfectants; b) studies conducted in countries in World Bank low- and middle-income country categories (2019 data)21; c) studies in which data were collected at households (e.g., not solely in health facilities or schools); d) studies including a quantitative measure of adoption; and e) an intervention or program start date. Titles and abstracts were screened for criteria 1–3; criteria 4–5 were confirmed during full text review. Cross-sectional studies were eligible if the start date of the chlorination intervention could be approximated. Because 1990 has been used as a baseline for measuring progress in global safe water access,23 we included all English language studies published from 1 January 1990 through 31 December 2021; we conducted our final search of this date range on 13 April 2022. Any non-English language studies identified during the hand search of prior systematic reviews were also eligible for title and abstract screening.
We extracted data related to the intervention or program design and measures of adoption. Where both self-reported and presence of chlorine were reported, we used the latter as the more objective measure. We categorized adoption as increasing if there was a point increase between the first and last measure of adoption, decreasing if there was a point decrease, and sustained if the change was percentage points. We determined 10 percentage points to be a reasonable and meaningful threshold to capture changes in adoption; previous work has modeled that a decline from 100% to 90% use negates nearly all health benefits of household water treatment.10 To visually illustrate these trends in plotted data, we fit a linear trend line fit to all adoption data points weighted by intervention group enrolled sample size. Given the expected heterogeneity in adoption measurement and reporting, we did not plan to report a pooled summary statistic. However, we chose to make two departures from our protocol after reviewing the available data and determining that additional analyses were possible. First, because we found comparable measures of adoption across studies, we calculated median values for adoption measures that used free or total chlorine. We calculated weighted medians by multiplying the number of observations of each group’s reported adoption by its enrolled sample size prior to calculation. Second, we additionally extracted data about which individuals, if specified, were specifically targeted for product usage instruction and were the primary implementer of the intervention at the household level. Our goal was to systematically extract data identifying upon whom the nonmonetary costs of household water treatment fall. Global water access data have established that women and girls are primarily responsible for water fetching,3 but less research has explicitly discussed the highly gendered household allocation of water treatment responsibilities.24,25
Our objective was to evaluate the adoption of chlorine POU products when provided directly to households. To separate household product use from less-than-perfect implementation fidelity, which may mean products do not reach households, we excluded program evaluations that included data from households that had not received chlorine POU products. These included, for example, large-scale programs that bundled chlorine POU promotion and distribution with antenatal care26–28 and humanitarian relief efforts that distributed chlorine POU products in the aftermath of disasters that damaged water infrastructure.29–31 We excluded studies of manual chlorine products that were installed outside of the household, that may have been associated with different barriers to and drivers of adoption (e.g., limited by availability at specific sources only, usage motivated by public peer pressure). Finally, although we did not prespecify a method of study quality assessment, we considered objective measurements of chlorine [i.e., free chlorine residual (FCR)] as higher quality data compared with self-reports. Adoption is not a primary outcome for any of the studies included in this review and only chlorine product arms (groups with different interventions within the same study) of trials are included; thus, many of the commonly used quality assessment criteria therefore do not apply (e.g., justified sample size, clearly defined outcome). Therefore, we did not exclude any studies owing to potential biases, which we acknowledge may be present. For example, response bias is a known issue with self-reported outcomes for which a respondent feels pressure to respond in a more socially acceptable32 or courteous way. These biases may be mitigated with objective outcomes, such as FCR measurements. An additional bias, unmitigated by objective outcomes, is the Hawthorne effect, which could result in overestimates of adoption because of respondents changing their behavior in reaction to being observed.33,34 Ultimately, we excluded one eligible study because data were reported in an unusable format.
To report adoption measures for crossover trials, we used the method as described by Albert et al.35 and pooled all households over the duration they experienced each product. For example, for Geremew et al.,36 we combined results from Group 1 crossover period 1 with Group 2 crossover period 2 to calculate adoption of a single product for all households over the duration of one crossover period. Depending on the order that households were assigned products, they may have experienced another chlorine product just prior. Where studies had multiple arms assigned the same chlorine POU product, Table 1 adoption calculations reflect pooled data across those arms. When possible, we used the number of units (e.g., households, children) measured at each time point so that each arm is appropriately weighted even if there was differential attrition between arms. Luby et al.37 was a follow-up study to Chiller et al.,38 and we included the follow-up study’s adoption as the last measured adoption in the sample. Our search identified a 6- to 12-month follow-up study39 to George et al.40 However, because it is unclear whether households had continued access to Aquatabs, which were provided for free in the original trial, we used only adoption data from the original trial.
Table 1.
Included studies of point-of-use chlorine water treatment with reported adoption, by product type.
| Product type | Reference | Setting | Study design | Enrolled households (or other specified unit) ()a | Indicator of adoption | Last measured adoption (%) | Time point at measurement | Change in adoption ()b |
|---|---|---|---|---|---|---|---|---|
| Flocculant–disinfectant | Albert et al. (b)35c | Kenya (rural) | RCT (crossover) | 400 | Self-report | 62 | 2 months | NA |
| Flocculant–disinfectant | Chiller et al.38 and Luby et al.37 | Guatemala (rural) | RCT | 268 | FCR | 44 and 1.5 | 10 wk and 8.5 months | − |
| Flocculant–disinfectant | Colindres et al.61 | Haiti (rural) | Cross-sectional study | 100 | FCR | 12 | 1 month | NA |
| Flocculant–disinfectant | Crump et al. (a)41c | Kenya (rural) | cRCT | 201 family compounds | FCR | 44 | Pooled (5 months) | NA |
| Flocculant–disinfectant | Doocy and Burnham55 | Liberia (IDP camp) | cRCT | 200 | FCR | 95 | Pooled (12 wk) | NA |
| Flocculant–disinfectant | Geremew et al. (b)36c | Ethiopia (rural) | cRCT (crossover) | 400 | Detectable free chlorine | 25 | 2 months | |
| Flocculant–disinfectant | Luoto et al. (c)42 | Bangladesh (urban) | RCT (crossover) | 600 | Detectable free chlorine | 3 | 6 weeks | NA |
| Flocculant–disinfectant | Norton et al.64 | Bangladesh (rural) | Nonrandomized trial | 105 women | FCR | 43 | Pooled (12 wk) | NA |
| Flocculant–disinfectant | Rangel et al. (b)44c | Guatemala (rural) | RCT | 60 | FCR | 83 | Pooled (3 wk) | NA |
| Flocculant–disinfectant | Reller et al. (b)45c | Guatemala (rural) | RCT | 199 | FCR | 30 | Pooled (9 months) | NA |
| Flocculant–disinfectant | Shaheed et al. (a)46c | Pakistan (rural) | RCT (crossover) | 247 | Total chlorine | 59 | 8 wk | − |
| Flocculant–disinfectant | Shaheed et al. (b)46c | Zambia (urban) | RCT (crossover) | 214 | Total chlorine | 18 | 8 wk | − |
| Liquid | Albert et al. (a)35c | Kenya (rural) | RCT (crossover) | 400 | Self-report | 76 | 2 months | NA |
| Liquid | Crump et al. (b)41c | Kenya (rural) | cRCT | 203 family compounds | FCR | 61 | Pooled (5 months) | NA |
| Liquid | Geremew et al. (a)36c | Ethiopia (rural) | cRCT (crossover) | 400 | Detectable free chlorine | 41 | 2 months | |
| Liquid | Humphrey et al.9 | Zimbabwe (rural) | cRCT | 2,035 womend | FCR | 58 | 12 months | NA |
| Liquid | Luby et al.82 | Pakistan (urban) | Trial (unclear if randomized) | 50 | FCR | 71 | Pooled (10 wk) | NA |
| Liquid | Luoto et al. (a)42c | Bangladesh (urban) | RCT (crossover) | 600 | Detectable free chlorine | 11 | 6 wk | NA |
| Liquid | Macy and Quick83 | Nicaragua (rural) | Nonrandomized trial | 100 | FCR | 52 | 3 months | NA |
| Liquid | Mellor et al.53 | Guatemala (rural) | RCT | 34 | FCR | 65 | 7 months | − |
| Liquid | Mengistie et al.84 | Ethiopia (rural) | cRCT | 286 | FCR | 77 | 12 wk | |
| Liquid | Murray et al.57 | Haiti (peri-urban) | Nonrandomized trial | 60 | FCR | 13 | 13 months | − |
| Liquid | Null et al.8 | Kenya (rural) | cRCT | 2,737 | Detectable free chlorine | 21 | 2 y | − |
| Liquid | Opryszko et al.48 | Afghanistan (rural) | cRCT | 607 | Self-report | 80 | 1 y | NA |
| Liquid | Potgieter et al. (a)43c | South Africa (rural) | RCT | 20 | Detectable free chlorine | 88 | 3 months | |
| Liquid | Potgieter et al. (b)43c | South Africa (rural) | RCT | 20 | Detectable free chlorine | 98 | 3 months | |
| Liquid | Quick et al.49 | Bolivia (urban) | RCT | 15 | FCR | 100 | 9 wk | |
| Liquid | Quick et al.51 | Bolivia (peri-urban) | RCT | 64 | Detectable total chlorine | 95 | 6 months | |
| Liquid | Quick et al.50 | Zambia (peri-urban) | Nonrandomized trial | 166 | Detectable total chlorine | 85 | 13 wk | |
| Liquid | Rangel et al. (a)44c | Guatemala (rural) | RCT | 20 | FCR | 83 | Pooled (3 wk) | NA |
| Liquid | Reller et al. (a)45c | Guatemala (rural) | RCT | 197 | FCR | 40 | Pooled (9 months) | NA |
| Liquid | Sobsey et al. (a)47c | Bangladesh (urban) | RCT | Detectable free chlorine | 89 | Pooled (8 months) | NA | |
| Liquid | Sobsey et al. (b)47c | Bolivia (peri-urban) | RCT | Detectable free chlorine | 77 | Pooled (6 months) | NA | |
| Liquid | Solomon et al.85 | Ethiopia (rural) | cRCT | 203 | FCR | 81 | Pooled (4 months) | NA |
| Liquid | Sugar et al.52 | Kenya (urban) | Program evaluation | 392 children | Smell of chlorine | 97 | Pooled (12 months) | NA |
| Tablet | Altmann et al.56 | Chade | cRCT | 850 children | FCR | 98 | 2 months | |
| Tablet | Boisson et al.62 | India (urban/rural) | RCT | 1,080 | FCR | 47 | 12 months | |
| Tablet | Clasen et al.54 | Bangladesh (urban) | RCT | 50 | FCR | 100 | 4 months | |
| Tablet | Ercumen et al.86 | Bangladesh (rural) | RCT | 600 | FCR | 79 | 1 y | − |
| Tablet | George et al. (a)39 | Bangladesh (urban) | cRCT | 84 | FCR | 94 | Pooled (9 d) | NA |
| Tablet | Jain et al.60 | Ghana (peri-urban) | RCT | 120 | FCR | 83 | 12 wk | |
| Tablet | Luby et al.7 | Bangladesh (rural) | cRCT | 2,086 compounds | FCR | 84 | 2 y | |
| Tablet | Luoto et al. (b)42 | Bangladesh (urban) | RCT (crossover) | 600 | Detectable free chlorine | 10 | 6 wk | NA |
| Tablet | Pickering et al.59 | Bangladesh (urban) | cRCT | 90 | Total chlorine | 55 | 10 months | − |
| Multiple | Blanton et al.58 | Kenya (rural) | Program evaluation | 662 | FCR | 18 | 13 months | |
| Granular | Tsai et al.63 | Haiti (rural) | cRCT | 447 | FCR | 27 | 180 d | − |
Note: Flocculant–disinfectant products included PuR, PureIt, Purifier of Water, and Bishan Gari (local brand in Ethiopia). Liquid chlorine products included sodium hypochlorite, WaterGuard, Klorin/Clorin, bleach, calcium hypochlorite solution, and electrochlorinator (for at-home sodium hypochlorite production). Tablet chlorine products included Aquatabs. Multiple products in a single study included PuR and WaterGuard. Granular chlorine products included Klorfasil. cRCT, cluster randomized controlled trial; FCR, free chlorine residual; IDP, internally displaced persons; NA, not applicable; RCT, randomized controlled trial.
Sample size was included for chlorine arm(s) only.
Change in adoption: point increase from first to last measure; – indicates point decrease; point change.
Albert et al.,35 Crump et al.,41 Geremew et al.,36 Shaheed et al.,46 Luoto et al.,42 Potgieter et al.,43 Rangel et al.44 Reller et al.,45 and Sobsey et al.47 had multiple eligible chlorine intervention arms that are separately listed because they had different settings or chlorine products. Separate arms (referred to here as intervention groups) are indicated with letters in parentheses. We combined intervention arms with the same product, even if other intervention components differed. Studies in which measurement across multiple time points are presented as a pooled statistic are indicated as such, with the study duration in parentheses.
FCR was measured in only 752 households; self-reported adoption across the entire sample was 87%.
Urban, rural, or peri-urban was not specified and could not be inferred from the main text of the paper.
Results
Our search identified 8,617 unique results. After reviewing all available titles and abstracts, we obtained 127 full-text articles to assess using full eligibility criteria. This step yielded 36 eligible texts, including 28 cluster or individually randomized controlled trials, 1 cross-sectional study, 2 program evaluations, 4 nonrandomized trials, and 1 trial in which method of intervention assignment was unspecified (Figure S1). Four studies had a crossover design, and studies were conducted in 16 countries (Table 1). Nine studies had multiple intervention arms or were crossover trials with different chlorine POU products35,36,41–45 or were conducted in more than one country.46,47 We categorized each product- or site-specific group within each study as a separate intervention group, and the results of each unique intervention group are separately listed (Table 1). We pooled data into a single intervention group if a single study had multiple arms that used the same chlorine product in the same setting but had different additional components (e.g., in combination with a safe storage container, handwashing stations, or latrines).7,8,43–45,48
Studies were conducted in rural (), urban (), and peri-urban () settings in addition to three studies in multiple settings, one in an internally displaced persons (IDP) camp, and one with an unspecified setting description. The enrolled sample size (of chlorine POU intervention groups) ranged from 15 households49 to 2,737 households.8 Nearly all provided the POU products for free for the duration of the study. The most common chlorine POU products were WaterGuard (liquid sodium hypochlorite by Population Services International), PuR (flocculant–disinfectant with calcium hypochlorite by Procter & Gamble), and Aquatabs [sodium dichloroisocyanurate (NaDCC) tablets by Medentech].
Defining and Measuring Adoption
A variety of metrics were used to assess product adoption, and various terms were used to describe adoption, including uptake, use/usage, adherence, and compliance. Here, we chose the terms adoption and use, rather than adherence or compliance, so as not to suggest a failure of the end user but rather of the product or its implementation. The most common reported indicator of adoption was the proportion of households with stored drinking water having a FCR greater than or equal to a specified threshold, typically , but as high as . The choice of threshold can significantly change conclusions about adoption. Using a threshold of , Altmann et al. reported that 51% of households adhered to chlorine POU treatment, compared with 98% when using a threshold of (their instrument limit of detection).56 Six studies defined adoption as the presence of “detectable” free or total chlorine without specifying a detection limit,8,36,42,43,47,50,51 and one study used the smell of chlorine in stored water because no test instruments were available.52 Two studies measured only self-reported adoption, which was defined as use “during the previous two weeks”48 or undefined.35 All others measured free or total chlorine as either a primary or secondary measure of adoption. Self-reported adoption was higher than FCR-confirmed adoption in studies that reported both.9,42 Quick et al. defined adoption as “any detectable total chlorine residual,” but they also measured and reported FCR.50 Over four time points, the percentage of households with detectable total chlorine ranged from 72% to 95%, in contrast to 55% to 81% with FCR . Eleven studies reported a single pooled measure of adoption across their entire study duration; studies that reported time point-specific measures included between 1 and 24 adoption measurements (across all intervention groups, ). Of the adoption results reported in Table 1, 18 were measured at unannounced visits, 2 at announced visits, 24 were not specified as either, and 2 were from studies that had both announced and unannounced visits. Measures of variance, such as standard deviation or range, were typically not reported with adoption results.
Observed Levels of Adoption Across Studies
Final measured adoption was highly variable and was not associated with study length (Figure 1). Across all intervention groups, there were 18,480 observations (each study-level final adoption data point times the enrolled group sample size). The studies reporting FCR-confirmed ( or ) adoption at any time point ranged from 1.5%37 to 100%.49,53,54 Of the studies that confirmed adoption with either free or total chlorine, eight groups had adoption40,43,49,51,52,54–56 and two groups had adoption at the final time point measured.37,42 Sugar et al. also reported with adoption defined as having the smell of chlorine in stored water.52 The rest reported adoption ranging from 10% to 90% at the final time point measured. With the exception of Luby et al.7 and Null et al.,8 which included 2 y of follow-up, study durations were months (Figure 1).
Figure 1.
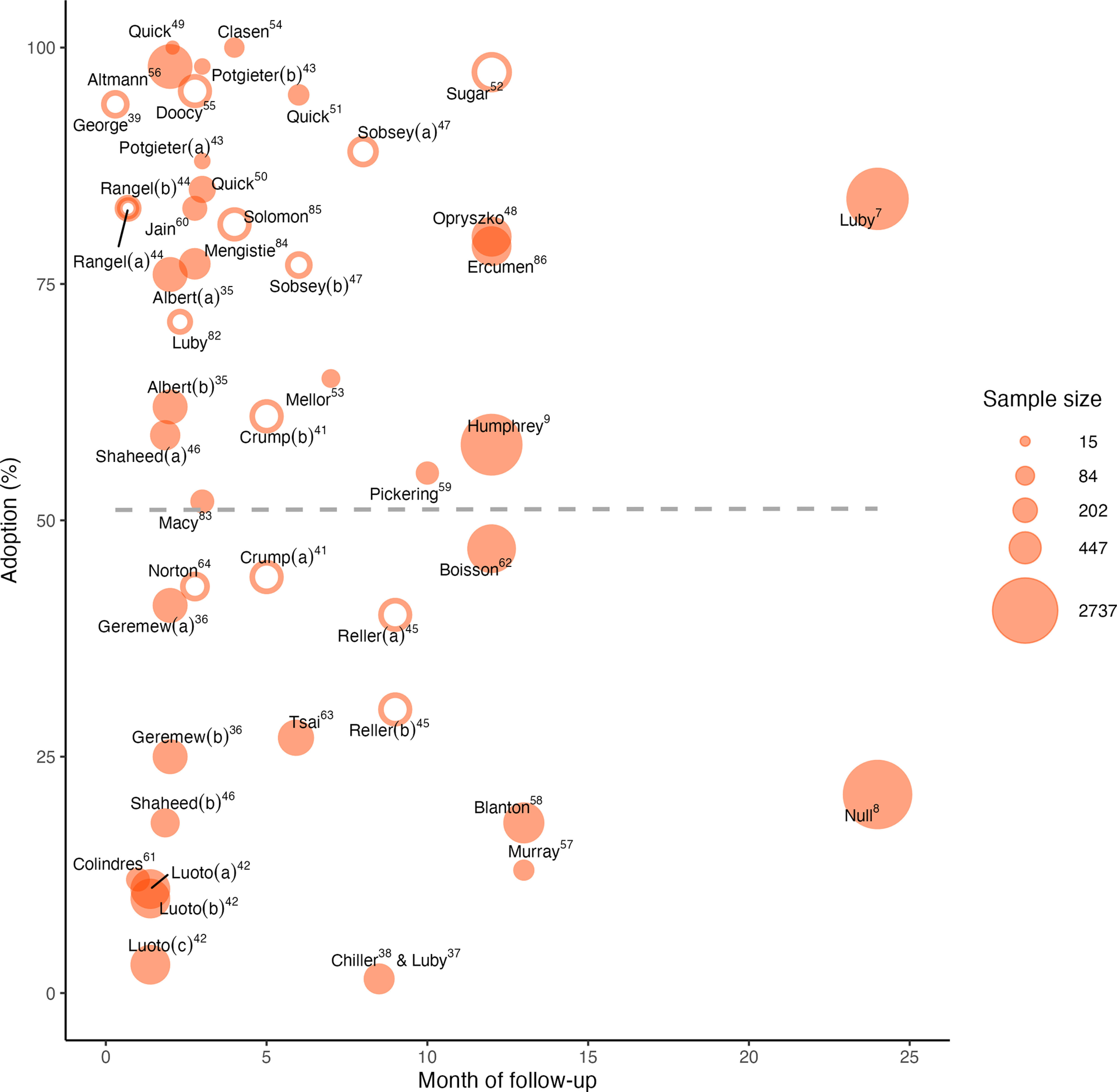
Measured product adoption at the last follow-up. The point sizes are scaled to indicate relative sample size [of the group(s) receiving chlorine only]. Open circles indicate that the data point is reported as multiple adoption measures pooled over the months of follow-up up until the time point shown. Closed circles are a single time point result. Letters in parentheses indicate different intervention groups within a single study. Opryszko et al.48 and Albert et al.35 used self-reported adoption; Sugar et al.52 used the smell of chlorine in stored water as adoption; the rest used either free or total chlorine to measure adoption. The dashed line shows a linear trend line using adoption measures at last follow-up weighted by sample size. Data are from Table 1, columns “Time point at measurement” and “Last measured adoption.” Note that papers are indicated by first author only.
Changing Adoption Over Time
On average across all intervention groups, adoption declined slightly over time (Figure 2), although some groups had increasing or sustained adoption. When restricting to studies months in duration, adoption remained stable on average. Among 23 groups that reported multiple time point-specific measures, adoption increased in 4 chlorine intervention groups, decreased in 9 groups, and was sustained in 10 groups. The total pooled sample size was similar for studies with increasing and sustained adoption, but there were approximately one-third as many observations across all studies with decreasing adoption. There were 52,349 observations (group average multiplied by enrolled sample size) from studies that reported one or more single time point adoption measurements and provided products entirely for free (Figure 2), which included one study with self-reported adoption.48 However, we note that data from months after intervention delivery were from only two related studies (i.e., WASH Benefits Bangladesh7 and WASH Benefits Kenya8), and when these studies were excluded, the remaining data (total ) showed no change in adoption over time.
Figure 2.
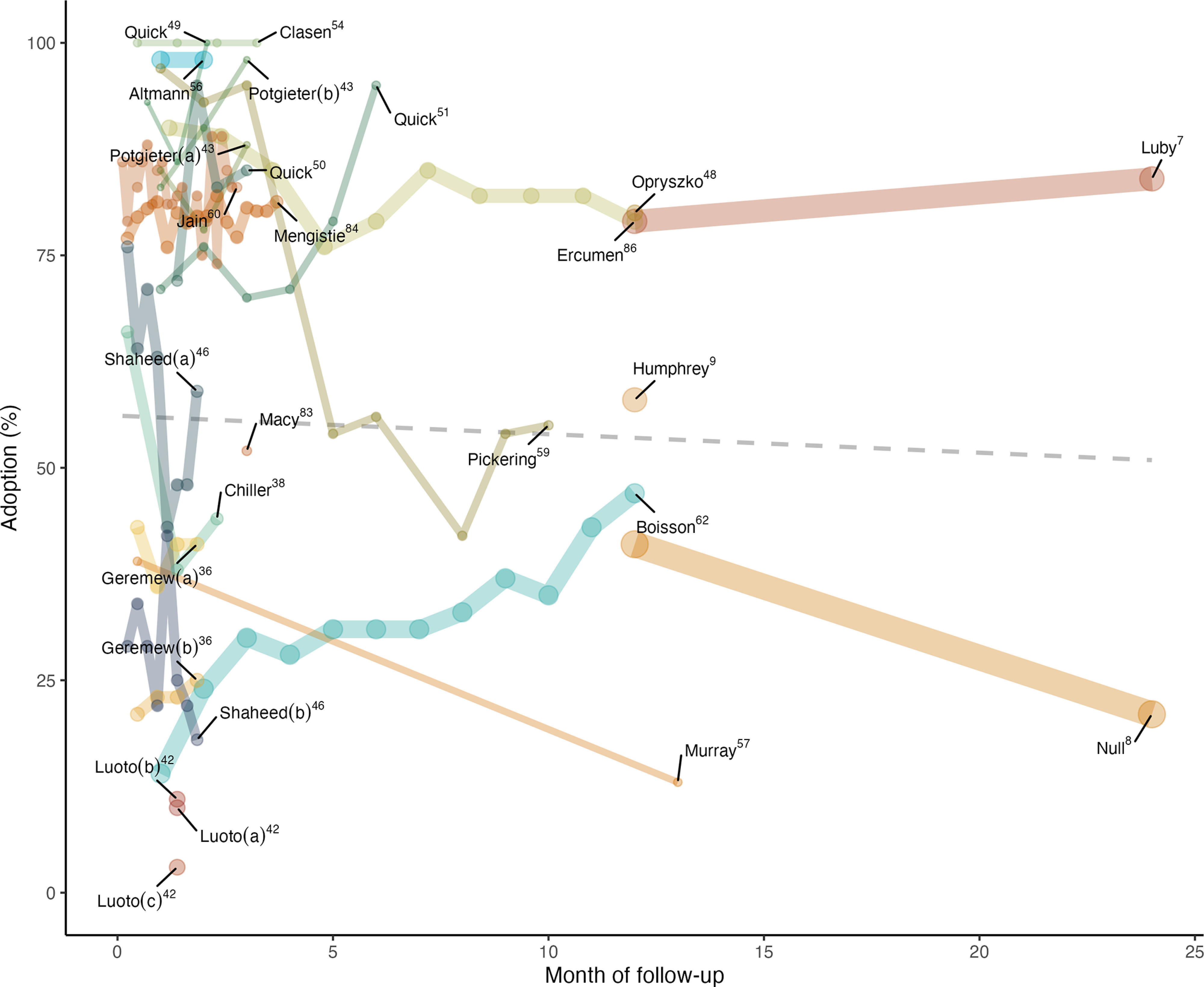
Reported product adoption over time after the start of intervention. The line width is scaled to indicate relative sample size [of the group(s) receiving chlorine only]. Letters in parentheses indicate different intervention groups within a single study. The studies in the graph are restricted to studies reporting one or more single time point measures of adoption and provided chlorine products entirely for free for the study duration. The latter restriction excludes Tsai et al.,63 Blanton et al.,58 Mellor et al.,53 and Luby et al.37 (which provided the final time point follow-up to Chiller et al.38). Among the studies included here, only Opryszko et al.48 had self-reported adoption. The dashed line shows a linear trend line using all adoption measures weighted by sample size. Data are available in Excel Tables S1 and S2. Note that papers are indicated by first author only.
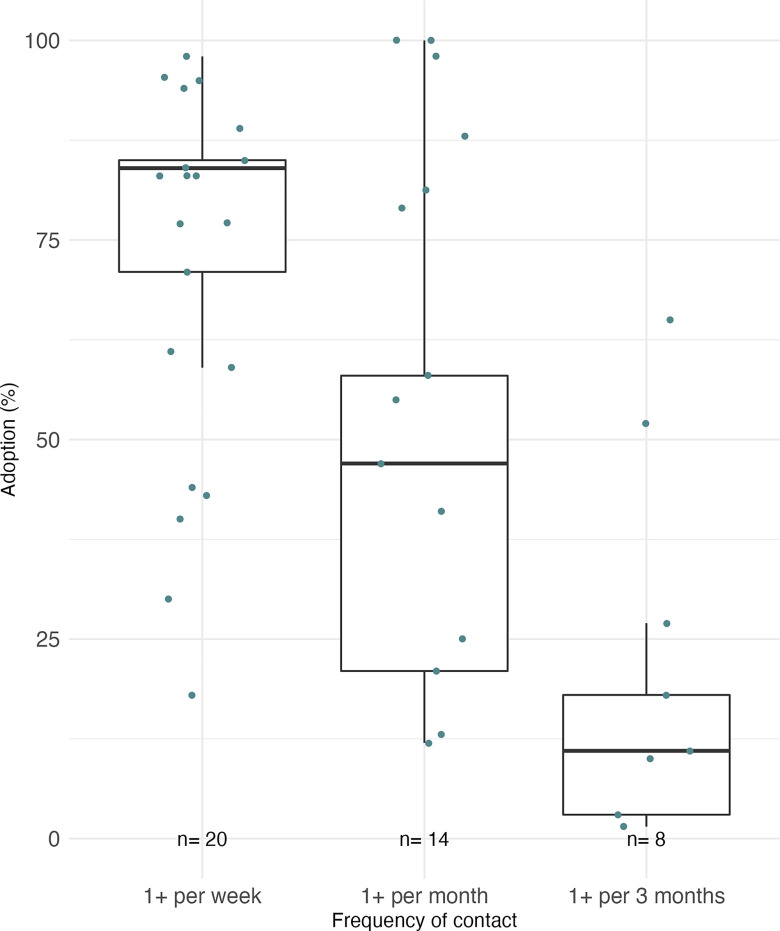
Contact Frequency between Participants and Study Staff
Adoption was positively associated with contact frequency between study participants and study staff across studies (Figure 3, Figure S3) and within studies as well. Restricting to studies that used free or total chlorine–confirmed use, adoption ranged from a sample-size-weighted median of 84%, when households were visited one or more times per week by study staff (20 groups, total ), to 47%, when visits were once or more per month (14 groups, total ), to 11% with less frequent visits (8 groups, total ) (Figure 3). In a cluster randomized controlled trial in urban Dhaka,59 the intervention included promotional visits every 2 wk for the first half of the 10-month study, and adoption was when promotions were ongoing. After these visits concluded, free delivery of Aquatabs and water quality testing continued, but adoption quickly dropped by and remained relatively stable (42%–56% from months 5–10). In a 2-y cluster randomized controlled trial in rural Kenya, Null et al.8 observed adoption decline by between year 1, during which households received monthly promotional visits, and year 2, when households were visited approximately every other month.
Figure 3.
Weighted box plots showing the relationship between contact frequency over the study and final measured adoption, restricted to only groups that used free chlorine residual (FCR) or total chlorine to measure adoption. This restriction excludes Opryszko et al.,48 Albert et al.,35 and Sugar et al.52 Dots show each group average. Groups were weighted by sample size and 25th, 50th, and 75th percentiles of the pooled data are displayed above as the midline and box limits. The whiskers extend to data within 1.5 times the interquartile range (IQR) above and below the 75th and 25th percentiles. This figure includes studies that reported single time point and pooled adoption measures. Summary data are presented in Table S1.
Type of Chlorine Product and Adoption
Across all 46 intervention groups, 23 received liquid chlorine products, including branded products, such as WaterGuard and Clorin/Klorin, and generic sodium hypochlorite. One group received a locally mixed calcium hypochlorite solution.49 Twelve groups received flocculant–disinfectant products, most commonly PuR brand, but one study in Ethiopia used a local product called Bishan Gari.36 Nine groups received tablets, all Aquatabs brand. Among groups that used FCR or total chlorine to measure adoption, tablet chlorine product interventions had the highest adoption (9 groups; total ; weighted ), followed by liquid products (20 groups, total ; weighted ), then flocculant–disinfectants (11 groups, total ; weighted ) (Figures S2 and S4).
Barriers to Use
Reasons for nonuse of products, reported by respondents, were provided for only 23 of the 46 intervention groups. This suggests that, much of the time, the reasons for low adoption are poorly understood simply because the relevant data are not systematically collected. Bad taste, smell, or appearance of treated water was identified by one or more households in 17/23 intervention groups (74%), and lack of time was identified in 10/23 groups (43%). Although most of the studies provided products for free, 4/23 groups (17%) identified price or availability as a barrier to repurchase and continued use. Often, however, each of these reasons was reported by a small proportion of households. Several studies emphasized reasons for use rather than nonuse, reporting instead that households preferred treated water41,55,59–61 or that households felt the time required to treat the water was worth it.45
Taste and Smell Concerns
The included studies suggest that taste and smell concerns can be a reason for nonuse, but they are not universal barriers. In Ethiopia, the majority (66% of ) of households said they disliked the chlorine taste.36 In rural South Africa, use of 3.5% sodium hypochlorite was slightly higher than use of a 1% solution, although households in the former group did mention disliking the taste of water and the sample size was small.43 However, in both studies that evaluated chlorine POU products in humanitarian crisis settings, respondents reported that they preferred the taste of the chlorinated water over the untreated water,55,61 as did respondents in some households in nonemergency situations.60 In urban Bangladesh, where FCR-confirmed use of Aquatabs, WaterGuard, and PuR was 10%, 11%, and 3%, respectively, only around half of respondents (across 1,737 household visits) said, unprompted, that taste and smell were obstacles to use.42
The success that blinded studies have had in blinding participants to treatment assignment also suggests that taste and smell are not the overwhelming problems sometimes ascribed to chlorination. Two blinded, placebo-controlled trials with Aquatabs60,62 found no difference between placebo and chlorine arm respondents in their beliefs about their group assignment. Jain et al.60 found that 16% of respondents (, reporting both placebo and chlorine arms together), said the tablets made their water taste better, compared with 2% and 1% reporting bad smell and taste, respectively. However, Boisson et al.62 found higher dissatisfaction with taste and smell among the intervention group compared with the placebo group.
Price of Chlorine POU Products
All but three studies provided the chlorine POU products free to respondents for the study duration, and one study37 reported data from households 6 months following the conclusion of the original study,38 after which households could continue to purchase the product on their own. Blanton et al.58 provided rural Kenyan schoolchildren with free samples of PuR to take home to parents, after which they could repurchase the widely available products in the markets. In rural Haiti, Tsai et al.63 provided half of respondents with a free trial of Klorfasil, a granular chlorine POU product, followed by the opportunity to purchase at a subsidized price. The other half received no free trial. Although over half of total respondents repurchased the product, of 236 respondents at the final follow-up had FCR in stored water. In Mexico, Mellor et al.53 provided 34 households with a free bottle of sodium hypochlorite, with the option to later purchase a 6-month supply for USD $3.14 from a local distributor; half a year later, 65% of 20 available households had FCR in stored water. In rural Guatemala, 93% (430/462) of households reported that they would be willing to pay half the market price for PuR (USD $0.14 to treat of water), but only 1.5% (7/462) had FCR in stored water.37
Gender and the Time Cost of Chlorine POU Interventions
The time required to treat the water was the second-most reported reason for nonuse by respondents, and this time burden was usually placed on women. Women were the primary respondents in 36/46 groups (78%), targeted for inclusion as either the primary caretaker of children years of age or the individual in charge of household water management. In one study in rural Afghanistan, intervention messaging was targeted to female caretakers, but nearly half of households allowed only males to participate as respondents.48 In the remaining studies, the gender of respondents was not addressed. In Guatemala, Luby et al. observed very low (1.5% of stored water with FCR 8.5 months after the start of intervention) sustained use of PuR for drinking water treatment, and respondents reported lack of time as one reason for nonuse.37 The authors observed: “Female heads of household already spent substantial time collecting water and on other innumerable household tasks required for family survival in a low-income setting. Using the flocculant–disinfectant required extra steps for water treatment and extra time spent washing the filter cloths.”37
Three studies provided instructions that included time estimates for treatment steps, with per week of active time spent stirring and filtering required for median reported product use.44,55,64 This doubles when including wait time required for disinfectant contact. Norton et al.64 provided step-by-step instructions for treatment with flocculant–disinfectant, which included a 5-min stirring step, a 5-min settling step, then filtering through a cloth before letting the filtered water sit for 20 min for disinfectant contact time. Respondents, all women, used a median of 11 (range: 0–48) flocculant–disinfectant sachets per week. Assuming 10 min of active time required, from stirring to filtering, that comes to 110 (range: 0–480) min per week spent actively treating water. Including the 20-min wait time, that increases to 330 (range: 0–1,440) min weekly spent treating and waiting for water before safe use. Rangel et al.44 reported three 30-s stirring and 5-min waiting periods before filtering through a cloth: of active time required. Ninety-four percent (94/100) of respondents were female and the reported median daily household drinking water consumption was . Assuming once daily treatment, with each sachet treating , that comes to 119 min of active time allocated to water treatment per week. Other estimates of daily household water volumes used were much higher. Altmann et al. estimated that families would need to purify of water per day, and the intervention provided sufficient tablets for 3 months of daily treatment of this volume.56 Doocy and Burnham55 estimated 40 min as the total time required to treat water with PuR, including the stirring, filtering, and waiting steps, although this was not reported as too time consuming by participants in an IDP camp.
Institutional Intervention Settings
Although most studies were in households, a handful of interventions introduced in non-household settings achieved high adoption (Figure S5). Three chlorine POU interventions delivered at health facilities in combination with treatment for cholera,40 severe acute malnutrition,56 and pediatric HIV care52 resulted in adoption ranging from 94% to 99% observed at household follow-up visits. George et al.40 did a randomized controlled trial to evaluate a hospital-based intervention that included Aquatabs to reduce the spread of cholera from patients to household members in urban Bangladesh. The intervention included a week of households visits (94% adoption; 308/327 household visits), and data from 6- to 12-months later suggest that the intervention may have increased use of household water treatment overall, even if not specifically of chlorine products. Sugar et al.52 evaluated a program that distributed water storage containers, hypochlorite solution for drinking water treatment, soap, and insecticide-treated bed nets in a program designed to reduce diarrhea and malaria among children living with HIV in peri-urban Mombasa, Kenya. Adoption at household follow-up visits, 97% (1,314/1,350 visits) on average, was assessed by chlorine odor in stored water. Altmann et al.56 did a cluster randomized controlled trial to evaluate the benefits of a water, sanitation, and hygiene package added to clinic-based treatment of severe acute malnutrition in Chad. The 2-month multicomponent intervention included two home visits, and 98%–99% of households (1,373 observations across visits) had FCR at monthly visits.
One school-based program resulted in a moderate but sustained increase in chlorine POU product use relative to baseline. Blanton et al.58 evaluated a school-based program that delivered drinking water and handwashing infrastructure in schools in rural Kenya, PuR flocculant–disinfectant for drinking water treatment, WaterGuard for handwashing water (which children sometimes drank), and educational comic books and samples of PuR for children to take home to their parents. Use of either WaterGuard or PuR, as confirmed by FCR , was 21% (134/644 households) at 4 months and 18% (96/536 households) at the 13-month follow-up. The program did not include regular household visits or products beyond an initial sample of three free sachets of PuR, but it was in a setting with mass media promoting the products.
Humanitarian Intervention Settings
Two included studies reported high adoption in humanitarian settings.55,61 Colindres et al.61 interviewed 100 households that had received free PuR following a 2004 tropical storm in Haiti. Marketing of PuR was through the radio, community demonstrations, and word of mouth from community leaders and neighbors. Although nearly all (97%) of the 100 respondents said that “PuR-treated water appears, tastes, smells, and is healthier,” stated that they would be willing to pay the product’s market price. Doocy and Burnham55 did a 12-wk trial of PuR with a water storage container in an IDP camp in Liberia. Additional free sachets were provided at weekly diarrhea monitoring visits; households were additionally visited weekly for unscheduled water quality testing. Across all 1,551 weekly water testing visit measures, 95% had FCR present, with the lowest adoption in the first week (90%). FCR was in 85% of visits. The study additionally included focus group discussions with participants, who reported that they preferred the taste of the chlorinated water over untreated water and that they noticed less diarrhea in their households.
Discussion
In this systematic review, we found a wide range in adoption of chlorine POU product use. On average, adoption declined over time, but the relatively short follow-up in most of the included studies limits our understanding of the long-term use of chlorine POU products. Notably, our search strategy selected for closely monitored trials vs. programs. The trials are more likely to have a short duration and intensive promotion, given the resources required for intervention studies. An important implication is that the observed levels of use described here are overestimates of the adoption likely to be observed long-term in programs that cannot continue the contact frequency of high-intensity interventions.
There was no standard definition for adoption of chlorine POU water treatment across the reviewed studies, but the proportion of households with FCR above a threshold on unannounced visits was the reported indicator most likely to capture both correct and consistent use. Although authors did not always explain the choice of FCR threshold that indicated adoption, align with widely used drinking water guidelines. World Health Organization Guidelines for Drinking Water recommend that a minimum FCR (and maximum ) be present at the point of delivery.65 The Sphere Handbook, used in humanitarian response, also recommends FCR at the point of delivery for household water,66 although chlorine decay modeling suggests that may not be sufficient in refugee camps where high heat causes more rapid chlorine decay and water and sanitation conditions are especially poor.67 Using these guidelines, FCR as a metric of adoption may indicate that water is safely protected in typical settings, but FCR decays over time and thus underestimates usage. The observation by Quick et al.50 that adoption measured by total chlorine was higher than that measured by free chlorine suggests that, although not all households were dosing as instructed, more households may have been consistently using chlorine than would be suggested by FCR testing only.50 Therefore, compared with FCR, total chlorine may be a more appropriate indicator of use. Objective measures such as FCR and total chlorine are preferable to self-reported usage, which is subject to social desirability and courtesy bias and varies widely in its definition across studies (e.g., used “since yesterday”42 to “last two weeks”48). Given that correct and consistent water treatment is required to realize health benefits,10,11 self-reported usage defined as, for example, “during the last two weeks,” may be uninformative. Because of this nonstandardized measurement and reporting, claims of “high” adoption will provide little information without clearly defining the indicators that are used. In addition, when reporting product use, we assert that single time point measurements are more informative than measures pooled over the duration of a study because of the variability in adoption over time and because pooled measures across time points do not allow adoption to be linked to outcomes measured at single time points.
We found a positive association between contact frequency and adoption, suggesting that weekly contact between households and study staff is necessary to sustain high adoption. Studies that contacted participants once per month had a sample-size-weighted median adoption of ; adoption dropped off substantially to 11% among those studies with less frequent contact. This finding makes sense in the context of health behavior change theories.14 Each contact with study staff, for any reason, provides households with a reminder or nudge to action,68 increasing the likelihood of habit formation, and this has important implications for health interventions. A recent article that reviewed POU safe water interventions and health impacts found that interventions with demonstrated reductions in diarrheal illness had higher frequency of contact between participants and study staff at levels often considered infeasible at large scales.69 Efforts to replicate and scale household water treatment interventions that have been successful in trials must consider whether they have the field staff resources required to achieve weekly contact with participants; otherwise, our findings suggest that very low adoption levels should be expected.
We also noted higher adoption for tablet products, compared with liquid or flocculant–disinfectant products. Tablets have greater ease of use and convenience compared with liquid products,70 which may require measuring out the correct dose and require more product for dosing use because they are typically diluted to around 1%. Flocculant–disinfectants, because they require separate mixing and filtering steps, also require more effort for use than tablets. In settings where high contact frequency is possible, or in humanitarian, emergency, or outbreak situations, where we found that adoption was typically higher, these results suggest that tablet products may be more effective in achieving high levels of use, compared with liquid or flocculant–disinfectant products.
The evidence to date suggests it is unrealistic to rely solely on household-level treatment to realize the benefits of safe water at the necessary scales. The historical public health benefits of centrally treated piped water1 are often cited as evidence of the importance of safe water interventions. However, in this utility model, in which water is effectively treated at a centralized facility and then distributed through pressurized pipe networks to in-home taps, the responsibilities for correct, consistent, and sustained use are not on individuals in households. The in-effect 100% adoption provided by effective centralized systems contrasts starkly with the adoption observed in real-world evaluations of household water treatment products. At the same time, the infrastructure limitations that first motivated household water treatment approaches are changing. Since 2000, more than people have gained access to piped water,3 and passive, in-line chlorination technologies are one example of safe water solutions that are increasingly compatible with this piped infrastructure. In urban Bangladesh, where researchers have generally observed low adoption to chlorine POU product use,42,71 a decentralized, passive, system-level chlorination technology had high acceptability and reduced child diarrhea by nearly 25%.72 This approach is closer to the centralized utility model in that the burden of treatment is not on individuals.
There is some demand for chlorine POU products, however, and it would be a mistake to dismiss the results of household water treatment trials as evidence that household water treatment should never be implemented. Chlorine POU provision at health facilities and in an IDP camp achieved adoption, suggesting that settings in which health risks are front-of-mind may motivate increased use of chlorine POU products.40,52,55,56 Even with lower sustained adoption, chlorine POU may still be a worthwhile investment in some settings. Ahuja et al. calculated that a 20%–40% reduction in child diarrhea, on par with pooled effect estimates across studies with adoption,12 makes chlorine POU a cost-effective health intervention. In urban Bangladesh, around half of households continued to use freely provided Aquatabs to treat their water for several months after promotional visits ended although water quality testing continued.59 In Kenya, where mass media promotion of household water treatment was ongoing and products were already widely available in markets, a school-based program to provide targeted education and promotion through students resulted in a sustained, although moderate, increase in use of chlorine POU products.58 Nearly all included studies provided chlorine entirely for free; of the few that followed up with households after encouraging them to purchase products, none found high sustained adoption. This makes sense in the context of demand for similar essential health products, for which demand drastically declines with increasing price and payment does not increase use after purchase.73 In some settings, the level of sustained demand is unclear because households may not have long-term access to the products that are so intensively promoted in shorter-term trials, although there may be other sustained and beneficial changes to household safe water behaviors.39 Factors such as continued communitywide messaging and access to products may be key to sustaining adoption, at least among households who do not view cost as a barrier to use. In rural Haiti, of participants in a long-running, nongovernmental organization-supported safe water enterprise could easily purchase low-cost chlorine and had chlorine residual in stored water, compared with 10% of nonparticipants.74 Although household water treatment with chlorine products may not be a universal solution, it can play an important supporting role in providing safe water in settings where it is promoted and available.
One aspect that remains neglected in chlorine POU evaluations is the gendered time and labor cost of household water treatment. We found that the time required to treat the water was identified as a barrier to water treatment by respondents, the majority of whom were women who were targeted because of their roles as household water managers and primary caretakers of young children. The nonmonetary costs, particularly on mothers, of interventions designed to improve child well-being are often unacknowledged and implicitly set to zero.75 Although the burden of water fetching on women and girls is widely acknowledged and even quantified in global statistics,3 the gendered work of household water treatment receives little attention. In settings with “innumerable household tasks required for family survival,”37 the nonmonetary costs of household water treatment challenge the notion that chlorine POU treatment is simply the cost of a bottle of diluted bleach. The household burdens placed on women and girls in low-income settings are added to the everyday stresses of poverty, described by Mullainathan and Shafir as a “bandwidth tax”76 and further discussed in relation to safe water by Ray and Smith.77 In other words, when daily survival is a struggle, even an extra 30 min a day to chlorinate and wait for water can be burdensome. These are tasks that behavioral economists have alluded to as small hassles, seemingly minor but very real barriers in the everyday lives of the poor.78 These issues are not unique to chlorine POU products—other POU options such as boiling,79 solar disinfection,80 and filters81 all require time and labor for use and maintenance.
There are some limitations to our review. First, our inclusion criteria excluded some studies that are relevant for understanding the use of chlorine POU products, including large-scale programs bundled with antenatal care, disaster relief efforts, and social marketing campaigns (see the “Methods” section). Second, we did not address user preferences for chlorine POU when other POU options are available, nor did we examine (relative) adoption of other POU methods. Burt et al. did not report adoption for individual chlorine products and was therefore excluded from our review, but study respondents ranked and preferred both boiling and pot filters over WaterGuard and PuR, although self-reported adoption of all POU methods was high (average 85% and 91% across two sites).24 Luoto et al. observed very low adoption ( self-reported) across all POU products, but use was slightly higher for siphon filters compared with Aquatabs, WaterGuard, and PuR.42 The results from these studies indicate that non-chlorine products may be preferred over chlorine products, when available, but also that if adoption of chlorine POU is very low, adoption of non-chlorine POU is likely to be similar, and vice versa.
Our review has several strengths. First, we designed a broad search strategy to capture the loosely defined construct of adoption of household water treatment. Although high adoption is an important determinant of health impact, it is not measured or reported in any standardized way, in contrast to the increasingly standardized primary health outcomes that are common across these studies. Our approach allowed us to systematically identify available adoption data in the literature. Second, in studies where they were available, we extracted multiple adoption data points and frequency of contact between participants and study staff. This allowed us to observe changing product use over time within studies and to link adoption with intensity of behavior promotion and staff visits. Third, we extracted and emphasized the available data on the gendered burden of POU adoption, showing that so-called low-cost chlorination products are as low cost as they are in part because no value is assigned to intra-household care work.
We were motivated to conduct this systematic review in part because recent large-scale trials that included chlorine POU interventions had small or no effects on child health outcomes that have been linked to safe water consumption. At the same time, the historical public health benefit of chlorinating water supplies is undisputed. A key difference between these two modes of water access is the reliance on systems vs. households to implement the treatment, and although there is a nonzero sustained demand for chlorine POU products, the evidence to date suggests that this approach will not achieve the widespread public health benefits of system-level safe water solutions. Where appropriate infrastructure exists, the safe water community should enhance efforts toward evaluating, implementing, and maintaining system-level treatment options. The effectiveness of chlorination for safe water depends as much on the mode of delivery as it does on the disinfection efficacy of the chlorine itself.
Supplementary Material
Acknowledgments
We thank F. Goddard for his valuable contributions to the review protocol and C.D. Elmera for excellent research assistance.
Y.S.C. was supported by the National Science Foundation Graduate Research Fellowship Program under grant DGE 1752814. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
References
- 1.Cutler D, Miller G. 2005. The role of public health improvements in health advances: the twentieth-century United States. Demography 42(1):1–22, PMID: , 10.1353/dem.2005.0002. [DOI] [PubMed] [Google Scholar]
- 2.White GC. 2010. White’s Handbook of Chlorination and Alternative Disinfectants. Hoboken, NJ: Wiley. [Google Scholar]
- 3.United Nations Children’s Fund, World Health Organization. 2019. Progress on Household Drinking Water, Sanitation and Hygiene 2000–2017: Special Focus on Inequalities. https://apps.who.int/iris/bitstream/handle/10665/329370/9789241516235-eng.pdf [accessed 10 July 2021].
- 4.Mintz E, Bartram J, Lochery P, Wegelin M. 2001. Not just a drop in the bucket: expanding access to point-of-use water treatment systems. Am J Public Health 91(10):1565–1570, PMID: , 10.2105/ajph.91.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt WP, Cairncross S. 2009. Household water treatment in poor populations: is there enough evidence for scaling up now? Environ Sci Technol 43(4):986–992, PMID: , 10.1021/es802232w. [DOI] [PubMed] [Google Scholar]
- 6.Clasen T, Bartram J, Colford J, Luby S, Quick R, Sobsey M. 2009. Comment on “Household water treatment in poor populations: is there enough evidence for scaling up now?” Environ Sci Technol 43(14):5542–5544, PMID: , 10.1021/es9008147. [DOI] [PubMed] [Google Scholar]
- 7.Luby SP, Rahman M, Arnold BF, Unicomb L, Ashraf S, Winch PJ, et al. . 2018. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Bangladesh: a cluster randomised controlled trial. Lancet Glob Health 6(3):e302–e315, PMID: , 10.1016/S2214-109X(17)30490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Null C, Stewart CP, Pickering AJ, Dentz HN, Arnold BF, Arnold CD, et al. . 2018. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Kenya: a cluster-randomised controlled trial. Lancet Glob Health 6(3):e316–e329, PMID: , 10.1016/S2214-109X(18)30005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humphrey JH, Mbuya MNN, Ntozini R, Moulton LH, Stoltzfus RJ, Tavengwa NV, et al. . 2019. Independent and combined effects of improved water, sanitation, and hygiene, and improved complementary feeding, on child stunting and anaemia in rural Zimbabwe: a cluster-randomised trial. Lancet Glob Health 7(1):e132–e147, PMID: , 10.1016/S2214-109X(18)30374-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown J, Clasen T. 2012. High adherence is necessary to realize health gains from water quality interventions. PLoS One 7(5):e36735, PMID: , 10.1371/journal.pone.0036735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enger KS, Nelson KL, Rose JB, Eisenberg JNS. 2013. The joint effects of efficacy and compliance: a study of household water treatment effectiveness against childhood diarrhea. Water Res 47(3):1181–1190, PMID: , 10.1016/j.watres.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 12.Arnold BF, Colford JM Jr.. 2007. Treating water with chlorine at point-of-use to improve water quality and reduce child diarrhea in developing countries: a systematic review and meta-analysis. Am J Trop Med Hyg 76(2):354–364, PMID: , 10.4269/ajtmh.2007.76.354. [DOI] [PubMed] [Google Scholar]
- 13.Clasen TF, Alexander KT, Sinclair D, Boisson S, Peletz R, Chang HH, et al. . 2015. Interventions to improve water quality for preventing diarrhoea. Cochrane Database Syst Rev 2015(10):CD004794, PMID: , 10.1002/14651858.CD004794.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dreibelbis R, Winch PJ, Leontsini E, Hulland KR, Ram PK, Unicomb L, et al. . 2013. The Integrated Behavioural Model for Water, Sanitation, and Hygiene: a systematic review of behavioural models and a framework for designing and evaluating behaviour change interventions in infrastructure-restricted settings. BMC Public Health 13(1):1015, PMID: , 10.1186/1471-2458-13-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown J, Hayashi MAL, Eisenberg JNS. 2019. The critical role of compliance in delivering health gains from environmental health interventions. Am J Trop Med Hyg 100(4):777–779, PMID: , 10.4269/ajtmh.18-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiebelkorn AP, Person B, Quick RE, Vindigni SM, Jhung M, Bowen A, et al. . 2012. Systematic review of behavior change research on point-of-use water treatment interventions in countries categorized as low- to medium-development on the human development index. Soc Sci Med 75(4):622–633, PMID: , 10.1016/j.socscimed.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Reygadas F, Gruber JS, Dreizler L, Nelson KL, Ray I. 2018. Measuring user compliance and cost effectiveness of safe drinking water programs: a cluster-randomized study of household ultraviolet disinfection in rural Mexico. Am J Trop Med Hyg 98(3):824–834, PMID: , 10.4269/ajtmh.17-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parvez SM, Azad R, Rahman M, Unicomb L, Ram PK, Naser AM, et al. . 2018. Achieving optimal technology and behavioral uptake of single and combined interventions of water, sanitation hygiene and nutrition, in an efficacy trial (WASH benefits) in rural Bangladesh. Trials 19(1):358, PMID: , 10.1186/s13063-018-2710-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535, PMID: , 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolf J, Hunter PR, Freeman MC, Cumming O, Clasen T, Bartram J, et al. . 2018. Impact of drinking water, sanitation and handwashing with soap on childhood diarrhoeal disease: updated meta-analysis and meta-regression. Trop Med Int Health 23(5):508–525, PMID: , 10.1111/tmi.13051. [DOI] [PubMed] [Google Scholar]
- 21.World Bank. 2023. World Bank country and lending groups—country classification. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
- 22.Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, Colford JM Jr.. 2005. Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: a systematic review and meta-analysis. Lancet Infect Dis 5(1):42–52. [Database], PMID: , 10.1016/S1473-3099(04)01253-8. [DOI] [PubMed] [Google Scholar]
- 23.United Nations General Assembly. 2001. Road Map Towards the Implementation of the United Nations Millennium Declaration: Report of the Secretary-General. A/56/326. New York, NY: United Nations. [Google Scholar]
- 24.Burt Z, Njee RM, Mbatia Y, Msimbe V, Brown J, Clasen TF, et al. . 2017. User preferences and willingness to pay for safe drinking water: experimental evidence from rural Tanzania. Soc Sci Med 173:63–71, PMID: , 10.1016/j.socscimed.2016.11.031. [DOI] [PubMed] [Google Scholar]
- 25.Ray I. 2007. Women, water, and development. Annu Rev Environ Resour 32(1):421–449, 10.1146/annurev.energy.32.041806.143704. [DOI] [Google Scholar]
- 26.Loharikar A, Russo E, Sheth A, Menon M, Kudzala A, Tauzie B, et al. . 2013. Long-term impact of integration of household water treatment and hygiene promotion with antenatal services on maternal water treatment and hygiene practices in Malawi. Am J Trop Med Hyg 88(2):267–274, PMID: , 10.4269/ajtmh.2012.11-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matanock A, Anderson T, Ayers T, Likicho L, Wamimbi R, Lu X, et al. . 2016. Integrating water treatment into antenatal care: impact on use of maternal health services and household water treatment by mothers—rural Uganda, 2013. Am J Trop Med Hyg 94(5):1150–1156, PMID: , 10.4269/ajtmh.15-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheth AN, Russo ET, Menon M, Wannemuehler K, Weinger M, Kudzala AC, et al. . 2010. Impact of the integration of water treatment and handwashing incentives with antenatal services on hygiene practices of pregnant women in Malawi. Am J Trop Med Hyg 83(6):1315–1321, PMID: , 10.4269/ajtmh.2010.10-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lantagne DS, Clasen TF. 2012. Use of household water treatment and safe storage methods in acute emergency response: case study results from Nepal, Indonesia, Kenya, and Haiti. Environ Sci Technol 46(20):11352–11360, PMID: , 10.1021/es301842u. [DOI] [PubMed] [Google Scholar]
- 30.Lantagne D, Clasen T. 2013. Effective use of household water treatment and safe storage in response to the 2010 Haiti earthquake. Am J Trop Med Hyg 89(3):426–433, PMID: , 10.4269/ajtmh.13-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mong Y, Kaiser R, Ibrahim D, Rasoatiana , Razafimbololona L, Quick RE. 2001. Impact of the safe water system on water quality in cyclone-affected communities in Madagascar. Am J Public Health 91(10):1577–1579, PMID: , 10.2105/ajph.91.10.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van de Mortel TF. 2008. Faking it: social desirability response bias in self-report research. Aust J Adv Nurs 25:40–48.http://hdl.handle.net/10072/56416 [accessed 10 July 2021]. [Google Scholar]
- 33.Arnold BF, Khush RS, Ramaswamy P, Rajkumar P, Durairaj N, Ramaprabha P, et al. . 2015. Reactivity in rapidly collected hygiene and toilet spot check measurements: a cautionary note for longitudinal studies. Am J Trop Med Hyg 92(1):159–162, PMID: , 10.4269/ajtmh.14-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adair JG. 1984. The Hawthorne effect: a reconsideration of the methodological artifact. J Appl Psychol 69(2):334–345, 10.1037/0021-9010.69.2.334. [DOI] [Google Scholar]
- 35.Albert J, Luoto J, Levine D. 2010. End-user preferences for and performance of competing POU water treatment technologies among the rural poor of Kenya. Environ Sci Technol 44(12):4426–4432, PMID: , 10.1021/es1000566. [DOI] [PubMed] [Google Scholar]
- 36.Geremew A, Mengistie B, Mellor J, Lantagne DS, Alemayehu E, Sahilu G. 2019. Consistent point-of-use water chlorination among households using unimproved water sources and treatment preference in eastern Ethiopia. Int J Environ Health Res 29(6):686–701, PMID: , 10.1080/09603123.2019.1569210. [DOI] [PubMed] [Google Scholar]
- 37.Luby SP, Mendoza C, Keswick BH, Chiller TM, Hoekstra RM. 2008. Difficulties in bringing point-of-use water treatment to scale in rural Guatemala. Am J Trop Med Hyg 78(3):382–387, PMID: , 10.4269/ajtmh.2008.78.382. [DOI] [PubMed] [Google Scholar]
- 38.Chiller TM, Mendoza CE, Lopez MB, Alvarez M, Hoekstra RM, Keswick BH, et al. . 2006. Reducing diarrhoea in Guatemalan children: randomized controlled trial of flocculant–disinfectant for drinking-water. Bull World Health Organ 84(1):28–35, PMID: , 10.2471/blt.04.016980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.George CM, Jung DS, Saif-Ur-Rahman KM, Monira S, Sack DA, Rashid MU, et al. . 2016. Sustained uptake of a hospital-based handwashing with soap and water treatment intervention (Cholera-Hospital-Based Intervention for 7 days [CHoBI7]): a randomized controlled trial. Am J Trop Med Hyg 94(2):428–436, PMID: , 10.4269/ajtmh.15-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.George CM, Monira S, Sack DA, Rashid MU, Saif-Ur-Rahman KM, Mahmud T, et al. . 2016. Randomized controlled trial of hospital-based hygiene and water treatment intervention (CHoBI7) to reduce cholera. Emerg Infect Dis 22(2):233–241, PMID: , 10.3201/eid2202.151175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crump JA, Otieno PO, Slutsker L, Keswick BH, Rosen DH, Hoekstra RM, et al. . 2005. Household based treatment of drinking water with flocculant-disinfectant for preventing diarrhoea in areas with turbid source water in rural western Kenya: cluster randomised controlled trial. BMJ 331(7515):478, PMID: , 10.1136/bmj.38512.618681.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luoto J, Najnin N, Mahmud M, Albert J, Islam MS, Luby S, et al. . 2011. What point-of-use water treatment products do consumers use? Evidence from a randomized controlled trial among the urban poor in Bangladesh. PLoS One 6(10):e26132, PMID: , 10.1371/journal.pone.0026132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Potgieter N, Becker PJ, Ehlers MM. 2009. Evaluation of the CDC safe water-storage intervention to improve the microbiological quality of point-of-use drinking water in rural communities in South Africa. Water SA 35(4), 10.4314/wsa.v35i4.76810. [DOI] [Google Scholar]
- 44.Rangel JM, Lopez B, Mejia MA, Mendoza C, Luby S. 2003. A novel technology to improve drinking water quality: a microbiological evaluation of in-home flocculation and chlorination in rural Guatemala. J Water Health 1(1):15–22, PMID: , 10.2166/wh.2003.0003. [DOI] [PubMed] [Google Scholar]
- 45.Reller ME, Mendoza CE, Lopez MB, Alvarez M, Hoekstra RM, Olson CA, et al. . 2003. A randomized controlled trial of household-based flocculant–disinfectant drinking water treatment for diarrhea prevention in rural Guatemala. Am J Trop Med Hyg 69(4):411–419, PMID: , 10.4269/ajtmh.2003.69.411. [DOI] [PubMed] [Google Scholar]
- 46.Shaheed A, Rathore S, Bastable A, Bruce J, Cairncross S, Brown J. 2018. Adherence to point-of-use water treatment over short-term implementation: parallel crossover trials of flocculation–disinfection sachets in Pakistan and Zambia. Environ Sci Technol 52(11):6601–6609, PMID: , 10.1021/acs.est.8b00167. [DOI] [PubMed] [Google Scholar]
- 47.Sobsey MD, Handzel T, Venczel L. 2003. Chlorination and safe storage of household drinking water in developing countries to reduce waterborne disease. Water Sci Technol 47(3):221–228, PMID: , 10.2166/wst.2003.0199. [DOI] [PubMed] [Google Scholar]
- 48.Opryszko MC, Majeed SW, Hansen PM, Myers JA, Baba D, Thompson RE, et al. . 2010. Water and hygiene interventions to reduce diarrhoea in rural Afghanistan: a randomized controlled study. J Water Health 8(4):687–702, PMID: , 10.2166/wh.2010.121. [DOI] [PubMed] [Google Scholar]
- 49.Quick RE, Venczel LV, González O, Mintz ED, Highsmith AK, Espada A, et al. . 1996. Narrow-mouthed water storage vessels and in situ chlorination in a Bolivian community: a simple method to improve drinking water quality. Am J Trop Med Hyg 54(5):511–516, PMID: , 10.4269/ajtmh.1996.54.511. [DOI] [PubMed] [Google Scholar]
- 50.Quick RE, Kimura A, Thevos A, Tembo M, Shamputa I, Hutwagner L, et al. . 2002. Diarrhea prevention through household-level water disinfection and safe storage in Zambia. Am J Trop Med Hyg 66(5):584–589, PMID: , 10.4269/ajtmh.2002.66.584. [DOI] [PubMed] [Google Scholar]
- 51.Quick RE, Venczel LV, Mintz ED, Soleto L, Aparicio J, Gironaz M, et al. . 1999. Diarrhoea prevention in Bolivia through point-of-use water treatment and safe storage: a promising new strategy. Epidemiol Infect 122(1):83–90, PMID: , 10.1017/s0950268898001782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sugar NR, Schilling KA, Kim S, Ahmed A, Ngui Muyanga D, Sivapalasingam S, et al. . 2017. Integrating household water treatment, hand washing, and insecticide-treated bed nets into pediatric HIV care in Mombasa, Kenya: impact on diarrhea and malaria risk. J Acquir Immune Defic Syndr 76(3):266–272, PMID: , 10.1097/QAI.0000000000001520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mellor JE, Kallman E, Oyanedel-Craver V, Smith JA. 2015. Comparison of three household water treatment technologies in San Mateo Ixtatán, Guatemala. J Environ Eng 141(5):04014085, 10.1061/(ASCE)EE.1943-7870.0000914. [DOI] [Google Scholar]
- 54.Clasen T, Saeed TF, Edmondson P, Boisson S, Shipin O. 2007. Household water treatment using sodium dichloroisocy anurate (NaDCC) tablets: a randomized, controlled trial to assess microbiological effectiveness in Bangladesh. Am J Trop Med Hyg 76(1):187–192, PMID: , 10.4269/ajtmh.2007.76.187. [DOI] [PubMed] [Google Scholar]
- 55.Doocy S, Burnham G. 2006. Point-of-use water treatment and diarrhoea reduction in the emergency context: an effectiveness trial in Liberia. Trop Med Int Health 11(10):1542–1552, PMID: , 10.1111/j.1365-3156.2006.01704.x. [DOI] [PubMed] [Google Scholar]
- 56.Altmann M, Altare C, van der Spek N, Barbiche JC, Dodos J, Bechir M, et al. . 2018. Effectiveness of a household water, sanitation and hygiene package on an outpatient program for severe acute malnutrition: a pragmatic cluster-randomized controlled trial in Chad. Am J Trop Med Hyg 98(4):1005–1012, PMID: , 10.4269/ajtmh.17-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murray AL, Napotnik JA, Rayner JS, Mendoza A, Mitro B, Norville J, et al. . 2020. Evaluation of consistent use, barriers to use, and microbiological effectiveness of three prototype household water treatment technologies in Haiti, Kenya, and Nicaragua. Sci Total Environ 718:134685, PMID: , 10.1016/j.scitotenv.2019.134685. [DOI] [PubMed] [Google Scholar]
- 58.Blanton E, Ombeki S, Oluoch GO, Mwaki A, Wannemuehler K, Quick R. 2010. Evaluation of the role of school children in the promotion of point-of-use water treatment and handwashing in schools and households—Nyanza province, western Kenya, 2007. Am J Trop Med Hyg 82(4):664–671, PMID: , 10.4269/ajtmh.2010.09-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pickering AJ, Crider Y, Amin N, Bauza V, Unicomb L, Davis J, et al. . 2015. Differences in field effectiveness and adoption between a novel automated chlorination system and household manual chlorination of drinking water in Dhaka, Bangladesh: a randomized controlled trial. PLoS One 10(3):e0118397, PMID: , 10.1371/journal.pone.0118397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jain S, Sahanoon OK, Blanton E, Schmitz A, Wannemuehler KA, Hoekstra RM, et al. . 2010. Sodium dichloroisocyanurate tablets for routine treatment of household drinking water in periurban Ghana: a randomized controlled trial. Am J Trop Med Hyg 82(1):16–22, PMID: , 10.4269/ajtmh.2010.08-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Colindres RE, Jain S, Bowen A, Mintz E, Domond P. 2007. After the flood: an evaluation of in-home drinking water treatment with combined flocculent-disinfectant following Tropical Storm Jeanne—Gonaives, Haiti, 2004. J Water Health 5(3):367–374, PMID: , 10.2166/wh.2007.032. [DOI] [PubMed] [Google Scholar]
- 62.Boisson S, Stevenson M, Shapiro L, Kumar V, Singh LP, Ward D, et al. . 2013. Effect of household-based drinking water chlorination on diarrhoea among children under five in Orissa, India: a double-blind randomised placebo-controlled trial. PLoS Med 10(8):e1001497, PMID: , 10.1371/journal.pmed.1001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsai FJ, Wu M, Lin CP. 2020. Does a free-trial approach increase purchase and use of a household water treatment and safe storage product in rural Haiti? Am J Trop Med Hyg 102(3):518–525, PMID: , 10.4269/ajtmh.19-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Norton DM, Rahman M, Shane AL, Hossain Z, Kulick RM, Bhuiyan MI, et al. . 2009. Flocculant–disinfectant point-of-use water treatment for reducing arsenic exposure in rural Bangladesh. Int J Environ Health Res 19(1):17–29, PMID: , 10.1080/09603120802272219. [DOI] [PubMed] [Google Scholar]
- 65.World Health Organization. 2017. Guidelines for Drinking Water Quality: Fourth Edition Incorporating the First Addendum. Geneva, Switzerland: World Health Organization. [PubMed] [Google Scholar]
- 66.Sphere Association. 2018. The Sphere Handbook: Humanitarian Charter and Minimum Standards in Humanitarian Response. 4th ed. Geneva, Switzerland: Sphere Association. https://spherestandards.org/wp-content/uploads/Sphere-Handbook-2018-EN.pdf [accessed 10 July 2021]. [Google Scholar]
- 67.Ali SI, Ali SS, Fesselet JF. 2021. Evidence-based chlorination targets for household water safety in humanitarian settings: recommendations from a multi-site study in refugee camps in South Sudan, Jordan, and Rwanda. Water Res 189:116642, PMID: , 10.1016/j.watres.2020.116642. [DOI] [PubMed] [Google Scholar]
- 68.Thaler RH, Sunstein CR. 2009. Nudge: Improving Decisions about Health, Wealth and Happiness. New York, NY: Penguin Books. [Google Scholar]
- 69.Pickering AJ, Null C, Winch PJ, Mangwadu G, Arnold BF, Prendergast AJ, et al. . 2019. The WASH benefits and SHINE trials: interpretation of WASH intervention effects on linear growth and diarrhoea. Lancet Glob Health 7(8):e1139–e1146, PMID: , 10.1016/S2214-109X(19)30268-2. [DOI] [PubMed] [Google Scholar]
- 70.Clasen T, Edmondson P. 2006. Sodium dichloroisocyanurate (NaDCC) tablets as an alternative to sodium hypochlorite for the routine treatment of drinking water at the household level. Int J Hyg Environ Health 209(2):173–181, PMID: , 10.1016/j.ijheh.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 71.Najnin N, Leder K, Qadri F, Forbes A, Unicomb L, Winch PJ, et al. . 2017. Impact of adding hand-washing and water disinfection promotion to oral cholera vaccination on diarrhoea-associated hospitalization in Dhaka, Bangladesh: evidence from a cluster randomized control trial. Int J Epidemiol 46(6):2056–2066, PMID: , 10.1093/ije/dyx187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pickering AJ, Crider Y, Sultana S, Swarthout J, Goddard FG, Anjerul Islam S, et al. . 2019. Effect of in-line drinking water chlorination at the point of collection on child diarrhoea in urban Bangladesh: a double-blind, cluster-randomised controlled trial. Lancet Glob Health 7(9):e1247–e1256, PMID: , 10.1016/S2214-109X(19)30315-8. [DOI] [PubMed] [Google Scholar]
- 73.Dupas P. 2014. Getting essential health products to their end users: subsidize, but how much? Science 345(6202):1279–1281, PMID: , 10.1126/science.1256973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harshfield E, Lantagne D, Turbes A, Null C. 2012. Evaluating the sustained health impact of household chlorination of drinking water in rural Haiti. Am J Trop Med Hyg 87(5):786–795, PMID: , 10.4269/ajtmh.2012.12-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Evans DK, Jakiela P, Knauer HA. 2021. The impact of early childhood interventions on mothers. Science 372(6544):794–796, PMID: , 10.1126/science.abg0132. [DOI] [PubMed] [Google Scholar]
- 76.Mullainathan S, Shafir E. 2013. Scarcity: Why Having Too Little Means So Much. New York, NY: Henry Holt and Co. [Google Scholar]
- 77.Ray I, Smith KR. 2021. Towards safe drinking water and clean cooking for all. Lancet Glob Health 9(3):e361–e365, PMID: , 10.1016/S2214-109X(20)30476-9. [DOI] [PubMed] [Google Scholar]
- 78.Bertrand M, Mullainathan S, Shafir E. 2006. Behavioral economics and marketing in aid of decision making among the poor. J Public Policy Mark 25(1):8–23, 10.1509/jppm.25.1.8. [DOI] [Google Scholar]
- 79.Rosa G, Miller L, Clasen T. 2010. Microbiological effectiveness of disinfecting water by boiling in rural Guatemala. Am J Trop Med Hyg 82(3):473–477, PMID: , 10.4269/ajtmh.2010.09-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rainey RC, Harding AK. 2005. Acceptability of solar disinfection of drinking water treatment in Kathmandu Valley, Nepal. Int J Environ Health Res 15(5):361–372, PMID: , 10.1080/09603120500289168. [DOI] [PubMed] [Google Scholar]
- 81.Pérez-Vidal A, Diaz-Gómez J, Castellanos-Rozo J, Usaquen-Perilla OL. 2016. Long-term evaluation of the performance of four point-of-use water filters. Water Res 98:176–182, PMID: , 10.1016/j.watres.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 82.Luby S, Agboatwalla M, Raza A, Sobel J, Mintz E, Baier K, et al. . 2001. A low-cost intervention for cleaner drinking water in Karachi, Pakistan. Int J Infect Dis 5(3):144–150, PMID: , 10.1016/S1201-9712(01)90089-X. [DOI] [PubMed] [Google Scholar]
- 83.Macy JT, Quick RE. 1998. Evaluation of a novel drinking water treatment and storage intervention in Nicaragua. Rev Panam Salud Publica 3(2):135–136, PMID: , 10.1590/s1020-49891998000200017. [DOI] [PubMed] [Google Scholar]
- 84.Mengistie B, Berhane Y, Worku A. 2013. Household water chlorination reduces incidence of diarrhea among under-five children in rural Ethiopia: a cluster randomized controlled trial. PLoS One 8(10):e77887, PMID: , 10.1371/journal.pone.0077887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Solomon ET, Robele S, Kloos H, Mengistie B. 2020. Effect of household water treatment with chlorine on diarrhea among children under the age of five years in rural areas of Dire Dawa, eastern Ethiopia: a cluster randomized controlled trial. Infect Dis Poverty 9(1):64, PMID: , 10.1186/s40249-020-00680-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ercumen A, Naser AM, Unicomb L, Arnold BF, Colford JM Jr, Luby SP. 2015. Effects of source- versus household contamination of tubewell water on child diarrhea in rural Bangladesh: a randomized controlled trial. PLoS One 10(3):e0121907, PMID: , 10.1371/journal.pone.0121907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.