At the dawn of the global severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) health crisis, practitioners observed anxiously and tried to comprehend how this new virus would affect patients. Within 1 month of the first cases reported in Wuhan, China, we began to understand that coronavirus disease-2019 (COVID-19) could extend beyond the pulmonary system and in many cases led to acute cardiac injury characterized by vascular inflammation, myocarditis, and arrhythmia (1,2). To make matters worse, the evidence that followed suggested that patients with underlying cardiovascular disease were at a higher risk for severe COVID-19 and death (3). Those of us in the adult congenital heart disease (ACHD) community became worried for our patients; this group had already overcome incredible medical, surgical, and psychosocial burdens due to the presence of congenital heart disease (CHD) in their young lives (4).
In the past year, we have relied on small studies (5-7) and inferred the potential risk of COVID-19 in ACHD (8,9), often hoping we were correct based upon minimal collective and anecdotal individual experience with the disease. It is therefore timely, that in this issue of the Journal, Broberg et al. (10) offer us a sizeable retrospective study of COVID-19 outcomes in 1,044 patients with ACHD from 58 centers in North America, South America, Europe, and Western Asia. The overarching goal of this study was to determine if there were risk factors associated with: 1) increased mortality in patients with ACHD with COVID-19; and 2) severe infection, as defined by the need for intensive care unit (ICU) admission, endotracheal intubation, acute respiratory distress syndrome, and/or renal replacement therapy.
The investigators should be commended for enrolling a robust number of patients with COVID-19 and ACHD. In total, 907 (87%) of the patients included in the study had laboratory confirmation of COVID-19 (polymerase chain reaction or antibody positivity). One of the challenges in outcomes-based research in ACHD is accounting for significant anatomic heterogeneity, which can complicate the likelihood that outcomes are truly accurate across the disease spectrum. To overcome this, the investigators classified CHD anatomy according to the single most complex heart defect present and counted each entry only once, according to the most recent U.S. guidelines: simple, moderate, or severely complex disease (11). Patients were also sub-stratified based upon their underlying physiological stage (A, B, C, D), consistent with recent guideline recommendations, to assess the functional impact of CHD (11). Finally, a third level of analysis was performed on age-based tertiles (18 to 27 years, 28 to 40 years, 41 years or older) in an attempt to determine if age itself affected COVID-19 outcomes in the population with ACHD.
In total, there were 179 (17%) hospital admissions (ICU in 67 [6.4%]) and 24 COVID-19–related inpatient deaths, resulting in an overall case/fatality ratio (in those tested) of 2.5% (95% confidence interval: 1.5% to 3.6%), which was similar to what was reported in non-ACHD cohorts (12). Within the mortality group, nearly one-half were cyanotic and approximately 17% had either pulmonary arterial hypertension (PAH) without cyanosis or a previous heart failure admission. Interestingly, a substantial number (n = 7; 30%) of patients who died had lesions of simple anatomic complexity, yet as stated by the investigators, remained at high risk for severe COVID-19 due to non-CHD causes, including diabetes, obesity, and older age. There were no deaths in patients with simple lesions and physiological stage A and/or B disease, implying that those who died with simple lesions also had advanced physiological stage (C and/or D). Therefore, it seems important to underscore that when evaluating the potential risk of a patient with ACHD for severe COVID-19, we cannot neglect non-ACHD–based risk factors because they may contribute to more severe COVID-19 and death, especially when combined with advanced physiological stage, as was seen in this study.
Arguably, one of the most important findings of this study was that anatomic complexity in and of itself did not portend a worse prognosis with COVID-19; however, it must be acknowledged that historical advances in care likely affected the current age-based heterogeneity in this group and perhaps confounded this outcome. Instead, a critical part of the story unfolded–patients with advanced physiological stages (C and D) had the highest risk of death with COVID-19 (3.9% and 7.9% vs. 0% and 1.1% in stages A and B, respectively). In particular, ACHD-specific risk factors that were found to be associated with severe COVID-19 and death included cyanosis, resting hypoxia, atrial arrhythmia, renal (end-organ) dysfunction, and clinically important heart failure (admissions). These clinical features are routinely assessed in assigning physiological stage, and therefore, begin to identify patients with ACHD who are at elevated risk for severe COVID-19, perhaps strictly due to their underlying CHD (so-called ACHD-specific risk factors). This is increasingly important as vaccination strategies evolve and priorities to protect those at high risk begin to emerge.
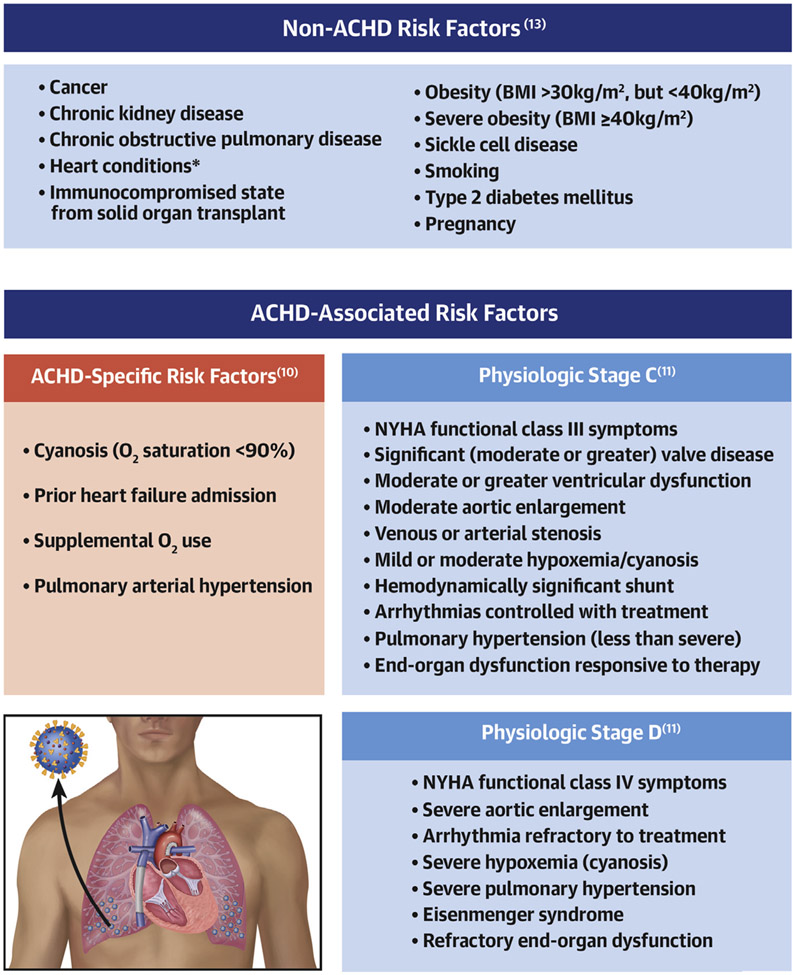
In the United States, the Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practice (ACIP) have made allocation recommendations for COVID-19 vaccine distribution (13). The recommendation prioritizes people with a high risk for exposure to COVID-19, such as health care personnel and long-term care facility residents, as well as those at high risk for severe disease, predominantly older age groups. However, the phase recommendation system (1a, 1b, 1c, 2) has not been clear on exactly what cardiovascular factors should be assessed to estimate individual risk for severe COVID-19. In particular, this comes into play in the phase 1c category, where recommendations are made for “persons aged 16-64 years with high-risk medical conditions.” In the original recommendation, the following conditions increase the risk for severe COVID-19: “cancer, chronic kidney disease, chronic obstructive pulmonary disease, heart conditions such as heart failure, coronary artery disease, or cardiomyopathies; immunocompromised state (weakened immune system) from solid organ transplant; obesity (body mass index [BMI] > 30 kg/m2 but < 40 kg/m2); severe obesity (BMI ≥ 40 kg/m2); sickle cell disease, smoking; type 2 diabetes mellitus; and pregnancy” (13). The issue of vaccine allocation is further complicated by state and local practice, which may not directly follow recommendations set forth by the CDC, and in many cases reference “congenital conditions” as potentially eligible for early vaccination.
It is likely intentional that the ACIP made broad recommendations for persons with underlying heart conditions, because this term encompasses a wide spectrum of disease and varying physiological and functional impact. However, as a community of ACHD practitioners, the “can has been kicked” to us, to help determine exactly which patients might be at risk for severe COVID-19. Although the investigators (10) did not set out to determine which patients with ACHD might benefit from early vaccination, this is one of the foremost questions in our minds as we aim to protect high-risk groups in an ethically responsible and data-driven way. The data presented highlight a few important points as we consider how to risk stratify patients with ACHD during the SARS-CoV-2 pandemic: 1) we cannot forget about non-ACHD COVID-19 risk factors, such as diabetes, age, and body mass index, because a sizeable group of patients with ACHD with simple CHD and advanced physiological class have succumbed to COVID-19 with these risk factors; and 2) we need to begin assessing ACHD-specific risk factors outlined by the investigators (10) that appear to be associated with severe COVID-19, including cyanosis, history of clinically important heart failure, resting hypoxia, advanced physiological stage, and PAH (Figure 1). Because of the relative association of these factors with case fatality, it may be reasonable to consider prioritizing patients with ACHD with ≥1 ACHD-specific severe COVID-19 risk factor. In the United States, in particular, this would clarify eligibility for a subset of patients with ACHD in phase 1c of the ACIP recommendation. However, as the pandemic persists, scientific data continues to evolve, and our understanding of this new disease in special populations becomes more informed. We can likely look forward to additional analyses of this cohort, as well as that from the EPOCH (European Collaboration for Prospective Outcome Research in Congenital Heart Disease) COVID-19 tracking project (9), both of which will continue to shape how we assess risk, therapeutically manage, and protect patients with ACHD from COVID-19.
FIGURE 1. Risk Factors for Severe COVID-19 in Patients With ACHD.
To assess risk factors for severe coronavirus disease-2019 (COVID-19) in adults with congenital heart disease (ACHD), it is important to evaluate non-ACHD risk factors as set forth by organizations such as the United States Centers for Disease Control and Prevention, as well as ACHD-associated risk factors (i.e., physiological stages C and D). In this study, new ACHD-specific risk factors (red box) are identified and should be considered when performing an overall assessment of individual risk for severe disease. *Heart conditions such as heart failure, coronary artery disease, or cardiomyopathies. BMI = body mass index; NYHA = New York Heart Association; O2 = oxygen.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
Dr. Bradley is supported by National Institutes of Health (grant HL148701). Dr. Cavus has reported that he has no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Editorials published in the Journal of the American College of Cardiology reflect the views of the authors and do not necessarily represent the views of JACC or the American College of Cardiology.
REFERENCES
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol 2020;5:831–40. [DOI] [PubMed] [Google Scholar]
- 3.Peng YD, Meng K, Guan HQ, et al. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV [in Chinese]. Zhonghua Xin Xue Guan Bing Za Zhi 2020;48:450–5. [DOI] [PubMed] [Google Scholar]
- 4.Rehan R, Kotchetkova I, Cordina R, Celermajer D. Adult congenital heart disease survivors at age 50 years: medical and psychosocial status. Heart Lung Circ 2021;30:261–6. [DOI] [PubMed] [Google Scholar]
- 5.Lewis MJ, Anderson BR, Fremed M, et al. Impact of coronavirus disease 2019 (COVID-19) on patients with congenital heart disease across the lifespan: the experience of an academic congenital heart disease center in New York City. J Am Heart Assoc 2020;9:e017580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohammadzadeh S, Mehrakizadeh A, Safari S, et al. Lessons Learnt from COVID-19 in adult congenital heart patient in Tehran: a survey-based study of prevention, exposure, susceptibility, and outcomes. Cardiol Young 2020:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabatino J, Ferrero P, Chessa M, et al. COVID-19 and congenital heart disease: results from a nationwide survey. J Clin Med 2020;9:1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radke RM, Frenzel T, Baumgartner H, Diller GP. Adult congenital heart disease and the COVID-19 pandemic. Heart (British Cardiac Society) 2020;106:1302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diller GP, Gatzoulis MA, Broberg CS, et al. Coronavirus disease 2019 in adults with congenital heart disease: a position paper from the ESC working group of adult congenital heart disease, and the International Society for Adult Congenital Heart Disease. Eur Heart J 2020. Dec. 12 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broberg CS, Kovacs AH, Sadeghi S, et al. COVID-19 in adults with congenital heart disease. J Am Coll Cardiol 2021;77:1644–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC Guideline for the management of adults with congenital heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139:e637–97. [DOI] [PubMed] [Google Scholar]
- 12.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020;20:533–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dooling K, Marin M, Wallace M, et al. The advisory committee on immuniqation practices’ updated interim recommendation for the allocation of COVID-19 vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep 2021;69:1657–60. [DOI] [PMC free article] [PubMed] [Google Scholar]